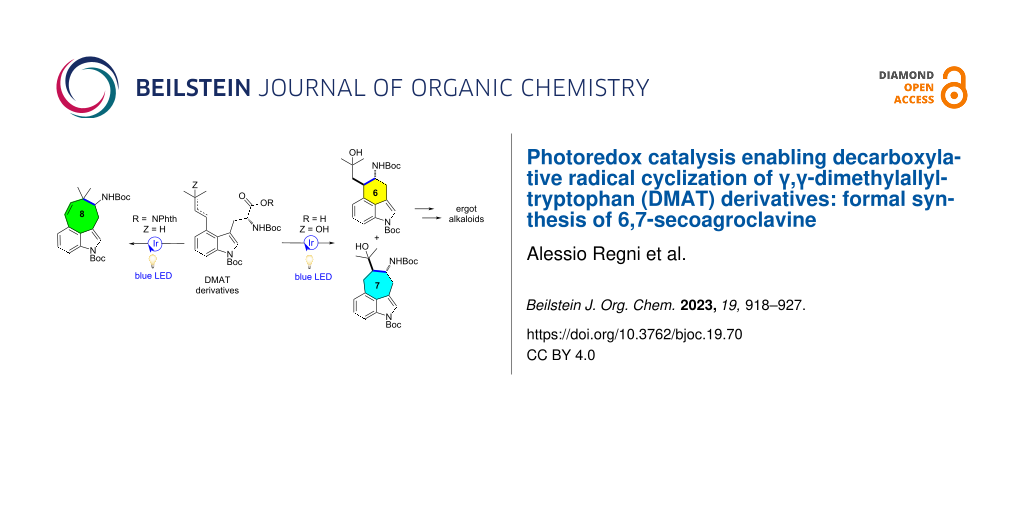
Abstract
An unusual photoredox-catalyzed radical decarboxylative cyclization cascade reaction of γ,γ-dimethylallyltryptophan (DMAT) derivatives containing unactivated alkene moieties has been developed, providing green and efficient access to various six-, seven-, and eight-membered ring 3,4-fused tricyclic indoles. This type of cyclization, which was hitherto very difficult to comprehend in ergot biosynthesis and to accomplish by more conventional procedures, enables the synthesis of ergot alkaloid precursors. In addition, this work describes a mild, environmentally friendly method to activate, reductively and oxidatively, natural carboxylic acids for decarboxylative C–C bond formation by exploiting the same photocatalyst.
Graphical Abstract
Introduction
Visible-light photoredox catalysis is rapidly changing the way organic chemists approach the design and synthesis of molecules by offering new synthetic disconnection opportunities that are usually more convergent, enabling a more diverse chemical space in a rapid fashion [1-15]. Currently, increasing numbers of synthetic chemists are developing photocatalytic processes to make molecules efficiently and in an environmentally friendly manner due to their intrinsic mildness and broad substrate compatibility [16-20]. This transformative synthetic tool often utilizes direct single-electron transfer (SET) between an electronically excited photoredox catalyst and an organic substrate, resulting in oxidation or reduction, to easily generate reactive open-shell radical species and/or intermediates. The substrate is consequently activated for bond cleavage, atom abstraction, or nucleophilic or electrophilic attack. After quenching, the oxidized or reduced photocatalyst regains or loses an electron to return to the starting ground state catalyst [21-26].
While early research has focused on methods for the functionalization of relatively simple hydrocarbons [27-30], developments in photoredox catalysis have gained traction recently as a viable strategy for the total synthesis of natural products [31-33], modification of biomacromolecules [34], and relatively complex pharmaceutical agents [35-38]. Photocatalysis tremendously enriches the synthetic compound library, providing a precious alternative to directly modify abundant natural substrates, including biomass, which usually requires a multistep process in conventional chemical synthesis [39-41]. Among the various widely available and abundant substrates for photocatalyzed reactions, natural and unnatural α-amino acids play a very important role, given their paramount importance across several chemistry fields as well as their ability to participate in either redox step of the catalytic cycle [42-45]. For example, the main use of α-amino acids in syntheses via photoredox catalysis is as readily available precursors of regioselective α-amino radicals by decarboxylative transformations, by oxidation of the carboxylate anion and/or reduction of the corresponding N-hydroxyphthalimide- (NHPI)-derived redox-active ester, although it destroys their stereochemical information [46-51]. In addition, the side-chains of aromatic amino acids (mainly electron-rich tryptophan and tyrosine) can be selectively targeted by photoredox catalysis to enable unprecedented modification of the amino acid. In this context, it is worth mentioning that the single-electron oxidation of the indole moiety in tryptophan provides the radical cation, which enables selective C-radical generation at the weaker benzylic position via a sequential electron transfer–proton transfer (ET/PT) [52-59].
With our ongoing interest of establishing new methods for the asymmetric synthesis of nonproteinogenic tryptophan derivatives as well as their associated indole alkaloid natural products [60-67], we became fascinated in exploring whether photoredox catalysis could be applied for the activation of such unnatural amino acids to expedite the development of completely new synthetic pathways. In particular, 4-dimethylallyltryptophan (DMAT) is of interest for the following reasons: 1) it functions as the central intermediate in the biosynthetic pathways leading to numerous prenylated indole alkaloids, such as ergot alkaloids in normal biosynthesis and clavicipitic acid in derailment biosynthesis [68-71]; and 2) the mechanism of the fundamental central C-ring formation of all ergot alkaloids, specifically the decarboxylative cyclization of DMAT, is still a puzzle even though a radical mechanism has been proposed (Figure 1a) [72,73].
Figure 1: (a) Transformations of DMAT to different classes of ergot alkaloids. (b) and (c) Strategies for the photoredox-catalyzed radical decarboxylative cyclization cascade reaction of DMAT derivatives (this work).
Figure 1: (a) Transformations of DMAT to different classes of ergot alkaloids. (b) and (c) Strategies for the...
Results and Discussion
Herein, we propose that visible light irradiation of the cationic iridium photocatalyst Ir[dF(CF3)ppy]2(dtbbpy)PF6 (E1/2*III/II = +1.21 V, E1/2III/II = −1.37; E1/2IV/*III = −0.89, E1/2 IV/III = +1.69 V) [74] would permit efficient radical generation and C(sp3)–C(sp3) bond formation either by challenging selective radical–radical cross-coupling or by radical addition to a π-bond, enabling a rare example of intramolecular decarboxylative cyclization with the formation of the 3,4-fused indole carbocycle rings (Figure 1b,c). In detail, the photocatalytic strategy for accessing the two C(sp3) radicals of DMAT derivatives envision the formation of a relatively stabilized allylic-benzylic carbon-centered radical by proton transfer from the oxidized indole radical cation [75], generated by SET from the activated photocatalyst. The α-amino radical generated by reductive decarboxylation of a DMAT derivative with a redox-active ester (−1.26 V to −1.37 V vs a saturated calomel electrode) would enable turnover of the photoredox cycle (Figure 1b). Alternatively, we envisioned a more established approach expecting the direct oxidative photoredox decarboxylation of the carboxylic acid/carboxylate (by SET from the activated photocatalyst) of DMAT to generate the α-aminoalkyl radical that might readily be captured/trapped intramolecularly with the C4-pendant prenyl side-chain previously oxidized [76]. Closure of the photoredox catalytic cycle would then involve SET reduction, and protonation would deliver the desired carbocyclic ring (Figure 1c). If this cyclization reaction could be realized in either way, it would shorten the synthetic route of ergot alkaloids and may offer new opportunities for drug discovery and biochemistry applications.
As natural and unnatural tryptophan-derived redox-active N-hydroxyphthalimide esters have already been used in photoredox catalysis, we decided to use them as the initial substrates [77-85]. To test this concept, we turned our attention to the synthesis of key intermediate 5 (Scheme 1). The synthesis began with protection of the indole nitrogen of the known compound 1, which is readily available from commercially available 4-bromoindole in one step [62]. Regioselective palladium-catalyzed prenylation of 2 with prenylboronic acid pinacol ester and subsequent hydrolysis with LiOH provided the linear prenylated acid 4 in good yield. Coupling acid 4 with N-hydroxyphthalimide using DCC and a catalytic amount of DMAP afforded the key intermediate 5 in 59% yield.
With compound 5 in hand, the required radical–radical coupling was investigated next, and some of the representative results are shown in Table S1 (see Supporting Information File 1). Irradiation from blue light-emitting diodes (LEDs) in the presence of 2 mol % of the photocatalyst [Ir(dF(CF3)ppy)2(dtbpy)]PF6 in CH2Cl2 at room temperature using our integrated photoreactor enabled efficient cyclization to give a decarboxylated compound with the correct mass (m/z 426.2) after 16 h. While we were delighted to find that the proposed radical–radical coupling in the synthesis of extracyclic fused indoles was feasible under these conditions, the observed reaction efficiency was poor (14–33% yield). However, on the 1H NMR spectrum, some unexpected signals were observed. The appearance of equilibrating species such as rotamers in the 1H NMR spectrum (see the variable-temperature NMR experiments in Supporting Information File 1, Figure S1) due to the protecting groups complicates the analysis of the reaction products. However, the olefinic signals were a pair of two doublets representing two vicinal vinylic protons [6.48 (d, J = 8.0 Hz, 1H), 6.29 (d, J = 8.0 Hz, 1H), 5.31 (d, J = 8.0 Hz, 1H), and 5.28 (d, J = 8.0 Hz, 1H)], strongly indicating that this product is not the desired structure 6’ but the eight-membered cycloalkene structure 6, shown in Scheme 2. Based on these results and previous reports on the benzylic and allylic C–H bond functionalization enabled by metallaphotoredox catalysis [86], we propose a tentative mechanism (Figure 2).
Scheme 2: Photoredox-catalyzed radical decarboxylative cyclization of 5.
Scheme 2: Photoredox-catalyzed radical decarboxylative cyclization of 5.
Figure 2: Proposed reaction mechanism for photoredox-catalyzed radical decarboxylative cyclization.
Figure 2: Proposed reaction mechanism for photoredox-catalyzed radical decarboxylative cyclization.
First, the radical cation I was generated via the oxidation of indole 5 by the excited Ir-based photocatalyst, followed by sequential regioselective proton transfer on the benzylic dimethylallyl unit C–H bond of the C4 side-chain, thereby generating II. Here, the radical is stabilized by both the indole ring and the Δ2-olefin. Next, the resonance-stabilized radical intermediate III was trapped by the active α-aminoalkyl radical, generated by reductive decarboxylation by Ir(II) produced in the photocatalytic cycle (which undergoes oxidation to afford the Ir(III) complex and to close the cycle), thus furnishing compound 6 comprised of an eight-membered ring. The related stabilization effect of the conjugated product 6 might be the thermodynamic driving force for this radical coupling. An alternative route (not showen) would be that, the α-amidoalkyl radical generated by reductive decarboxylation, could add in an 8-endo-trig manner (common in radical chemistry) to the alkene and the resulting radical could be oxidized to the cation by the oxidized form of the photocatalyst to close the photocatalytic cycle, followed by formation of the double bond. Even though no desired cyclized product was observed, an interesting aspect of this reaction was the access of an attractive, unusual, and highly functionalized 3,4-fused eight-membered tricyclic indole, whose ring closure would not have been possible or at least very difficult in the ground state [87-89].
Although not yet completely clarified, some previous studies on the detailed mode of closure of the C ring in ergot alkaloids from DMAT have been shown to involve, before decarboxylative cyclization, oxidation on the C4-prenyl chain to give the stable rearranged allyl alcohol and/or the relatively unstable diene [90,91]. In addition, these results support the hypothesis that the decarboxylative cyclization can occur through subsequent selective 6-exo-trig radical addition. It also has been reported that it is difficult to detect which intermediate is really involved, since they are easily interconvertible to each other by hydration or dehydration, i.e., a plausible precursor of the allylic alcohol would be the diene, and vice versa [90]. Since both 8 and 10 are easily obtainable from 2 by Mozoroki–Heck coupling with commercially available 2-methyl-3-buten-2-ol, ester hydrolysis (LiOH in THF/H2O), and, finally for 10, dehydration of the tertiary alcohol (mesylation and elimination) (Scheme 3), we decided to test their roles in the photoredox-catalyzed decarboxylative cyclization. With 8 and 10 in hand with the C4-prenyl side-chain already oxidized/functionalized, we recognized that this cyclization event would be triggered using their innate functionality, namely the α-amino carboxylate, through photoredox-mediated oxidative activation and CO2 extrusion, without the need for acid prefunctionalization to the redox-activated ester. Consequently, a technique involving direct generation of α-aminoalkyl radicals from free carboxylic acids of 8 and 10 under mild conditions would make the approach even more efficient and more biosimilar; nevertheless, issues regarding the regioselectivity of the ring formation could be raised, since both the 6-exo-trig and 7-endo-trig cyclization are both favorable, according to the Baldwin rules [92].
Scheme 3: Synthesis of tryptophan derivatives 8 and 10.
Scheme 3: Synthesis of tryptophan derivatives 8 and 10.
We began our investigation of the proposed decarboxylative cyclization by exposing the N-Boc derivative 8, Ir catalyst, and K2HPO4 in DMF to a 34 W blue LED lamp at room temperature (Table 1) [93-97]. To our delight, cyclization was observed under these preliminary conditions, albeit in low yield (35% yield) and low regioselectivity (1:1) (Table 1, entry 1). No regiocontrol was observed; but remarkably, the regioisomers exhibited distinct retention factors on silica gel, allowing 11 and 12 to be isolated separately in good yield as single trans diastereomers [98]. Reducing the substrate concentration increased efficiency while assisting in avoiding the oligomerization pathways (Table 1, entries 2 and 3). Higher photocatalyst loadings resulted in an increased yield (Table 1, entry 4). Control experiments showed that both the photocatalyst and light were essential for product production (Table 1, entries 6 and 7), despite the fact that the removal of base did not result in a significantly reduced efficiency (Table 1, entry 5). The regioselectivity outcome was explained by the relative stability of the intermediate radicals involved, with strong evidence of the importance of steric effects [99]. Indeed, while the addition of the nucleophilic α-amino radical to the α-styrenyl position affords the 6-membered ring (kinetic product via intramolecular 6-exo-trig ring closure) [100] the resulting radical is unstabilized, the 7-membered ring (obtained via intramolecular 7-endo-trig ring closure) may well be the thermodynamic product based on the more stabilized benzylic radical that is produced [101].
Table 1: Photoredox-catalyzed radical decarboxylative cyclization of 8.a
|
|
|||||
| Entry | Conditions | Concentration of 8 | Ir catalyst (mol %) | Ratio of 11/12b | Yield of 11 and 12c |
| 1 | as shown | 10 mM | 2 | 1:1 | 35% |
| 2 | as shown | 5 mM | 2 | 1:0.7 | 39% |
| 3 | as shown | 2.5 mM | 2 | 1:0.6 | 42% |
| 4 | as shown | 2.5 mM | 4 | 1:0.7 | 59% |
| 5 | no base | 2.5 mM | 4 | 1:0.7 | 53% |
| 6 | no photocatalyst | 2.5 mM | – | – | N.D.d |
| 7 | no light | 2.5 mM | 4 | – | N.D.d |
| 8 | DMSO instead of DMF | 2.5 mM | 4 | 0.7:1 | 33% |
| 9 | DCE instead of DMF | 2.5 mM | 4 | 1:0.7 | 40% |
aReaction conditions: 8 (0.1 mmol), K2HPO4 (0.12 mmol), catalyst (x mol %), solvent (4 mL), irradiation with 34 W blue LEDs for 60 h. bRatio of 11/12 was determined by 1H NMR analysis. cIsolated yields. dN.D., not detected.
As largely reported in the literature [102,103], radicals generated next to alcohols do not normally undergo β-elimination to give alkene/carbon–carbon double-bond formation and a hydroxyl radical (•OH). However, it is possible to transform an alcohol into a leaving group, in the radical sense, by converting it into a halide or pseudohalide derivative [104,105]. For alcohol 8, all attempts to make a better leaving group, including phenyl sulfone derivative, to have radical addition–fragmentation on the latter and most likely to shift the regioselectivity towards 6-exo-trig by a favorable interplay of polar effects [99] failed and furnished only the 1,3-diene 10. Unfortunately, when substrate 10 was subjected to the reaction conditions shown above, only tarry compounds were obtained; this result was probably due to the competitive cycloaddition and polymerization reactions and decomposition of the diene moiety, which is unstable and very sensitive to acidic and basic conditions [106].
As shown in Figure 3 and anticipated above, our proposed mechanism begins with visible-light irradiation of the photoredox catalyst [Ir(dF(CF3)ppy)2(dtbpy)]PF6 to access the excited state *[Ir(dF(CF3)ppy)2(dtbpy)]PF6, which can trigger SET oxidation of 8. Rapid decarboxylation leads to α-amino radical V (and the reduced photocatalyst), which is intercepted by the pendant double bond to forge the desired six-membered ring through a key C–C bond formation while furnishing secondary radical VI and the undesired seven-membered-ring compound VII. Closure of the photoredox catalytic cycle would then involve either SET reduction of the radical VI and VII (which upon protonation would deliver the desired product 11 and the undesired product 12), or an hydrogen-atom-transfer (HAT) process (which would not place a formal negative charge onto the molecule), where the hydrogen atom required for this possible final HAT step originates from the solvent (DMF) itself [107]. Therefore, we tested the reaction in N,N-dimethylformamide-d7 (DMF-d7), which showed almost quantitative deuterium incorporation. While this result was surprising, further studies into this complex mechanism are ongoing and will be reported in due course.
Figure 3: Proposed reaction mechanism for photoredox-catalyzed radical decarboxylative cyclization.
Figure 3: Proposed reaction mechanism for photoredox-catalyzed radical decarboxylative cyclization.
The synthetic potential and utility of this method was further demonstrated by the formal total synthesis of (±)-6,7-secoagroclavine (Scheme 4) [108-114]. Towards this end, compound 11 was methylated efficiently and selectively at the secondary amide by treatment with methyl iodide in DMF to afford compound 13. In additional two steps, intermediate 13 was transformed to (±)-6,7-secoagroclavine in enantiopure form, as reported previously by the Bisai group in 2018 [115]. All the spectroscopic data of 13 were in agreement with those reported in the literature, confirming that the radical addition reaction provided the trans amino group due to steric hindrance.
Scheme 4: Methylation of 11 and the formal total synthesis of (±)-6,7-secoagroclavine.
Scheme 4: Methylation of 11 and the formal total synthesis of (±)-6,7-secoagroclavine.
Conclusion
In summary, this work illustrates, once more, the synthetic potential of an Ir-polypyridyl complex as a photoredox catalyst that can efficiently convert visible light into chemical energy. In addition, this catalyst was applied to demonstrate the proposed radical mechanism involved in the biosynthetic formation of the central C ring of several DMAT derivatives. The results presented here lend strong credence to decarboxylation and cyclization to form the six-membered ring as well as the nature of the stable oxidized intermediates concerned. Moreover, unprecedented and functionalized 3,4-fused tricyclic indoles with medium-sized rings (seven and eight), which have been largely neglected in previous studies, can be synthesized by this new protocol. Notably, the reaction has been successfully applied in the formal synthesis of (±)-6,7-secoagroclavine, a key intermediate for a common synthetic route to ergot alkaloids, providing an advantageous synthetic method for this class of natural products. Further studies on the utility of the decarboxylative radical cyclization and their applications are currently being investigated in our laboratory.
Supporting Information
| Supporting Information File 1: Experimental and copies of spectra. | ||
| Format: PDF | Size: 2.3 MB | Download |
References
-
Nicewicz, D. A.; MacMillan, D. W. C. Science 2008, 322, 77–80. doi:10.1126/science.1161976
Return to citation in text: [1] -
Ischay, M. A.; Anzovino, M. E.; Du, J.; Yoon, T. P. J. Am. Chem. Soc. 2008, 130, 12886–12887. doi:10.1021/ja805387f
Return to citation in text: [1] -
Narayanam, J. M. R.; Tucker, J. W.; Stephenson, C. R. J. J. Am. Chem. Soc. 2009, 131, 8756–8757. doi:10.1021/ja9033582
Return to citation in text: [1] -
Narayanam, J. M. R.; Stephenson, C. R. J. Chem. Soc. Rev. 2011, 40, 102–113. doi:10.1039/b913880n
Return to citation in text: [1] -
Xuan, J.; Xiao, W.-J. Angew. Chem., Int. Ed. 2012, 51, 6828–6838. doi:10.1002/anie.201200223
Return to citation in text: [1] -
Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322–5363. doi:10.1021/cr300503r
Return to citation in text: [1] -
Schultz, D. M.; Yoon, T. P. Science 2014, 343, 1239176. doi:10.1126/science.1239176
Return to citation in text: [1] -
Ravelli, D.; Protti, S.; Fagnoni, M. Chem. Rev. 2016, 116, 9850–9913. doi:10.1021/acs.chemrev.5b00662
Return to citation in text: [1] -
Shaw, M. H.; Twilton, J.; MacMillan, D. W. C. J. Org. Chem. 2016, 81, 6898–6926. doi:10.1021/acs.joc.6b01449
Return to citation in text: [1] -
Tellis, J. C.; Kelly, C. B.; Primer, D. N.; Jouffroy, M.; Patel, N. R.; Molander, G. A. Acc. Chem. Res. 2016, 49, 1429–1439. doi:10.1021/acs.accounts.6b00214
Return to citation in text: [1] -
Marzo, L.; Pagire, S. K.; Reiser, O.; König, B. Angew. Chem., Int. Ed. 2018, 57, 10034–10072. doi:10.1002/anie.201709766
Return to citation in text: [1] -
McAtee, R. C.; McClain, E. J.; Stephenson, C. R. J. Trends Chem. 2019, 1, 111–125. doi:10.1016/j.trechm.2019.01.008
Return to citation in text: [1] -
Zhu, C.; Yue, H.; Chu, L.; Rueping, M. Chem. Sci. 2020, 11, 4051–4064. doi:10.1039/d0sc00712a
Return to citation in text: [1] -
Stephenson, C.; Yoon, T.; MacMillan, D. W. C. Visible Light Photocatalysis in Organic Chemistry; Wiley-VCH: Weinheim, Germany, 2018. doi:10.1002/9783527674145
Return to citation in text: [1] -
König, B., Ed. Chemical Photocatalysis, 2nd ed.; De Gruyter: Berlin, Germany, 2020. doi:10.1515/9783110576764
Return to citation in text: [1] -
Yoon, T. P.; Ischay, M. A.; Du, J. Nat. Chem. 2010, 2, 527–532. doi:10.1038/nchem.687
Return to citation in text: [1] -
Oelgemöller, M. Chem. Rev. 2016, 116, 9664–9682. doi:10.1021/acs.chemrev.5b00720
Return to citation in text: [1] -
Cambié, D.; Bottecchia, C.; Straathof, N. J. W.; Hessel, V.; Noël, T. Chem. Rev. 2016, 116, 10276–10341. doi:10.1021/acs.chemrev.5b00707
Return to citation in text: [1] -
Zhou, L.; Lokman Hossain, M.; Xiao, T. Chem. Rec. 2016, 16, 319–334. doi:10.1002/tcr.201500228
Return to citation in text: [1] -
Crisenza, G. E. M.; Melchiorre, P. Nat. Commun. 2020, 11, 803. doi:10.1038/s41467-019-13887-8
Return to citation in text: [1] -
Romero, N. A.; Nicewicz, D. A. Chem. Rev. 2016, 116, 10075–10166. doi:10.1021/acs.chemrev.6b00057
Return to citation in text: [1] -
Capaldo, L.; Ravelli, D.; Fagnoni, M. Chem. Rev. 2022, 122, 1875–1924. doi:10.1021/acs.chemrev.1c00263
Return to citation in text: [1] -
Cao, H.; Tang, X.; Tang, H.; Yuan, Y.; Wu, J. Chem Catal. 2021, 1, 523–598. doi:10.1016/j.checat.2021.04.008
Return to citation in text: [1] -
Bell, J. D.; Murphy, J. A. Chem. Soc. Rev. 2021, 50, 9540–9685. doi:10.1039/d1cs00311a
Return to citation in text: [1] -
Buzzetti, L.; Crisenza, G. E. M.; Melchiorre, P. Angew. Chem., Int. Ed. 2019, 58, 3730–3747. doi:10.1002/anie.201809984
Return to citation in text: [1] -
Arias-Rotondo, D. M.; McCusker, J. K. Chem. Soc. Rev. 2016, 45, 5803–5820. doi:10.1039/c6cs00526h
Return to citation in text: [1] -
Ciamician, G. Science 1912, 36, 385–394. doi:10.1126/science.36.926.385
Return to citation in text: [1] -
Klán, P.; Wirz, J. Photochemistry of Organic Compounds: From Concepts to Practice; John Wiley & Sons: Chichester, UK, 2009. doi:10.1002/9781444300017
Return to citation in text: [1] -
Hu, A.; Guo, J.-J.; Pan, H.; Zuo, Z. Science 2018, 361, 668–672. doi:10.1126/science.aat9750
Return to citation in text: [1] -
Laudadio, G.; Deng, Y.; van der Wal, K.; Ravelli, D.; Nuño, M.; Fagnoni, M.; Guthrie, D.; Sun, Y.; Noël, T. Science 2020, 369, 92–96. doi:10.1126/science.abb4688
Return to citation in text: [1] -
Nicholls, T. P.; Leonori, D.; Bissember, A. C. Nat. Prod. Rep. 2016, 33, 1248–1254. doi:10.1039/c6np00070c
Return to citation in text: [1] -
Liu, X.-Y.; Qin, Y. Acc. Chem. Res. 2019, 52, 1877–1891. doi:10.1021/acs.accounts.9b00246
Return to citation in text: [1] -
Pitre, S. P.; Overman, L. E. Chem. Rev. 2022, 122, 1717–1751. doi:10.1021/acs.chemrev.1c00247
Return to citation in text: [1] -
Lechner, V. M.; Nappi, M.; Deneny, P. J.; Folliet, S.; Chu, J. C. K.; Gaunt, M. J. Chem. Rev. 2022, 122, 1752–1829. doi:10.1021/acs.chemrev.1c00357
Return to citation in text: [1] -
Capaldo, L.; Quadri, L. L.; Ravelli, D. Green Chem. 2020, 22, 3376–3396. doi:10.1039/d0gc01035a
Return to citation in text: [1] -
Douglas, J. J.; Sevrin, M. J.; Stephenson, C. R. J. Org. Process Res. Dev. 2016, 20, 1134–1147. doi:10.1021/acs.oprd.6b00125
Return to citation in text: [1] -
Li, P.; Terrett, J. A.; Zbieg, J. R. ACS Med. Chem. Lett. 2020, 11, 2120–2130. doi:10.1021/acsmedchemlett.0c00436
Return to citation in text: [1] -
Candish, L.; Collins, K. D.; Cook, G. C.; Douglas, J. J.; Gómez-Suárez, A.; Jolit, A.; Keess, S. Chem. Rev. 2022, 122, 2907–2980. doi:10.1021/acs.chemrev.1c00416
Return to citation in text: [1] -
Nguyen, S. T.; Murray, P. R. D.; Knowles, R. R. ACS Catal. 2020, 10, 800–805. doi:10.1021/acscatal.9b04813
Return to citation in text: [1] -
Zhang, J. ChemSusChem 2018, 11, 3071–3080. doi:10.1002/cssc.201801370
Return to citation in text: [1] -
Scott, E.; Peter, F.; Sanders, J. Appl. Microbiol. Biotechnol. 2007, 75, 751–762. doi:10.1007/s00253-007-0932-x
Return to citation in text: [1] -
Liu, J.-Q.; Shatskiy, A.; Matsuura, B. S.; Kärkäs, M. D. Synthesis 2019, 51, 2759–2791. doi:10.1055/s-0037-1611852
Return to citation in text: [1] -
Bottecchia, C.; Noël, T. Chem. – Eur. J. 2019, 25, 26–42. doi:10.1002/chem.201803074
Return to citation in text: [1] -
Aguilar Troyano, F. J.; Merkens, K.; Anwar, K.; Gómez‐Suárez, A. Angew. Chem., Int. Ed. 2021, 60, 1098–1115. doi:10.1002/anie.202010157
Return to citation in text: [1] -
King, T. A.; Mandrup Kandemir, J.; Walsh, S. J.; Spring, D. R. Chem. Soc. Rev. 2021, 50, 39–57. doi:10.1039/d0cs00344a
Return to citation in text: [1] -
Zuo, Z.; MacMillan, D. W. C. J. Am. Chem. Soc. 2014, 136, 5257–5260. doi:10.1021/ja501621q
Return to citation in text: [1] -
Schwarz, J.; König, B. Green Chem. 2016, 18, 4743–4749. doi:10.1039/c6gc01101b
Return to citation in text: [1] -
Liao, L.-L.; Cao, G.-M.; Jiang, Y.-X.; Jin, X.-H.; Hu, X.-L.; Chruma, J. J.; Sun, G.-Q.; Gui, Y.-Y.; Yu, D.-G. J. Am. Chem. Soc. 2021, 143, 2812–2821. doi:10.1021/jacs.0c11896
Return to citation in text: [1] -
Li, Y.; Dai, C.; Xie, S.; Liu, P.; Sun, P. Org. Lett. 2021, 23, 5906–5910. doi:10.1021/acs.orglett.1c02014
Return to citation in text: [1] -
Xuan, J.; Zhang, Z.-G.; Xiao, W.-J. Angew. Chem., Int. Ed. 2015, 54, 15632–15641. doi:10.1002/anie.201505731
Return to citation in text: [1] -
Rahman, M.; Mukherjee, A.; Kovalev, I. S.; Kopchuk, D. S.; Zyryanov, G. V.; Tsurkan, M. V.; Majee, A.; Ranu, B. C.; Charushin, V. N.; Chupakhin, O. N.; Santra, S. Adv. Synth. Catal. 2019, 361, 2161–2214. doi:10.1002/adsc.201801331
Return to citation in text: [1] -
Schmittel, M.; Burghart, A. Angew. Chem., Int. Ed. Engl. 1997, 36, 2550–2589. doi:10.1002/anie.199725501
Return to citation in text: [1] -
Roth, H. G.; Romero, N. A.; Nicewicz, D. A. Synlett 2016, 27, 714–723. doi:10.1055/s-0035-1561297
Return to citation in text: [1] -
Yu, Y.; Zhang, L.-K.; Buevich, A. V.; Li, G.; Tang, H.; Vachal, P.; Colletti, S. L.; Shi, Z.-C. J. Am. Chem. Soc. 2018, 140, 6797–6800. doi:10.1021/jacs.8b03973
Return to citation in text: [1] -
Laroche, B.; Tang, X.; Archer, G.; Di Sanza, R.; Melchiorre, P. Org. Lett. 2021, 23, 285–289. doi:10.1021/acs.orglett.0c03735
Return to citation in text: [1] -
Rahimidashaghoul, K.; Klimánková, I.; Hubálek, M.; Matoušek, V.; Filgas, J.; Slavíček, P.; Slanina, T.; Beier, P. ChemPhotoChem 2021, 5, 43–50. doi:10.1002/cptc.202000214
Return to citation in text: [1] -
Lima, R. N.; Delgado, J. A. C.; Bernardi, D. I.; Berlinck, R. G. S.; Kaplaneris, N.; Ackermann, L.; Paixão, M. W. Chem. Commun. 2021, 57, 5758–5761. doi:10.1039/d1cc01822a
Return to citation in text: [1] -
Weng, Y.; Ding, B.; Liu, Y.; Song, C.; Chan, L.-Y.; Chiang, C.-W. Org. Lett. 2021, 23, 2710–2714. doi:10.1021/acs.orglett.1c00609
Return to citation in text: [1] -
Hoopes, C. R.; Garcia, F. J.; Sarkar, A. M.; Kuehl, N. J.; Barkan, D. T.; Collins, N. L.; Meister, G. E.; Bramhall, T. R.; Hsu, C.-H.; Jones, M. D.; Schirle, M.; Taylor, M. T. J. Am. Chem. Soc. 2022, 144, 6227–6236. doi:10.1021/jacs.1c10536
Return to citation in text: [1] -
Bartoccini, F.; Regni, A.; Retini, M.; Piersanti, G. Eur. J. Org. Chem. 2022, e202200315. doi:10.1002/ejoc.202200315
Return to citation in text: [1] -
Bartoccini, F.; Regni, A.; Retini, M.; Piersanti, G. Org. Biomol. Chem. 2021, 19, 2932–2940. doi:10.1039/d1ob00353d
Return to citation in text: [1] -
Bartoccini, F.; Fanini, F.; Retini, M.; Piersanti, G. Tetrahedron Lett. 2020, 61, 151923. doi:10.1016/j.tetlet.2020.151923
Return to citation in text: [1] [2] -
Bartoccini, F.; Venturi, S.; Retini, M.; Mari, M.; Piersanti, G. J. Org. Chem. 2019, 84, 8027–8034. doi:10.1021/acs.joc.9b00879
Return to citation in text: [1] -
Bartoccini, F.; Bartolucci, S.; Mari, M.; Piersanti, G. Org. Biomol. Chem. 2016, 14, 10095–10100. doi:10.1039/c6ob01791f
Return to citation in text: [1] -
Bartoccini, F.; Casoli, M.; Mari, M.; Piersanti, G. J. Org. Chem. 2014, 79, 3255–3259. doi:10.1021/jo500245s
Return to citation in text: [1] -
Lucarini, S.; Bartoccini, F.; Battistoni, F.; Diamantini, G.; Piersanti, G.; Righi, M.; Spadoni, G. Org. Lett. 2010, 12, 3844–3847. doi:10.1021/ol101527j
Return to citation in text: [1] -
Bartoccini, F.; Piersanti, G. Synthesis 2021, 53, 1396–1408. doi:10.1055/a-1340-3423
Return to citation in text: [1] -
Tasker, N. R.; Wipf, P. Biosynthesis, Total Synthesis, and Biological Profiles of Ergot Alkaloids. The Alkaloids: Chemistry and Biology; Academic Press: Cambridge, MA, USA, 2021; Vol. 85, pp 1–112. doi:10.1016/bs.alkal.2020.08.001
Return to citation in text: [1] -
Liu, H.; Jia, Y. Nat. Prod. Rep. 2017, 34, 411–432. doi:10.1039/c6np00110f
Return to citation in text: [1] -
McCabe, S. R.; Wipf, P. Org. Biomol. Chem. 2016, 14, 5894–5913. doi:10.1039/c6ob00878j
Return to citation in text: [1] -
Jakubczyk, D.; Cheng, J. Z.; O'Connor, S. E. Nat. Prod. Rep. 2014, 31, 1328–1338. doi:10.1039/c4np00062e
Return to citation in text: [1] -
Yao, Y.; An, C.; Evans, D.; Liu, W.; Wang, W.; Wei, G.; Ding, N.; Houk, K. N.; Gao, S.-S. J. Am. Chem. Soc. 2019, 141, 17517–17521. doi:10.1021/jacs.9b10217
Return to citation in text: [1] -
Ma, Y.; Yan, J.; Yang, L.; Yao, Y.; Wang, L.; Gao, S.-S.; Cui, C. Front. Bioeng. Biotechnol. 2022, 10, 1095464. doi:10.3389/fbioe.2022.1095464
And references cited therein.
Return to citation in text: [1] -
Lowry, M. S.; Goldsmith, J. I.; Slinker, J. D.; Rohl, R.; Pascal, R. A.; Malliaras, G. G.; Bernhard, S. Chem. Mater. 2005, 17, 5712–5719. doi:10.1021/cm051312+
Return to citation in text: [1] -
Walden, S. E.; Wheeler, R. A. J. Phys. Chem. 1996, 100, 1530–1535. doi:10.1021/jp951838p
Return to citation in text: [1] -
Ge, Y.; Wang, H.; Wang, H.-N.; Yu, S.-S.; Yang, R.; Chen, X.; Zhao, Q.; Chen, G. Org. Lett. 2021, 23, 370–375. doi:10.1021/acs.orglett.0c03867
Return to citation in text: [1] -
Yang, J.; Zhang, J.; Qi, L.; Hu, C.; Chen, Y. Chem. Commun. 2015, 51, 5275–5278. doi:10.1039/c4cc06344a
Return to citation in text: [1] -
Jin, Y.; Jiang, M.; Wang, H.; Fu, H. Sci. Rep. 2016, 6, 20068. doi:10.1038/srep20068
Return to citation in text: [1] -
Cheng, W.-M.; Shang, R.; Fu, Y. ACS Catal. 2017, 7, 907–911. doi:10.1021/acscatal.6b03215
Return to citation in text: [1] -
Cheng, W.-M.; Shang, R.; Fu, M.-C.; Fu, Y. Chem. – Eur. J. 2017, 23, 2537–2541. doi:10.1002/chem.201605640
Return to citation in text: [1] -
Liu, X.; Liu, Y.; Chai, G.; Qiao, B.; Zhao, X.; Jiang, Z. Org. Lett. 2018, 20, 6298–6301. doi:10.1021/acs.orglett.8b02791
Return to citation in text: [1] -
Proctor, R. S. J.; Davis, H. J.; Phipps, R. J. Science 2018, 360, 419–422. doi:10.1126/science.aar6376
Return to citation in text: [1] -
Xiao, Z.; Wang, L.; Wei, J.; Ran, C.; Liang, S. H.; Shang, J.; Chen, G.-Y.; Zheng, C. Chem. Commun. 2020, 56, 4164–4167. doi:10.1039/d0cc00451k
Return to citation in text: [1] -
He, S.; Li, H.; Chen, X.; Krylov, I. B.; Terent'ev, A. O.; Qu, L.; Yu, B. Chin. J. Org. Chem. 2021, 41, 4661–4689. doi:10.6023/cjoc202105041
Return to citation in text: [1] -
Dong, W.; Yen-Pon, E.; Li, L.; Bhattacharjee, A.; Jolit, A.; Molander, G. A. Nat. Chem. 2022, 14, 1068–1077. doi:10.1038/s41557-022-00979-0
Return to citation in text: [1] -
Yue, H.; Zhu, C.; Huang, L.; Dewanji, A.; Rueping, M. Chem. Commun. 2022, 58, 171–184. doi:10.1039/d1cc06285a
Return to citation in text: [1] -
Nemoto, T.; Harada, S.; Nakajima, M. Asian J. Org. Chem. 2018, 7, 1730–1742. doi:10.1002/ajoc.201800336
Return to citation in text: [1] -
Yuan, K.; Jia, Y. Chin. J. Org. Chem. 2018, 38, 2386–2399. doi:10.6023/cjoc201705058
Return to citation in text: [1] -
Connon, R.; Guiry, P. J. Tetrahedron Lett. 2020, 61, 151696. doi:10.1016/j.tetlet.2020.151696
Return to citation in text: [1] -
Kozikowski, A. P.; Chen, C.; Wu, J. P.; Shibuya, M.; Kim, C. G.; Floss, H. G. J. Am. Chem. Soc. 1993, 115, 2482–2488. doi:10.1021/ja00059a051
Return to citation in text: [1] [2] -
Wong, G.; Lim, L. R.; Tan, Y. Q.; Go, M. K.; Bell, D. J.; Freemont, P. S.; Yew, W. S. Nat. Commun. 2022, 13, 712. doi:10.1038/s41467-022-28386-6
Return to citation in text: [1] -
Jasperse, C. P.; Curran, D. P.; Fevig, T. L. Chem. Rev. 1991, 91, 1237–1286. doi:10.1021/cr00006a006
Return to citation in text: [1] -
Chu, L.; Ohta, C.; Zuo, Z.; MacMillan, D. W. C. J. Am. Chem. Soc. 2014, 136, 10886–10889. doi:10.1021/ja505964r
Return to citation in text: [1] -
Noble, A.; McCarver, S. J.; MacMillan, D. W. C. J. Am. Chem. Soc. 2015, 137, 624–627. doi:10.1021/ja511913h
Return to citation in text: [1] -
Xiao, T.; Li, L.; Zhou, L. J. Org. Chem. 2016, 81, 7908–7916. doi:10.1021/acs.joc.6b01620
Return to citation in text: [1] -
Ernouf, G.; Chirkin, E.; Rhyman, L.; Ramasami, P.; Cintrat, J.-C. Angew. Chem., Int. Ed. 2020, 59, 2618–2622. doi:10.1002/anie.201908951
Return to citation in text: [1] -
Li, J.-T.; Luo, J.-N.; Wang, J.-L.; Wang, D.-K.; Yu, Y.-Z.; Zhuo, C.-X. Nat. Commun. 2022, 13, 1778. doi:10.1038/s41467-022-29464-5
Return to citation in text: [1] -
It should be mentioned that racemization was found in products 11 and 12 from the chiral intermediate V, which is common in this radical coupling reaction. See reference [76].
Return to citation in text: [1] -
McCarver, S. J.; Qiao, J. X.; Carpenter, J.; Borzilleri, R. M.; Poss, M. A.; Eastgate, M. D.; Miller, M. M.; MacMillan, D. W. C. Angew. Chem., Int. Ed. 2017, 56, 728–732. doi:10.1002/anie.201608207
Return to citation in text: [1] [2] -
Blackwell, J. H.; Harris, G. R.; Smith, M. A.; Gaunt, M. J. J. Am. Chem. Soc. 2021, 143, 15946–15959. doi:10.1021/jacs.1c07402
Return to citation in text: [1] -
Lovett, G. H.; Sparling, B. A. Org. Lett. 2016, 18, 3494–3497. doi:10.1021/acs.orglett.6b01712
Return to citation in text: [1] -
Renaud, P.; Sibi, M. P., Eds. Radicals in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2001; Vol. 1 and 2. doi:10.1002/9783527618293
Return to citation in text: [1] -
Zard, S. Z. Radicals Reactions in Organic Synthesis; Oxford University Press: Oxford, UK, 2003.
Return to citation in text: [1] -
Beckwith, A. L. J.; Crich, D.; Duggan, P. J.; Yao, Q. Chem. Rev. 1997, 97, 3273–3312. doi:10.1021/cr950207o
Return to citation in text: [1] -
Crich, D.; Brebion, F.; Suk, D.-H. Top. Curr. Chem. 2006, 263, 1–38. doi:10.1007/128_024
Return to citation in text: [1] -
Hurtley, A. E.; Lu, Z.; Yoon, T. P. Angew. Chem., Int. Ed. 2014, 53, 8991–8994. doi:10.1002/anie.201405359
Return to citation in text: [1] -
Capaldo, L.; Ravelli, D. Eur. J. Org. Chem. 2017, 2056–2071. doi:10.1002/ejoc.201601485
Return to citation in text: [1] -
Yamada, F.; Makita, Y.; Somei, M. Heterocycles 2007, 72, 599–620. doi:10.3987/com-06-s(k)55
Return to citation in text: [1] -
Somei, M.; Yamada, F.; Ohnishi, H.; Makita, Y.; Kuriki, M. Heterocycles 1987, 26, 2823–2828. doi:10.3987/r-1987-11-2823
Return to citation in text: [1] -
Somei, M.; Ohnishi, H.; Shoken, Y. Chem. Pharm. Bull. 1986, 34, 677–681. doi:10.1248/cpb.34.677
Return to citation in text: [1] -
Oppolzer, W.; Grayson, J. I.; Wegmann, H.; Urrea, M. Tetrahedron 1983, 39, 3695–3705. doi:10.1016/s0040-4020(01)88608-7
Return to citation in text: [1] -
Somei, M.; Tsuchiya, M. Chem. Pharm. Bull. 1981, 29, 3145–3157. doi:10.1248/cpb.29.3145
Return to citation in text: [1] -
Somei, M.; Yamada, F.; Karasawa, Y.; Kaneko, C. Chem. Lett. 1981, 10, 615–618. doi:10.1246/cl.1981.615
Return to citation in text: [1] -
Natsume, M.; Muratake, H. Heterocycles 1980, 14, 1101–1105. doi:10.3987/r-1980-08-1101
Return to citation in text: [1] -
Chaudhuri, S.; Bhunia, S.; Roy, A.; Das, M. K.; Bisai, A. Org. Lett. 2018, 20, 288–291. doi:10.1021/acs.orglett.7b03683
Return to citation in text: [1]
| 100. | Blackwell, J. H.; Harris, G. R.; Smith, M. A.; Gaunt, M. J. J. Am. Chem. Soc. 2021, 143, 15946–15959. doi:10.1021/jacs.1c07402 |
| 101. | Lovett, G. H.; Sparling, B. A. Org. Lett. 2016, 18, 3494–3497. doi:10.1021/acs.orglett.6b01712 |
| 102. | Renaud, P.; Sibi, M. P., Eds. Radicals in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2001; Vol. 1 and 2. doi:10.1002/9783527618293 |
| 103. | Zard, S. Z. Radicals Reactions in Organic Synthesis; Oxford University Press: Oxford, UK, 2003. |
| 1. | Nicewicz, D. A.; MacMillan, D. W. C. Science 2008, 322, 77–80. doi:10.1126/science.1161976 |
| 2. | Ischay, M. A.; Anzovino, M. E.; Du, J.; Yoon, T. P. J. Am. Chem. Soc. 2008, 130, 12886–12887. doi:10.1021/ja805387f |
| 3. | Narayanam, J. M. R.; Tucker, J. W.; Stephenson, C. R. J. J. Am. Chem. Soc. 2009, 131, 8756–8757. doi:10.1021/ja9033582 |
| 4. | Narayanam, J. M. R.; Stephenson, C. R. J. Chem. Soc. Rev. 2011, 40, 102–113. doi:10.1039/b913880n |
| 5. | Xuan, J.; Xiao, W.-J. Angew. Chem., Int. Ed. 2012, 51, 6828–6838. doi:10.1002/anie.201200223 |
| 6. | Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322–5363. doi:10.1021/cr300503r |
| 7. | Schultz, D. M.; Yoon, T. P. Science 2014, 343, 1239176. doi:10.1126/science.1239176 |
| 8. | Ravelli, D.; Protti, S.; Fagnoni, M. Chem. Rev. 2016, 116, 9850–9913. doi:10.1021/acs.chemrev.5b00662 |
| 9. | Shaw, M. H.; Twilton, J.; MacMillan, D. W. C. J. Org. Chem. 2016, 81, 6898–6926. doi:10.1021/acs.joc.6b01449 |
| 10. | Tellis, J. C.; Kelly, C. B.; Primer, D. N.; Jouffroy, M.; Patel, N. R.; Molander, G. A. Acc. Chem. Res. 2016, 49, 1429–1439. doi:10.1021/acs.accounts.6b00214 |
| 11. | Marzo, L.; Pagire, S. K.; Reiser, O.; König, B. Angew. Chem., Int. Ed. 2018, 57, 10034–10072. doi:10.1002/anie.201709766 |
| 12. | McAtee, R. C.; McClain, E. J.; Stephenson, C. R. J. Trends Chem. 2019, 1, 111–125. doi:10.1016/j.trechm.2019.01.008 |
| 13. | Zhu, C.; Yue, H.; Chu, L.; Rueping, M. Chem. Sci. 2020, 11, 4051–4064. doi:10.1039/d0sc00712a |
| 14. | Stephenson, C.; Yoon, T.; MacMillan, D. W. C. Visible Light Photocatalysis in Organic Chemistry; Wiley-VCH: Weinheim, Germany, 2018. doi:10.1002/9783527674145 |
| 15. | König, B., Ed. Chemical Photocatalysis, 2nd ed.; De Gruyter: Berlin, Germany, 2020. doi:10.1515/9783110576764 |
| 31. | Nicholls, T. P.; Leonori, D.; Bissember, A. C. Nat. Prod. Rep. 2016, 33, 1248–1254. doi:10.1039/c6np00070c |
| 32. | Liu, X.-Y.; Qin, Y. Acc. Chem. Res. 2019, 52, 1877–1891. doi:10.1021/acs.accounts.9b00246 |
| 33. | Pitre, S. P.; Overman, L. E. Chem. Rev. 2022, 122, 1717–1751. doi:10.1021/acs.chemrev.1c00247 |
| 74. | Lowry, M. S.; Goldsmith, J. I.; Slinker, J. D.; Rohl, R.; Pascal, R. A.; Malliaras, G. G.; Bernhard, S. Chem. Mater. 2005, 17, 5712–5719. doi:10.1021/cm051312+ |
| 76. | Ge, Y.; Wang, H.; Wang, H.-N.; Yu, S.-S.; Yang, R.; Chen, X.; Zhao, Q.; Chen, G. Org. Lett. 2021, 23, 370–375. doi:10.1021/acs.orglett.0c03867 |
| 27. | Ciamician, G. Science 1912, 36, 385–394. doi:10.1126/science.36.926.385 |
| 28. | Klán, P.; Wirz, J. Photochemistry of Organic Compounds: From Concepts to Practice; John Wiley & Sons: Chichester, UK, 2009. doi:10.1002/9781444300017 |
| 29. | Hu, A.; Guo, J.-J.; Pan, H.; Zuo, Z. Science 2018, 361, 668–672. doi:10.1126/science.aat9750 |
| 30. | Laudadio, G.; Deng, Y.; van der Wal, K.; Ravelli, D.; Nuño, M.; Fagnoni, M.; Guthrie, D.; Sun, Y.; Noël, T. Science 2020, 369, 92–96. doi:10.1126/science.abb4688 |
| 75. | Walden, S. E.; Wheeler, R. A. J. Phys. Chem. 1996, 100, 1530–1535. doi:10.1021/jp951838p |
| 21. | Romero, N. A.; Nicewicz, D. A. Chem. Rev. 2016, 116, 10075–10166. doi:10.1021/acs.chemrev.6b00057 |
| 22. | Capaldo, L.; Ravelli, D.; Fagnoni, M. Chem. Rev. 2022, 122, 1875–1924. doi:10.1021/acs.chemrev.1c00263 |
| 23. | Cao, H.; Tang, X.; Tang, H.; Yuan, Y.; Wu, J. Chem Catal. 2021, 1, 523–598. doi:10.1016/j.checat.2021.04.008 |
| 24. | Bell, J. D.; Murphy, J. A. Chem. Soc. Rev. 2021, 50, 9540–9685. doi:10.1039/d1cs00311a |
| 25. | Buzzetti, L.; Crisenza, G. E. M.; Melchiorre, P. Angew. Chem., Int. Ed. 2019, 58, 3730–3747. doi:10.1002/anie.201809984 |
| 26. | Arias-Rotondo, D. M.; McCusker, J. K. Chem. Soc. Rev. 2016, 45, 5803–5820. doi:10.1039/c6cs00526h |
| 68. | Tasker, N. R.; Wipf, P. Biosynthesis, Total Synthesis, and Biological Profiles of Ergot Alkaloids. The Alkaloids: Chemistry and Biology; Academic Press: Cambridge, MA, USA, 2021; Vol. 85, pp 1–112. doi:10.1016/bs.alkal.2020.08.001 |
| 69. | Liu, H.; Jia, Y. Nat. Prod. Rep. 2017, 34, 411–432. doi:10.1039/c6np00110f |
| 70. | McCabe, S. R.; Wipf, P. Org. Biomol. Chem. 2016, 14, 5894–5913. doi:10.1039/c6ob00878j |
| 71. | Jakubczyk, D.; Cheng, J. Z.; O'Connor, S. E. Nat. Prod. Rep. 2014, 31, 1328–1338. doi:10.1039/c4np00062e |
| 108. | Yamada, F.; Makita, Y.; Somei, M. Heterocycles 2007, 72, 599–620. doi:10.3987/com-06-s(k)55 |
| 109. | Somei, M.; Yamada, F.; Ohnishi, H.; Makita, Y.; Kuriki, M. Heterocycles 1987, 26, 2823–2828. doi:10.3987/r-1987-11-2823 |
| 110. | Somei, M.; Ohnishi, H.; Shoken, Y. Chem. Pharm. Bull. 1986, 34, 677–681. doi:10.1248/cpb.34.677 |
| 111. | Oppolzer, W.; Grayson, J. I.; Wegmann, H.; Urrea, M. Tetrahedron 1983, 39, 3695–3705. doi:10.1016/s0040-4020(01)88608-7 |
| 112. | Somei, M.; Tsuchiya, M. Chem. Pharm. Bull. 1981, 29, 3145–3157. doi:10.1248/cpb.29.3145 |
| 113. | Somei, M.; Yamada, F.; Karasawa, Y.; Kaneko, C. Chem. Lett. 1981, 10, 615–618. doi:10.1246/cl.1981.615 |
| 114. | Natsume, M.; Muratake, H. Heterocycles 1980, 14, 1101–1105. doi:10.3987/r-1980-08-1101 |
| 16. | Yoon, T. P.; Ischay, M. A.; Du, J. Nat. Chem. 2010, 2, 527–532. doi:10.1038/nchem.687 |
| 17. | Oelgemöller, M. Chem. Rev. 2016, 116, 9664–9682. doi:10.1021/acs.chemrev.5b00720 |
| 18. | Cambié, D.; Bottecchia, C.; Straathof, N. J. W.; Hessel, V.; Noël, T. Chem. Rev. 2016, 116, 10276–10341. doi:10.1021/acs.chemrev.5b00707 |
| 19. | Zhou, L.; Lokman Hossain, M.; Xiao, T. Chem. Rec. 2016, 16, 319–334. doi:10.1002/tcr.201500228 |
| 20. | Crisenza, G. E. M.; Melchiorre, P. Nat. Commun. 2020, 11, 803. doi:10.1038/s41467-019-13887-8 |
| 72. | Yao, Y.; An, C.; Evans, D.; Liu, W.; Wang, W.; Wei, G.; Ding, N.; Houk, K. N.; Gao, S.-S. J. Am. Chem. Soc. 2019, 141, 17517–17521. doi:10.1021/jacs.9b10217 |
| 73. |
Ma, Y.; Yan, J.; Yang, L.; Yao, Y.; Wang, L.; Gao, S.-S.; Cui, C. Front. Bioeng. Biotechnol. 2022, 10, 1095464. doi:10.3389/fbioe.2022.1095464
And references cited therein. |
| 115. | Chaudhuri, S.; Bhunia, S.; Roy, A.; Das, M. K.; Bisai, A. Org. Lett. 2018, 20, 288–291. doi:10.1021/acs.orglett.7b03683 |
| 42. | Liu, J.-Q.; Shatskiy, A.; Matsuura, B. S.; Kärkäs, M. D. Synthesis 2019, 51, 2759–2791. doi:10.1055/s-0037-1611852 |
| 43. | Bottecchia, C.; Noël, T. Chem. – Eur. J. 2019, 25, 26–42. doi:10.1002/chem.201803074 |
| 44. | Aguilar Troyano, F. J.; Merkens, K.; Anwar, K.; Gómez‐Suárez, A. Angew. Chem., Int. Ed. 2021, 60, 1098–1115. doi:10.1002/anie.202010157 |
| 45. | King, T. A.; Mandrup Kandemir, J.; Walsh, S. J.; Spring, D. R. Chem. Soc. Rev. 2021, 50, 39–57. doi:10.1039/d0cs00344a |
| 52. | Schmittel, M.; Burghart, A. Angew. Chem., Int. Ed. Engl. 1997, 36, 2550–2589. doi:10.1002/anie.199725501 |
| 53. | Roth, H. G.; Romero, N. A.; Nicewicz, D. A. Synlett 2016, 27, 714–723. doi:10.1055/s-0035-1561297 |
| 54. | Yu, Y.; Zhang, L.-K.; Buevich, A. V.; Li, G.; Tang, H.; Vachal, P.; Colletti, S. L.; Shi, Z.-C. J. Am. Chem. Soc. 2018, 140, 6797–6800. doi:10.1021/jacs.8b03973 |
| 55. | Laroche, B.; Tang, X.; Archer, G.; Di Sanza, R.; Melchiorre, P. Org. Lett. 2021, 23, 285–289. doi:10.1021/acs.orglett.0c03735 |
| 56. | Rahimidashaghoul, K.; Klimánková, I.; Hubálek, M.; Matoušek, V.; Filgas, J.; Slavíček, P.; Slanina, T.; Beier, P. ChemPhotoChem 2021, 5, 43–50. doi:10.1002/cptc.202000214 |
| 57. | Lima, R. N.; Delgado, J. A. C.; Bernardi, D. I.; Berlinck, R. G. S.; Kaplaneris, N.; Ackermann, L.; Paixão, M. W. Chem. Commun. 2021, 57, 5758–5761. doi:10.1039/d1cc01822a |
| 58. | Weng, Y.; Ding, B.; Liu, Y.; Song, C.; Chan, L.-Y.; Chiang, C.-W. Org. Lett. 2021, 23, 2710–2714. doi:10.1021/acs.orglett.1c00609 |
| 59. | Hoopes, C. R.; Garcia, F. J.; Sarkar, A. M.; Kuehl, N. J.; Barkan, D. T.; Collins, N. L.; Meister, G. E.; Bramhall, T. R.; Hsu, C.-H.; Jones, M. D.; Schirle, M.; Taylor, M. T. J. Am. Chem. Soc. 2022, 144, 6227–6236. doi:10.1021/jacs.1c10536 |
| 106. | Hurtley, A. E.; Lu, Z.; Yoon, T. P. Angew. Chem., Int. Ed. 2014, 53, 8991–8994. doi:10.1002/anie.201405359 |
| 39. | Nguyen, S. T.; Murray, P. R. D.; Knowles, R. R. ACS Catal. 2020, 10, 800–805. doi:10.1021/acscatal.9b04813 |
| 40. | Zhang, J. ChemSusChem 2018, 11, 3071–3080. doi:10.1002/cssc.201801370 |
| 41. | Scott, E.; Peter, F.; Sanders, J. Appl. Microbiol. Biotechnol. 2007, 75, 751–762. doi:10.1007/s00253-007-0932-x |
| 60. | Bartoccini, F.; Regni, A.; Retini, M.; Piersanti, G. Eur. J. Org. Chem. 2022, e202200315. doi:10.1002/ejoc.202200315 |
| 61. | Bartoccini, F.; Regni, A.; Retini, M.; Piersanti, G. Org. Biomol. Chem. 2021, 19, 2932–2940. doi:10.1039/d1ob00353d |
| 62. | Bartoccini, F.; Fanini, F.; Retini, M.; Piersanti, G. Tetrahedron Lett. 2020, 61, 151923. doi:10.1016/j.tetlet.2020.151923 |
| 63. | Bartoccini, F.; Venturi, S.; Retini, M.; Mari, M.; Piersanti, G. J. Org. Chem. 2019, 84, 8027–8034. doi:10.1021/acs.joc.9b00879 |
| 64. | Bartoccini, F.; Bartolucci, S.; Mari, M.; Piersanti, G. Org. Biomol. Chem. 2016, 14, 10095–10100. doi:10.1039/c6ob01791f |
| 65. | Bartoccini, F.; Casoli, M.; Mari, M.; Piersanti, G. J. Org. Chem. 2014, 79, 3255–3259. doi:10.1021/jo500245s |
| 66. | Lucarini, S.; Bartoccini, F.; Battistoni, F.; Diamantini, G.; Piersanti, G.; Righi, M.; Spadoni, G. Org. Lett. 2010, 12, 3844–3847. doi:10.1021/ol101527j |
| 67. | Bartoccini, F.; Piersanti, G. Synthesis 2021, 53, 1396–1408. doi:10.1055/a-1340-3423 |
| 107. | Capaldo, L.; Ravelli, D. Eur. J. Org. Chem. 2017, 2056–2071. doi:10.1002/ejoc.201601485 |
| 35. | Capaldo, L.; Quadri, L. L.; Ravelli, D. Green Chem. 2020, 22, 3376–3396. doi:10.1039/d0gc01035a |
| 36. | Douglas, J. J.; Sevrin, M. J.; Stephenson, C. R. J. Org. Process Res. Dev. 2016, 20, 1134–1147. doi:10.1021/acs.oprd.6b00125 |
| 37. | Li, P.; Terrett, J. A.; Zbieg, J. R. ACS Med. Chem. Lett. 2020, 11, 2120–2130. doi:10.1021/acsmedchemlett.0c00436 |
| 38. | Candish, L.; Collins, K. D.; Cook, G. C.; Douglas, J. J.; Gómez-Suárez, A.; Jolit, A.; Keess, S. Chem. Rev. 2022, 122, 2907–2980. doi:10.1021/acs.chemrev.1c00416 |
| 104. | Beckwith, A. L. J.; Crich, D.; Duggan, P. J.; Yao, Q. Chem. Rev. 1997, 97, 3273–3312. doi:10.1021/cr950207o |
| 105. | Crich, D.; Brebion, F.; Suk, D.-H. Top. Curr. Chem. 2006, 263, 1–38. doi:10.1007/128_024 |
| 34. | Lechner, V. M.; Nappi, M.; Deneny, P. J.; Folliet, S.; Chu, J. C. K.; Gaunt, M. J. Chem. Rev. 2022, 122, 1752–1829. doi:10.1021/acs.chemrev.1c00357 |
| 46. | Zuo, Z.; MacMillan, D. W. C. J. Am. Chem. Soc. 2014, 136, 5257–5260. doi:10.1021/ja501621q |
| 47. | Schwarz, J.; König, B. Green Chem. 2016, 18, 4743–4749. doi:10.1039/c6gc01101b |
| 48. | Liao, L.-L.; Cao, G.-M.; Jiang, Y.-X.; Jin, X.-H.; Hu, X.-L.; Chruma, J. J.; Sun, G.-Q.; Gui, Y.-Y.; Yu, D.-G. J. Am. Chem. Soc. 2021, 143, 2812–2821. doi:10.1021/jacs.0c11896 |
| 49. | Li, Y.; Dai, C.; Xie, S.; Liu, P.; Sun, P. Org. Lett. 2021, 23, 5906–5910. doi:10.1021/acs.orglett.1c02014 |
| 50. | Xuan, J.; Zhang, Z.-G.; Xiao, W.-J. Angew. Chem., Int. Ed. 2015, 54, 15632–15641. doi:10.1002/anie.201505731 |
| 51. | Rahman, M.; Mukherjee, A.; Kovalev, I. S.; Kopchuk, D. S.; Zyryanov, G. V.; Tsurkan, M. V.; Majee, A.; Ranu, B. C.; Charushin, V. N.; Chupakhin, O. N.; Santra, S. Adv. Synth. Catal. 2019, 361, 2161–2214. doi:10.1002/adsc.201801331 |
| 99. | McCarver, S. J.; Qiao, J. X.; Carpenter, J.; Borzilleri, R. M.; Poss, M. A.; Eastgate, M. D.; Miller, M. M.; MacMillan, D. W. C. Angew. Chem., Int. Ed. 2017, 56, 728–732. doi:10.1002/anie.201608207 |
| 62. | Bartoccini, F.; Fanini, F.; Retini, M.; Piersanti, G. Tetrahedron Lett. 2020, 61, 151923. doi:10.1016/j.tetlet.2020.151923 |
| 76. | Ge, Y.; Wang, H.; Wang, H.-N.; Yu, S.-S.; Yang, R.; Chen, X.; Zhao, Q.; Chen, G. Org. Lett. 2021, 23, 370–375. doi:10.1021/acs.orglett.0c03867 |
| 77. | Yang, J.; Zhang, J.; Qi, L.; Hu, C.; Chen, Y. Chem. Commun. 2015, 51, 5275–5278. doi:10.1039/c4cc06344a |
| 78. | Jin, Y.; Jiang, M.; Wang, H.; Fu, H. Sci. Rep. 2016, 6, 20068. doi:10.1038/srep20068 |
| 79. | Cheng, W.-M.; Shang, R.; Fu, Y. ACS Catal. 2017, 7, 907–911. doi:10.1021/acscatal.6b03215 |
| 80. | Cheng, W.-M.; Shang, R.; Fu, M.-C.; Fu, Y. Chem. – Eur. J. 2017, 23, 2537–2541. doi:10.1002/chem.201605640 |
| 81. | Liu, X.; Liu, Y.; Chai, G.; Qiao, B.; Zhao, X.; Jiang, Z. Org. Lett. 2018, 20, 6298–6301. doi:10.1021/acs.orglett.8b02791 |
| 82. | Proctor, R. S. J.; Davis, H. J.; Phipps, R. J. Science 2018, 360, 419–422. doi:10.1126/science.aar6376 |
| 83. | Xiao, Z.; Wang, L.; Wei, J.; Ran, C.; Liang, S. H.; Shang, J.; Chen, G.-Y.; Zheng, C. Chem. Commun. 2020, 56, 4164–4167. doi:10.1039/d0cc00451k |
| 84. | He, S.; Li, H.; Chen, X.; Krylov, I. B.; Terent'ev, A. O.; Qu, L.; Yu, B. Chin. J. Org. Chem. 2021, 41, 4661–4689. doi:10.6023/cjoc202105041 |
| 85. | Dong, W.; Yen-Pon, E.; Li, L.; Bhattacharjee, A.; Jolit, A.; Molander, G. A. Nat. Chem. 2022, 14, 1068–1077. doi:10.1038/s41557-022-00979-0 |
| 98. | It should be mentioned that racemization was found in products 11 and 12 from the chiral intermediate V, which is common in this radical coupling reaction. See reference [76]. |
| 99. | McCarver, S. J.; Qiao, J. X.; Carpenter, J.; Borzilleri, R. M.; Poss, M. A.; Eastgate, M. D.; Miller, M. M.; MacMillan, D. W. C. Angew. Chem., Int. Ed. 2017, 56, 728–732. doi:10.1002/anie.201608207 |
| 92. | Jasperse, C. P.; Curran, D. P.; Fevig, T. L. Chem. Rev. 1991, 91, 1237–1286. doi:10.1021/cr00006a006 |
| 93. | Chu, L.; Ohta, C.; Zuo, Z.; MacMillan, D. W. C. J. Am. Chem. Soc. 2014, 136, 10886–10889. doi:10.1021/ja505964r |
| 94. | Noble, A.; McCarver, S. J.; MacMillan, D. W. C. J. Am. Chem. Soc. 2015, 137, 624–627. doi:10.1021/ja511913h |
| 95. | Xiao, T.; Li, L.; Zhou, L. J. Org. Chem. 2016, 81, 7908–7916. doi:10.1021/acs.joc.6b01620 |
| 96. | Ernouf, G.; Chirkin, E.; Rhyman, L.; Ramasami, P.; Cintrat, J.-C. Angew. Chem., Int. Ed. 2020, 59, 2618–2622. doi:10.1002/anie.201908951 |
| 97. | Li, J.-T.; Luo, J.-N.; Wang, J.-L.; Wang, D.-K.; Yu, Y.-Z.; Zhuo, C.-X. Nat. Commun. 2022, 13, 1778. doi:10.1038/s41467-022-29464-5 |
| 90. | Kozikowski, A. P.; Chen, C.; Wu, J. P.; Shibuya, M.; Kim, C. G.; Floss, H. G. J. Am. Chem. Soc. 1993, 115, 2482–2488. doi:10.1021/ja00059a051 |
| 91. | Wong, G.; Lim, L. R.; Tan, Y. Q.; Go, M. K.; Bell, D. J.; Freemont, P. S.; Yew, W. S. Nat. Commun. 2022, 13, 712. doi:10.1038/s41467-022-28386-6 |
| 90. | Kozikowski, A. P.; Chen, C.; Wu, J. P.; Shibuya, M.; Kim, C. G.; Floss, H. G. J. Am. Chem. Soc. 1993, 115, 2482–2488. doi:10.1021/ja00059a051 |
| 86. | Yue, H.; Zhu, C.; Huang, L.; Dewanji, A.; Rueping, M. Chem. Commun. 2022, 58, 171–184. doi:10.1039/d1cc06285a |
| 87. | Nemoto, T.; Harada, S.; Nakajima, M. Asian J. Org. Chem. 2018, 7, 1730–1742. doi:10.1002/ajoc.201800336 |
| 88. | Yuan, K.; Jia, Y. Chin. J. Org. Chem. 2018, 38, 2386–2399. doi:10.6023/cjoc201705058 |
| 89. | Connon, R.; Guiry, P. J. Tetrahedron Lett. 2020, 61, 151696. doi:10.1016/j.tetlet.2020.151696 |
© 2023 Regni et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.