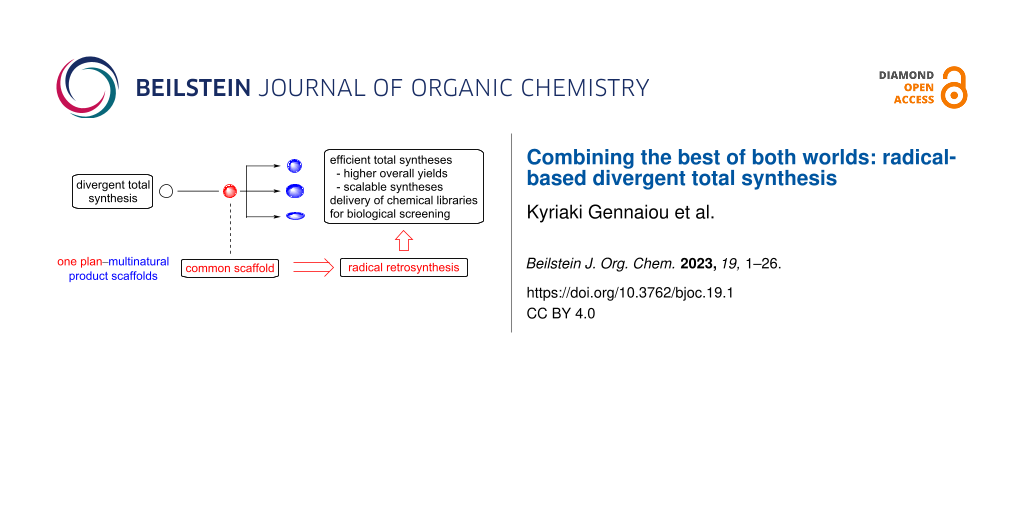
Abstract
A mature science, combining the art of the total synthesis of complex natural structures and the practicality of delivering highly diverged lead compounds for biological screening, is the constant aim of the organic chemistry community. Delivering natural lead compounds became easier during the last two decades, with the evolution of green chemistry and the concepts of atom economy and protecting-group-free synthesis dominating the field of total synthesis. In this new era, total synthesis is moving towards natural efficacy by utilizing both the biosynthetic knowledge of divergent synthesis and the latest developments in radical chemistry. This contemporary review highlights recent total syntheses that incorporate the best of both worlds.
Graphical Abstract
Introduction
Societal needs push sciences into new directions, as the urge for new pharmaceutical leads grows, in order to counteract global health challenges. Following this trend, total synthesis has been remodeled from the purely academic quest and display of human abilities to synthetically achieve natural complexity [1] to a modern science addressing the need for the supply of natural products and congeners for biological screening.
The era of scalability [2] in total synthesis prompts researchers in this field to make use of more direct retrosynthetic disconnections with the aid of “radical” retrosynthetic analysis, as the advancement in the area now allows to harness one-electron power in a highly chemoselective manner [3]. The development of persistent radicals [4] as synthons in chemical synthesis, coupled with the advancements in generating and manipulating transient radicals [5] as cross-coupling partners in an array of chemical reactions, gives access to a wide variety of “new” retrosynthetic disconnections. As radical disconnections are gaining ground, more sophisticated retrosyntheses of natural products are unlocked, enriching thus their synthetic scalability [6,7]. A direct comparison of a classic vs a radical approach highlights the complementarity and, more often than not, the superiority of the latter, which is proven in the number of steps and the overall yield, hence establishing it as highly appealing for further development (Scheme 1).
Scheme 1: The power of radical retrosynthesis and the tactic of divergent total synthesis.
Scheme 1: The power of radical retrosynthesis and the tactic of divergent total synthesis.
In order to identify the new pharmaceutical leads of tomorrow, drug discovery relies on the available chemical space rising from existing chemical libraries. But how big should this chemical space be, so as to actually address our needs? A general consensus has emerged, supporting that “it is not actually the library size but rather the library diversity in terms of molecular structure and function which is fundamental for a successful drug discovery” [8]. And this is where divergent total synthesis might help. Divergent total synthesis is an old but yet underdeveloped strategy, utilizing the conceptual advantages of biosynthetic routes that allow multi-target natural product synthesis through a unified synthetic plan [9,10]. Based on the logic of divergent synthesis, common synthetic scaffolds, which are regarded as the points of diversity of the synthetic plan, lie at the heart of retrosynthetic design. Radical disconnections on common scaffolds, in accordance to the trends of green chemistry [11] and the concepts of atom economy [12] and protecting-group-free synthesis [13], are gradually drawing more and more the interest of organic chemists as a sustainable way to deliver structurally diverse chemical libraries for biological screening. The current review is focusing on selected examples utilizing a radical-based divergent total synthesis approach, excluding electrochemical methods for generating radicals. An exhaustive review on radical total synthesis or divergent total synthesis lies beyond the scope of this review, and the readers are advised to refer to excellent reviews on these topics [6,10,14]. This review covers the years 2018–2022.
Review
Radical-based divergent synthesis
Commonly, the most successful divergent plans apply where the natural molecular complexity is rich. Not surprisingly, most of the divergent total syntheses carried out thus far are performed on terpenoid and alkaloid targets, utilizing common synthetic intermediates closely related to the biosynthetic origins of the family. On the other hand, radical retrosynthetic disconnections on common scaffolds are much less predictable and rarely similar due to the plethora of radical chemical transformations available nowadays.
Although radicals stopped being confronted as “scientific curiosities” in the late 1960s, when radical initiators and organomercury reagents were developed as reliable reaction partners (Giese reaction) [15], it was not until the mid-1980s, at which point they appeared as key reaction players in total synthesis (Figure 1). The change in the perception that radicals cannot be selectively used took place with the introduction of tin hydrides in organic synthesis. Apart from the lower toxicity compared to organomercury reagents, the stability and longevity of tin-centered radicals allowed better propagation of radical chain reactions [16]. Based on their reactivity, major contributions in carbon-centered radical formation followed, consequently unlocking highly predictable intramolecular reactions, deoxygenation protocols (Barton–McCombie reaction) [17], etc. Other reagents that majorly contributed were samarium diiodide for the generation of radicals from carbonyl reduction [18] but also manganese(III) acetate as a convenient one-electron oxidant [19]. The next twenty years, the field continued to flourish mainly by way of the decipherment of hydrogen atom transfer (HAT) mechanisms, which led to the establishment of several reactions of transition metal hydrides (Fe, Co, Mn, etc) with alkenes (e.g., Mukaiyama hydration) [20].
Figure 1: Evolution of radical chemistry for organic synthesis.
Figure 1: Evolution of radical chemistry for organic synthesis.
The last decade saw the development of milder methods for generating carbon-centered radicals as the advancement of their reactivity in cross-coupling reactions, the concept of photoredox catalysis [21], and electrochemistry [22] all refuelled the field, allowing for more practical radical disconnections for total synthesis.
Divergent synthesis of pyrone diterpenes
(Baran 2018) [23]: The modestly sized family of pyrone diterpenes exhibits a wide range of bioactivities, ranging from immunosuppressive to hypertensive properties [24-26] depending on the subtle substituents in the periphery of a decalin core (Scheme 2). In 2018, the Baran group reported the divergent total synthesis of several pyrone diterpene natural products, relying solely on one-electron-based retrosynthesis. The group recognized the disadvantages that stemmed from prior 2-electron disconnections, namely the complicated C–C bond formations and the necessity for excessive functional group manipulations but also the unavailability of a unified divergent plan for this class of diterpenoids. As an alternative, they proposed nickel-mediated decarboxylative Giese reactions and decarboxylative radical zinc-mediated cross-coupling reactions of redox-active esters, established from previous works of the group [27,28], for the key C–C bonds of the diverse congeners. To this end, a hypothetical intermediate 3 was envisioned for their synthesis (Scheme 2). The synthetic variant of 3 was designed as the common scaffold 16, bearing the appropriate substitution for sequential revelation of carboxylic acid moieties. The highly congested decalin core of common scaffold 16 was obtained by a modified electrochemical polycyclization of polyene 14 (prepared in two steps) in multigram quantities [23]. The reaction employed a divided cell with substoichiometric amounts of magnesium(II) acetate (0.5 equiv) and catalytic copper(II) 3,5-diisopropylsalicylate (0.02 equiv) to allow the redox radical cyclization of polyene in 42% yield. A Tsuji allylation using achiral H-PHOX followed to produce 16, without being isolated. From this point of divergence, Baran’s group managed to reveal the requisite phthalimide carboxylates for each precursor of the diverse natural products and transformed it carrying out Giese reactions or nickel-catalyzed radical coupling to 13, 20, 25, and 28, few steps away from the total syntheses of sesquicillin A (18), subglutinols A and B (19 and 24) and higginsianin A (23, Scheme 2).
Scheme 2: Divergent total synthesis of α-pyrone-diterpenoids (Baran).
Scheme 2: Divergent total synthesis of α-pyrone-diterpenoids (Baran).
Merged chemoenzymatic and radical synthesis of oxidized pyrone meroterpenoids
(Renata 2020) [29]: In 2020, a different approach was conceptualized by Renata’s group to access various oxidized members of pyrone meroterpenoids. The divergent plan of Renata’s group depended on the development of a highly chemoselective, chemoenzymatic 3-hydroxylation of sclareolide (29) and (−)-sclareol (43, Scheme 3 and Scheme 4). The group began by conducting a brief survey of several P450 BM3 mutants, deducing that the variant 1857 V328A (BM3 MERO1) was able to achieve high conversion of sclareolide (29) to the hydroxylated counterpart 30 in >95% yield. Based on this success, the group employed a radical disconnection approach of several 3-hydroxylated pyrone meroterpenoids on sclareolide (29). Key reaction of this strategy was the formal [3 + 3] cycloaddition, catalyzed by phosphoric acid 33, followed by addition of a pyrone residue 32 to sclareolide-derived aldehyde 31, which served as the common synthetic intermediate for the synthesis (Scheme 3) [30]. HAT reductions of the C9–C11 alkene followed to deliver arisugacin F (35), phenylpyropene C (36), pyripyropene E (38), and phenylpyropene F (41). The steric bulk of the manganese catalyst employed suppressed the undesired reaction with tetrasubstituted alkenes and led to the exclusive reaction of the desired trisubstituted alkene due to stabilization of the incipient radical at C9. Furthermore, HAT reduction served to only deliver the thermodynamic product of the trans-decalin. Similarly, the C9–C11 alkene can serve as an ideal handle to C11-hydroxylated products, such as 42, through a Mukaiyama hydration [20] to furnish natural complexity.
Scheme 3: Divergent synthesis of pyrone diterpenoids by merged chemoenzymatic and radical synthesis (part I, Renata).
Scheme 3: Divergent synthesis of pyrone diterpenoids by merged chemoenzymatic and radical synthesis (part I, ...
Scheme 4: Divergent synthesis of pyrone diterpenoids by merged chemoenzymatic and radical synthesis (part II, Renata).
Scheme 4: Divergent synthesis of pyrone diterpenoids by merged chemoenzymatic and radical synthesis (part II,...
A similar approach was devised for the synthesis of modified meroterpenoids chevalone A (52), taondiol (53), decaturin E (54), and stypodiol (57, Scheme 4). For this purpose, tricycle 45 was prepared from compound 44 in gram-scale quantities by HAT–Giese coupling, followed by reductive cleavage of the lactone moiety with LiI. Enzymatic hydroxylation by the BM3 MERO1 variant worked equally well to provide the 3-hydroxylated product 46. Photochemical radical decarboxylation of the formed mercaptopyridine derivative and radical capture by iodoform led to common scaffolds 47 and diene 48 after subsequent elimination. Those molecules serve as templates for Ni-based radical-based sp3–sp2 coupling and single-electron transfer (SET)-based [3 + 2] coupling, respectively (Scheme 4). Initial attempts to realize the [3 + 2] radical coupling with CAN led to competitive oxidation of the C3 alcohol to the respective ketone. Increasing the equivalents of pyrone led to 83% of 54. On the other hand, employment of the same conditions to phenol 55 resulted only in the oxidation of the phenol. A more controlled delivery of electrons was realized by applying an electrochemical method to provide the desired coupling towards 56 in 62% yield. Radical reduction by Mn(dpm)3 afforded stypodiol (57) after BBr3-mediated deprotection. Nickel-catalyzed coupling under a Weix procedure [31] was selected in order to elaborate the cores of taondiol (53) and chevalone A (52) as the radical cross-coupling employing redox-active esters of carboxylic acid 46 proved unsuccessful. The coupling was followed by an acid-catalyzed cyclization to yield the pyrone core of the natural products. The divergent plan described provided various meroterpenoids in 7–12 steps, comprising one of the most concise methods to attain this class of compounds, highlighting the power of merged biocatalytic and radical tactics [32].
(+)-Yahazunol (61) and related meroterpenoids
(Li 2018) [33]: In 2018, Li’s group reported a divergent plan for the synthesis of drimane-type hydroquinone meroterpenoids. This class of compounds possesses versatile bioactivities, ranging from anticancer and anti-HIV to antifungal properties, with minor modifications on the decoration of either the hydroquinone or the terpene part of the secondary metabolite [34]. The group applied a semisynthetic plan starting from (−)-sclareol (43) to access the common synthetic intermediate of (+)-yahazunol (61), inspired by prior work of Baran’s group on divergent synthesis of meroterpenoids utilizing boronosclareolide (Scheme 5) [35]. Li’s group instead utilized compound 58, readily available by the oxidative degradation of (−)-sclareol (43) [36] as the precursor to a photolabile Barton ester 59. When the latter was irradiated at 250 W in the presence of benzoquinone, a decarboxylated coupling occurred, yielding semiquinone 60, few steps away from the common scaffold 61. Following this protocol, researchers managed to synthesize more than 4 g of the natural product (+)-yahazunol (61). (+)-Yahazunol (61) can be readily transformed to several members of meroterpenoids of the class, either by Friedel−Crafts reactions or common oxidative manipulations.
Scheme 5: Divergent synthesis of drimane-type hydroquinone meroterpenoids (Li).
Scheme 5: Divergent synthesis of drimane-type hydroquinone meroterpenoids (Li).
Total synthesis of dysideanone B (75) and dysiherbol A (79)
(Lu 2021) [37]: Dysideanone B (75), isolated from the South China Sea sponge Dysidea avara, possesses an unprecedented 6/6/6/6-fused tetracycle with interesting anticancer properties against HeLa and HepG2 cancer cell lines (Scheme 6) [38]. The structurally similar dysiherbols 79 and 80, bearing a 6/6/5/6-fused tetracycle instead, were reported to possess NF-kB-inhibitory activity and anticancer activity against NCI H-929 cancer cell lines (Scheme 6) [39]. In 2021 Lu’s group reported the total synthesis of members of both meroterpenoid families based on a highly chemoselective α-alkylation in the thermodynamic position of a Wieland−Miescher ketone derivative 68 with benzyl bromide 69. Despite the challenging O- and C7-alkylations that required suppression, the desired C9-alkylation was achieved in 72% yield under thermodynamically controlled conditions (t-BuOK in THF at −40 °C). This coupled the terpene and the aromatic moieties present in these natural products and provided the common synthetic intermediate 70 (Scheme 6). The diverse tetracycles were accessed either via an intramolecular radical cyclization of the reduced congener 73 or through a Heck reaction of intermediate 71. Reaction of 73 with Bu3SnH in the presence of AIBN as initiator provided the tetracyclic core of dysideanones. The late introduction of an ethoxy group completed the total synthesis of dysideanone B (75) and “dysideanone F” (76). Ring closure to the dysiherbol scaffold was a much more challenging task as the classic conditions of the Heck reaction to common scaffold 70 proved unsuccessful. Screening of several reaction conditions on different analogues led to the conclusion that reduction of the C8-carbonyl side and acetal deprotection to 71 are essential in order to create the 6/6/5/6-carbocycle in the presence of Pd2(dba)3, SPhos, and Et3N in 86% yield. Reduction of the double bond with Pd/C followed by dual Stille coupling for the introduction of two methyl groups and Mukaiyama hydration utilizing Mn(dpm)3 and PhSiH3 furnished the misassigned structure for dysiherbol A (79). A revised structure was finally assigned after deprotection with BBr3 to complete the first total synthesis of dysiherbol A (79).
Scheme 6: Divergent synthesis of natural products isolated from Dysidea avara (Lu).
Scheme 6: Divergent synthesis of natural products isolated from Dysidea avara (Lu).
Total syntheses of (+)-jungermatrobrunin A (89) and related congeners
(Lei 2019) [40]: The ent-kaurane diterpenoids constitute a highly diverse class of structurally complex natural products possessing promising biological profiles, including anticancer, antifungal, and antiviral activities [41]. The highly diverse nature of the family makes a divergent synthesis extremely challenging, even for closely related members. Biosynthetically, the jungermannenone natural products have been proposed to derive from ent-kaurane diterpenoids through carbocationic rearrangements [42]. Jungermatrobrunin A (89) [43] bears a highly oxidized scaffold with a unique bicyclo[3.2.1]octene backbone and an unprecedented peroxide bridge (Scheme 7). Natural product (−)-1α,6α-diacetoxyjungermannenone C (88) [43] was projected by Lei’s group as the common scaffold for the divergent synthesis of this class. Finally, the closely related congener 90 [43] was envisaged to originate by a radical rearrangement of the common scaffold 88.
Scheme 7: Divergent synthesis of kaurene-type terpenoids (Lei).
Scheme 7: Divergent synthesis of kaurene-type terpenoids (Lei).
Initially, Lei’s group unfolded the synthesis of 83 on a decagram scale, utilizing an asymmetric conjugate reaction of commercially available 81 and 82 using Fletcher’s protocol (94% ee) [44]. A subsequent intramolecular arylation in the α-position of the ketone of 83, catalyzed by a Pd(II)–NHC [45], followed by methylation, provided cis-decalin 84 (Scheme 7). Appropriate redox modifications allowed the delivery of trans-decalin 85 in a gram-scale quantity. Birch reduction of the electron-rich aromatic ring, followed by propargylic addition and functional group interconversion (FGI) provided dienyne 86. Compound 86, under the previously developed radical reductive cyclization for 1,6-dienyne cyclization (using Bu3SnH and AIBN) [46], led to the construction of the key bicyclo[3.2.1]octene carbocyclic core of jungermatrobrunin, which was further elaborated to 87 in up to 61% yield, after alkene cleavage by OsO4 and NaIO4. The described reductive radical cyclization can be scaled up to 2 g without substantial decrease of the product yield. FGI, followed by methylenation provided the common scaffold 88. Further elaboration of 88 to natural products 90 and 89 was accomplished by UV irradiation at 365 nm in MeOH and by utilizing singlet oxygen (using rose Bengal) in MeCN/pyridine, 40:1, respectively. Interestingly, irradiation at 365 nm even in the presence of photosensitizer and O2 failed to furnish (+)-jungermatrobrunin A (89), and 90 was obtained as the sole product, albeit in low yield (14%). Attempts to optimize the yield always afforded recovered 88, hinting at a potential equilibrium between 88 and 90.
Total syntheses of magninoids and guignardones
(Lou 2021) [47]: Magninoids and guignardones are two classes of biogenetically related meroterpenoids, bearing a highly substituted cyclopentane moiety and a 6-oxabicyclo[3.2.1]octane fragment [48,49]. These classes exhibit diverse biological properties, such as potent inhibition of 11-β-hydroxysteroid dehydrogenase type I and inhibition of Candida albicans [48]. Although earlier syntheses have been reported recently for magninoids [50,51], Lou’s group envisioned a divergent plan based on a late-stage bioinspired semipinacol rearrangement–cyclization of common synthetic intermediates 94 and 95 (Scheme 8). Compound 94 was obtained in three steps, with the key step being the Suzuki–Miyaura coupling of appropriately functionalized precursors 91 and 92 using Romo and co-worker’s protocol [52]. Reaction of 94 under PPTS acidic conditions initiated a semipinacol rearrangement leading to 95, followed by subsequent cyclization to natural products guignardone A (96) and C (97). This process involved 1,2-allyl migration and C–O bond formation through a semipinacol rearrangement and a cyclodehydration cascade reaction (Scheme 8).
Scheme 8: Divergent synthesis of 6-oxabicyclo[3.2.1]octane meroterpenoids (Lou).
Scheme 8: Divergent synthesis of 6-oxabicyclo[3.2.1]octane meroterpenoids (Lou).
Following the same rationale, 94 was diverted to produce 100 after basic deprotection of the nonisolated 95. The radical oxidation of the former in the presence of dioxygen and sunlight or a catalytic amount of Mn(OAc)3 led to the creation of the compounds 101 and 102. FGI, followed by the cleavage of the hydroperoxide bond and final dehydration by Burgess reagent provided the total syntheses of magninoids A (104) and C (103, Scheme 8).
Divergent total synthesis of crinipellins
(Xie and Ding 2022) [53]: Crinipellins are highly congested tetraquinane natural products comprising 6–10 stereogenic centers, three of which are consecutive all-carbon quaternary carbon atoms [54-56]. Preliminary biological screening of this family revealed notable antibacterial and anticancer activities due to the α-methylene lactone moiety they bear [57]. Recently, in order to synthesize the common core present in crinipellins, Xie and Ding’s groups developed an approach using an unprecedented ring distortion. Their strategy consisted of a metal-catalyzed HAT to the exo-Δ-alkene of the 5/5/6/5 tetracycle 109, so as to subsequently favor a Dowd–Beckwith rearrangement [58] towards the tetraquinane skeleton of 112 (Scheme 9). The synthesis commenced with the generation of 107 from cyclopentenone 105 and aryl aldehyde 106 in a three-step sequence. An oxidative dearomatization induced a [5 + 2] cycloaddition–pinacol rearrangement of 107 to 109, according to previous studies of the same group (Scheme 9) [59-61]. The key HAT-mediated rearrangement was realized in an impressive yield of 95% to obtain 112 on a gram-scale, when cobalt complex C6 was used in the presence of PhSiH3 and TBHP in isopropanol. Further modifications of 112 led to the common scaffold 113 in 47% yield, which could be readily transformed to several crinipellin natural products by chemoselective redox reactions.
Scheme 9: Divergent synthesis of crinipellins by radical-mediated Dowd–Backwith rearrangement (Xie and Ding).
Scheme 9: Divergent synthesis of crinipellins by radical-mediated Dowd–Backwith rearrangement (Xie and Ding).
Divergent total synthesis of Galbulimima alkaloids
(Shenvi 2022) [62]: Members of the Galbulimima alkaloids extracted from rainforest canopy trees were found to possess neuroactive properties, such as antagonistic activity at muscarinic receptors [63], psychotropic activity, and antiplasmodic activity [64]. Their structural diversity, consisting of different connectivities between piperidine and decalin domains, is especially difficult to be divergently accessed. Shenvi’s group recognized that an aromatic congener within this class could be traced back to aromatic common intermediate 9 (Scheme 10). Despite the simplicity, the most obvious disconnections, such as an anionic enone conjugated addition and a direct cationic Friedel–Crafts reaction failed. Highlighting the power of radical disconnection, the group thought of utilizing a β-keto carbon-centered radical to circumvent the unsuccessful Friedel–Crafts reaction. Prior reports implicated β-keto radical formation in the ring opening of siloxycyclopropanes with photoinduced electron transfer (PET) to 1,4-dicyanonaphthalene [65]. Inspired by reports on dual photoredox and Ni-catalytic cross-coupling platforms [66], the group considered a system in which a photoexcited catalyst oxidatively cleaves a siloxycyclopropane with endo selectivity [67], leading to aryl–nickel capture and reductive elimination. Thus, when substrates 121 and 122 were photoirradiated with blue LED light at 45 °C in the presence of lutidine base, 7 mol % organic photocatalyst 4CzIPN, 30 mol % NiBr2, and 30 mol % bpy provided 57% of 9. Intramolecular Friedel–Crafts reaction by Et2AlCl and HFIP complex led to 123, possessing the correct connectivity for the divergent synthesis of the family. Choreographically executed sequential reduction steps allowed the total synthesis of GB13 (8), himgaline (126), and GB22 (125) in only one third of the number of steps of prior syntheses (Scheme 10).
Scheme 10: Divergent total synthesis of Galbulimima alkaloids (Shenvi).
Scheme 10: Divergent total synthesis of Galbulimima alkaloids (Shenvi).
Concise syntheses of eburnane alkaloids
(Qin 2018) [68,69]: Eburnane indole alkaloids comprise a highly diverse class of natural products mainly distributed in Southeast Asia and China [70]. Compounds of this class are traditionally used for detoxification and as anti-inflammatory agents in Chinese medicine [71]. Qin and co-workers reported the asymmetric total syntheses of several eburnane alkaloids. Therein, they relied on one of their previous discoveries, namely a photoredox-catalytic nitrogen-centered radical cascade [72], which has resulted in the impressive collective total synthesis of 33 alkaloids of three different classes of indole natural products (please see the inset of Scheme 11 for concise representation). Specifically, this included (–)-eburnaminol (132), (+)-larutenine (133), (–)-terengganensine B (134), and (–)-strempeliopine (136), as well as the asymmetric formal total synthesis of (–)-terengganensine A (not shown, Scheme 11). The requisite common synthetic intermediate 129 for the cascade was accessed by an acid-promoted condensation of chiral aldehyde 127 and Boc-protected amine 128, followed by zinc reduction of the nitro group and subsequent protection of the amine by a tosyl group in 27% overall yield. Irradiating 129 with blue light at 30 W in the presence of 1 mol % of [Ir(dtbbpy)(ppy)2]PF6 and 5 equiv of KHCO3 in THF resulted in the radical formation of the tetracyclic core of 130 in 75% yield as a mixture of two diastereoisomers (dr = 3:2) that were both used to access natural products. Impressively, the protocol allowed the installation of three rings and the stereoselective introduction of chiral centers at C2 and C21 for the final targets. With regard to the mechanism, it is hypothesized that it commences with the formation of a nitrogen-centered radical. The carbon radical 139 is then formed after the aforementioned nitrogen radical attacks the enamide group. The α-amide positioning is theorized to improve drastically the radical stability, nucleophilicity, and selectivity of 139 [73]. Furnishing of the common scaffold 130 can be carried out via an attack of intermediates of this type (e.g., 139) on Michael acceptors. Tosyl group deprotection of 130, followed by selenium anhydride oxidation and catalytic reduction of the amide using Wilkinson’s catalyst provided diastereoisomeric indole 131. Careful manipulation of the nitrile and alcohol side chains allowed selective cyclizations to the nitrogen atom of the indole core to conclude the total syntheses of 132–134. Samarium diiodide-mediated reductive cyclization of aldehyde 135, obtained also from 131, provided the pentacyclic core of (−)-strempeliopine (136) as a single diastereoisomer in 65% yield. Then, Barton’s radical deoxygenation resulted in the total synthesis of 136. Further, FGI of both diastereoisomers of 130 allowed the formal synthesis of (−)-terengganensine A (not shown) under the same divergent plan (Scheme 11).
Scheme 11: Divergent synthesis of eburnane alkaloids (Qin).
Scheme 11: Divergent synthesis of eburnane alkaloids (Qin).
Divergent total synthesis of (−)-pseudocopsinine (149) and (−)-minovincinine (150)
(Boger 2020) [74]: (−)-Pseudocopsinine (149) was isolated from Vinca erecta, with a structure related to the Aspidosperma alkaloids, containing an additional C20–C2 bond (Scheme 12) [75]. In 2020, Boger’s group reported the first total synthesis of (−)-pseudocopsinine (149) and (−)-minovincinine (150) from a common intermediate 146, featuring a late-stage HAT strategy to assemble the highly congested carbocyclic core of these natural products (Scheme 12). Based on earlier studies of the group on the total synthesis of vinblastine and related natural congeners [76], the authors realized that a late-stage formation of the C20–C2 bond would be highly strategic to provide the greatest simplification to these targets. The Aspidosperma skeleton 146 of both natural products was accessed in a single step from 145 through a scalable tandem [4 + 2]–[3 + 2] cascade in 74–84% yield in gram-scale quantities, known from previous studies [77]. Compound 145 was readily prepared in four steps from N-benzyltryptamine and 4-(2-t-butyldimethylsilyloxy)pent-4-enoic acid, requiring only two purification steps [77]. FGI of 146 led to (−)-enantiomer 147, which serves as the radical point od divergence of this plan. HAT-initiated transannular free-radical cyclization of (−)-enantiomer 147 according to Baran’s protocols [78] provided the benzyl-protected (−)-pseudocopsinine 148 in 60% yield, when 147 was treated with PhSiH3 in the presence of Fe(acac)3. Notably, the reaction provided a diastereoselectivity of 3:1 for the formation of the C20-stereocenter and exclusive formation of the C3-center. Key to this success is the low level of Fe(III)–H generation, thus minimizing intermediate radical reduction. The observed diastereoselectivity can be rationalized by referring to earlier mechanistic studies [79]. The same (−)-configured intermediate 147 was utilized in a HAT-initiated oxidation to access (−)-minovincinine (150) in 38% yield after deprotection (Scheme 12). Interestingly, the classic Mukaiyama conditions using Co(acac)2 with PhSiH3 provided compound 152 as the only isomer which, upon reduction, led to the exclusive formation of the compound epi-minovincinine (151). Replacement of Co(acac)2 with Co complex A suppressed the formation of 152 and provided the desired 150 and the isomer as an almost 1:1 mixture.
Scheme 12: Divergent synthesis of Aspidosperma alkaloids (Boger).
Scheme 12: Divergent synthesis of Aspidosperma alkaloids (Boger).
Syntheses of (−)-FR901483 (160) and (+)-TAN1251C (162)
(Gaunt 2020) [80]: Nitrogen-spirocyclic natural products consist a common class of important pharmaceutical candidates. FR901483, which was isolated from the fermentation broth of Cladobotryum sp. No. 11231, exhibits impressively potent immunosuppressant activity. This has resulted in extensive synthetic efforts towards the compound, in order to meet the needs for the supply as a potential therapeutic for the treatment of arthritis, Crohn’s disease, and organ transplant rejection [81]. The TAN1251 natural products, on the other hand, show potent activity as muscarinic antagonists, with potential applications as antispasmodic and antiulcer agents [82]. Despite the synthetic efforts on these natural products [83-86], in 2020, Gaunt’s group recognized a novel common synthetic intermediate in the structure of spirolactam 157 to access the family (Scheme 13). To synthesize it, they conjectured that a tyrosine amino acid, a cyclohexadione derivative, and a nonracemic dehydroalanine derivative could be effectively combined to build the core structure, using an already known iridium-photocatalyzed radical reaction [87]. Indeed, when ʟ-tyrosine methyl ester (154), 1,4-cyclohexanedione monoethylene acetal (155), and dehydroalanine derivative 156 were allowed to react in the presence of TFA, molecular sieves, 1 mol % of fac-Ir(ppy)3, and Hantzsch ester under blue LED irradiation at 40 W, this resulted in the formation of spirolactam 157 in 73% yield (Scheme 13). The reaction is estimated to take place initially with the one electron reduction to α-amino radical 164. This step is thought to be facilitated after TFA protonates the formed imine. Afterwards, radical addition of 164 to 156, generates an α-carbonyl species. A HAT from Hantzsch ester, which takes place diastereoselectively from the more accessible face, afforded the lactone 158. Spirolactam 157 can effortlessly be produced after the cyclization of the aforementioned lactone. Redox manipulations from this point on brought about the total synthesis of (−)-FR901483 (160) through an aldol reaction, and an intramolecular condensation resulted in the synthesis of (+)-TAN1251C (162, Scheme 13).
Scheme 13: Photoredox based synthesis of (−)-FR901483 (160) and (+)-TAN1251C (162, Gaunt).
Scheme 13: Photoredox based synthesis of (−)-FR901483 (160) and (+)-TAN1251C (162, Gaunt).
Divergent synthesis of bipolamine alkaloids
(Maimone 2022) [88]: Bipolamines were isolated from the fungi Curvularia sp. IFB Z10 and Bipolaris maydis in 2014 and were reported to possess antibacterial activity against a small panel of both gram-positive and -negative bacteria [89,90]. Interestingly, their chemical structure bears no resemblance to recognize antibiotics and their mechanism of action remains unknown. Based on the knowledge gained from the first total synthesis of (−)-curvulamine (171) [91], Maimone’s group leveraged their plan for accessing several members of this class (Scheme 14). The challenge this group had to address in this particular case was the high acid sensitivity and oxidative fragility of pyrrole intermediates. As common synthetic intermediate, the group utilized compound 170, readily available in gram-scale quantity, through a modified previously reported sequence [91]. The synthesis of the sterically constrained tetracyclic core of 170 relied initially on the photochemical radical cyclization of iodide 167 at 390 nm in the presence of NaHCO3 in CH3CN/t-BuOH, 5:1 to provide 168 in 55% yield (Scheme 14) [91]. Alkylation of the tetracycle, followed by epimerization of the C2 center and radical deoxygenation, or alternatively SN2 etherification, provided the common scaffold 170. The latter can serve as ideal diversification point to access (−)-curvulamine (171) by CBS reduction, bipolamines D (173) and E (172) by additional BH3·DMS hydroboration, and bipolamine G (174) initially by dihydroxylation of the alkene moiety with osmium tetroxide, followed by acidic etherification and reduction. Finally, bipolamine I (176) was obtained from 169 via a samarium diiodide reduction of the mesylate, followed by sodium borohydride reduction of the ketone, hydroboration, and base-mediated cyclization.
Scheme 14: Divergent synthesis of bipolamines (Maimone).
Scheme 14: Divergent synthesis of bipolamines (Maimone).
Flow-controlled divergent synthesis of aporphine and morphinandienone natural products
(Felpin 2022) [92]: Reticuline-type alkaloid oxidative coupling is a well-established biosynthetic pathway that produces important pharmaceutical structures [93], such as (+)-corytuberine, (−)-codeine, (−)-morphine, (+)-sebiferine (181), etc., depending on the regioselectivity of the coupling (Scheme 15) [94]. During this process, two major families of natural compounds are formed, namely the aporphine and the morphinandienone alkaloids. Mimicking the selectivity of the natural process in laboratory setups commonly proves tricky, producing an irreproducible yield of isomers for both classes. Recently, Felpin’s group reported the flow-controlled divergent synthesis of aporphine and morphinandienone alkaloids based on biomimetic common scaffolds (e.g., 180) using hypervalent iodine(III) reagents. Capitalizing on previously reported mechanistic investigations, they assumed that 180 can rearrange to glaucine (183) through the erythrinadienone intermediate 182. On contrary, common scaffold 180 should hydrolyze to sebiferine-type scaffolds in the presence of water. Taking these results into account, the group exploited the ability of HFIP to stabilize the radical cation formed by PIFA and BF3·EtO2 [95,96] to selectively produce aporphine natural products, while the use of PIDA or PIFA in the presence of BF3·OEt or TMSOTf in wet CH3CN allows to diverge the synthesis to morphinandienone natural products (e.g., 181, Scheme 15). The flow reaction was performed in a reaction coil at room temperature. Two reaction loops were used. The first one was loaded with the substrate and the second with PIFA and BF3·EtO2, while HFIP was used as the solvent. The two streams were mixed in a T-mixer, equipped with a 250 μL frit, to ensure efficient mixing. Under the optimized conditions, the method provided aporphine products in good to moderate yield, depending on the substrates used. Altering the solvent to wet CH3CN allowed the efficient delivery of morphindienone compounds.
Scheme 15: Flow chemistry divergency between aporphine and morphinandione alkaloids (Felpin).
Scheme 15: Flow chemistry divergency between aporphine and morphinandione alkaloids (Felpin).
Pyrroloazocine natural products
(Echavarren 2018) [97]: In 2018, Echavarren’s group reported the divergent synthesis of several pyrroloazocine alkaloids [98-100]. Preliminary biological screening indicates that members of this class are able to overcome multidrug resistance in vincristine resistant cells [98-100]. To access the common scaffold 188, the group relied on an intramolecular gold-photocatalyzed radical-mediated cyclization of an α-keto radical to the pendant indole core, reported earlier in the total synthesis of lundurines A–C (Scheme 16) [101]. The authors postulate that photoexcitation of [(dppmAuCl)2] with 365 nm light serves as initiator for radical generation in the brominated position of 186, prepared after following a 7-step sequence. The cyclization of the formed radical is 6-exo-trig and leads to the formation of a benzyl radical that is further oxidized to 188. From this common scaffold, the group managed to access several natural products of the class, majorly by utilizing the ability of conjugated alkenes to be further oxidized, and thus producing the respective benzylic cation. Intramolecular cyclization in the cationic position under participation of the methyl ester function provided the core for (+)-grandilodine C (191) and (+)-lapidilectine B (192), while allylation of the benzylic position allowed oxidative decomposition to the core of 194 and 195. FGI followed to complete targets 196–200 (Scheme 16).
Scheme 16: Divergent synthesis of pyrroloazocine natural products (Echavarren).
Scheme 16: Divergent synthesis of pyrroloazocine natural products (Echavarren).
Pyrroloindoline natural products
(Knowles 2018) [102]: In 2018, Knowles’ group demonstrated the ability of TEMPO to act as a trap for radical cations arising from the single-electron oxidation of protected tryptamine starting materials. The utilization of a chiral phosphate base is essential for the formation of a hydrogen bond between phosphate and tryptamines, allowing the decrease of the oxidation potential. This concept was used for the synthesis of pyrroloindoline natural products (Scheme 17). Thus, upon irradiation, iridium polypyridyl photocatalyst allowed the oxidation of the phosphate complex 207 to radical cation 206, which can be readily trapped by TEMPO, and hence stabilizing the imine and allowing cyclization with the pendant amine to form the pyrroloindoline core 210 in 81% yield and 93% ee. The latter can serve as a common scaffold to access an array of pyrroloindoline natural products but also synthetic analogues (Scheme 17). Oxidation of 210 by a second iridium photocatalyst yields benzyl cation 213, which can undergo nucleophilic attack by tryptamine derivatives to allow the total synthesis of (−)-psychotriasine (202), (−)-calycanthidine (203), and (−)-chimonanthine (204).
Scheme 17: Using TEMPO to stabilize radicals for the divergent synthesis of pyrroloindoline natural products (Knowles).
Scheme 17: Using TEMPO to stabilize radicals for the divergent synthesis of pyrroloindoline natural products (...
Synthesis of structurally diverse lignans
(Zhu, 2022) [103]: Lignans are structurally diverse natural compounds generated biosynthetically by the oxidative dimerization of phenylpropanoids [104]. Despite the wide oxidative diversity, classic lignans bearing a C8–C8’ bond can be biosynthetically traced back to coniferyl alcohol (Scheme 18). Commonly, lignans possess important pharmacological properties including antimicrobial, anti-inflammatory, immunosuppressive activities, etc. [105]. At the same time, some members have been recognized as potent topoisomerase inhibitors and have been used as anticancer drugs [106]. To access the rich diversity of this class, Zhu’s group recently applied a Fukuzumi salt ([Mes–Acr–Me]BF4)-mediated photochemical oxidation of dicinnamyl ether derivative 225 in the presence of appropriate additives (Scheme 18). According to the postulated mechanism, the reaction is initiated by an SET of the dicinnamyl ether substrate to Fukuzumi’s salt 233, leading to radical cation 216. Earlier findings of the same group [107] revealed that substitution on the aryl groups is the determinant factor for either 8,8’-cis- or 8,8’-trans-cyclization to furan heterocycle cation 218, which serves as the hypothetical common scaffold of the plan. Diverting this mechanistic route to different lignans is possible by introducing nucleophilic additives (e.g., MeOH), oxidants (e.g., Cu(OTFA)2), or quenchers (e.g., PhSSPh) to the reaction mixture. When monosubstitution of the aryl group is present, the formed radical cation, the product of the photooxidation of the cinnamyl ether, readily cyclizes to cyclobutene radical cation 217. The latter cleaves the benzylic C–C bond to produce the 1,4-radical cation cis-218. On the other hand, when polysubstitution with methoxy groups is present, the cation in 216 is delocalized, inhibiting the production of cyclobutene 217. Thus, radical cyclization according to the Beckwith–Houk model [108,109] via transition states TSI and TSII would take place, leading to the intermediate trans-218. In the presence of external nucleophiles (e.g., MeOH), the cation can be trapped, leading to substitution in the 7-position, while the radical is postulated to be oxidized to a cation, followed by a Friedel–Crafts reaction to the final product 222. When an excess of nucleophile is employed, radical 223 is favored, leading to either monosubstitution or disubstitution with external nucleophiles, depending on the presence of oxidant or reductant in the reaction mixture. Based on this plan, Zhu’s group managed to synthesize a rich number of lignans and congeners, such as aglacin A (229), β-ΟΗ-aglacin E (227), α-ΟΗ-aglacin F (228), brassilignan (232), etc.
Scheme 18: Radical pathway for preparation of lignans (Zhu).
Scheme 18: Radical pathway for preparation of lignans (Zhu).
Diverse synthesis of highly oxidized dibenzocyclooctadiene (DBCOD)-type lignans
Lumb (2021) [110]: Extracts of Schisandraceae are rich sources of highly oxidized DBCOD lignans with interesting biological properties [111]. Designing their divergent plan on postulated biosynthetic steps, Lumb’s group managed to efficiently prepare DBCOD derivatives 238 bearing the appropriate handles for late-stage radical formation (Scheme 19). Their success relied on the strategic design of linear precursors 239, bearing the appropriate substitution for the minimization of 1,3-allylic strain to enable Suzuki coupling for biaryl formation as a single atropisomer. The optimized conditions for this transformation utilize Buchwald’s catalyst (SPhos and Pd-based G2 precatalyst) in conjunction with K3PO4. With DBCOD bearing carboxylic acid handles at the 19-position in hands, the group proceeded with the generation of requisite radical 243 from the respective phthalimide ester under photocatalyzed conditions, either with [Ir(dtbpy)(ppy)2](PF6) or [Ru(bpy)3](PF6)2 in the presence of base. The reaction provided a good yield of the cyclized products kadsulignan E (235) and heteroclitin J (236) depending on the appropriate substitution of DBCODs. Selection of radical termination at the 3- and 1-positions, respectively, can be engineered by the strategic incorporation of a TES protecting group at the 1-position (see 243) for heteroclitin J (236). Further treatment of heteroclitin J (236) with ozone provided the selective formation of taiwankadsurins A and B (237a,b) by initial oxidative cleavage of the electron-rich aromatic ring and subsequent formation of the lactole ring. Heteroclitin J (236) has also been transformed to kadsuphilol G (245) by basic deprotection of the acetyl and benzoyl groups, followed by intramolecular cyclization and angelate esterification. Also, the differently redox-active DBCOD bearing an angelate functional group enabled the synthesis of kadsuphilin N (234). The synthetic sequence utilizes Fu’s protocol for palladium-mediated photocatalysis (using Pd(PPh3)2Cl2 in combination with xantphos) [112] towards 244, followed by Mukaiyama hydration with the aid of Mn(dpm)3, dioxygen, and PhSiH3 for the synthesis of kadsuphilin N (234).
Scheme 19: Divergent synthesis of DBCOD lignans (Lumb).
Scheme 19: Divergent synthesis of DBCOD lignans (Lumb).
Conclusion
The utility of radical retrosynthetic disconnections in natural product synthesis is highlighted in practice, day after day, when shorter and scalable syntheses are coming into light. Combining these advantages with the power of divergent synthesis provides a yet underdeveloped strategy to address the challenges that insufficient supply of pharmaceutical leads poses, enriching the chemical libraries with natural scaffolds for biological screening. The evidenced increase of divergent radical syntheses in the last few years indicates that this approach is here to change the way chemists will practice total synthesis in the future.
Funding
This work was supported by the project “OPENSCREEN-GR” (MIS 5002691), which is implemented under the Action “Reinforcement of the Research and Innovation Infrastructure”, funded by the Operational Program “Competitiveness, Entrepreneurship and Innovation” (NSRF 2014-2020) and cofinanced by Greece and the European Union (European Regional Development Fund).
References
-
Nicolaou, K. C.; Vourloumis, D.; Winssinger, N.; Baran, P. S. Angew. Chem., Int. Ed. 2000, 39, 44–122. doi:10.1002/(sici)1521-3773(20000103)39:1<44::aid-anie44>3.0.co;2-l
Return to citation in text: [1] -
Kuttruff, C. A.; Eastgate, M. D.; Baran, P. S. Nat. Prod. Rep. 2014, 31, 419–432. doi:10.1039/c3np70090a
Return to citation in text: [1] -
Smith, J. M.; Harwood, S. J.; Baran, P. S. Acc. Chem. Res. 2018, 51, 1807–1817. doi:10.1021/acs.accounts.8b00209
Return to citation in text: [1] -
Leifert, D.; Studer, A. Angew. Chem., Int. Ed. 2020, 59, 74–108. doi:10.1002/anie.201903726
Return to citation in text: [1] -
Romero, K. J.; Galliher, M. S.; Pratt, D. A.; Stephenson, C. R. J. Chem. Soc. Rev. 2018, 47, 7851–7866. doi:10.1039/c8cs00379c
Return to citation in text: [1] -
Bonjoch, J.; Diaba, F. Eur. J. Org. Chem. 2020, 5070–5100. doi:10.1002/ejoc.202000391
Return to citation in text: [1] [2] -
Hung, K.; Hu, X.; Maimone, T. J. Nat. Prod. Rep. 2018, 35, 174–202. doi:10.1039/c7np00065k
Return to citation in text: [1] -
Follmann, M.; Briem, H.; Steinmeyer, A.; Hillisch, A.; Schmitt, M. H.; Haning, H.; Meier, H. Drug Discovery Today 2019, 24, 668–672. doi:10.1016/j.drudis.2018.12.003
Return to citation in text: [1] -
Anagnostaki, E. E.; Zografos, A. L. Chem. Soc. Rev. 2012, 41, 5613–5625. doi:10.1039/c2cs35080g
Return to citation in text: [1] -
Li, L.; Chen, Z.; Zhang, X.; Jia, Y. Chem. Rev. 2018, 118, 3752–3832. doi:10.1021/acs.chemrev.7b00653
Return to citation in text: [1] [2] -
Li, C.-J.; Trost, B. M. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 13197–13202. doi:10.1073/pnas.0804348105
Return to citation in text: [1] -
Gaich, T.; Baran, P. S. J. Org. Chem. 2010, 75, 4657–4673. doi:10.1021/jo1006812
Return to citation in text: [1] -
Young, I. S.; Baran, P. S. Nat. Chem. 2009, 1, 193–205. doi:10.1038/nchem.216
Return to citation in text: [1] -
Galliher, M. S.; Roldan, B. J.; Stephenson, C. R. J. Chem. Soc. Rev. 2021, 50, 10044–10057. doi:10.1039/d1cs00411e
Return to citation in text: [1] -
Giese, B.; Meister, J. Chem. Ber. 1977, 110, 2588–2600. doi:10.1002/cber.19771100717
Return to citation in text: [1] -
Giese, B.; Rupaner, R. Synthesis 1988, 219–221. doi:10.1055/s-1988-27517
Return to citation in text: [1] -
Herrmann, J. M.; König, B. Eur. J. Org. Chem. 2013, 7017–7027. doi:10.1002/ejoc.201300657
Return to citation in text: [1] -
Nicolaou, K. C.; Ellery, S. P.; Chen, J. S. Angew. Chem., Int. Ed. 2009, 48, 7140–7165. doi:10.1002/anie.200902151
Return to citation in text: [1] -
Snider, B. B. Chem. Rev. 1996, 96, 339–364. doi:10.1021/cr950026m
Return to citation in text: [1] -
Shevick, S. L.; Wilson, C. V.; Kotesova, S.; Kim, D.; Holland, P. L.; Shenvi, R. A. Chem. Sci. 2020, 11, 12401–12422. doi:10.1039/d0sc04112b
Return to citation in text: [1] [2] -
Pitre, S. P.; Overman, L. E. Chem. Rev. 2022, 122, 1717–1751. doi:10.1021/acs.chemrev.1c00247
Return to citation in text: [1] -
Novaes, L. F. T.; Liu, J.; Shen, Y.; Lu, L.; Meinhardt, J. M.; Lin, S. Chem. Soc. Rev. 2021, 50, 7941–8002. doi:10.1039/d1cs00223f
Return to citation in text: [1] -
Merchant, R. R.; Oberg, K. M.; Lin, Y.; Novak, A. J. E.; Felding, J.; Baran, P. S. J. Am. Chem. Soc. 2018, 140, 7462–7465. doi:10.1021/jacs.8b04891
Return to citation in text: [1] [2] -
Engel, B.; Erkel, G.; Anke, T.; Sterner, O. J. Antibiot. 1998, 51, 518–521. doi:10.7164/antibiotics.51.518
Return to citation in text: [1] -
Uchida, R.; Imasato, R.; Yamaguchi, Y.; Masuma, R.; Shiomi, K.; Tomoda, H.; Ōmura, S. J. Antibiot. 2005, 58, 804–809. doi:10.1038/ja.2005.107
Return to citation in text: [1] -
Lee, J. C.; Lobkovsky, E.; Pliam, N. B.; Strobel, G.; Clardy, J. J. Org. Chem. 1995, 60, 7076–7077. doi:10.1021/jo00127a001
Return to citation in text: [1] -
Qin, T.; Cornella, J.; Li, C.; Malins, L. R.; Edwards, J. T.; Kawamura, S.; Maxwell, B. D.; Eastgate, M. D.; Baran, P. S. Science 2016, 352, 801–805. doi:10.1126/science.aaf6123
Return to citation in text: [1] -
Qin, T.; Malins, L. R.; Edwards, J. T.; Merchant, R. R.; Novak, A. J. E.; Zhong, J. Z.; Mills, R. B.; Yan, M.; Yuan, C.; Eastgate, M. D.; Baran, P. S. Angew. Chem., Int. Ed. 2017, 56, 260–265. doi:10.1002/anie.201609662
Return to citation in text: [1] -
Li, J.; Li, F.; King-Smith, E.; Renata, H. Nat. Chem. 2020, 12, 173–179. doi:10.1038/s41557-019-0407-6
Return to citation in text: [1] -
Hubert, C.; Moreau, J.; Batany, J.; Duboc, A.; Hurvois, J.-P.; Renaud, J.-L. Adv. Synth. Catal. 2008, 350, 40–42. doi:10.1002/adsc.200700375
Return to citation in text: [1] -
Everson, D. A.; Shrestha, R.; Weix, D. J. J. Am. Chem. Soc. 2010, 132, 920–921. doi:10.1021/ja9093956
Return to citation in text: [1] -
Hyster, T. K. Synlett 2020, 31, 248–254. doi:10.1055/s-0037-1611818
Return to citation in text: [1] -
Zhang, S.; Wang, X.; Hao, J.; Li, D.; Csuk, R.; Li, S. J. Nat. Prod. 2018, 81, 2010–2017. doi:10.1021/acs.jnatprod.8b00310
Return to citation in text: [1] -
Shan, W.-G.; Ying, Y.-M.; Ma, L.-F.; Zhan, Z.-J. Drimane-Related Merosesquiterpenoids, a Promising Library of Metabolites for Drug Development. In Studies in Natural Products Chemistry; Attar-ur-Rahman, Ed.; Elsevier: Amsterdam, Netherlands, 2015; Vol. 45, pp 147–215. doi:10.1016/b978-0-444-63473-3.00006-x
Return to citation in text: [1] -
Dixon, D. D.; Lockner, J. W.; Zhou, Q.; Baran, P. S. J. Am. Chem. Soc. 2012, 134, 8432–8435. doi:10.1021/ja303937y
Return to citation in text: [1] -
Barrero, A. F.; Alvarez‐Manzaneda, E. J.; Chahboun, R.; Arteaga, A. F. Synth. Commun. 2004, 34, 3631–3643. doi:10.1081/scc-200031056
Return to citation in text: [1] -
Chong, C.; Zhang, Q.; Ke, J.; Zhang, H.; Yang, X.; Wang, B.; Ding, W.; Lu, Z. Angew. Chem., Int. Ed. 2021, 60, 13807–13813. doi:10.1002/anie.202100541
Return to citation in text: [1] -
Jiao, W.-H.; Xu, T.-T.; Yu, H.-B.; Chen, G.-D.; Huang, X.-J.; Yang, F.; Li, Y.-S.; Han, B.-N.; Liu, X.-Y.; Lin, H.-W. J. Nat. Prod. 2014, 77, 346–350. doi:10.1021/np4009392
Return to citation in text: [1] -
Jiao, W.-H.; Shi, G.-H.; Xu, T.-T.; Chen, G.-D.; Gu, B.-B.; Wang, Z.; Peng, S.; Wang, S.-P.; Li, J.; Han, B.-N.; Zhang, W.; Lin, H.-W. J. Nat. Prod. 2016, 79, 406–411. doi:10.1021/acs.jnatprod.5b01079
Return to citation in text: [1] -
Wu, J.; Kadonaga, Y.; Hong, B.; Wang, J.; Lei, X. Angew. Chem., Int. Ed. 2019, 58, 10879–10883. doi:10.1002/anie.201903682
Return to citation in text: [1] -
Sun, H.-D.; Huang, S.-X.; Han, Q.-B. Nat. Prod. Rep. 2006, 23, 673–698. doi:10.1039/b604174d
Return to citation in text: [1] -
Li, L.-M.; Li, G.-Y.; Xiao, W.-L.; Zhou, Y.; Li, S.-H.; Huang, S.-X.; Han, Q.-B.; Ding, L.-S.; Lou, L.-G.; Sun, H.-D. Tetrahedron Lett. 2006, 47, 5187–5190. doi:10.1016/j.tetlet.2006.05.025
Return to citation in text: [1] -
Qu, J.-B.; Zhu, R.-L.; Zhang, Y.-L.; Guo, H.-F.; Wang, X.-N.; Xie, C.-F.; Yu, W.-T.; Ji, M.; Lou, H.-X. J. Nat. Prod. 2008, 71, 1418–1422. doi:10.1021/np8003062
Return to citation in text: [1] [2] [3] -
Maksymowicz, R. M.; Roth, P. M. C.; Fletcher, S. P. Nat. Chem. 2012, 4, 649–654. doi:10.1038/nchem.1394
Return to citation in text: [1] -
Xiao, Z.-K.; Shao, L.-X. Synthesis 2012, 44, 711–716. doi:10.1055/s-0031-1289698
Return to citation in text: [1] -
Liu, W.; Li, H.; Cai, P.-J.; Wang, Z.; Yu, Z.-X.; Lei, X. Angew. Chem., Int. Ed. 2016, 55, 3112–3116. doi:10.1002/anie.201511659
Return to citation in text: [1] -
Zong, Y.; Xu, Z.-J.; Zhu, R.-X.; Su, A.-H.; Liu, X.-Y.; Zhu, M.-Z.; Han, J.-J.; Zhang, J.-Z.; Xu, Y.-L.; Lou, H.-X. Angew. Chem., Int. Ed. 2021, 60, 15286–15290. doi:10.1002/anie.202104182
Return to citation in text: [1] -
Chen, K.; Zhang, X.; Sun, W.; Liu, J.; Yang, J.; Chen, C.; Liu, X.; Gao, L.; Wang, J.; Li, H.; Luo, Z.; Xue, Y.; Zhu, H.; Zhang, Y. Org. Lett. 2017, 19, 5956–5959. doi:10.1021/acs.orglett.7b02955
Return to citation in text: [1] [2] -
Yuan, W. H.; Liu, M.; Jiang, N.; Guo, Z. K.; Ma, J.; Zhang, J.; Song, Y. C.; Tan, R. X. Eur. J. Org. Chem. 2010, 6348–6353. doi:10.1002/ejoc.201000916
Return to citation in text: [1] -
Yan, Z.; Zhao, C.; Gong, J.; Yang, Z. Org. Lett. 2020, 22, 1644–1647. doi:10.1021/acs.orglett.0c00241
Return to citation in text: [1] -
Zhang, Y.-A.; Milkovits, A.; Agarawal, V.; Taylor, C. A.; Snyder, S. A. Angew. Chem., Int. Ed. 2021, 60, 11127–11132. doi:10.1002/anie.202016178
Return to citation in text: [1] -
Tao, Y.; Reisenauer, K.; Taube, J. H.; Romo, D. Angew. Chem., Int. Ed. 2019, 58, 2734–2738. doi:10.1002/anie.201812909
Return to citation in text: [1] -
Zhao, Y.; Hu, J.; Chen, R.; Xiong, F.; Xie, H.; Ding, H. J. Am. Chem. Soc. 2022, 144, 2495–2500. doi:10.1021/jacs.1c13370
Return to citation in text: [1] -
Anke, T.; Heim, J.; Knoch, F.; Mocek, U.; Steffan, B.; Steglich, W. Angew. Chem., Int. Ed. Engl. 1985, 24, 709–711. doi:10.1002/anie.198507091
Return to citation in text: [1] -
Li, Y.-Y.; Shen, Y.-M. Helv. Chim. Acta 2010, 93, 2151–2157. doi:10.1002/hlca.200900470
Return to citation in text: [1] -
Rohr, M.; Oleinikov, K.; Jung, M.; Sandjo, L. P.; Opatz, T.; Erkel, G. Bioorg. Med. Chem. 2017, 25, 514–522. doi:10.1016/j.bmc.2016.11.016
Return to citation in text: [1] -
Kupka, J.; Anke, T.; Oberwinkler, F.; Schramm, G.; Steglich, W. J. Antibiot. 1979, 32, 130–135. doi:10.7164/antibiotics.32.130
Return to citation in text: [1] -
Dowd, P.; Zhang, W. Chem. Rev. 1993, 93, 2091–2115. doi:10.1021/cr00022a007
Return to citation in text: [1] -
He, C.; Hu, J.; Wu, Y.; Ding, H. J. Am. Chem. Soc. 2017, 139, 6098–6101. doi:10.1021/jacs.7b02746
Return to citation in text: [1] -
Gao, J.; Rao, P.; Xu, K.; Wang, S.; Wu, Y.; He, C.; Ding, H. J. Am. Chem. Soc. 2020, 142, 4592–4597. doi:10.1021/jacs.0c00308
Return to citation in text: [1] -
Wang, B.; Liu, Z.; Tong, Z.; Gao, B.; Ding, H. Angew. Chem., Int. Ed. 2021, 60, 14892–14896. doi:10.1002/anie.202104410
Return to citation in text: [1] -
Landwehr, E. M.; Baker, M. A.; Oguma, T.; Burdge, H. E.; Kawajiri, T.; Shenvi, R. A. Science 2022, 375, 1270–1274. doi:10.1126/science.abn8343
Return to citation in text: [1] -
Miller, J. H.; Aagaard, P. J.; Gibson, V. A.; McKinney, M. J. Pharmacol. Exp. Ther. 1992, 263, 663–667.
Return to citation in text: [1] -
Collins, D. J.; Culvenor, C. C. J.; Lamberton, J. A.; Loder, J. W.; Price, J. R. Plants for Medicines: A Chemical and Pharmacological Survey of Plants in the Australian Region; CSIRO Publishing: Clayton, Australia, 1990. doi:10.1071/9780643101203
Return to citation in text: [1] -
Rinderhagen, H.; Waske, P. A.; Mattay, J. Tetrahedron 2006, 62, 6589–6593. doi:10.1016/j.tet.2006.03.060
Return to citation in text: [1] -
Milligan, J. A.; Phelan, J. P.; Badir, S. O.; Molander, G. A. Angew. Chem., Int. Ed. 2019, 58, 6152–6163. doi:10.1002/anie.201809431
Return to citation in text: [1] -
Varabyeva, N.; Barysevich, M.; Aniskevich, Y.; Hurski, A. Org. Lett. 2021, 23, 5452–5456. doi:10.1021/acs.orglett.1c01795
Return to citation in text: [1] -
Liu, X.-Y.; Qin, Y. Acc. Chem. Res. 2019, 52, 1877–1891. doi:10.1021/acs.accounts.9b00246
Return to citation in text: [1] -
Zhou, Q.; Dai, X.; Song, H.; He, H.; Wang, X.; Liu, X.-Y.; Qin, Y. Chem. Commun. 2018, 54, 9510–9512. doi:10.1039/c8cc05374j
Return to citation in text: [1] -
Kam, T.-S. Alkaloids from Malaysian Flora. In Alkaloids: Chemical and Biological Perspectives; Pelletier, S. W., Ed.; Pergamon Press: Oxford, UK, 1999; p 350.
Return to citation in text: [1] -
Yap, W.-S.; Gan, C.-Y.; Sim, K.-S.; Lim, S.-H.; Low, Y.-Y.; Kam, T.-S. J. Nat. Prod. 2016, 79, 230–239. doi:10.1021/acs.jnatprod.5b00992
Return to citation in text: [1] -
Wang, X.; Xia, D.; Qin, W.; Zhou, R.; Zhou, X.; Zhou, Q.; Liu, W.; Dai, X.; Wang, H.; Wang, S.; Tan, L.; Zhang, D.; Song, H.; Liu, X.-Y.; Qin, Y. Chem 2017, 2, 803–816. doi:10.1016/j.chempr.2017.04.007
Return to citation in text: [1] -
Alabugin, I. V.; Harris, T. Chem 2017, 2, 753–755. doi:10.1016/j.chempr.2017.05.018
Return to citation in text: [1] -
Zeng, X.; Shukla, V.; Boger, D. L. J. Org. Chem. 2020, 85, 14817–14826. doi:10.1021/acs.joc.0c02493
Return to citation in text: [1] -
Abdurakhimova, N.; Yuldashev, P. K.; Yunusov, S. Y. Chem. Nat. Compd. 1967, 3, 263–266. doi:10.1007/bf00574630
Return to citation in text: [1] -
Ishikawa, H.; Colby, D. A.; Seto, S.; Va, P.; Tam, A.; Kakei, H.; Rayl, T. J.; Hwang, I.; Boger, D. L. J. Am. Chem. Soc. 2009, 131, 4904–4916. doi:10.1021/ja809842b
Return to citation in text: [1] -
Campbell, E. L.; Zuhl, A. M.; Liu, C. M.; Boger, D. L. J. Am. Chem. Soc. 2010, 132, 3009–3012. doi:10.1021/ja908819q
Return to citation in text: [1] [2] -
Lo, J. C.; Kim, D.; Pan, C.-M.; Edwards, J. T.; Yabe, Y.; Gui, J.; Qin, T.; Gutiérrez, S.; Giacoboni, J.; Smith, M. W.; Holland, P. L.; Baran, P. S. J. Am. Chem. Soc. 2017, 139, 2484–2503. doi:10.1021/jacs.6b13155
Return to citation in text: [1] -
Kim, D.; Rahaman, S. M. W.; Mercado, B. Q.; Poli, R.; Holland, P. L. J. Am. Chem. Soc. 2019, 141, 7473–7485. doi:10.1021/jacs.9b02117
Return to citation in text: [1] -
Reich, D.; Trowbridge, A.; Gaunt, M. J. Angew. Chem., Int. Ed. 2020, 59, 2256–2261. doi:10.1002/anie.201912010
Return to citation in text: [1] -
Sakamoto, K.; Tsujii, E.; Abe, F.; Nakanishi, T.; Yamashita, M.; Shigematsu, N.; Izumi, S.; Okuhara, M. J. Antibiot. 1996, 49, 37–44. doi:10.7164/antibiotics.49.37
Return to citation in text: [1] -
Shirafuji, H.; Tsubotani, S.; Ishimaru, T.; Harada, S. Compound tan-1251, its derivatives, their production and use. WO Patent WO1991013887, Sept 19, 1991.
Return to citation in text: [1] -
Scheffler, G.; Seike, H.; Sorensen, E. J. Angew. Chem., Int. Ed. 2000, 39, 4593–4596. doi:10.1002/1521-3773(20001215)39:24<4593::aid-anie4593>3.0.co;2-x
Return to citation in text: [1] -
Ousmer, M.; Braun, N. A.; Bavoux, C.; Perrin, M.; Ciufolini, M. A. J. Am. Chem. Soc. 2001, 123, 7534–7538. doi:10.1021/ja016030z
Return to citation in text: [1] -
Carson, C. A.; Kerr, M. A. Org. Lett. 2009, 11, 777–779. doi:10.1021/ol802870c
Return to citation in text: [1] -
Snider, B. B.; Lin, H. Org. Lett. 2000, 2, 643–646. doi:10.1021/ol991401q
Return to citation in text: [1] -
Trowbridge, A.; Reich, D.; Gaunt, M. J. Nature 2018, 561, 522–527. doi:10.1038/s41586-018-0537-9
Return to citation in text: [1] -
Xuan, J.; Machicao, P. A.; Haelsig, K. T.; Maimone, T. J. Angew. Chem., Int. Ed. 2022, 61, e202209457. doi:10.1021/ol991401q
Return to citation in text: [1] -
Han, W. B.; Lu, Y. H.; Zhang, A. H.; Zhang, G. F.; Mei, Y. N.; Jiang, N.; Lei, X.; Song, Y. C.; Ng, S. W.; Tan, R. X. Org. Lett. 2014, 16, 5366–5369. doi:10.1021/ol502572g
Return to citation in text: [1] -
Han, W. B.; Zhang, A. H.; Deng, X. Z.; Lei, X.; Tan, R. X. Org. Lett. 2016, 18, 1816–1819. doi:10.1021/acs.orglett.6b00549
Return to citation in text: [1] -
Haelsig, K. T.; Xuan, J.; Maimone, T. J. J. Am. Chem. Soc. 2020, 142, 1206–1210. doi:10.1021/jacs.9b12546
Return to citation in text: [1] [2] [3] -
Tra, B. B. J.; Abollé, A.; Coefard, V.; Felpin, F.-X. Eur. J. Org. Chem. 2022, e202200301. doi:10.1002/ejoc.202200301
Return to citation in text: [1] -
Phillipson, J. D.; Roberts, M. F.; Zenk, M. H., Eds. The Chemistry and Biology of Isoquinoline Alkaloids; Springer: Berlin, Heidelberg, 1985. doi:10.1007/978-3-642-70128-3
Return to citation in text: [1] -
Beaudoin, G. A. W.; Facchini, P. J. Planta 2014, 240, 19–32. doi:10.1007/s00425-014-2056-8
Return to citation in text: [1] -
Dohi, T.; Ito, M.; Yamaoka, N.; Morimoto, K.; Fujioka, H.; Kita, Y. Tetrahedron 2009, 65, 10797–10815. doi:10.1016/j.tet.2009.10.040
Return to citation in text: [1] -
Dohi, T.; Yamaoka, N.; Kita, Y. Tetrahedron 2010, 66, 5775–5785. doi:10.1016/j.tet.2010.04.116
Return to citation in text: [1] -
Miloserdov, F. M.; Kirillova, M. S.; Muratore, M. E.; Echavarren, A. M. J. Am. Chem. Soc. 2018, 140, 5393–5400. doi:10.1021/jacs.7b13484
Return to citation in text: [1] -
Awang, K.; Sévenet, T.; Hamid, A.; Hadi, A.; David, B.; Païs, M. Tetrahedron Lett. 1992, 33, 2493–2496. doi:10.1016/s0040-4039(00)92223-8
Return to citation in text: [1] [2] -
Awang, K.; Sévenet, T.; Païs, M.; Hadi, A. H. A. J. Nat. Prod. 1993, 56, 1134–1139. doi:10.1021/np50097a018
Return to citation in text: [1] [2] -
Yap, W.-S.; Gan, C.-Y.; Low, Y.-Y.; Choo, Y.-M.; Etoh, T.; Hayashi, M.; Komiyama, K.; Kam, T.-S. J. Nat. Prod. 2011, 74, 1309–1312. doi:10.1021/np200008g
Return to citation in text: [1] [2] -
Kirillova, M. S.; Muratore, M. E.; Dorel, R.; Echavarren, A. M. J. Am. Chem. Soc. 2016, 138, 3671–3674. doi:10.1021/jacs.6b01428
Return to citation in text: [1] -
Gentry, E. C.; Rono, L. J.; Hale, M. E.; Matsuura, R.; Knowles, R. R. J. Am. Chem. Soc. 2018, 140, 3394–3402. doi:10.1021/jacs.7b13616
Return to citation in text: [1] -
Xiang, J.-C.; Fung, C.; Wang, Q.; Zhu, J. Nat. Commun. 2022, 13, 3481. doi:10.1038/s41467-022-31000-4
Return to citation in text: [1] -
Teponno, R. B.; Kusari, S.; Spiteller, M. Nat. Prod. Rep. 2016, 33, 1044–1092. doi:10.1039/c6np00021e
Return to citation in text: [1] -
Saleem, M.; Kim, H. J.; Ali, M. S.; Lee, Y. S. Nat. Prod. Rep. 2005, 22, 696–716. doi:10.1039/b514045p
Return to citation in text: [1] -
Zhang, X.; Rakesh, K. P.; Shantharam, C. S.; Manukumar, H. M.; Asiri, A. M.; Marwani, H. M.; Qin, H.-L. Bioorg. Med. Chem. 2018, 26, 340–355. doi:10.1016/j.bmc.2017.11.026
Return to citation in text: [1] -
Xiang, J.-C.; Wang, Q.; Zhu, J. Angew. Chem., Int. Ed. 2020, 59, 21195–21202. doi:10.1002/anie.202007548
Return to citation in text: [1] -
Beckwith, A. L. J.; Schiesser, C. H. Tetrahedron 1985, 41, 3925–3941. doi:10.1016/s0040-4020(01)97174-1
Return to citation in text: [1] -
Spellmeyer, D. C.; Houk, K. N. J. Org. Chem. 1987, 52, 959–974. doi:10.1021/jo00382a001
Return to citation in text: [1] -
Huang, Z.; Lumb, J.-P. Nat. Chem. 2021, 13, 24–32. doi:10.1038/s41557-020-00603-z
Return to citation in text: [1] -
Zhu, P.; Li, J.; Fu, X.; Yu, Z. Phytomedicine 2019, 59, 152760. doi:10.1016/j.phymed.2018.11.020
Return to citation in text: [1] -
Wang, G.-Z.; Shang, R.; Fu, Y. Org. Lett. 2018, 20, 888–891. doi:10.1021/acs.orglett.8b00023
Return to citation in text: [1]
| 79. | Kim, D.; Rahaman, S. M. W.; Mercado, B. Q.; Poli, R.; Holland, P. L. J. Am. Chem. Soc. 2019, 141, 7473–7485. doi:10.1021/jacs.9b02117 |
| 80. | Reich, D.; Trowbridge, A.; Gaunt, M. J. Angew. Chem., Int. Ed. 2020, 59, 2256–2261. doi:10.1002/anie.201912010 |
| 77. | Campbell, E. L.; Zuhl, A. M.; Liu, C. M.; Boger, D. L. J. Am. Chem. Soc. 2010, 132, 3009–3012. doi:10.1021/ja908819q |
| 78. | Lo, J. C.; Kim, D.; Pan, C.-M.; Edwards, J. T.; Yabe, Y.; Gui, J.; Qin, T.; Gutiérrez, S.; Giacoboni, J.; Smith, M. W.; Holland, P. L.; Baran, P. S. J. Am. Chem. Soc. 2017, 139, 2484–2503. doi:10.1021/jacs.6b13155 |
| 77. | Campbell, E. L.; Zuhl, A. M.; Liu, C. M.; Boger, D. L. J. Am. Chem. Soc. 2010, 132, 3009–3012. doi:10.1021/ja908819q |
| 88. | Xuan, J.; Machicao, P. A.; Haelsig, K. T.; Maimone, T. J. Angew. Chem., Int. Ed. 2022, 61, e202209457. doi:10.1021/ol991401q |
| 83. | Scheffler, G.; Seike, H.; Sorensen, E. J. Angew. Chem., Int. Ed. 2000, 39, 4593–4596. doi:10.1002/1521-3773(20001215)39:24<4593::aid-anie4593>3.0.co;2-x |
| 84. | Ousmer, M.; Braun, N. A.; Bavoux, C.; Perrin, M.; Ciufolini, M. A. J. Am. Chem. Soc. 2001, 123, 7534–7538. doi:10.1021/ja016030z |
| 85. | Carson, C. A.; Kerr, M. A. Org. Lett. 2009, 11, 777–779. doi:10.1021/ol802870c |
| 86. | Snider, B. B.; Lin, H. Org. Lett. 2000, 2, 643–646. doi:10.1021/ol991401q |
| 87. | Trowbridge, A.; Reich, D.; Gaunt, M. J. Nature 2018, 561, 522–527. doi:10.1038/s41586-018-0537-9 |
| 81. | Sakamoto, K.; Tsujii, E.; Abe, F.; Nakanishi, T.; Yamashita, M.; Shigematsu, N.; Izumi, S.; Okuhara, M. J. Antibiot. 1996, 49, 37–44. doi:10.7164/antibiotics.49.37 |
| 82. | Shirafuji, H.; Tsubotani, S.; Ishimaru, T.; Harada, S. Compound tan-1251, its derivatives, their production and use. WO Patent WO1991013887, Sept 19, 1991. |
| 92. | Tra, B. B. J.; Abollé, A.; Coefard, V.; Felpin, F.-X. Eur. J. Org. Chem. 2022, e202200301. doi:10.1002/ejoc.202200301 |
| 93. | Phillipson, J. D.; Roberts, M. F.; Zenk, M. H., Eds. The Chemistry and Biology of Isoquinoline Alkaloids; Springer: Berlin, Heidelberg, 1985. doi:10.1007/978-3-642-70128-3 |
| 91. | Haelsig, K. T.; Xuan, J.; Maimone, T. J. J. Am. Chem. Soc. 2020, 142, 1206–1210. doi:10.1021/jacs.9b12546 |
| 91. | Haelsig, K. T.; Xuan, J.; Maimone, T. J. J. Am. Chem. Soc. 2020, 142, 1206–1210. doi:10.1021/jacs.9b12546 |
| 89. | Han, W. B.; Lu, Y. H.; Zhang, A. H.; Zhang, G. F.; Mei, Y. N.; Jiang, N.; Lei, X.; Song, Y. C.; Ng, S. W.; Tan, R. X. Org. Lett. 2014, 16, 5366–5369. doi:10.1021/ol502572g |
| 90. | Han, W. B.; Zhang, A. H.; Deng, X. Z.; Lei, X.; Tan, R. X. Org. Lett. 2016, 18, 1816–1819. doi:10.1021/acs.orglett.6b00549 |
| 91. | Haelsig, K. T.; Xuan, J.; Maimone, T. J. J. Am. Chem. Soc. 2020, 142, 1206–1210. doi:10.1021/jacs.9b12546 |
| 112. | Wang, G.-Z.; Shang, R.; Fu, Y. Org. Lett. 2018, 20, 888–891. doi:10.1021/acs.orglett.8b00023 |
| 111. | Zhu, P.; Li, J.; Fu, X.; Yu, Z. Phytomedicine 2019, 59, 152760. doi:10.1016/j.phymed.2018.11.020 |
| 97. | Miloserdov, F. M.; Kirillova, M. S.; Muratore, M. E.; Echavarren, A. M. J. Am. Chem. Soc. 2018, 140, 5393–5400. doi:10.1021/jacs.7b13484 |
| 98. | Awang, K.; Sévenet, T.; Hamid, A.; Hadi, A.; David, B.; Païs, M. Tetrahedron Lett. 1992, 33, 2493–2496. doi:10.1016/s0040-4039(00)92223-8 |
| 99. | Awang, K.; Sévenet, T.; Païs, M.; Hadi, A. H. A. J. Nat. Prod. 1993, 56, 1134–1139. doi:10.1021/np50097a018 |
| 100. | Yap, W.-S.; Gan, C.-Y.; Low, Y.-Y.; Choo, Y.-M.; Etoh, T.; Hayashi, M.; Komiyama, K.; Kam, T.-S. J. Nat. Prod. 2011, 74, 1309–1312. doi:10.1021/np200008g |
| 94. | Beaudoin, G. A. W.; Facchini, P. J. Planta 2014, 240, 19–32. doi:10.1007/s00425-014-2056-8 |
| 95. | Dohi, T.; Ito, M.; Yamaoka, N.; Morimoto, K.; Fujioka, H.; Kita, Y. Tetrahedron 2009, 65, 10797–10815. doi:10.1016/j.tet.2009.10.040 |
| 96. | Dohi, T.; Yamaoka, N.; Kita, Y. Tetrahedron 2010, 66, 5775–5785. doi:10.1016/j.tet.2010.04.116 |
| 53. | Zhao, Y.; Hu, J.; Chen, R.; Xiong, F.; Xie, H.; Ding, H. J. Am. Chem. Soc. 2022, 144, 2495–2500. doi:10.1021/jacs.1c13370 |
| 54. | Anke, T.; Heim, J.; Knoch, F.; Mocek, U.; Steffan, B.; Steglich, W. Angew. Chem., Int. Ed. Engl. 1985, 24, 709–711. doi:10.1002/anie.198507091 |
| 55. | Li, Y.-Y.; Shen, Y.-M. Helv. Chim. Acta 2010, 93, 2151–2157. doi:10.1002/hlca.200900470 |
| 56. | Rohr, M.; Oleinikov, K.; Jung, M.; Sandjo, L. P.; Opatz, T.; Erkel, G. Bioorg. Med. Chem. 2017, 25, 514–522. doi:10.1016/j.bmc.2016.11.016 |
| 52. | Tao, Y.; Reisenauer, K.; Taube, J. H.; Romo, D. Angew. Chem., Int. Ed. 2019, 58, 2734–2738. doi:10.1002/anie.201812909 |
| 1. | Nicolaou, K. C.; Vourloumis, D.; Winssinger, N.; Baran, P. S. Angew. Chem., Int. Ed. 2000, 39, 44–122. doi:10.1002/(sici)1521-3773(20000103)39:1<44::aid-anie44>3.0.co;2-l |
| 5. | Romero, K. J.; Galliher, M. S.; Pratt, D. A.; Stephenson, C. R. J. Chem. Soc. Rev. 2018, 47, 7851–7866. doi:10.1039/c8cs00379c |
| 65. | Rinderhagen, H.; Waske, P. A.; Mattay, J. Tetrahedron 2006, 62, 6589–6593. doi:10.1016/j.tet.2006.03.060 |
| 4. | Leifert, D.; Studer, A. Angew. Chem., Int. Ed. 2020, 59, 74–108. doi:10.1002/anie.201903726 |
| 3. | Smith, J. M.; Harwood, S. J.; Baran, P. S. Acc. Chem. Res. 2018, 51, 1807–1817. doi:10.1021/acs.accounts.8b00209 |
| 63. | Miller, J. H.; Aagaard, P. J.; Gibson, V. A.; McKinney, M. J. Pharmacol. Exp. Ther. 1992, 263, 663–667. |
| 2. | Kuttruff, C. A.; Eastgate, M. D.; Baran, P. S. Nat. Prod. Rep. 2014, 31, 419–432. doi:10.1039/c3np70090a |
| 64. | Collins, D. J.; Culvenor, C. C. J.; Lamberton, J. A.; Loder, J. W.; Price, J. R. Plants for Medicines: A Chemical and Pharmacological Survey of Plants in the Australian Region; CSIRO Publishing: Clayton, Australia, 1990. doi:10.1071/9780643101203 |
| 11. | Li, C.-J.; Trost, B. M. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 13197–13202. doi:10.1073/pnas.0804348105 |
| 59. | He, C.; Hu, J.; Wu, Y.; Ding, H. J. Am. Chem. Soc. 2017, 139, 6098–6101. doi:10.1021/jacs.7b02746 |
| 60. | Gao, J.; Rao, P.; Xu, K.; Wang, S.; Wu, Y.; He, C.; Ding, H. J. Am. Chem. Soc. 2020, 142, 4592–4597. doi:10.1021/jacs.0c00308 |
| 61. | Wang, B.; Liu, Z.; Tong, Z.; Gao, B.; Ding, H. Angew. Chem., Int. Ed. 2021, 60, 14892–14896. doi:10.1002/anie.202104410 |
| 9. | Anagnostaki, E. E.; Zografos, A. L. Chem. Soc. Rev. 2012, 41, 5613–5625. doi:10.1039/c2cs35080g |
| 10. | Li, L.; Chen, Z.; Zhang, X.; Jia, Y. Chem. Rev. 2018, 118, 3752–3832. doi:10.1021/acs.chemrev.7b00653 |
| 62. | Landwehr, E. M.; Baker, M. A.; Oguma, T.; Burdge, H. E.; Kawajiri, T.; Shenvi, R. A. Science 2022, 375, 1270–1274. doi:10.1126/science.abn8343 |
| 8. | Follmann, M.; Briem, H.; Steinmeyer, A.; Hillisch, A.; Schmitt, M. H.; Haning, H.; Meier, H. Drug Discovery Today 2019, 24, 668–672. doi:10.1016/j.drudis.2018.12.003 |
| 57. | Kupka, J.; Anke, T.; Oberwinkler, F.; Schramm, G.; Steglich, W. J. Antibiot. 1979, 32, 130–135. doi:10.7164/antibiotics.32.130 |
| 6. | Bonjoch, J.; Diaba, F. Eur. J. Org. Chem. 2020, 5070–5100. doi:10.1002/ejoc.202000391 |
| 7. | Hung, K.; Hu, X.; Maimone, T. J. Nat. Prod. Rep. 2018, 35, 174–202. doi:10.1039/c7np00065k |
| 68. | Liu, X.-Y.; Qin, Y. Acc. Chem. Res. 2019, 52, 1877–1891. doi:10.1021/acs.accounts.9b00246 |
| 69. | Zhou, Q.; Dai, X.; Song, H.; He, H.; Wang, X.; Liu, X.-Y.; Qin, Y. Chem. Commun. 2018, 54, 9510–9512. doi:10.1039/c8cc05374j |
| 70. | Kam, T.-S. Alkaloids from Malaysian Flora. In Alkaloids: Chemical and Biological Perspectives; Pelletier, S. W., Ed.; Pergamon Press: Oxford, UK, 1999; p 350. |
| 66. | Milligan, J. A.; Phelan, J. P.; Badir, S. O.; Molander, G. A. Angew. Chem., Int. Ed. 2019, 58, 6152–6163. doi:10.1002/anie.201809431 |
| 67. | Varabyeva, N.; Barysevich, M.; Aniskevich, Y.; Hurski, A. Org. Lett. 2021, 23, 5452–5456. doi:10.1021/acs.orglett.1c01795 |
| 75. | Abdurakhimova, N.; Yuldashev, P. K.; Yunusov, S. Y. Chem. Nat. Compd. 1967, 3, 263–266. doi:10.1007/bf00574630 |
| 76. | Ishikawa, H.; Colby, D. A.; Seto, S.; Va, P.; Tam, A.; Kakei, H.; Rayl, T. J.; Hwang, I.; Boger, D. L. J. Am. Chem. Soc. 2009, 131, 4904–4916. doi:10.1021/ja809842b |
| 73. | Alabugin, I. V.; Harris, T. Chem 2017, 2, 753–755. doi:10.1016/j.chempr.2017.05.018 |
| 74. | Zeng, X.; Shukla, V.; Boger, D. L. J. Org. Chem. 2020, 85, 14817–14826. doi:10.1021/acs.joc.0c02493 |
| 71. | Yap, W.-S.; Gan, C.-Y.; Sim, K.-S.; Lim, S.-H.; Low, Y.-Y.; Kam, T.-S. J. Nat. Prod. 2016, 79, 230–239. doi:10.1021/acs.jnatprod.5b00992 |
| 72. | Wang, X.; Xia, D.; Qin, W.; Zhou, R.; Zhou, X.; Zhou, Q.; Liu, W.; Dai, X.; Wang, H.; Wang, S.; Tan, L.; Zhang, D.; Song, H.; Liu, X.-Y.; Qin, Y. Chem 2017, 2, 803–816. doi:10.1016/j.chempr.2017.04.007 |
| 31. | Everson, D. A.; Shrestha, R.; Weix, D. J. J. Am. Chem. Soc. 2010, 132, 920–921. doi:10.1021/ja9093956 |
| 33. | Zhang, S.; Wang, X.; Hao, J.; Li, D.; Csuk, R.; Li, S. J. Nat. Prod. 2018, 81, 2010–2017. doi:10.1021/acs.jnatprod.8b00310 |
| 40. | Wu, J.; Kadonaga, Y.; Hong, B.; Wang, J.; Lei, X. Angew. Chem., Int. Ed. 2019, 58, 10879–10883. doi:10.1002/anie.201903682 |
| 41. | Sun, H.-D.; Huang, S.-X.; Han, Q.-B. Nat. Prod. Rep. 2006, 23, 673–698. doi:10.1039/b604174d |
| 38. | Jiao, W.-H.; Xu, T.-T.; Yu, H.-B.; Chen, G.-D.; Huang, X.-J.; Yang, F.; Li, Y.-S.; Han, B.-N.; Liu, X.-Y.; Lin, H.-W. J. Nat. Prod. 2014, 77, 346–350. doi:10.1021/np4009392 |
| 39. | Jiao, W.-H.; Shi, G.-H.; Xu, T.-T.; Chen, G.-D.; Gu, B.-B.; Wang, Z.; Peng, S.; Wang, S.-P.; Li, J.; Han, B.-N.; Zhang, W.; Lin, H.-W. J. Nat. Prod. 2016, 79, 406–411. doi:10.1021/acs.jnatprod.5b01079 |
| 36. | Barrero, A. F.; Alvarez‐Manzaneda, E. J.; Chahboun, R.; Arteaga, A. F. Synth. Commun. 2004, 34, 3631–3643. doi:10.1081/scc-200031056 |
| 37. | Chong, C.; Zhang, Q.; Ke, J.; Zhang, H.; Yang, X.; Wang, B.; Ding, W.; Lu, Z. Angew. Chem., Int. Ed. 2021, 60, 13807–13813. doi:10.1002/anie.202100541 |
| 34. | Shan, W.-G.; Ying, Y.-M.; Ma, L.-F.; Zhan, Z.-J. Drimane-Related Merosesquiterpenoids, a Promising Library of Metabolites for Drug Development. In Studies in Natural Products Chemistry; Attar-ur-Rahman, Ed.; Elsevier: Amsterdam, Netherlands, 2015; Vol. 45, pp 147–215. doi:10.1016/b978-0-444-63473-3.00006-x |
| 35. | Dixon, D. D.; Lockner, J. W.; Zhou, Q.; Baran, P. S. J. Am. Chem. Soc. 2012, 134, 8432–8435. doi:10.1021/ja303937y |
| 42. | Li, L.-M.; Li, G.-Y.; Xiao, W.-L.; Zhou, Y.; Li, S.-H.; Huang, S.-X.; Han, Q.-B.; Ding, L.-S.; Lou, L.-G.; Sun, H.-D. Tetrahedron Lett. 2006, 47, 5187–5190. doi:10.1016/j.tetlet.2006.05.025 |
| 43. | Qu, J.-B.; Zhu, R.-L.; Zhang, Y.-L.; Guo, H.-F.; Wang, X.-N.; Xie, C.-F.; Yu, W.-T.; Ji, M.; Lou, H.-X. J. Nat. Prod. 2008, 71, 1418–1422. doi:10.1021/np8003062 |
| 43. | Qu, J.-B.; Zhu, R.-L.; Zhang, Y.-L.; Guo, H.-F.; Wang, X.-N.; Xie, C.-F.; Yu, W.-T.; Ji, M.; Lou, H.-X. J. Nat. Prod. 2008, 71, 1418–1422. doi:10.1021/np8003062 |
| 48. | Chen, K.; Zhang, X.; Sun, W.; Liu, J.; Yang, J.; Chen, C.; Liu, X.; Gao, L.; Wang, J.; Li, H.; Luo, Z.; Xue, Y.; Zhu, H.; Zhang, Y. Org. Lett. 2017, 19, 5956–5959. doi:10.1021/acs.orglett.7b02955 |
| 50. | Yan, Z.; Zhao, C.; Gong, J.; Yang, Z. Org. Lett. 2020, 22, 1644–1647. doi:10.1021/acs.orglett.0c00241 |
| 51. | Zhang, Y.-A.; Milkovits, A.; Agarawal, V.; Taylor, C. A.; Snyder, S. A. Angew. Chem., Int. Ed. 2021, 60, 11127–11132. doi:10.1002/anie.202016178 |
| 47. | Zong, Y.; Xu, Z.-J.; Zhu, R.-X.; Su, A.-H.; Liu, X.-Y.; Zhu, M.-Z.; Han, J.-J.; Zhang, J.-Z.; Xu, Y.-L.; Lou, H.-X. Angew. Chem., Int. Ed. 2021, 60, 15286–15290. doi:10.1002/anie.202104182 |
| 48. | Chen, K.; Zhang, X.; Sun, W.; Liu, J.; Yang, J.; Chen, C.; Liu, X.; Gao, L.; Wang, J.; Li, H.; Luo, Z.; Xue, Y.; Zhu, H.; Zhang, Y. Org. Lett. 2017, 19, 5956–5959. doi:10.1021/acs.orglett.7b02955 |
| 49. | Yuan, W. H.; Liu, M.; Jiang, N.; Guo, Z. K.; Ma, J.; Zhang, J.; Song, Y. C.; Tan, R. X. Eur. J. Org. Chem. 2010, 6348–6353. doi:10.1002/ejoc.201000916 |
| 45. | Xiao, Z.-K.; Shao, L.-X. Synthesis 2012, 44, 711–716. doi:10.1055/s-0031-1289698 |
| 46. | Liu, W.; Li, H.; Cai, P.-J.; Wang, Z.; Yu, Z.-X.; Lei, X. Angew. Chem., Int. Ed. 2016, 55, 3112–3116. doi:10.1002/anie.201511659 |
| 43. | Qu, J.-B.; Zhu, R.-L.; Zhang, Y.-L.; Guo, H.-F.; Wang, X.-N.; Xie, C.-F.; Yu, W.-T.; Ji, M.; Lou, H.-X. J. Nat. Prod. 2008, 71, 1418–1422. doi:10.1021/np8003062 |
| 44. | Maksymowicz, R. M.; Roth, P. M. C.; Fletcher, S. P. Nat. Chem. 2012, 4, 649–654. doi:10.1038/nchem.1394 |
| 105. | Saleem, M.; Kim, H. J.; Ali, M. S.; Lee, Y. S. Nat. Prod. Rep. 2005, 22, 696–716. doi:10.1039/b514045p |
| 106. | Zhang, X.; Rakesh, K. P.; Shantharam, C. S.; Manukumar, H. M.; Asiri, A. M.; Marwani, H. M.; Qin, H.-L. Bioorg. Med. Chem. 2018, 26, 340–355. doi:10.1016/j.bmc.2017.11.026 |
| 103. | Xiang, J.-C.; Fung, C.; Wang, Q.; Zhu, J. Nat. Commun. 2022, 13, 3481. doi:10.1038/s41467-022-31000-4 |
| 104. | Teponno, R. B.; Kusari, S.; Spiteller, M. Nat. Prod. Rep. 2016, 33, 1044–1092. doi:10.1039/c6np00021e |
| 101. | Kirillova, M. S.; Muratore, M. E.; Dorel, R.; Echavarren, A. M. J. Am. Chem. Soc. 2016, 138, 3671–3674. doi:10.1021/jacs.6b01428 |
| 102. | Gentry, E. C.; Rono, L. J.; Hale, M. E.; Matsuura, R.; Knowles, R. R. J. Am. Chem. Soc. 2018, 140, 3394–3402. doi:10.1021/jacs.7b13616 |
| 98. | Awang, K.; Sévenet, T.; Hamid, A.; Hadi, A.; David, B.; Païs, M. Tetrahedron Lett. 1992, 33, 2493–2496. doi:10.1016/s0040-4039(00)92223-8 |
| 99. | Awang, K.; Sévenet, T.; Païs, M.; Hadi, A. H. A. J. Nat. Prod. 1993, 56, 1134–1139. doi:10.1021/np50097a018 |
| 100. | Yap, W.-S.; Gan, C.-Y.; Low, Y.-Y.; Choo, Y.-M.; Etoh, T.; Hayashi, M.; Komiyama, K.; Kam, T.-S. J. Nat. Prod. 2011, 74, 1309–1312. doi:10.1021/np200008g |
| 17. | Herrmann, J. M.; König, B. Eur. J. Org. Chem. 2013, 7017–7027. doi:10.1002/ejoc.201300657 |
| 18. | Nicolaou, K. C.; Ellery, S. P.; Chen, J. S. Angew. Chem., Int. Ed. 2009, 48, 7140–7165. doi:10.1002/anie.200902151 |
| 15. | Giese, B.; Meister, J. Chem. Ber. 1977, 110, 2588–2600. doi:10.1002/cber.19771100717 |
| 13. | Young, I. S.; Baran, P. S. Nat. Chem. 2009, 1, 193–205. doi:10.1038/nchem.216 |
| 110. | Huang, Z.; Lumb, J.-P. Nat. Chem. 2021, 13, 24–32. doi:10.1038/s41557-020-00603-z |
| 6. | Bonjoch, J.; Diaba, F. Eur. J. Org. Chem. 2020, 5070–5100. doi:10.1002/ejoc.202000391 |
| 10. | Li, L.; Chen, Z.; Zhang, X.; Jia, Y. Chem. Rev. 2018, 118, 3752–3832. doi:10.1021/acs.chemrev.7b00653 |
| 14. | Galliher, M. S.; Roldan, B. J.; Stephenson, C. R. J. Chem. Soc. Rev. 2021, 50, 10044–10057. doi:10.1039/d1cs00411e |
| 107. | Xiang, J.-C.; Wang, Q.; Zhu, J. Angew. Chem., Int. Ed. 2020, 59, 21195–21202. doi:10.1002/anie.202007548 |
| 12. | Gaich, T.; Baran, P. S. J. Org. Chem. 2010, 75, 4657–4673. doi:10.1021/jo1006812 |
| 108. | Beckwith, A. L. J.; Schiesser, C. H. Tetrahedron 1985, 41, 3925–3941. doi:10.1016/s0040-4020(01)97174-1 |
| 109. | Spellmeyer, D. C.; Houk, K. N. J. Org. Chem. 1987, 52, 959–974. doi:10.1021/jo00382a001 |
| 21. | Pitre, S. P.; Overman, L. E. Chem. Rev. 2022, 122, 1717–1751. doi:10.1021/acs.chemrev.1c00247 |
| 20. | Shevick, S. L.; Wilson, C. V.; Kotesova, S.; Kim, D.; Holland, P. L.; Shenvi, R. A. Chem. Sci. 2020, 11, 12401–12422. doi:10.1039/d0sc04112b |
| 30. | Hubert, C.; Moreau, J.; Batany, J.; Duboc, A.; Hurvois, J.-P.; Renaud, J.-L. Adv. Synth. Catal. 2008, 350, 40–42. doi:10.1002/adsc.200700375 |
| 20. | Shevick, S. L.; Wilson, C. V.; Kotesova, S.; Kim, D.; Holland, P. L.; Shenvi, R. A. Chem. Sci. 2020, 11, 12401–12422. doi:10.1039/d0sc04112b |
| 23. | Merchant, R. R.; Oberg, K. M.; Lin, Y.; Novak, A. J. E.; Felding, J.; Baran, P. S. J. Am. Chem. Soc. 2018, 140, 7462–7465. doi:10.1021/jacs.8b04891 |
| 29. | Li, J.; Li, F.; King-Smith, E.; Renata, H. Nat. Chem. 2020, 12, 173–179. doi:10.1038/s41557-019-0407-6 |
| 24. | Engel, B.; Erkel, G.; Anke, T.; Sterner, O. J. Antibiot. 1998, 51, 518–521. doi:10.7164/antibiotics.51.518 |
| 25. | Uchida, R.; Imasato, R.; Yamaguchi, Y.; Masuma, R.; Shiomi, K.; Tomoda, H.; Ōmura, S. J. Antibiot. 2005, 58, 804–809. doi:10.1038/ja.2005.107 |
| 26. | Lee, J. C.; Lobkovsky, E.; Pliam, N. B.; Strobel, G.; Clardy, J. J. Org. Chem. 1995, 60, 7076–7077. doi:10.1021/jo00127a001 |
| 27. | Qin, T.; Cornella, J.; Li, C.; Malins, L. R.; Edwards, J. T.; Kawamura, S.; Maxwell, B. D.; Eastgate, M. D.; Baran, P. S. Science 2016, 352, 801–805. doi:10.1126/science.aaf6123 |
| 28. | Qin, T.; Malins, L. R.; Edwards, J. T.; Merchant, R. R.; Novak, A. J. E.; Zhong, J. Z.; Mills, R. B.; Yan, M.; Yuan, C.; Eastgate, M. D.; Baran, P. S. Angew. Chem., Int. Ed. 2017, 56, 260–265. doi:10.1002/anie.201609662 |
| 22. | Novaes, L. F. T.; Liu, J.; Shen, Y.; Lu, L.; Meinhardt, J. M.; Lin, S. Chem. Soc. Rev. 2021, 50, 7941–8002. doi:10.1039/d1cs00223f |
| 23. | Merchant, R. R.; Oberg, K. M.; Lin, Y.; Novak, A. J. E.; Felding, J.; Baran, P. S. J. Am. Chem. Soc. 2018, 140, 7462–7465. doi:10.1021/jacs.8b04891 |
© 2023 Gennaiou et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.