Abstract
The chemistry of hypervalent iodine (HVI) reagents has gathered increased attention towards the synthesis of a wide range of chemical structures. HVI reagents are characterized by their diverse reactivity as oxidants and electrophilic reagents. In addition, they are inexpensive, non-toxic and considered to be environmentally friendly. An important application of HVI reagents is the synthesis of halogenated cyclic compounds, in particular, the intramolecular HVI-mediated halocyclisation of alkenes using carbon, oxygen, nitrogen or sulfur nucleophiles. Herein, we describe the examples reported in the literature, which are organised by the different halogens involved and the internal nucleophiles.
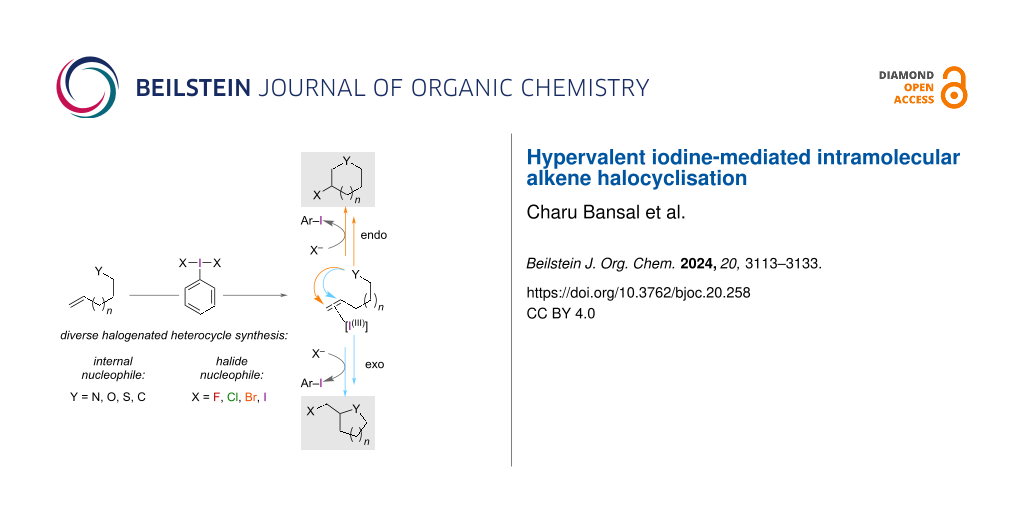
Graphical Abstract
Introduction
Halogenated carbocyclic and heterocyclic compounds are present in many active pharmaceutical ingredients [1,2]. The intramolecular halocyclisation of alkenes mediated by HVI(III) reagents allow access to a range of halogenated cyclic scaffolds in a cost effective and selective, one-pot transformation. Pharmaceutical uses for bio-active cyclic molecules accessible by I(III) reagents are plentiful; anticancer drugs can be formed from the basis of pyrrolo[2,3-b]indoles 1 [3,4], 2-oxazolines 2 [5,6], dihydrofuran 3 [7,8], and spirocyclic scaffolds 4 [9,10] (Figure 1). Halogenated cyclised structures have also been found to exhibit medicinal and pharmaceutical properties, including antibacterial [11], antibiotic [12], and enzyme inhibition [13] among others.
Figure 1: Example bioactive compounds containing cyclic scaffolds potentially accessible by HVI chemistry.
Figure 1: Example bioactive compounds containing cyclic scaffolds potentially accessible by HVI chemistry.
The general mechanism for the HVI-mediated halocyclisation of alkenes proceeds firstly through the coordination of an alkene by the HVI reagent, which activates it toward intramolecular attack by an internal nucleophile. Following this, substitution of the iodane(III) can occur from the nucleophilic halide in solution to reveal the halo-cyclised product (Figure 2).
Figure 2: A general mechanism for HVI-mediated endo- or exo-halocyclisation.
Figure 2: A general mechanism for HVI-mediated endo- or exo-halocyclisation.
In this review, we have collected and described the HVI-mediated halocyclisation reactions reported in the literature for each halide. We have organised the examples firstly by the halide nucleophile, and then secondly by the internal heteroatom nucleophile involved in the cyclisation. The selectivity (chemo-, regio-, stereo-) of the reaction, which is influenced by the type of HVI reagent, the nature of the substrates employed and the proposed mechanism from the authors are all described. The halocyclisation of alkenes to make halogenated carbo or heterocycles is yet to be covered by a review, which is the vacancy this review aims to fill. The synthetic uses of HVI reagents [14-16], their involvement in heterocycle synthesis [17-19], and alkene functionalisation [20,21], have each been well-reviewed elsewhere.
Review
Hypervalent iodine-mediated fluorocyclisation
Fluorine can substantially improve the activity of biologically relevant molecules [22], and compounds containing fluorine have seen huge success in medicine and agrochemicals, with over 30% of small molecule drugs [23,24] and 16% of pesticides [25] now containing fluorine atoms. A range of synthetically important fluorinated hetero- and carbocycles can be synthesized mildly and effectively using HVI reagents.
Nitrogen nucleophiles
A metal-free synthesis of β-fluorinated piperidines was reported in 2012 by Meng, Li and co-workers (Scheme 1) [26]. The authors describe a reaction using PhI(OPiv)2 as oxidant with HF·pyridine as the source of fluoride and BF3·OEt2 as activator. A range of unsaturated amines 5 were cyclised to racemic β-fluorinated piperidines 6. Good yields were reported for all compounds except those with substituents present on the alkene. Homologation of the carbon chain from 5 to 6 carbons gave both 6- and 7-membered rings in poor yield with α preference for 7-membered rings 7 in a ratio of 1:9.3.
Scheme 1: Metal-free synthesis of β-fluorinated piperidines 6. Ts = tosyl.
Scheme 1: Metal-free synthesis of β-fluorinated piperidines 6. Ts = tosyl.
The authors proposed two mechanisms for the reaction (Scheme 1). In pathway A (top), the alkene-activated iodonium is formed, intramolecular attack of nitrogen forms the 6-membered ring A before an SN2 reaction with the fluoride ion to displace PhI. In pathway B (bottom), the nitrogen is oxidised by the iodane, generating an electrophilic intermediate B. Nucleophilic attack by the double bond subsequently forms the 6-membered ring intermediate C, which is either immediately attacked by fluoride to form both cis and trans products or stabilised by the tosyl group and subsequently attacked to form only the cis product in an SN2 reaction.
Liu and co-workers reported a palladium-catalysed intramolecular aminofluorination of unactivated alkenes [27] (Scheme 2) in the presence of PhI(OPiv)2, AgF and MgSO4 as an oxidant, source of fluorine and additive, respectively. Racemic β-fluorinated piperidines 6 were synthesised in excellent yields, under mild conditions. A small amount of amino carboxylation side-product was determined to have been additionally produced from the reaction. A range of other alkenes were cyclised in good yields, demonstrating the scope of the reaction. The authors proposed an alternative reaction mechanism to those already described, in which trans-aminopalladation of the alkene, mediated by Pd(II), occurs with intramolecular attack of the nitrogen on the terminal carbon, generating a 6-membered ring A (Scheme 2). The Pd(II) intermediate is oxidised by PhI(OPiv)2/AgF, forming Pd(IV). Formation of the product can occur either by reductive elimination by Pd(IV) or SN2 nucleophilic attack by fluorine with concomitant palladium reduction. Reductive elimination of the Pd(II) intermediate forms the C‒F bond to give predominantly the trans product, but this pathway competes with a less favourable SN2 nucleophilic attack by fluorine to form the cis product. However, a mechanism entirely mediated by the I(III) HVI reagent, with the Pd(OAc)2 only acting as a Lewis acid to activate the HVI reagent, was not ruled out.
Scheme 2: Intramolecular aminofluorination of unactivated alkenes with a palladium catalyst.
Scheme 2: Intramolecular aminofluorination of unactivated alkenes with a palladium catalyst.
In 2013, Nevado and co-workers used (R,R)- and (S,S)-tert-butyl lactate iodotoluene difluoride (8) for the aminofluorination of alkenes toward the synthesis of enantiomerically pure β-fluorinated piperidines 6 (Scheme 3) [28]. A range of enantiomerically pure fluorinated piperidines were synthesised in moderate to excellent yields and enantiomeric excesses. In addition to the synthesis of 6-membered rings, 7-membered hexenamines 7 were synthesised. To the authors’ surprise, reaction conditions for the synthesis of β-fluorinated piperidines 6 afforded no product. The reaction instead proceeded with addition of dichloro(pyridine-2-carboxylato)gold(III) complex in combination with silver triflimide, AgNTf2. A range of β-fluoroazepanes 7 were successfully synthesised with high enantiomeric purity in good yields. A mechanism for the synthesis of β-fluorinated piperidines was proposed by the authors (Scheme 3). Activation of the HVI reagent by H-bonding leads to ligand exchange to give an aminofluoro iodonium intermediate A. Cyclisation occurs via nitrogen attack on the alkene to then give aziridinium intermediate B. Subsequent nucleophilic attack by fluoride on the more substituted carbon that is more cationic leads to the product.
Scheme 3: Aminofluorination of alkenes in the synthesis of enantiomerically pure β-fluorinated piperidines. PMB = para-methoxybenzene, athe ee value was determined after Cbz group was replaced with tosyl group, bthe value in brackets corresponds to the ee value after crystallization. PG = protecting group.
Scheme 3: Aminofluorination of alkenes in the synthesis of enantiomerically pure β-fluorinated piperidines. P...
Synthesis of β-fluorinated piperidines 6 with in situ-generated HVI reagent was reported in 2014 by Kita, Shibata and co-workers (Scheme 4) [29]. Using difluoroiodotoluene 10, formed in situ from 4-iodotoluene, pyridine·HF and m-CPBA, intramolecular aminofluorination of a range of unsaturated amines formed β-fluorinated piperidines 6 and 3-fluoroazepanes 7 in good yields. Again, yields only significantly fell with substrates containing substituents on the alkene. Depending on the length of the alkyl chain, both 6- and 7-membered rings were formed. The use of a chiral aryl iodide was tested, which gave products with low enantiomeric excess. However, these preliminary trials represent the first example of a catalytic, enantioselective HVI-mediated fluorocyclisation.
Scheme 4: Synthesis of β-fluorinated piperidines.
Scheme 4: Synthesis of β-fluorinated piperidines.
The authors proposed a mechanism (Scheme 4) for this reaction that involved iodoarene difluoride 10 being generated from iodosylarene 9 (ArI=O) and HF, with iodosylarene itself generated by aryl iodide and m-CPBA. Ligand exchange of iodoarene difluoride with nitrogen and reaction with the alkene forms aziridinium intermediate A which, after nucleophilic attack by fluoride, forms the product.
Li reported a haloamination of unsaturated amines in 2014 (Scheme 5) [30], to form fluorinated piperidines 6 using PhI(OAc)2 as an oxidant and BF3·OEt2 as the source of fluoride. Fluorocyclisations gave lower yields compared to other halocyclisations reported by the authors. Aminoacetylation of the alkene competed with the aminofluorination to form 3-acetoxypiperidines 11. Other sources of fluoride were tested, with metal fluoride salts giving no or trace products. The authors reported that only 6-membered rings were formed, with a range of substituents on the β-carbon of the alkene. The authors proposed a mechanism for the reaction, in which the alkene is activated by PhI(OAc)2, followed by intramolecular nucleophilic attack of nitrogen and displacement of iodobenzene by fluoride.
Scheme 5: Intramolecular fluoroaminations of unsaturated amines published by Li.
Scheme 5: Intramolecular fluoroaminations of unsaturated amines published by Li.
The intramolecular aminofluorination of unsaturated amines using a structural analogue of Togni’s reagent, 1-fluoro-3,3-dimethylbenziodoxole (12), was reported in 2015 by Szabó (Scheme 6) [31]. With catalytic Zn(BF4)2, 1-fluoro-3,3- dimethylbenziodoxole mediated the formation of fluorinated piperidines 6 and hexanamines 7 in high yields. A range of unsaturated amines with substituents on both carbons of the alkene were successfully cyclised in similarly good yields. However, increased substitution on the alkene resulted in an increased reaction time, with 9 hours required for disubstituted alkenes compared to 3 hours for non-substituted alkenes. With extended chain lengths, 5-, 6- and 7-membered aza-heterocycles were synthesised in good yields.
Scheme 6: Intramolecular aminofluorination of unsaturated amines using 1-fluoro-3,3-dimethylbenziodoxole (12). PG = protecting group.
Scheme 6: Intramolecular aminofluorination of unsaturated amines using 1-fluoro-3,3-dimethylbenziodoxole (12)...
The authors proposed a mechanism for the fluorocyclisation reactions (Scheme 6), which relies on the activation of the fluoro-iodane reagent 12 with the zinc catalyst. The activation enables better orbital overlap to occur with the π bond of the alkene to form an iodonium species. Nucleophilic attack occurs on the least hindered carbon of the iodonium, before displacement of the HVI by fluoride to give the product.
Zhang and co-workers reported the intramolecular aminofluorination of unsaturated amines using an HVI reagent generated in situ from iodosylbenzene 9 (Scheme 7) [32]. The authors reported the synthesis of 3-fluoropyrrolidines 14 with BF3·OEt2 as a source of fluoride for the intramolecular aminofluorination of homoallylic amines 13. The authors explored the reaction with various protecting groups on nitrogen and substituents on the alkene and alkyl chain. Substrates with p-tolylsulfonyl (Ts), p-nitrobenzenesulfonyl (Ns) and benzenesulfonyl (Bs) protecting groups were cyclised in high yields to the corresponding 3-fluoropyrrolidine derivatives 14. A range of unsaturated amines were successfully cyclised. However, substrates with substituents on the alkene gave low yields of product. A mechanism was proposed involving the activation of iodosylbenzene 9 with BF3·Et2O to form an HVI intermediate that activates the alkene to form an iodonium species. Intramolecular nucleophilic attack of nitrogen, elimination of PhI and attack by fluoride then forms the product.
Scheme 7: 3-fluoropyrrolidine synthesis. aDiastereomeric ratio (cis/trans) determined by 19F NMR analysis.
Scheme 7: 3-fluoropyrrolidine synthesis. aDiastereomeric ratio (cis/trans) determined by 19F NMR analysis.
Kitamura and co-workers additionally reported a synthesis of 3-fluoropyrrolidines 14 in 2017 using an alternative source of fluoride (Scheme 8) [33]. The authors used either PhI(OAc)2 or PhI(OCOCF3)2 as oxidants and pyridine·HF as a source of fluoride. This alternative reagent system was used due to concerns with the long-term stability of iodosylbenzene and unwanted reactions of BF3·Et2O with other reagents. In addition, a catalytic system was reported that employed 20 mol % iodotoluene with 1 equivalent of m-CPBA as terminal oxidant. The authors proposed that difluoroiodobenzene 10 is formed in situ, which is activated by HF. Two possible mechanisms were given for the synthesis of pyrrolidines 14, which are the same two proposed for the synthesis of piperidines 6 (Scheme 1). Either an alkene-activated iodonium is formed or an activated electrophilic nitrogen is generated from interaction with the HVI, that is then attacked by the alkene.
Scheme 8: Kitamura’s synthesis of 3-fluoropyrrolidines. Values in parentheses represent the cis:trans ratio.
Scheme 8: Kitamura’s synthesis of 3-fluoropyrrolidines. Values in parentheses represent the cis:trans ratio.
While the fluoroamination of alkenes to form 4-membered azetidines has not been reported, an exo fluorocyclisation to form 3-membered aziridines with an adjacent fluorine was reported by Jacobsen in 2018 (Scheme 9) [34]. The synthesis from the styrenyl starting materials is stereoselective, giving the syn-diasteroisomer in high yields. A chiral iodoarene catalyst 16 was employed, along with a stoichiometric sacrificial oxidant, to give good to excellent levels of enantioselectivity. This elegant strategy led to a variety of β-fluorinated tosylated aziridines 15 with good tolerance to styrenyl arenes with electron-withdrawing groups on them. The yields of product dropped off significantly when the ring did not contain an electron-withdrawing group on it.
Scheme 9: Jacobsen’s enantio- and diastereoselective protocol for the synthesis of syn-β-fluoroaziridines 15.
Scheme 9: Jacobsen’s enantio- and diastereoselective protocol for the synthesis of syn-β-fluoroaziridines 15.
In 2023, Šmit and co-workers conducted an in-depth mechanistic study on the cyclisation of alkenyl N-tosylamides using BF3-activated aryl iodane(III) carboxylates to create 3-fluoropiperidines [35]. The challenges faced relate to selectivity due to competing carboxyaminations (18, 18’), rather than fluoroamination (17, 17’), and difficulties in controlling the diastereoselectivity. The authors studied the stereo-, regio, and chemoselectivity in both cyclic and acyclic substrates. It was observed that the use of acyclic iodane reagents 19 and 20 predominantly led to products with β-stereochemistry, whereas the cyclic iodanes 21 and 22 favour pathways leading to α-stereochemistry (Scheme 10). The selectivity of the reaction was also found to be influenced by the presence of electrolytes like TBABF4, and the ligand attached to I(III). Carboxyfluorination was observed, in which a ligand from the iodane, e.g., OAc, OPiv or o-I-OBz, adds and was found to compete with fluoroamination. The level of this chemoselectivity was dependent on the iodane ligand: OPiv was more selective for aminofluorination than OAc, which was proposed to be due to differences in basicity and nucleophilicity (Scheme 10).
Scheme 10: Different HVI reagents lead to different diastereoselectivity in aminofluorination competing with carboxyfluorination products. Mechanistic studies of the fluoroamination of acyclic substrates showing the competing carboxyfluorination products. AIF = aziridinium ion formation, AIO = aziridinium ion opening, RLC = reductive ligand coupling.
Scheme 10: Different HVI reagents lead to different diastereoselectivity in aminofluorination competing with c...
Detailed mechanistic studies were carried out using multinuclear NMR spectroscopy, deuterium labelling, rearrangements on stereodefined substrates, and structural analyses (NMR and X-ray) of the reaction products. RT-NMR-derived data strongly supported a pathway of alkene activation by the iodane, as opposed to the formation of an N-I(III) adduct. The presence of 5-exo-products, with support of a deuterium labelling experiment, also ruled out the possibility of an N-activation pathway. Therefore, the proposed mechanism involves BF3-coordinated I(III) iodane forming iodiranium(III) ions with the alkene, followed by diastereo-determining 5-exo-cyclisation. These transiently formed pyrrolidine intermediates A can undergo further transformations (Scheme 10), depending on their structure and the iodane reagent being used. For example, higher yields of 3-fluoropiperidine products 6 were observed when using cyclic iodane reagents 21 and 22 (Scheme 10), which was suggested to be because a reductive ligand coupling (RLC) pathway would be suppressed due to reduced fluxionality of the carboxylate ligand on I(III). These important findings are expected to enhance the use of aryl iodane(III)-dicarboxylates for constructing fluorinated azaheterocycles with improved selectivity and control.
Oxygen nucleophiles
In 2000, Hara and co-workers reported the fluorocyclisation of unsaturated alcohols and carboxylic acids promoted by HVI reagents (Scheme 11) [36]. Using 4-tolyl difluoroiodane 10 as the reaction promoter, and pyridine·6HF as a source of fluoride, a range of unsaturated alcohols 23 to fluorinated tetrahydrofurans 25 and tetrahydropyrans 26. Unsaturated carboxylic acids 24 was also cyclised to form 5-membered fluorinated lactones 27.
Scheme 11: Fluorocyclisation of unsaturated alcohols and carboxylic acids to make tetrahydrofurans, fluoromethyl-γ-lactones and tetrahydropyrans. The ratio of stereoisomers is shown in parentheses.
Scheme 11: Fluorocyclisation of unsaturated alcohols and carboxylic acids to make tetrahydrofurans, fluorometh...
The mechanisms proposed for the cyclisation of unsaturated alcohols and carboxylic acids both proceed first through the activation of the alkene by the iodane (Scheme 11). The internal oxygen nucleophile and fluoride then sequentially attack the activated alkene, to either form 5- or 6-membered furan or pyran heterocycles depending on the chain length in the substrate. The pyran ring is only formed from the alcohol starting material, presumably because the oxygen is reactive enough to displace the iodane to form the oxonium species A. When the carboxylic acid is used, the oxygen in the lactone intermediate is less reactive and so substitution of the iodane by fluoride is more favourable and the branched product is formed.
In addition to aminofluorination, Szabó also reported the oxyfluorination of alkenes in 2015 [31]. Under identical conditions to the aminofluorination using 1-fluoro-3,3-dimethylbenziodoxole (12) with Zn(BF4)2 catalyst, unsaturated alcohols were cyclised to fluorinated tetrahydropyrans 26 and oxepanes 28 (Scheme 12) in 1–2 hours in good yields.
Scheme 12: Oxyfluorination of unsaturated alcohols.
Scheme 12: Oxyfluorination of unsaturated alcohols.
Gulder and co-workers reported a mild, metal free-synthesis of fluorobenzoxazepines 30 in 2016 (Scheme 13) [37]. Using 1-fluoro-3,3-dimethylbenziodoxole (12) and 4 Å molecular sieves, a range of benzamides 29 were successfully cyclised in good yields. The sustainability of the reaction was improved by regeneration of 1-fluoro-3,3- dimethylbenziodoxole (12) in 91% yield from isolated benzyl alcohol after the fluorination reaction. A mechanism of the reaction was proposed (Scheme 13) in which the iodane-activated alkene is attacked by fluoride and the aromatic ring, to form a fluorinated phenonium intermediate A. The product is formed following a 6-endo cyclisation with nucleophilic attack from oxygen to provide the fluoro-benzoxazepines 30.
Scheme 13: Synthesis and mechanism of fluoro-benzoxazepines.
Scheme 13: Synthesis and mechanism of fluoro-benzoxazepines.
In 2015, Stuart and co-workers reported an intramolecular fluorocyclisation of unsaturated carboxylic acids 24 (Scheme 14) [38]. Using 1-fluoro-3,3-dimethylbenziodoxole (12) as an oxidant with AgBF4 and 4 Å molecular sieves to prevent water competing with fluoride as the nucleophile, a range of unsaturated carboxylic acids were successfully cyclised to fluorinated lactones 27 in good yields. The authors proposed a mechanism for fluorolactonization (Scheme 14) whereby AgBF4 first activates the fluoroiodane 12 for alkene coordination. Intramolecular nucleophilic attack of oxygen on the more substituted carbon forms the cyclised intermediate A and eliminates fluoride. Phenonium intermediate B is formed with elimination of the iodoarene and subsequent attack of fluoride forms the product.
Scheme 14: Intramolecular fluorocyclisation of unsaturated carboxylic acids. Yield of isolated product within parentheses.
Scheme 14: Intramolecular fluorocyclisation of unsaturated carboxylic acids. Yield of isolated product within ...
In 2017, Kitamura and co-workers reported the synthesis of fluorinated tetrahydrofurans 25 and butyrolactone 27 (Scheme 15) [33]. Unsaturated alcohols 23 and 3-butenoic acid (24) were competent starting materials, using PhI(OPiv)2 as an oxidant and pyridine·HF as a source of fluoride. Unsaturated alcohols gave moderate yields of racemic fluorinated tetrahydrofurans 26. Moderate yields were also reported for the fluorocyclisation of 3-butenoic acid (24) to form the fluorinated butyrolactone 28.
Scheme 15: Synthesis of fluorinated tetrahydrofurans and butyrolactone.
Scheme 15: Synthesis of fluorinated tetrahydrofurans and butyrolactone.
The preparation of fluorinated oxazolines was reported in 2018 by Gilmour and co-workers (Scheme 16) [39]. p-TolIF2 is formed in situ from p-iodotoluene and Selectfluor in a 4.5:1 HF:amine solution, which is obtained by combining Et3N·3HF and pyridine·HF. A range of N-allyl carboxamides 31 were successfully cyclised forming fluoromethyl-2-oxazolines 32 in good yields.
Scheme 16: Synthesis of fluorinated oxazolines 32. aReaction time increased to 40 hours. Yields refer to isolated values whilst NMR yields are given in parentheses (19F NMR using ethyl fluoroacetate as an internal standard).
Scheme 16: Synthesis of fluorinated oxazolines 32. aReaction time increased to 40 hours. Yields refer to isola...
The synthesis of fluorinated oxazolines 32 was also reported using an electrochemical approach in 2019 by Waldvogel and co-workers (Scheme 17) [40]. The authors used electrochemical oxidation to form p-tolyl-difluoro-λ3-iodane 10 on the anode using an undivided cell with platinium electrodes in a 1:1 solution of CH2Cl2 and Et3N·5HF. The in situ formation of this unstable HVI reagent avoided the requirement for it to be isolated. It was used either in a 1-step in-cell procedure with alkene, or in a 2-step, ex-cell approach [41], in which the substrate was added after the electrolysis, thereby avoiding any competing electrochemical oxidation of the substrate. A range of N-allylcarboxamides 31 were cyclised to fluorinated oxazolines 32 in moderate to very good yields. Poor yields, however, were reported with electron-withdrawing aryl groups on the substrate. Intramolecular nucleophilic attack from oxygen onto the activated alkene forms the oxazoline A, and SN2 substitution with the fluoride ion displaces iodotoluene to form the product.
Scheme 17: Electrochemical synthesis of fluorinated oxazolines.
Scheme 17: Electrochemical synthesis of fluorinated oxazolines.
An electrochemical approach was also reported by Lennox and co-workers for the synthesis of chromanes 34 (Scheme 18) [42]. The authors reported using p-tolyl-difluoro-λ3-iodane 10, formed via electrochemical oxidation of 4-iodotoluene at the anode, in a 5:6 HF:amine mixture to cyclise a range of phenolic ethers 33. Tolerance for substituents on both the aromatic ring and the alkene were shown, although the electronic requirements were quite narrow for reaction success. As the arene ring attacks the activated alkene, if it is too electron-poor then it is not reactive enough. Moreover, if it is too electron-rich, then it preferentially oxidises via a single electron transfer mechanism which deactivates the ring as a nucleophile.
Scheme 18: Electrochemical synthesis of chromanes.
Scheme 18: Electrochemical synthesis of chromanes.
The synthesis of fluorinated oxazepanes 36 was reported by Ding and co-workers in 2021 (Scheme 19) [43]. Using fluoro benziodoxole 12 as oxidant and Zn(BF4)2 as a catalytic activator, a range of fluorinated oxazepanes were synthesised from allylamino ethanols 35 in good yields.
Scheme 19: Synthesis of fluorinated oxazepanes.
Scheme 19: Synthesis of fluorinated oxazepanes.
In 2021, Jiang and co-workers reported catalytic asymmetric aminofluorination using BF3·Et2O with a chiral aryliodide 16 catalyst (Scheme 20) [44]. The study successfully obtained various chiral fluorinated oxazine products 38 with high enantioselectivity (up to >99% ee) and diastereoselectivity (up to >20:1 dr). Control experiments showed that using Py·9HF or Et3N·3HF as the fluoride source did not yield the desired fluorooxazines, highlighting the role of BF3·Et2O as both a fluoride reagent and an activating reagent of iodosylbenzene. Different chiral iodide catalysts were studied, revealing that the substituents of the catalysts significantly influenced the stereochemistry of the reaction. Linear chiral catalysts were found to offer higher stereoselectivity compared to spiro-catalysts. Under optimized conditions, the asymmetric aminofluorination of N-cinnamylbenzamides 37 using BF3·Et2O as the fluorine reagent demonstrated good yields and high stereoselectivity (Scheme 20). The scope of the reaction was probed, showcasing the versatility and applicability of the method in synthesizing chiral fluorinated oxazines. The authors proposed the mechanism of the catalytic asymmetric nucleophilic fluorination to involve the activation of iodosylbenzene by BF3·Et2O which is then attacked by a nucleophile. The use of chiral iodine catalysts is essential for controlling the stereochemistry of the reaction. The specific arrangement of the catalyst influences the orientation of this nucleophilic attack as supported by density functional theory (DFT) calculations.
Scheme 20: Enantioselective oxy-fluorination with a chiral aryliodide catayst.
Scheme 20: Enantioselective oxy-fluorination with a chiral aryliodide catayst.
In 2022, Xu, Zhang, Zhu and co-workers reported a method to catalytically synthesise 5‑fluoro-2-aryloxazolines 39 by utilising BF3·Et2O as the fluoride source and activating reagent (Scheme 21) [6]. The synthesis of these derivatives was achieved with high efficiency, resulting in good to excellent yields of up to 95% within a short time-frame of 10 minutes. Treatment of N-(2-phenylallyl)benzamides with 10 equivalents of BF3·Et2O, iodobenzene, m-CPBA in dichloromethane (DCM) at 0 °C resulted in the formation of the oxazoline product (Scheme 21). DFT calculations indicated several steps in the mechanism, including ligand coupling, oxidative addition, intermolecular nucleophilic attack, 1,2-aryl migration, reductive elimination, and intramolecular nucleophilic attack. This approach offers a rapid and effective way to produce 5-fluoro-2-aryloxazoline compounds, which are valuable building blocks in organic synthesis.
Scheme 21: Catalytic synthesis of 5‑fluoro-2-aryloxazolines using BF3·Et2O as a source of fluoride and an activating reagent.
Scheme 21: Catalytic synthesis of 5‑fluoro-2-aryloxazolines using BF3·Et2O as a source of fluoride and an acti...
Carbon nucleophiles
In addition to intramolecular aminofluorination and oxyfluorination, Szabó and co-workers reported alkene carbofluorination in 2015 (Scheme 22) [31]. Using 1-fluoro-3,3-dimethylbenziodoxole (12) and [Cu(MeCN)4]BF4 as a catalyst to activate it, the authors reported the synthesis of fluorinated cyclopentane products 41 from alkenyl malonate derivatives 40. The malonate nucleophile required longer reaction times of 8 hours compared to 1–3 hours for aminofluorination and oxyfluorination, however good yields were reported.
Scheme 22: Intramolecular carbofluorination of alkenes.
Scheme 22: Intramolecular carbofluorination of alkenes.
Hypervalent iodine-mediated chlorocyclisation
Although less common than fluorine in biologically active compounds, chlorine-containing molecules have interest in drug discovery, with over 250 chloro-containing drugs presently available [43]. The introduction of a chlorine atom into biologically active compounds for use in pharmaceuticals and agrochemicals has been shown to greatly increase the potency of a compound [45,46] and can be considered a bioisostere for a methyl group. Chlorination through HVI approaches provides a safe and mild approach to chlorinated cyclic compounds.
Nitrogen nucleophiles
Intramolecular chlorocyclisation promoted by PhI(OAc)2 was reported by Liu and Li in 2014 alongside their intramolecular fluorocyclisation (Scheme 23) [30]. The authors reported the formation of 5- and 6-membered chlorinated azaheterocycles 42 from unsaturated amines, using PhI(OAc)2 as an oxidant and pyridinium chloride as a chlorine source. Substrates with a range of substituents on the alkyl chain were cyclised in good yields, yet introduction of substituents on the alkene led to a reduction of yield. The authors proposed a standard mechanism for the reaction (Scheme 23) in which PhI(OAc)2 activates the alkene, intramolecular attack of nitrogen forms the cyclised intermediate and a chloride ion displaces iodobenzene.
Scheme 23: Intramolecular chlorocyclisation of unsaturated amines.
Scheme 23: Intramolecular chlorocyclisation of unsaturated amines.
In 2015, Cariou, Dodd and co-workers reported the synthesis of chlorinated cyclic guanidines 44 (Scheme 24) [47]. Using Koser’s reagent as an oxidant with LiCl as a source of chloride, a range of unsaturated guanidines 43 were cyclised forming 5- or 6-membered rings in good yields. The change in ring size was proposed to be due to the position of the positive charge on the activated alkene. The carbon with the higher substitution has the greater positive charge and therefore undergoes nucleophilic attack by nitrogen. The authors proposed two possible mechanisms for the reaction (Scheme 24). Firstly, a chloronium ion is generated by HVI and LiCl followed by intramolecular nucleophilic attack by nitrogen to form the heterocycle. Secondly, oxidation of the unsaturated guanidine forms an intermediate aziridinium A with subsequent nucleophilic attack by chloride to form the product.
Scheme 24: Synthesis of chlorinated cyclic guanidines 44.
Scheme 24: Synthesis of chlorinated cyclic guanidines 44.
A metal-free chlorocyclisation of indole derivatives was reported by Yu and co-workers in 2019 (Scheme 25) [45]. The authors reported the use of 1-chloro-1,2-benziodoxol-3-one (45) as a single reagent to form 6- or 7-membered rings under mild conditions in DCM at room temperature. A range of substituents on the aromatic ring were tested with electron-withdrawing groups resulting in lower yields compared to electron-donating groups. The proposed mechanism for the reaction involved formation of a chloronium ion and nucleophilic attack from nitrogen on the less sterically-hindered carbon, forming a cyclic intermediate A (Scheme 25). Chloride is subsequently eliminated with formation of an enamine B that reacts with a second equivalent of 1-chloro-1,2-benziodoxol-3-one (45) to afford the product 46.
Scheme 25: Synthesis of chlorinated pyrido[2,3-b]indoles 46.
Scheme 25: Synthesis of chlorinated pyrido[2,3-b]indoles 46.
Oxygen nucleophiles
Liu and Li reported the chlorolactonization and chloroetherification (Scheme 26) of 2,2-diphenylpent-4-enoic acid (47’) and 2,2-diphenyl-4-penten-1-ol (47), respectively, using PhI(OAc)2 as an oxidant and pyridinium chloride as a chlorine source. This was an expansion of their scope that used nitrogen nucleophiles [30].
Scheme 26: Chlorolactonization and chloroetherification reactions.
Scheme 26: Chlorolactonization and chloroetherification reactions.
In 2015, Li and co-workers reported the synthesis of chloromethyloxazolines 49 [48] (Scheme 27). Using PhI(OAc)2 as an oxidant and TMSCl as a source of chloride and activator, a range of N-allyl carboxamides 31 were successfully cyclised, forming 5-chloromethyl-2-aryloxazolines 49 in good yields. A mechanism for the reaction was proposed by the authors (Scheme 27), whereby a TMS-adduct A of the amide is formed, alkene activation and cyclisation from oxygen forms the cyclised intermediate B, then displacement of PhI by chloride gives the product.
Scheme 27: Proposed mechanism for the synthesis of chloromethyl oxazolines 49.
Scheme 27: Proposed mechanism for the synthesis of chloromethyl oxazolines 49.
Chai, Jiang, Zhu and co-workers reported the synthesis of various halogenated 1,3-oxazine 50 and 2-oxazoline derivatives 51 using boron trihalides as the halogen source [6,49]. They found that the choice of halogen source influences the reaction outcomes. With the use of BCl3 (Scheme 28), N-cinnamylbenzamides 52 were transformed to give the corresponding chlorinated dihydro-[1,3]-oxazines 50 in good to excellent isolated yields [49]. When various substituted N-(2-phenylallyl)benzamides (52) were tolerated, it led to the formation of chlorinated 2-oxazolines 51 in good to excellent yields. When BF3 was used as the halogen source in the author’s previous work (Scheme 21) [6], it led to the formation of different products compared to when BCl3 was utilized, suggesting a different mechanism is operative.
Scheme 28: Oxychlorination to form oxazine and oxazoline heterocycles promoted by BCl3.
Scheme 28: Oxychlorination to form oxazine and oxazoline heterocycles promoted by BCl3.
Hypervalent iodine-mediated bromocyclisation
Bromocyclisation promoted by HVI reagents allows for a mild, metal-free synthesis of various cyclic functional groups and avoids the use of highly toxic and corrosive bromine. Approaches using this approach are outlined below.
Nitrogen nucleophiles
In 2007, aminobromocyclisation of homoallylic sulfonamides 53 was reported by Fan, Wang and co-workers (Scheme 29) [50]. Using PhI(OAc)2 as an oxidant with KBr as the bromine source and Bu4NBr as a reaction promoter, racemic brominated pyrrolidines 54 were synthesised from a range of homoallylic sulfonamides 53 in excellent yields under mild conditions at room temperature. A mechanism was suggested by the authors (Scheme 29), whereby ligand exchange on PhI(OAc)2 with a bromide ion forms unstable PhIOAcBr. Elimination of bromoacetate then gives a reactive electrophilic bromine source, which forms a bromonium intermediate A after reaction with the alkene. Intramolecular nucleophilic attack from nitrogen forms the product 54.
Scheme 29: Aminobromocyclisation of homoallylic sulfonamides 53. The cis:trans ratios based on the 1H NMR of the corresponding products are given in parentheses.
Scheme 29: Aminobromocyclisation of homoallylic sulfonamides 53. The cis:trans ratios based on the 1H NMR of t...
Chiba and co-worker reported the synthesis of cyclic imines using a one-pot protocol involving Grignard addition to a cyano group followed by PhI(OAc)2 (Scheme 30) [51]. The authors used p-tolylmagnesium bromide for both the arylation of the unsaturated carbonitriles 55 and as a bromide source. Bromocyclisation was achieved using PhI(OAc)2, which formed a range of 5- and 6-membered bromomethyl cyclic imines 56 in good yields from unsaturated imines 57.
Scheme 30: Synthesis of cyclic imines 45.
Scheme 30: Synthesis of cyclic imines 45.
Xia and co-workers reported the bromocyclisation of indole derivatives 58 (Scheme 31) using PIDA and CuBr2 as the oxidant and bromide source, respectively [52]. Racemic pyrrolo[2,3-b]indoles 59 were synthesised in up to quantitative yields under mild reaction conditions at room temperature. A range of other indole derivatives were cyclised in similarly good yields demonstrating the scope of the reaction.
Scheme 31: Synthesis of brominated pyrrolo[2,3-b]indoles 59.
Scheme 31: Synthesis of brominated pyrrolo[2,3-b]indoles 59.
Li and Liu reported the bromoamidation of alkenes in 2014 (Scheme 32) [30]. Using PhI(OAc)2 as an oxidant and LiBr as a source of bromine, a range of unsaturated amines were successfully cyclised to from 5- and 6-membered aza-heterocycles 60 under mild conditions at room temperature.
Cariou, Dodd and co-workers reported in 2015 the synthesis of brominated cyclic guanidines 61 (Scheme 33) alongside their chlorinated cyclic guanidines 44 (vide supra) [47]. Koser’s reagent was employed with LiBr to form 5- and 6-membered brominated cyclic guanidines 61 and 61’ in good yields from allylic guanidines 43. A range of substrates with substituents on the terminal alkene were investigated, which were successfully cyclised to brominated 5- or 6-membered rings in good yields.
Scheme 33: Synthesis of brominated cyclic guanidines 61 and 61’.
Scheme 33: Synthesis of brominated cyclic guanidines 61 and 61’.
The intramolecular bromocyclisation of N-oxyureas was also reported by Cariou and co-workers in 2019 (Scheme 34) [53]. From the same starting material, the authors reported the synthesis of both oxazolidinone oximes 63 and N-hydroxylated ureas 64 depending on the reagent system used. Formation of oxazolidinone oximes 63 occurred using PhI(OCOCF3)2 (PIFA) as an oxidant with pyridine·HBr and the MgO additive. The oxybromocyclisation of a range of unsaturated N-alkoxyureas 62 occurred rapidly in 10 minutes at room temperature in acetonitrile with good yields. Formation of the N-hydroxylated ureas 64 occurred using PhI(OPiv)2 and TBABr, with MgO as an additive to trap acetic acid. Aminobromocylization of a range of unsaturated N-alkoxyureas 62 was less successful with longer reactions times up to 1 hours required and poorer yields afforded. The rationale for the difference in mechanism was attributed to the oxycyclisation to yield oxazolidinone oximes 63 occurring through an ionic mechanism, whereas the aminocyclisation takes place through a radical manifold, a difference that is triggered by the difference in HVI reagent used.
Scheme 34: Intramolecular bromocyclisation of N-oxyureas.
Scheme 34: Intramolecular bromocyclisation of N-oxyureas.
In 2023, Du and co-workers reported a method for synthesizing 3-bromoindoles via a cascade oxidative cyclisation–halogenation encompassing oxidative C−N/C−Br bond formation, and utilising phenyliodine(III) diacetate (PIDA) in combination with LiBr in HFIP (Scheme 35) [54]. The reaction of 2-alkenylanilines 65 with PIDA and LiBr resulted in the successful synthesis of various 3-bromoindoles 66 in high yields obtained under the optimised reaction conditions, highlighting the efficiency of the synthetic protocol. The proposed mechanism suggests that the reactive AcO–Br species is formed in situ from the reaction of PIDA and LiBr.
Scheme 35: The formation of 3-bromoindoles.
Scheme 35: The formation of 3-bromoindoles.
Oxygen nucleophiles
A novel use of HVI reagents that promotes bromocyclization was reported by Braddock and co-workers in 2006 (Scheme 36) [55]. The authors reported the use of a bromoiodinane, formed in situ from ortho-substituted amidine iodobenzene 67 and N-bromosuccinimide (NBS), which promoted the intramolecular bromolactonisation of unsaturated acids 68. The authors investigated the oxidation using a variety of ortho-substituted iodobenzenes. Increasing the nucleophilicity of the groups at the ortho-substituted positions of iodobenzene gave increased yields of cyclised product. Both 5- and 6-membered bromolactone products 69 and 70 were formed with 5-exo ring closure preferred.
Scheme 36: Bromolactonisation of unsaturated acids 68.
Scheme 36: Bromolactonisation of unsaturated acids 68.
In addition to the synthesis of 5-chloromethyl-2-oxazolines 49, Li and co-workers reported the preparation of 5-bromomethyl-2-oxazolines 71 (Scheme 37) [48]. Treatment of a range of N-allyl carboxamides 31 with PhI(OAc)2 and TMSBr formed 5-bromomethyl-2-oxazolines 71 in excellent yields.
Scheme 37: Synthesis of 5-bromomethyl-2-oxazolines.
Scheme 37: Synthesis of 5-bromomethyl-2-oxazolines.
In 2015, Wang and co-workers reported the bromocyclisation of allylamino alcohols 72 to give chiral morpholines 73 (Scheme 38) [56]. Using an amino acid-derived chiral HVI reagent with KBr and NaOAc, a range of chiral 2,3,6-trisubstituted bromomethylmorpholines 73 were synthesised in excellent yields and diastereoselectivities that ranged from nothing to excellent depending on the substrate. The enantioselectivity of the reaction was not measured. The authors suggested a mechanism for the reaction (Scheme 38) in which activation of the alkene and intramolecular attack of oxygen through a 6-exo-trig mechanism followed by SN2 reaction with bromide eliminates the chiral aryliodide to form the product 73.
Scheme 38: Synthesis of brominated chiral morpholines.
Scheme 38: Synthesis of brominated chiral morpholines.
In 2018, Liu, Ling and co-workers reported bromoenol-cyclisation of unsaturated dicarbonyls 74 (Scheme 39) [57]. Using PhI(OAc)2 as an oxidant and TMSBr as a source of bromine and reaction promoter, a range of bromomethyldihydrofurans 75 were cyclised in good yields. Both 5- and 6-membered rings were formed, with homologation of the unsaturated chain. The authors initially proposed two alternative mechanisms for the reaction (Scheme 39). Either the reaction of PhI(OAc)2 (PIDA) with TMSBr generates BrOAc, which forms a bromonium ion A with the alkene, followed by intramolecular nucleophilic attack of oxygen to form the cyclic product 75. Alternatively, the alkene could be activated after coordination to PhI(OAc)2, then intramolecular nucleophilic attack from oxygen and nucleophilic attack by bromide forms the final product. NMR studies of PIDA and the substrate indicated that there was no interaction between them, thereby discounting this second pathway and thereby providing support for the former pathway (Scheme 39).
Scheme 39: Bromoenolcyclisation of unsaturated dicarbonyl groups.
Scheme 39: Bromoenolcyclisation of unsaturated dicarbonyl groups.
In 2023, Chai, Jiang, Zhu and co-workers included the oxybromination of alkenes to form brominated dihydro-[1,3]-oxazines 76 and 2-oxazoline 77 derivative (Scheme 40) [6,49], alongside their chlorination examples. Optimized reaction conditions were developed with BBr3 as bromide source and activating reagent, which led to the formation of the brominated oxazines 76 and 2-oxazoline 77 in very good to excellent yields. The authors found that when substituted N-(2-phenylallyl)benzamides 52 were tolerated, it led to the formation of brominated 2-oxazolines in excellent yields. The structures were also assigned by X-ray crystallography.
Scheme 40: Brominated oxazines and oxazolines with BBr3.
Scheme 40: Brominated oxazines and oxazolines with BBr3.
Sulfur nucleophiles
In 2015, Li and co-workers reported the synthesis of 5-bromomethyl-2-phenylthiazoline (79, Scheme 41) [48]. Sulfur was used as the internal nucleophile instead of nitrogen, as previously reported by the authors in the formation of oxazolines. Using PhI(OAc)2 with TMSBr as an oxidant and source of bromine respectively, N-allylbenzothioamide (78) was cyclised to form 5-bromomethtyl-2-phenylthiazoline (79) in a good yield.
Scheme 41: Synthesis of 5-bromomethtyl-2-phenylthiazoline.
Scheme 41: Synthesis of 5-bromomethtyl-2-phenylthiazoline.
Hypervalent iodine-mediated iodocyclisation
Reactions involving iodocyclisation mediated by HVI compounds are less prevalent compared to other halocyclisations. The use of PhI(OAc)2 with KI or TMSI as the iodide source has been reported to promote iodocyclisation in unsaturated compounds with internal nucleophiles.
Nitrogen nucleophiles
In addition to both chloro- and bromoaminations, Liu and Li reported intramolecular iodoamidiation of unsaturated amines in 2014 (Scheme 42) [30]. Using PIDA as an oxidant and KI as a source of iodide, iodinated pyrrolidines 80 were synthesized in excellent yields under mild conditions of CH2Cl2 at room temperature. A range of unsaturated amines were successfully cyclised to form 5-membered rings. Lower yields were observed with substituted alkenes. A mechanism was proposed by the authors identical to both chloro- and bromoaminations previously reported.
Scheme 42: Intramolecular iodoamination of unsaturated amines.
Scheme 42: Intramolecular iodoamination of unsaturated amines.
Du and co-workers described the formation of 3-iodoindoles 81 in their 2023 report that also demonstrated the formation of 3-bromoindoles 66 (Scheme 43) [54]. In this instance, KI was used as the iodide source with PIDA in HFIP. The mechanism proposed was the same as that for the bromoindoles (Scheme 35).
Oxygen nucleophiles
In addition to chloroamidation and chlorolactonization, iodoetherification of 2,2-diphenyl-4-penten-1-carboxylic acid (47') and 2,2-diphenyl-4-penten-1-ol (47) was reported by Liu and Li in 2014 (Scheme 26) [30]. The authors reported using PhI(OAc)2 and KI in the synthesis of iodonated γ-butyrolactone 83 and iodomethyltetrahydrofuran 82 in excellent yields (Scheme 44).
Scheme 44: Iodoetherification of 2,2-diphenyl-4-penten-1-carboxylic acid (47’) and 2,2-diphenyl-4-penten-1-ol (47).
Scheme 44: Iodoetherification of 2,2-diphenyl-4-penten-1-carboxylic acid (47’) and 2,2-diphenyl-4-penten-1-ol (...
Li and co-workers reported the synthesis of 5-iodomethyl-2-aryloxazolines 84 in addition to the synthesis of 5-chloromethyl-2-aryloxazolines 49 and 5-bromomethyl-2-aryloxzaolines 71 (Scheme 27) [48]. Treatment of a N-allyl carboxamides 31 with PhI(OCOCH3)2 as an oxidant and TMSI as a source of iodide formed a range of 5-iodomethyl-2-aryloxazolines 84 in good yields (Scheme 45).
Scheme 45: Synthesis of 5-iodomethyl-2-oxazolines.
Scheme 45: Synthesis of 5-iodomethyl-2-oxazolines.
In addition to the synthesis of brominated morpholines, Wang and co-workers reported the synthesis of chiral iodinated morpholines 85 (Scheme 46) [56]. Using an amino acid-derived chiral iodine(III) reagent with KI and NaOAc, a range of allylamino alcohols 72 were cyclised in excellent yields, again without reporting the enantioselectivity of the reaction.
Scheme 46: Synthesis of chiral iodinated morpholines. aFrom the ʟ-form of the amino acid starting material. The dr values were determined by 1H NMR given in parentheses.
Scheme 46: Synthesis of chiral iodinated morpholines. aFrom the ʟ-form of the amino acid starting material. Th...
As well as bromoenol cyclisation (Scheme 39), Liu, Ling and co-workers reported iodoenolcyclisation of unsaturated dicarbonyl compounds 74 in 2018 (Scheme 47) [57]. Using PhI(OAc)2 and TMSI, a range of polysubstituted iodomethyldihydrofurans 86 were successfully synthesised in good yields. From NMR measurements, the authors proposed the formation of an iodonium intermediate.
Scheme 47: Iodoenolcyclisation of unsaturated dicarbonyl compounds 74.
Scheme 47: Iodoenolcyclisation of unsaturated dicarbonyl compounds 74.
Sulphur nucleophiles
Li and co-workers reported the synthesis of 5-iodomethyl-2-phenylthiazoline (87) in 2015 in addition to the synthesis of 5-bromomethtyl-2-thiazoline (Scheme 41), using sulfur as an internal nucleophile (Scheme 48) [48]. Using PhI(OAc)2 with TMSI, N-allylbenzothioamide (78) was cyclised to form 87 in excellent yield.
Scheme 48: Synthesis of 5-iodomethtyl-2-phenylthiazoline (87).
Scheme 48: Synthesis of 5-iodomethtyl-2-phenylthiazoline (87).
Conclusion
The HVI-mediated halocyclization of alkenes is an important approach that yields a broad variety of heterocycles under mild and efficient oxidative conditions. The internal nucleophile, length of the carbon chain and halide can be designed such that a very broad range of 5-, 6- and 7-membered heterocycles can be accessed. The identity of the HVI reagent has also shown to dictate the regioselectivity of the reaction. Future research efforts should focus on further developing access to new heterocycles, as well as designing better systems to incorporate high levels of diastereoselectivity and enantioselectivity into chiral halogenated heterocycles.
Data Availability Statement
Data sharing is not applicable as no new data was generated or analyzed in this study.
References
-
Gerebtzoff, G.; Li‐Blatter, X.; Fischer, H.; Frentzel, A.; Seelig, A. ChemBioChem 2004, 5, 676–684. doi:10.1002/cbic.200400017
Return to citation in text: [1] -
Hernandes, M. Z.; Cavalcanti, S. M. T.; Moreira, D. R. M.; de Azevedo Junior, W. F.; Leite, A. C. L. Curr. Drug Targets 2010, 11, 303–314. doi:10.2174/138945010790711996
Return to citation in text: [1] -
Depew, K. M.; Marsden, S. P.; Zatorska, D.; Zatorski, A.; Bornmann, W. G.; Danishefsky, S. J. J. Am. Chem. Soc. 1999, 121, 11953–11963. doi:10.1021/ja991558d
Return to citation in text: [1] -
Li Petri, G.; Spanò, V.; Spatola, R.; Holl, R.; Raimondi, M. V.; Barraja, P.; Montalbano, A. Eur. J. Med. Chem. 2020, 208, 112783. doi:10.1016/j.ejmech.2020.112783
Return to citation in text: [1] -
Li, Q.; Woods, K. W.; Claiborne, A.; Gwaltney, II, S. L.; Barr, K. J.; Liu, G.; Gehrke, L.; Credo, R. B.; Hui, Y. H.; Lee, J.; Warner, R. B.; Kovar, P.; Nukkala, M. A.; Zielinski, N. A.; Tahir, S. K.; Fitzgerald, M.; Kim, K. H.; Marsh, K.; Frost, D.; Ng, S.-C.; Rosenberg, S.; Sham, H. L. Bioorg. Med. Chem. Lett. 2002, 12, 465–469. doi:10.1016/s0960-894x(01)00759-4
Return to citation in text: [1] -
Chai, H.; Zhen, X.; Wang, X.; Qi, L.; Qin, Y.; Xue, J.; Xu, Z.; Zhang, H.; Zhu, W. ACS Omega 2022, 7, 19988–19996. doi:10.1021/acsomega.2c01791
Return to citation in text: [1] [2] [3] [4] [5] -
Zhang, Y.; Zhong, H.; Wang, T.; Geng, D.; Zhang, M.; Li, K. Eur. J. Med. Chem. 2012, 48, 69–80. doi:10.1016/j.ejmech.2011.11.036
Return to citation in text: [1] -
Nangunuri, B. G.; Shirke, R. P.; Kim, M.-h. Org. Biomol. Chem. 2023, 21, 960–965. doi:10.1039/d2ob02077g
Return to citation in text: [1] -
Singh, F. V.; Kole, P. B.; Mangaonkar, S. R.; Shetgaonkar, S. E. Beilstein J. Org. Chem. 2018, 14, 1778–1805. doi:10.3762/bjoc.14.152
Return to citation in text: [1] -
Bora, D.; Kaushal, A.; Shankaraiah, N. Eur. J. Med. Chem. 2021, 215, 113263. doi:10.1016/j.ejmech.2021.113263
Return to citation in text: [1] -
Bahrin, L. G.; Hopf, H.; Jones, P. G.; Sarbu, L. G.; Babii, C.; Mihai, A. C.; Stefan, M.; Birsa, L. M. Beilstein J. Org. Chem. 2016, 12, 1065–1071. doi:10.3762/bjoc.12.100
Return to citation in text: [1] -
Pirrung, M. C.; Tumey, L. N.; McClerren, A. L.; Raetz, C. R. H. J. Am. Chem. Soc. 2003, 125, 1575–1586. doi:10.1021/ja0209114
Return to citation in text: [1] -
Goossens, F.; Vanhoof, G.; De Meester, I.; Augustyns, K.; Borloo, M.; Tourwe, D.; Haemers, A.; Scharpé, S. Eur. J. Biochem. 1997, 250, 177–183. doi:10.1111/j.1432-1033.1997.00177.x
Return to citation in text: [1] -
Wang, X.; Studer, A. Acc. Chem. Res. 2017, 50, 1712–1724. doi:10.1021/acs.accounts.7b00148
Return to citation in text: [1] -
Singh, F. V.; Shetgaonkar, S. E.; Krishnan, M.; Wirth, T. Chem. Soc. Rev. 2022, 51, 8102–8139. doi:10.1039/d2cs00206j
Return to citation in text: [1] -
Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547
Return to citation in text: [1] -
Sun, J.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Rep. Org. Chem. 2016, 6, 25–45. doi:10.2147/roc.s84894
Return to citation in text: [1] -
Alam, M. M.; Hussien, M.; Bollikolla, H. B.; Seema, V.; Dubasi, N.; Amanullah, M.; Varala, R. J. Heterocycl. Chem. 2023, 60, 1326–1355. doi:10.1002/jhet.4627
Return to citation in text: [1] -
Reddy Kandimalla, S.; Prathima Parvathaneni, S.; Sabitha, G.; Subba Reddy, B. V. Eur. J. Org. Chem. 2019, 1687–1714. doi:10.1002/ejoc.201801469
Return to citation in text: [1] -
Li, X.; Chen, P.; Liu, G. Beilstein J. Org. Chem. 2018, 14, 1813–1825. doi:10.3762/bjoc.14.154
Return to citation in text: [1] -
Lee, J. H.; Choi, S.; Hong, K. B. Molecules 2019, 24, 2634. doi:10.3390/molecules24142634
Return to citation in text: [1] -
Smart, B. E. J. Fluorine Chem. 2001, 109, 3–11. doi:10.1016/s0022-1139(01)00375-x
Return to citation in text: [1] -
Xu, Z.; Yang, Z.; Liu, Y.; Lu, Y.; Chen, K.; Zhu, W. J. Chem. Inf. Model. 2014, 54, 69–78. doi:10.1021/ci400539q
Return to citation in text: [1] -
Marshall, C. M.; Federice, J. G.; Bell, C. N.; Cox, P. B.; Njardarson, J. T. J. Med. Chem. 2024, 67, 11622–11655. doi:10.1021/acs.jmedchem.4c01122
Return to citation in text: [1] -
Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. iScience 2020, 23, 101467. doi:10.1016/j.isci.2020.101467
Return to citation in text: [1] -
Wang, Q.; Zhong, W.; Wei, X.; Ning, M.; Meng, X.; Li, Z. Org. Biomol. Chem. 2012, 10, 8566–8569. doi:10.1039/c2ob26664d
Return to citation in text: [1] -
Wu, T.; Yin, G.; Liu, G. J. Am. Chem. Soc. 2009, 131, 16354–16355. doi:10.1021/ja9076588
Return to citation in text: [1] -
Kong, W.; Feige, P.; de Haro, T.; Nevado, C. Angew. Chem., Int. Ed. 2013, 52, 2469–2473. doi:10.1002/anie.201208471
Return to citation in text: [1] -
Suzuki, S.; Kamo, T.; Fukushi, K.; Hiramatsu, T.; Tokunaga, E.; Dohi, T.; Kita, Y.; Shibata, N. Chem. Sci. 2014, 5, 2754–2760. doi:10.1039/c3sc53107d
Return to citation in text: [1] -
Liu, G.-Q.; Li, Y.-M. J. Org. Chem. 2014, 79, 10094–10109. doi:10.1021/jo501739j
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Yuan, W.; Szabó, K. J. Angew. Chem. 2015, 127, 8653–8657. doi:10.1002/ange.201503373
Return to citation in text: [1] [2] [3] -
Cui, J.; Jia, Q.; Feng, R.-Z.; Liu, S.-S.; He, T.; Zhang, C. Org. Lett. 2014, 16, 1442–1445. doi:10.1021/ol500238k
Return to citation in text: [1] -
Kitamura, T.; Miyake, A.; Muta, K.; Oyamada, J. J. Org. Chem. 2017, 82, 11721–11726. doi:10.1021/acs.joc.7b01266
Return to citation in text: [1] [2] -
Mennie, K. M.; Banik, S. M.; Reichert, E. C.; Jacobsen, E. N. J. Am. Chem. Soc. 2018, 140, 4797–4802. doi:10.1021/jacs.8b02143
Return to citation in text: [1] -
Pavlović, R. Z.; Kop, T. J.; Nešić, M.; Stepanović, O.; Wang, X.; Todorović, N.; Rodić, M. V.; Šmit, B. M. J. Org. Chem. 2023, 88, 10946–10959. doi:10.1021/acs.joc.3c00944
Return to citation in text: [1] -
Sawaguchi, M.; Hara, S.; Fukuhara, T.; Yoneda, N. J. Fluorine Chem. 2000, 104, 277–280. doi:10.1016/s0022-1139(00)00241-4
Return to citation in text: [1] -
Ulmer, A.; Brunner, C.; Arnold, A. M.; Pöthig, A.; Gulder, T. Chem. – Eur. J. 2016, 22, 3660–3664. doi:10.1002/chem.201504749
Return to citation in text: [1] -
Geary, G. C.; Hope, E. G.; Stuart, A. M. Angew. Chem., Int. Ed. 2015, 54, 14911–14914. doi:10.1002/anie.201507790
Return to citation in text: [1] -
Scheidt, F.; Thiehoff, C.; Yilmaz, G.; Meyer, S.; Daniliuc, C. G.; Kehr, G.; Gilmour, R. Beilstein J. Org. Chem. 2018, 14, 1021–1027. doi:10.3762/bjoc.14.88
Return to citation in text: [1] -
Haupt, J. D.; Berger, M.; Waldvogel, S. R. Org. Lett. 2019, 21, 242–245. doi:10.1021/acs.orglett.8b03682
Return to citation in text: [1] -
Coppock, S. B.; Lennox, A. J. J. Curr. Opin. Electrochem. 2022, 35, 101069. doi:10.1016/j.coelec.2022.101069
Return to citation in text: [1] -
Doobary, S.; Poole, D. L.; Lennox, A. J. J. J. Org. Chem. 2021, 86, 16095–16103. doi:10.1021/acs.joc.1c01946
Return to citation in text: [1] -
Yang, S.; Shi, S.; Chen, Y.; Ding, Z. J. Org. Chem. 2021, 86, 14004–14010. doi:10.1021/acs.joc.1c00159
Return to citation in text: [1] [2] -
Zhu, W.; Zhen, X.; Wu, J.; Cheng, Y.; An, J.; Ma, X.; Liu, J.; Qin, Y.; Zhu, H.; Xue, J.; Jiang, X. Nat. Commun. 2021, 12, 3957. doi:10.1038/s41467-021-24278-3
Return to citation in text: [1] -
Jiang, X.; Zhu, W.; Yang, L.; Zheng, Z.; Yu, C. Eur. J. Org. Chem. 2019, 2268–2274. doi:10.1002/ejoc.201801842
Return to citation in text: [1] [2] -
Chiodi, D.; Ishihara, Y. J. Med. Chem. 2023, 66, 5305–5331. doi:10.1021/acs.jmedchem.2c02015
Return to citation in text: [1] -
Daniel, M.; Blanchard, F.; Nocquet-Thibault, S.; Cariou, K.; Dodd, R. H. J. Org. Chem. 2015, 80, 10624–10633. doi:10.1021/acs.joc.5b01750
Return to citation in text: [1] [2] -
Liu, G.-Q.; Yang, C.-H.; Li, Y.-M. J. Org. Chem. 2015, 80, 11339–11350. doi:10.1021/acs.joc.5b01832
Return to citation in text: [1] [2] [3] [4] [5] -
Qin, Y.; Qi, L.; Zhen, X.; Wang, X.; Chai, H.; Ma, X.; Jiang, X.; Cai, X.; Zhu, W. J. Org. Chem. 2023, 88, 4359–4371. doi:10.1021/acs.joc.2c02967
Return to citation in text: [1] [2] [3] -
Fan, R.; Wen, F.; Qin, L.; Pu, D.; Wang, B. Tetrahedron Lett. 2007, 48, 7444–7447. doi:10.1016/j.tetlet.2007.08.085
Return to citation in text: [1] -
Sanjaya, S.; Chiba, S. Tetrahedron 2011, 67, 590–596. doi:10.1016/j.tet.2010.11.060
Return to citation in text: [1] -
Tu, D.; Ma, L.; Tong, X.; Deng, X.; Xia, C. Org. Lett. 2012, 14, 4830–4833. doi:10.1021/ol302158h
Return to citation in text: [1] -
Peilleron, L.; Retailleau, P.; Cariou, K. Adv. Synth. Catal. 2019, 361, 5160–5169. doi:10.1002/adsc.201901135
Return to citation in text: [1] -
Zhao, B.; Li, X.; Wang, X.; Jiang, L.; Li, Z.; Du, Y. J. Org. Chem. 2023, 88, 1493–1503. doi:10.1021/acs.joc.2c02480
Return to citation in text: [1] [2] -
Braddock, D. C.; Cansell, G.; Hermitage, S. A. Chem. Commun. 2006, 2483–2485. doi:10.1039/b604130b
Return to citation in text: [1] -
Kishore Vandavasi, J.; Hu, W.-P.; Chandru Senadi, G.; Chen, H.-T.; Chen, H.-Y.; Hsieh, K.-C.; Wang, J.-J. Adv. Synth. Catal. 2015, 357, 2788–2794. doi:10.1002/adsc.201500177
Return to citation in text: [1] [2] -
Liu, J.; Liu, Q.-Y.; Fang, X.-X.; Liu, G.-Q.; Ling, Y. Org. Biomol. Chem. 2018, 16, 7454–7460. doi:10.1039/c8ob02161a
Return to citation in text: [1] [2]
| 38. | Geary, G. C.; Hope, E. G.; Stuart, A. M. Angew. Chem., Int. Ed. 2015, 54, 14911–14914. doi:10.1002/anie.201507790 |
| 33. | Kitamura, T.; Miyake, A.; Muta, K.; Oyamada, J. J. Org. Chem. 2017, 82, 11721–11726. doi:10.1021/acs.joc.7b01266 |
| 39. | Scheidt, F.; Thiehoff, C.; Yilmaz, G.; Meyer, S.; Daniliuc, C. G.; Kehr, G.; Gilmour, R. Beilstein J. Org. Chem. 2018, 14, 1021–1027. doi:10.3762/bjoc.14.88 |
| 31. | Yuan, W.; Szabó, K. J. Angew. Chem. 2015, 127, 8653–8657. doi:10.1002/ange.201503373 |
| 43. | Yang, S.; Shi, S.; Chen, Y.; Ding, Z. J. Org. Chem. 2021, 86, 14004–14010. doi:10.1021/acs.joc.1c00159 |
| 44. | Zhu, W.; Zhen, X.; Wu, J.; Cheng, Y.; An, J.; Ma, X.; Liu, J.; Qin, Y.; Zhu, H.; Xue, J.; Jiang, X. Nat. Commun. 2021, 12, 3957. doi:10.1038/s41467-021-24278-3 |
| 6. | Chai, H.; Zhen, X.; Wang, X.; Qi, L.; Qin, Y.; Xue, J.; Xu, Z.; Zhang, H.; Zhu, W. ACS Omega 2022, 7, 19988–19996. doi:10.1021/acsomega.2c01791 |
| 42. | Doobary, S.; Poole, D. L.; Lennox, A. J. J. J. Org. Chem. 2021, 86, 16095–16103. doi:10.1021/acs.joc.1c01946 |
| 43. | Yang, S.; Shi, S.; Chen, Y.; Ding, Z. J. Org. Chem. 2021, 86, 14004–14010. doi:10.1021/acs.joc.1c00159 |
| 40. | Haupt, J. D.; Berger, M.; Waldvogel, S. R. Org. Lett. 2019, 21, 242–245. doi:10.1021/acs.orglett.8b03682 |
| 41. | Coppock, S. B.; Lennox, A. J. J. Curr. Opin. Electrochem. 2022, 35, 101069. doi:10.1016/j.coelec.2022.101069 |
| 45. | Jiang, X.; Zhu, W.; Yang, L.; Zheng, Z.; Yu, C. Eur. J. Org. Chem. 2019, 2268–2274. doi:10.1002/ejoc.201801842 |
| 46. | Chiodi, D.; Ishihara, Y. J. Med. Chem. 2023, 66, 5305–5331. doi:10.1021/acs.jmedchem.2c02015 |
| 30. | Liu, G.-Q.; Li, Y.-M. J. Org. Chem. 2014, 79, 10094–10109. doi:10.1021/jo501739j |
| 47. | Daniel, M.; Blanchard, F.; Nocquet-Thibault, S.; Cariou, K.; Dodd, R. H. J. Org. Chem. 2015, 80, 10624–10633. doi:10.1021/acs.joc.5b01750 |
| 50. | Fan, R.; Wen, F.; Qin, L.; Pu, D.; Wang, B. Tetrahedron Lett. 2007, 48, 7444–7447. doi:10.1016/j.tetlet.2007.08.085 |
| 51. | Sanjaya, S.; Chiba, S. Tetrahedron 2011, 67, 590–596. doi:10.1016/j.tet.2010.11.060 |
| 49. | Qin, Y.; Qi, L.; Zhen, X.; Wang, X.; Chai, H.; Ma, X.; Jiang, X.; Cai, X.; Zhu, W. J. Org. Chem. 2023, 88, 4359–4371. doi:10.1021/acs.joc.2c02967 |
| 6. | Chai, H.; Zhen, X.; Wang, X.; Qi, L.; Qin, Y.; Xue, J.; Xu, Z.; Zhang, H.; Zhu, W. ACS Omega 2022, 7, 19988–19996. doi:10.1021/acsomega.2c01791 |
| 48. | Liu, G.-Q.; Yang, C.-H.; Li, Y.-M. J. Org. Chem. 2015, 80, 11339–11350. doi:10.1021/acs.joc.5b01832 |
| 6. | Chai, H.; Zhen, X.; Wang, X.; Qi, L.; Qin, Y.; Xue, J.; Xu, Z.; Zhang, H.; Zhu, W. ACS Omega 2022, 7, 19988–19996. doi:10.1021/acsomega.2c01791 |
| 49. | Qin, Y.; Qi, L.; Zhen, X.; Wang, X.; Chai, H.; Ma, X.; Jiang, X.; Cai, X.; Zhu, W. J. Org. Chem. 2023, 88, 4359–4371. doi:10.1021/acs.joc.2c02967 |
| 45. | Jiang, X.; Zhu, W.; Yang, L.; Zheng, Z.; Yu, C. Eur. J. Org. Chem. 2019, 2268–2274. doi:10.1002/ejoc.201801842 |
| 30. | Liu, G.-Q.; Li, Y.-M. J. Org. Chem. 2014, 79, 10094–10109. doi:10.1021/jo501739j |
| 30. | Liu, G.-Q.; Li, Y.-M. J. Org. Chem. 2014, 79, 10094–10109. doi:10.1021/jo501739j |
| 47. | Daniel, M.; Blanchard, F.; Nocquet-Thibault, S.; Cariou, K.; Dodd, R. H. J. Org. Chem. 2015, 80, 10624–10633. doi:10.1021/acs.joc.5b01750 |
| 52. | Tu, D.; Ma, L.; Tong, X.; Deng, X.; Xia, C. Org. Lett. 2012, 14, 4830–4833. doi:10.1021/ol302158h |
| 1. | Gerebtzoff, G.; Li‐Blatter, X.; Fischer, H.; Frentzel, A.; Seelig, A. ChemBioChem 2004, 5, 676–684. doi:10.1002/cbic.200400017 |
| 2. | Hernandes, M. Z.; Cavalcanti, S. M. T.; Moreira, D. R. M.; de Azevedo Junior, W. F.; Leite, A. C. L. Curr. Drug Targets 2010, 11, 303–314. doi:10.2174/138945010790711996 |
| 9. | Singh, F. V.; Kole, P. B.; Mangaonkar, S. R.; Shetgaonkar, S. E. Beilstein J. Org. Chem. 2018, 14, 1778–1805. doi:10.3762/bjoc.14.152 |
| 10. | Bora, D.; Kaushal, A.; Shankaraiah, N. Eur. J. Med. Chem. 2021, 215, 113263. doi:10.1016/j.ejmech.2021.113263 |
| 26. | Wang, Q.; Zhong, W.; Wei, X.; Ning, M.; Meng, X.; Li, Z. Org. Biomol. Chem. 2012, 10, 8566–8569. doi:10.1039/c2ob26664d |
| 6. | Chai, H.; Zhen, X.; Wang, X.; Qi, L.; Qin, Y.; Xue, J.; Xu, Z.; Zhang, H.; Zhu, W. ACS Omega 2022, 7, 19988–19996. doi:10.1021/acsomega.2c01791 |
| 49. | Qin, Y.; Qi, L.; Zhen, X.; Wang, X.; Chai, H.; Ma, X.; Jiang, X.; Cai, X.; Zhu, W. J. Org. Chem. 2023, 88, 4359–4371. doi:10.1021/acs.joc.2c02967 |
| 7. | Zhang, Y.; Zhong, H.; Wang, T.; Geng, D.; Zhang, M.; Li, K. Eur. J. Med. Chem. 2012, 48, 69–80. doi:10.1016/j.ejmech.2011.11.036 |
| 8. | Nangunuri, B. G.; Shirke, R. P.; Kim, M.-h. Org. Biomol. Chem. 2023, 21, 960–965. doi:10.1039/d2ob02077g |
| 27. | Wu, T.; Yin, G.; Liu, G. J. Am. Chem. Soc. 2009, 131, 16354–16355. doi:10.1021/ja9076588 |
| 5. | Li, Q.; Woods, K. W.; Claiborne, A.; Gwaltney, II, S. L.; Barr, K. J.; Liu, G.; Gehrke, L.; Credo, R. B.; Hui, Y. H.; Lee, J.; Warner, R. B.; Kovar, P.; Nukkala, M. A.; Zielinski, N. A.; Tahir, S. K.; Fitzgerald, M.; Kim, K. H.; Marsh, K.; Frost, D.; Ng, S.-C.; Rosenberg, S.; Sham, H. L. Bioorg. Med. Chem. Lett. 2002, 12, 465–469. doi:10.1016/s0960-894x(01)00759-4 |
| 6. | Chai, H.; Zhen, X.; Wang, X.; Qi, L.; Qin, Y.; Xue, J.; Xu, Z.; Zhang, H.; Zhu, W. ACS Omega 2022, 7, 19988–19996. doi:10.1021/acsomega.2c01791 |
| 23. | Xu, Z.; Yang, Z.; Liu, Y.; Lu, Y.; Chen, K.; Zhu, W. J. Chem. Inf. Model. 2014, 54, 69–78. doi:10.1021/ci400539q |
| 24. | Marshall, C. M.; Federice, J. G.; Bell, C. N.; Cox, P. B.; Njardarson, J. T. J. Med. Chem. 2024, 67, 11622–11655. doi:10.1021/acs.jmedchem.4c01122 |
| 56. | Kishore Vandavasi, J.; Hu, W.-P.; Chandru Senadi, G.; Chen, H.-T.; Chen, H.-Y.; Hsieh, K.-C.; Wang, J.-J. Adv. Synth. Catal. 2015, 357, 2788–2794. doi:10.1002/adsc.201500177 |
| 3. | Depew, K. M.; Marsden, S. P.; Zatorska, D.; Zatorski, A.; Bornmann, W. G.; Danishefsky, S. J. J. Am. Chem. Soc. 1999, 121, 11953–11963. doi:10.1021/ja991558d |
| 4. | Li Petri, G.; Spanò, V.; Spatola, R.; Holl, R.; Raimondi, M. V.; Barraja, P.; Montalbano, A. Eur. J. Med. Chem. 2020, 208, 112783. doi:10.1016/j.ejmech.2020.112783 |
| 25. | Ogawa, Y.; Tokunaga, E.; Kobayashi, O.; Hirai, K.; Shibata, N. iScience 2020, 23, 101467. doi:10.1016/j.isci.2020.101467 |
| 57. | Liu, J.; Liu, Q.-Y.; Fang, X.-X.; Liu, G.-Q.; Ling, Y. Org. Biomol. Chem. 2018, 16, 7454–7460. doi:10.1039/c8ob02161a |
| 14. | Wang, X.; Studer, A. Acc. Chem. Res. 2017, 50, 1712–1724. doi:10.1021/acs.accounts.7b00148 |
| 15. | Singh, F. V.; Shetgaonkar, S. E.; Krishnan, M.; Wirth, T. Chem. Soc. Rev. 2022, 51, 8102–8139. doi:10.1039/d2cs00206j |
| 16. | Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547 |
| 20. | Li, X.; Chen, P.; Liu, G. Beilstein J. Org. Chem. 2018, 14, 1813–1825. doi:10.3762/bjoc.14.154 |
| 21. | Lee, J. H.; Choi, S.; Hong, K. B. Molecules 2019, 24, 2634. doi:10.3390/molecules24142634 |
| 55. | Braddock, D. C.; Cansell, G.; Hermitage, S. A. Chem. Commun. 2006, 2483–2485. doi:10.1039/b604130b |
| 13. | Goossens, F.; Vanhoof, G.; De Meester, I.; Augustyns, K.; Borloo, M.; Tourwe, D.; Haemers, A.; Scharpé, S. Eur. J. Biochem. 1997, 250, 177–183. doi:10.1111/j.1432-1033.1997.00177.x |
| 22. | Smart, B. E. J. Fluorine Chem. 2001, 109, 3–11. doi:10.1016/s0022-1139(01)00375-x |
| 48. | Liu, G.-Q.; Yang, C.-H.; Li, Y.-M. J. Org. Chem. 2015, 80, 11339–11350. doi:10.1021/acs.joc.5b01832 |
| 12. | Pirrung, M. C.; Tumey, L. N.; McClerren, A. L.; Raetz, C. R. H. J. Am. Chem. Soc. 2003, 125, 1575–1586. doi:10.1021/ja0209114 |
| 53. | Peilleron, L.; Retailleau, P.; Cariou, K. Adv. Synth. Catal. 2019, 361, 5160–5169. doi:10.1002/adsc.201901135 |
| 11. | Bahrin, L. G.; Hopf, H.; Jones, P. G.; Sarbu, L. G.; Babii, C.; Mihai, A. C.; Stefan, M.; Birsa, L. M. Beilstein J. Org. Chem. 2016, 12, 1065–1071. doi:10.3762/bjoc.12.100 |
| 17. | Sun, J.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Rep. Org. Chem. 2016, 6, 25–45. doi:10.2147/roc.s84894 |
| 18. | Alam, M. M.; Hussien, M.; Bollikolla, H. B.; Seema, V.; Dubasi, N.; Amanullah, M.; Varala, R. J. Heterocycl. Chem. 2023, 60, 1326–1355. doi:10.1002/jhet.4627 |
| 19. | Reddy Kandimalla, S.; Prathima Parvathaneni, S.; Sabitha, G.; Subba Reddy, B. V. Eur. J. Org. Chem. 2019, 1687–1714. doi:10.1002/ejoc.201801469 |
| 54. | Zhao, B.; Li, X.; Wang, X.; Jiang, L.; Li, Z.; Du, Y. J. Org. Chem. 2023, 88, 1493–1503. doi:10.1021/acs.joc.2c02480 |
| 30. | Liu, G.-Q.; Li, Y.-M. J. Org. Chem. 2014, 79, 10094–10109. doi:10.1021/jo501739j |
| 28. | Kong, W.; Feige, P.; de Haro, T.; Nevado, C. Angew. Chem., Int. Ed. 2013, 52, 2469–2473. doi:10.1002/anie.201208471 |
| 29. | Suzuki, S.; Kamo, T.; Fukushi, K.; Hiramatsu, T.; Tokunaga, E.; Dohi, T.; Kita, Y.; Shibata, N. Chem. Sci. 2014, 5, 2754–2760. doi:10.1039/c3sc53107d |
| 54. | Zhao, B.; Li, X.; Wang, X.; Jiang, L.; Li, Z.; Du, Y. J. Org. Chem. 2023, 88, 1493–1503. doi:10.1021/acs.joc.2c02480 |
| 30. | Liu, G.-Q.; Li, Y.-M. J. Org. Chem. 2014, 79, 10094–10109. doi:10.1021/jo501739j |
| 48. | Liu, G.-Q.; Yang, C.-H.; Li, Y.-M. J. Org. Chem. 2015, 80, 11339–11350. doi:10.1021/acs.joc.5b01832 |
| 30. | Liu, G.-Q.; Li, Y.-M. J. Org. Chem. 2014, 79, 10094–10109. doi:10.1021/jo501739j |
| 31. | Yuan, W.; Szabó, K. J. Angew. Chem. 2015, 127, 8653–8657. doi:10.1002/ange.201503373 |
| 37. | Ulmer, A.; Brunner, C.; Arnold, A. M.; Pöthig, A.; Gulder, T. Chem. – Eur. J. 2016, 22, 3660–3664. doi:10.1002/chem.201504749 |
| 35. | Pavlović, R. Z.; Kop, T. J.; Nešić, M.; Stepanović, O.; Wang, X.; Todorović, N.; Rodić, M. V.; Šmit, B. M. J. Org. Chem. 2023, 88, 10946–10959. doi:10.1021/acs.joc.3c00944 |
| 36. | Sawaguchi, M.; Hara, S.; Fukuhara, T.; Yoneda, N. J. Fluorine Chem. 2000, 104, 277–280. doi:10.1016/s0022-1139(00)00241-4 |
| 33. | Kitamura, T.; Miyake, A.; Muta, K.; Oyamada, J. J. Org. Chem. 2017, 82, 11721–11726. doi:10.1021/acs.joc.7b01266 |
| 57. | Liu, J.; Liu, Q.-Y.; Fang, X.-X.; Liu, G.-Q.; Ling, Y. Org. Biomol. Chem. 2018, 16, 7454–7460. doi:10.1039/c8ob02161a |
| 34. | Mennie, K. M.; Banik, S. M.; Reichert, E. C.; Jacobsen, E. N. J. Am. Chem. Soc. 2018, 140, 4797–4802. doi:10.1021/jacs.8b02143 |
| 48. | Liu, G.-Q.; Yang, C.-H.; Li, Y.-M. J. Org. Chem. 2015, 80, 11339–11350. doi:10.1021/acs.joc.5b01832 |
| 31. | Yuan, W.; Szabó, K. J. Angew. Chem. 2015, 127, 8653–8657. doi:10.1002/ange.201503373 |
| 48. | Liu, G.-Q.; Yang, C.-H.; Li, Y.-M. J. Org. Chem. 2015, 80, 11339–11350. doi:10.1021/acs.joc.5b01832 |
| 32. | Cui, J.; Jia, Q.; Feng, R.-Z.; Liu, S.-S.; He, T.; Zhang, C. Org. Lett. 2014, 16, 1442–1445. doi:10.1021/ol500238k |
| 56. | Kishore Vandavasi, J.; Hu, W.-P.; Chandru Senadi, G.; Chen, H.-T.; Chen, H.-Y.; Hsieh, K.-C.; Wang, J.-J. Adv. Synth. Catal. 2015, 357, 2788–2794. doi:10.1002/adsc.201500177 |
© 2024 Bansal et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.