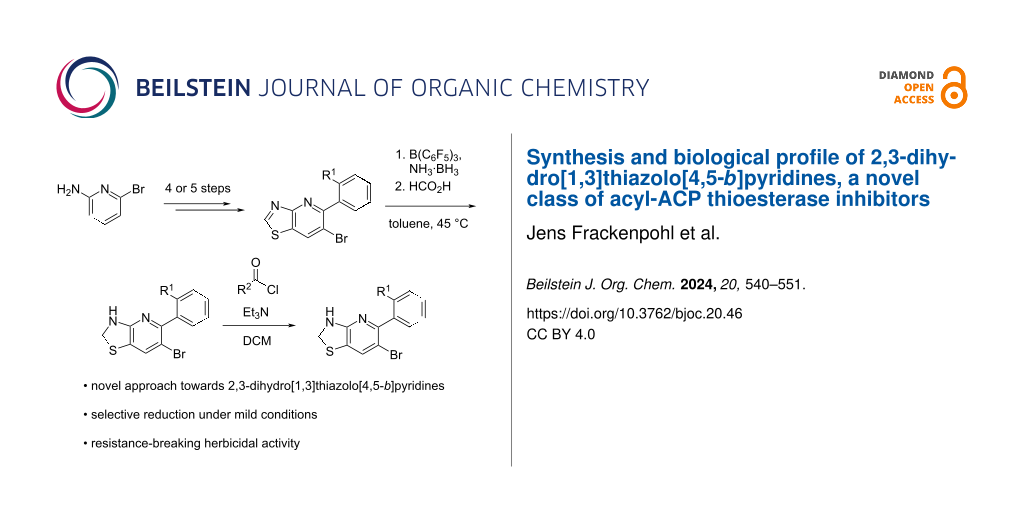
Abstract
The present work covers novel herbicidal lead structures that contain a 2,3-dihydro[1,3]thiazolo[4,5-b]pyridine scaffold as structural key feature carrying a substituted phenyl side chain. These new compounds show good acyl-ACP thioesterase inhibition in line with strong herbicidal activity against commercially important weeds in broadacre crops, e.g., wheat and corn. The desired substituted 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines were prepared via an optimized BH3-mediated reduction involving tris(pentafluorophenyl)borane as a strong Lewis acid. Remarkably, greenhouse trials showed that some of the target compounds outlined herein display promising control of grass weed species in preemergence application, combined with a dose response window that enables partial selectivity in certain crops.
Graphical Abstract
Introduction
The presence of weed infestations exerts a high strain on food production around the globe by depleting resources for the crops and facilitating the transmission of diseases [1]. Although herbicides remain the most effective solution for weed control due to the associated efficiency and simplicity, they face multiple challenges, such as the emergence and growth of resistant weed populations. It is therefore essential that crop protection research acts rapidly to provide farmers with new solutions that enable them to fight back against resistant weed species [2]. Nevertheless, discovering novel and commercially viable modes of action within the timeframe needed to significantly impact the control of resistant weeds is a demanding task. Thus, we analyzed several herbicidal modes of action with emphasis on the structural diversity of small-molecule ligands. In this context, acyl-acyl carrier protein (acyl-ACP) thioesterase inhibitors have shown a remarkable variability. Fatty acid thioesterase (FAT) enzymes represent a family of proteins exclusively found in higher plants. They mediate the release of fatty acids from the plastids to the endoplasmic reticulum, where they are utilized for the synthesis of acyl lipids that are essential components for various physiological and defensive processes [3-6]. As this enzyme target does not exist in other kingdoms, structure–activity relationship (SAR) studies on selective inhibitors reduce the prevalence of undesired effects, such as toxicity in mammals [4]. Despite being employed in the field for over three decades, the mode of action of preemergence herbicide cinmethylin (1, Scheme 1) has remained unknown until 2018. At that time, the binding affinity to enzyme targets, e.g., acyl-ACP thioesterases, belonging to the protein family of FATs, was demonstrated by using co-crystallization, fluorescence-based thermal shift assays, and chemoproteomics techniques [3]. Likewise, methiozolin (2) is a recently assigned FAT inhibitor that has shown good results in selectively controlling grass weeds in both cool and warm seasons [7-9]. Recently, it has been shown that several herbicides bearing a gem-dimethylbenzylamide motif, e.g., cumyluron (3a) and oxaziclomefone (3b), previously exhibiting an unknown mode of action, control weeds due to the inhibition of FAT [10]. In search for further chemical entities that can control resistant weed species via the inhibition of FAT, we were interested in exploring a compound class containing a 1,8-naphthyridine core that was first reported by BASF, e.g., compound 4 [11].
Scheme 1: Selected known inhibitors 1–3 of acyl-ACP thioesterases (belonging to the protein family of FATs) and new lead structures 4–7a.
Scheme 1: Selected known inhibitors 1–3 of acyl-ACP thioesterases (belonging to the protein family of FATs) a...
In contrast to bicyclic cinmethylin (1) and methiozolin (2), substituted 1,8-naphthyridine 4 does not contain any stereocenters but still displays promising efficacy against grass weeds. Further considering the rather low molecular weight (220 g/mol) and structural simplicity, compound 4 is a highly attractive initial lead structure with ample space for structural variations. By formally replacing one pyridine moiety of 1,8-naphthyridine 4 by a five-membered thiazole unit, we have identified thiazolo[4,5-b]pyridine 5 as a strong inhibitor of acyl-ACP thioesterase, which has further been confirmed via an X-ray co-crystal structure [12]. Additionally, greenhouse trials have shown that thiazolopyridine 5 and a large number of closely related analogues display excellent control of grass weed species in preemergence applications [13,14]. Independently, researchers at Syngenta have shown that the pyridine unit in the 1,8-naphthyridine scaffold can also be formally substituted by an isothiazole group, as can be seen in isothiazolo[3,4-b]pyridine 6 [15].
Thus, several bicyclic heteroaromatic motifs containing two nitrogen atoms serve as structural surrogates of cinmethylin (1), bearing a substituted 7-oxabicyclo[2.2.1]heptane scaffold [16]. Based on the findings outlined above and based on other plant-specific modes of action, it is plausible that FAT inhibitors encompass a broader range of structural motifs [16,17]. Herein, we present our approach to complement heteroaromatic lead structures 4–6 by introducing a nonaromatic motif via preparation of the novel 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 7.
Results and Discussion
Although the 2,3-dihydro[1,3]thiazolo[4,5-b]pyridine scaffold looks relatively simple at a first glance, it displays a very different reactivity compared to the parent naphthyridine series. Likewise, 1,8-naphthyridines are easily accessed in high yield and on a multigram scale via Friedländer synthesis [18]. This was in clear contrast to the intermediate thiazolo[4,5-b]pyridines and the desired 2,3-dihydro[1,3]thiazolo[4,5-b]pyridine that we wanted to access, with approaches to prepare the thiazolo[4,5-b]pyridines using the Friedländer synthesis often being met with failure or disappointingly low product yield [12]. We thus particularly emphasized on upscaling, facile workup, and a robust yield for each step. This was due to the potential need for the preparation of multigram quantities of the most active compounds for advanced biological testing. Pleasingly, our four-step approach using a potassium O-ethyl dithiocarbonate-mediated formation of thio intermediates 11a–c (thiol–thione tautomers) with subsequent sulfur removal using iron powder in acetic acid [19] proceeded smoothly to afford thiazolopyridines 12a–c in good yield. This allowed us to circumvent the previously employed alkylation–oxidation–reduction sequences (Scheme 2) [12]. Thereupon, we recognized that we could introduce two halogen atoms in the halogenation step and carry one through to the end of the synthetic route, which enabled us to introduce a methyl substituent in this position. Likewise, methyl-substituted thiazolo[4,5-b]pyridines 5, 15a, and 15c were synthesized using an optimized Suzuki coupling and served together with compounds 12a–c as key intermediates to explore different reagents and conditions to prepare 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 7a–c and 13a–c via a late-stage reduction (Scheme 2 and Table 1).
Scheme 2: Preparation of 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 7a–c and 13a–c via iron-mediated sulfur removal and subsequent reduction. dppf = 1,1'-bis(diphenylphosphino)ferrocene, SPhos = 2-dicyclohexylphosphino-2',6'-dimethoxybiphenyl, APDTC = ammonium pyrrolidinedithiocarbamate.
Scheme 2: Preparation of 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 7a–c and 13a–c via iron-mediated sulfur rem...
Table 1: Preparation of 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 7a–c via reduction of the thiazole moiety: optimization of the reaction conditions.a
|
|
||||||||
| entry | R1 | reagents | solvent | T, °C | T, h | 7a–c, %b | 17a–c, %b | 18a–c, %b |
| 1 | CH3 | H2, Pd/C, 2–20 bar | MeOH | 20 | 4 | — | — | — |
| 2 | F | H2, Pd/C, 4–35 bar | MeOH | 20 | 6 | — | — | — |
| 3 | F | N2H4, Pd/C | EtOH | 80 | 6 | — | — | — |
| 4 | F | B2(OH)4 | H2O | 80 | 6 | — | — | — |
| 5 | F | Bu4NBH4 | THF | 20 | 5 | — | — | — |
| 6 | F | Bu4NBH4 | THF | 70 | 5 | 5 | — | 48 |
| 7 | H | Bu4NBH4 | dioxane | 80 | 5 | 8 | — | 59 |
| 8 | F | NaCNBH3, AcOH | MeOH | 60 | 5 | 4 | — | 15 |
| 9 | F | SiCl3H | toluene | 110 | 3 | — | — | 35 |
| 10 | CH3 | SiEt3H | dioxane | 80 | 3 | — | — | 43 |
| 11 | F | NH3-BH3 | toluene | 80 | 4 | 5 | 4 | 12 |
| 12 | F | NH3⋅BH3, B(C6F5)3 | toluene | 80 | 4 | 16 | 33 | 18 |
| 13 | CH3 | NH3⋅BH3, B(C6F5)3 | toluene | 80 | 7 | 8 | 29 | 30 |
| 14 | CH3 | NH3⋅BH3, B(C6F5)3 | toluene | 45 | 6 | 4 | 51 | 3 |
| 15 | H | NH3⋅BH3, B(C6F5)3, HCO2H | toluene | 45 | 7 | 64c | — | 2 |
| 16 | F | NH3⋅BH3, B(C6F5)3, HCO2H | toluene | 45 | 5 | 66c | — | 5 |
| 17 | CH3 |
NH3⋅BH3, B(C6F5)3,
HCO2H |
toluene | 45 | 7 | 59c | — | 4 |
aAll reactions in the optimization phase were carried out using 0.2 mmol of 5, 15a, and 15c, respectively. bDetermined by analytical HPLC. cIsolated yield after silica gel column chromatography.
Whilst several synthetic approaches towards 2,3-dihydro-1,3-benzothiazoles involving the hydrogenation of 1,3-benzothiazoles have been described [20], the corresponding preparation of 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines remained unexplored to our great surprise. Thus, we investigated the conversion of [1,3]thiazolo[4,5-b]pyridines 5 (R1 = F), 15a (R1 = H), and 15c (R1 = CH3) into 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 7a–c thoroughly, with the aim to establish a practicable and robust synthetic route enabling us to carry out a broad SAR study. Initial attempts to prepare 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 7a and 7b using hydrogen and palladium on charcoal under elevated pressure did not show any conversion of the starting material (Table 1, entries 1 and 2). Correspondingly, [1,3]thiazolo[4,5-b]pyridine 5 remained unchanged upon application of methods that had been successfully utilized in the hydrogenation of 1,3-benzothiazoles, involving diboronic acid or hydrazine hydrate as key reagents [21] in protic solvents at an elevated temperature (Table 1, entries 3 and 4). Whilst tetrabutylammonium borohydride [22] at room temperature did not lead to a conversion of the starting material 5 either, a trace amount of desired 2,3-dihydro[1,3]thiazolo[4,5-b]pyridine 7b was formed at elevated temperature, accompanied by disulfide 18b as the main product (Table 1, entries 5 and 6). This result indicated that borohydride reagents were able to activate the thiazole moiety in [1,3]thiazolo[4,5-b]pyridines, leaving the pyridine unit unchanged. While sodium cyanoborohydride afforded a comparable result, albeit with lower conversion, the use of silane reagents at elevated temperature [23] led to the cleavage of the thiazole ring, furnishing disulfides 18b and 18c exclusively (Table 1, entries 7–10). Interestingly, the reaction of 5 with ammonia borane at elevated temperature in toluene [20] furnished three reaction products with a low yield since 2,3-dihydro[1,3]thiazolo[4,5-b]pyridine 7b was formed together with disulfide 18b and aminoborane 17b (Table 1, entry 11). We thus evaluated B(C6F5)3 as a nonmetallic catalyst to activate ammonia borane in the reductive hydrogenation of the C=N-bond in [1,3]thiazolo[4,5-b]pyridines 5 and 15c. In line with reports on the hydrogenation of quinolines and indoles [24], pyridines [25], and imines [26], the reactions of 5 and 15c with ammonia borane (3 equiv) in the presence of a catalytic amount of B(C6F5)3 in toluene as an aprotic solvent at a temperature of 80 °C afforded aminoboranes 17b (R1 = F) and 17c (R1 = CH3) as main products along with desired target compounds 7b and 7c (Table 1, entries 12 and 13). However, disulfides were still formed as side products in a significant amount. Pleasingly, the undesired thiazole cleavage could successfully be minimized by reducing the reaction temperature to 45 °C, furnishing aminoborane 17c in 51% yield (Table 1, entry 14). The borane group could be cleaved off easily by the subsequent treatment of 17c with formic acid in acetonitrile, affording 2,3-dihydro[1,3]thiazolo[4,5-b]pyridine 7c as the only reaction product. By applying this optimized two-step procedure to [1,3]thiazolo[4,5-b]pyridines 5, 15a, and 15c, the desired 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 7a–c were prepared in good yield (Table 1, entries 15–17, 59–66% isolated yield), enabling us to investigate the biological profiles as well as the reactions with acyl chlorides to form amides 16a–f (Scheme 2) [27]. These acylations proceeded cleanly under mild conditions, using the corresponding acyl chloride together with triethylamine as a suitable base in DCM.
Furthermore, we evaluated the tolerance of [1,3]thiazolo[4,5-b]pyridines 12a–c, containing a bromine atom, towards the optimized B(C6F5)3-mediated reduction. In good accordance with the results obtained for dimethylated [1,3]thiazolo[4,5-b]pyridine 15c, the corresponding aminoboranes 17d and 17e were formed when 6-bromo[1,3]thiazolo[4,5-b]pyridine 12c was treated with ammonia borane in toluene at 45 °C in the presence of a catalytic amount of B(C6F5)3 (Scheme 3). Whilst diphenyl analogue 17f was isolated as a side product upon arylation with B(C6F5)3, debromination was only observed in traces. Both aminoboranes 17d and 17e were then cleaved separately in clean conversions using formic acid to afford the desired substituted 6-bromo-5-(2-tolyl)-2,3-dihydrothiazolo[4,5-b]pyridine (13c, 54% combined isolated yield). As shown for N-acylated target compounds 16a–f, the acylation of 6-bromo-2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 13a–c, affording target compounds 14a–c, proceeded under mild conditions with a suitable acyl chloride reagent and triethylamine as base in DCM. It was not necessary to add a further base to activate the thiazoline nitrogen atom. Following the aforementioned two-step procedure, more than 15 unprecedented 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines bearing different substituents were obtained for biological and biochemical tests.
Scheme 3: Evaluation of potential side reactions in the borane-mediated preparation of 2,3-dihydro[1,3]thiazolo[4,5-b]pyridine 13c.
Scheme 3: Evaluation of potential side reactions in the borane-mediated preparation of 2,3-dihydro[1,3]thiazo...
Converting the thiazole moiety into a thiazoline unit had a measurable impact on several physicochemical parameters, such as LogP and water solubility. Whilst thiazolo[4,5-b]pyridine 5 afforded a moderate water solubility of 49 mg/L, paired with a LogP of 2.28 (pH 2.3), the corresponding 2,3-dihydro[1,3]thiazolo[4,5-b]pyridine 7b had a higher water solubility of 173 mg/L and a lower LogP of 1.59 (pH 2.3). However, the lipophilicity of the new 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines was highly dependent on the substituents. For example, the brominated analogs 13b and 13c showed considerably higher LogP values of 2.88 (i.e., 13b) and 3.17 (i.e., 13c). We were thus curious to see how the structural change from a heteroaromatic thiazole unit to a partially saturated thiazoline moiety affected the in vitro and in vivo efficacy of the target compounds.
All compounds that were prepared to explore the SAR of substituted 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines, i.e., 13a–c, and 7a–c, the acylated analogues 14a–c and 16a–f, as well as selected aminoboranes 17d and 17e, were tested for target affinity in dedicated in vitro tests, as well as for herbicidal effects in vivo upon preemergence application to plants. Based on our experience with thiazolopyridine-based FAT inhibitors [12,13], five representative grass weeds (ALOMY, ECHCG, LOLRI, POAAN, and SETVI) were chosen as model plants to assess initial preemergence activity using a dose rate of 320 g/ha, whereas in vitro tests were carried out using FAT A, isolated from duckweed (Lemna paucicostata, LEMPA, Lp). As outlined in Table 2, entries 19 and 20, cinmethylin (1) and methiozolin (2) proved to be suitable commercial reference compounds. They showed good and broad control of grass weeds in various test systems, albeit with incomplete control of the commercially important grass weed LOLRI, paired with insufficient control of ECHCG by methiozolin (2) in our greenhouse tests. Furthermore, we used thiazolo[4,5-b]pyridine 5 as a strong internal standard to assess how modification of the thiazole moiety would affected the biological activity. It is worth noting that we emphasized investigating the preemergence efficacy as this application type is still important for cereals.
Table 2: Preemergence in vivo efficacy screening of 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 7a–c and 13a–c as well as of N-acylated analogs 14a–c and 16a–f against selected monocotyledon weeds, and binding affinity to FAT A from LEMPA.
|
|
|||||||||
| entry | compound | R1 | R2 | pI50a | ALOMYb,c | ECHCGb,c | POAANb,c | SETVIb,c | LOLRIb,c |
| 1 | 7a | H | H | 5.9 | 5 | 5 | 5 | 5 | 5 |
| 2 | 7b | F | H | 6.1 | 5 | 5 | 5 | 5 | 5 |
| 3 | 7c | CH3 | H | 6.3 | 5 | 5 | 5 | 5 | 5 |
| 4 | 13a | H | H | 5.7 | 4 | 5 | 5 | 5 | 3 |
| 5 | 13b | F | H | 7.3 | 5 | 5 | 5 | 5 | 5 |
| 6 | 13c | CH3 | H | 4.5 | 1 | 5 | 1 | 5 | 1 |
| 7 | 14a | H | Ac | 5.5 | 4 | 5 | 5 | 5 | 5 |
| 8 | 14b | F | iBtrd | 4.7 | 3 | 3 | 3 | 5 | 2 |
| 9 | 14c | CH3 | Ac | 4.3 | 3 | 3 | 3 | 5 | 1 |
| 10 | 16a | H | Ac | 5.5 | 4 | 5 | 5 | 5 | 5 |
| 11 | 16b | H | iBtr | 4.7 | 3 | 3 | 5 | 5 | 4 |
| 12 | 16c | F | Ac | 5.3 | 4 | 4 | 5 | 5 | 4 |
| 13 | 16d | F | iBtr | 4.7 | 3 | 5 | 5 | 5 | 4 |
| 14 | 16e | CH3 | Ac | 4.1 | 3 | 3 | 5 | 4 | 3 |
| 15 | 16f | CH3 | iBtr | 4.8 | 3 | 5 | 5 | 5 | 3 |
| 16 | 17d | CH3 | BH2 | 4.8 | 2 | 5 | 3 | 5 | 3 |
| 17 | 17e | CH3 | BX2e | <4.0 | 1 | 3 | 3 | 5 | 1 |
| 18 | 5 | F | — | 7.2 | 5 | 5 | 5 | 5 | 5 |
| 19 | 1 | 6.8 | 5 | 5 | 4 | 4 | 1 | ||
| 20 | 2 | 7.1 | 4 | 1 | 5 | 5 | 1 | ||
aIn vitro inhibition of FAT A (from LEMPA). bn = 10, i.e., 10 monocotyledonous weed seeds were grown per pot. cDose rate 320 g/ha. Efficacy values are given based on a rating scale by final visual experts’ assessment of green mass, i.e., 5 ≥ 90% inhibition, 4 = 70–89% inhibition, 3 = 50–69% inhibition, 2 = 40–49% inhibition, 1 = 21–39% inhibition, and — < 20% inhibition. Cinmethylin (1), methiozolin (2), and thiazolo[4,5-b]pyridine 5 were used as comparative internal standards. dIsobutyryl. eBX2 = 1,3,2,4-diazadiboretidin-2-amine.
Firstly, we investigated 6-methyl-2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 7a–c containing a variously substituted phenyl moiety. Moderate receptor affinity towards FAT A isolated from LEMPA could be observed for all three target compounds 7a–c (pI50 5.9–6.3, Table 2, entries 1–3). However, this was paired with strong in vivo efficacy on the level of internal standard 5 and exceeding the results obtained for cinmethylin (1) and methiozolin (2). Whilst compounds 7a–c afforded complete control of all tested weeds at an application rate of 320 g/ha, the corresponding 6-bromo analogues 13a–c showed higher sensitivity towards changes in the phenyl moiety. 6-Bromo-5-phenyl-2,3-dihydrothiazolo[4,5-b]pyridine (13a) showed only partial control of ALOMY and LOLRI, combined with moderate target affinity (pI50 5.7), whereas compound 13c, bearing an o-tolyl substituent, provided insufficient control of three weeds, i.e., ALOMY, POAAN, and LOLRI (Table 2, entry 6), in line with a surprisingly weak target affinity (pI50 4.5). Remarkably, 6-bromo-5-(2-fluorophenyl)-2,3-dihydro[1,3]thiazolo[4,5-b]pyridine (13b) exhibited the highest affinity towards FAT A of all new analogues (pI50 7.3), paired with full control of all tested weeds (Table 2, entry 5). As shown by the results outlined for 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 7a–c and 13a–c (Table 2, entries 1–6) our approach to introduce a thiazoline moiety via hydrogenation was well tolerated with respect to in vivo efficacy, albeit paired with reduced target affinity except for 13b. Likewise, the N-acylated 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 14a–c and 16a–f afforded a moderate target affinity in line with moderate to good control of the tested weeds (Table 2, entries 7–15). Interestingly, N-acylated analogues 14a and 16a, both bearing an unsubstituted phenyl substituent, delivered the best in vivo results, fully controlling four grass weeds, including commercially important LOLRI, and only showing partial control of ALOMY (Table 2, entries 7 and 10). Thus, both N-acylated compounds afforded nearly the same level of in vivo efficacy as the parent 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 13a and 7a. Aminoboranes 17d and 17e also showed partial control of the tested grass weeds, albeit on a significantly lower level. Accordingly, the target binding affinities were considerably lower than those measured for the strongest analogues 7b, 7c, and 13b.
To gain further insights into the biological profile, we chose compounds 7b, 7c, and 13b with promising initial in vivo activity as representatives of our new class of FAT inhibitors to assess the activity in advanced greenhouse tests (i.e., more replicates, larger pots, and lower application rate). Emphasis was put on the efficacy against resistant weeds and on crop selectivity profiles. We thus expanded our investigations to resistant monocotyledon weed species, i.e., resistant blackgrass (ALOMY_R, also known as black twitch or slender foxtail) and ryegrass (LOLSS_R), together with nonresistant ALOMY, LOLRI, APESV, and BROTE as commercially relevant target weeds, and wheat (TRZAS) as the crop (Figure 1).
![[1860-5397-20-46-1]](/bjoc/content/figures/1860-5397-20-46-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Preemergence efficacy of 2,3-dihydro[1,3]thiazolo[4,5-b]pyridine-based FAT inhibitors 7b, 7c, and 13b as well as internal standards cinmethylin (1) and methiozolin (2) against selected resistant and nonresistant monocotyledon weeds in wheat at application rates of 200 and 50 g/ha in advanced greenhouse trials, e.g., LOLSS_R and ALOMY_R (replicates: 10 plants per pot).
Figure 1: Preemergence efficacy of 2,3-dihydro[1,3]thiazolo[4,5-b]pyridine-based FAT inhibitors 7b, 7c, and 1...
Whilst all three new 2,3-dihydro[1,3]thiazolo[4,5-b]pyridine-based FAT inhibitors exceeded the efficacy of commercial standard methiozolin (2), target compound 7b controlled grass weeds on the same level or slightly better than cinmethylin (1). Accordingly, application of 7c, 13b, and 2 resulted in low crop damage in wheat (in particular at an application rate of 50 g/ha), whereas compounds 7b and 1 exhibited higher crop damage in the test systems on a comparable level. Methiozolin (2) only showed moderate control of nonresistant ALOMY (85% at 200 g/ha and 20% at 50 g/ha vs more than 90% control at both application rates by 7b, Figure 1). Likewise, 2 had only marginal effects against ALOMY_R and LOLSS_R. Whilst 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 7c and 13b showed weak to moderate efficacy against ALOMY_R (strongly dependent on the application rate), o-fluorophenyl analogue 7b exhibited strong control of this resistant, commercially important grass weed. Remarkably, all three 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 7b, 7c, and 13b afforded very good control of the second resistant monocotyledon weed LOLSS_R, reaching an efficacy level of above 90% at both application rates. Considering the low crop damage of 7c and 13b, their strong control of sensitive and resistant lolium species emphasizes their potential as lead structures to identify tailored solutions for sustainable lolium control in relevant countries (e.g., Australia). In line with good control of other grass weeds (APESV and BROTE, Figure 1), target compound 7b showed the most promising spectrum of efficacy of all new test compounds, being on the same level or slightly better than 1. Hence, 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines represent a propitious class of herbicidal lead structures with the potential to control resistant grass weeds. To complement our investigations that focused on relevant monocotyledon weeds in wheat, including resistant species, we tested the three selected 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 7b, 7c, and 13b against commercially relevant weeds in corn, e.g., crabgrass or red fingergrass (DIGSA) and goosegrass or crowsfoot grass (ELEIN), together with Johnson grass (SORHA) and broad-leaved signal grass (BRAPP). We used 1 and 5 as standards to assess the potential of the new lead structures. Both standards showed sufficient crop selectivity only at the lower application rate of 50 g/ha. [1,3]Thiazolo[4,5-b]pyridine 5 showed good control of all tested warm-season weeds, whilst 1 showed only insufficient control of BRAPP, ELEIN, and SORHA at the lower application rate of 50 g/ha. 2,3-Dihydro[1,3]thiazolo[4,5-b]pyridines 7b, 7c, and 13b showed good crop selectivity at both application rates in our advanced preemergence greenhouse test, affording low damage in corn and only marginal damage in soy (Figure 2). However, 7c showed a moderate damage of 20% in both crops at the higher application rate of 150 g/ha. Remarkably, 2,3-dihydro[1,3]thiazolo[4,5-b]pyridine 7b afforded good efficacy at both dose rates against all five monocotyledon weeds, including BRAPP, ELEIN, and SORHA, which were not sufficiently controlled by 1. Whilst the structurally closely related compounds 7b and 5 showed comparable efficacy against all tested weeds, 7b afforded considerably lower crop damage at the higher application rate in corn and at both application rates in soy compared to 5 (Figure 2). Furthermore, 7b, 7c, and 13b provided full control of ELEIN, one of the most difficult turfgrass weeds to control in the tropics and warmer temperate zones, emphasizing the potential of novel FAT inhibitors to contribute to integrated weed and resistance management.
![[1860-5397-20-46-2]](/bjoc/content/figures/1860-5397-20-46-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Preemergence efficacy of 2,3-dihydro[1,3]thiazolo[4,5-b]pyridine-based FAT inhibitors 7b, 7c, and 13b as well as internal standards cinmethylin (1) and [1,3]thiazolo[4,5-b]pyridine 5 against selected warm-season monocotyledon weeds in corn and soy at application rates of 150 and 50 g/ha in advanced greenhouse trials, e.g., BRAPP and ELEIN (replicates: 10 plants per pot).
Figure 2: Preemergence efficacy of 2,3-dihydro[1,3]thiazolo[4,5-b]pyridine-based FAT inhibitors 7b, 7c, and 1...
Conclusion
The agrochemical work outlined herein covers a series of novel herbicidal lead structures that contain a 2,3-dihydro[1,3]thiazolo[4,5-b]pyridine unit as the essential structural feature, with all of them carrying an o-substituted phenyl group. Inspired by earlier work in our group focusing on substituted thiazolo[4,5-b]pyridines that showed promising inhibition of the plant-specific enzyme FAT, we explored the selective late-stage conversion into the corresponding 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines via different reduction methods. Noteworthy, substituted 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines had remained almost entirely unexplored prior to our investigations. Likewise, we identified an optimized BH3⋅NH3-mediated reduction involving tris(pentafluorophenyl)borane as a strong Lewis acid and subsequent treatment with formic acid as the most suitable conditions to prepare the desired 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines. It is worth noting that this reduction proved to be thiazole-specific and gave access to a broad range of desired target compounds. Several substituted 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines showed promising herbicidal activity against commercially important grass weeds in preemergence greenhouse tests in line with competitive application rates and hints for crop selectivity, particularly in wheat and soy. Furthermore, the new heterocyclic lead structures have the potential to mitigate and affect weeds that have become resistant towards commercial herbicides, such as resistant blackgrass (ALOMY_R, also known as slender foxtail or black twitch) and ryegrass (LOLSS_R). Remarkably, 2,3-dihydro[1,3]thiazolo[4,5-b]pyridine 7b turned out to be superior to market standards (i.e., 1 and 2 in wheat) in terms of overall efficacy and resistance breaking potential. Halogen-free target compound 7c also showed strong efficacy against commercially important weeds, in particular resistant ryegrass (LOLSS_R), combined with promising crop safety. In our view, these results underline that chemical innovation using isostere concepts and addressing unusual structural features is a useful tool to broaden the structural scope of modern agrochemical research and to address sustainability goals, e.g., overcoming herbicide resistance and meeting demanding environmental safety goals.
Experimental
Synthesis
Representative procedure for the synthesis of 6-bromo-5-(2-fluorophenyl)-2,3-dihydro[1,3]thiazolo[4,5-b]pyridine (13b): To a stirred mixture of 6-bromopyridin-2-amine (8, 10.00 g, 56.88 mmol, 1.00 equiv), 2-fluorophenylboronic acid (9.52 g, 65.98 mmol, 1.16 equiv), and Na2CO3 (12.06 g, 113.8 mmol, 2.00 equiv) in a mixture of 1,4-dioxane (80 mL) and water (80 mL) at room temperature was added Pd(dppf)Cl2 (1.67 g, 2.28 mmol, 0.04 equiv), and the mixture was stirred at 80 °C for 3 h. Thereafter, the reaction mixture was cooled to room temperature, diluted with water, and extracted thoroughly with ethyl acetate. The combined organic layer was washed with brine, dried over Na2SO4, filtered, and concentrated under reduced pressure. The remaining residue was purified via column chromatography (gradient ethyl acetate/hexane) to afford 6-(2-fluorophenyl)pyridin-2-ylamine (9b, 9.98 g, 93%). 1H NMR (400 MHz, CDCl3, δ) 7.92–7.88 (m, 1H), 7.53–7.49 (m, 1H), 7.36–7.31 (m, 1H), 7.25–7.20 (m, 1H), 7.16–7.10 (m, 1H), 6.50–6.48 (d, 1H), 4.53–4.44 (br s, 2H, NH2).
6-(2-Fluorophenyl)pyridin-2-ylamine (9b, 9.98 g, 52.49 mmol, 1.0 equiv) was dissolved in acetonitrile (140 mL) and cooled to 0 °C. Thereafter, N-bromosuccinimide (20.56 g, 115.49 mmol, 2.2 equiv) was added carefully. The reaction mixture was warmed to room temperature and stirred for 4 h. Subsequently, the reaction mixture was diluted with water, and the resulting solid was filtered off. The solid was washed thoroughly with water and dried to afford 3,5-dibromo-6-(2-fluorophenyl)pyridin-2-ylamine (10b, 17.62 g, 97%) as an orange solid. 1H NMR (400 MHz, CDCl3, δ) 7.92 (s, 1H), 7.44–7.33 (m, 2H), 7.25–7.20 (m, 1H), 7.17–7.12 (m, 1H), 5.07–4.98 (br s, 2H, NH2).
To a stirred solution of 3,5-dibromo-6-(2-fluorophenyl)pyridin-2-ylamine (10b, 17.62 g, 50.93 mmol, 1.0 equiv) in DMF (120 mL) at room temperature was added potassium O-ethyl dithiocarbonate (18.52 g, 112.04 mmol, 2.2 equiv). The resulting mixture was heated at reflux for 7 h. Thereafter, the reaction mixture was cooled to room temperature, poured onto ice water, and acidified with 2 N HCl. The obtained precipitate was filtered, washed with water, collected, and dried under reduced pressure to afford 11b as thiol–thione tautomer consisting of 6-bromo-5-(2-fluorophenyl)[1,3]thiazolo[4,5-b]pyridine-2-thiol and 6-bromo-5-(2-fluorophenyl)[1,3]thiazolo[4,5-b]pyridine-2(3H)thione (17.20 g, 97%). 1H NMR (400 MHz, CDCl3, δ) 9.96 (br s, 1H), 8.01 (s, 1H), 7.50–7.44 (m, 1H), 7.41–7.38 (m, 1H), 7.29–7.25 (m, 1H), 7.19–7.15 (m, 1H).
The thiol–thione tautomer 11b (14.55 g, 42.64 mmol, 1.0 equiv) was dissolved in acetic acid (200 mL), and iron powder (35.71 g, 639.61 mmol, 15 equiv) was carefully added in portions. The resulting reaction mixture was stirred at 100 °C for 10 h. After full conversion (indicated by LC–MS), the reaction mixture was cooled to 60 °C, and the iron powder was filtered off. The remaining solution was diluted with water, and the resulting precipitate was filtered, washed with water, and dried under reduced pressure. The remaining crude residue was redissolved in DCM, then water was added, followed by thorough extraction. The combined organic layer was dried over magnesium sulfate, filtered, and dried under reduced pressure. The remaining residue was purified via column chromatography (gradient ethyl acetate/hexane) to afford 6-bromo-5-(2-fluorophenyl)[1,3]thiazolo[4,5-b]pyridine (12b, 8.75 g, 63%). 1H NMR (400 MHz, CDCl3, δ) 9.32 (s, 1H), 8.64 (s, 1H), 7.54–7.45 (m, 2H), 7.31–7.27 (m, 1H), 7.21–7.16 (m, 1H).
6-Bromo-5-(2-fluorophenyl)[1,3]thiazolo[4,5-b]pyridine (12b, 1,000 mg, 3.07 mmol, 1.0 equiv) was dissolved in absolute toluene (10 mL) in an oven-dried round-bottom flask under argon, to which ammonia borane (285 mg, 9.20 mmol, 3.0 equiv) and B(C6F5)3 (79 mg, 0.15 mmol, 0.05 equiv) were added. The resulting reaction mixture was stirred at a temperature of 45 °C for 5 h and then concentrated under reduced pressure. The remaining residue was redissolved in acetonitrile, formic acid was added, and the reaction mixture was stirred at room temperature for 2 h. The phases were separated via phase separator, and the organic layer was concentrated under reduced pressure. The remaining crude product was purified via column chromatography (gradient ethyl acetate/hexane) to afford 6-bromo-5-(2-fluorophenyl)-2,3-dihydro[1,3]thiazolo[4,5-b]pyridine (13b, 596 mg, 59%). 1H NMR (600 MHz, CDCl3, δ) 4.56 (s, 2H), 6.88 (br s, 1H), 7.11–7.16 (m, 1H), 7.21 (td, J = 7.5; 1.1 Hz, 1H), 7.31 (s, 1H), 7.34 (td, J = 7.4; 1.9 Hz, 1H), 7.36–7.41 (m, 1H); 13C NMR (151 MHz, CDCl3, δ) 49.0 (CH2), 108.1 (C), 115.7 (CH), 115.9 (CH), 123.5 (C), 124.0 (CH), 127.7 (d, C), 130.4 (CH), 131.1 (CH), 146.2 (C), 158.7 (C), 160.3 (d, C); HRESIMS (m/z): [M + H]+ calcd for C12H9BrFN2S, 310.9671; found, 310.9654.
5-(2-Fluorophenyl)-6-methyl-2,3-dihydro[1,3]thiazolo[4,5-b]pyridine (7b): 6-Bromo-5-(2-fluorophenyl)[1,3]thiazolo[4,5-b]pyridine (12b, 1.88 g, 4.56 mmol, 1.0 equiv), methylboronic acid (1.13 g, 18.24 mmol, 4.0 equiv), potassium phosphate (1.94 g, 9.12 mmol, 2.0 equiv), palladium(II) acetate (103 mg, 0.46 mmol, 0.1 equiv), and 2-dicyclohexylphosphino-2’,6’-dimethoxybiphenyl (579 mg, 1.37 mmol, 0.3 equiv) were dissolved in absolute toluene (40 mL) in an oven-dried round-bottom flask under argon. The resulting reaction mixture was stirred at reflux for 3.5 h. After cooling to room temperature, water (80 mL) and toluene (20 mL) were added, followed by addition of ammonium pyrrolidinedithiocarbamate (328 mg, 2.00 mmol, 0.44 equiv). The reaction mixture was stirred for 1 h at room temperature, and the organic layer was washed with saturated sodium hydrogencarbonate solution, dried over magnesium sulfate, filtered, and concentrated under reduced pressure. The remaining residue was purified via column chromatography (gradient ethyl acetate/hexane) to afford 5-(2-fluorophenyl)-6-methyl[1,3]thiazolo[4,5-b]pyridine (5, 900 mg, 81%). 1H NMR (400 MHz, CDCl3, δ) 9.25 (s, 1H), 8.23 (s, 1H), 7.55–7.51 (m, 1H), 7.47–7.41 (m, 1H), 7.30–7.27 (m, 1H), 7.19–7.15 (m, 1H), 2.40 (s, 3H).
5-(2-Fluorophenyl)-6-methyl[1,3]thiazolo[4,5-b]pyridine (5, 320 mg, 1.23 mmol, 1.0 equiv) was dissolved in absolute toluene (10 mL) in an oven-dried round-bottom flask under argon, to which ammonia borane (114 mg, 3.69 mmol, 3.0 equiv) and B(C6F5)3 (32 mg, 0.06 mmol, 0.05 equiv) were added. The resulting reaction mixture was stirred at a temperature of 45 °C for 5 h and then concentrated under reduced pressure. The remaining residue was redissolved in acetonitrile, formic acid was added, and the reaction mixture was stirred at room temperature for further 45 min. The phases were separated via phase separator, and the organic layer was concentrated under reduced pressure. The remaining crude product was purified via column chromatography (gradient ethyl acetate/hexane) to afford 5-(2-fluorophenyl)-6-methyl-2,3-dihydro[1,3]thiazolo[4,5-b]pyridine (7b, 62%). 1H NMR (600 MHz, CDCl3, δ) 2.00 (s, 3H), 4.55 (s, 2H), 6.61 (br s, 1H), 7.07 (s, 1H), 7.09–7.12 (m, 1H), 7.19–7.21 (m, 1H), 7.32–7.36 (m, 2H); 13C NMR (151 MHz, CDCl3, δ) 17.96 (CH3), 17.99 (CH3), 48.8 (CH2), 115.6 (CH), 115.7 (CH), 121.0 (C), 121.8 (C), 124.15 (CH), 124.18 (CH), 129.59 (CH), 129.64 (CH), 130.2 (CH), 131.40 (CH), 131.43 (CH), 145.6 (C), 158.9 (C), 159.2 (C), 160.5 (C); HRESIMS (m/z): [M + H]+ calcd for C13H12FN2S, 247.0705; found, 247.0724.
General procedure for the synthesis of N-acylated 6-methyl-2,3-dihydro[1,3]thiazolo[4,5-b]pyridines 16a–f: The corresponding acyl chloride (0.31 mmol, 1.1 equiv) and triethylamine (0.09 mL, 0.63 mmol, 2.2 equiv) were added to a stirred solution of the corresponding 6-methyl-5-phenyl-2,3-dihydro[1,3]thiazolo[4,5-b]pyridine 7a–c (0.28 mmol, 1.00 equiv) in absolute DCM (5 mL). The resulting reaction mixture was stirred at room temperature for 30–120 min, followed by dilution with DCM and water, and subsequent extraction and phase separation. The aqueous layer was thoroughly extracted with DCM, and the combined organic layer was dried over anhydrous MgSO4, filtered, and concentrated under reduced pressure. The remaining crude product was purified via column chromatography (gradient ethyl acetate/heptane) to afford the corresponding desired target compound 16a–f, for example, 1-[5-(2-fluorophenyl)-6-methyl[1,3]thiazolo[4,5-b]pyridin-3(2H)-yl]ethanone (16c, 150 mg, 84%). 1H NMR (600 MHz, CDCl3, δ) 2.16 (d, J = 2.2 Hz, 3H), 2.61 (s, 2H), 5.32 (s, 2H), 7.12–7.15 (m, 1H), 7.21–7.24 (m, 1H), 7.34 (s, 1H), 7.37–7.49 (m, 2H); 13C NMR (151 MHz, CDCl3, δ) 18.41 (CH3), 18.44 (CH3), 24.7 (CH3), 49.3 (CH2), 115.7 (CH), 115.8 (CH), 124.09 (CH), 124.12 (CH), 125.2 (C), 127.8 (C), 129.9 (CH), 130.0 (CH), 131.37 (CH), 131.40 (CH), 132.1 (CH), 147.0 (C), 149.6 (C), 158.9 (C), 160.6 (C), 170.6 (C); HRESIMS (m/z): [M + H]+ calcd for C15H14FN2OS, 289.0811; found, 289.0806.
Biology and biochemistry
In vivo greenhouse screening: Seeds of mono- and dicotyledonous weed plants and crop plants were sown in plastic or organic planting pots in sandy loam and covered with soil (replicates: n = 10, i.e., 10 monocotyledonous weed seeds were grown per pot or n = 5, i.e., 5 dicotyledonous weed seeds were grown per pot). The 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines described above (e.g., 7a–c, 13a–c, 14a–c, 16a–f, and 17a–b), formulated in the form of wettable powder (WP) , were applied to the surface of the covering soil as aqueous suspension or emulsion, with the addition of 0.5% of an additive at an application rate of 600 L of water/ha (converted). Following treatment, the pots were placed in a greenhouse and kept under optimum growth conditions for the test plants. The test plants were placed in the greenhouse for ca. three weeks under optimum growth conditions, and then the effect of the preparations was assessed visually in comparison to untreated control plants (herbicidal effect: 100% = plants died off, 0% = as untreated control plants). Efficacy values were given based on a rating scale by final visual experts’ assessment of green mass, i.e., 5 = ≥90% inhibition, 4 = 70–89% inhibition, 3 = 50–69% inhibition, 2 = 40–49% inhibition, 1 = 21–39% inhibition, and — = <20% inhibition. Advanced screening was carried out with or as emulsifiable concentrate formulations, three replicate pots, and a standardized number of seeds per pot depending on the plant species (10 seeds for corn and wheat spectrum). The harmful plants and crops used in greenhouse tests were the following species: Alopecurus myosuroides (ALOMY), resistant Alopecurus myosuroides (ALOMY_R, origin: Germany), Apera spica-venti (APESV), Brachiaria platyphylla (BRAPP), Bromus tectorum (BROTE), Echinochloa crus-galli (ECHCG), Digitaria sanguinalis (DIGSA), Eleusine indica (ELEIN), Glycine max (GLXMA), Lolium rigidum (LOLRI), Lolium sp. (LOLSS), resistant Lolium sp. (LOLSS_R, origin: France), Poa annua (POAAN), Setaria viridis (SETVI), Sorghum halepense (SORHA), Triticum aestivum (TRZAS), and Zea mays (ZEAMX).
LpFAT A expression and purification: The fat a03 gene from Lemna paucicostata, in which the N-terminal amino acids representing the chloroplast transit peptide were replaced by an N-terminal 6xHis-tag, was cloned into a pET24 vector [3]. The LpFAT A protein was expressed in E. coli BL21Star(DE3) cells. 5 mL of an overnight culture of E. coli cells grown in LB medium with 100 µg/mL carbenicillin were used to inoculate 0.5 L of autoinduction medium containing 100 µg/mL carbenicillin [28]. The bacteria were grown at 37 °C and 120 rpm for about 4.5 h to reach OD600 = 0.6 and then further cultivated at 21 °C overnight. The bacteria were harvested by centrifugation (20 min, 6,000g) and stored frozen at −80 °C. LpFAT A protein was purified using the Ni-NTA Fast Start Kit (Qiagen GmbH, Germany) according to the instructions of the manufacturer. Active fractions were pooled together, and the buffer was exchanged into 25 mM potassium phosphate buffer pH 7.3 containing 10% glycerol with PD10 columns (GE Healthcare). Aliquots of the protein solution were frozen in liquid nitrogen and stored at −80 °C.
LpFAT A fluorescence polarization assay: Fluorescence polarization (FP) competition assays were performed at room temperature in black 96-well microtiter plates (Greiner, Catalog No. 655900). The assay mixture contained 25 mM potassium phosphate buffer pH 7.3, 200 mM NaCl, 0.01% Triton X-100, 2 nM fluorescent tracer, 0.4 µg of purified LpFAT A protein and different amounts of the test compound in a total volume of 100 µL. FP was measured with a BMG CLARIOstar microtiter plate reader using the FP filter set for fluorescein (Ex 482-16, Em 530-40, LP504). FP is the difference between wells containing LpFAT A and wells containing only tracer. The pI50 values were calculated from plots of inhibition values vs test compound concentration using Model 205 of the ID Business Solutions Ltd Xlfit software suite. The FAT A binding fluorescent tracer was synthesized from (2S,4S)-4-[(2,6-difluorophenyl)methoxymethyl]-4-ethyl-2-methyl-N-(prop-2-ynylcarbamoyl)-1,3-dioxolane-2-carboxamide [3] and FAM azide, 5-isomer (Broadpharm BP-22544, San Diego, CA) by click chemistry [29] and was purified by flash column chromatography on silica gel.
Supporting Information
| Supporting Information File 1: General synthetic procedures, characterization of all target compounds, methods for biological and biochemical testing, and scans of 1H and 13C NMR spectra of the new 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines. | ||
| Format: PDF | Size: 7.1 MB | Download |
Acknowledgements
We would like to thank Susanne Ries, Gudrun Fey, Andreas Uhl, Peter Zöllner, and Martin Annau for valuable analytical support. R. L. M. and K.-Y. K. would like to thank the Herbicide Innovation Partnership (HIP) between the Grains Research and Development Corporation (GRDC) and Bayer AG for a Postdoctoral Research Fellowship.
References
-
Busi, R.; Dayan, F. E.; Francis, I.; Goggin, D.; Lerchl, J.; Porri, A.; Powles, S. B.; Sun, C.; Beckie, H. J. Pest Manage. Sci. 2020, 76, 2601–2608. doi:10.1002/ps.5798
Return to citation in text: [1] -
Dayan, F. E. Plants 2019, 8, 341. doi:10.3390/plants8090341
Return to citation in text: [1] -
Campe, R.; Hollenbach, E.; Kämmerer, L.; Hendriks, J.; Höffken, H. W.; Kraus, H.; Lerchl, J.; Mietzner, T.; Tresch, S.; Witschel, M.; Hutzler, J. Pestic. Biochem. Physiol. 2018, 148, 116–125. doi:10.1016/j.pestbp.2018.04.006
Return to citation in text: [1] [2] [3] [4] -
Kalinger, R. S.; Pulsifer, I. P.; Hepworth, S. R.; Rowland, O. Lipids 2020, 55, 435–455. doi:10.1002/lipd.12226
Return to citation in text: [1] [2] -
Salas, J. J.; Ohlrogge, J. B. Arch. Biochem. Biophys. 2002, 403, 25–34. doi:10.1016/s0003-9861(02)00017-6
Return to citation in text: [1] -
Johnen, P.; Zimmermann, S.; Betz, M.; Hendriks, J.; Zimmermann, A.; Marnet, M.; De, I.; Zimmermann, G.; Kibat, C.; Cornaciu, I.; Mariaule, V.; Pica, A.; Clavel, D.; Márquez, J. A.; Witschel, M. Pest Manage. Sci. 2022, 78, 3620–3629. doi:10.1002/ps.7004
Return to citation in text: [1] -
Brabham, C.; Johnen, P.; Hendriks, J.; Betz, M.; Zimmermann, A.; Gollihue, J.; Serson, W.; Kempinski, C.; Barrett, M. Weed Sci. 2021, 69, 18–30. doi:10.1017/wsc.2020.87
Return to citation in text: [1] -
Kim, J.-H.; Seo, J.-S.; An, J.-Y.; Kwon, Y.-S.; Hwang, K.-H.; Koo, S.-J.; Kim, J.-H. Bull. Environ. Contam. Toxicol. 2020, 105, 656–664. doi:10.1007/s00128-020-02976-w
Return to citation in text: [1] -
Koo, S.-J.; Hwang, K.-H.; Jeon, M.-S.; Kim, S.-H.; Lim, J.; Lee, D.-G.; Cho, N.-G. Pest Manage. Sci. 2014, 70, 156–162. doi:10.1002/ps.3541
Return to citation in text: [1] -
Messelhäuser, M. H.; Saile, M.; Sievernich, B.; Gerhards, R. Plant, Soil Environ. 2021, 67, 46–54. doi:10.17221/586/2020-pse
Return to citation in text: [1] -
Bratz, M.; Meyer, N.; Koenig, H.; Walter, H.; Gerber, M.; Westphalen, K.-O. Substituierte Naphthyridine und deren Verwendung. German Patent DE4405712A1, Aug 24, 1995.
Return to citation in text: [1] -
Abel, S. A. G.; Alnafta, N.; Asmus, E.; Bollenbach-Wahl, B.; Braun, R.; Dittgen, J.; Endler, A.; Frackenpohl, J.; Freigang, J.; Gatzweiler, E.; Heinemann, I.; Helmke, H.; Laber, B.; Lange, G.; Machettira, A.; McArthur, G.; Müller, T.; Odaybat, M.; Reingruber, A. M.; Roth, S.; Rosinger, C. H.; Schmutzler, D.; Schulte, W.; Stoppel, R.; Tiebes, J.; Volpin, G.; Barber, D. M. J. Agric. Food Chem. 2023, 71, 18212–18226. doi:10.1021/acs.jafc.3c02490
Return to citation in text: [1] [2] [3] [4] -
Barber, D. M.; Helmke, H.; Braun, R.; Tiebes, J.; Machettira, A. B.; Asmus, E.; Rosinger, C. H.; Gatzweiler, E.; Schmutzler, D.; Frackenpohl, J.; Reingruber, A. M.; Bollenbach-Wahl, B.; Dittgen, J. Substituted pyrrolidin-2-ones, salts thereof and their use as herbicidally active substances. WO Patent WO2021/204589, Oct 14, 2021.
Return to citation in text: [1] [2] -
Barber, D. M.; Braun, R.; Frackenpohl, J.; Heinemann, I.; Schmutzler, D.; Reingruber, A. M.; Bollenbach-Wahl, B.; Dittgen, J.; Roth, S. Substituted thiazolopyridines, salts thereof and their use as herbicidally active substances. WO Patent WO2023/036706, March 16, 2023.
Return to citation in text: [1] -
Scutt, J. N.; Seden, P. T.; Wailes, J. S. Isothiazolo[3,4-b]pyridines as herbicides. WO Patent WO2023/156398, Aug 24, 2023.
Return to citation in text: [1] -
Barber, D. M. J. Agric. Food Chem. 2022, 70, 11075–11090. doi:10.1021/acs.jafc.1c07910
Return to citation in text: [1] [2] -
Frackenpohl, J.; Abel, S. A. G.; Alnafta, N.; Barber, D. M.; Bojack, G.; Brant, N. Z.; Helmke, H.; Mattison, R. L. J. Agric. Food Chem. 2023, 71, 18141–18168. doi:10.1021/acs.jafc.3c01809
Return to citation in text: [1] -
Marco-Contelles, J.; Pérez-Mayoral, E.; Samadi, A.; Carreiras, M. d. C.; Soriano, E. Chem. Rev. 2009, 109, 2652–2671. doi:10.1021/cr800482c
Return to citation in text: [1] -
Seganish, W. M.; Brumfield, S. N.; Lim, J.; Matasi, J. J.; Mcelroy, W. T.; Tulshian, D. B.; Lavey, B. J.; Altman, M. D.; Gibeau, C. R.; Lampe, J. W.; Methot, J.; Zhu, L. Aminopyrimidinones as interleukin receptor-associated kinase inhibitors. WO Patent WO2013/066729, May 10, 2013.
Return to citation in text: [1] -
Pang, M.; Chen, J.-Y.; Zhang, S.; Liao, R.-Z.; Tung, C.-H.; Wang, W. Nat. Commun. 2020, 11, 1249. doi:10.1038/s41467-020-15118-x
Return to citation in text: [1] [2] -
Xia, Y.-T.; Sun, X.-T.; Zhang, L.; Luo, K.; Wu, L. Chem. – Eur. J. 2016, 22, 17151–17155. doi:10.1002/chem.201604503
Return to citation in text: [1] -
Forlani, L.; Ferrara, A.; Lugli, A.; Todesco, P. E. J. Chem. Soc., Perkin Trans. 2 1994, 1703–1707. doi:10.1039/p29940001703
Return to citation in text: [1] -
Okamoto, H.; Kobutani, T.; Kato, S. Bull. Chem. Soc. Jpn. 1992, 65, 674–678. doi:10.1246/bcsj.65.674
Return to citation in text: [1] -
Ding, F.; Zhang, Y.; Zhao, R.; Jiang, Y.; Bao, R. L.-Y.; Lin, K.; Shi, L. Chem. Commun. 2017, 53, 9262–9264. doi:10.1039/c7cc04709f
Return to citation in text: [1] -
Liu, Z.-Y.; Wen, Z.-H.; Wang, X.-C. Angew. Chem., Int. Ed. 2017, 56, 5817–5820. doi:10.1002/anie.201702304
Return to citation in text: [1] -
He, Y.; Nie, W.; Xue, Y.; Hu, Q. RSC Adv. 2021, 11, 20961–20969. doi:10.1039/d1ra02399c
Return to citation in text: [1] -
Frackenpohl, J.; Barber, D. M.; Braun, R.; Brown, R. W.; Heinemann, I.; Kallus, C.; Dittgen, J.; Reingruber, A. M.; Bollenbach-Wahl, B.; Gatzweiler, E. Substituted 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines, salts thereof and their use as herbicidally active substances. WO Patent WO2023/036707, March 16, 2023.
Return to citation in text: [1] -
Studier, F. W. Protein Expression Purif. 2005, 41, 207–234. doi:10.1016/j.pep.2005.01.016
Return to citation in text: [1] -
Punna, S.; Kaltgrad, E.; Finn, M. G. Bioconjugate Chem. 2005, 16, 1536–1541. doi:10.1021/bc0501496
Return to citation in text: [1]
| 3. | Campe, R.; Hollenbach, E.; Kämmerer, L.; Hendriks, J.; Höffken, H. W.; Kraus, H.; Lerchl, J.; Mietzner, T.; Tresch, S.; Witschel, M.; Hutzler, J. Pestic. Biochem. Physiol. 2018, 148, 116–125. doi:10.1016/j.pestbp.2018.04.006 |
| 28. | Studier, F. W. Protein Expression Purif. 2005, 41, 207–234. doi:10.1016/j.pep.2005.01.016 |
| 3. | Campe, R.; Hollenbach, E.; Kämmerer, L.; Hendriks, J.; Höffken, H. W.; Kraus, H.; Lerchl, J.; Mietzner, T.; Tresch, S.; Witschel, M.; Hutzler, J. Pestic. Biochem. Physiol. 2018, 148, 116–125. doi:10.1016/j.pestbp.2018.04.006 |
| 1. | Busi, R.; Dayan, F. E.; Francis, I.; Goggin, D.; Lerchl, J.; Porri, A.; Powles, S. B.; Sun, C.; Beckie, H. J. Pest Manage. Sci. 2020, 76, 2601–2608. doi:10.1002/ps.5798 |
| 3. | Campe, R.; Hollenbach, E.; Kämmerer, L.; Hendriks, J.; Höffken, H. W.; Kraus, H.; Lerchl, J.; Mietzner, T.; Tresch, S.; Witschel, M.; Hutzler, J. Pestic. Biochem. Physiol. 2018, 148, 116–125. doi:10.1016/j.pestbp.2018.04.006 |
| 12. | Abel, S. A. G.; Alnafta, N.; Asmus, E.; Bollenbach-Wahl, B.; Braun, R.; Dittgen, J.; Endler, A.; Frackenpohl, J.; Freigang, J.; Gatzweiler, E.; Heinemann, I.; Helmke, H.; Laber, B.; Lange, G.; Machettira, A.; McArthur, G.; Müller, T.; Odaybat, M.; Reingruber, A. M.; Roth, S.; Rosinger, C. H.; Schmutzler, D.; Schulte, W.; Stoppel, R.; Tiebes, J.; Volpin, G.; Barber, D. M. J. Agric. Food Chem. 2023, 71, 18212–18226. doi:10.1021/acs.jafc.3c02490 |
| 4. | Kalinger, R. S.; Pulsifer, I. P.; Hepworth, S. R.; Rowland, O. Lipids 2020, 55, 435–455. doi:10.1002/lipd.12226 |
| 19. | Seganish, W. M.; Brumfield, S. N.; Lim, J.; Matasi, J. J.; Mcelroy, W. T.; Tulshian, D. B.; Lavey, B. J.; Altman, M. D.; Gibeau, C. R.; Lampe, J. W.; Methot, J.; Zhu, L. Aminopyrimidinones as interleukin receptor-associated kinase inhibitors. WO Patent WO2013/066729, May 10, 2013. |
| 3. | Campe, R.; Hollenbach, E.; Kämmerer, L.; Hendriks, J.; Höffken, H. W.; Kraus, H.; Lerchl, J.; Mietzner, T.; Tresch, S.; Witschel, M.; Hutzler, J. Pestic. Biochem. Physiol. 2018, 148, 116–125. doi:10.1016/j.pestbp.2018.04.006 |
| 4. | Kalinger, R. S.; Pulsifer, I. P.; Hepworth, S. R.; Rowland, O. Lipids 2020, 55, 435–455. doi:10.1002/lipd.12226 |
| 5. | Salas, J. J.; Ohlrogge, J. B. Arch. Biochem. Biophys. 2002, 403, 25–34. doi:10.1016/s0003-9861(02)00017-6 |
| 6. | Johnen, P.; Zimmermann, S.; Betz, M.; Hendriks, J.; Zimmermann, A.; Marnet, M.; De, I.; Zimmermann, G.; Kibat, C.; Cornaciu, I.; Mariaule, V.; Pica, A.; Clavel, D.; Márquez, J. A.; Witschel, M. Pest Manage. Sci. 2022, 78, 3620–3629. doi:10.1002/ps.7004 |
| 16. | Barber, D. M. J. Agric. Food Chem. 2022, 70, 11075–11090. doi:10.1021/acs.jafc.1c07910 |
| 17. | Frackenpohl, J.; Abel, S. A. G.; Alnafta, N.; Barber, D. M.; Bojack, G.; Brant, N. Z.; Helmke, H.; Mattison, R. L. J. Agric. Food Chem. 2023, 71, 18141–18168. doi:10.1021/acs.jafc.3c01809 |
| 18. | Marco-Contelles, J.; Pérez-Mayoral, E.; Samadi, A.; Carreiras, M. d. C.; Soriano, E. Chem. Rev. 2009, 109, 2652–2671. doi:10.1021/cr800482c |
| 12. | Abel, S. A. G.; Alnafta, N.; Asmus, E.; Bollenbach-Wahl, B.; Braun, R.; Dittgen, J.; Endler, A.; Frackenpohl, J.; Freigang, J.; Gatzweiler, E.; Heinemann, I.; Helmke, H.; Laber, B.; Lange, G.; Machettira, A.; McArthur, G.; Müller, T.; Odaybat, M.; Reingruber, A. M.; Roth, S.; Rosinger, C. H.; Schmutzler, D.; Schulte, W.; Stoppel, R.; Tiebes, J.; Volpin, G.; Barber, D. M. J. Agric. Food Chem. 2023, 71, 18212–18226. doi:10.1021/acs.jafc.3c02490 |
| 15. | Scutt, J. N.; Seden, P. T.; Wailes, J. S. Isothiazolo[3,4-b]pyridines as herbicides. WO Patent WO2023/156398, Aug 24, 2023. |
| 11. | Bratz, M.; Meyer, N.; Koenig, H.; Walter, H.; Gerber, M.; Westphalen, K.-O. Substituierte Naphthyridine und deren Verwendung. German Patent DE4405712A1, Aug 24, 1995. |
| 16. | Barber, D. M. J. Agric. Food Chem. 2022, 70, 11075–11090. doi:10.1021/acs.jafc.1c07910 |
| 10. | Messelhäuser, M. H.; Saile, M.; Sievernich, B.; Gerhards, R. Plant, Soil Environ. 2021, 67, 46–54. doi:10.17221/586/2020-pse |
| 29. | Punna, S.; Kaltgrad, E.; Finn, M. G. Bioconjugate Chem. 2005, 16, 1536–1541. doi:10.1021/bc0501496 |
| 7. | Brabham, C.; Johnen, P.; Hendriks, J.; Betz, M.; Zimmermann, A.; Gollihue, J.; Serson, W.; Kempinski, C.; Barrett, M. Weed Sci. 2021, 69, 18–30. doi:10.1017/wsc.2020.87 |
| 8. | Kim, J.-H.; Seo, J.-S.; An, J.-Y.; Kwon, Y.-S.; Hwang, K.-H.; Koo, S.-J.; Kim, J.-H. Bull. Environ. Contam. Toxicol. 2020, 105, 656–664. doi:10.1007/s00128-020-02976-w |
| 9. | Koo, S.-J.; Hwang, K.-H.; Jeon, M.-S.; Kim, S.-H.; Lim, J.; Lee, D.-G.; Cho, N.-G. Pest Manage. Sci. 2014, 70, 156–162. doi:10.1002/ps.3541 |
| 13. | Barber, D. M.; Helmke, H.; Braun, R.; Tiebes, J.; Machettira, A. B.; Asmus, E.; Rosinger, C. H.; Gatzweiler, E.; Schmutzler, D.; Frackenpohl, J.; Reingruber, A. M.; Bollenbach-Wahl, B.; Dittgen, J. Substituted pyrrolidin-2-ones, salts thereof and their use as herbicidally active substances. WO Patent WO2021/204589, Oct 14, 2021. |
| 14. | Barber, D. M.; Braun, R.; Frackenpohl, J.; Heinemann, I.; Schmutzler, D.; Reingruber, A. M.; Bollenbach-Wahl, B.; Dittgen, J.; Roth, S. Substituted thiazolopyridines, salts thereof and their use as herbicidally active substances. WO Patent WO2023/036706, March 16, 2023. |
| 21. | Xia, Y.-T.; Sun, X.-T.; Zhang, L.; Luo, K.; Wu, L. Chem. – Eur. J. 2016, 22, 17151–17155. doi:10.1002/chem.201604503 |
| 12. | Abel, S. A. G.; Alnafta, N.; Asmus, E.; Bollenbach-Wahl, B.; Braun, R.; Dittgen, J.; Endler, A.; Frackenpohl, J.; Freigang, J.; Gatzweiler, E.; Heinemann, I.; Helmke, H.; Laber, B.; Lange, G.; Machettira, A.; McArthur, G.; Müller, T.; Odaybat, M.; Reingruber, A. M.; Roth, S.; Rosinger, C. H.; Schmutzler, D.; Schulte, W.; Stoppel, R.; Tiebes, J.; Volpin, G.; Barber, D. M. J. Agric. Food Chem. 2023, 71, 18212–18226. doi:10.1021/acs.jafc.3c02490 |
| 20. | Pang, M.; Chen, J.-Y.; Zhang, S.; Liao, R.-Z.; Tung, C.-H.; Wang, W. Nat. Commun. 2020, 11, 1249. doi:10.1038/s41467-020-15118-x |
| 27. | Frackenpohl, J.; Barber, D. M.; Braun, R.; Brown, R. W.; Heinemann, I.; Kallus, C.; Dittgen, J.; Reingruber, A. M.; Bollenbach-Wahl, B.; Gatzweiler, E. Substituted 2,3-dihydro[1,3]thiazolo[4,5-b]pyridines, salts thereof and their use as herbicidally active substances. WO Patent WO2023/036707, March 16, 2023. |
| 12. | Abel, S. A. G.; Alnafta, N.; Asmus, E.; Bollenbach-Wahl, B.; Braun, R.; Dittgen, J.; Endler, A.; Frackenpohl, J.; Freigang, J.; Gatzweiler, E.; Heinemann, I.; Helmke, H.; Laber, B.; Lange, G.; Machettira, A.; McArthur, G.; Müller, T.; Odaybat, M.; Reingruber, A. M.; Roth, S.; Rosinger, C. H.; Schmutzler, D.; Schulte, W.; Stoppel, R.; Tiebes, J.; Volpin, G.; Barber, D. M. J. Agric. Food Chem. 2023, 71, 18212–18226. doi:10.1021/acs.jafc.3c02490 |
| 13. | Barber, D. M.; Helmke, H.; Braun, R.; Tiebes, J.; Machettira, A. B.; Asmus, E.; Rosinger, C. H.; Gatzweiler, E.; Schmutzler, D.; Frackenpohl, J.; Reingruber, A. M.; Bollenbach-Wahl, B.; Dittgen, J. Substituted pyrrolidin-2-ones, salts thereof and their use as herbicidally active substances. WO Patent WO2021/204589, Oct 14, 2021. |
| 25. | Liu, Z.-Y.; Wen, Z.-H.; Wang, X.-C. Angew. Chem., Int. Ed. 2017, 56, 5817–5820. doi:10.1002/anie.201702304 |
| 26. | He, Y.; Nie, W.; Xue, Y.; Hu, Q. RSC Adv. 2021, 11, 20961–20969. doi:10.1039/d1ra02399c |
| 20. | Pang, M.; Chen, J.-Y.; Zhang, S.; Liao, R.-Z.; Tung, C.-H.; Wang, W. Nat. Commun. 2020, 11, 1249. doi:10.1038/s41467-020-15118-x |
| 24. | Ding, F.; Zhang, Y.; Zhao, R.; Jiang, Y.; Bao, R. L.-Y.; Lin, K.; Shi, L. Chem. Commun. 2017, 53, 9262–9264. doi:10.1039/c7cc04709f |
| 22. | Forlani, L.; Ferrara, A.; Lugli, A.; Todesco, P. E. J. Chem. Soc., Perkin Trans. 2 1994, 1703–1707. doi:10.1039/p29940001703 |
| 23. | Okamoto, H.; Kobutani, T.; Kato, S. Bull. Chem. Soc. Jpn. 1992, 65, 674–678. doi:10.1246/bcsj.65.674 |
© 2024 Frackenpohl et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.