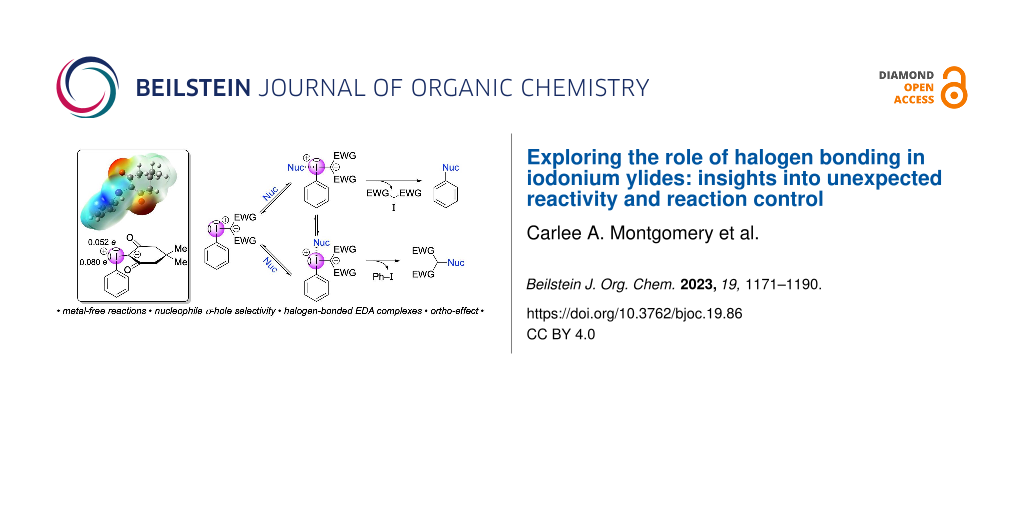
Abstract
Halogen bonding is commonly found with iodine-containing molecules, and it arises when Lewis bases interact with iodine’s σ-holes. Halogen bonding and σ-holes have been encountered in numerous monovalent and hypervalent iodine-containing compounds, and in 2022 σ-holes were computationally confirmed and quantified in the iodonium ylide subset of hypervalent iodine compounds. In light of this new discovery, this article provides an overview of the reactions of iodonium ylides in which halogen bonding has been invoked. Herein, we summarize key discoveries and mechanistic proposals from the early iodonium ylide literature that invoked halogen bonding-type mechanisms, as well as recent reports of reactions between iodonium ylides and Lewis basic nucleophiles in which halogen bonding has been specifically invoked. The reactions discussed herein are organized to enable the reader to build an understanding of how halogen bonding might impact yield and chemoselectivity outcomes in reactions of iodonium ylides. Areas of focus include nucleophile σ-hole selectivity, and how ylide structural modifications and intramolecular halogen bonding (e.g., the ortho-effect) can improve ylide stability or solubility, and alter reaction outcomes.

Graphical Abstract
Introduction
Iodonium ylides are a subset of hypervalent iodine (HVI) reagents that were first reported in 1957 by Neiland [1]. These have since been investigated under a variety of thermal, photochemical, radical and transition metal-catalyzed conditions [2], and they have been successfully employed in numerous reactions such as metallocarbene chemistry [3-8], cycloadditions [9-14], and radiofluorinations [15-18]. As with most HVI reagents, reactions of iodonium ylides are often described using terminologies (e.g., ylide transfer, coupling or reductive elimination) more commonly associated with transition metal-mediated chemistry; however, halogen- or σ-hole bonding has recently emerged as a credible explanation for the diverse reactivity that iodonium ylides undergo. σ-Hole bonding theory offers a means to explain the occurrence of transition metal-free reactions in processes that are typically metal-mediated, as well as the reactions observed between iodonium ylide-Lewis base pairs, including single electron transfers and proton transfers. As iodonium ylides exhibit two σ-holes, they offer two potential sites for halogen bonding to occur, potentially resulting in two different ligand coupling outcomes. While most of their reactions expel an iodoarene to produce functionalized β-dicarbonyl motifs, ligand coupling with the arene motif is also possible, such as in the (radio)fluorination of iodonium ylides. Finally, intramolecular σ-hole bonding offers the potential to alter the physical properties (e.g., stability, solubility, UV–vis absorption) of an ylide, as well as bias a Lewis base’s σ-hole selectivity through σ-hole blocking, which represent emerging avenues for tuning an ylide’s reactivity and improving its reaction outcomes.
Review
1 Halogen bonding
1.1 σ-Holes in halogen bonding
The history of halogen bonding dates back to the 19th century [19,20], and yet our knowledge and understanding of this non-covalent force is continuously growing, encompassing more and more classes of organic molecules. There was no generally accepted name until it was coined “halogen bonding” by Dumas et al. in 1978 [21,22], and only in 2013 did it receive an official IUPAC definition [23]. Iodine is the largest and most polarizable of the halogens, and as such it predominantly engages in halogen bonding (I > Br > Cl >> F) [24]. Halogen bonding was first observed by Guthrie in the 1860s as an attraction between ammonia and molecular iodine [19]. The 20th century saw halogen bonding extended to organohalides such as diiodoacetylene [25,26], and also saw the first X-ray crystallographic evidence of a ‘halogen molecular bridge’ between molecular bromine and 1,4-dioxane [27,28]. In 1953, the first instance of halogen bonding in a hypervalent iodine-containing molecule was reported [29], and this set the stage for decades of subsequent investigation.
To fully understand halogen bonding, a thorough understanding of the properties of σ-hole bonding is necessary. A σ-hole bond is the non-covalent inter- or intramolecular interaction between the σ-hole of a group IV–VII atom with the electron-rich site of Lewis bases such as anions, hydrides, or even π electrons [24,30]. Halogen bonding is a subset of this bonding classification, represented by the generic bonding pattern shown in Figure 1, where R is the host atom or functional group to which the halogen is covalently bound, where X is the halogen atom possessing the σ-hole (halogen bond donor), and where Y is the Lewis base (halogen bond acceptor) [31].
Figure 1: Generic representation of halogen bonding.
Figure 1: Generic representation of halogen bonding.
σ-Holes arise from anisotropic covalent bonds between the halogen and its host, where polarization of the halogen’s electronic charge is directed towards the covalent bond [24]. This results in a phenomenon called polar flattening, resulting in a non-spherical distribution of electron density on the halogen’s surface, with a positive electrostatic potential located along the extension of the covalent bond, and with a lateral belt of negative electrostatic potential [31-35]. The resulting region of diminished electron density is dominated by the electrostatic contributions from the nucleus over those from its electrons and results in a σ-hole [24,36-39]. This anisotropic electron distribution manifests itself in many ways, such as the L-shaped packing observed with diatomic halogens resulting from the secondary bonding between the positive end of one halogen and the negative end of another [40].
σ-Holes can be assessed both qualitatively and quantitatively, enabling predictions of their properties and comparisons of their non-covalent halogen bonding interactions [41]. A molecular electrostatic potential (MEP) map, taken as the 0.001 au density envelope, is typically used for qualitative σ-hole depictions [42,43]. Conversely, the electrostatic potentials at specific points on the atom’s surface can be quantitatively determined through either computation or diffraction techniques [44,45]. Computation of the surface’s electrostatic potential extrema (VS,max and VS,min) are based on Equation 1 [31], which considers the contributions of both the nucleus and the electrons at a point in space (r), where ZA is the charge on nucleus A, located at RA, and where ρ(r) is the molecule’s electron density. A positive electrostatic potential (VS,max) value signifies a dominating nuclear contribution and a positive σ-hole.
The electrostatic strength of a σ-hole typically increases with polarizability within the same group (I > Br > Cl >> F) [41,46,47]; however, the electron attracting abilities of the host [31,46], the electronegativity of the halogen atom [41] and, the degree of spn-hybridization in the covalent σR–X bond can also influence σ-hole strength [32,48]. Should the halogen possess two covalent bonds, two σ-holes would result with the stronger situated opposite the more electron-attracting group [24]. σ-Holes are also highly directional, with near linear halogen bond-acceptor approach angles that typically fall between 160–180° [39,49,50], and with halogen bond lengths that are typically less than or equal to the sum of the atomic Van der Waals radii of the engaged atoms [33]. The strength of a subsequent halogen bond is influenced by the magnitudes of the positive electrostatic potential (VS,max) of the donor and the negative electrostatic potential (VS,min) of the acceptor [46,48,51,52], but also by other factors [53-57] including hydrogen bonding [31,48], solvent polarity [47,58], the Lewis basicity of the acceptor [46,50] and by sterics [46]. As with any interatomic interaction, the energy of a halogen bond is influenced by multiple factors including polarization, electrostatic attraction, charge transfer, dispersion and molecular orbital interactions [59-63]. The charge transfer nature (donation into the σ* orbital of the R–X bond) of the halogen bond leads to elongation and weakening in the host/donor R–X bond, and an accompanied decrease in the HOMO–LUMO gap which may be experimentally observed as a red-shift in the vibrational frequency [46,47,64,65].
1.2 Halogen bonding in monovalent iodine
The σ-holes expressed by both molecular iodine and other organoiodine compounds have been evaluated extensively through both qualitative and quantitative means. Methods of evaluation have included X-ray crystallography and spectroscopy (e.g., microwave, IR, Raman, NMR, NQR), as well as through computational determination of their electrostatic VS,max potentials (Figure 2), their halogen bond lengths and their interaction energies [66-71]. Comparing the VS,max of select iodine-containing molecules shows that the electrostatic potential for molecular iodine (I-1) was 0.049 e, significantly greater than that of iodobenzene (I-2, 0.027 e) [70,72], though identical to that of trifluoroiodomethane (I-3, 0.049 e) [70,71]. As expected, given the ability of the host atom to directly influence the strength of the σ-hole, a significantly increased VS,max value of 0.090 e was observed for iodine monofluoride (I-4) [72]. Since halogen- and hydrogen bonding exhibit numerous similar attributes, halogen bonding has also proven to be a viable alternative in methodologies that rely on hydrogen bond initiation. For example, both halogen- and hydrogen bonding can be used in supramolecular chemistry as the binding mechanism in photoresponsive receptors [73-82]. Organocatalysis is also a common point of intersection for halogen- and hydrogen bonding, and this has been thoroughly explored using monovalent iodine catalysts [83-85].
Figure 2: Quantitative evaluation of σ-holes in monovalent iodine-containing compounds; and, qualitative molecular electrostatic potential (MEP) of I-3 from −0.052 to 0.052 e showing a single σ-hole (dark blue) [86].
Figure 2: Quantitative evaluation of σ-holes in monovalent iodine-containing compounds; and, qualitative mole...
1.3 Halogen bonding in hypervalent iodine complexes
Similar to monovalent iodine compounds, a diverse selection of hypervalent iodine compounds have also been assessed in silico to determine the strengths of their σ-holes (Figure 3). Togni’s CF3-benziodoxole reagent (I-5, 0.029 e) possessed the weakest σ-hole from among those analyzed, consistent with monovalent iodobenzene (I-2) [72]. Difluoroiodobenzene (I-6, 0.042 e) and dichloroiodobenzene (I-7, 0.060 e) showed similar potentials with I-2 and I-3, with the weaker σ-hole found on the more covalent difluoroiodoarene species I-6 [72,87]. The symmetric diaryliodonium salts I-8 and I-9 each presented two σ-holes, with the acyclic variant’s potentials being slightly different (0.18 e and 0.19 e) due to its non-planarity, whereas the cyclic, planar variant’s potentials were each 0.20 e [88,89]. The strength of the σ-holes for I-8 and I-9 were far greater than those of other HVI reagents, which was unsurprising given that its iodine atom was positively charged. Halogen-bonded adducts of these have also been observed, which further illustrated the existence and properties of σ-holes in such HVI compounds. For example, I-7-pyr was characterized by X-ray crystallography and by computational methods, where molecular orbital (MO) analysis showed that pyridine was in fact interacting with the LUMO+1 MO of I-7, corresponding to the σ* orbital oriented along the I–C bond axis [87]. Lüthi et al. quantified the symmetry-adapted perturbation theory (SAPT) interaction energies of halogen bonded acetonitrile complexes of HVI molecules [72], and Huber et al. used 1H NMR titrations and isothermal titration calorimetry (ITC) to experimentally determine interactions energies for I-9 complexes (e.g., I-9-lig, −6.3 kcal/mol), they also discovered that simultaneous binding to both σ-holes was possible [90]. Given these observations, it is unsurprising that HVI compounds have also played an active role in halogen bond-based organocatalysis [91-97].
Figure 3: Quantitative evaluation of σ-holes in hypervalent iodine-containing molecules; and, qualitative MEP map of I-8 from −0.199 to 0.199 e showing two σ-holes (dark blue) [86].
Figure 3: Quantitative evaluation of σ-holes in hypervalent iodine-containing molecules; and, qualitative MEP...
2 Halogen bonding in iodonium ylides
2.1 Quantification of σ-holes in iodonium ylides
Until recently, there were no quantitative evaluations of σ-holes in iodonium ylides [98,99], even though halogen bonding had been invoked for years when describing reactions of such ylides. In 2022, Murphy et al. used computational analysis to assess iodonium ylides for their σ-holes, and found that two of these existed, with one situated opposite the arene and the other opposite the β-dicarbonyl motif (Figure 4) [100]. In the dimethyl malonate-derived ylide I-10, the σ-hole opposite the β-dicarbonyl was stronger with an electrostatic potential of 0.084 e, compared to the 0.049 e found for that opposite the arene. The same pattern was observed for the acetylacetone-derived ylide I-11, which possessed potentials of 0.075 e and 0.048 e. The cyclic dimedone-derived ylide I-12 showed similar potentials of 0.080 e and 0.052 e, as did the Meldrum’s acid-derived ylide I-13 (0.085 e and 0.057 e). Whether the ylide was cyclic or acyclic, the σ-hole opposite the β-dicarbonyl motif was consistently greater in strength due to its higher electron affinity. While this trend was also true with the poly-substituted ylide I-14, the electron-rich arene significantly offset the electropositivity on the iodine, resulting in two σ-holes of diminished strength (0.040 e, 0.031 e).
Figure 4: Quantitative evaluation of σ-holes in iodonium ylides; and, qualitative MEP map of I-12 from −0.083 to 0.083 e, showing strong (dark blue) and weaker (light blue) σ-holes [86].
Figure 4: Quantitative evaluation of σ-holes in iodonium ylides; and, qualitative MEP map of I-12 from −0.083...
Despite the historically limited appreciation and understanding of σ-holes in iodonium ylides, many examples exist where inter- or intramolecular halogen bonding has been invoked to provide a meaningful explanation of unexpected reactivity, of reagent stability, or as a reaction control element. Examples of these include spontaneous cycloaddition reactions that occur without transition metal catalysts, the unexpected initiation of single electron transfer (SET) processes or photochemical transformations, and even proton transfers that appear to defy pKa limitations. The reaction pathways followed by iodonium ylides and Lewis basic reaction partners, from the initial adducts they form to the different products they produce, has long been a cause for discussion. This concept was elegantly illustrated by Kobayashi and Takemoto (Scheme 1) [101], who suggested that an ylide 1 may halogen bond with a nucleophile using either of its σ-holes to generate intermediates 2 or 3, where the cis-oriented ligands may reductively eliminate to generate either a functionalized β-dicarbonyl (4, most common) or a substituted arene (5, rare). Interestingly, when the first iodonium ylide was synthesized by Neiland and co-workers in 1957, they also evaluated its decomposition [1,102]. They discovered that an iodonium ylide (e.g., 6/I-12) reacts with hydrochloric acid to produce a chlorinated β-dicarbonyl 7, opposite their expectation that the C–I bond with the lower electron density would be cleaved.
Scheme 1: Outline of possible reaction pathways between iodonium ylides and Lewis basic nucleophiles (top); and, reaction between 6 and HCl to give 7, as an example of β-dicarbonyl functionalization.
Scheme 1: Outline of possible reaction pathways between iodonium ylides and Lewis basic nucleophiles (top); a...
While both of these reaction pathways are viable, the iodonium ylide literature to-date has shown that the vast majority of reactions follow the reductive elimination pathway to produce 4. However, a clear exception to this is in reactions with nucleophilic fluoride or [18F]fluoride, which reductively eliminate to exclusively generate fluoroarenes (e.g., 5). The rationale for these preferential outcomes is not understood, and may derive from rapidly equilibrating species (e.g., 2 and 3) reacting under Curtin–Hammett control, from hard/soft principles dictating the Lewis base’s selectivity with either the stronger (e.g., 3) or weaker (e.g., 2) σ-hole, or possibly from other contributing factors.
2.2 Halogen bond-initiated reactions: early discoveries
Iodonium ylides have long been employed as metallocarbene precursors, and Müller et al. contributed significant evidence that their reactions with olefins took place within the coordination sphere of the metal [103-106]. However, numerous inconsistencies have been observed when comparing the outcomes of diazo- and iodonium ylide-based metallocarbene reactions, especially during metal-free control experiments, which led researchers to propose alternative, carbene-free reaction pathways for iodonium ylides. This was first reported by Hadjiarapoglou, Varvoglis and co-workers [107-112] and Moriarty et al. [113-115], who observed metal-free cycloadditions between iodonium ylides and olefins in the absence of a transition metal catalyst. These reactions proceeded under mild conditions without catalyst, where the byproducts typically associated with free carbene formation (e.g., dimerization or rearrangement) were not observed (Scheme 2). In 1988, Hadjiarapoglou was investigating transition metal- and photocatalyzed intermolecular cyclopropanations between ylide 8 and norbornene, and found indane 9 was produced in 74% yield after six days at room temperature in the absence of catalyst (Scheme 2a) [107]. At that time, the authors did not articulate any theory as to how the reaction might initiate under such mild conditions, though they recognized that the decomposition of 8 into a free carbene would not have been feasible. In 1989, Moriarty was investigating the intramolecular cyclopropanation of 10 under copper-catalysis, presuming that the reaction would proceed through a metallocarbene intermediate [113]. However, a control experiment showed the reaction to also be viable without catalyst, from which the expected product of a free carbene-derived Wolff-type rearrangement was not observed (Scheme 2b). Likewise, Gallos et al. were investigating the intramolecular cyclopropanation of ylide 12, and found that the metal-free reaction proceeded with identical yield and diastereoselectivity as did their rhodium- and copper-catalyzed reactions (Scheme 2c) [116]. These results were in stark contrast to those observed for the ylide’s diazo counterparts, which did not react without a catalyst, and which gave the opposite diastereoselectivity with copper and rhodium.
Scheme 2: Metal-free cyclopropanations of iodonium ylides, either as intermolecular (a) or intramolecular processes (b, c).
Scheme 2: Metal-free cyclopropanations of iodonium ylides, either as intermolecular (a) or intramolecular pro...
Inspired by ionic pathways previously proposed for hypervalent iodine compounds [9,117-119], Moriarty proposed a carbene-free mechanism for the formation of 11 [113]. In this, the nucleophilic olefin first attacked the electrophilic iodine center to form zwitterion 15, which closed to produce iodocycle 16 and then underwent reductive elimination of iodobenzene to give 11 (Figure 5, left). This was also the pathway subsequently suggested by Gallos et al. [116] for the synthesis of 13/14 from 12. In 2003, Hadjiarapoglou further investigated the intermolecular reaction between 8 and cyclopentene under thermal, metal-free conditions, and offered a related mechanistic proposal (Figure 5, right) for the formation of 20 [110]. Given that their reaction proceeded almost equally well under metal-free conditions as under Rh2(OAc)4 catalysis (3.5 h, 40 °C, 31% 20), and that free carbene formation was unlikely at this temperature, they believed that the reaction was likely initiated by either single electron transfer between the reagents (not shown), or by electrophilic addition of the olefin onto the ylide, forming intermediate adduct 17. This was followed by formation of iodocycle 18, from which reductive elimination of iodobenzene gave 19, and extrusion of SO2 gave 20.
Figure 5: Zwitterionic mechanism for intramolecular cyclopropanation of iodonium ylides (left); and, stepwise intermolecular cycloadditions with olefins (right).
Figure 5: Zwitterionic mechanism for intramolecular cyclopropanation of iodonium ylides (left); and, stepwise...
In 2010, Moriarty revisited their earlier transformation and reported that the metal-free intramolecular cyclopropanation of 10 to 11 could be achieved in an improved 95% yield by performing the reaction at room temperature rather than 0 °C [11]. The reaction was also viable with a selection of other mono- and bicyclic olefin motifs, undergoing intramolecular cyclopropanations in moderate to high yields (Scheme 3). They also conducted a mechanistic investigation to better understand this metal-free pathway, again on the premise that a free carbene intermediate was not viable under such mild conditions. The initially proposed ionic pathway (Figure 5, left) was abandoned as solvent effects had little influence on the reaction rate, and since no Wagner–Meerwein rearrangement products were detected with bicyclic olefin precursors. Radical-based pathways were also discredited because ESR spectra of reaction mixtures showed no evidence of unpaired electrons. Instead, a concerted cycloaddition pathway was proposed for the formation of iodocyclobutane 23 (Figure 6), which would again be followed by reductive elimination of iodobenzene to give the cyclopropane 22a.
Scheme 3: Metal-free intramolecular cyclopropanation of iodonium ylides.
Scheme 3: Metal-free intramolecular cyclopropanation of iodonium ylides.
Figure 6: Concerted cycloaddition pathway for the metal-free, intramolecular cyclopropanation of iodonium ylides.
Figure 6: Concerted cycloaddition pathway for the metal-free, intramolecular cyclopropanation of iodonium yli...
Interestingly, Hadjiarapoglou also revisited their investigation of iodonium ylide cycloadditions and their associated mechanisms [120], using diphenylketene as a new reaction partner with dimedone iodonium ylide 6 (analogous to earlier work reported by Koser in 1975 [9]). Investigations by both Koser and Hadjiarapoglou were performed using identical substrates and solvents (albeit at different concentrations), and yet different product compositions were obtained. Hadjiarapoglou reported that ylide 6 reacted with diphenylketene to give 24 in 58% yield, as well as 25 in 42% yield (Scheme 4). In the earlier study, Koser recovered 24 and 27, and they proposed an ionic mechanism wherein the ylide acted as a nucleophile, giving 26 as the initial intermediate (Figure 7, top). To account for the differing products observed, Hadjiarapoglou instead suggested that an electrophilic addition pathway was operative, producing halogen-bonded adducts 28/28’ which could cyclize on the acylium at either carbon or oxygen, to eventually produce 24 and 25 after reductive elimination of iodobenzene (Figure 7, bottom).
Scheme 4: Reaction of ylide 6 with diphenylketene to form lactone 24 and 25.
Scheme 4: Reaction of ylide 6 with diphenylketene to form lactone 24 and 25.
Figure 7: Nucleophilic (top) and electrophilic (bottom) addition pathways proposed by Koser and Hadjiarapoglou for the cyclization of 6 with diphenylketene.
Figure 7: Nucleophilic (top) and electrophilic (bottom) addition pathways proposed by Koser and Hadjiarapoglo...
Based on these initial results and their corresponding mechanistic proposals, iodine was presumed to play a central role in these metal-free processes. Various cycloaddition reactions of iodonium ylides that were typically associated with metallocarbene-mediated processes were also operative in the absence of catalysts. The mild reaction conditions precluded researchers from proposing analogous free-carbene mediated reactions, and they instead proposed iodine to have engaged with Lewis basic ligands and coordinated their coupling within its ligand sphere. An ylide’s positively charged iodine was consistently proposed to accept ligands, via either concerted or ionic steps, ultimately leading to an iodocycle that underwent reductive elimination of an iodoarene.
2.3 Halogen bonding-initiated reactions: recent discoveries
In recent years, additional processes have emerged that involve halogen bond-based adducts between iodonium ylides and Lewis bases, and with these have also emerged more complex theories as to why such adducts evolve differently. As previously stated, the strength of a halogen bond interaction is impacted by various factors (e.g., electrostatics, charge transfer abilities, dispersion), where the extent of their influence depends on both the halogen bond donor and acceptor [61]. Beyond simply considering the strength of these bonds, other characteristics of this interaction should directly impact the outcomes of the reactions that they undergo. For instance, Mulliken’s initial description of an intermolecular halogen bond was of a charge transfer complex (also known as an electron donor–acceptor complex) [121], and this bonding description has recently been used to support proposals for single electron transfer (SET) reaction pathways between iodonium ylides and various halogen bond acceptors. Alternatively, halogen-bonded complexes of iodonium ylides could lead to changes in an ylide’s UV–vis absorption profile, or to its overall basicity or nucleophilicity, both of which could lead new and unexpected reactivity.
The first example that specifically invoked electron donor–acceptor complexes of iodonium ylides was reported in 2018 by Wang and co-workers [122]. They disclosed a reaction between acyclic iodonium ylides (e.g., 31/ I-10) and tertiary amines 32, which produced indoline rings 33 by forging two new C–C bonds between the ylidic carbon and unactivated positions on the amine (Scheme 5). In 2020, they disclosed a more complex variant of this reaction that reacted 31 with secondary amines 34, which produced densely functionalized N-heterocycles 35 that incorporated two of the ylide’s β-dicarbonyl motifs (Scheme 6) [123].
Scheme 5: Indoline synthesis from acyclic iodonium ylide 31 and tertiary amines.
Scheme 5: Indoline synthesis from acyclic iodonium ylide 31 and tertiary amines.
Scheme 6: N-Heterocycle synthesis from acyclic iodonium ylide 31 and secondary amines.
Scheme 6: N-Heterocycle synthesis from acyclic iodonium ylide 31 and secondary amines.
Though these reactions were conducted at 70 °C, free carbenes were not involved. Both TEMPO and 1,4-dinitrobenzene inhibited the formation of 33 and 35, implying that single electron-transfer processes were occurring. Also, a competition experiment between 32 and [D6]-32 gave a competitive intermolecular kinetic isotope effect of 9.5 that suggested an N-Me proton abstraction was the rate determining step. Given this, the authors proposed that electron donor–acceptor (EDA) complex 36 was initially formed between 32 and a sacrificial equivalent of 31, and that 36 underwent a SET to give radical anion 37 and radical cation 38 (Figure 8). While one equivalent of the ylide orchestrated a series of proton transfer (PT) and SET events leading to malonate, the second equivalent of 31 coupled with the activated amine and cyclized to produce 33. The formation of 35 in their latter study was mechanistically analogous to this proposal; however, a third equivalent of ylide 31 was necessary to facilitate an additional series of PT and SET events.
Figure 8: Proposed mechanism for the formation of 33a from iodonium ylides and amines, involving an initial halogen bonded EDA complex 36.
Figure 8: Proposed mechanism for the formation of 33a from iodonium ylides and amines, involving an initial h...
Xuan, Li and co-workers further investigated the coupling between iodonium ylides and tertiary amines, and showed that this thermal reaction could instead be initiated by blue light photocatalysis (Scheme 7) [124]. Therein, they investigated the iodoarene motif of the ylide, and while ylide 31 gave 33a in 32% yield, significantly lower than the previously obtained 56%, when the ortho-anisyl ylide 39 was used it gave 33a in a much improved 72% yield. The yields of other reactions that employed various amine and ylide derivatives were consistent with those previously obtained (Scheme 5). Control experiments showed that the reaction failed in the dark at room temperature, and they concluded that blue light activation of the initially-formed EDA complex (analogous to 36) promoted the onset of SET events. As this latter protocol also required two equivalents of ylide 39, the authors proposed a near identical mechanism to that of Wang (Figure 8). Strong evidence for formation of an initial EDA complex was found when measuring the UV–vis absorption spectra of 39, alone and in the presence of 32. The latter showed a significant absorption red shift, which implied a structural change in 39 had occurred, which was attributed to halogen bond complex formation.
Scheme 7: Indoline synthesis from acyclic iodonium ylides 39 and tertiary amines under blue light photocatalysis.
Scheme 7: Indoline synthesis from acyclic iodonium ylides 39 and tertiary amines under blue light photocataly...
In 2019, the Murphy group reported the first transformation induced by blue LED irradiation of cyclic and acyclic iodonium ylides (e.g., 6) and alkenes, which generated cyclopropanes 40 in yields up to 96% (Scheme 8) [125]. UV–vis absorption spectra of various ylides were generated both computationally and experimentally, and these revealed that ylide excitation should occur in the blue light region, inducing a HOMO–LUMO excitation within the ylide. The LUMO was a molecular orbital situated primarily on iodine, and its natural population analysis revealed that 0.26 e of electron density was transferred to iodine, resulting in a 1,2-diradical (41*, Figure 9). In their mechanistic proposal, they presumed that this excited species would not have been sufficiently long-lived to encounter the styrene (41*→43*). Instead, they invoked an initial halogen-bonded complex 42, whose irradiation with blue light was proposed to produce acyclic excited state 43*, where possible bond rotation could account for the convergence of alkene isomers into a single product (40c). Radical coupling would then lead to the typical iodocyclobutane intermediate 44, from which reductive elimination of iodobenzene would generate 40.
Scheme 8: Metal-free cycloproponation of iodonium ylides under blue LED irradiation. aUsing trans-β-methylstyrene. bUsing cis-β-methylstyrene.
Scheme 8: Metal-free cycloproponation of iodonium ylides under blue LED irradiation. aUsing trans-β-methylsty...
Figure 9: Proposed mechanism of the cyclopropanation between iodonium ylides and alkenes under blue LED irradiation.
Figure 9: Proposed mechanism of the cyclopropanation between iodonium ylides and alkenes under blue LED irrad...
In 2021, Sen and Gremaud disclosed a blue LED-mediated formal C–H insertion reaction between iodonium ylides (e.g., 31) and pyrroles (e.g., 45), indoles and furans, producing malonate-substituted heterocycles 46 (Scheme 9) [126]. The authors discounted a free carbene-based C–H insertion because conducting the reaction in the presence of the radical trap phenyl N-tert-butyl nitrone (PBN) and the radical scavenger TEMPO resulted in decreased yields and isolation of their iodonium ylide adducts. Additional kinetic isotope effect studies revealed that the C2–H bond of pyrrole did not participate in the rate-determining step. This led the authors to propose a multi-step reaction mechanism, where irradiation of an initial halogen-bonded EDA complex 47 led directly to iodocyclobutane 48 (Figure 10). Reductive elimination of iodobenzene then generated a strained, cyclopropane-fused bicycle 49 that rearranged into the isolated C–H insertion product 46a.
Scheme 9: Formal C–H alkylation of iodonium ylides by nucleophilic heterocycles under blue LED irradiation.
Scheme 9: Formal C–H alkylation of iodonium ylides by nucleophilic heterocycles under blue LED irradiation.
Figure 10: Proposed mechanism of the formal C–H insertion of pyrrole under blue LED irradiation.
Figure 10: Proposed mechanism of the formal C–H insertion of pyrrole under blue LED irradiation.
In addition to Neiland’s disclosure of the reactions between iodonium ylides and hydrochloric acid (Scheme 1) [1,102], others have also reported that both strong acids (e.g., TsOH, TFA, and trichloroacetic acid) and even weak acids (e.g., HF, Et3N·3HF, and AcOH) react with iodonium ylides to give formal X–H insertions [127-130]. In 2022, Murphy et al. disclosed a computational and experimental investigation into the reaction scope and the mechanism by which such X–H insertion reactions could occur (Scheme 10) [100]. Systematic evaluation of acidic compounds showed that carboxylic acids (pKa ≈ 5; 85–99% yield) and thiophenols (pKa ≈ 7; 50f, 59%) were especially viable, whereas electron-rich phenols (pKa ≈ 10; 50d, <15% yield) were not. Though electron-poor phenols (pKa ≈ 8; 50e, 64–74% yield) were suitable, they were unreactive in the presence of a carboxylic acid (50c). If these insertions proceed via initial protonation of the iodonium ylide to produce 51 (Figure 11), its high acidity (pKa ≈ 0) [131,132] would suggest that only strong acids should undergo this reaction; however, the reaction was also viable with weakly acidic compounds (pKa < 8). There was no evidence of free carbenes being involved, and competition experiments showed that both electron-rich and electron-poor benzoic acids reacted faster than benzoic acid, which suggested that both proton acidity and the Lewis basicity of the X–H molecule were important. This led the authors to propose an initial halogen-bonded complex 52 (consistent with the rate acceleration seen for more Lewis basic reactants), which would weaken the X–H bond and simultaneously strengthen the basicity of the ylidic carbanion. Transfer of the proton would give 53, from which expulsion of iodoarene would generate the formal X–H insertion products 50.
Scheme 10: X–H insertions between iodonium ylides and carboxylic acids, phenols and thiophenols.
Scheme 10: X–H insertions between iodonium ylides and carboxylic acids, phenols and thiophenols.
Figure 11: Mechanistic proposal for the X–H insertion reactions of iodonium ylides.
Figure 11: Mechanistic proposal for the X–H insertion reactions of iodonium ylides.
In these recent reports, researchers commonly invoked EDA complexes between iodonium ylides and their Lewis basic reaction partners. This served to pre-complex reactants such that they can undergo intramolecular reactions within the iodine’s ligand sphere. These EDA complexes have been proposed to then undergo single electron transfers from the Lewis base to the ylide, under both thermal or blue LED irradiation conditions, leading to C–H insertion products. Irradiating EDA complexes between alkenes and iodonium ylides with blue light was believed to induce intramolecular iodocyclobutane formation, followed by cyclopropane formation. These reactions were believed to either involve 1,2-diradicals on the ylide, which could engage the complexed alkene, or to involve direct cycloaddition between the ylide and alkene. Murphy’s report of formal X–H insertions with iodonium ylides was similarly proposed to initiate upon complex formation with a Lewis base. Adduct formation was believed to both increase the acidity of halogen bond acceptor’s attached protons, as well as increase the basicity of the ylide’s β-dicarbonyl motif, thereby enabling unexpected reactivity. The reactions available to iodonium ylides have extended well beyond the traditional roles of metallocarbene precursors, where halogen bonding is a key element of modern mechanistic proposals.
2.4 Radiofluorination of iodonium ylides
In the preceding sections, the halogen-bonded complexes between iodonium ylides and Lewis basic reactants all decomposed to furnish functionalized β-dicarbonyl motifs. This is despite there being two available σ-holes for complex formation, and two pathways by which reductive elimination (e.g., with the β-dicarbonyl or arene) could occur (see Scheme 1). A prominent example of the coupling occurring between the Lewis base and the arene is in reactions with fluoride, which has been translated to enable the radiofluorination of non-activated arenes. While this fluorination reaction was first reported in 2010 [133], the subsequent collection of works by Vasdev and Liang led to an improved methodology appropriate for the time and temperature requirements of radiofluorination reactions, and led to a mechanistic proposal dependent on σ-hole selective halogen bonding [134]. Vasdev and Liang modified the iodonium ylide’s β-dicarbonyl skeleton (Scheme 11), and while derivatives of barbituric acid (54a) and Meldrum’s acid (54b) were moderately effective, replacing the gem-dimethyl with spirocyclic motifs 54c–e provided [18F]fluorobiphenyl 55 in 47–85% yield [134]. Decomposition studies showed that this spirocyclic alkyl motif increased the stability of the ylide, which was essential given the elevated temperatures used in radiofluorination. They then prepared and tested a series of spirocyclopentane-derived ylides 56, and were able to radiofluorinate a series of electron-rich and electron-poor arenes 57, including hindered ortho-substituted derivatives (Scheme 12). Further derivatization of the spirocyclic motif led to SPIAd-derived iodonium ylides 58, whose radiofluorinations showed improved radiochemical conversions with a variety of arene derivatives (Scheme 13) [16].
Scheme 11: Radiofluorination of biphenyl using iodonium ylides 54a–e derived from various β-dicarbonyl auxiliaries.
Scheme 11: Radiofluorination of biphenyl using iodonium ylides 54a–e derived from various β-dicarbonyl auxilia...
Scheme 12: Radiofluorination of arenes using spirocycle-derived iodonium ylides 56.
Scheme 12: Radiofluorination of arenes using spirocycle-derived iodonium ylides 56.
Scheme 13: Radiofluorination of arenes using SPIAd-derived iodonium ylides 58.
Scheme 13: Radiofluorination of arenes using SPIAd-derived iodonium ylides 58.
Computational investigations were conducted to better understand these reactions, and it was determined that the changing alkyl motif (e.g., dimethyl, cyclopentyl, adamantyl) had minimal impact on the activation energy of the fluorination reactions. The reaction coordinate was calculated for Meldrum’s acid-derived iodonium ylide (60/I-13) (Figure 12, inset), which showed a 5.1 kcal/mol energy difference between rapidly equilibrating halogen-bonded fluoride adducts. The lower energy adduct IntA had fluoride residing in the stronger σ-hole opposite the β-dicarbonyl (syn to the arene), whereas the higher energy adduct had fluoride residing in the weaker σ-hole (syn to the β-dicarbonyl) [16]. These calculations showed that a reductive elimination event was operative from both IntA/IntB, with a >25 kcal/mol energy difference between transitions states of the observed fluorination (TSA, path A) versus that of the alternate Csp3–F reductive elimination pathway (TSB, path B). These computations are consistent with all known experimental observation, as over 100 iodonium ylides have been tested in these fluorination reactions and none have reacted via this alternative decomposition pathway.
Figure 12: Calculated reaction coordinate for the radiofluorination of iodonium ylide 60.
Figure 12: Calculated reaction coordinate for the radiofluorination of iodonium ylide 60.
Vasdev and Liang also assessed the effect of ortho-substituents on iodonium ylides undergoing radiofluorination, similar to that encountered with diaryliodonium salts [135-138]. They prepared a series of ylides (61a–d) with substituents situated ortho- or para- to iodine, and found the radiochemical conversions (RCCs) of the ortho- or para-alkyl derivatives to be consistent at 37% (62b) and 35% (62a), respectively (Scheme 14) [139]. However, when an ortho-alkoxy substituent (61c) was used, a significantly improved 69% RCC (51% radiochemical yield) was achieved for 62c, despite it being derived from an electron-rich, deactivated arene. Improved results were further realized for 61d, in which the oxygen was one atom removed from the arene, which gave 62d in a 90% RCC and an 82% RCY. These results offered important insight into the hierarchy of fluorination selectivity and pointed directly to secondary bonding occurring between the Lewis basic ether and a σ-hole on the electropositive iodine (Scheme 14, inset). These results suggest that with iodonium ylides, ortho-substituents impose an electronic effect (compare 62a vs 62b and 62b vs 62c), contrary to the steric effect observed for radiofluorination of diaryliodonium salts [136,140]. Incorporation of a Lewis basic ether that could participate in secondary bonding with iodine was critical, and further improvements were realized when this Lewis base was not deactivating the ring (62c vs 62d).
Scheme 14: Radiofluorination of iodonium ylides possessing various ortho- and para-substituents on the iodoarene.
Scheme 14: Radiofluorination of iodonium ylides possessing various ortho- and para-substituents on the iodoare...
From these examples, the evidence suggests that σ-holes are playing an important role in the outcomes of (radio)fluorination reactions. Computational evidence suggests an energetic preference for fluorine adducts with the stronger σ-hole, reminiscent of hard/soft acid and base principles, where better electrostatic stabilization is achieved when the hard fluoride interacts with the stronger σ-hole. Structural modification of the β-dicarbonyl auxiliary has led to improved outcomes, due to increased ylide stability rather than decreasing activation energies of the fluorination reaction. There are, however, other conflicting pieces of evidence that indicate these reactions are much more complex. For example, in earlier computational studies of anisyl-derived iodonium ylides 63a/b, Vasdev and Liang found a steric explanation for the ortho-substituent effect (Figure 13), contrary to that observed for 62a/b. They showed how the ortho-methoxy group in 63a induced an energetically-disfavoured twist in the fluorine adduct’s conformation (INTA’), raising its energy over that of 63b and decreasing the energy barrier between it and the reductive elimination transition state TSA’. Additionally, the role of the halogen bond acceptor in 61d is also poorly understood. Intramolecular halogen bonds might be expected to block the proximal (strong) σ-hole and prevent fluoride addition at that site (64a, Figure 14); however, computational results suggested that adduct formation at the strong σ-hole was key to the chemoselective reductive elimination of the fluoroarene. From this, we might infer that adduct equilibration was occurring (e.g., 64b,c) to properly situate the fluorine prior to the reductive elimination event (refer to Figure 14).
Figure 13: Difference in Gibbs activation energy for ortho- or para-anisyl derived iodonium ylides 63a and 63b.
Figure 13: Difference in Gibbs activation energy for ortho- or para-anisyl derived iodonium ylides 63a and 63b....
Figure 14: Proposed equilibration of intermediates to transit between 64a (the initial adduct formed between 61d and fluoride) and 64c, to properly situate fluorine for reductive elimination.
Figure 14: Proposed equilibration of intermediates to transit between 64a (the initial adduct formed between 6...
2.5 Intramolecular halogen bonding in iodonium ylides
Halogen bonding was encountered as numerous intra- and intermolecular processes throughout the preceding sections, and was often formed between the ylide and the intended nucleophile (e.g., alkene, amine or halide) of the reaction. However, intramolecular halogen bonding was also observed with other non-reacting Lewis bases, where it altered an ylide’s stability and even impacted the chemoselectivity of its subsequent reactions. Examples of this include the ortho-substituted iodoarene-derived ylides used in blue LED photoreactions between ylides and amines (Scheme 7) and radiofluorinations (Scheme 14), where ortho-ether moieties positively influenced the reaction’s outcome. Additional examples of intramolecular secondary interactions also exist in the literature, in which ortho-substituents on iodoarene-derived ylides have been key to tuning reactivity and improving reaction outcomes.
Zhdankin et al. first reported on this concept, testing ortho-alkoxy-substituted iodoarene-derived iodonium ylides for improved solubility and stability [5,6,141]. ortho-Iodoanisole-derived ylide 39 was subject to a rhodium-catalyzed cyclopropanation reaction with styrene, and the results were compared with Charette’s 2009 work on 31 (Scheme 15) [142]. While both ylides were effective as metallocarbene precursors, the o-anisyl derivative 39 gave an improved 90% yield of 65, compared to the 80% yield achieved with ylide 31 under identical reaction conditions. Zhdankin also assessed the cyclopropenation of phenylacetylene, in which 39 gave 66 in 68% yield, which was again a significant improvement over the 37% achieved by Müller in 1995 with ylide 31 [3]. The crystal structure of 39 was also reported and it showed clear secondary halogen bonding that engaged both of iodine’s σ-holes, as both intramolecular (2.9 Å I1–O1) and intermolecular (2.9 Å (I1–O2) interactions (Figure 15). The authors’ explanation for the improved reactivity of o-anisyl derivative 39 was based on its improved solubility in dichloromethane. The contact distances were both within the Van der Waals radii criteria for confirming non-covalent interactions [33], which likely contributed to the ylide’s increased stability. As the Lewis basic ortho-methoxy was engaged with the ylide’s stronger σ-hole, it likely resulted in weaker intermolecular interactions that diminished its aggregation ability and increased its solubility. However, the full impact of this ortho-methoxy group was unclear, as it could also have improved the nucleophilicity of the carbanion (see 52, Figure 11), facilitating the initial metallocarbene forming event.
Scheme 15: Comparison of 31 and ortho-methoxy iodonium ylide 39 in rhodium-catalyzed cyclopropanation and cyclopropenation reactions.
Scheme 15: Comparison of 31 and ortho-methoxy iodonium ylide 39 in rhodium-catalyzed cyclopropanation and cycl...
![[1860-5397-19-86-15]](/bjoc/content/figures/1860-5397-19-86-15.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 15: X-ray crystal structure of dimeric 39 [6], (CCDC# 893474) [143,144].
Figure 15: X-ray crystal structure of dimeric 39 [6], (CCDC# 893474) [143,144].
In 2019, Takemoto et al. investigated ortho-groups as a means to enhance the stability and solubility of ylide precursors, and they envisioned how blocking one of iodine’s σ-holes might enhance the reaction’s chemoselectivity. Specifically, they investigated the coupling reactions between diazonium and iodonium ylides and the soft Lewis base thioamide 67 [145], intending to achieve C–S bond formation with the β-dicarbonyl, producing 68 (Scheme 16). They compared a series of ylide precursors and found that when diazo compound 69 was reacted with 67 (with or without a transition-metal catalyst), no reaction occurred. Conversely, iodonium ylides 31, 70 and 71 all reacted with 67 to produce 68 in 28–81% yield. A significant improvement was realized when an ortho-ether substituent was present (31 vs 70), and the highest yield was found with ortho-nitro ylide derivative 71.
Scheme 16: Enaminone synthesis using diazonium and iodonium ylides.
Scheme 16: Enaminone synthesis using diazonium and iodonium ylides.
Computational studies, using abridged ylide 72 and thioacetamide as model substrates, were also performed to better understand the mechanism, specifically exploring the role of the nitro group and the reductive elimination of the iodoarene (Figure 16). They found 72 to possess secondary intramolecular halogen bonding between the nitro group and iodine’s proximal (stronger) σ-hole. The reaction initiated by selectively forming adduct 73 between the thioamide and the ylide, which possessed both halogen- and hydrogen intermolecular bonds. This adduct decomposed by reductive elimination of the iodoarene via a three-membered transition state (TS), coupling the syn thioamide and β-dicarbonyl ligands to give thiocarbenium ion 74. The authors determined similar activation energies (≈20 kcal/mol) when comparing ylides 31 and 70 in this reductive elimination step, which suggested that the ortho-nitro group of 72 (and 71, by extension) played a role other than lowering the activation energy of this step. Given that other optimized halogen- and hydrogen-bonded conformations were found between thioamide and these ylides (e.g., 75), they surmised that the nitro group was best suited to blocking the proximal σ-hole, thereby preventing non-productive reaction pathways (e.g., thioamide arylation) from occurring.
Figure 16: Transition state calculations for enaminone synthesis from iodonium ylides and thioamides.
Figure 16: Transition state calculations for enaminone synthesis from iodonium ylides and thioamides.
Takemoto and Kobayashi have theorized that an equilibrium should exist between Lewis-basic nucleophiles and iodonium ylides, where the nucleophile could halogen bond to either σ-hole, and that this could lead to distinct reductive elimination pathways (see Scheme 1) [101]. To probe this theory, they prepared a series β-dicarbonyl-derived iodonium ylides (76a–f) possessing a σ-hole-blocking ortho-t-BuSO2 group on the iodoarene (Scheme 17). A natural-bond-orbital (NBO) analysis conducted on hexafluoroacetylacetone-derived ylide 76c identified three distinct lone pair interactions between the sulfonyl oxygens and the σ* orbital of the I–C bond, at energies of 0.79, 3.15, and 4.73 kcal/mol. Also found were intermolecular interactions between the lone pairs of either the carbonyl or sulfonyl with the other I–C bond σ* orbital, at energies of 0.70 and 0.29 kcal/mol, respectively. These interactions were presumed to have contributed to the increased stability of such ylides. X-ray crystal structures of ylides 76c and 76e confirmed these interactions as intramolecular halogen bonds at lengths of 2.7 Å (Figure 17), and showed intermolecular halogen bonds with iodine’s remaining σ-hole, at bond lengths of 3.3 Å or 3.4 Å. Though the σ-hole syn to the arene was blocked, when these six ylides were subject to photoirradiation with N-methylpyrrole, products corresponding to both β-dicarbonyl- (77, expected) and arene- (78, unexpected) functionalization were observed. When malonate-derived ylide 76a was reacted with N-methylpyrrole, it produced 77a in 61% yield. This presumably occurred via a reaction sequence analogous to that proposed by Sen and Gremaud for reactions between ylides and 2-methylpyrrole, involving irradiation of an initially-formed EDA complex (see Figure 10). However, the remaining ylides 76b–f all reacted to produce arene-coupled 78b–f as the sole product in 27–62% yield. The formation of these two product classes supports their hypothesis that two halogen bond-initiated reactions are possible with such ylides. As the σ-hole syn to the arene was blocked, one might have expected these ylides to be predisposed to forming 77 exclusively, but this was not observed. Similarly, one might have expected these ylides to display consistent chemoselectivity, but this was also not observed. The reasons for this “anomalous” reactivity remain unclear, as it is unprecedented that changing the β-dicarbonyl motif of an ylide would flip its ligand coupling selectivity. This could be attributed to a range of factors including disfavoured sterics in the reductive elimination or higher energy UV photoirradiation enabling unknown reactivity pathways, and it points to an unknown effect of changing an ylide’s β-dicarbonyl motif. Nonetheless, these results represent the first examples (beyond halogenation) where the arene of an ylide has undergone coupling with a Lewis basic reactant.
Scheme 17: The reaction between ylides 73a–f and N-methylpyrrole under 365 nm UV irradiation.
Scheme 17: The reaction between ylides 73a–f and N-methylpyrrole under 365 nm UV irradiation.
Figure 17: Crystal structures of 76c (top) and 76e (bottom) [101], (CCDC# 2104180 & 2104181) [143,144].
Figure 17: Crystal structures of 76c (top) and 76e (bottom) [101], (CCDC# 2104180 & 2104181) [143,144].
Collectively, these results show the clear benefits of intramolecular halogen bonding in achieving increased solubility and stability of iodonium ylides. When Lewis basic motifs were incorporated ortho- to the iodine on the arene, these engaged with the ylide’s stronger σ-hole to block this site and diminish the ylide’s aggregation potential. However, intramolecular σ-hole blocking by halogen bond acceptors appears to be reversible, as these σ-holes do participate in reactions with extraneous Lewis basic reactants, presumably via equilibration between initially-formed adducts. And while the impact of these halogen bond acceptors on subsequent mechanistic events remains unknown, these represent an easily modified structural feature that may prove instrumental to discovering new reactivity patterns for iodonium ylides.
Conclusion
Iodonium ylides are an important subset of hypervalent iodine compounds [146-148], and these have received increased attention from the synthetic community over the past decade. In addition to conventional cycloaddition or metallocarbene-based reactions of iodonium ylides, their scope has recently expanded to include X–H insertions based on single electron transfer (SET) mechanisms, cycloadditions based on blue LED photochemistry and radiofluorinations of unactivated arene motifs. In these reports, mechanistic proposals commonly treat the hypervalent iodine atom as being metal-like, orchestrating ligand couplings within its ligand sphere. Iodonium ylides were recently confirmed to possess two σ-holes of differing strengths, and theories about these ylides react now explicitly invoke halogen bonding and EDA complexes between iodine and Lewis basic reactants. Many of these theories are still in their infancy, including whether hard/soft acid and base principles may be relevant to matching σ-hole selectivity (such as in radiofluorination), and whether the ortho-Lewis base effect is merely one of ylide stabilization, or if this also influences σ-hole selectivity and ultimately the reaction’s chemoselectivity. Through this review, we hope to have given researchers a concise summary of halogen bonding in iodonium ylides, how they are operative in their mechanisms, and provided a compelling case for continued investigation of this versatile compound class.
Funding
This work was supported by the Natural Sciences and Engineering Research Council (NSERC) of Canada and the University of Waterloo. CAM acknowledges the Ontario Graduate Scholarship (OGS) for graduate funding. This research was also enabled in part by support provided by Compute Ontario (https://www.computeontario.ca) and Compute Canada (https://www.computecanada.ca).
References
-
Gudrinietse, E.; Neiland, O.; Vanag, G. J. Gen. Chem. USSR 1957, 27, 2777–2780.
Return to citation in text: [1] [2] [3] -
Yusubov, M. S.; Yoshimura, A.; Zhdankin, V. V. ARKIVOC 2016, No. 1, 342–374. doi:10.3998/ark.5550190.p009.732
Return to citation in text: [1] -
Müller, P.; Fernandez, D. Helv. Chim. Acta 1995, 78, 947–958. doi:10.1002/hlca.19950780417
Return to citation in text: [1] [2] -
Murphy, G. K.; West, F. G. Org. Lett. 2006, 8, 4359–4361. doi:10.1021/ol061772o
Return to citation in text: [1] -
Goudreau, S. R.; Marcoux, D.; Charette, A. B. J. Org. Chem. 2009, 74, 470–473. doi:10.1021/jo802208q
Return to citation in text: [1] [2] -
Zhu, C.; Yoshimura, A.; Ji, L.; Wei, Y.; Nemykin, V. N.; Zhdankin, V. V. Org. Lett. 2012, 14, 3170–3173. doi:10.1021/ol301268j
Return to citation in text: [1] [2] [3] -
Bosnidou, A.-E.; Kalpogiannaki, D.; Karanestora, S.; Nixas, J. A.; Hadjiarapoglou, L. P. J. Org. Chem. 2015, 80, 1279–1283. doi:10.1021/jo502611x
Return to citation in text: [1] -
Hayasi, Y.; Okada, T.; Kawanisi, M. Bull. Chem. Soc. Jpn. 1970, 43, 2506–2511. doi:10.1246/bcsj.43.2506
Return to citation in text: [1] -
Koser, G. F.; Yu, S.-M. J. Org. Chem. 1975, 40, 1166–1168. doi:10.1021/jo00896a037
Return to citation in text: [1] [2] [3] -
Gololobov, Y. G.; Golding, I. R.; Galkina, M. A.; Lokshin, B. V.; Garbuzova, I. A.; Petrovskii, P. V.; Starikova, Z. A.; Averkiev, B. B. Russ. Chem. Bull. 2006, 55, 883–891. doi:10.1007/s11172-006-0347-3
Return to citation in text: [1] -
Moriarty, R. M.; Tyagi, S.; Kinch, M. Tetrahedron 2010, 66, 5801–5810. doi:10.1016/j.tet.2010.05.005
Return to citation in text: [1] [2] -
Huang, H.; Yang, Y.; Zhang, X.; Zeng, W.; Liang, Y. Tetrahedron Lett. 2013, 54, 6049–6052. doi:10.1016/j.tetlet.2013.08.096
Return to citation in text: [1] -
Chelli, S.; Troshin, K.; Mayer, P.; Lakhdar, S.; Ofial, A. R.; Mayr, H. J. Am. Chem. Soc. 2016, 138, 10304–10313. doi:10.1021/jacs.6b05768
Return to citation in text: [1] -
Tao, J.; Estrada, C. D.; Murphy, G. K. Chem. Commun. 2017, 53, 9004–9007. doi:10.1039/c7cc04859a
Return to citation in text: [1] -
Chassé, M.; Pees, A.; Lindberg, A.; Liang, S. H.; Vasdev, N. Chem. Rec. 2023, e202300072. doi:10.1002/tcr.202300072
Return to citation in text: [1] -
Rotstein, B. H.; Wang, L.; Liu, R. Y.; Patteson, J.; Kwan, E. E.; Vasdev, N.; Liang, S. H. Chem. Sci. 2016, 7, 4407–4417. doi:10.1039/c6sc00197a
Return to citation in text: [1] [2] [3] -
Liang, S. H.; Wang, L.; Stephenson, N. A.; Rotstein, B. H.; Vasdev, N. Nat. Protoc. 2019, 14, 1530–1545. doi:10.1038/s41596-019-0149-3
Return to citation in text: [1] -
Calderwood, S.; Collier, T. L.; Gouverneur, V.; Liang, S. H.; Vasdev, N. J. Fluorine Chem. 2015, 178, 249–253. doi:10.1016/j.jfluchem.2015.08.006
Return to citation in text: [1] -
Guthrie, F. J. Chem. Soc. 1863, 16, 239–244. doi:10.1039/js8631600239
Return to citation in text: [1] [2] -
Remsen, I.; Norris, J. F. Am. Chem. J. 1896, 18, 90–96.
Return to citation in text: [1] -
Dumas, J.-M.; Kern, M.; Janier-Dubry, J. L. Bull. Soc. Chim. Fr. 1976, 1785–1787.
Return to citation in text: [1] -
Dumas, J.-M.; Peurichard, H.; Gomel, M. J. Chem. Res., Synop. 1978, 54–57.
Return to citation in text: [1] -
Desiraju, G. R.; Ho, P. S.; Kloo, L.; Legon, A. C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Pure Appl. Chem. 2013, 85, 1711–1713. doi:10.1351/pac-rec-12-05-10
Return to citation in text: [1] -
Politzer, P.; Murray, J. S.; Clark, T. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. doi:10.1039/c3cp00054k
Return to citation in text: [1] [2] [3] [4] [5] -
Bent, H. A. Chem. Rev. 1968, 68, 587–648. doi:10.1021/cr60255a003
Return to citation in text: [1] -
Gagnaux, P.; Susz, B. P. Helv. Chim. Acta 1960, 43, 948–956. doi:10.1002/hlca.19600430403
Return to citation in text: [1] -
Hassel, O.; Hvoslef, J. Acta Chem. Scand. 1954, 8, 873. doi:10.3891/acta.chem.scand.08-0873
Return to citation in text: [1] -
Hassel, O.; Rømming, C. Q. Rev., Chem. Soc. 1962, 16, 1–18. doi:10.1039/qr9621600001
Return to citation in text: [1] -
Archer, E. M.; van Schalkwyk, T. G. Acta Crystallogr. 1953, 6, 88–92. doi:10.1107/s0365110x53000193
Return to citation in text: [1] -
Lu, Y.; Zou, J.; Wang, H.; Yu, Q.; Zhang, H.; Jiang, Y. J. Phys. Chem. A 2005, 109, 11956–11961. doi:10.1021/jp0547360
Return to citation in text: [1] -
Decato, D. A.; John, E. A.; Berryman, O. B. Halogen Bonding: An Introduction. In Halogen Bonding in Solution; Huber, S., Ed.; Wiley‐VCH Verlag GmbH & Co. KGaA: Weinheim, 2021; pp 1–41. doi:10.1002/9783527825738.ch1
Return to citation in text: [1] [2] [3] [4] [5] -
Clark, T.; Hennemann, M.; Murray, J. S.; Politzer, P. J. Mol. Model. 2007, 13, 291–296. doi:10.1007/s00894-006-0130-2
Return to citation in text: [1] [2] -
Politzer, P.; Murray, J. S. Crystals 2017, 7, 212. doi:10.3390/cryst7070212
Return to citation in text: [1] [2] [3] -
Sedlak, R.; Kolář, M. H.; Hobza, P. J. Chem. Theory Comput. 2015, 11, 4727–4732. doi:10.1021/acs.jctc.5b00687
Return to citation in text: [1] -
Brinck, T.; Murray, J. S.; Politzer, P. Int. J. Quantum Chem. 1992, 44, 57–64. doi:10.1002/qua.560440709
Return to citation in text: [1] -
σ-Holes may appear neutral or even negatively charged for less polarizable or more electronegative halogens (e.g., fluorine). Alternatively, when there is significant polarization, the entire atomic surface may appear positive, which is typically seen with strongly electon withdrawing hosts. See references [37-39].
Return to citation in text: [1] -
Varadwaj, A.; Marques, H. M.; Varadwaj, P. R. Molecules 2019, 24, 379. doi:10.3390/molecules24030379
Return to citation in text: [1] [2] -
Politzer, P.; Murray, J. S.; Concha, M. C. J. Mol. Model. 2007, 13, 643–650. doi:10.1007/s00894-007-0176-9
Return to citation in text: [1] [2] -
Shields, Z. P.; Murray, J. S.; Politzer, P. Int. J. Quantum Chem. 2010, 110, 2823–2832. doi:10.1002/qua.22787
Return to citation in text: [1] [2] [3] -
Tsirelson, V. G.; Zhou, P. F.; Tang, T.-H.; Bader, R. F. W. Acta Crystallogr., Sect. A: Found. Crystallogr. 1995, 51, 143–153. doi:10.1107/s0108767394009463
Return to citation in text: [1] -
Murray, J. S.; Riley, K. E.; Politzer, P.; Clark, T. Aust. J. Chem. 2010, 63, 1598–1607. doi:10.1071/ch10259
Return to citation in text: [1] [2] [3] -
Politzer, P.; Murray, J. S. ChemPhysChem 2013, 14, 278–294. doi:10.1002/cphc.201200799
Return to citation in text: [1] -
Bader, R. F. W.; Carroll, M. T.; Cheeseman, J. R.; Chang, C. J. Am. Chem. Soc. 1987, 109, 7968–7979. doi:10.1021/ja00260a006
Return to citation in text: [1] -
Stewart, R. F. Chem. Phys. Lett. 1979, 65, 335–342. doi:10.1016/0009-2614(79)87077-3
Return to citation in text: [1] -
Stevens, E. D.; Klain, C. L. Charge Density Studies of Drug Molecules. In The Application of Charge Density Research to Chemistry and Drug Design; Jeffrey, G. A.; Piniella, J. F., Eds.; Springer US: Boston, MA, 1991; pp 319–336. doi:10.1007/978-1-4615-3700-7_13
Return to citation in text: [1] -
Mohajeri, A.; Pakiari, A. H.; Bagheri, N. Chem. Phys. Lett. 2009, 467, 393–397. doi:10.1016/j.cplett.2008.11.017
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Costa, P. J. Phys. Sci. Rev. 2017, 2, 20170136.
Return to citation in text: [1] [2] [3] -
Murray, J. S.; Lane, P.; Politzer, P. J. Mol. Model. 2009, 15, 723–729. doi:10.1007/s00894-008-0386-9
Return to citation in text: [1] [2] [3] -
Ramasubbu, N.; Parthasarathy, R.; Murray-Rust, P. J. Am. Chem. Soc. 1986, 108, 4308–4314. doi:10.1021/ja00275a012
Return to citation in text: [1] -
Lu, Y.; Wang, Y.; Zhu, W. Phys. Chem. Chem. Phys. 2010, 12, 4543–4551. doi:10.1039/b926326h
Return to citation in text: [1] [2] -
Clark, T.; Heßelmann, A. Phys. Chem. Chem. Phys. 2018, 20, 22849–22855. doi:10.1039/c8cp03079k
Return to citation in text: [1] -
Huber, S. M.; Jimenez-Izal, E.; Ugalde, J. M.; Infante, I. Chem. Commun. 2012, 48, 7708–7710. doi:10.1039/c2cc33304j
Return to citation in text: [1] -
σ-Hole bond strength trends are inconsistent across the periodic table, but do appear to be influenced by several factors. For example, the strongest σ-hole bond may change from halogen to pnicogen atoms, depending on the σ-hole bond acceptor. See references [54-57].
Return to citation in text: [1] -
Bartashevich, E. V.; Matveychuk, Y. V.; Mukhitdinova, S. E.; Sobalev, S. A.; Khrenova, M. G.; Tsirelson, V. G. Theor. Chem. Acc. 2020, 139, 26. doi:10.1007/s00214-019-2534-y
Return to citation in text: [1] [2] -
Dong, W.; Li, Q.; Scheiner, S. Molecules 2018, 23, 1681. doi:10.3390/molecules23071681
Return to citation in text: [1] [2] -
Bauzá, A.; Quiñonero, D.; Deyà, P. M.; Frontera, A. CrystEngComm 2013, 15, 3137–3144. doi:10.1039/c2ce26741a
Return to citation in text: [1] [2] -
Liu, N.; Li, Q.; McDowell, S. A. C. Front. Chem. (Lausanne, Switz.) 2020, 8, 608486. doi:10.3389/fchem.2020.608486
Return to citation in text: [1] [2] -
Lu, Y.; Li, H.; Zhu, X.; Zhu, W.; Liu, H. J. Phys. Chem. A 2011, 115, 4467–4475. doi:10.1021/jp111616x
Return to citation in text: [1] -
Reed, A. E.; Curtiss, L. A.; Weinhold, F. Chem. Rev. 1988, 88, 899–926. doi:10.1021/cr00088a005
Return to citation in text: [1] -
Riley, K. E.; Murray, J. S.; Fanfrlík, J.; Řezáč, J.; Solá, R. J.; Concha, M. C.; Ramos, F. M.; Politzer, P. J. Mol. Model. 2013, 19, 4651–4659. doi:10.1007/s00894-012-1428-x
Return to citation in text: [1] -
Deepa, P.; Sedlak, R.; Hobza, P. Phys. Chem. Chem. Phys. 2014, 16, 6679–6686. doi:10.1039/c4cp00055b
Return to citation in text: [1] [2] -
Robidas, R.; Reinhard, D. L.; Legault, C. Y.; Huber, S. M. Chem. Rec. 2021, 21, 1912–1927. doi:10.1002/tcr.202100119
Return to citation in text: [1] -
Rosokha, S. V.; Stern, C. L.; Ritzert, J. T. Chem. – Eur. J. 2013, 19, 8774–8788. doi:10.1002/chem.201300577
Return to citation in text: [1] -
Blue shifting has also been reported as a result of halogen bonding, however, it is far less common. See reference [65].
Return to citation in text: [1] -
Murray, J. S.; Concha, M. C.; Lane, P.; Hobza, P.; Politzer, P. J. Mol. Model. 2008, 14, 699–704. doi:10.1007/s00894-008-0307-y
Return to citation in text: [1] [2] -
Stephens, S. L.; Walker, N. R.; Legon, A. C. Phys. Chem. Chem. Phys. 2011, 13, 20736–20744. doi:10.1039/c1cp21854a
Return to citation in text: [1] -
Hauchecorne, D.; Nagels, N.; van der Veken, B. J.; Herrebout, W. A. Phys. Chem. Chem. Phys. 2012, 14, 681–690. doi:10.1039/c1cp22771h
Return to citation in text: [1] -
Sladojevich, F.; McNeill, E.; Börgel, J.; Zheng, S.-L.; Ritter, T. Angew. Chem., Int. Ed. 2015, 54, 3712–3716. doi:10.1002/anie.201410954
Return to citation in text: [1] -
Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. Chem. Rev. 2016, 116, 2478–2601. doi:10.1021/acs.chemrev.5b00484
Return to citation in text: [1] -
Kolář, M. H.; Hobza, P. Chem. Rev. 2016, 116, 5155–5187. doi:10.1021/acs.chemrev.5b00560
Return to citation in text: [1] [2] [3] -
Bundhun, A.; Ramasami, P.; Murray, J. S.; Politzer, P. J. Mol. Model. 2013, 19, 2739–2746. doi:10.1007/s00894-012-1571-4
Return to citation in text: [1] [2] -
Pinto de Magalhães, H.; Togni, A.; Lüthi, H. P. J. Org. Chem. 2017, 82, 11799–11805. doi:10.1021/acs.joc.7b01716
Return to citation in text: [1] [2] [3] [4] [5] -
Hua, Y.; Flood, A. H. J. Am. Chem. Soc. 2010, 132, 12838–12840. doi:10.1021/ja105793c
Return to citation in text: [1] -
Parks, F. C.; Liu, Y.; Debnath, S.; Stutsman, S. R.; Raghavachari, K.; Flood, A. H. J. Am. Chem. Soc. 2018, 140, 17711–17723. doi:10.1021/jacs.8b10538
Return to citation in text: [1] -
Kerckhoffs, A.; Moss, I.; Langton, M. J. Chem. Commun. 2023, 59, 51–54. doi:10.1039/d2cc05199k
Return to citation in text: [1] -
Kerckhoffs, A.; Langton, M. J. Chem. Sci. 2020, 11, 6325–6331. doi:10.1039/d0sc02745f
Return to citation in text: [1] -
Choi, Y. R.; Kim, G. C.; Jeon, H.-G.; Park, J.; Namkung, W.; Jeong, K.-S. Chem. Commun. 2014, 50, 15305–15308. doi:10.1039/c4cc07560a
Return to citation in text: [1] -
Ryan, S. T. J.; del Barrio, J.; Suardíaz, R.; Ryan, D. F.; Rosta, E.; Scherman, O. A. Angew. Chem., Int. Ed. 2016, 55, 16096–16100. doi:10.1002/anie.201607693
Return to citation in text: [1] -
Docker, A.; Shang, X.; Yuan, D.; Kuhn, H.; Zhang, Z.; Davis, J. J.; Beer, P. D.; Langton, M. J. Angew. Chem., Int. Ed. 2021, 60, 19442–19450. doi:10.1002/anie.202107748
Return to citation in text: [1] -
Kokan, Z.; Chmielewski, M. J. J. Am. Chem. Soc. 2018, 140, 16010–16014. doi:10.1021/jacs.8b08689
Return to citation in text: [1] -
Wezenberg, S. J.; Vlatković, M.; Kistemaker, J. C. M.; Feringa, B. L. J. Am. Chem. Soc. 2014, 136, 16784–16787. doi:10.1021/ja510700j
Return to citation in text: [1] -
Villarón, D.; Siegler, M. A.; Wezenberg, S. J. Chem. Sci. 2021, 12, 3188–3193. doi:10.1039/d0sc06686a
Return to citation in text: [1] -
Sutar, R. L.; Huber, S. M. ACS Catal. 2019, 9, 9622–9639. doi:10.1021/acscatal.9b02894
Return to citation in text: [1] -
Bulfield, D.; Huber, S. M. Chem. – Eur. J. 2016, 22, 14434–14450. doi:10.1002/chem.201601844
Return to citation in text: [1] -
Saito, M.; Kobayashi, Y.; Tsuzuki, S.; Takemoto, Y. Angew. Chem., Int. Ed. 2017, 56, 7653–7657. doi:10.1002/anie.201703641
Return to citation in text: [1] -
Molecular electrostatic potential (MEP) maps were calculated de-novo using previously-reported methods. See reference [100] for details.
Return to citation in text: [1] [2] [3] -
Poynder, T. B.; Chamorro Orué, A. I.; Tania; Sharp-Bucknall, L.; Flynn, M. T.; Wilson, D. J. D.; Athukorala Arachchige, K. S.; Clegg, J. K.; Dutton, J. L. Chem. Commun. 2021, 57, 4970–4973. doi:10.1039/d1cc01567b
Return to citation in text: [1] [2] -
Soldatova, N. S.; Postnikov, P. S.; Suslonov, V. V.; Kissler, T. Y.; Ivanov, D. M.; Yusubov, M. S.; Galmés, B.; Frontera, A.; Kukushkin, V. Y. Org. Chem. Front. 2020, 7, 2230–2242. doi:10.1039/d0qo00678e
Return to citation in text: [1] -
Cavallo, G.; Murray, J. S.; Politzer, P.; Pilati, T.; Ursini, M.; Resnati, G. IUCrJ 2017, 4, 411–419. doi:10.1107/s2052252517004262
Return to citation in text: [1] -
Heinen, F.; Engelage, E.; Cramer, C. J.; Huber, S. M. J. Am. Chem. Soc. 2020, 142, 8633–8640. doi:10.1021/jacs.9b13309
Return to citation in text: [1] -
Zhang, Y.; Han, J.; Liu, Z.-J. RSC Adv. 2015, 5, 25485–25488. doi:10.1039/c5ra00209e
Return to citation in text: [1] -
Heinen, F.; Engelage, E.; Dreger, A.; Weiss, R.; Huber, S. M. Angew. Chem., Int. Ed. 2018, 57, 3830–3833. doi:10.1002/anie.201713012
Return to citation in text: [1] -
Wolf, J.; Huber, F.; Erochok, N.; Heinen, F.; Guérin, V.; Legault, C. Y.; Kirsch, S. F.; Huber, S. M. Angew. Chem., Int. Ed. 2020, 59, 16496–16500. doi:10.1002/anie.202005214
Return to citation in text: [1] -
Haraguchi, R.; Nishikawa, T.; Kanazawa, A.; Aoshima, S. Macromolecules 2020, 53, 4185–4192. doi:10.1021/acs.macromol.0c00823
Return to citation in text: [1] -
Heinen, F.; Reinhard, D. L.; Engelage, E.; Huber, S. M. Angew. Chem., Int. Ed. 2021, 60, 5069–5073. doi:10.1002/anie.202013172
Return to citation in text: [1] -
Yunusova, S. N.; Novikov, A. S.; Soldatova, N. S.; Vovk, M. A.; Bolotin, D. S. RSC Adv. 2021, 11, 4574–4583. doi:10.1039/d0ra09640g
Return to citation in text: [1] -
To, A. J.; Murphy, G. K. New J. Chem. 2022, 46, 15313–15320. doi:10.1039/d2nj02731c
Return to citation in text: [1] -
Yoshida, Y.; Fujimura, T.; Mino, T.; Sakamoto, M. Adv. Synth. Catal. 2022, 364, 1091–1098. doi:10.1002/adsc.202101380
Return to citation in text: [1] -
Lang, T.; Zhang, X.; Meng, L.; Zeng, Y. Struct. Chem. 2016, 27, 927–937. doi:10.1007/s11224-015-0676-7
Return to citation in text: [1] -
Montgomery, C. A.; Jameel, I.; Cuzzucoli, F.; Chidley, T.; Hopkins, W. S.; Murphy, G. K. Chem. – Eur. J. 2022, 28, e202202029. doi:10.1002/chem.202202029
Return to citation in text: [1] [2] [3] -
Kimura, T.; Hamada, S.; Furuta, T.; Takemoto, Y.; Kobayashi, Y. Crystals 2021, 11, 1085. doi:10.3390/cryst11091085
Return to citation in text: [1] [2] [3] -
Neiland, O.; Vanag, G. Proc. Acad. Sci. USSR 1960, 130, 19.
Return to citation in text: [1] [2] -
Müller, P.; Boléa, C. Helv. Chim. Acta 2001, 84, 1093–1111. doi:10.1002/1522-2675(20010516)84:5<1093::aid-hlca1093>3.0.co;2-t
Return to citation in text: [1] -
Müller, P.; Boléa, C. Synlett 2000, 826–828. doi:10.1055/s-2000-6697
Return to citation in text: [1] -
Müller, P.; Boléa, C. Molecules 2001, 6, 258–266. doi:10.3390/60300258
Return to citation in text: [1] -
Müller, P. Acc. Chem. Res. 2004, 37, 243–251. doi:10.1021/ar0202619
Return to citation in text: [1] -
Hadjiarapoglou, L. P.; Varvoglis, A.; Alcock, N. W.; Pike, G. A. J. Chem. Soc., Perkin Trans. 1 1988, 2839–2846. doi:10.1039/p19880002839
Return to citation in text: [1] [2] -
Adam, W.; Bosio, S. G.; Gogonas, E. P.; Hadjiarapoglou, L. P. Synthesis 2002, 2084–2090. doi:10.1055/s-2002-34376
Return to citation in text: [1] -
Adam, W.; Gogonas, E. P.; Hadjiarapoglou, L. P. Eur. J. Org. Chem. 2003, 1064–1068. doi:10.1002/ejoc.200390156
Return to citation in text: [1] -
Adam, W.; Gogonas, E. P.; Hadjiarapoglou, L. P. J. Org. Chem. 2003, 68, 9155–9158. doi:10.1021/jo035362e
Return to citation in text: [1] [2] -
Adam, W.; Gogonas, E. P.; Hadjiarapoglou, L. P. Tetrahedron 2003, 59, 7929–7934. doi:10.1016/j.tet.2003.07.011
Return to citation in text: [1] -
Adam, W.; Gogonas, E. P.; Hadjiarapoglou, L. P. Synlett 2003, 1165–1169. doi:10.1055/s-2003-39894
Return to citation in text: [1] -
Moriarty, R. M.; Prakash, O.; Vaid, R. K.; Zhao, L. J. Am. Chem. Soc. 1989, 111, 6443–6444. doi:10.1021/ja00198a078
Return to citation in text: [1] [2] [3] -
Moriarty, R. M.; May, E. J.; Prakash, O. Tetrahedron Lett. 1997, 38, 4333–4336. doi:10.1016/s0040-4039(97)00967-2
Return to citation in text: [1] -
Moriarty, R. M.; Kim, J.; Guo, L. Tetrahedron Lett. 1993, 34, 4129–4132. doi:10.1016/s0040-4039(00)60509-9
Return to citation in text: [1] -
Gallos, J. K.; Koftis, T. V.; Massen, Z. S.; Dellios, C. C.; Mourtzinos, I. T.; Coutouli-Argyropoulou, E.; Koumbis, A. E. Tetrahedron 2002, 58, 8043–8053. doi:10.1016/s0040-4020(02)01002-5
Return to citation in text: [1] [2] -
Papadopoulou, M.; Spyroudis, S.; Varvoglis, A. J. Org. Chem. 1985, 50, 1509–1511. doi:10.1021/jo00209a029
Return to citation in text: [1] -
Spyroudis, S. P. J. Org. Chem. 1986, 51, 3453–3456. doi:10.1021/jo00368a009
Return to citation in text: [1] -
Hadjiarapoglou, L. P. Tetrahedron Lett. 1987, 28, 4449–4450. doi:10.1016/s0040-4039(00)96535-3
Return to citation in text: [1] -
Antos, A.; Elemes, Y.; Michaelides, A.; Nyxas, J. A.; Skoulika, S.; Hadjiarapoglou, L. P. J. Org. Chem. 2012, 77, 10949–10954. doi:10.1021/jo3020787
Return to citation in text: [1] -
Mulliken, R. S. J. Am. Chem. Soc. 1952, 74, 811–824. doi:10.1021/ja01123a067
Return to citation in text: [1] -
Zhao, Z.; Luo, Y.; Liu, S.; Zhang, L.; Feng, L.; Wang, Y. Angew. Chem., Int. Ed. 2018, 57, 3792–3796. doi:10.1002/anie.201800389
Return to citation in text: [1] -
Zhao, Z.; Kong, X.; Wang, W.; Hao, J.; Wang, Y. Org. Lett. 2020, 22, 230–233. doi:10.1021/acs.orglett.9b04213
Return to citation in text: [1] -
Zhao, Y.-R.; Li, L.; Xuan, J. Adv. Synth. Catal. 2023, 365, 110–115. doi:10.1002/adsc.202201085
Return to citation in text: [1] -
Chidley, T.; Jameel, I.; Rizwan, S.; Peixoto, P. A.; Pouységu, L.; Quideau, S.; Hopkins, W. S.; Murphy, G. K. Angew. Chem., Int. Ed. 2019, 58, 16959–16965. doi:10.1002/anie.201908994
Return to citation in text: [1] -
Sar, S.; Das, R.; Barman, D.; Latua, P.; Guha, S.; Gremaud, L.; Sen, S. Org. Biomol. Chem. 2021, 19, 7627–7632. doi:10.1039/d1ob01219c
Return to citation in text: [1] -
Gondo, K.; Kitamura, T. Molecules 2012, 17, 6625–6632. doi:10.3390/molecules17066625
Return to citation in text: [1] -
Pongratz, E.; Kappe, T. Monatsh. Chem. 1984, 115, 231–242. doi:10.1007/bf00798414
Return to citation in text: [1] -
Geary, G. C.; Hope, E. G.; Singh, K.; Stuart, A. M. RSC Adv. 2015, 5, 16501–16506. doi:10.1039/c4ra15733h
Return to citation in text: [1] -
Neilands, O. Zh. Org. Khim. 1965, 1, 1858–1862.
Return to citation in text: [1] -
Kalnin, S. V.; Neiland, O. Y. Zh. Org. Khim. 1971, 7, 1606.
Return to citation in text: [1] -
Neiland, O. Y.; Karele, B. Y. Zh. Org. Khim. 1971, 7, 1611.
Return to citation in text: [1] -
Satyamurthy, N.; Barrio, J. R. No-carrier-added nucleophilic [F-18]fluorination of aromatic compounds. WO Pat. WO2010117435A2, Oct 14, 2010.
Return to citation in text: [1] -
Rotstein, B. H.; Stephenson, N. A.; Vasdev, N.; Liang, S. H. Nat. Commun. 2014, 5, 4365. doi:10.1038/ncomms5365
Return to citation in text: [1] [2] -
Shah, A.; Pike, V. W.; Widdowson, D. A. J. Chem. Soc., Perkin Trans. 1 1998, 2043–2046. doi:10.1039/a802349b
Return to citation in text: [1] -
Wang, B.; Graskemper, J. W.; Qin, L.; DiMagno, S. G. Angew. Chem., Int. Ed. 2010, 49, 4079–4083. doi:10.1002/anie.201000695
Return to citation in text: [1] [2] -
Grushin, V. V. Acc. Chem. Res. 1992, 25, 529–536. doi:10.1021/ar00023a007
Return to citation in text: [1] -
Grushin, V. V.; Demkina, I. I.; Tolstaya, T. P. J. Chem. Soc., Perkin Trans. 2 1992, 505–511. doi:10.1039/p29920000505
Return to citation in text: [1] -
Wang, L.; Jacobson, O.; Avdic, D.; Rotstein, B. H.; Weiss, I. D.; Collier, L.; Chen, X.; Vasdev, N.; Liang, S. H. Angew. Chem., Int. Ed. 2015, 54, 12777–12781. doi:10.1002/anie.201505927
Return to citation in text: [1] -
Ross, T. L.; Ermert, J.; Hocke, C.; Coenen, H. H. J. Am. Chem. Soc. 2007, 129, 8018–8025. doi:10.1021/ja066850h
Return to citation in text: [1] -
Zhdankin, V. V.; Protasiewicz, J. D. Coord. Chem. Rev. 2014, 275, 54–62. doi:10.1016/j.ccr.2014.04.007
Return to citation in text: [1] -
Zhu, C.; Yoshimura, A.; Solntsev, P.; Ji, L.; Wei, Y.; Nemykin, V. N.; Zhdankin, V. V. Chem. Commun. 2012, 48, 10108–10110. doi:10.1039/c2cc35708a
Return to citation in text: [1] -
Groom, C. R.; Bruno, I. J.; Lightfoot, M. P.; Ward, S. C. Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater. 2016, 72, 171–179. doi:10.1107/s2052520616003954
See for crystal structures of 39 (CCDC #893474), 76c (CCDC #2104180) and 76d (CCDC #2104181), accessed from the Cambridge Structural Database.
Return to citation in text: [1] [2] -
Momma, K.; Izumi, F. J. Appl. Crystallogr. 2011, 44, 1272–1276. doi:10.1107/s0021889811038970
Return to citation in text: [1] [2] -
Saito, M.; Kobayashi, Y.; Takemoto, Y. Chem. – Eur. J. 2019, 25, 10314–10318. doi:10.1002/chem.201902699
Return to citation in text: [1] -
Varvoglis, A. The organic chemistry of polycoordinated iodine; VCH: New York, N.Y., 1992.
Return to citation in text: [1] -
Wirth, T. Hypervalent Iodine Chemistry: Modern Developments in Organic Synthesis; Springer Berlin Heidelberg: Berlin, 2003.
Return to citation in text: [1] -
Zhdankin, V. V., Ed. Hypervalent Iodine Chemistry: Preparation, Structure, and Synthetic Applications of Polyvalent Iodine Compounds; John Wiley & Sons, 2013; pp 468 ff. doi:10.1002/9781118341155
Return to citation in text: [1]
| 121. | Mulliken, R. S. J. Am. Chem. Soc. 1952, 74, 811–824. doi:10.1021/ja01123a067 |
| 122. | Zhao, Z.; Luo, Y.; Liu, S.; Zhang, L.; Feng, L.; Wang, Y. Angew. Chem., Int. Ed. 2018, 57, 3792–3796. doi:10.1002/anie.201800389 |
| 9. | Koser, G. F.; Yu, S.-M. J. Org. Chem. 1975, 40, 1166–1168. doi:10.1021/jo00896a037 |
| 61. | Deepa, P.; Sedlak, R.; Hobza, P. Phys. Chem. Chem. Phys. 2014, 16, 6679–6686. doi:10.1039/c4cp00055b |
| 120. | Antos, A.; Elemes, Y.; Michaelides, A.; Nyxas, J. A.; Skoulika, S.; Hadjiarapoglou, L. P. J. Org. Chem. 2012, 77, 10949–10954. doi:10.1021/jo3020787 |
| 1. | Gudrinietse, E.; Neiland, O.; Vanag, G. J. Gen. Chem. USSR 1957, 27, 2777–2780. |
| 102. | Neiland, O.; Vanag, G. Proc. Acad. Sci. USSR 1960, 130, 19. |
| 125. | Chidley, T.; Jameel, I.; Rizwan, S.; Peixoto, P. A.; Pouységu, L.; Quideau, S.; Hopkins, W. S.; Murphy, G. K. Angew. Chem., Int. Ed. 2019, 58, 16959–16965. doi:10.1002/anie.201908994 |
| 126. | Sar, S.; Das, R.; Barman, D.; Latua, P.; Guha, S.; Gremaud, L.; Sen, S. Org. Biomol. Chem. 2021, 19, 7627–7632. doi:10.1039/d1ob01219c |
| 123. | Zhao, Z.; Kong, X.; Wang, W.; Hao, J.; Wang, Y. Org. Lett. 2020, 22, 230–233. doi:10.1021/acs.orglett.9b04213 |
| 124. | Zhao, Y.-R.; Li, L.; Xuan, J. Adv. Synth. Catal. 2023, 365, 110–115. doi:10.1002/adsc.202201085 |
| 134. | Rotstein, B. H.; Stephenson, N. A.; Vasdev, N.; Liang, S. H. Nat. Commun. 2014, 5, 4365. doi:10.1038/ncomms5365 |
| 134. | Rotstein, B. H.; Stephenson, N. A.; Vasdev, N.; Liang, S. H. Nat. Commun. 2014, 5, 4365. doi:10.1038/ncomms5365 |
| 100. | Montgomery, C. A.; Jameel, I.; Cuzzucoli, F.; Chidley, T.; Hopkins, W. S.; Murphy, G. K. Chem. – Eur. J. 2022, 28, e202202029. doi:10.1002/chem.202202029 |
| 131. | Kalnin, S. V.; Neiland, O. Y. Zh. Org. Khim. 1971, 7, 1606. |
| 132. | Neiland, O. Y.; Karele, B. Y. Zh. Org. Khim. 1971, 7, 1611. |
| 133. | Satyamurthy, N.; Barrio, J. R. No-carrier-added nucleophilic [F-18]fluorination of aromatic compounds. WO Pat. WO2010117435A2, Oct 14, 2010. |
| 127. | Gondo, K.; Kitamura, T. Molecules 2012, 17, 6625–6632. doi:10.3390/molecules17066625 |
| 128. | Pongratz, E.; Kappe, T. Monatsh. Chem. 1984, 115, 231–242. doi:10.1007/bf00798414 |
| 129. | Geary, G. C.; Hope, E. G.; Singh, K.; Stuart, A. M. RSC Adv. 2015, 5, 16501–16506. doi:10.1039/c4ra15733h |
| 130. | Neilands, O. Zh. Org. Khim. 1965, 1, 1858–1862. |
| 37. | Varadwaj, A.; Marques, H. M.; Varadwaj, P. R. Molecules 2019, 24, 379. doi:10.3390/molecules24030379 |
| 38. | Politzer, P.; Murray, J. S.; Concha, M. C. J. Mol. Model. 2007, 13, 643–650. doi:10.1007/s00894-007-0176-9 |
| 39. | Shields, Z. P.; Murray, J. S.; Politzer, P. Int. J. Quantum Chem. 2010, 110, 2823–2832. doi:10.1002/qua.22787 |
| 100. | Montgomery, C. A.; Jameel, I.; Cuzzucoli, F.; Chidley, T.; Hopkins, W. S.; Murphy, G. K. Chem. – Eur. J. 2022, 28, e202202029. doi:10.1002/chem.202202029 |
| 146. | Varvoglis, A. The organic chemistry of polycoordinated iodine; VCH: New York, N.Y., 1992. |
| 147. | Wirth, T. Hypervalent Iodine Chemistry: Modern Developments in Organic Synthesis; Springer Berlin Heidelberg: Berlin, 2003. |
| 148. | Zhdankin, V. V., Ed. Hypervalent Iodine Chemistry: Preparation, Structure, and Synthetic Applications of Polyvalent Iodine Compounds; John Wiley & Sons, 2013; pp 468 ff. doi:10.1002/9781118341155 |
| 65. | Murray, J. S.; Concha, M. C.; Lane, P.; Hobza, P.; Politzer, P. J. Mol. Model. 2008, 14, 699–704. doi:10.1007/s00894-008-0307-y |
| 54. | Bartashevich, E. V.; Matveychuk, Y. V.; Mukhitdinova, S. E.; Sobalev, S. A.; Khrenova, M. G.; Tsirelson, V. G. Theor. Chem. Acc. 2020, 139, 26. doi:10.1007/s00214-019-2534-y |
| 55. | Dong, W.; Li, Q.; Scheiner, S. Molecules 2018, 23, 1681. doi:10.3390/molecules23071681 |
| 56. | Bauzá, A.; Quiñonero, D.; Deyà, P. M.; Frontera, A. CrystEngComm 2013, 15, 3137–3144. doi:10.1039/c2ce26741a |
| 57. | Liu, N.; Li, Q.; McDowell, S. A. C. Front. Chem. (Lausanne, Switz.) 2020, 8, 608486. doi:10.3389/fchem.2020.608486 |
| 143. |
Groom, C. R.; Bruno, I. J.; Lightfoot, M. P.; Ward, S. C. Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater. 2016, 72, 171–179. doi:10.1107/s2052520616003954
See for crystal structures of 39 (CCDC #893474), 76c (CCDC #2104180) and 76d (CCDC #2104181), accessed from the Cambridge Structural Database. |
| 144. | Momma, K.; Izumi, F. J. Appl. Crystallogr. 2011, 44, 1272–1276. doi:10.1107/s0021889811038970 |
| 135. | Shah, A.; Pike, V. W.; Widdowson, D. A. J. Chem. Soc., Perkin Trans. 1 1998, 2043–2046. doi:10.1039/a802349b |
| 136. | Wang, B.; Graskemper, J. W.; Qin, L.; DiMagno, S. G. Angew. Chem., Int. Ed. 2010, 49, 4079–4083. doi:10.1002/anie.201000695 |
| 137. | Grushin, V. V. Acc. Chem. Res. 1992, 25, 529–536. doi:10.1021/ar00023a007 |
| 138. | Grushin, V. V.; Demkina, I. I.; Tolstaya, T. P. J. Chem. Soc., Perkin Trans. 2 1992, 505–511. doi:10.1039/p29920000505 |
| 139. | Wang, L.; Jacobson, O.; Avdic, D.; Rotstein, B. H.; Weiss, I. D.; Collier, L.; Chen, X.; Vasdev, N.; Liang, S. H. Angew. Chem., Int. Ed. 2015, 54, 12777–12781. doi:10.1002/anie.201505927 |
| 16. | Rotstein, B. H.; Wang, L.; Liu, R. Y.; Patteson, J.; Kwan, E. E.; Vasdev, N.; Liang, S. H. Chem. Sci. 2016, 7, 4407–4417. doi:10.1039/c6sc00197a |
| 16. | Rotstein, B. H.; Wang, L.; Liu, R. Y.; Patteson, J.; Kwan, E. E.; Vasdev, N.; Liang, S. H. Chem. Sci. 2016, 7, 4407–4417. doi:10.1039/c6sc00197a |
| 90. | Heinen, F.; Engelage, E.; Cramer, C. J.; Huber, S. M. J. Am. Chem. Soc. 2020, 142, 8633–8640. doi:10.1021/jacs.9b13309 |
| 91. | Zhang, Y.; Han, J.; Liu, Z.-J. RSC Adv. 2015, 5, 25485–25488. doi:10.1039/c5ra00209e |
| 92. | Heinen, F.; Engelage, E.; Dreger, A.; Weiss, R.; Huber, S. M. Angew. Chem., Int. Ed. 2018, 57, 3830–3833. doi:10.1002/anie.201713012 |
| 93. | Wolf, J.; Huber, F.; Erochok, N.; Heinen, F.; Guérin, V.; Legault, C. Y.; Kirsch, S. F.; Huber, S. M. Angew. Chem., Int. Ed. 2020, 59, 16496–16500. doi:10.1002/anie.202005214 |
| 94. | Haraguchi, R.; Nishikawa, T.; Kanazawa, A.; Aoshima, S. Macromolecules 2020, 53, 4185–4192. doi:10.1021/acs.macromol.0c00823 |
| 95. | Heinen, F.; Reinhard, D. L.; Engelage, E.; Huber, S. M. Angew. Chem., Int. Ed. 2021, 60, 5069–5073. doi:10.1002/anie.202013172 |
| 96. | Yunusova, S. N.; Novikov, A. S.; Soldatova, N. S.; Vovk, M. A.; Bolotin, D. S. RSC Adv. 2021, 11, 4574–4583. doi:10.1039/d0ra09640g |
| 97. | To, A. J.; Murphy, G. K. New J. Chem. 2022, 46, 15313–15320. doi:10.1039/d2nj02731c |
| 72. | Pinto de Magalhães, H.; Togni, A.; Lüthi, H. P. J. Org. Chem. 2017, 82, 11799–11805. doi:10.1021/acs.joc.7b01716 |
| 1. | Gudrinietse, E.; Neiland, O.; Vanag, G. J. Gen. Chem. USSR 1957, 27, 2777–2780. |
| 15. | Chassé, M.; Pees, A.; Lindberg, A.; Liang, S. H.; Vasdev, N. Chem. Rec. 2023, e202300072. doi:10.1002/tcr.202300072 |
| 16. | Rotstein, B. H.; Wang, L.; Liu, R. Y.; Patteson, J.; Kwan, E. E.; Vasdev, N.; Liang, S. H. Chem. Sci. 2016, 7, 4407–4417. doi:10.1039/c6sc00197a |
| 17. | Liang, S. H.; Wang, L.; Stephenson, N. A.; Rotstein, B. H.; Vasdev, N. Nat. Protoc. 2019, 14, 1530–1545. doi:10.1038/s41596-019-0149-3 |
| 18. | Calderwood, S.; Collier, T. L.; Gouverneur, V.; Liang, S. H.; Vasdev, N. J. Fluorine Chem. 2015, 178, 249–253. doi:10.1016/j.jfluchem.2015.08.006 |
| 103. | Müller, P.; Boléa, C. Helv. Chim. Acta 2001, 84, 1093–1111. doi:10.1002/1522-2675(20010516)84:5<1093::aid-hlca1093>3.0.co;2-t |
| 104. | Müller, P.; Boléa, C. Synlett 2000, 826–828. doi:10.1055/s-2000-6697 |
| 105. | Müller, P.; Boléa, C. Molecules 2001, 6, 258–266. doi:10.3390/60300258 |
| 106. | Müller, P. Acc. Chem. Res. 2004, 37, 243–251. doi:10.1021/ar0202619 |
| 9. | Koser, G. F.; Yu, S.-M. J. Org. Chem. 1975, 40, 1166–1168. doi:10.1021/jo00896a037 |
| 10. | Gololobov, Y. G.; Golding, I. R.; Galkina, M. A.; Lokshin, B. V.; Garbuzova, I. A.; Petrovskii, P. V.; Starikova, Z. A.; Averkiev, B. B. Russ. Chem. Bull. 2006, 55, 883–891. doi:10.1007/s11172-006-0347-3 |
| 11. | Moriarty, R. M.; Tyagi, S.; Kinch, M. Tetrahedron 2010, 66, 5801–5810. doi:10.1016/j.tet.2010.05.005 |
| 12. | Huang, H.; Yang, Y.; Zhang, X.; Zeng, W.; Liang, Y. Tetrahedron Lett. 2013, 54, 6049–6052. doi:10.1016/j.tetlet.2013.08.096 |
| 13. | Chelli, S.; Troshin, K.; Mayer, P.; Lakhdar, S.; Ofial, A. R.; Mayr, H. J. Am. Chem. Soc. 2016, 138, 10304–10313. doi:10.1021/jacs.6b05768 |
| 14. | Tao, J.; Estrada, C. D.; Murphy, G. K. Chem. Commun. 2017, 53, 9004–9007. doi:10.1039/c7cc04859a |
| 3. | Müller, P.; Fernandez, D. Helv. Chim. Acta 1995, 78, 947–958. doi:10.1002/hlca.19950780417 |
| 4. | Murphy, G. K.; West, F. G. Org. Lett. 2006, 8, 4359–4361. doi:10.1021/ol061772o |
| 5. | Goudreau, S. R.; Marcoux, D.; Charette, A. B. J. Org. Chem. 2009, 74, 470–473. doi:10.1021/jo802208q |
| 6. | Zhu, C.; Yoshimura, A.; Ji, L.; Wei, Y.; Nemykin, V. N.; Zhdankin, V. V. Org. Lett. 2012, 14, 3170–3173. doi:10.1021/ol301268j |
| 7. | Bosnidou, A.-E.; Kalpogiannaki, D.; Karanestora, S.; Nixas, J. A.; Hadjiarapoglou, L. P. J. Org. Chem. 2015, 80, 1279–1283. doi:10.1021/jo502611x |
| 8. | Hayasi, Y.; Okada, T.; Kawanisi, M. Bull. Chem. Soc. Jpn. 1970, 43, 2506–2511. doi:10.1246/bcsj.43.2506 |
| 101. | Kimura, T.; Hamada, S.; Furuta, T.; Takemoto, Y.; Kobayashi, Y. Crystals 2021, 11, 1085. doi:10.3390/cryst11091085 |
| 2. | Yusubov, M. S.; Yoshimura, A.; Zhdankin, V. V. ARKIVOC 2016, No. 1, 342–374. doi:10.3998/ark.5550190.p009.732 |
| 1. | Gudrinietse, E.; Neiland, O.; Vanag, G. J. Gen. Chem. USSR 1957, 27, 2777–2780. |
| 102. | Neiland, O.; Vanag, G. Proc. Acad. Sci. USSR 1960, 130, 19. |
| 24. | Politzer, P.; Murray, J. S.; Clark, T. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. doi:10.1039/c3cp00054k |
| 100. | Montgomery, C. A.; Jameel, I.; Cuzzucoli, F.; Chidley, T.; Hopkins, W. S.; Murphy, G. K. Chem. – Eur. J. 2022, 28, e202202029. doi:10.1002/chem.202202029 |
| 23. | Desiraju, G. R.; Ho, P. S.; Kloo, L.; Legon, A. C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Pure Appl. Chem. 2013, 85, 1711–1713. doi:10.1351/pac-rec-12-05-10 |
| 86. | Molecular electrostatic potential (MEP) maps were calculated de-novo using previously-reported methods. See reference [100] for details. |
| 21. | Dumas, J.-M.; Kern, M.; Janier-Dubry, J. L. Bull. Soc. Chim. Fr. 1976, 1785–1787. |
| 22. | Dumas, J.-M.; Peurichard, H.; Gomel, M. J. Chem. Res., Synop. 1978, 54–57. |
| 86. | Molecular electrostatic potential (MEP) maps were calculated de-novo using previously-reported methods. See reference [100] for details. |
| 19. | Guthrie, F. J. Chem. Soc. 1863, 16, 239–244. doi:10.1039/js8631600239 |
| 20. | Remsen, I.; Norris, J. F. Am. Chem. J. 1896, 18, 90–96. |
| 98. | Yoshida, Y.; Fujimura, T.; Mino, T.; Sakamoto, M. Adv. Synth. Catal. 2022, 364, 1091–1098. doi:10.1002/adsc.202101380 |
| 99. | Lang, T.; Zhang, X.; Meng, L.; Zeng, Y. Struct. Chem. 2016, 27, 927–937. doi:10.1007/s11224-015-0676-7 |
| 107. | Hadjiarapoglou, L. P.; Varvoglis, A.; Alcock, N. W.; Pike, G. A. J. Chem. Soc., Perkin Trans. 1 1988, 2839–2846. doi:10.1039/p19880002839 |
| 113. | Moriarty, R. M.; Prakash, O.; Vaid, R. K.; Zhao, L. J. Am. Chem. Soc. 1989, 111, 6443–6444. doi:10.1021/ja00198a078 |
| 107. | Hadjiarapoglou, L. P.; Varvoglis, A.; Alcock, N. W.; Pike, G. A. J. Chem. Soc., Perkin Trans. 1 1988, 2839–2846. doi:10.1039/p19880002839 |
| 108. | Adam, W.; Bosio, S. G.; Gogonas, E. P.; Hadjiarapoglou, L. P. Synthesis 2002, 2084–2090. doi:10.1055/s-2002-34376 |
| 109. | Adam, W.; Gogonas, E. P.; Hadjiarapoglou, L. P. Eur. J. Org. Chem. 2003, 1064–1068. doi:10.1002/ejoc.200390156 |
| 110. | Adam, W.; Gogonas, E. P.; Hadjiarapoglou, L. P. J. Org. Chem. 2003, 68, 9155–9158. doi:10.1021/jo035362e |
| 111. | Adam, W.; Gogonas, E. P.; Hadjiarapoglou, L. P. Tetrahedron 2003, 59, 7929–7934. doi:10.1016/j.tet.2003.07.011 |
| 112. | Adam, W.; Gogonas, E. P.; Hadjiarapoglou, L. P. Synlett 2003, 1165–1169. doi:10.1055/s-2003-39894 |
| 113. | Moriarty, R. M.; Prakash, O.; Vaid, R. K.; Zhao, L. J. Am. Chem. Soc. 1989, 111, 6443–6444. doi:10.1021/ja00198a078 |
| 114. | Moriarty, R. M.; May, E. J.; Prakash, O. Tetrahedron Lett. 1997, 38, 4333–4336. doi:10.1016/s0040-4039(97)00967-2 |
| 115. | Moriarty, R. M.; Kim, J.; Guo, L. Tetrahedron Lett. 1993, 34, 4129–4132. doi:10.1016/s0040-4039(00)60509-9 |
| 110. | Adam, W.; Gogonas, E. P.; Hadjiarapoglou, L. P. J. Org. Chem. 2003, 68, 9155–9158. doi:10.1021/jo035362e |
| 11. | Moriarty, R. M.; Tyagi, S.; Kinch, M. Tetrahedron 2010, 66, 5801–5810. doi:10.1016/j.tet.2010.05.005 |
| 113. | Moriarty, R. M.; Prakash, O.; Vaid, R. K.; Zhao, L. J. Am. Chem. Soc. 1989, 111, 6443–6444. doi:10.1021/ja00198a078 |
| 116. | Gallos, J. K.; Koftis, T. V.; Massen, Z. S.; Dellios, C. C.; Mourtzinos, I. T.; Coutouli-Argyropoulou, E.; Koumbis, A. E. Tetrahedron 2002, 58, 8043–8053. doi:10.1016/s0040-4020(02)01002-5 |
| 116. | Gallos, J. K.; Koftis, T. V.; Massen, Z. S.; Dellios, C. C.; Mourtzinos, I. T.; Coutouli-Argyropoulou, E.; Koumbis, A. E. Tetrahedron 2002, 58, 8043–8053. doi:10.1016/s0040-4020(02)01002-5 |
| 9. | Koser, G. F.; Yu, S.-M. J. Org. Chem. 1975, 40, 1166–1168. doi:10.1021/jo00896a037 |
| 117. | Papadopoulou, M.; Spyroudis, S.; Varvoglis, A. J. Org. Chem. 1985, 50, 1509–1511. doi:10.1021/jo00209a029 |
| 118. | Spyroudis, S. P. J. Org. Chem. 1986, 51, 3453–3456. doi:10.1021/jo00368a009 |
| 119. | Hadjiarapoglou, L. P. Tetrahedron Lett. 1987, 28, 4449–4450. doi:10.1016/s0040-4039(00)96535-3 |
| 24. | Politzer, P.; Murray, J. S.; Clark, T. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. doi:10.1039/c3cp00054k |
| 39. | Shields, Z. P.; Murray, J. S.; Politzer, P. Int. J. Quantum Chem. 2010, 110, 2823–2832. doi:10.1002/qua.22787 |
| 49. | Ramasubbu, N.; Parthasarathy, R.; Murray-Rust, P. J. Am. Chem. Soc. 1986, 108, 4308–4314. doi:10.1021/ja00275a012 |
| 50. | Lu, Y.; Wang, Y.; Zhu, W. Phys. Chem. Chem. Phys. 2010, 12, 4543–4551. doi:10.1039/b926326h |
| 59. | Reed, A. E.; Curtiss, L. A.; Weinhold, F. Chem. Rev. 1988, 88, 899–926. doi:10.1021/cr00088a005 |
| 60. | Riley, K. E.; Murray, J. S.; Fanfrlík, J.; Řezáč, J.; Solá, R. J.; Concha, M. C.; Ramos, F. M.; Politzer, P. J. Mol. Model. 2013, 19, 4651–4659. doi:10.1007/s00894-012-1428-x |
| 61. | Deepa, P.; Sedlak, R.; Hobza, P. Phys. Chem. Chem. Phys. 2014, 16, 6679–6686. doi:10.1039/c4cp00055b |
| 62. | Robidas, R.; Reinhard, D. L.; Legault, C. Y.; Huber, S. M. Chem. Rec. 2021, 21, 1912–1927. doi:10.1002/tcr.202100119 |
| 63. | Rosokha, S. V.; Stern, C. L.; Ritzert, J. T. Chem. – Eur. J. 2013, 19, 8774–8788. doi:10.1002/chem.201300577 |
| 46. | Mohajeri, A.; Pakiari, A. H.; Bagheri, N. Chem. Phys. Lett. 2009, 467, 393–397. doi:10.1016/j.cplett.2008.11.017 |
| 47. | Costa, P. J. Phys. Sci. Rev. 2017, 2, 20170136. |
| 64. | Blue shifting has also been reported as a result of halogen bonding, however, it is far less common. See reference [65]. |
| 65. | Murray, J. S.; Concha, M. C.; Lane, P.; Hobza, P.; Politzer, P. J. Mol. Model. 2008, 14, 699–704. doi:10.1007/s00894-008-0307-y |
| 46. | Mohajeri, A.; Pakiari, A. H.; Bagheri, N. Chem. Phys. Lett. 2009, 467, 393–397. doi:10.1016/j.cplett.2008.11.017 |
| 50. | Lu, Y.; Wang, Y.; Zhu, W. Phys. Chem. Chem. Phys. 2010, 12, 4543–4551. doi:10.1039/b926326h |
| 46. | Mohajeri, A.; Pakiari, A. H.; Bagheri, N. Chem. Phys. Lett. 2009, 467, 393–397. doi:10.1016/j.cplett.2008.11.017 |
| 31. | Decato, D. A.; John, E. A.; Berryman, O. B. Halogen Bonding: An Introduction. In Halogen Bonding in Solution; Huber, S., Ed.; Wiley‐VCH Verlag GmbH & Co. KGaA: Weinheim, 2021; pp 1–41. doi:10.1002/9783527825738.ch1 |
| 48. | Murray, J. S.; Lane, P.; Politzer, P. J. Mol. Model. 2009, 15, 723–729. doi:10.1007/s00894-008-0386-9 |
| 47. | Costa, P. J. Phys. Sci. Rev. 2017, 2, 20170136. |
| 58. | Lu, Y.; Li, H.; Zhu, X.; Zhu, W.; Liu, H. J. Phys. Chem. A 2011, 115, 4467–4475. doi:10.1021/jp111616x |
| 46. | Mohajeri, A.; Pakiari, A. H.; Bagheri, N. Chem. Phys. Lett. 2009, 467, 393–397. doi:10.1016/j.cplett.2008.11.017 |
| 48. | Murray, J. S.; Lane, P.; Politzer, P. J. Mol. Model. 2009, 15, 723–729. doi:10.1007/s00894-008-0386-9 |
| 51. | Clark, T.; Heßelmann, A. Phys. Chem. Chem. Phys. 2018, 20, 22849–22855. doi:10.1039/c8cp03079k |
| 52. | Huber, S. M.; Jimenez-Izal, E.; Ugalde, J. M.; Infante, I. Chem. Commun. 2012, 48, 7708–7710. doi:10.1039/c2cc33304j |
| 53. | σ-Hole bond strength trends are inconsistent across the periodic table, but do appear to be influenced by several factors. For example, the strongest σ-hole bond may change from halogen to pnicogen atoms, depending on the σ-hole bond acceptor. See references [54-57]. |
| 54. | Bartashevich, E. V.; Matveychuk, Y. V.; Mukhitdinova, S. E.; Sobalev, S. A.; Khrenova, M. G.; Tsirelson, V. G. Theor. Chem. Acc. 2020, 139, 26. doi:10.1007/s00214-019-2534-y |
| 55. | Dong, W.; Li, Q.; Scheiner, S. Molecules 2018, 23, 1681. doi:10.3390/molecules23071681 |
| 56. | Bauzá, A.; Quiñonero, D.; Deyà, P. M.; Frontera, A. CrystEngComm 2013, 15, 3137–3144. doi:10.1039/c2ce26741a |
| 57. | Liu, N.; Li, Q.; McDowell, S. A. C. Front. Chem. (Lausanne, Switz.) 2020, 8, 608486. doi:10.3389/fchem.2020.608486 |
| 66. | Stephens, S. L.; Walker, N. R.; Legon, A. C. Phys. Chem. Chem. Phys. 2011, 13, 20736–20744. doi:10.1039/c1cp21854a |
| 67. | Hauchecorne, D.; Nagels, N.; van der Veken, B. J.; Herrebout, W. A. Phys. Chem. Chem. Phys. 2012, 14, 681–690. doi:10.1039/c1cp22771h |
| 68. | Sladojevich, F.; McNeill, E.; Börgel, J.; Zheng, S.-L.; Ritter, T. Angew. Chem., Int. Ed. 2015, 54, 3712–3716. doi:10.1002/anie.201410954 |
| 69. | Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. Chem. Rev. 2016, 116, 2478–2601. doi:10.1021/acs.chemrev.5b00484 |
| 70. | Kolář, M. H.; Hobza, P. Chem. Rev. 2016, 116, 5155–5187. doi:10.1021/acs.chemrev.5b00560 |
| 71. | Bundhun, A.; Ramasami, P.; Murray, J. S.; Politzer, P. J. Mol. Model. 2013, 19, 2739–2746. doi:10.1007/s00894-012-1571-4 |
| 70. | Kolář, M. H.; Hobza, P. Chem. Rev. 2016, 116, 5155–5187. doi:10.1021/acs.chemrev.5b00560 |
| 72. | Pinto de Magalhães, H.; Togni, A.; Lüthi, H. P. J. Org. Chem. 2017, 82, 11799–11805. doi:10.1021/acs.joc.7b01716 |
| 70. | Kolář, M. H.; Hobza, P. Chem. Rev. 2016, 116, 5155–5187. doi:10.1021/acs.chemrev.5b00560 |
| 71. | Bundhun, A.; Ramasami, P.; Murray, J. S.; Politzer, P. J. Mol. Model. 2013, 19, 2739–2746. doi:10.1007/s00894-012-1571-4 |
| 88. | Soldatova, N. S.; Postnikov, P. S.; Suslonov, V. V.; Kissler, T. Y.; Ivanov, D. M.; Yusubov, M. S.; Galmés, B.; Frontera, A.; Kukushkin, V. Y. Org. Chem. Front. 2020, 7, 2230–2242. doi:10.1039/d0qo00678e |
| 89. | Cavallo, G.; Murray, J. S.; Politzer, P.; Pilati, T.; Ursini, M.; Resnati, G. IUCrJ 2017, 4, 411–419. doi:10.1107/s2052252517004262 |
| 87. | Poynder, T. B.; Chamorro Orué, A. I.; Tania; Sharp-Bucknall, L.; Flynn, M. T.; Wilson, D. J. D.; Athukorala Arachchige, K. S.; Clegg, J. K.; Dutton, J. L. Chem. Commun. 2021, 57, 4970–4973. doi:10.1039/d1cc01567b |
| 72. | Pinto de Magalhães, H.; Togni, A.; Lüthi, H. P. J. Org. Chem. 2017, 82, 11799–11805. doi:10.1021/acs.joc.7b01716 |
| 72. | Pinto de Magalhães, H.; Togni, A.; Lüthi, H. P. J. Org. Chem. 2017, 82, 11799–11805. doi:10.1021/acs.joc.7b01716 |
| 87. | Poynder, T. B.; Chamorro Orué, A. I.; Tania; Sharp-Bucknall, L.; Flynn, M. T.; Wilson, D. J. D.; Athukorala Arachchige, K. S.; Clegg, J. K.; Dutton, J. L. Chem. Commun. 2021, 57, 4970–4973. doi:10.1039/d1cc01567b |
| 83. | Sutar, R. L.; Huber, S. M. ACS Catal. 2019, 9, 9622–9639. doi:10.1021/acscatal.9b02894 |
| 84. | Bulfield, D.; Huber, S. M. Chem. – Eur. J. 2016, 22, 14434–14450. doi:10.1002/chem.201601844 |
| 85. | Saito, M.; Kobayashi, Y.; Tsuzuki, S.; Takemoto, Y. Angew. Chem., Int. Ed. 2017, 56, 7653–7657. doi:10.1002/anie.201703641 |
| 86. | Molecular electrostatic potential (MEP) maps were calculated de-novo using previously-reported methods. See reference [100] for details. |
| 72. | Pinto de Magalhães, H.; Togni, A.; Lüthi, H. P. J. Org. Chem. 2017, 82, 11799–11805. doi:10.1021/acs.joc.7b01716 |
| 73. | Hua, Y.; Flood, A. H. J. Am. Chem. Soc. 2010, 132, 12838–12840. doi:10.1021/ja105793c |
| 74. | Parks, F. C.; Liu, Y.; Debnath, S.; Stutsman, S. R.; Raghavachari, K.; Flood, A. H. J. Am. Chem. Soc. 2018, 140, 17711–17723. doi:10.1021/jacs.8b10538 |
| 75. | Kerckhoffs, A.; Moss, I.; Langton, M. J. Chem. Commun. 2023, 59, 51–54. doi:10.1039/d2cc05199k |
| 76. | Kerckhoffs, A.; Langton, M. J. Chem. Sci. 2020, 11, 6325–6331. doi:10.1039/d0sc02745f |
| 77. | Choi, Y. R.; Kim, G. C.; Jeon, H.-G.; Park, J.; Namkung, W.; Jeong, K.-S. Chem. Commun. 2014, 50, 15305–15308. doi:10.1039/c4cc07560a |
| 78. | Ryan, S. T. J.; del Barrio, J.; Suardíaz, R.; Ryan, D. F.; Rosta, E.; Scherman, O. A. Angew. Chem., Int. Ed. 2016, 55, 16096–16100. doi:10.1002/anie.201607693 |
| 79. | Docker, A.; Shang, X.; Yuan, D.; Kuhn, H.; Zhang, Z.; Davis, J. J.; Beer, P. D.; Langton, M. J. Angew. Chem., Int. Ed. 2021, 60, 19442–19450. doi:10.1002/anie.202107748 |
| 80. | Kokan, Z.; Chmielewski, M. J. J. Am. Chem. Soc. 2018, 140, 16010–16014. doi:10.1021/jacs.8b08689 |
| 81. | Wezenberg, S. J.; Vlatković, M.; Kistemaker, J. C. M.; Feringa, B. L. J. Am. Chem. Soc. 2014, 136, 16784–16787. doi:10.1021/ja510700j |
| 82. | Villarón, D.; Siegler, M. A.; Wezenberg, S. J. Chem. Sci. 2021, 12, 3188–3193. doi:10.1039/d0sc06686a |
| 6. | Zhu, C.; Yoshimura, A.; Ji, L.; Wei, Y.; Nemykin, V. N.; Zhdankin, V. V. Org. Lett. 2012, 14, 3170–3173. doi:10.1021/ol301268j |
| 143. |
Groom, C. R.; Bruno, I. J.; Lightfoot, M. P.; Ward, S. C. Acta Crystallogr., Sect. B: Struct. Sci., Cryst. Eng. Mater. 2016, 72, 171–179. doi:10.1107/s2052520616003954
See for crystal structures of 39 (CCDC #893474), 76c (CCDC #2104180) and 76d (CCDC #2104181), accessed from the Cambridge Structural Database. |
| 144. | Momma, K.; Izumi, F. J. Appl. Crystallogr. 2011, 44, 1272–1276. doi:10.1107/s0021889811038970 |
| 3. | Müller, P.; Fernandez, D. Helv. Chim. Acta 1995, 78, 947–958. doi:10.1002/hlca.19950780417 |
| 5. | Goudreau, S. R.; Marcoux, D.; Charette, A. B. J. Org. Chem. 2009, 74, 470–473. doi:10.1021/jo802208q |
| 6. | Zhu, C.; Yoshimura, A.; Ji, L.; Wei, Y.; Nemykin, V. N.; Zhdankin, V. V. Org. Lett. 2012, 14, 3170–3173. doi:10.1021/ol301268j |
| 141. | Zhdankin, V. V.; Protasiewicz, J. D. Coord. Chem. Rev. 2014, 275, 54–62. doi:10.1016/j.ccr.2014.04.007 |
| 142. | Zhu, C.; Yoshimura, A.; Solntsev, P.; Ji, L.; Wei, Y.; Nemykin, V. N.; Zhdankin, V. V. Chem. Commun. 2012, 48, 10108–10110. doi:10.1039/c2cc35708a |
| 136. | Wang, B.; Graskemper, J. W.; Qin, L.; DiMagno, S. G. Angew. Chem., Int. Ed. 2010, 49, 4079–4083. doi:10.1002/anie.201000695 |
| 140. | Ross, T. L.; Ermert, J.; Hocke, C.; Coenen, H. H. J. Am. Chem. Soc. 2007, 129, 8018–8025. doi:10.1021/ja066850h |
| 31. | Decato, D. A.; John, E. A.; Berryman, O. B. Halogen Bonding: An Introduction. In Halogen Bonding in Solution; Huber, S., Ed.; Wiley‐VCH Verlag GmbH & Co. KGaA: Weinheim, 2021; pp 1–41. doi:10.1002/9783527825738.ch1 |
| 24. | Politzer, P.; Murray, J. S.; Clark, T. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. doi:10.1039/c3cp00054k |
| 29. | Archer, E. M.; van Schalkwyk, T. G. Acta Crystallogr. 1953, 6, 88–92. doi:10.1107/s0365110x53000193 |
| 24. | Politzer, P.; Murray, J. S.; Clark, T. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. doi:10.1039/c3cp00054k |
| 30. | Lu, Y.; Zou, J.; Wang, H.; Yu, Q.; Zhang, H.; Jiang, Y. J. Phys. Chem. A 2005, 109, 11956–11961. doi:10.1021/jp0547360 |
| 25. | Bent, H. A. Chem. Rev. 1968, 68, 587–648. doi:10.1021/cr60255a003 |
| 26. | Gagnaux, P.; Susz, B. P. Helv. Chim. Acta 1960, 43, 948–956. doi:10.1002/hlca.19600430403 |
| 101. | Kimura, T.; Hamada, S.; Furuta, T.; Takemoto, Y.; Kobayashi, Y. Crystals 2021, 11, 1085. doi:10.3390/cryst11091085 |
| 27. | Hassel, O.; Hvoslef, J. Acta Chem. Scand. 1954, 8, 873. doi:10.3891/acta.chem.scand.08-0873 |
| 28. | Hassel, O.; Rømming, C. Q. Rev., Chem. Soc. 1962, 16, 1–18. doi:10.1039/qr9621600001 |
| 145. | Saito, M.; Kobayashi, Y.; Takemoto, Y. Chem. – Eur. J. 2019, 25, 10314–10318. doi:10.1002/chem.201902699 |
| 101. | Kimura, T.; Hamada, S.; Furuta, T.; Takemoto, Y.; Kobayashi, Y. Crystals 2021, 11, 1085. doi:10.3390/cryst11091085 |
| 40. | Tsirelson, V. G.; Zhou, P. F.; Tang, T.-H.; Bader, R. F. W. Acta Crystallogr., Sect. A: Found. Crystallogr. 1995, 51, 143–153. doi:10.1107/s0108767394009463 |
| 31. | Decato, D. A.; John, E. A.; Berryman, O. B. Halogen Bonding: An Introduction. In Halogen Bonding in Solution; Huber, S., Ed.; Wiley‐VCH Verlag GmbH & Co. KGaA: Weinheim, 2021; pp 1–41. doi:10.1002/9783527825738.ch1 |
| 32. | Clark, T.; Hennemann, M.; Murray, J. S.; Politzer, P. J. Mol. Model. 2007, 13, 291–296. doi:10.1007/s00894-006-0130-2 |
| 33. | Politzer, P.; Murray, J. S. Crystals 2017, 7, 212. doi:10.3390/cryst7070212 |
| 34. | Sedlak, R.; Kolář, M. H.; Hobza, P. J. Chem. Theory Comput. 2015, 11, 4727–4732. doi:10.1021/acs.jctc.5b00687 |
| 35. | Brinck, T.; Murray, J. S.; Politzer, P. Int. J. Quantum Chem. 1992, 44, 57–64. doi:10.1002/qua.560440709 |
| 24. | Politzer, P.; Murray, J. S.; Clark, T. Phys. Chem. Chem. Phys. 2013, 15, 11178–11189. doi:10.1039/c3cp00054k |
| 36. | σ-Holes may appear neutral or even negatively charged for less polarizable or more electronegative halogens (e.g., fluorine). Alternatively, when there is significant polarization, the entire atomic surface may appear positive, which is typically seen with strongly electon withdrawing hosts. See references [37-39]. |
| 37. | Varadwaj, A.; Marques, H. M.; Varadwaj, P. R. Molecules 2019, 24, 379. doi:10.3390/molecules24030379 |
| 38. | Politzer, P.; Murray, J. S.; Concha, M. C. J. Mol. Model. 2007, 13, 643–650. doi:10.1007/s00894-007-0176-9 |
| 39. | Shields, Z. P.; Murray, J. S.; Politzer, P. Int. J. Quantum Chem. 2010, 110, 2823–2832. doi:10.1002/qua.22787 |
| 41. | Murray, J. S.; Riley, K. E.; Politzer, P.; Clark, T. Aust. J. Chem. 2010, 63, 1598–1607. doi:10.1071/ch10259 |
| 32. | Clark, T.; Hennemann, M.; Murray, J. S.; Politzer, P. J. Mol. Model. 2007, 13, 291–296. doi:10.1007/s00894-006-0130-2 |
| 48. | Murray, J. S.; Lane, P.; Politzer, P. J. Mol. Model. 2009, 15, 723–729. doi:10.1007/s00894-008-0386-9 |
| 41. | Murray, J. S.; Riley, K. E.; Politzer, P.; Clark, T. Aust. J. Chem. 2010, 63, 1598–1607. doi:10.1071/ch10259 |
| 46. | Mohajeri, A.; Pakiari, A. H.; Bagheri, N. Chem. Phys. Lett. 2009, 467, 393–397. doi:10.1016/j.cplett.2008.11.017 |
| 47. | Costa, P. J. Phys. Sci. Rev. 2017, 2, 20170136. |
| 31. | Decato, D. A.; John, E. A.; Berryman, O. B. Halogen Bonding: An Introduction. In Halogen Bonding in Solution; Huber, S., Ed.; Wiley‐VCH Verlag GmbH & Co. KGaA: Weinheim, 2021; pp 1–41. doi:10.1002/9783527825738.ch1 |
| 46. | Mohajeri, A.; Pakiari, A. H.; Bagheri, N. Chem. Phys. Lett. 2009, 467, 393–397. doi:10.1016/j.cplett.2008.11.017 |
| 44. | Stewart, R. F. Chem. Phys. Lett. 1979, 65, 335–342. doi:10.1016/0009-2614(79)87077-3 |
| 45. | Stevens, E. D.; Klain, C. L. Charge Density Studies of Drug Molecules. In The Application of Charge Density Research to Chemistry and Drug Design; Jeffrey, G. A.; Piniella, J. F., Eds.; Springer US: Boston, MA, 1991; pp 319–336. doi:10.1007/978-1-4615-3700-7_13 |
| 31. | Decato, D. A.; John, E. A.; Berryman, O. B. Halogen Bonding: An Introduction. In Halogen Bonding in Solution; Huber, S., Ed.; Wiley‐VCH Verlag GmbH & Co. KGaA: Weinheim, 2021; pp 1–41. doi:10.1002/9783527825738.ch1 |
| 41. | Murray, J. S.; Riley, K. E.; Politzer, P.; Clark, T. Aust. J. Chem. 2010, 63, 1598–1607. doi:10.1071/ch10259 |
| 42. | Politzer, P.; Murray, J. S. ChemPhysChem 2013, 14, 278–294. doi:10.1002/cphc.201200799 |
| 43. | Bader, R. F. W.; Carroll, M. T.; Cheeseman, J. R.; Chang, C. J. Am. Chem. Soc. 1987, 109, 7968–7979. doi:10.1021/ja00260a006 |
© 2023 Montgomery and Murphy; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.