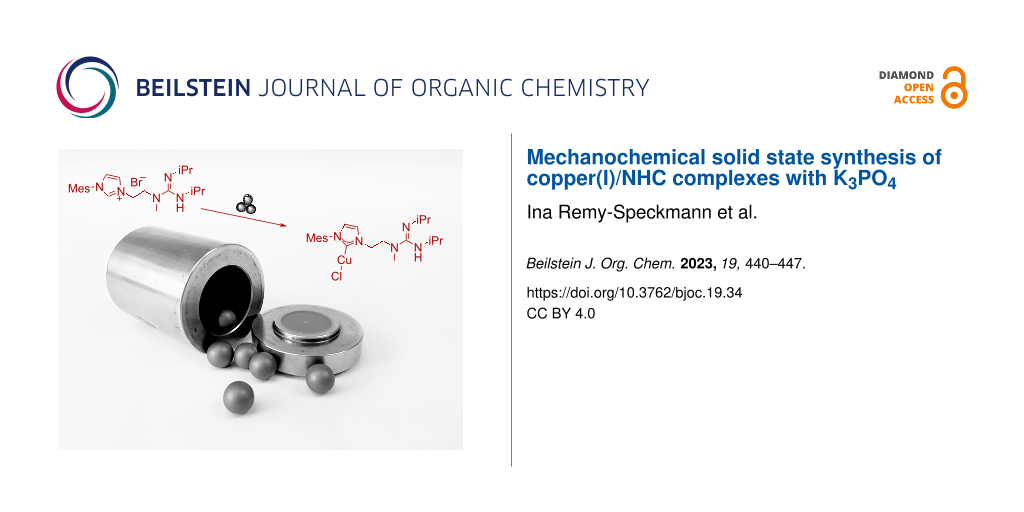
Abstract
A protocol for the mechanochemical synthesis of copper(I)/N-heterocyclic carbene complexes using cheap and readily available K3PO4 as base has been developed. This method employing a ball mill is amenable to typical simple copper(I)/NHC complexes but also to a sophisticated copper(I)/N-heterocyclic carbene complex bearing a guanidine moiety. In this way, the present approach circumvents commonly employed silver(I) complexes which are associated with significant and undesired waste formation and the excessive use of solvents. The resulting bifunctional catalyst has been shown to be active in a variety of reduction/hydrogenation transformations employing dihydrogen as terminal reducing agent.
Graphical Abstract
Introduction
Prominent goals of green chemistry heralded for synthetic chemistry are minimization or ideally the complete prevention of chemical waste. In this vein, the use of innocuous chemicals, replacement of hazardous reagents, atom efficient reactions and overall safer chemical processes are desirable [1,2]. Therefore, one current challenge for syntheses is the development of green and environmentally friendly routes to access value-added products.
One important way to more economical syntheses is the concept of catalysis to avoid stoichiometric amounts of reactants and to design reactions more atom efficient [1-3]. However, the focus has seldomly been on the preparative methods to access the required catalysts themselves. As case in point, we decided to re-investigate the synthesis of copper(I)/N-heterocyclic carbene (NHC) complexes, which are broadly applicable catalysts for a wide variety of transformations [4-6]. While generally there are many different synthetic routes to transition metal/NHC complexes [7-15] not all of them are applicable to the preparation of copper(I)/NHC compounds (Scheme 1) [5,6,13,16-19]. Generally, the so-called direct routes via the appropriate imidazol(in)ium salt, a copper precursor and a suitably strong base (e.g., NaH, NaOt-Bu, KHMDS or n-BuLi) [20-26] are challenging for copper complexes 2 as they tend to give low yields (Scheme 1a) [5,6,13,16-19]. One elegant protocol employing K2CO3 as weak base in combination with copper(I) salts for simple copper(I)/NHC complexes has been disclosed (Scheme 1b) [27]. While this variant is the method of choice due to its simplicity and practicability, other alternatives have to be sought in cases where these direct synthesis approaches fail: On the one hand, the so-called “built-in base” route relies on the use of Cu2O which can be directly reacted with a suitable NHC precursor 1 (Scheme 1c) [28]. In any case, the most common approach hinges upon the use of the preliminary preparation of an intermediate silver(I)/NHC complex followed by facile transmetallation to copper(I) (Scheme 1d). In some cases, this transmetallation step is carried out in situ [14,15,29-32]. Notably, these generally successful synthetic routes produce a considerable amount of transition metal waste (next to the inherent use of solvents) and are therefore in misalignment with the principles of green chemistry.
Scheme 1: General synthetic routes to copper(I)/NHC complexes (X = Cl, Br).
Scheme 1: General synthetic routes to copper(I)/NHC complexes (X = Cl, Br).
Syntheses via mechanochemical methods offer elegant and atom-economic alternatives to liquid state synthesis approaches [33-43]. In accordance with the in situ transmetallation route in liquid state synthesis, a one-pot two-step procedure in a ball mill was discovered (Scheme 1e) [44]. The possibility of synthesizing copper(I)/NHC complexes in the ball mill is promising due to the avoidance of organic solvents. Direct approaches from the NHC-precursor to the copper(I)/NHC complex not undergoing the transmetallation step have been disclosed (Scheme 1f) [45-47]. These approaches are an elegant alternative to the transmetallation route performing without unwanted transition metal waste. Two possible direct mechanochemical pathways have been presented for the synthesis of copper(I)/NHC complexes: First, the complexes can be synthesized by milling the ligand precursor 1 with metallic copper powder in air [45]. Another mechanochemical pathway was published using K2CO3 as a base and copper(I) chloride [46,47]. This latter procedure is practical, avoids the use of solvents, and relies on an abundant and cheap base.
We have recently disclosed an ester reduction with H2 as terminal reducing agent utilizing bifunctional copper(I)/NHC complex 5 bearing a guanidine moiety as additional catalytic unit [48]. This catalyst acts by employing the copper(I)/NHC complex for H2 activation on the one hand and by using the guanidine subunit for simultaneous organocatalytic activation of the ester on the other hand. Following a previously established synthetic pathway [49], we have found that transmetallation via silver(I)/NHC complex 4 was the only viable synthetic entry point to this sophisticated bifunctional catalyst (Scheme 2) [10,12,14,50]. First, the silver(I)/NHC complex 4 had to be synthesized and isolated prior to transmetallation with copper(I) chloride [48,49]. The required formation of silver(I) complex 4 diminishes the overall yield of copper complex 5. As an additional disadvantage, the silver(I) byproducts have to be carefully removed in order to maintain reproducible results in subsequent catalytic hydrogenations [48]. We deemed this synthetic route unattractive with regards to sustainable synthesis due to the silver waste generated in the process and sought to replace the transmetallation route with a more atom economic approach to circumvent these problems.
Scheme 2: Preparation of sophisticated Cu(I)/NHC complexes: Synthesis of bifunctional catalyst 5 via transmetallation. Mes = mesityl [48,49].
Scheme 2: Preparation of sophisticated Cu(I)/NHC complexes: Synthesis of bifunctional catalyst 5 via transmet...
Results and Discussion
We therefore examined different approaches to avoid the transmetallation step (4→5) and to establish a protocol for the direct synthesis of 5 in solution from imidazolium salt 3 (Table 1, liquid state approaches) [48]. The use of strong bases such as n-BuLi or NaOt-Bu (in various equivalents, Table 1, entries 1 and 2) or weak bases (Et3N, K2CO3 or K3PO4, Table 1, entries 3–5) either did give no conversion to 5 at all or delivered a catalytically inactive complex, which we assign to a CO2 adduct of 5 (Table 1, entry 4) [51,52]. We hypothesize that in this CO2 adduct, the guanidine moiety is unavailable to perform its assisting part in catalysis through hydrogen-bonding interaction [48]. As additional evidence to support the formation of the CO2 adduct of 5, we can show that bubbling of CO2 through a solution of 5 leads to catalytically inactive complexes (see Supporting Information File 1 for details). This also supports the notion that during catalytic ester hydrogenation, the guanidinium moiety acts as a hydrogen bond donor to the esters [48]. The formation of a CO2 adduct hinders the ability to form hydrogen bonds. Furthermore, utilizing Cu2O for a “built-in base” approach did not give complex 5 (Table 1, entry 6).
Table 1: Attempted direct synthesis of bifunctional catalyst 5 from imidazolinium salt 3: liquid and solid state approaches.
|
|
||||
| Entry | Reagents | Conditions | Results | |
| Liquid state aproaches [20] | ||||
| 1 | strong bases | 1.00 equiv 3, 1.10 equiv CuCl, 3.00 equiv n-BuLi | THF, 0 °C → rt, 16 h | no formation of 5 |
| 2 | 1.00 equiv 3, 1.10 equiv CuCl, 1.05/2.00/3.00/5.00 equiv NaOt-Bu | THF, rt, 16 h | no formation of 5 | |
| 3 | weak bases | 1.00 equiv 3, 1.10 equiv CuCl, 3.00 equiv NEt3 | THF, 0 °C → rt, 16 h | no formation of 5 |
| 4 | 1.00 equiv 3, 1.00 equiv CuCl, 2.00 equiv K2CO3 | acetone, 60 °C, 16 h | formation of catalytically inactive 5∙CO2 | |
| 5 | 1.00 equiv 3, 1.00 equiv CuCl, 2.00 equiv K3PO4 | acetone, 60 °C, 16 h | no formation of 5 | |
| 6 | “built-in base” approach | 1.00 equiv 3, 2.0 equiv Cu2O | CH2Cl2, 4 Å MS, 60 °C, 16 h | no formation of 5 |
| Solid state approaches (steel vessel (12 mL), 6 steel balls (1 cm diameter) if not noted otherwise) | ||||
| 7 | strong bases | 1.0 equiv 3, 1.0 equiv CuCl, 1.5 equiv NaOt-Bu | 450 rpm, 4 h | no formation of 5 |
| 8 | 1.0 equiv 3, 1.0 equiv CuCl, 1.5 equiv NaOH | 450 rpm, 4 h | no formation of 5 | |
| 9 | 1.0 equiv 3, 1.0 equiv CuCl, 1.5 equiv KHMDS | 450 rpm, 4 h | no formation of 5 | |
| 10 | 1.0 equiv 3, 1.0 equiv CuCl, 1.5 equiv NaH | gastight zirconia vessel (45 mL), 6 zirconia balls (1.5 cm diameter), 450 rpm, 4 h | formation of 5 observed, inseparable mixture of products | |
| 11 | no added base [46] | 1.0 equiv 3, 1.0 equiv CuCl | 450 rpm, 4 h | formation of [3][CuClBr]– observed by HRMS |
| 12 | “built-in base” approach | 1.0 equiv 3, 0.5 equiv Cu2O | 450 rpm, 4 h | no formation of 5 |
| 13 | weak base | 1.0 equiv 3, 1.0 equiv CuCl, 1.5 equiv K2CO3 | 450 rpm, 4 h | 44% of 5 |
| 14 | 1.0 equiv 3, 1.0 equiv CuCl, 1.5 equiv K3PO4 | 450 rpm, 4 h | 91% of 5 | |
Since our attempts to establish direct synthetic routes to 5 from 3 in liquid state were not fruitful, we turned our attention to the mechanochemical synthesis of bifunctional catalyst 5, based on two recent reports on preparation of copper(I)/NHC complexes [45,47].
All mechanochemical syntheses were carried out in a planetary ball mill and the vessel was loaded in an argon-filled glovebox. Copper(I) chloride, imidazolium salt 3 and the appropriate base were mixed (in a molar ratio of 1.0:1.0:1.5, respectively) and ground for 4 hours. Afterwards purification included dissolving the crude product in CH2Cl2, filtration over a PTFE syringe filter and concentrating the filtrate under reduced pressure. Employing strong bases such as KHMDS, NaOt-Bu or NaOH did not lead to the desired product (Table 1, entries 7–9). All three approaches have in common that the conjugated acid of the added base is a liquid. In the literature, the improvement of mechanochemical syntheses by addition of small amounts of a liquid have been reported (LAG, liquid-assisted grinding) [43]. However, in our case, the formation of small amounts of liquid during the milling process lead to agglutination of the remaining solids and therefore insufficient homogenization of the reaction mixture. This gave a mixture of compounds, in which the envisaged complex 5 could not be identified.
A different approach was made using sodium hydride as a base (Table 1, entry 10). Instead of small amounts of liquid, here, deprotonation leads to the formation of dihydrogen. Hence, another gastight mill was utilized for this approach. Unfortunately, a successful synthesis of 5 directly from 3 was not possible under these conditions: NMR analysis of the resulting mixture indicated the presence of 5, but also of unwanted side-products that could not be identified. Purification of 5 from this complex mixture turned out not to be feasible. Further modifications of the milling conditions did not lead to the elimination of these side-products, therefore the experiments with NaH as a base were discontinued. As a side comment, the addition of no base at all led to the formation of the imidazolinium cuprate ([3][CuClBr]−, Table 1, entry 11) [46]. The direct transition of the “built-in base” approach conditions to mechanochemical synthesis (copper(I) oxide and imidazolium salt 3 as starting materials), lead to no formation of 5 (Table 1, entry 12).
The use of K2CO3 for the mechanochemical synthesis of copper(I)/NHC complexes [46,47] was feasible for the preparation of 5 (Table 1, entry 13). Importantly, we found that extending on this concept also K3PO4 could be employed equally well while giving significantly higher yields than the previous protocol [46,47] (Table 1, entry 14). All of the approaches discussed here are attractive due to the use of copper(I) chloride as the copper source. Interestingly, the use of K2CO3, which led to the formation of a catalytically inactive postulated CO2 adduct of 5 in the liquid state synthesis, did lead to catalytically active 5 in the mechanochemical approach. In a similar vein, the different outcome with K3PO4 as a base (which led to no catalytically active complexes in the liquid state synthesis) was surprising, as in the ball mill, clean and catalytically active copper(I) complex 5 was obtained. To avoid the possible formation of the catalytically inactive CO2 adduct when employing K2CO3 for the synthesis of 5 we decided to use K3PO4 for subsequent investigations (see also below, Table 2). Even though the imidazolium bromide salt 3 was employed in combination with CuCl as copper(I) precursor, elemental analysis of 5 clearly supported the formation of 5 as a chloride salt (see Supporting Information File 1).
Table 2: Synthesis of standard Cu(I)/NHC-complexes using K3PO4 as a weak base (standard procedure: steel vessel (12 mL), 6 steel balls (1 cm diameter), 450 rpm, 4 h). (dimer = [(NHC)2Cu+]Cl−).
For the optimized protocol, the starting materials were mixed in a steel vessel and ground at 450 rpm for a total time of four hours. After ball milling, an off-white powder was obtained which gave complex 5 in very good yield of 91% after extraction with CH2Cl2 and filtration. NMR analysis of 5 matched previously reported data [48,49] and showed no side products. It has to be mentioned that complex 5 synthesized via the mechanochemical route is isolated as a CH2Cl2 adduct (5/CH2Cl2 = 1:1) as confirmed by NMR spectroscopy and elemental analysis. If complex 5 is formed via the liquid state synthesis [48,49], also a CH2Cl2 adduct is isolated, albeit with a 5/CH2Cl2 ratio of 2:1.
In order to demonstrate the general applicability of the K3PO4-based protocol for the mechanochemical synthesis of copper(I)/NHC complexes, we decided to prepare the most common copper(I)/NHC complexes 7a–d [5,6] employing our method (Table 2). When the corresponding imidazoli(ni)um salts 6a–d were submitted to the standard protocol, complexes 7a–d were obtained with acceptable yields, with similar yields compared to previous methods. In some cases, the homoleptic cationic copper(I) complexes [(NHC)2Cu]+CuCl2− were observed as side products [48,53].
We decided to directly compare complex 5 from mechanochemical synthesis (5bm) with its counterpart from the liquid state transmetallation route (5ls) in catalysis. We found that 5bm was catalytically active, however displaying slightly diminished activity in general most likely due to different adduct ratio inhibiting the catalytic activity (Scheme 3). This was established using the standard reactions for catalytic hydrogenations with copper(I)/NHC complexes [4]. In this vein, we tested complex 5 from solid and liquid phase synthesis in the catalytic hydrogenation of esters, carbonyl compounds and in the semihydrogenation of alkynes. In the catalytic hydrogenation of ethyl benzoate (8) lower overall conversion to benzyl alcohol (9) and lower yield was found with 5bm (65% conv. and 53% yield with 5bm, in comparison to 100% conv. and 80% yield with 5ls; Scheme 3a). We hypothesize that the higher amount of CH2Cl2 as part of the prepared complex, which is not a suitable solvent for catalytic ester reduction with H2 [48], led to lower catalyst activity. Possible coordination of residual phosphate to the guanidine moiety was excluded as analysis by 31P NMR experiments. The copper(I)-catalyzed 1,2-reduction of functionalized ester 10 was also successfully achieved using the ball mill synthesized bifunctional catalyst 5bm, again with slightly diminished yields and conversions.
Scheme 3: Application of bifunctional catalyst 5 in copper(I)-catalyzed hydrogenations: comparison of 5 prepared by solid state/ball milling (5bm) and liquid state (5ls) synthesis. Standard conditions: Substrate (0.40 mmol), 10 mol % 5, 1.1 equiv NaOt-Bu, 1.3 equiv 15-crown-5, 100 bar H2, 1,4-dioxane (3 mL), 70 °C, 24 h.
Scheme 3: Application of bifunctional catalyst 5 in copper(I)-catalyzed hydrogenations: comparison of 5 prepa...
Application of the ball mill-synthesized complex 5bm in the alkyne semihydrogenation of tolane (12) gave (Z)-stilbene (13) with full stereoselectivity in good yield (86%, Scheme 3b). Noteworthy, the complex 5 was never evaluated in this reported reaction. Therefore, 5bm behaves similarly to other copper(I)/NHC complexes in this transformation [54-60]. The catalytic 1,2-reduction of carbonyl compounds is mainstay for copper(I)/NHC complexes [61-67], which is why we also tested 5bm in these transformations: The 1,2-reduction of benzaldehyde (14) and acetophenone (15) proceeded with good yields (Scheme 3c). No aldol addition for the acetophenone substrate has been observed although working under strongly basic conditions [68,69].
Conclusion
In conclusion, we have disclosed a practical approach to a sophisticated bifunctional copper(I)/NHC complex based on a mechanochemical protocol. This operationally simple synthetic route circumvents the previously necessary use of surplus transition metal reagents and therefore diminishes unwanted waste formation. The new protocol presented here is based on K3PO4 and has successfully displayed the activity of the resulting catalyst in a variety of hydrogenative transformations. We show that the new protocol is also amenable to the synthesis of other standard copper(I)/NHC complexes. Our results do not only add to the wide area of applications of mechanochemical synthesis but also showcase that transition metal complexes bearing additional functional groups can be prepared with a ball milling synthesis. We think that our protocol could be useful for the atom economic preparation of other complex catalysts, which are difficult or wasteful to be prepared by typical liquid state synthesis methods.
Experimental
Mechanochemical synthesis procedure for 5: The product was synthesized using a Fritsch Pulverisette 7 classic line, a high-energy planetary ball mill. The starting materials 1-(2-(2,3-diisopropyl-1-methylguanidino)ethyl)-3-mesityl-1H-imidazol-3-ium bromide (3, 75 mg, 0.16 mmol, 1.0 equiv), CuCl (17 mg, 0.16 mmol, 1.0 equiv) and K3PO4 (53 mg, 0.25 mmol, 1.5 equiv) were filled into a 12 mL steel vessel equipped with six steel balls (1 cm diameter). The beaker was sealed in an argon-filled glovebox. Milling was carried out with 450 rpm for a total of four hours. After each hour the milling was paused for 30 minutes to avoid overheating of the machine. The raw product was obtained as an off-white powder after milling. The ground product was mixed with CH2Cl2 (3 mL) and the resulting suspension was filtered over a PTFE syringe filter (0.45 μm). The filtrate was concentrated under reduced pressure. The product 5 was obtained as the CH2Cl2 adduct as a colourless solid (86 mg, 0.15 mmol, 91%).
Supporting Information
| Supporting Information File 1: General procedures, experimental details, analytical data and copies of NMR spectra. | ||
| Format: PDF | Size: 2.8 MB | Download |
Acknowledgements
Prof. Dr. Martin Oestreich (TU Berlin) is kindly thanked for generous support. Some parts of this work have been published in the dissertation theses by Dr. Birte M. Zimmermann and Dr. Ina Remy-Speckmann (both TU Berlin).
Funding
This research was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy EXC 2008-390540038-UniSysCat, and through an Emmy Noether Fellowship for J.F.T. (TE1101/2-1). M.G. is supported by a fellowship by the Einstein Center for Catalysis (EC2).
References
-
Sheldon, R. A. Chem. Soc. Rev. 2012, 41, 1437–1451. doi:10.1039/c1cs15219j
Return to citation in text: [1] [2] -
Anastas, P.; Eghbali, N. Chem. Soc. Rev. 2010, 39, 301–312. doi:10.1039/b918763b
Return to citation in text: [1] [2] -
Trost, B. M. Angew. Chem., Int. Ed. Engl. 1995, 34, 259–281. doi:10.1002/anie.199502591
Return to citation in text: [1] -
Thiel, N. O.; Pape, F.; Teichert, J. F. Homogeneous Hydrogenation with Copper Catalysts. In Homogeneous Hydrogenation with Non-Precious Catalysts; Teichert, J. F., Ed.; Wiley-VCH: Weinheim, Germany, 2019; pp 87–109. doi:10.1002/9783527814237.ch4
Return to citation in text: [1] [2] -
Lazreg, F.; Nahra, F.; Cazin, C. S. J. Coord. Chem. Rev. 2015, 293–294, 48–79. doi:10.1016/j.ccr.2014.12.019
Return to citation in text: [1] [2] [3] [4] -
Egbert, J. D.; Cazin, C. S. J.; Nolan, S. P. Catal. Sci. Technol. 2013, 3, 912–926. doi:10.1039/c2cy20816d
Return to citation in text: [1] [2] [3] [4] -
Scattolin, T.; Nolan, S. P. Trends Chem. 2020, 2, 721–736. doi:10.1016/j.trechm.2020.06.001
Return to citation in text: [1] -
Peris, E. Chem. Rev. 2018, 118, 9988–10031. doi:10.1021/acs.chemrev.6b00695
Return to citation in text: [1] -
Hameury, S.; de Frémont, P.; Braunstein, P. Chem. Soc. Rev. 2017, 46, 632–733. doi:10.1039/c6cs00499g
Return to citation in text: [1] -
Hopkinson, M. N.; Richter, C.; Schedler, M.; Glorius, F. Nature 2014, 510, 485–496. doi:10.1038/nature13384
Return to citation in text: [1] [2] -
Brown, J. M.; Dixneuf, P. H.; Fürstner, A.; Hegedus, L. S.; Hofmann, P.; Knochel, P.; van Koten, G.; Murai, S.; Reetz, M. In N-Heterocyclic Carbenes in Transition Metal Catalysis; Glorius, F., Ed.; Topics in Organometallic Chemistry, Vol. 21; Springer: Berlin, Germany, 2007. doi:10.1007/978-3-540-36930-1
Return to citation in text: [1] -
Díez-González, S.; Nolan, S. P. Coord. Chem. Rev. 2007, 251, 874–883. doi:10.1016/j.ccr.2006.10.004
Return to citation in text: [1] [2] -
Danopoulos, A. A.; Simler, T.; Braunstein, P. Chem. Rev. 2019, 119, 3730–3961. doi:10.1021/acs.chemrev.8b00505
Return to citation in text: [1] [2] [3] -
Pape, F.; Teichert, J. F. Eur. J. Org. Chem. 2017, 4206–4229. doi:10.1002/ejoc.201700124
Return to citation in text: [1] [2] [3] -
Nolan, S. P. N‐Heterocyclic Carbenes in Synthesis; Wiley-VCH: Weinheim, Germany, 2006. doi:10.1002/9783527609451
Return to citation in text: [1] [2] -
Budagumpi, S.; Keri, R. S.; Achar, G.; Brinda, K. N. Adv. Synth. Catal. 2020, 362, 970–997. doi:10.1002/adsc.201900859
Return to citation in text: [1] [2] -
Lin, J. C. Y.; Huang, R. T. W.; Lee, C. S.; Bhattacharyya, A.; Hwang, W. S.; Lin, I. J. B. Chem. Rev. 2009, 109, 3561–3598. doi:10.1021/cr8005153
Return to citation in text: [1] [2] -
Nahra, F.; Gómez-Herrera, A.; Cazin, C. S. J. Dalton Trans. 2017, 46, 628–631. doi:10.1039/c6dt03687b
Return to citation in text: [1] [2] -
Yong, X.; Thurston, R.; Ho, C.-Y. Synthesis 2019, 51, 2058–2080. doi:10.1055/s-0037-1611751
Return to citation in text: [1] [2] -
Arduengo, A. J., III; Harlow, R. L.; Kline, M. J. Am. Chem. Soc. 1991, 113, 361–363. doi:10.1021/ja00001a054
Return to citation in text: [1] [2] -
Arduengo, A. J., III; Dias, H. V. R.; Calabrese, J. C.; Davidson, F. Organometallics 1993, 12, 3405–3409. doi:10.1021/om00033a009
Return to citation in text: [1] -
Raubenheimer, H. G.; Cronje, S.; Olivier, P. J. J. Chem. Soc., Dalton Trans. 1995, 313–316. doi:10.1039/dt9950000313
Return to citation in text: [1] -
Hu, X.; Castro-Rodriguez, I.; Olsen, K.; Meyer, K. Organometallics 2004, 23, 755–764. doi:10.1021/om0341855
Return to citation in text: [1] -
Díez-González, S.; Kaur, H.; Zinn, F. K.; Stevens, E. D.; Nolan, S. P. J. Org. Chem. 2005, 70, 4784–4796. doi:10.1021/jo050397v
Return to citation in text: [1] -
Michon, C.; Ellern, A.; Angelici, R. J. Inorg. Chim. Acta 2006, 359, 4549–4556. doi:10.1016/j.ica.2006.07.019
Return to citation in text: [1] -
Díez-González, S.; Escudero-Adán, E. C.; Benet-Buchholz, J.; Stevens, E. D.; Slawin, A. M. Z.; Nolan, S. P. Dalton Trans. 2010, 39, 7595–7606. doi:10.1039/c0dt00218f
Return to citation in text: [1] -
Santoro, O.; Collado, A.; Slawin, A. M. Z.; Nolan, S. P.; Cazin, C. S. J. Chem. Commun. 2013, 49, 10483–10485. doi:10.1039/c3cc45488f
Return to citation in text: [1] -
Citadelle, C. A.; Le Nouy, E.; Bisaro, F.; Slawin, A. M. Z.; Cazin, C. S. J. Dalton Trans. 2010, 39, 4489–4491. doi:10.1039/c0dt00128g
Return to citation in text: [1] -
Van Veldhuizen, J. J.; Campbell, J. E.; Giudici, R. E.; Hoveyda, A. H. J. Am. Chem. Soc. 2005, 127, 6877–6882. doi:10.1021/ja050179j
Return to citation in text: [1] -
Brown, M. K.; May, T. L.; Baxter, C. A.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2007, 46, 1097–1100. doi:10.1002/anie.200604511
Return to citation in text: [1] -
Lee, Y.; Akiyama, K.; Gillingham, D. G.; Brown, M. K.; Hoveyda, A. H. J. Am. Chem. Soc. 2008, 130, 446–447. doi:10.1021/ja0782192
Return to citation in text: [1] -
May, T. L.; Brown, M. K.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2008, 47, 7358–7362. doi:10.1002/anie.200802910
Return to citation in text: [1] -
Beillard, A.; Quintin, F.; Gatignol, J.; Retailleau, P.; Renaud, J.-L.; Gaillard, S.; Métro, T.-X.; Lamaty, F.; Bantreil, X. Dalton Trans. 2020, 49, 12592–12598. doi:10.1039/d0dt00410c
Return to citation in text: [1] -
Beillard, A.; Bantreil, X.; Métro, T.-X.; Martinez, J.; Lamaty, F. Chem. Rev. 2019, 119, 7529–7609. doi:10.1021/acs.chemrev.8b00479
Return to citation in text: [1] -
Zaky, R.; Fekri, A. Appl. Organomet. Chem. 2019, e4786. doi:10.1002/aoc.4786
Return to citation in text: [1] -
Andersen, J.; Mack, J. Green Chem. 2018, 20, 1435–1443. doi:10.1039/c7gc03797j
Return to citation in text: [1] -
Howard, J. L.; Cao, Q.; Browne, D. L. Chem. Sci. 2018, 9, 3080–3094. doi:10.1039/c7sc05371a
Return to citation in text: [1] -
Tan, D.; Friščić, T. Eur. J. Org. Chem. 2018, 18–33. doi:10.1002/ejoc.201700961
Return to citation in text: [1] -
Hernández, J. G.; Bolm, C. J. Org. Chem. 2017, 82, 4007–4019. doi:10.1021/acs.joc.6b02887
Return to citation in text: [1] -
Rightmire, N. R.; Hanusa, T. P. Dalton Trans. 2016, 45, 2352–2362. doi:10.1039/c5dt03866a
Return to citation in text: [1] -
Beillard, A.; Bantreil, X.; Métro, T.-X.; Martinez, J.; Lamaty, F. Dalton Trans. 2016, 45, 17859–17866. doi:10.1039/c6dt03564g
Return to citation in text: [1] -
Beillard, A.; Golliard, E.; Gillet, V.; Bantreil, X.; Métro, T.-X.; Martinez, J.; Lamaty, F. Chem. – Eur. J. 2015, 21, 17614–17617. doi:10.1002/chem.201503472
Return to citation in text: [1] -
Friščić, T.; Mottillo, C.; Titi, H. M. Angew. Chem., Int. Ed. 2020, 59, 1018–1029. doi:10.1002/anie.201906755
Return to citation in text: [1] [2] -
Beillard, A.; Bantreil, X.; Métro, T.-X.; Martinez, J.; Lamaty, F. New J. Chem. 2017, 41, 1057–1063. doi:10.1039/c6nj02895k
Return to citation in text: [1] -
Beillard, A.; Métro, T.-X.; Bantreil, X.; Martinez, J.; Lamaty, F. Chem. Sci. 2017, 8, 1086–1089. doi:10.1039/c6sc03182j
Return to citation in text: [1] [2] [3] [4] -
Pisanò, G.; Cazin, C. S. J. Green Chem. 2020, 22, 5253–5256. doi:10.1039/d0gc01923b
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] -
Pisanò, G.; Cazin, C. S. J. ACS Sustainable Chem. Eng. 2021, 9, 9625–9631. doi:10.1021/acssuschemeng.1c00556
Return to citation in text: [1] [2] [3] [4] [5] -
Zimmermann, B. M.; Ngoc, T. T.; Tzaras, D.-I.; Kaicharla, T.; Teichert, J. F. J. Am. Chem. Soc. 2021, 143, 16865–16873. doi:10.1021/jacs.1c09626
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] -
Tai, C.-C.; Yu, M.-S.; Chen, Y.-L.; Chuang, W.-H.; Lin, T.-H.; Yap, G. P. A.; Ong, T.-G. Chem. Commun. 2014, 50, 4344–4346. doi:10.1039/c4cc00550c
Return to citation in text: [1] [2] [3] [4] [5] -
Poyatos, M.; Mata, J. A.; Peris, E. Chem. Rev. 2009, 109, 3677–3707. doi:10.1021/cr800501s
Return to citation in text: [1] -
Grayson, D. H.; Ishikawa, T.; Kato, M.; Kobayashi, Y.; Leow, D.; Nagasawa, K.; Nájera, C.; Odagi, M.; Rozas, I.; Shaw, J. W.; Takemoto, Y.; Tan, C.-H.; Yus, M. In Guanidines as Reagents and Catalysts; Selig, P., Ed.; Topics in Heterocyclic Chemistry, Vol. 50; Springer International Publishing: Cham, Switzerland, 2017. doi:10.1007/978-3-319-52725-3
Return to citation in text: [1] -
del Amo, V.; Capitão, R. M.; Concellón, C.; von Eßen, C.; Göb, C. R.; González, E. R. P.; Herres-Pawlis, S.; Himmel, H.-J.; Hoffmann, A.; Mannsperger, J.; Metz, A.; Oppel, I. M.; Rösener, T.; Santo, R. D. E.; Stanek, J. In Guanidines as Reagents and Catalysts II; Selig, P., Ed.; Topics in Heterocyclic Chemistry, Vol. 51; Springer International Publishing: Cham, Switzerland, 2017. doi:10.1007/978-3-319-53013-0
Return to citation in text: [1] -
Kuehn, L.; Eichhorn, A. F.; Marder, T. B.; Radius, U. J. Organomet. Chem. 2019, 881, 25–33. doi:10.1016/j.jorganchem.2018.11.032
Return to citation in text: [1] -
Semba, K.; Kameyama, R.; Nakao, Y. Synlett 2015, 26, 318–322. doi:10.1055/s-0034-1379896
Return to citation in text: [1] -
Pape, F.; Thiel, N. O.; Teichert, J. F. Chem. – Eur. J. 2015, 21, 15934–15938. doi:10.1002/chem.201501739
Return to citation in text: [1] -
Thiel, N. O.; Teichert, J. F. Org. Biomol. Chem. 2016, 14, 10660–10666. doi:10.1039/c6ob02271e
Return to citation in text: [1] -
Wakamatsu, T.; Nagao, K.; Ohmiya, H.; Sawamura, M. Organometallics 2016, 35, 1354–1357. doi:10.1021/acs.organomet.6b00126
Return to citation in text: [1] -
Pape, F.; Teichert, J. Synthesis 2017, 49, 2470–2482. doi:10.1055/s-0036-1590112
Return to citation in text: [1] -
Thiel, N. O.; Kemper, S.; Teichert, J. F. Tetrahedron 2017, 73, 5023–5028. doi:10.1016/j.tet.2017.05.029
Return to citation in text: [1] -
Brechmann, L. T.; Teichert, J. F. Synthesis 2020, 52, 2483–2496. doi:10.1055/s-0040-1707185
Return to citation in text: [1] -
Mahoney, W. S.; Stryker, J. M. J. Am. Chem. Soc. 1989, 111, 8818–8823. doi:10.1021/ja00206a008
Return to citation in text: [1] -
Chen, J.-X.; Daeuble, J. F.; Brestensky, D. M.; Stryker, J. M. Tetrahedron 2000, 56, 2153–2166. doi:10.1016/s0040-4020(99)01098-4
Return to citation in text: [1] -
Chen, J.-X.; Daeuble, J. F.; Stryker, J. M. Tetrahedron 2000, 56, 2789–2798. doi:10.1016/s0040-4020(00)00133-2
Return to citation in text: [1] -
Shimizu, H.; Igarashi, D.; Kuriyama, W.; Yusa, Y.; Sayo, N.; Saito, T. Org. Lett. 2007, 9, 1655–1657. doi:10.1021/ol070289q
Return to citation in text: [1] -
Shimizu, H.; Nagasaki, I.; Matsumura, K.; Sayo, N.; Saito, T. Acc. Chem. Res. 2007, 40, 1385–1393. doi:10.1021/ar700101x
Return to citation in text: [1] -
Junge, K.; Wendt, B.; Addis, D.; Zhou, S.; Das, S.; Fleischer, S.; Beller, M. Chem. – Eur. J. 2011, 17, 101–105. doi:10.1002/chem.201002311
Return to citation in text: [1] -
Trose, M.; Lazreg, F.; Chang, T.; Nahra, F.; Cordes, D. B.; Slawin, A. M. Z.; Cazin, C. S. J. ACS Catal. 2017, 7, 238–242. doi:10.1021/acscatal.6b02723
Return to citation in text: [1] -
Mahrwald, R., Ed. Modern Aldol Reactions; Wiley-VCH: Weinheim, Germany, 2004. doi:10.1002/9783527619566
Return to citation in text: [1] -
Aggarwal, V. K.; Arai, N.; Bergin, E.; Buitrago Sanatnilla, A.; Carreira, E. M. In Science of Synthesis: Stereoselective Synthesis Vol. 2; Molander, G., Ed.; Thieme: Stuttgart, Germany, 2011.
Return to citation in text: [1]
| 45. | Beillard, A.; Métro, T.-X.; Bantreil, X.; Martinez, J.; Lamaty, F. Chem. Sci. 2017, 8, 1086–1089. doi:10.1039/c6sc03182j |
| 47. | Pisanò, G.; Cazin, C. S. J. ACS Sustainable Chem. Eng. 2021, 9, 9625–9631. doi:10.1021/acssuschemeng.1c00556 |
| 43. | Friščić, T.; Mottillo, C.; Titi, H. M. Angew. Chem., Int. Ed. 2020, 59, 1018–1029. doi:10.1002/anie.201906755 |
| 46. | Pisanò, G.; Cazin, C. S. J. Green Chem. 2020, 22, 5253–5256. doi:10.1039/d0gc01923b |
| 1. | Sheldon, R. A. Chem. Soc. Rev. 2012, 41, 1437–1451. doi:10.1039/c1cs15219j |
| 2. | Anastas, P.; Eghbali, N. Chem. Soc. Rev. 2010, 39, 301–312. doi:10.1039/b918763b |
| 5. | Lazreg, F.; Nahra, F.; Cazin, C. S. J. Coord. Chem. Rev. 2015, 293–294, 48–79. doi:10.1016/j.ccr.2014.12.019 |
| 6. | Egbert, J. D.; Cazin, C. S. J.; Nolan, S. P. Catal. Sci. Technol. 2013, 3, 912–926. doi:10.1039/c2cy20816d |
| 13. | Danopoulos, A. A.; Simler, T.; Braunstein, P. Chem. Rev. 2019, 119, 3730–3961. doi:10.1021/acs.chemrev.8b00505 |
| 16. | Budagumpi, S.; Keri, R. S.; Achar, G.; Brinda, K. N. Adv. Synth. Catal. 2020, 362, 970–997. doi:10.1002/adsc.201900859 |
| 17. | Lin, J. C. Y.; Huang, R. T. W.; Lee, C. S.; Bhattacharyya, A.; Hwang, W. S.; Lin, I. J. B. Chem. Rev. 2009, 109, 3561–3598. doi:10.1021/cr8005153 |
| 18. | Nahra, F.; Gómez-Herrera, A.; Cazin, C. S. J. Dalton Trans. 2017, 46, 628–631. doi:10.1039/c6dt03687b |
| 19. | Yong, X.; Thurston, R.; Ho, C.-Y. Synthesis 2019, 51, 2058–2080. doi:10.1055/s-0037-1611751 |
| 46. | Pisanò, G.; Cazin, C. S. J. Green Chem. 2020, 22, 5253–5256. doi:10.1039/d0gc01923b |
| 47. | Pisanò, G.; Cazin, C. S. J. ACS Sustainable Chem. Eng. 2021, 9, 9625–9631. doi:10.1021/acssuschemeng.1c00556 |
| 5. | Lazreg, F.; Nahra, F.; Cazin, C. S. J. Coord. Chem. Rev. 2015, 293–294, 48–79. doi:10.1016/j.ccr.2014.12.019 |
| 6. | Egbert, J. D.; Cazin, C. S. J.; Nolan, S. P. Catal. Sci. Technol. 2013, 3, 912–926. doi:10.1039/c2cy20816d |
| 7. | Scattolin, T.; Nolan, S. P. Trends Chem. 2020, 2, 721–736. doi:10.1016/j.trechm.2020.06.001 |
| 8. | Peris, E. Chem. Rev. 2018, 118, 9988–10031. doi:10.1021/acs.chemrev.6b00695 |
| 9. | Hameury, S.; de Frémont, P.; Braunstein, P. Chem. Soc. Rev. 2017, 46, 632–733. doi:10.1039/c6cs00499g |
| 10. | Hopkinson, M. N.; Richter, C.; Schedler, M.; Glorius, F. Nature 2014, 510, 485–496. doi:10.1038/nature13384 |
| 11. | Brown, J. M.; Dixneuf, P. H.; Fürstner, A.; Hegedus, L. S.; Hofmann, P.; Knochel, P.; van Koten, G.; Murai, S.; Reetz, M. In N-Heterocyclic Carbenes in Transition Metal Catalysis; Glorius, F., Ed.; Topics in Organometallic Chemistry, Vol. 21; Springer: Berlin, Germany, 2007. doi:10.1007/978-3-540-36930-1 |
| 12. | Díez-González, S.; Nolan, S. P. Coord. Chem. Rev. 2007, 251, 874–883. doi:10.1016/j.ccr.2006.10.004 |
| 13. | Danopoulos, A. A.; Simler, T.; Braunstein, P. Chem. Rev. 2019, 119, 3730–3961. doi:10.1021/acs.chemrev.8b00505 |
| 14. | Pape, F.; Teichert, J. F. Eur. J. Org. Chem. 2017, 4206–4229. doi:10.1002/ejoc.201700124 |
| 15. | Nolan, S. P. N‐Heterocyclic Carbenes in Synthesis; Wiley-VCH: Weinheim, Germany, 2006. doi:10.1002/9783527609451 |
| 48. | Zimmermann, B. M.; Ngoc, T. T.; Tzaras, D.-I.; Kaicharla, T.; Teichert, J. F. J. Am. Chem. Soc. 2021, 143, 16865–16873. doi:10.1021/jacs.1c09626 |
| 48. | Zimmermann, B. M.; Ngoc, T. T.; Tzaras, D.-I.; Kaicharla, T.; Teichert, J. F. J. Am. Chem. Soc. 2021, 143, 16865–16873. doi:10.1021/jacs.1c09626 |
| 53. | Kuehn, L.; Eichhorn, A. F.; Marder, T. B.; Radius, U. J. Organomet. Chem. 2019, 881, 25–33. doi:10.1016/j.jorganchem.2018.11.032 |
| 4. | Thiel, N. O.; Pape, F.; Teichert, J. F. Homogeneous Hydrogenation with Copper Catalysts. In Homogeneous Hydrogenation with Non-Precious Catalysts; Teichert, J. F., Ed.; Wiley-VCH: Weinheim, Germany, 2019; pp 87–109. doi:10.1002/9783527814237.ch4 |
| 5. | Lazreg, F.; Nahra, F.; Cazin, C. S. J. Coord. Chem. Rev. 2015, 293–294, 48–79. doi:10.1016/j.ccr.2014.12.019 |
| 6. | Egbert, J. D.; Cazin, C. S. J.; Nolan, S. P. Catal. Sci. Technol. 2013, 3, 912–926. doi:10.1039/c2cy20816d |
| 45. | Beillard, A.; Métro, T.-X.; Bantreil, X.; Martinez, J.; Lamaty, F. Chem. Sci. 2017, 8, 1086–1089. doi:10.1039/c6sc03182j |
| 46. | Pisanò, G.; Cazin, C. S. J. Green Chem. 2020, 22, 5253–5256. doi:10.1039/d0gc01923b |
| 47. | Pisanò, G.; Cazin, C. S. J. ACS Sustainable Chem. Eng. 2021, 9, 9625–9631. doi:10.1021/acssuschemeng.1c00556 |
| 48. | Zimmermann, B. M.; Ngoc, T. T.; Tzaras, D.-I.; Kaicharla, T.; Teichert, J. F. J. Am. Chem. Soc. 2021, 143, 16865–16873. doi:10.1021/jacs.1c09626 |
| 49. | Tai, C.-C.; Yu, M.-S.; Chen, Y.-L.; Chuang, W.-H.; Lin, T.-H.; Yap, G. P. A.; Ong, T.-G. Chem. Commun. 2014, 50, 4344–4346. doi:10.1039/c4cc00550c |
| 1. | Sheldon, R. A. Chem. Soc. Rev. 2012, 41, 1437–1451. doi:10.1039/c1cs15219j |
| 2. | Anastas, P.; Eghbali, N. Chem. Soc. Rev. 2010, 39, 301–312. doi:10.1039/b918763b |
| 3. | Trost, B. M. Angew. Chem., Int. Ed. Engl. 1995, 34, 259–281. doi:10.1002/anie.199502591 |
| 45. | Beillard, A.; Métro, T.-X.; Bantreil, X.; Martinez, J.; Lamaty, F. Chem. Sci. 2017, 8, 1086–1089. doi:10.1039/c6sc03182j |
| 48. | Zimmermann, B. M.; Ngoc, T. T.; Tzaras, D.-I.; Kaicharla, T.; Teichert, J. F. J. Am. Chem. Soc. 2021, 143, 16865–16873. doi:10.1021/jacs.1c09626 |
| 49. | Tai, C.-C.; Yu, M.-S.; Chen, Y.-L.; Chuang, W.-H.; Lin, T.-H.; Yap, G. P. A.; Ong, T.-G. Chem. Commun. 2014, 50, 4344–4346. doi:10.1039/c4cc00550c |
| 28. | Citadelle, C. A.; Le Nouy, E.; Bisaro, F.; Slawin, A. M. Z.; Cazin, C. S. J. Dalton Trans. 2010, 39, 4489–4491. doi:10.1039/c0dt00128g |
| 33. | Beillard, A.; Quintin, F.; Gatignol, J.; Retailleau, P.; Renaud, J.-L.; Gaillard, S.; Métro, T.-X.; Lamaty, F.; Bantreil, X. Dalton Trans. 2020, 49, 12592–12598. doi:10.1039/d0dt00410c |
| 34. | Beillard, A.; Bantreil, X.; Métro, T.-X.; Martinez, J.; Lamaty, F. Chem. Rev. 2019, 119, 7529–7609. doi:10.1021/acs.chemrev.8b00479 |
| 35. | Zaky, R.; Fekri, A. Appl. Organomet. Chem. 2019, e4786. doi:10.1002/aoc.4786 |
| 36. | Andersen, J.; Mack, J. Green Chem. 2018, 20, 1435–1443. doi:10.1039/c7gc03797j |
| 37. | Howard, J. L.; Cao, Q.; Browne, D. L. Chem. Sci. 2018, 9, 3080–3094. doi:10.1039/c7sc05371a |
| 38. | Tan, D.; Friščić, T. Eur. J. Org. Chem. 2018, 18–33. doi:10.1002/ejoc.201700961 |
| 39. | Hernández, J. G.; Bolm, C. J. Org. Chem. 2017, 82, 4007–4019. doi:10.1021/acs.joc.6b02887 |
| 40. | Rightmire, N. R.; Hanusa, T. P. Dalton Trans. 2016, 45, 2352–2362. doi:10.1039/c5dt03866a |
| 41. | Beillard, A.; Bantreil, X.; Métro, T.-X.; Martinez, J.; Lamaty, F. Dalton Trans. 2016, 45, 17859–17866. doi:10.1039/c6dt03564g |
| 42. | Beillard, A.; Golliard, E.; Gillet, V.; Bantreil, X.; Métro, T.-X.; Martinez, J.; Lamaty, F. Chem. – Eur. J. 2015, 21, 17614–17617. doi:10.1002/chem.201503472 |
| 43. | Friščić, T.; Mottillo, C.; Titi, H. M. Angew. Chem., Int. Ed. 2020, 59, 1018–1029. doi:10.1002/anie.201906755 |
| 45. | Beillard, A.; Métro, T.-X.; Bantreil, X.; Martinez, J.; Lamaty, F. Chem. Sci. 2017, 8, 1086–1089. doi:10.1039/c6sc03182j |
| 27. | Santoro, O.; Collado, A.; Slawin, A. M. Z.; Nolan, S. P.; Cazin, C. S. J. Chem. Commun. 2013, 49, 10483–10485. doi:10.1039/c3cc45488f |
| 44. | Beillard, A.; Bantreil, X.; Métro, T.-X.; Martinez, J.; Lamaty, F. New J. Chem. 2017, 41, 1057–1063. doi:10.1039/c6nj02895k |
| 46. | Pisanò, G.; Cazin, C. S. J. Green Chem. 2020, 22, 5253–5256. doi:10.1039/d0gc01923b |
| 5. | Lazreg, F.; Nahra, F.; Cazin, C. S. J. Coord. Chem. Rev. 2015, 293–294, 48–79. doi:10.1016/j.ccr.2014.12.019 |
| 6. | Egbert, J. D.; Cazin, C. S. J.; Nolan, S. P. Catal. Sci. Technol. 2013, 3, 912–926. doi:10.1039/c2cy20816d |
| 13. | Danopoulos, A. A.; Simler, T.; Braunstein, P. Chem. Rev. 2019, 119, 3730–3961. doi:10.1021/acs.chemrev.8b00505 |
| 16. | Budagumpi, S.; Keri, R. S.; Achar, G.; Brinda, K. N. Adv. Synth. Catal. 2020, 362, 970–997. doi:10.1002/adsc.201900859 |
| 17. | Lin, J. C. Y.; Huang, R. T. W.; Lee, C. S.; Bhattacharyya, A.; Hwang, W. S.; Lin, I. J. B. Chem. Rev. 2009, 109, 3561–3598. doi:10.1021/cr8005153 |
| 18. | Nahra, F.; Gómez-Herrera, A.; Cazin, C. S. J. Dalton Trans. 2017, 46, 628–631. doi:10.1039/c6dt03687b |
| 19. | Yong, X.; Thurston, R.; Ho, C.-Y. Synthesis 2019, 51, 2058–2080. doi:10.1055/s-0037-1611751 |
| 46. | Pisanò, G.; Cazin, C. S. J. Green Chem. 2020, 22, 5253–5256. doi:10.1039/d0gc01923b |
| 47. | Pisanò, G.; Cazin, C. S. J. ACS Sustainable Chem. Eng. 2021, 9, 9625–9631. doi:10.1021/acssuschemeng.1c00556 |
| 20. | Arduengo, A. J., III; Harlow, R. L.; Kline, M. J. Am. Chem. Soc. 1991, 113, 361–363. doi:10.1021/ja00001a054 |
| 21. | Arduengo, A. J., III; Dias, H. V. R.; Calabrese, J. C.; Davidson, F. Organometallics 1993, 12, 3405–3409. doi:10.1021/om00033a009 |
| 22. | Raubenheimer, H. G.; Cronje, S.; Olivier, P. J. J. Chem. Soc., Dalton Trans. 1995, 313–316. doi:10.1039/dt9950000313 |
| 23. | Hu, X.; Castro-Rodriguez, I.; Olsen, K.; Meyer, K. Organometallics 2004, 23, 755–764. doi:10.1021/om0341855 |
| 24. | Díez-González, S.; Kaur, H.; Zinn, F. K.; Stevens, E. D.; Nolan, S. P. J. Org. Chem. 2005, 70, 4784–4796. doi:10.1021/jo050397v |
| 25. | Michon, C.; Ellern, A.; Angelici, R. J. Inorg. Chim. Acta 2006, 359, 4549–4556. doi:10.1016/j.ica.2006.07.019 |
| 26. | Díez-González, S.; Escudero-Adán, E. C.; Benet-Buchholz, J.; Stevens, E. D.; Slawin, A. M. Z.; Nolan, S. P. Dalton Trans. 2010, 39, 7595–7606. doi:10.1039/c0dt00218f |
| 14. | Pape, F.; Teichert, J. F. Eur. J. Org. Chem. 2017, 4206–4229. doi:10.1002/ejoc.201700124 |
| 15. | Nolan, S. P. N‐Heterocyclic Carbenes in Synthesis; Wiley-VCH: Weinheim, Germany, 2006. doi:10.1002/9783527609451 |
| 29. | Van Veldhuizen, J. J.; Campbell, J. E.; Giudici, R. E.; Hoveyda, A. H. J. Am. Chem. Soc. 2005, 127, 6877–6882. doi:10.1021/ja050179j |
| 30. | Brown, M. K.; May, T. L.; Baxter, C. A.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2007, 46, 1097–1100. doi:10.1002/anie.200604511 |
| 31. | Lee, Y.; Akiyama, K.; Gillingham, D. G.; Brown, M. K.; Hoveyda, A. H. J. Am. Chem. Soc. 2008, 130, 446–447. doi:10.1021/ja0782192 |
| 32. | May, T. L.; Brown, M. K.; Hoveyda, A. H. Angew. Chem., Int. Ed. 2008, 47, 7358–7362. doi:10.1002/anie.200802910 |
| 46. | Pisanò, G.; Cazin, C. S. J. Green Chem. 2020, 22, 5253–5256. doi:10.1039/d0gc01923b |
| 47. | Pisanò, G.; Cazin, C. S. J. ACS Sustainable Chem. Eng. 2021, 9, 9625–9631. doi:10.1021/acssuschemeng.1c00556 |
| 48. | Zimmermann, B. M.; Ngoc, T. T.; Tzaras, D.-I.; Kaicharla, T.; Teichert, J. F. J. Am. Chem. Soc. 2021, 143, 16865–16873. doi:10.1021/jacs.1c09626 |
| 49. | Tai, C.-C.; Yu, M.-S.; Chen, Y.-L.; Chuang, W.-H.; Lin, T.-H.; Yap, G. P. A.; Ong, T.-G. Chem. Commun. 2014, 50, 4344–4346. doi:10.1039/c4cc00550c |
| 49. | Tai, C.-C.; Yu, M.-S.; Chen, Y.-L.; Chuang, W.-H.; Lin, T.-H.; Yap, G. P. A.; Ong, T.-G. Chem. Commun. 2014, 50, 4344–4346. doi:10.1039/c4cc00550c |
| 4. | Thiel, N. O.; Pape, F.; Teichert, J. F. Homogeneous Hydrogenation with Copper Catalysts. In Homogeneous Hydrogenation with Non-Precious Catalysts; Teichert, J. F., Ed.; Wiley-VCH: Weinheim, Germany, 2019; pp 87–109. doi:10.1002/9783527814237.ch4 |
| 10. | Hopkinson, M. N.; Richter, C.; Schedler, M.; Glorius, F. Nature 2014, 510, 485–496. doi:10.1038/nature13384 |
| 12. | Díez-González, S.; Nolan, S. P. Coord. Chem. Rev. 2007, 251, 874–883. doi:10.1016/j.ccr.2006.10.004 |
| 14. | Pape, F.; Teichert, J. F. Eur. J. Org. Chem. 2017, 4206–4229. doi:10.1002/ejoc.201700124 |
| 50. | Poyatos, M.; Mata, J. A.; Peris, E. Chem. Rev. 2009, 109, 3677–3707. doi:10.1021/cr800501s |
| 48. | Zimmermann, B. M.; Ngoc, T. T.; Tzaras, D.-I.; Kaicharla, T.; Teichert, J. F. J. Am. Chem. Soc. 2021, 143, 16865–16873. doi:10.1021/jacs.1c09626 |
| 54. | Semba, K.; Kameyama, R.; Nakao, Y. Synlett 2015, 26, 318–322. doi:10.1055/s-0034-1379896 |
| 55. | Pape, F.; Thiel, N. O.; Teichert, J. F. Chem. – Eur. J. 2015, 21, 15934–15938. doi:10.1002/chem.201501739 |
| 56. | Thiel, N. O.; Teichert, J. F. Org. Biomol. Chem. 2016, 14, 10660–10666. doi:10.1039/c6ob02271e |
| 57. | Wakamatsu, T.; Nagao, K.; Ohmiya, H.; Sawamura, M. Organometallics 2016, 35, 1354–1357. doi:10.1021/acs.organomet.6b00126 |
| 58. | Pape, F.; Teichert, J. Synthesis 2017, 49, 2470–2482. doi:10.1055/s-0036-1590112 |
| 59. | Thiel, N. O.; Kemper, S.; Teichert, J. F. Tetrahedron 2017, 73, 5023–5028. doi:10.1016/j.tet.2017.05.029 |
| 60. | Brechmann, L. T.; Teichert, J. F. Synthesis 2020, 52, 2483–2496. doi:10.1055/s-0040-1707185 |
| 20. | Arduengo, A. J., III; Harlow, R. L.; Kline, M. J. Am. Chem. Soc. 1991, 113, 361–363. doi:10.1021/ja00001a054 |
| 46. | Pisanò, G.; Cazin, C. S. J. Green Chem. 2020, 22, 5253–5256. doi:10.1039/d0gc01923b |
| 48. | Zimmermann, B. M.; Ngoc, T. T.; Tzaras, D.-I.; Kaicharla, T.; Teichert, J. F. J. Am. Chem. Soc. 2021, 143, 16865–16873. doi:10.1021/jacs.1c09626 |
| 48. | Zimmermann, B. M.; Ngoc, T. T.; Tzaras, D.-I.; Kaicharla, T.; Teichert, J. F. J. Am. Chem. Soc. 2021, 143, 16865–16873. doi:10.1021/jacs.1c09626 |
| 48. | Zimmermann, B. M.; Ngoc, T. T.; Tzaras, D.-I.; Kaicharla, T.; Teichert, J. F. J. Am. Chem. Soc. 2021, 143, 16865–16873. doi:10.1021/jacs.1c09626 |
| 51. | Grayson, D. H.; Ishikawa, T.; Kato, M.; Kobayashi, Y.; Leow, D.; Nagasawa, K.; Nájera, C.; Odagi, M.; Rozas, I.; Shaw, J. W.; Takemoto, Y.; Tan, C.-H.; Yus, M. In Guanidines as Reagents and Catalysts; Selig, P., Ed.; Topics in Heterocyclic Chemistry, Vol. 50; Springer International Publishing: Cham, Switzerland, 2017. doi:10.1007/978-3-319-52725-3 |
| 52. | del Amo, V.; Capitão, R. M.; Concellón, C.; von Eßen, C.; Göb, C. R.; González, E. R. P.; Herres-Pawlis, S.; Himmel, H.-J.; Hoffmann, A.; Mannsperger, J.; Metz, A.; Oppel, I. M.; Rösener, T.; Santo, R. D. E.; Stanek, J. In Guanidines as Reagents and Catalysts II; Selig, P., Ed.; Topics in Heterocyclic Chemistry, Vol. 51; Springer International Publishing: Cham, Switzerland, 2017. doi:10.1007/978-3-319-53013-0 |
| 48. | Zimmermann, B. M.; Ngoc, T. T.; Tzaras, D.-I.; Kaicharla, T.; Teichert, J. F. J. Am. Chem. Soc. 2021, 143, 16865–16873. doi:10.1021/jacs.1c09626 |
| 61. | Mahoney, W. S.; Stryker, J. M. J. Am. Chem. Soc. 1989, 111, 8818–8823. doi:10.1021/ja00206a008 |
| 62. | Chen, J.-X.; Daeuble, J. F.; Brestensky, D. M.; Stryker, J. M. Tetrahedron 2000, 56, 2153–2166. doi:10.1016/s0040-4020(99)01098-4 |
| 63. | Chen, J.-X.; Daeuble, J. F.; Stryker, J. M. Tetrahedron 2000, 56, 2789–2798. doi:10.1016/s0040-4020(00)00133-2 |
| 64. | Shimizu, H.; Igarashi, D.; Kuriyama, W.; Yusa, Y.; Sayo, N.; Saito, T. Org. Lett. 2007, 9, 1655–1657. doi:10.1021/ol070289q |
| 65. | Shimizu, H.; Nagasaki, I.; Matsumura, K.; Sayo, N.; Saito, T. Acc. Chem. Res. 2007, 40, 1385–1393. doi:10.1021/ar700101x |
| 66. | Junge, K.; Wendt, B.; Addis, D.; Zhou, S.; Das, S.; Fleischer, S.; Beller, M. Chem. – Eur. J. 2011, 17, 101–105. doi:10.1002/chem.201002311 |
| 67. | Trose, M.; Lazreg, F.; Chang, T.; Nahra, F.; Cordes, D. B.; Slawin, A. M. Z.; Cazin, C. S. J. ACS Catal. 2017, 7, 238–242. doi:10.1021/acscatal.6b02723 |
| 48. | Zimmermann, B. M.; Ngoc, T. T.; Tzaras, D.-I.; Kaicharla, T.; Teichert, J. F. J. Am. Chem. Soc. 2021, 143, 16865–16873. doi:10.1021/jacs.1c09626 |
| 49. | Tai, C.-C.; Yu, M.-S.; Chen, Y.-L.; Chuang, W.-H.; Lin, T.-H.; Yap, G. P. A.; Ong, T.-G. Chem. Commun. 2014, 50, 4344–4346. doi:10.1039/c4cc00550c |
| 68. | Mahrwald, R., Ed. Modern Aldol Reactions; Wiley-VCH: Weinheim, Germany, 2004. doi:10.1002/9783527619566 |
| 69. | Aggarwal, V. K.; Arai, N.; Bergin, E.; Buitrago Sanatnilla, A.; Carreira, E. M. In Science of Synthesis: Stereoselective Synthesis Vol. 2; Molander, G., Ed.; Thieme: Stuttgart, Germany, 2011. |
© 2023 Remy-Speckmann et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.