Abstract
Nine new fluorinated analogues were synthesised by late-stage functionalisation using Diversinate™ chemistry on the Open Source Malaria (OSM) triazolopyrazine scaffold (Series 4). The structures of all analogues were fully characterised by NMR, UV and MS data analysis; three triazolopyrazines were confirmed by X-ray crystal structure analysis. The inhibitory activity of all compounds against the growth of the malaria parasite Plasmodium falciparum (3D7 and Dd2 strains) and the cytotoxicity against a human embryonic kidney (HEK293) cell line were tested. Some of the compounds demonstrated moderate antimalarial activity with IC50 values ranging from 0.2 to >80 µM; none of the compounds displayed any cytotoxicity against HEK293 cells at 80 µM. Antimalarial activity was significantly reduced when C-8 of the triazolopyrazine scaffold was substituted with CF3 and CF2H moieties, whereas incorporation of a CF2Me group at the same position completely abolished antiplasmodial effects.
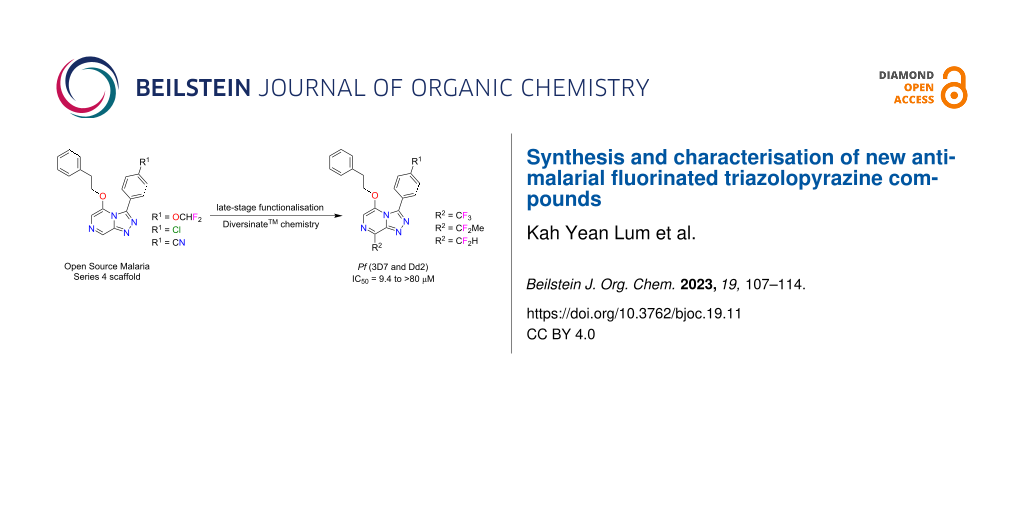
Graphical Abstract
Introduction
Malaria is an infectious disease caused by Plasmodium parasites and is a major global threat to human health. The WHO World Malaria Report 2021, estimates 241 million cases of malaria and 627,000 deaths globally in 2020, an increase of 12% from the previous year [1]. The increase was mainly from countries in the WHO African region, which accounted for about 95% of malaria cases and deaths, and was associated with service disruptions during the COVID-19 pandemic [1]. Infants and young children are at a disproportionately high risk of severe malaria and death, as 80% of deaths in this region were children under five [1]. Whilst there are drugs available for the treatment of malaria infections, most have now succumbed to parasite drug resistance and thus reduced clinical efficacy [2,3]. Consequently, new antiplasmodial drugs with novel malaria targets are urgently needed to combat the global problem of parasite drug resistance. For more than 10 years, the Open Source Malaria (OSM) consortium [4] has had an interest in identifying and developing novel antimalarial compounds that belong to a variety of chemotypes, one of which includes the 1,2,4-triazolo[4,3-a]pyrazine scaffold [5]. This particular series, known as OSM Series 4, has demonstrated significant potency against various strains of Plasmodium falciparum (Pf) with IC50 values as low as 16 nM. The series also showed decent in vitro human liver microsome and human hepatocyte stability, with hepatic intrinsic clearance of <8.1 µL/min/mg [5]. Furthermore, minimal poly-pharmacology and cytotoxicity have been identified for this series to date, giving confidence in its specificity and tolerability, and thus supporting on-going efforts towards the continued development of this unique antimalarial structure class [5]. Through investigations into the mechanism of action of OSM Series 4 compounds, it has been suggested that this nitrogen-rich chemotype inhibits the ATPase, PfATP4 [6]. PfATP4 functions as a Na+/H+-ATPase, which allows the malaria parasite to regulate Na+ to maintain cell homeostasis [7-9]. Interfering with this process means the parasite is unable to regulate Na+ [10], resulting in a significant increase in the acid load of the cell, which can lead to parasite growth inhibition and ultimately parasite death [8,9]. One of the current Series 4 aims includes lead optimisation to improve solubility and metabolic stability while retaining potency [5]. Late-stage functionalisation (LSF) is a strategy involving the use of C–H bonds as chemical handles for the introduction of various functional groups, which has been widely employed by medicinal chemists to generate new analogues of lead compounds without the need for de novo synthesis [5]. Baran et al. has developed an operationally simple, radical-based functionalisation strategy that allows direct transformation of C–H bonds to C–C bonds in a practical manner [11]. This strategy involves the utilisation of sodium and zinc sulfinate-based reagents (marketed by Merck as Diversinates™) to functionalise heteroaromatic C–H bonds of unprotected systems in a variety of solvents at room temperature and without the requirement of an inert atmosphere or solvent purification [11-13].
In our previous work on OSM Series 4 scaffolds [14], we had undertaken some preliminary investigations into the use of commercially available Diversinate™ reagents and showed the bicyclic nitrogen-rich core of Series 4 was amenable to this chemistry, with radical sulfinate substitution occurring with high-selectivity at C-8 and in respectable yields. This paper reports additional and more thorough Diversinate™ studies on three phenethyl ether substituted triazolopyrazine scaffolds, with a particular focus on incorporating the fluoro fragments -CF3, -CF2H and -CF2Me into the OSM Series 4 structures via LSF chemistry. The new library of triazolopyrazines were all evaluated in vitro for antimalarial activity and cytotoxicity.
Results and Discussion
Previous structure–activity relationship (SAR) studies reported that any substitution at the C8 position of Series 4 triazolopyrazines can lower the potency for P. falciparum [14-16]. However, a recent preliminary SAR study identified that substitution at the C8 position with trifluoromethane and difluoroethane moieties using Diversinate™ chemistry increased the potency of the parent scaffold (compound 2), suggesting the potential of these fluoroalkyl groups for improving the potency of other promising leads within the OSM project [14]. Fluorine-containing compounds have exhibited wide applications in pharmaceuticals and agrochemicals – approximately 20% of marketed drugs are fluoro-pharmaceuticals, while for agrochemicals, 53% are fluoro-compounds [17,18]. In recent decades, the introduction of fluorine or a fluorinated functional group into organic compounds has become increasingly prevalent in drug design and development, as fluorine substitution can greatly influence drug potency, pharmacokinetic and pharmacodynamic properties [19]. Therefore, in this study we undertook additional LSF investigations by introduction of fluoroalkyl groups to OSM leads with the aim to probe the SAR of 8-fluoroalkylated triazolopyrazine derivatives and further improve their potency.
Based on the existing SAR data for the C3 position of the triazolopyrazine core, substituents with a phenyl ring containing alkyl, cyano, nitro, or halogenated groups at the para-position were crucial for activity [5,16,20]. Thus, compounds 1–3 with a para-phenyl-OCHF2, -Cl or -CN substituent at the C3 position of the triazole were selected as scaffolds in this study. In addition, the reported SAR data also indicated that the use of an ether linker on the pyrazine ring, with a two methylene unit chain length between the heterocyclic core and benzylic substituent, improved the potency of these compounds [16]. Hence, scaffolds 1–3 were then converted into a series of ether-linked triazolopyrazines with phenethyl alcohol or ethanol using the standard nucleophilic displacement method as previously described (Scheme 1) [14,16]. Structures of synthesised compounds 4–9 were determined using 1D/2D NMR and HRMS (Supporting Information File 1, S6–S23). Crystals of compounds 5 and 6 were also analysed by X-ray crystallography studies (Supporting Information File 1, S51, S52, and S54), which confirmed the structure assignment. Compounds 4–6 were known OSM compounds that displayed good selective activity with IC50 values of <1 µM [16,21], whereas 7–9 are new ether derivatives without a phenyl ring that were synthesised for SAR evaluation.
Scheme 1: Synthesis of ether triazolopyrazine derivatives 4–9.
Scheme 1: Synthesis of ether triazolopyrazine derivatives 4–9.
The incorporation of fluoroalkyl groups at the C8 position of three OSM leads (4–6) was performed using Diversinate™ chemistry following the previously described method (Scheme 2) [14]. The Diversinate™ reagents used in this study were zinc trifluoromethanesulfinate (TFMS), sodium 1,1-difluoroethanesulfinate (DFES) and zinc difluoromethanesulfinate (DFMS). In brief, a mixture of the respective scaffold, Diversinate™ (2 equiv), and TFA (5 equiv) in DMSO/CH2Cl2/H2O (5:5:2) was stirred for 30 min at room temperature and cooled to 4 °C. Then, aqueous tert-butyl hydroperoxide (TBHP, 70%, 3 equiv) was slowly added over 5 min and stirring continued for 1 h. The mixture was slowly warmed to room temperature with stirring for another 24 h. The products were isolated and purified using C18 and phenyl HPLC (MeOH/H2O/0.1% TFA).
Scheme 2: Synthesis of fluorinated triazolopyrazine compounds (10–18) via Diversinate™ chemistry.
Scheme 2: Synthesis of fluorinated triazolopyrazine compounds (10–18) via Diversinate™ chemistry.
The structures of all fluorinated triazolopyrazine compounds (10–18) were fully characterised using 1D/2D NMR and HRMS (Supporting Information File 1, S24–S50). The structure elucidation studies on a fluorinated triazolopyrazine are detailed below. The 1H NMR spectrum of 18 in CDCl3 (Supporting Information File 1, S49) revealed signals corresponding to two methylenes [δH 2.99 (H-18) and 4.60 (H-17)] and 10 aromatic protons [δH 7.49 (H-6), 7.68 (H-11, H-15), 7.64 (H-12, H-14), 6.90 (H-20, H-24), 7.26 (H-21, H-22, H-23)]. A triplet resonating at δH 7.06 represents the proton located in the difluoromethyl group (H-25), with a 1JHF coupling constant of 53.4 Hz. The 13C NMR spectrum revealed a 2JCF triplet splitting (27.3 Hz) for δC 137.5, which allowed the assignment of C-8. The substitution of a difluoromethyl moiety at the C8 position of the triazolopyrazine ring was further supported by HMBC correlations of H-25 to δC 137.5 (C-8) and δC 144.6 (C-9). Detailed analysis of the HMBC spectrum also confirmed the presence of the phenyl ether sidechain at C-5 of the pyrazine ring, based on correlations from the aromatic protons δH 6.90 (H-20, H-24) to δC 34.3 (C-18), and δH 4.60 (H-17) to δC 135.5 (C-19) and 145.0 (C-5). This assignment was further confirmed by ROE correlations from H-17 to H-20, H-24 and H-6 (δH 7.49). The presence of a benzonitrile ring at the C-3 position was supported by the HMBC correlations of aromatic protons δH 7.68 (H-11, H-15) to C-3 (δC 146.2), and δH 7.64 (H-12, H-14) to C-16 (δC 118.0). Furthermore, crystals obtained for compound 18 were analysed by X-ray crystallographic studies and confirmed the NMR-based structure assignment. Key COSY, HMBC, and ROESY correlations, and ORTEP drawing of compound 18 are shown in Figure 1.
![[1860-5397-19-11-1]](/bjoc/content/figures/1860-5397-19-11-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Key HMBC, COSY and ROESY correlations, and ORTEP drawing of 18.
Figure 1: Key HMBC, COSY and ROESY correlations, and ORTEP drawing of 18.
All compounds were tested for their antimalarial activity against P. falciparum 3D7 (chloroquine-sensitive strain) and Dd2 (chloroquine, pyrimethamine and mefloquine drug-resistant strain) (Table 1). In terms of the cLogP values of these compounds, an increase in hydrophobicity did not improve the potency. A similar trend was also observed in a previous study [20], where an increase in the hydrophobicity of several triazolopyrazine derivatives resulted in significant drops in antimalarial activity. However, the same paper also commented that the cLogP values showed no significant correlation with experimental potency when compared to other Series 4 triazolopyrazines [20]. In addition, consistent with reported SAR data [14,16,21], the ether-linked compounds 4–6 exhibited strong activity with IC50 values of 0.2–1.2 µM, whereas compounds 7–9 with the removal of the phenyl ring from the ether methylene group resulted in a loss of potency at the tested concentrations. For fluorinated compounds, previous studies [14] reported that the introduction of CF3 and CF2Me groups at the C-8 position of scaffold 2 improved the antimalarial activity. In particular, compounds with a CF2Me moiety showed a 7.3-fold improvement in potency (IC50 = 1.7 µM) compared to the parent scaffold 2 (IC50 = 12.6 µM) [14]. Herein, ether-linked triazolopyrazine scaffolds with CF3 or CF2H moieties at the C-8 position displayed weak antimalarial activity, whereas incorporation of a CF2Me group completely abolished the effect at the tested concentrations, in comparison to the parent scaffolds 4–6. These data suggest that substituents at the C-5 position of the triazolopyrazine core appear to influence the antimalarial activity of C-8 fluoroalkyl-substituted compounds. Additional investigations on other OSM leads are warranted to further expand the SAR surrounding the 8-position of Series 4 triazolopyrazines with fluoroalkyl substituents or other functional groups. In addition, the replacement of H-8 by small electron-withdrawing groups appeared to be detrimental for activity in Series 4 compounds.
Table 1: Biological data for triazolopyrazine analogues 1–18.
| Compound | cLogP |
Pf 3D7a
IC50 ± SD µM |
Pf Dd2b
IC50 ± SD µM |
SI for 3D7c | SI for Dd2c |
| 1 | 1.7 | 45.20 ± 5.42d | 34.48 ± 4.46d | >2 | >2 |
| 2 | 2.2 | 18.91 ± 0.63d | 17.08 ± 0.43d | >4 | >4 |
| 3 | 1.4 | 35.46 ± 3.33d | 26.16 ± 1.01d | >2 | >3 |
| 4 | 3.2 | 0.24 ± 0.01 | 0.26 ± 0.01 | >329 | >312 |
| 5 | 3.6 | 0.20 ± 0.02 | 0.22 ± 0.01 | >396 | >364 |
| 6 | 2.8 | 1.15 ± 0.05 | 1.17 ± 0.09 | >70 | >68 |
| 7 | 1.7 | e | e | – | – |
| 8 | 2.1 | 2.72 ± 0.03 | 2.91 ± 0.18 | >29 | >27 |
| 9 | 1.4 | NA | NA | – | – |
| 10 | 4.1 | 11.35 ± 1.45d | 17.08 ± 0.71d | >7 | >4 |
| 11 | 4.1 | NA | NA | – | – |
| 12 | 3.9 | 16.38 ± 1.13d | 19.55 ± 0.42d | >5 | >4 |
| 13 | 4.5 | 9.43 ± 0.44 | 10.24 ± 0.75 | >9 | >8 |
| 14 | 4.6 | NA | NA | – | – |
| 15 | 4.3 | 18.77 ± 0.21d | 14.29 ± 2.26d | >4 | >6 |
| 16 | 3.7 | 22.53 ± 1.03d | 45.09 ± 6.58d | >4 | >2 |
| 17 | 3.8 | f | f | – | – |
| 18 | 3.6 | 31.04 ± 2.37d | 58.56 ± 1.68d | >3 | >1 |
| Control |
Pf 3D7a
IC50 ± SD nM |
Pf Dd2b
IC50 ± SD nM |
SI for 3D7c | SI for Dd2c | |
| Pyrimethamine | 15.20 ± 1.20 | f | >657 | – | |
| Artesunate | 2.90 ± 0.10 | 2.40 ± 0.10 | >857 | >1051 | |
| Puromycin | 79.00 ± 5.70 | 101.80 ± 9.50 | 127 | 98 | |
a3D7 = P. falciparum (chloroquine-sensitive strain); bDd2 = P. falciparum (chloroquine, pyrimethamine and mefloquine drug-resistant strain); cAll compounds 1–18 and controls tested for cytotoxicity against human embryonic kidney cells (HEK293) in order to determine selectivity index (SI) using the formula: SI = HEK293 IC50/parasite IC50, all compounds were inactive towards HEK293 at the top dose of 80 µM; dEstimated IC50 as a plateau of inhibition was not reached; SD = standard deviation. All cLogP values were calculated using the open-source program DataWarrior [22]. e<81% inhibition observed at the top dose of 80 µM; f<33% inhibition observed at the top dose of 80 µM; NA = not active at the top dose of 80 µM.
Conclusion
Three selected Series 4 triazolopyrazine scaffolds (1–3) were converted to three known (4–6) and three new ether-linked derivatives (7–9). H-8 of the known OSM leads 4–6 was subsequently substituted with three different fluoroalkyl moieties using Diversinate™ chemistry that resulted in the synthesis of nine new fluorinated triazolopyrazines (10–19). The antimalarial data indicated that substitution of H-8 of ether-linked triazolopyrazines with fluoroalkyl moieties led to a reduction or loss of activity at the tested concentrations. These data indicate that additional medicinal chemistry efforts involving H-8 replacement with fluoroalkyl groups for this OSM series is not warranted.
Experimental
General procedure for Diversinate™ derivatisations on triazolopyrazine scaffolds. In a manner analogous to [14], the scaffold (0.1 mmol) was dissolved in DMSO/CH2Cl2 (1:1, 250 µL:250 µL) before the addition of Diversinate™ (0.2 mmol, 2 equiv), TFA (40 μL, 0.5 mmol, 5 equiv) and filtered H2O (100 μL). The reaction mixture was stirred for 30 min at room temperature, cooled to 4 °C then 70% TBHP solution (41 μL, 0.3 mmol, 3 equiv) was slowly added over 5 min and left to stir for 1 h at 4 °C. The mixture was then slowly warmed to room temperature with stirring for 24 h. The crude reaction mixture was dried down initially under nitrogen then reduced under vacuum before being re-dissolved in MeOH/CH2Cl2 (1:1, 500 µL:500 µL) then preadsorbed to C18-bonded silica (≈1 g). The resulting material was packed into a guard cartridge that was subsequently attached to a semipreparative C18-bonded silica HPLC column. Isocratic conditions of 30% MeOH/70% H2O (0.1% TFA) were held for the first 1 min, followed by a linear gradient to 100% MeOH (0.1% TFA) over 59 min, all at a flow rate of 9 mL/min. Sixty fractions (60 × 1 min) were collected from the start of the HPLC run. Fractions containing UV-active material were analysed by 1H NMR spectroscopy and LCMS, and relevant fractions with desired products were combined. In order to afford the desired products in sufficient purity (>95%) for biological testing, the combined fractions were further purified using semipreparative phenyl HPLC with isocratic conditions of 50% MeOH/50% H2O (0.1% TFA) held for the first 1 min, followed by a linear gradient to 80% MeOH/20% H2O (0.1% TFA) over 59 min, all at a flow rate of 9 mL/min.
3-(4-(Difluoromethoxy)phenyl)-5-phenethoxy-8-(trifluoromethyl)-[1,2,4]triazolo[4,3-a]pyrazine (10). White amorphous solid (6 mg, 12%); UV (MeOH) λmax (log ε): 243 (3.94), 324 (3.50) nm; 1H NMR (800 MHz, CDCl3) δH 2.96 (t, J = 6.6 Hz, 2H, H-18), 4.54 (t, J = 6.6 Hz, 2H, H-17), 6.58 (t, J = 73.5 Hz, 1H, H-16), 6.86 (m, 2H, H-20, H-24), 7.19 (m, 2H, H-12, H-14), 7.22 (m, 1H, H-22), 7.23 (m, 2H, H-21, H-23), 7.37 (s, 1H, H-6), 7.62 (m, 2H, H-11, H-15); 13C NMR (200 MHz, CDCl3) δC 34.5 (C-18), 72.1 (C-17), 106.9 (C-6), 115.6 (t, J = 262.1 Hz, C-16), 118.9 (C-12, C-14), 120.2 (q, J = 273.3 Hz, C-25), 124.2 (C-10), 127.4 (C-22), 128.5 (C-20, C-24), 128.9 (C-21, C-23), 132.6 (C-11, C-15), 133.1 (q, J = 38.8 Hz, C-8), 135.8 (C-19), 144.2 (C-9), 145.8 (C-5), 147.2 (C-3), 152.7 (t, J = 2.7 Hz, C-13); LRESIMS m/z: 451 [M + H]+, 473 [M + Na]+, 923 [2M + Na]+; HRESIMS (m/z): [M + H]+ calcd for C21H16F5N4O2, 451.1188; found, 451.1195.
8-(1,1-Difluoroethyl)-3-(4-(difluoromethoxy)phenyl)-5-phenethoxy-[1,2,4]triazolo[4,3-a]pyrazine (11). Yellow amorphous solid (23 mg, 51%); UV (MeOH) λmax (log ε): 240 (4.30), 317 (3.88) nm; 1H NMR (800 MHz, CDCl3) δH 2.27 (t, J = 18.7 Hz, 3H, H-26), 2.94 (t, J = 6.5 Hz, 2H, H-18), 4.48 (t, J = 6.5 Hz, 2H, H-17), 6.56 (t, J = 72.1 Hz, 1H, H-16), 6.86 (m, 2H, H-20, H-24), 7.18 (m, 2H, H-12, H-14), 7.22 (m, 1H, H-22), 7.23 (m, 2H, H-21, H-23), 7.30 (s, 1H, H-6), 7.62 (m, 2H, H-11, H-15); 13C NMR (200 MHz, CDCl3) δC 23.2 (t, J = 26.3 Hz, C-26), 34.5 (C-18), 71.7 (C-17), 106.6 (C-6), 115.6 (t, J = 260.3 Hz, C-16), 118.8 (C-12, C-14), 119.7 (t, J = 241.1 Hz, C-25), 124.7 (C-10), 127.3 (C-22), 128.6 (C-20, C-24), 128.9 (C-21, C-23), 132.6 (C-11, C-15), 140.2 (t, J = 31.3 Hz, C-8), 136.1 (C-19), 144.8 (C-9), 144.9 (C-5), 146.8 (C-3), 152.6 (t, J = 2.8 Hz, C-13); LRESIMS m/z: 447 [M + H]+, 915 [2M + Na]+; HRESIMS (m/z): [M + H]+ calcd for C22H19F4N4O2, 447.1439; found, 447.1457.
3-(4-(Difluoromethoxy)phenyl)-8-(difluoromethyl)-5-phenethoxy-[1,2,4]triazolo[4,3-a]pyrazine (12). White amorphous solid (14 mg, 31%); UV (MeOH) λmax (log ε): 242 (4.16), 321 (3.73) nm; 1H NMR (800 MHz, CDCl3) δH 2.96 (t, J = 6.5 Hz, 2H, H-18), 4.52 (t, J = 6.5 Hz, 2H, H-17), 6.57 (t, J = 73.0 Hz, 1H, H-16), 6.86 (m, 2H, H-20, H-24), 7.09, (t, J = 54.0 Hz, 1H, H-25), 7.18 (m, 2H, H-12, H-14), 7.21 (m, 1H, H-22), 7.22 (m, 2H, H-21, H-23), 7.34 (s, 1H, H-6), 7.61 (m, 2H, H-11, H-15); 13C NMR (200 MHz, CDCl3) δC 34.4 (C-18), 71.9 (C-17), 107.5 (C-6), 115.6 (t, J = 262.2 Hz, C-16), 118.8 (C-12, C-14), 111.5 (t, J = 241.3 Hz, C-25), 124.4 (C-10), 127.2 (C-22), 128.5 (C-20, C-24), 128.8 (C-21, C-23), 132.6 (C-11, C-15), 137.5 (t, J = 26.4 Hz, C-8), 136.0 (C-19), 145.0 (C-9), 145.2 (C-5), 147.0 (C-3), 152.7 (t, J = 2.7 Hz, C-13); LRESIMS m/z: 433 [M + H]+, 887 [2M + Na]+; HRESIMS (m/z) [M + H]+ calcd for C21H17F4N4O2, 433.1282; found, 433.1282.
3-(4-Chlorophenyl)-5-phenethoxy-8-(trifluoromethyl)-[1,2,4]triazolo[4,3-a]pyrazine (13). White amorphous solid (3 mg, 6%); UV (MeOH) λmax (log ε): 244 (3.69), 286 (3.19), 322 (3.22) nm; 1H NMR (800 MHz, CDCl3) δH 2.98 (t, J = 6.6 Hz, 2H, H-17), 4.55 (t, J = 6.6 Hz, 2H, H-16), 6.88 (m, 2H, H-19, H-23), 7.24 (m, 1H, H-21), 7.25 (m, 2H, H-20, H-22), 7.39 (s, 1H, H-6), 7.42 (m, 2H, H-12, H-14), 7.54 (m, 2H, H-11, H-15); 13C NMR (200 MHz, CDCl3) δC 34.5 (C-17), 72.2 (C-16), 107.0 (C-6), 120.0 (q, J = 274.4 Hz, C-24), 125.4 (C-10), 127.4 (C-21), 128.3 (C-12, C-14), 128.5 (C-19, C-23), 129.0 (C-20, C-22), 132.2 (C-11, C-15), 133.2 (q, J = 38.9 Hz, C-8), 135.7 (C-18), 137.1 (C-13), 144.1 (C-9), 145.8 (C-5), 147.2 (C-3); LRESIMS m/z 419 [M + H]+, 441 [M + Na]+, 859 [2M + Na]+; HRESIMS (m/z) [M + H]+ calcd for C20H15ClF3N4O, 419.0881; found, 419.0887.
3-(4-Chlorophenyl)-8-(1,1-difluoroethyl)-5-phenethoxy-[1,2,4]triazolo[4,3-a]pyrazine (14). Yellow amorphous solid (18 mg, 44%); UV (MeOH) λmax (log ε): 243 (4.23), 286 (3.81), 317 (3.80) nm; 1H NMR (800 MHz, CDCl3) δH 2.26 (t, J = 18.9 Hz, 3H, H-25), 2.95 (t, J = 6.5 Hz, 2H, H-17), 4.49 (t, J = 6.5 Hz, 2H, H-16), 6.88 (m, 2H, H-19, H-23), 7.23 (m, 1H, H-21), 7.24 (m, 2H, H-20, H-22), 7.30 (s, 1H, H-6), 7.40 (m, 2H, H-12, H-14), 7.53 (m, 2H, H-11, H-15); 13C NMR (200 MHz, CDCl3) δC 23.1 (t, J = 26.2 Hz, C-25), 34.5 (C-17), 71.7 (C-16), 106.7 (C-6), 119.7 (t, J = 240.3 Hz, C-24), 126.0 (C-10), 127.3 (C-21), 128.2 (C-12, C-14), 128.6 (C-19, C-23), 128.9 (C-20, C-22), 132.2 (C-11, C-15), 136.0 (C-18), 136.7 (C-13), 140.2 (t, J = 31.7 Hz, C-8), 144.8 (C-5), 144.9 (C-9), 146.8 (C-3); LRESIMS m/z: 415 [M + H]+, 851 [2M + Na]+; HRESIMS (m/z): [M + H]+ calcd for C21H18ClF2N4O, 415.1132, found, 415.1141.
3-(4-Chlorophenyl)-8-(difluoromethyl)-5-phenethoxy-[1,2,4]triazolo[4,3-a]pyrazine (15). White amorphous solid (11 mg, 28%); UV (MeOH) λmax (log ε): 244 (4.26), 286 (3.82), 319 (3.83) nm; 1H NMR (800 MHz, CDCl3) δH 2.97 (t, J = 6.5 Hz, 2H, H-17), 4.54 (t, J = 6.5 Hz, 2H, H-16), 6.87 (m, 2H, H-19, H-23), 7.05 (t, J = 53.5 Hz, 1H, H-24), 7.22 (m, 1H, H-21), 7.23 (m, 2H, H-20, H-22), 7.40 (s, 1H, H-6), 7.40 (m, 2H, H-12, H-14), 7.52 (m, 2H, H-11, H-15); 13C NMR (200 MHz, CDCl3) δC 34.4 (C-17), 72.1 (C-16), 107.8 (C-6), 111.6 (t, J = 241.8 Hz, C-24), 125.1 (C-10), 127.3 (C-21), 128.3 (C-12, C-14), 128.5 (C-19, C-23), 128.9 (C-20, C-22), 132.1 (C-11, C-15), 135.8 (C-18), 137.1 (C-13), 137.3 (t, J = 26.6 Hz, C-8), 144.5 (C-9), 145.2 (C-5), 147.0 (C-3); LRESIMS m/z: 401 [M + H]+, 823 [2M + Na]+; HRESIMS (m/z): [M + H]+ calcd for C20H16ClF2N4O, 401.0975; found, 401.0978.
4-(5-Phenethoxy-8-(trifluoromethyl)-[1,2,4]triazolo[4,3-a]pyrazin-3-yl)benzonitrile (16). White amorphous solid (5 mg, 11%); UV (MeOH) λmax (log ε): 226 (4.26), 249 (4.20), 297 (3.95) nm; 1H NMR (800 MHz, CDCl3) δH 2.99 (t, J = 6.5 Hz, 2H, H-18), 4.62 (t, J = 6.5 Hz, 2H, H-17), 6.90 (m, 2H, H-20, H-24), 7.26 (m, 1H, H-22), 7.27 (m, 2H, H-21, H-23), 7.47 (s, 1H, H-6), 7.64 (m, 2H, H-12, H-14), 7.68 (m, 2H, H-11, H-15); 13C NMR (200 MHz, CDCl3) δC 34.2 (C-18), 72.1 (C-17), 107.6 (C-6), 114.3 (C-13), 118.1 (C-16), 120.0 (q, J = 274.6 Hz, C-25), 127.6 (C-22), 128.3 (C-20, C-24), 129.1 (C-21, C-23), 131.1 (C-10), 131.5 (C-11, C-15), 131.6 (C-12, C-14), 133.1 (q, J = 39.1 Hz, C-8), 135.4 (C-19), 144.1 (C-9), 145.6 (C-5), 146.4 (C-3); LRESIMS m/z: 410 [M + H]+, 432 [M + Na]+; HRESIMS (m/z): [M + H]+ calcd for C21H15F3N5O, 410.1223; found, 410.1221.
4-(8-(1,1-Difluoroethyl)-5-phenethoxy-[1,2,4]triazolo[4,3-a]pyrazin-3-yl)benzonitrile (17). Yellow amorphous solid (23 mg, 56%); UV (MeOH) λmax (log ε): 226 (4.41), 250 (4.33), 294 (4.15) nm; 1H NMR (800 MHz, CDCl3) δH 2.27 (t, J = 18.7 Hz, 3H, H-26), 2.97 (t, J = 6.5 Hz, 2H, H-18), 4.55 (t, J = 6.5 Hz, 2H, H-17), 6.90 (m, 2H, H-20, H-24), 7.27 (m, 1H, H-22), 7.27 (m, 2H, H-21, H-23), 7.38 (s, 1H, H-6), 7.64 (m, 2H, H-12, H-14), 7.69 (m, 2H, H-11, H-15); 13C NMR (200 MHz, CDCl3) δC 23.1 (t, J = 26.4 Hz, C-26), 34.3 (C-18), 71.5 (C-17), 107.0 (C-6), 114.0 (C-13), 118.3 (C-16), 119.7 (t, J = 240.3 Hz, C-25), 127.5 (C-22), 128.4 (C-20, C-24), 129.0 (C-21, C-23), 131.46 (C-12, C-14), 131.51 (C-11, C-15), 131.9 (C-10), 135.7 (C-19), 140.4 (t, J = 31.7 Hz, C-8), 144.7 (C-5), 145.0 (C-9), 146.1 (C-3); LRESIMS m/z: 406 [M + H]+, 428 [M + Na]+; HRESIMS (m/z): [M + H]+ calcd for C22H18F2N5O, 406.1474; found, 406.1473.
4-(8-(Difluoromethyl)-5-phenethoxy-[1,2,4]triazolo[4,3-a]pyrazin-3-yl)benzonitrile (18). White crystalline solid (11 mg, 27%); mp 109–111 °C; UV (MeOH) λmax (log ε): 227 (4.56), 248 (4.48), 299 (4.28) nm; 1H NMR (800 MHz, CDCl3) δH 2.99 (t, J = 6.5 Hz, 2H, H-18), 4.60 (t, J = 6.5 Hz, 2H, H-17), 6.90 (m, 2H, H-20, H-24), 7.06, (t, J = 53.4 Hz, 1H, H-25), 7.26 (m, 1H, H-22), 7.26 (m, 2H, H-21, H-23), 7.49 (s, 1H, H-6), 7.64 (m, 2H, H-12, H-14), 7.68 (m, 2H, H-11, H-15); 13C NMR (200 MHz, CDCl3) δC 34.3 (C-18), 71.9 (C-17), 108.2 (C-6), 111.6 (t, J = 241.7 Hz, C-25), 114.3 (C-13), 118.0 (C-16), 127.5 (C-22), 128.3 (C-20, C-24), 129.0 (C-21, C-23), 131.0 (C-10), 131.5 (C-11, C-15), 131.6 (C-12, C-14), 135.5 (C-19), 137.5 (t, J = 27.3 Hz, C-8), 144.6 (C-9), 145.0 (C-5), 146.2 (C-3); LRESIMS m/z: 392 [M + H]+, 414 [M + Na]+, 805 [2M + Na]+; HRESIMS (m/z): [M + H]+ calcd for C21H16F2N5O, 392.1317; found, 392.1317.
Supporting Information
| Supporting Information File 1: General experimental procedures, NMR spectra and characterisation data for all new triazolopyrazine compounds and X-ray crystallography data for compounds 5, 6 and 18. | ||
| Format: PDF | Size: 2.5 MB | Download |
Acknowledgements
The authors would like to thank Dr. Sandra Duffy from the Discovery Biology team for technical assistance with the assays. The authors wish to thank and acknowledge the Australian Red Cross Blood Bank for the provision of fresh red blood cells, without which antiplasmodial testing could not have been performed.
References
-
World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021.
Return to citation in text: [1] [2] [3] -
Dondorp, A. M.; Nosten, F.; Yi, P.; Das, D.; Phyo, A. P.; Tarning, J.; Lwin, K. M.; Ariey, F.; Hanpithakpong, W.; Lee, S. J.; Ringwald, P.; Silamut, K.; Imwong, M.; Chotivanich, K.; Lim, P.; Herdman, T.; An, S. S.; Yeung, S.; Singhasivanon, P.; Day, N. P. J.; Lindegardh, N.; Socheat, D.; White, N. J. N. Engl. J. Med. 2009, 361, 455–467. doi:10.1056/nejmoa0808859
Return to citation in text: [1] -
Balikagala, B.; Fukuda, N.; Ikeda, M.; Katuro, O. T.; Tachibana, S.-I.; Yamauchi, M.; Opio, W.; Emoto, S.; Anywar, D. A.; Kimura, E.; Palacpac, N. M. Q.; Odongo-Aginya, E. I.; Ogwang, M.; Horii, T.; Mita, T. N. Engl. J. Med. 2021, 385, 1163–1171. doi:10.1056/nejmoa2101746
Return to citation in text: [1] -
Open Source Malaria Project wiki. Open Source Malaria Project/ Series 4. https://github.com/OpenSourceMalaria/Series4/wiki (accessed Aug 20, 2022).
Return to citation in text: [1] -
Open Source Malaria Series 4: The Triazolopyrazine (TP) Series. https://openwetware.org/wiki/OpenSourceMalaria:Triazolopyrazine_(TP%29_Series (accessed Aug 20, 2022).
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Gilson, P. R.; Kumarasingha, R.; Thompson, J.; Zhang, X.; Penington, J. S.; Kalhor, R.; Bullen, H. E.; Lehane, A. M.; Dans, M. G.; de Koning-Ward, T. F.; Holien, J. K.; Soares da Costa, T. P.; Hulett, M. D.; Buskes, M. J.; Crabb, B. S.; Kirk, K.; Papenfuss, A. T.; Cowman, A. F.; Abbott, B. M. Sci. Rep. 2019, 9, 10292. doi:10.1038/s41598-019-46500-5
Return to citation in text: [1] -
Vaidya, A. B.; Morrisey, J. M.; Zhang, Z.; Das, S.; Daly, T. M.; Otto, T. D.; Spillman, N. J.; Wyvratt, M.; Siegl, P.; Marfurt, J.; Wirjanata, G.; Sebayang, B. F.; Price, R. N.; Chatterjee, A.; Nagle, A.; Stasiak, M.; Charman, S. A.; Angulo-Barturen, I.; Ferrer, S.; Belén Jiménez-Díaz, M.; Martínez, M. S.; Gamo, F. J.; Avery, V. M.; Ruecker, A.; Delves, M.; Kirk, K.; Berriman, M.; Kortagere, S.; Burrows, J.; Fan, E.; Bergman, L. W. Nat. Commun. 2014, 5, 5521. doi:10.1038/ncomms6521
Return to citation in text: [1] -
Spillman, N. J.; Kirk, K. Int. J. Parasitol.: Drugs Drug Resist. 2015, 5, 149–162. doi:10.1016/j.ijpddr.2015.07.001
Return to citation in text: [1] [2] -
Rosling, J. E. O.; Ridgway, M. C.; Summers, R. L.; Kirk, K.; Lehane, A. M. J. Biol. Chem. 2018, 293, 13327–13337. doi:10.1074/jbc.ra118.003640
Return to citation in text: [1] [2] -
Open Source Malaria Project wiki. Mechanism of Action: Possible PfATP4 Activity Deduced from Parasite Ion Regulation Assays. https://github.com/OpenSourceMalaria/Series4/wiki/Mechanism-of-Action%3A-Possible-PfATP4-Activity-Deduced-from-Parasite-Ion-Regulation-Assays (accessed Aug 20, 2022).
Return to citation in text: [1] -
Ji, Y.; Brueckl, T.; Baxter, R. D.; Fujiwara, Y.; Seiple, I. B.; Su, S.; Blackmond, D. G.; Baran, P. S. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 14411–14415. doi:10.1073/pnas.1109059108
Return to citation in text: [1] [2] -
Fujiwara, Y.; Dixon, J. A.; O’Hara, F.; Funder, E. D.; Dixon, D. D.; Rodriguez, R. A.; Baxter, R. D.; Herlé, B.; Sach, N.; Collins, M. R.; Ishihara, Y.; Baran, P. S. Nature 2012, 492, 95–99. doi:10.1038/nature11680
Return to citation in text: [1] -
Fujiwara, Y.; Dixon, J. A.; Rodriguez, R. A.; Baxter, R. D.; Dixon, D. D.; Collins, M. R.; Blackmond, D. G.; Baran, P. S. J. Am. Chem. Soc. 2012, 134, 1494–1497. doi:10.1021/ja211422g
Return to citation in text: [1] -
Johnson, D. J. G.; Jenkins, I. D.; Huxley, C.; Coster, M. J.; Lum, K. Y.; White, J. M.; Avery, V. M.; Davis, R. A. Molecules 2021, 26, 2421. doi:10.3390/molecules26092421
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] [9] -
Korsik, M.; Tse, E. G.; Smith, D. G.; Lewis, W.; Rutledge, P. J.; Todd, M. H. J. Org. Chem. 2020, 85, 13438–13452. doi:10.1021/acs.joc.0c01045
Return to citation in text: [1] -
Tse, E. G.-O. Open Source Malaria: Potent Triazolopyrazine-Based Antiplasmodium Agents that Probe an Important Mechanism of Action; The University of Sydney: Sydney, Australia, 2019. doi:10.25910/5d7af492b2c63
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. iScience 2020, 23. doi:10.1016/j.isci.2020.101467
Return to citation in text: [1] -
Inoue, M.; Sumii, Y.; Shibata, N. ACS Omega 2020, 5, 10633–10640. doi:10.1021/acsomega.0c00830
Return to citation in text: [1] -
Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258
Return to citation in text: [1] -
Tse, E. G.; Houston, S. D.; Williams, C. M.; Savage, G. P.; Rendina, L. M.; Hallyburton, I.; Anderson, M.; Sharma, R.; Walker, G. S.; Obach, R. S.; Todd, M. H. J. Med. Chem. 2020, 63, 11585–11601. doi:10.1021/acs.jmedchem.0c00746
Return to citation in text: [1] [2] [3] -
Ubels, J. M. Synthesis of Novel Triazolopyrazines as Candidate Antimalarials; The University of Sydney: Sydney, Australia, 2014.
Return to citation in text: [1] [2] -
Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. J. Chem. Inf. Model. 2015, 55, 460–473. doi:10.1021/ci500588j
Return to citation in text: [1]
| 20. | Tse, E. G.; Houston, S. D.; Williams, C. M.; Savage, G. P.; Rendina, L. M.; Hallyburton, I.; Anderson, M.; Sharma, R.; Walker, G. S.; Obach, R. S.; Todd, M. H. J. Med. Chem. 2020, 63, 11585–11601. doi:10.1021/acs.jmedchem.0c00746 |
| 14. | Johnson, D. J. G.; Jenkins, I. D.; Huxley, C.; Coster, M. J.; Lum, K. Y.; White, J. M.; Avery, V. M.; Davis, R. A. Molecules 2021, 26, 2421. doi:10.3390/molecules26092421 |
| 16. | Tse, E. G.-O. Open Source Malaria: Potent Triazolopyrazine-Based Antiplasmodium Agents that Probe an Important Mechanism of Action; The University of Sydney: Sydney, Australia, 2019. doi:10.25910/5d7af492b2c63 |
| 21. | Ubels, J. M. Synthesis of Novel Triazolopyrazines as Candidate Antimalarials; The University of Sydney: Sydney, Australia, 2014. |
| 14. | Johnson, D. J. G.; Jenkins, I. D.; Huxley, C.; Coster, M. J.; Lum, K. Y.; White, J. M.; Avery, V. M.; Davis, R. A. Molecules 2021, 26, 2421. doi:10.3390/molecules26092421 |
| 1. | World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. |
| 4. | Open Source Malaria Project wiki. Open Source Malaria Project/ Series 4. https://github.com/OpenSourceMalaria/Series4/wiki (accessed Aug 20, 2022). |
| 11. | Ji, Y.; Brueckl, T.; Baxter, R. D.; Fujiwara, Y.; Seiple, I. B.; Su, S.; Blackmond, D. G.; Baran, P. S. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 14411–14415. doi:10.1073/pnas.1109059108 |
| 2. | Dondorp, A. M.; Nosten, F.; Yi, P.; Das, D.; Phyo, A. P.; Tarning, J.; Lwin, K. M.; Ariey, F.; Hanpithakpong, W.; Lee, S. J.; Ringwald, P.; Silamut, K.; Imwong, M.; Chotivanich, K.; Lim, P.; Herdman, T.; An, S. S.; Yeung, S.; Singhasivanon, P.; Day, N. P. J.; Lindegardh, N.; Socheat, D.; White, N. J. N. Engl. J. Med. 2009, 361, 455–467. doi:10.1056/nejmoa0808859 |
| 3. | Balikagala, B.; Fukuda, N.; Ikeda, M.; Katuro, O. T.; Tachibana, S.-I.; Yamauchi, M.; Opio, W.; Emoto, S.; Anywar, D. A.; Kimura, E.; Palacpac, N. M. Q.; Odongo-Aginya, E. I.; Ogwang, M.; Horii, T.; Mita, T. N. Engl. J. Med. 2021, 385, 1163–1171. doi:10.1056/nejmoa2101746 |
| 11. | Ji, Y.; Brueckl, T.; Baxter, R. D.; Fujiwara, Y.; Seiple, I. B.; Su, S.; Blackmond, D. G.; Baran, P. S. Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 14411–14415. doi:10.1073/pnas.1109059108 |
| 12. | Fujiwara, Y.; Dixon, J. A.; O’Hara, F.; Funder, E. D.; Dixon, D. D.; Rodriguez, R. A.; Baxter, R. D.; Herlé, B.; Sach, N.; Collins, M. R.; Ishihara, Y.; Baran, P. S. Nature 2012, 492, 95–99. doi:10.1038/nature11680 |
| 13. | Fujiwara, Y.; Dixon, J. A.; Rodriguez, R. A.; Baxter, R. D.; Dixon, D. D.; Collins, M. R.; Blackmond, D. G.; Baran, P. S. J. Am. Chem. Soc. 2012, 134, 1494–1497. doi:10.1021/ja211422g |
| 1. | World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. |
| 5. | Open Source Malaria Series 4: The Triazolopyrazine (TP) Series. https://openwetware.org/wiki/OpenSourceMalaria:Triazolopyrazine_(TP%29_Series (accessed Aug 20, 2022). |
| 1. | World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021. |
| 5. | Open Source Malaria Series 4: The Triazolopyrazine (TP) Series. https://openwetware.org/wiki/OpenSourceMalaria:Triazolopyrazine_(TP%29_Series (accessed Aug 20, 2022). |
| 6. | Gilson, P. R.; Kumarasingha, R.; Thompson, J.; Zhang, X.; Penington, J. S.; Kalhor, R.; Bullen, H. E.; Lehane, A. M.; Dans, M. G.; de Koning-Ward, T. F.; Holien, J. K.; Soares da Costa, T. P.; Hulett, M. D.; Buskes, M. J.; Crabb, B. S.; Kirk, K.; Papenfuss, A. T.; Cowman, A. F.; Abbott, B. M. Sci. Rep. 2019, 9, 10292. doi:10.1038/s41598-019-46500-5 |
| 10. | Open Source Malaria Project wiki. Mechanism of Action: Possible PfATP4 Activity Deduced from Parasite Ion Regulation Assays. https://github.com/OpenSourceMalaria/Series4/wiki/Mechanism-of-Action%3A-Possible-PfATP4-Activity-Deduced-from-Parasite-Ion-Regulation-Assays (accessed Aug 20, 2022). |
| 14. | Johnson, D. J. G.; Jenkins, I. D.; Huxley, C.; Coster, M. J.; Lum, K. Y.; White, J. M.; Avery, V. M.; Davis, R. A. Molecules 2021, 26, 2421. doi:10.3390/molecules26092421 |
| 5. | Open Source Malaria Series 4: The Triazolopyrazine (TP) Series. https://openwetware.org/wiki/OpenSourceMalaria:Triazolopyrazine_(TP%29_Series (accessed Aug 20, 2022). |
| 8. | Spillman, N. J.; Kirk, K. Int. J. Parasitol.: Drugs Drug Resist. 2015, 5, 149–162. doi:10.1016/j.ijpddr.2015.07.001 |
| 9. | Rosling, J. E. O.; Ridgway, M. C.; Summers, R. L.; Kirk, K.; Lehane, A. M. J. Biol. Chem. 2018, 293, 13327–13337. doi:10.1074/jbc.ra118.003640 |
| 5. | Open Source Malaria Series 4: The Triazolopyrazine (TP) Series. https://openwetware.org/wiki/OpenSourceMalaria:Triazolopyrazine_(TP%29_Series (accessed Aug 20, 2022). |
| 14. | Johnson, D. J. G.; Jenkins, I. D.; Huxley, C.; Coster, M. J.; Lum, K. Y.; White, J. M.; Avery, V. M.; Davis, R. A. Molecules 2021, 26, 2421. doi:10.3390/molecules26092421 |
| 5. | Open Source Malaria Series 4: The Triazolopyrazine (TP) Series. https://openwetware.org/wiki/OpenSourceMalaria:Triazolopyrazine_(TP%29_Series (accessed Aug 20, 2022). |
| 7. | Vaidya, A. B.; Morrisey, J. M.; Zhang, Z.; Das, S.; Daly, T. M.; Otto, T. D.; Spillman, N. J.; Wyvratt, M.; Siegl, P.; Marfurt, J.; Wirjanata, G.; Sebayang, B. F.; Price, R. N.; Chatterjee, A.; Nagle, A.; Stasiak, M.; Charman, S. A.; Angulo-Barturen, I.; Ferrer, S.; Belén Jiménez-Díaz, M.; Martínez, M. S.; Gamo, F. J.; Avery, V. M.; Ruecker, A.; Delves, M.; Kirk, K.; Berriman, M.; Kortagere, S.; Burrows, J.; Fan, E.; Bergman, L. W. Nat. Commun. 2014, 5, 5521. doi:10.1038/ncomms6521 |
| 8. | Spillman, N. J.; Kirk, K. Int. J. Parasitol.: Drugs Drug Resist. 2015, 5, 149–162. doi:10.1016/j.ijpddr.2015.07.001 |
| 9. | Rosling, J. E. O.; Ridgway, M. C.; Summers, R. L.; Kirk, K.; Lehane, A. M. J. Biol. Chem. 2018, 293, 13327–13337. doi:10.1074/jbc.ra118.003640 |
| 22. | Sander, T.; Freyss, J.; von Korff, M.; Rufener, C. J. Chem. Inf. Model. 2015, 55, 460–473. doi:10.1021/ci500588j |
| 14. | Johnson, D. J. G.; Jenkins, I. D.; Huxley, C.; Coster, M. J.; Lum, K. Y.; White, J. M.; Avery, V. M.; Davis, R. A. Molecules 2021, 26, 2421. doi:10.3390/molecules26092421 |
| 14. | Johnson, D. J. G.; Jenkins, I. D.; Huxley, C.; Coster, M. J.; Lum, K. Y.; White, J. M.; Avery, V. M.; Davis, R. A. Molecules 2021, 26, 2421. doi:10.3390/molecules26092421 |
| 14. | Johnson, D. J. G.; Jenkins, I. D.; Huxley, C.; Coster, M. J.; Lum, K. Y.; White, J. M.; Avery, V. M.; Davis, R. A. Molecules 2021, 26, 2421. doi:10.3390/molecules26092421 |
| 15. | Korsik, M.; Tse, E. G.; Smith, D. G.; Lewis, W.; Rutledge, P. J.; Todd, M. H. J. Org. Chem. 2020, 85, 13438–13452. doi:10.1021/acs.joc.0c01045 |
| 16. | Tse, E. G.-O. Open Source Malaria: Potent Triazolopyrazine-Based Antiplasmodium Agents that Probe an Important Mechanism of Action; The University of Sydney: Sydney, Australia, 2019. doi:10.25910/5d7af492b2c63 |
| 14. | Johnson, D. J. G.; Jenkins, I. D.; Huxley, C.; Coster, M. J.; Lum, K. Y.; White, J. M.; Avery, V. M.; Davis, R. A. Molecules 2021, 26, 2421. doi:10.3390/molecules26092421 |
| 20. | Tse, E. G.; Houston, S. D.; Williams, C. M.; Savage, G. P.; Rendina, L. M.; Hallyburton, I.; Anderson, M.; Sharma, R.; Walker, G. S.; Obach, R. S.; Todd, M. H. J. Med. Chem. 2020, 63, 11585–11601. doi:10.1021/acs.jmedchem.0c00746 |
| 14. | Johnson, D. J. G.; Jenkins, I. D.; Huxley, C.; Coster, M. J.; Lum, K. Y.; White, J. M.; Avery, V. M.; Davis, R. A. Molecules 2021, 26, 2421. doi:10.3390/molecules26092421 |
| 16. | Tse, E. G.-O. Open Source Malaria: Potent Triazolopyrazine-Based Antiplasmodium Agents that Probe an Important Mechanism of Action; The University of Sydney: Sydney, Australia, 2019. doi:10.25910/5d7af492b2c63 |
| 16. | Tse, E. G.-O. Open Source Malaria: Potent Triazolopyrazine-Based Antiplasmodium Agents that Probe an Important Mechanism of Action; The University of Sydney: Sydney, Australia, 2019. doi:10.25910/5d7af492b2c63 |
| 21. | Ubels, J. M. Synthesis of Novel Triazolopyrazines as Candidate Antimalarials; The University of Sydney: Sydney, Australia, 2014. |
| 5. | Open Source Malaria Series 4: The Triazolopyrazine (TP) Series. https://openwetware.org/wiki/OpenSourceMalaria:Triazolopyrazine_(TP%29_Series (accessed Aug 20, 2022). |
| 16. | Tse, E. G.-O. Open Source Malaria: Potent Triazolopyrazine-Based Antiplasmodium Agents that Probe an Important Mechanism of Action; The University of Sydney: Sydney, Australia, 2019. doi:10.25910/5d7af492b2c63 |
| 20. | Tse, E. G.; Houston, S. D.; Williams, C. M.; Savage, G. P.; Rendina, L. M.; Hallyburton, I.; Anderson, M.; Sharma, R.; Walker, G. S.; Obach, R. S.; Todd, M. H. J. Med. Chem. 2020, 63, 11585–11601. doi:10.1021/acs.jmedchem.0c00746 |
| 16. | Tse, E. G.-O. Open Source Malaria: Potent Triazolopyrazine-Based Antiplasmodium Agents that Probe an Important Mechanism of Action; The University of Sydney: Sydney, Australia, 2019. doi:10.25910/5d7af492b2c63 |
| 17. | Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. iScience 2020, 23. doi:10.1016/j.isci.2020.101467 |
| 18. | Inoue, M.; Sumii, Y.; Shibata, N. ACS Omega 2020, 5, 10633–10640. doi:10.1021/acsomega.0c00830 |
| 19. | Gillis, E. P.; Eastman, K. J.; Hill, M. D.; Donnelly, D. J.; Meanwell, N. A. J. Med. Chem. 2015, 58, 8315–8359. doi:10.1021/acs.jmedchem.5b00258 |
© 2023 Lum et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.