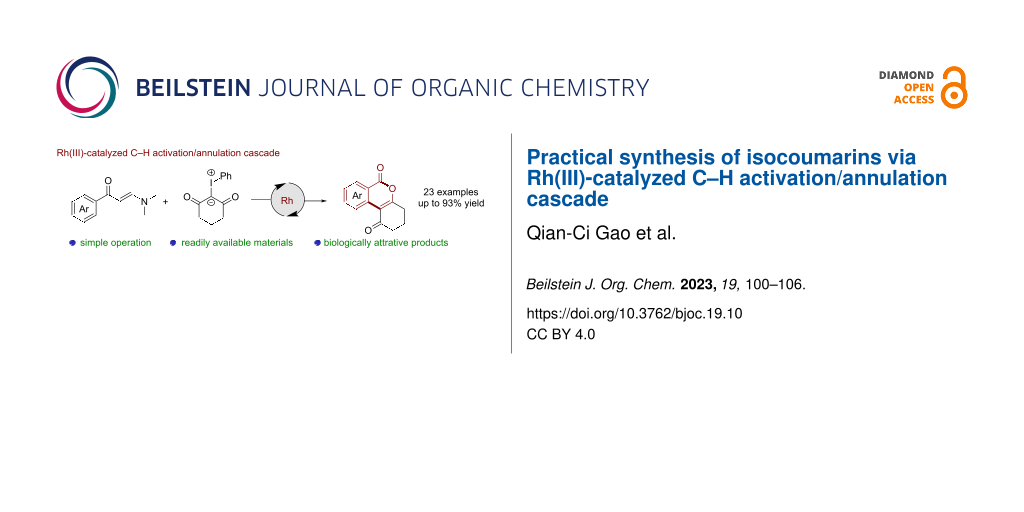
Abstract
Herein, we report an unprecedented Rh(III)-catalyzed C–H activation/annulation cascade of readily available enaminones with iodonium ylides towards the convenient synthesis of isocoumarins. This coupling system proceeds in useful chemical yields (up to 93%) via a cascade C–H activation, Rh-carbenoid migratory insertion and acid-promoted intramolecular annulation. The success of gram-scale reaction and diverse functionalization of isocoumarins demonstrated the synthetic utility of this protocol.
Graphical Abstract
Introduction
Isocoumarins are an important structural motif in many naturally occurring lactones isolated from bacterial strains, molds, and plants, exhibiting a wide range of pharmacological properties such as antibacterial, antitumor, and anti-HIV activities (Scheme 1a) [1-5]. Fascinated by their versatile properties, researchers were prompted to develop efficient methods for the synthesis of isocoumarin scaffolds. Traditional synthetic strategies including 1) intramolecular cyclization of 2-alkenyl benzoic acids or o‑alkynylbenzoates (Scheme 1b, I) [6-10], 2) oxidation of isochromans (Scheme 1b, II) [11,12], or 3) metal-catalyzed cross-coupling/cyclization of 1,2-difunctionalized arenes with alkynes or carbon monoxide (Scheme 1b, III) [13-16], have been widely applied for the assembly of isocoumarins over the past decades. Recently, the transition-metal-catalyzed ortho C–H activation/annulation of benzoic acids has emerged as an attractive approach towards isocoumarins [17,18]. Pioneering examples relying on the Pd, Ru, and Ir-catalyzed C–H cross coupling of benzoic acids with alkenes and alkynes were realized by the groups of Miura [19], Lee [20], Ackermann [21], Zhang [22], Jiang [23], and Jeganmohan [24] et al. Despite these impressive achievements, established methods often require the use of stoichiometric oxidants or harsh conditions, thus limiting their broad applicability. Consequently, it is still highly desirable to exploit innovative and convenient methods for the rapid construction of isocoumarins.
Scheme 1: Significance of isocoumarins (a), classic methods for the synthesis of isocoumarins (b) and reaction design (c).
Scheme 1: Significance of isocoumarins (a), classic methods for the synthesis of isocoumarins (b) and reactio...
Enaminones are bench stable and easily available, which have been established as versatile synthetic building blocks for the synthesis of cyclic scaffolds [25]. In 2016, Zhu et al. reported the first example of a Rh-catalyzed C–H functionalization of enaminones with alkynes and α-diazo-β-ketoesters to access naphthalenes [26]. Very recently, the same group developed an efficient Rh(III)-catalyzed C–H cross-coupling of enaminones with diazodicarbonyls for the divergent construction of isocoumarins and naphthalenes [27]. Moreover, Loh et al. disclosed a Rh-catalyzed formal [4 + 2] cycloaddition of enaminones with diazocarbonyls [28]. Compared with highly sensitive diazo compounds, iodonium ylides are known to show ready availability and good stability [29,30]. Our group has recently demonstrated that iodonium ylides can be used as carbene precursors in the Rh-catalyzed [4 + 2] cyclization of pyrazolidinones [31]. During the preparation of manuscript, the group of Li reported a similar Rh(III)-catalyzed [3 + 3] annulation of enaminones with iodonium ylides [32]. Inspired by the collected contributions [26-28] and based on our ongoing research in C–H activation [33-35], we recently wondered whether it might be possible to couple iodonium ylides with enaminones in a Rh(III)-catalyzed C–H activation/annulation cascade reaction for the rapid construction of isocoumarins (Scheme 1c).
Results and Discussion
Our initial experiment was performed with enaminone 1a and iodonium ylide 1b in the presence of [Cp*RhCl2]2 (5 mol %) as the catalyst, AgSbF6 (10 mol %) and KOAc (50 mol %) as additives in 1,2-dichloroethane (DCE) at 100 °C for 16 hours. To our delight, the desired isocoumarin 3aa was obtained in 42% yield (Table 1, entry 1). Then, the influence of solvents has been subsequently investigated. As a result, DCE proved to be the optimal solvent, while other commonly used solvents such as toluene, dioxane, and ethanol gave inferior yields (Table 1, entries 2–4, 14–39%). Further screening of bases did not improve the outcome of the product, whereas 63% yield of 3aa was obtained when acetic acid was added into the reaction system (Table 1, entry 8). Increasing the amount of acetic acid significantly improved the reaction efficiency delivering product 3aa in 80% yield (Table 1, entry 9). The choice of a suitable catalyst was the key factor for the success of this reaction since only a trace amount of 3aa can be obtained by using [{Ru(p-cymene)Cl2}]2 as a catalyst (Table 1, entry 10).
Table 1: Optimization of the reaction conditionsa.
|
|
||||
| entry | catalyst | additive | solvent | yieldb |
| 1 | [Cp*RhCl2]2 | KOAc | DCE | 42 |
| 2 | [Cp*RhCl2]2 | KOAc | toluene | 39 |
| 3 | [Cp*RhCl2]2 | KOAc | dioxane | 16 |
| 4 | [Cp*RhCl2]2 | KOAc | EtOH | 14 |
| 5 | [Cp*RhCl2]2 | Cs(OPiv)2 | DCE | 47 |
| 6 | [Cp*RhCl2]2 | Cs(OAc)2 | DCE | 51 |
| 7 | [Cp*RhCl2]2 | K2CO3 | DCE | 27 |
| 8 | [Cp*RhCl2]2 | AcOH | DCE | 63 |
| 9c | [Cp*RhCl2]2 | AcOH | DCE | 80 |
| 10 | [{Ru(p-cymene)Cl2}]2 | AcOH | DCE | trace |
aStandard conditions: 1a (0.2 mmol), 2a (0.6 mmol), catalyst (5 mol %), AgSbF6 (10 mol %), additive (0.5 equiv), solvent (2.0 mL) at 100 °C for 16 h. bIsolated yields. cAcOH (5.0 equiv) was used.
With the optimal conditions in hand, we then investigated the scope of this Rh-catalyzed C–H activation/annulation cascade reaction. As shown in Scheme 2, a range of functionalized enaminones were compatible with this Rh-catalytic system, furnishing the corresponding isocoumarin products in satisfying yields. For example, enaminones bearing electron-donating (Me, OEt and t-Bu), as well as electron-withdrawing groups (F, Cl, Br, I, CF3, CN and NO2) at ortho, meta or para-positions were well tolerated in this transformation, delivering a variety of structurally diverse isocoumarins in an efficient manner (3aa–qa, 43–82%). It is worth mentioning that the tolerance of halogen substituents (Cl, Br and I) may open up a new opportunity for further transition-metal-catalyzed cross-coupling reactions. Sensitive groups, such as ester, trifluoromethyl and nitro substituents, were retained unchanged in the final products (3ja, 3ka, 3ma, and 3na). Also the strongly coordinating thioether substituent proved to be suitable for this protocol, providing the desired product 3ea in 76% yield. Moreover, under the standard conditions, 3-thienyl and 2-naphthyl-substituted enaminones were smoothly coupled with iodonium ylide 1b to give the expected isocoumarins 3ra and 3sa in 60% and 78%, respectively.
Next, we sought to test the generality of this reaction with respect to iodonium ylides. As outlined in Scheme 3, iodonium ylides featuring dimethyl, methyl, and phenyl groups underwent the current reaction efficiently, delivering the desired products 3ab–ae in moderate to good yields (43–93%).
To demonstrate the synthetic utility of this methodology, a gram-scale synthesis of isocoumarin 3ia was firstly performed. Under the optimal conditions, the desired product 3ia was successfully obtained in 84% yield (1.1 g) via a simple recrystallization from the reaction mixture (Scheme 4a). In the presence of hydroxylamine hydrochloride, the carbonyl group of the ketone can be selectively converted into an oxime product 4 (Scheme 4b, 71% yield). In addition, the reaction of the isocoumarin 3ia with p-toluenesulfonyl hydrazide proceeded smoothly to deliver hydrazone 5 in 66% yield (Scheme 4b, right). Of note, oxime and hydrazone compounds are versatile synthetic building blocks, which have been widely applied in transition-metal-catalyzed cross-coupling reactions and radical transformations [36-38].
Scheme 4: Gram-scale reaction (a) and synthetic transformation (b).
Scheme 4: Gram-scale reaction (a) and synthetic transformation (b).
Based on the literature precedents [27] and our previous work [33-35], a mechanism for this Rh-catalyzed C–H activation/annulation reaction was proposed and depicted in Scheme 5. In the presence of AgSbF6, dimeric [Cp*RhCl2]2 transforms into the active Rh catalyst. Subsequently, the oxygen atom of the enaminone is coordinated to the Rh catalyst, following by a Rh(III)-promoted ortho C–H activation to form a five-membered ruthenacycle 1-A. Then, the reaction of the iodonium ylide with intermediate 1-A generates a Rh-carbenoid intermediate 1-B, which undergoes a rapid migratory insertion to give intermediate 1-C. The protonation of 1-C produces the intermediate 1-D with the regeneration of the active Rh catalyst for the next catalytic cycle. Under acidic conditions, the further protonation of compound 1-D delivers an imine intermediate 1-E, which undergoes an intramolecular annulation to give 1-F. The final isocoumarin product 3ba can be generated from 1-F by elimination of imine 1-G [39]. Finally, the rapid hydrolysis of the resulting 1-G gives rise to acetaldehyde and dimethylamine as byproducts.
Conclusion
In summary, an efficient Rh-catalyzed C–H activation/annulation reaction of enaminones with iodonium ylides has been developed. This reaction features simple operation and readily available substrates, enabling the rapid construction of biologically attractive isocoumarins in useful to good yields. The success of a gram-scale reaction and diverse functionalization of the isocoumarin products highlight the tremendous synthetic potential of this methodology in chemical synthesis and drug discovery.
Experimental
A 10 mL screw-cap vial was charged with enaminone 1 (0.2 mmol), iodonium ylide 2 (0.6 mmol), [Cp*RhCl2]2 (6.2 mg, 5 mol %), AgSbF6 (6.9 mg, 10 mol %), HOAc (60.0 mg, 1.0 mmol) and DCE (2 mL) under N2 atmosphere. Then, the reaction mixture was stirred at 100 °C for 16 h. The crude product was purified by flash chromatography on silica gel (petroleum ether/ethyl acetate 5:1) directly to give the desired products 3. (Note: a heating module was used for the preparation of isocoumarin products 3.)
Supporting Information
| Supporting Information File 1: Experimental and copies of spectra. | ||
| Format: PDF | Size: 4.4 MB | Download |
References
-
Barry, R. D. Chem. Rev. 1964, 64, 229–260. doi:10.1021/cr60229a002
Return to citation in text: [1] -
Tianpanich, K.; Prachya, S.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. J. Nat. Prod. 2011, 74, 79–81. doi:10.1021/np1003752
Return to citation in text: [1] -
Chaudhary, N. K.; Pitt, J. I.; Lacey, E.; Crombie, A.; Vuong, D.; Piggott, A. M.; Karuso, P. J. Nat. Prod. 2018, 81, 1517–1526. doi:10.1021/acs.jnatprod.7b00816
Return to citation in text: [1] -
Bai, M.; Zheng, C.-J.; Huang, G.-L.; Mei, R.-Q.; Wang, B.; Luo, Y.-P.; Zheng, C.; Niu, Z.-G.; Chen, G.-Y. J. Nat. Prod. 2019, 82, 1155–1164. doi:10.1021/acs.jnatprod.8b00866
Return to citation in text: [1] -
Cai, R.; Wu, Y.; Chen, S.; Cui, H.; Liu, Z.; Li, C.; She, Z. J. Nat. Prod. 2018, 81, 1376–1383. doi:10.1021/acs.jnatprod.7b01018
Return to citation in text: [1] -
Yao, T.; Larock, R. C. J. Org. Chem. 2003, 68, 5936–5942. doi:10.1021/jo034308v
Return to citation in text: [1] -
Mallampudi, N. A.; Reddy, G. S.; Maity, S.; Mohapatra, D. K. Org. Lett. 2017, 19, 2074–2077. doi:10.1021/acs.orglett.7b00673
Return to citation in text: [1] -
Chen, B.; Ma, S. Org. Lett. 2013, 15, 3884–3887. doi:10.1021/ol401625t
Return to citation in text: [1] -
Faizi, D. J.; Issaian, A.; Davis, A. J.; Blum, S. A. J. Am. Chem. Soc. 2016, 138, 2126–2129. doi:10.1021/jacs.5b12989
Return to citation in text: [1] -
Xing, L.; Zhang, Y.; Li, B.; Du, Y. Org. Lett. 2019, 21, 1989–1993. doi:10.1021/acs.orglett.9b00047
Return to citation in text: [1] -
Finney, L. C.; Mitchell, L. J.; Moody, C. J. Green Chem. 2018, 20, 2242–2249. doi:10.1039/c7gc03741d
Return to citation in text: [1] -
Xia, Q.; Wang, Q.; Yan, C.; Dong, J.; Song, H.; Li, L.; Liu, Y.; Wang, Q.; Liu, X.; Song, H. Chem. – Eur. J. 2017, 23, 10871–10877. doi:10.1002/chem.201701755
Return to citation in text: [1] -
Bera, S.; Mondal, P.; Sarkar, D.; Pathi, V. B.; Pakrashy, S.; Datta, A.; Banerji, B. J. Org. Chem. 2021, 86, 7069–7077. doi:10.1021/acs.joc.1c00311
Return to citation in text: [1] -
Pati, B. V.; Banjare, S. K.; Das Adhikari, G. K.; Nanda, T.; Ravikumar, P. C. Org. Lett. 2022, 24, 5651–5656. doi:10.1021/acs.orglett.2c01901
Return to citation in text: [1] -
Li, Y.; Wang, Q.; Yang, X.; Xie, F.; Li, X. Org. Lett. 2017, 19, 3410–3413. doi:10.1021/acs.orglett.7b01365
Return to citation in text: [1] -
Subramanian, V.; Batchu, V. R.; Barange, D.; Pal, M. J. Org. Chem. 2005, 70, 4778–4783. doi:10.1021/jo050440e
Return to citation in text: [1] -
Luo, F. Chin. J. Org. Chem. 2019, 39, 3084. doi:10.6023/cjoc201905027
Return to citation in text: [1] -
Pichette Drapeau, M.; Gooßen, L. J. Chem. – Eur. J. 2016, 22, 18654–18677. doi:10.1002/chem.201603263
Return to citation in text: [1] -
Miura, M.; Tsuda, T.; Satoh, T.; Pivsa-Art, S.; Nomura, M. J. Org. Chem. 1998, 63, 5211–5215. doi:10.1021/jo980584b
Return to citation in text: [1] -
Nandi, D.; Ghosh, D.; Chen, S.-J.; Kuo, B.-C.; Wang, N. M.; Lee, H. M. J. Org. Chem. 2013, 78, 3445–3451. doi:10.1021/jo400174w
Return to citation in text: [1] -
Warratz, S.; Kornhaaß, C.; Cajaraville, A.; Niepötter, B.; Stalke, D.; Ackermann, L. Angew. Chem., Int. Ed. 2015, 54, 5513–5517. doi:10.1002/anie.201500600
Return to citation in text: [1] -
Hong, C.; Yu, S.; Liu, Z.; Zhang, Y. Org. Lett. 2022, 24, 815–820. doi:10.1021/acs.orglett.1c03992
Return to citation in text: [1] -
Jiang, G.; Li, J.; Zhu, C.; Wu, W.; Jiang, H. Org. Lett. 2017, 19, 4440–4443. doi:10.1021/acs.orglett.7b01919
Return to citation in text: [1] -
Chinnagolla, R. K.; Jeganmohan, M. Chem. Commun. 2012, 48, 2030–2032. doi:10.1039/c2cc16916a
Return to citation in text: [1] -
Chen, X. Y.; Zhang, X.; Wan, J.-P. Org. Biomol. Chem. 2022, 20, 2356–2369. doi:10.1039/d2ob00126h
Return to citation in text: [1] -
Zhou, S.; Wang, J.; Wang, L.; Song, C.; Chen, K.; Zhu, J. Angew. Chem., Int. Ed. 2016, 55, 9384–9388. doi:10.1002/anie.201603943
Return to citation in text: [1] [2] -
Wu, W.; Wu, X.; Fan, S.; Zhu, J. Org. Lett. 2022, 24, 7850–7855. doi:10.1021/acs.orglett.2c03288
Return to citation in text: [1] [2] [3] -
Zhou, S.; Yan, B.-W.; Fan, S.-X.; Tian, J.-S.; Loh, T.-P. Org. Lett. 2018, 20, 3975–3979. doi:10.1021/acs.orglett.8b01540
Return to citation in text: [1] [2] -
Zhao, Y.-R.; Li, L.; Xu, G.-Y.; Xuan, J. Adv. Synth. Catal. 2022, 364, 506–511. doi:10.1002/adsc.202101144
Return to citation in text: [1] -
Jiang, Y.; Li, P.; Zhao, J.; Liu, B.; Li, X. Org. Lett. 2020, 22, 7475–7479. doi:10.1021/acs.orglett.0c02618
Return to citation in text: [1] -
Li, R.; Hou, Y.-X.; Xu, J.-H.; Gao, Y.; Hu, X.-Q. Org. Chem. Front. 2022, 9, 2181–2186. doi:10.1039/d2qo00144f
Return to citation in text: [1] -
Yang, Z.; Liu, C.; Lei, J.; Zhou, Y.; Gao, X.; Li, Y. Chem. Commun. 2022, 58, 13483–13486. doi:10.1039/d2cc05899e
Return to citation in text: [1] -
Hu, X.-Q.; Liu, Z.-K.; Hou, Y.-X.; Zhang, G.; Gao, Y. Chem. Commun. 2021, 57, 1113–1116. doi:10.1039/d0cc07573f
Return to citation in text: [1] [2] -
Hu, X.-Q.; Liu, Z.-K.; Hou, Y.-X.; Xu, J.-H.; Gao, Y. Org. Lett. 2021, 23, 6332–6336. doi:10.1021/acs.orglett.1c02131
Return to citation in text: [1] [2] -
Gao, Y.; Nie, J.; Li, Y.; Li, X.; Chen, Q.; Huo, Y.; Hu, X.-Q. Org. Lett. 2020, 22, 2600–2605. doi:10.1021/acs.orglett.0c00539
Return to citation in text: [1] [2] -
Xia, Y.; Wang, J. Chem. Soc. Rev. 2017, 46, 2306–2362. doi:10.1039/c6cs00737f
Return to citation in text: [1] -
Chen, J.-R.; Hu, X.-Q.; Lu, L.-Q.; Xiao, W.-J. Chem. Soc. Rev. 2016, 45, 2044–2056. doi:10.1039/c5cs00655d
Return to citation in text: [1] -
Cai, B.; Xuan, J. Chin. J. Org. Chem. 2021, 41, 4565. doi:10.6023/cjoc202109040
Return to citation in text: [1] -
Kumar, S.; Nunewar, S.; Kanchupalli, V. Asian J. Org. Chem. 2022, 11, e202100689. doi:10.1002/ajoc.202100689
Return to citation in text: [1]
| 26. | Zhou, S.; Wang, J.; Wang, L.; Song, C.; Chen, K.; Zhu, J. Angew. Chem., Int. Ed. 2016, 55, 9384–9388. doi:10.1002/anie.201603943 |
| 27. | Wu, W.; Wu, X.; Fan, S.; Zhu, J. Org. Lett. 2022, 24, 7850–7855. doi:10.1021/acs.orglett.2c03288 |
| 28. | Zhou, S.; Yan, B.-W.; Fan, S.-X.; Tian, J.-S.; Loh, T.-P. Org. Lett. 2018, 20, 3975–3979. doi:10.1021/acs.orglett.8b01540 |
| 31. | Li, R.; Hou, Y.-X.; Xu, J.-H.; Gao, Y.; Hu, X.-Q. Org. Chem. Front. 2022, 9, 2181–2186. doi:10.1039/d2qo00144f |
| 32. | Yang, Z.; Liu, C.; Lei, J.; Zhou, Y.; Gao, X.; Li, Y. Chem. Commun. 2022, 58, 13483–13486. doi:10.1039/d2cc05899e |
| 1. | Barry, R. D. Chem. Rev. 1964, 64, 229–260. doi:10.1021/cr60229a002 |
| 2. | Tianpanich, K.; Prachya, S.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. J. Nat. Prod. 2011, 74, 79–81. doi:10.1021/np1003752 |
| 3. | Chaudhary, N. K.; Pitt, J. I.; Lacey, E.; Crombie, A.; Vuong, D.; Piggott, A. M.; Karuso, P. J. Nat. Prod. 2018, 81, 1517–1526. doi:10.1021/acs.jnatprod.7b00816 |
| 4. | Bai, M.; Zheng, C.-J.; Huang, G.-L.; Mei, R.-Q.; Wang, B.; Luo, Y.-P.; Zheng, C.; Niu, Z.-G.; Chen, G.-Y. J. Nat. Prod. 2019, 82, 1155–1164. doi:10.1021/acs.jnatprod.8b00866 |
| 5. | Cai, R.; Wu, Y.; Chen, S.; Cui, H.; Liu, Z.; Li, C.; She, Z. J. Nat. Prod. 2018, 81, 1376–1383. doi:10.1021/acs.jnatprod.7b01018 |
| 17. | Luo, F. Chin. J. Org. Chem. 2019, 39, 3084. doi:10.6023/cjoc201905027 |
| 18. | Pichette Drapeau, M.; Gooßen, L. J. Chem. – Eur. J. 2016, 22, 18654–18677. doi:10.1002/chem.201603263 |
| 28. | Zhou, S.; Yan, B.-W.; Fan, S.-X.; Tian, J.-S.; Loh, T.-P. Org. Lett. 2018, 20, 3975–3979. doi:10.1021/acs.orglett.8b01540 |
| 13. | Bera, S.; Mondal, P.; Sarkar, D.; Pathi, V. B.; Pakrashy, S.; Datta, A.; Banerji, B. J. Org. Chem. 2021, 86, 7069–7077. doi:10.1021/acs.joc.1c00311 |
| 14. | Pati, B. V.; Banjare, S. K.; Das Adhikari, G. K.; Nanda, T.; Ravikumar, P. C. Org. Lett. 2022, 24, 5651–5656. doi:10.1021/acs.orglett.2c01901 |
| 15. | Li, Y.; Wang, Q.; Yang, X.; Xie, F.; Li, X. Org. Lett. 2017, 19, 3410–3413. doi:10.1021/acs.orglett.7b01365 |
| 16. | Subramanian, V.; Batchu, V. R.; Barange, D.; Pal, M. J. Org. Chem. 2005, 70, 4778–4783. doi:10.1021/jo050440e |
| 29. | Zhao, Y.-R.; Li, L.; Xu, G.-Y.; Xuan, J. Adv. Synth. Catal. 2022, 364, 506–511. doi:10.1002/adsc.202101144 |
| 30. | Jiang, Y.; Li, P.; Zhao, J.; Liu, B.; Li, X. Org. Lett. 2020, 22, 7475–7479. doi:10.1021/acs.orglett.0c02618 |
| 11. | Finney, L. C.; Mitchell, L. J.; Moody, C. J. Green Chem. 2018, 20, 2242–2249. doi:10.1039/c7gc03741d |
| 12. | Xia, Q.; Wang, Q.; Yan, C.; Dong, J.; Song, H.; Li, L.; Liu, Y.; Wang, Q.; Liu, X.; Song, H. Chem. – Eur. J. 2017, 23, 10871–10877. doi:10.1002/chem.201701755 |
| 26. | Zhou, S.; Wang, J.; Wang, L.; Song, C.; Chen, K.; Zhu, J. Angew. Chem., Int. Ed. 2016, 55, 9384–9388. doi:10.1002/anie.201603943 |
| 39. | Kumar, S.; Nunewar, S.; Kanchupalli, V. Asian J. Org. Chem. 2022, 11, e202100689. doi:10.1002/ajoc.202100689 |
| 6. | Yao, T.; Larock, R. C. J. Org. Chem. 2003, 68, 5936–5942. doi:10.1021/jo034308v |
| 7. | Mallampudi, N. A.; Reddy, G. S.; Maity, S.; Mohapatra, D. K. Org. Lett. 2017, 19, 2074–2077. doi:10.1021/acs.orglett.7b00673 |
| 8. | Chen, B.; Ma, S. Org. Lett. 2013, 15, 3884–3887. doi:10.1021/ol401625t |
| 9. | Faizi, D. J.; Issaian, A.; Davis, A. J.; Blum, S. A. J. Am. Chem. Soc. 2016, 138, 2126–2129. doi:10.1021/jacs.5b12989 |
| 10. | Xing, L.; Zhang, Y.; Li, B.; Du, Y. Org. Lett. 2019, 21, 1989–1993. doi:10.1021/acs.orglett.9b00047 |
| 27. | Wu, W.; Wu, X.; Fan, S.; Zhu, J. Org. Lett. 2022, 24, 7850–7855. doi:10.1021/acs.orglett.2c03288 |
| 22. | Hong, C.; Yu, S.; Liu, Z.; Zhang, Y. Org. Lett. 2022, 24, 815–820. doi:10.1021/acs.orglett.1c03992 |
| 24. | Chinnagolla, R. K.; Jeganmohan, M. Chem. Commun. 2012, 48, 2030–2032. doi:10.1039/c2cc16916a |
| 27. | Wu, W.; Wu, X.; Fan, S.; Zhu, J. Org. Lett. 2022, 24, 7850–7855. doi:10.1021/acs.orglett.2c03288 |
| 21. | Warratz, S.; Kornhaaß, C.; Cajaraville, A.; Niepötter, B.; Stalke, D.; Ackermann, L. Angew. Chem., Int. Ed. 2015, 54, 5513–5517. doi:10.1002/anie.201500600 |
| 25. | Chen, X. Y.; Zhang, X.; Wan, J.-P. Org. Biomol. Chem. 2022, 20, 2356–2369. doi:10.1039/d2ob00126h |
| 33. | Hu, X.-Q.; Liu, Z.-K.; Hou, Y.-X.; Zhang, G.; Gao, Y. Chem. Commun. 2021, 57, 1113–1116. doi:10.1039/d0cc07573f |
| 34. | Hu, X.-Q.; Liu, Z.-K.; Hou, Y.-X.; Xu, J.-H.; Gao, Y. Org. Lett. 2021, 23, 6332–6336. doi:10.1021/acs.orglett.1c02131 |
| 35. | Gao, Y.; Nie, J.; Li, Y.; Li, X.; Chen, Q.; Huo, Y.; Hu, X.-Q. Org. Lett. 2020, 22, 2600–2605. doi:10.1021/acs.orglett.0c00539 |
| 20. | Nandi, D.; Ghosh, D.; Chen, S.-J.; Kuo, B.-C.; Wang, N. M.; Lee, H. M. J. Org. Chem. 2013, 78, 3445–3451. doi:10.1021/jo400174w |
| 33. | Hu, X.-Q.; Liu, Z.-K.; Hou, Y.-X.; Zhang, G.; Gao, Y. Chem. Commun. 2021, 57, 1113–1116. doi:10.1039/d0cc07573f |
| 34. | Hu, X.-Q.; Liu, Z.-K.; Hou, Y.-X.; Xu, J.-H.; Gao, Y. Org. Lett. 2021, 23, 6332–6336. doi:10.1021/acs.orglett.1c02131 |
| 35. | Gao, Y.; Nie, J.; Li, Y.; Li, X.; Chen, Q.; Huo, Y.; Hu, X.-Q. Org. Lett. 2020, 22, 2600–2605. doi:10.1021/acs.orglett.0c00539 |
| 19. | Miura, M.; Tsuda, T.; Satoh, T.; Pivsa-Art, S.; Nomura, M. J. Org. Chem. 1998, 63, 5211–5215. doi:10.1021/jo980584b |
| 23. | Jiang, G.; Li, J.; Zhu, C.; Wu, W.; Jiang, H. Org. Lett. 2017, 19, 4440–4443. doi:10.1021/acs.orglett.7b01919 |
| 36. | Xia, Y.; Wang, J. Chem. Soc. Rev. 2017, 46, 2306–2362. doi:10.1039/c6cs00737f |
| 37. | Chen, J.-R.; Hu, X.-Q.; Lu, L.-Q.; Xiao, W.-J. Chem. Soc. Rev. 2016, 45, 2044–2056. doi:10.1039/c5cs00655d |
| 38. | Cai, B.; Xuan, J. Chin. J. Org. Chem. 2021, 41, 4565. doi:10.6023/cjoc202109040 |
© 2023 Gao et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.