Abstract
Presented here is the design, synthesis, and study of a variety of novel hydrogen-bonding-capable π-conjugated N-heteroacenes, 1,4-dihydropyrazino[2,3-b]quinoxaline-2,3-diones (DPQDs). The DPQDs were accessed from the corresponding weakly hydrogen-bonding dicyanopyrazinoquinoxaline (DCPQ) suspensions with excess potassium hydroxide, resulting in moderate to good yields. Both families of compounds were analyzed by UV–vis and NMR spectroscopy, where the consequences of hydrogen bonding capability could be assessed through the structure–property studies. Conversion of the DCPQs into hydrogen-bonding capable DPQDs results in modulation of frontier MO energies, higher molar extinction coefficients, enhanced crystallinity, and on-average higher thermal stability (where in some cases the 5% weight loss temperature is increased by up to 100 °C). Single crystal X-ray diffraction data could be obtained for three DPQDs. One reveals pairwise hydrogen bonding in the solid state as well as a herringbone packing arrangement rendering it a promising candidate for additional studies in the context of organic optoelectronic devices.

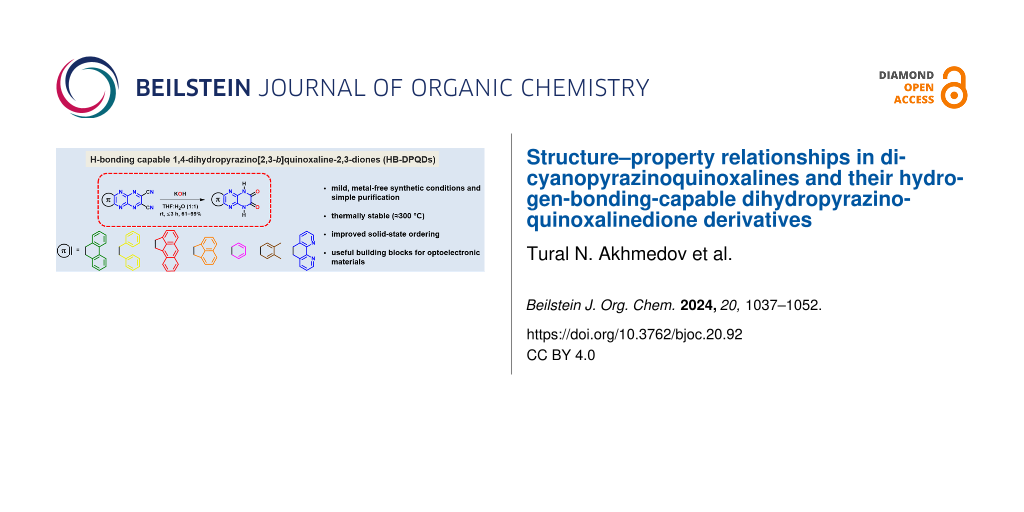
Graphical Abstract
Introduction
The role of weak intermolecular interactions in tuning the properties of organic semiconductors has garnered significant attention in the past two decades, owing to their profound implications on device performance [1]. Among these interactions, hydrogen bonding (H-bonding, HB) as a highly directional noncovalent interaction can influence the structural, electronic, and optoelectronic properties of bulk materials [2,3]. Hydrogen bonding plays a crucial role in molecular ordering in solid-state organic semiconductors, thereby dictating charge transport pathways as well as carrier mobilities of both electrons and holes [4-6]. Furthermore, strong hydrogen bonding interactions effectively modulate energy levels of semiconducting materials, affecting the bandgap and charge injection/extraction processes [7]. The additional merits of H-bonding designs in organic optoelectronic materials include higher thermal stability, synergistic stabilizing effects with π-stacking interactions, etc. [8].
Acenes and N-heteroacenes are two prominent π-conjugated scaffolds for both n- and p-type organic semiconductors [9-12]. The Würthner and Meijer groups presented an early example of the utility of hydrogen bonding in the fabrication of organic semiconductor devices. A thin-film device architecture was developed consisting of oligo(p-phenylenevinylene) (OPV) and perylenebisimide (PBI) components connected laterally through H-bonding and self-assembled orthogonally through π–π interactions [13]. Mixtures of PBI and OPV successfully exhibited ambipolar charge transport depending on processing conditions as a result of the co-assembled morphology.
Our group utilized a supramolecular approach in the solar cell arena. π-Conjugated linear and branched oligothiophenes appended to H-bonding capable phthalhydrazide (PH) were prepared [14,15]. These compounds demonstrated a power conversion efficiency (PCE) twice as high as that of non-hydrogen bonding controls upon photovoltaic device fabrication with the electron acceptor C60. Subsequently, the design was extended to “ditopic” systems with diverse HB-capable units such as PH, 2,4-diamino-1,3,5-triazine (DAT), and barbiturate (B) that can form trimeric and hexameric “rosettes”, respectively [16].
The Sokolowski and Głowacki groups have extensively developed H-bonded systems including dyes and pigments utilized in organic field-effect transistor (OFET) devices [17-20]. In two different studies, Głowacki reported H-bonding capable quinacridone and epindolidione-based semiconductors within OFET devices that offer moderate to excellent hole transport mobilities (1.5 × 10−3 and 1.5 cm2/Vs, respectively) [21]. In 2012, Miao et al. reported an H-bonding capable 1,4-dihydropyrazinopyrazine fragment within N-heteroacenes [22]. The X-ray analysis of single crystals revealed the formation of highly-ordered ribbons constructed via intermolecular N–H∙∙∙N interactions. However, vacuum-deposited films afforded poor hole transport. In separate work, Bunz et al. reported the serendipitous and understated synthesis of an H-bonding capable 1,4-dihydropyrazino[2,3-b]quinoxaline-2,3-dione (Figure 1a) [23]. In 2018, Takeda et al. reported the synthesis, electronic characteristics, and liquid-crystalline properties of several electron-accepting acenes, including 1,4-dihydropyrazinoquinoxalinediones (Figure 1b). The incorporation of strong hydrogen-bonding interactions facilitated the formation of a highly ordered liquid-crystalline phase within these systems [24]. These molecules are particularly intriguing as they bear resemblance to Głowacki’s compounds discussed above. Reported here is a robust structure–property relationship study of these types of molecules with a particular interest in their optoelectronic properties.
Figure 1: Chemical structures of H-bonding N-heteroacenes synthesized by Miao et al. and Bunz et al. (a) [22,23]. Preparation of electron-accepting azaacene derivatives as liquid-crystalline materials by Takeda et al. (b) [24]. Transformation of Yamashita’s DCPQs to H-bonding capable DPQD derivatives discussed in this work (c) [25].
Figure 1: Chemical structures of H-bonding N-heteroacenes synthesized by Miao et al. and Bunz et al. (a) [22,23]. Pr...
Primary motivation for our effort comes from the work of Yamashita et al. who explored the OFET behavior of a library of electron-deficient dicyanopyrazinoquinoxaline (DCPQ) compounds [25]. The computations predicted low-lying LUMO levels, around −4.0 eV. However, the molecules exhibited poor n-type FET behavior and electron transport characteristics (μe = 1 × 10−8 to 3.6 × 10−6 cm2/Vs). Structurally, the combination of cyano groups and an electron-deficient pyrazinoquinoxaline creates an extremely electron poor π-system. Given our previous work and that of others, in 2013 we reasoned [26] that DCPQs could be efficiently transformed to tautomerically active, H-bonding capable 1,4-dihydropyrazino[2,3-b]quinoxaline-2,3-diones (Figure 1b, DPQDs) via nucleophilic aromatic substitution (SNAr) at the ipso-CN positions. Here, the lactim–lactam tautomerization of DPQDs to arrive at the more stable 2,3-dione lactam form would mirror our prior work with phthalhydrazide (PH) and is also consistent with the work later reported by Takeda et al. We additionally envisioned that the DCPQs would serve as valuable comparators as the H-bonding capable DPQDs were studied.
Reported here is the synthesis of a library of dicyanopyrazinoquinoxalines (DCPQs) 1a–7a and subsequent mild one-step synthesis of hydrogen-bonding dihydropyrazinoquinoxalinedione (DPQDs) 1b–7b. The structure–property relationships have been established within and between the two families using optical measurements. Moreover, the incorporation of H-bonding functionality exhibits a noteworthy impact on the thermal stabilities (analyzed by TGA) of the compounds. The single-crystal X-ray diffraction of DPQD 2b exhibits a desirable herringbone solid-state arrangement for a potential OFET application. Complementary gas-phase computational analysis has provided insight into the electronic structures of both families which is related to the optical properties studied in solution.
Results and Discussion
Synthesis of DCPQs and DPQDs
Compounds 12 and 13 are two major building blocks to obtain dicyanopyrazinoquinoxalines (DCPQs) 1a–7a. The synthesis proceeded through highly scalable reactions (over 10-gram scale) using diaminomaleonitrile (15) as a starting material in accordance with the procedure in the literature (Scheme 1) [27]. The first step is the acylation with oxalyl chloride to yield 14 followed by a reaction with SOCl2 in the presence of cat. DMF to obtain dichloropyrazine 13. The solvent 1,4-dioxane is crucial to the synthesis and purification in the initial two steps as other solvents such as THF make the purification process more intricate. This sequence is followed by SNAr with ammonia (29% v/v) to obtain building block diaminopyrazine 12.
Scheme 1: Synthesis of dicyanopyrazinoquinoxaline derivatives 1a–7a.
Scheme 1: Synthesis of dicyanopyrazinoquinoxaline derivatives 1a–7a.
The synthesis of Yamashita’s DCPQs 1a (BenzCN) and 2a (XyICN) began via SNAr reaction of commercially available o-phenylenediamines 11a and 11b with building block 13 to afford dihydropyrazine derivatives 8 and 9, respectively, as precipitates in 1,4-dioxane solution. The reaction generates two equivalents of HCl, adding a slight excess of diamines to sequester released HCl proved to be pivotal in achieving better yields. Oxidation with two equivalents of DDQ provided the comparator DCPQs 1a and 2a in 89% and 87% yield, respectively.
The synthesis of DCPQ 3a was attempted rigorously under different conditions using building blocks 1,2-aceanthrylenedione and 12, as detailed in Table S1 (Supporting Information File 1). However, an inseparable unknown impurity was observed along with the desired product 3a in all cases when analyzed by 1H NMR. Under strict temperature and time control (80 °C for 72 hours) using 2.5 equiv of 1,2-aceanthrylenedione, the reaction provided pure 3a product in moderate yield (41%). Subsequent recrystallization with boiling DMSO provided pristine red-brown crystals of 3a. The synthesis of DCPQ 4a was simple and proceeded with the condensation of a 1:1 mixture of acenaphthenequinone and 12 under standard boiling acetic acid conditions, as reported in Scheme 1. The crude product could be collected via vacuum filtration, followed by additional purification via recrystallization from boiling DMF to afford golden crystals of 4a in moderate yields.
In order to access 5a and 6a, a modified procedure from the literature was implemented [28]. To synthesize 6a, the condensation was performed with 9,10-phenanthrenequinone, building block 12 in the presence of glacial CH3COOH, trifluoracetic acid, and 1,4-dioxane at reflux. Then, recrystallization from DMF and sublimation under ambient pressure worked well for the purification step to achieve an excellent 80% yield. To our delight, 5a did not require any additional purification under the same reaction conditions.
Next, several unsuccessful synthetic efforts were made to access 7a using starting material 7e and building block 12, listed in Table S2 (Supporting Information File 1). First, the starting material 7e was obtained using 1,10-phenanthroline under harsh conditions as shown in Scheme 3 [29]. The same condensation reaction that worked for structurally similar 6a, produced a poorly separable mixture in the case of 7a. In the course of optimizing this reaction under different conditions, additional novel products were isolated in moderate yields (Scheme 2, Table S2, Supporting Information File 1). A variety of bis-alkoxylated products 16a–d was obtained in moderate yields upon using different polar solvents such as methanol, ethanol, ethylene glycol, and diethylene glycol in the presence of excess triethylamine (Scheme 2). These products provide evidence for the in situ formation of DCPQ 7a and demonstrate its ability to undergo trapping with various nucleophiles through an SNAr mechanism.
Scheme 2: Synthesis of bis-alkoxy-substituted π-conjugated phenanthrolines 16a, 16b, 16c, and 16d.
Scheme 2: Synthesis of bis-alkoxy-substituted π-conjugated phenanthrolines 16a, 16b, 16c, and 16d.
An alternate strategy was employed to access 7a as depicted in Scheme 3. The synthetic strategy first involved the access of dihydropyrazine precursor 10 which then underwent oxidation in the final step. Next, 7e was easily converted to (Z,E)-bis-oxime derivative 7d in satisfactory yield. The oxime stereochemistry shown is presumably secured due to the favorable hydrogen bonding (O–H∙∙∙N) between the oxime units; this idea is supported by 1H NMR spectroscopy which shows the presence of two distinct hydroxyl proton signals. The reduction of 7d in the presence of Pd/C and hydrazine monohydrate afforded 1,10-phenanthroline-5,6-diamine (7c) in moderate yield. The condensation of 7c with 13 in the presence of 1,4-dioxane yielded poorly soluble 10. Unfortunately, several oxidation attempts to access target 7a failed presumably due to the insolubility of 10 even in high polarity solvents, listed in Table S3 (Supporting Information File 1).
Scheme 3: An alternative synthetic route to access 7a.
Scheme 3: An alternative synthetic route to access 7a.
The H-bonding capable dihydropyrazinoquinoxaline diones (DPQDs) were obtained by a SNAr mechanism involving the corresponding DCPQ derivatives. Based on numerous examples in the literature, it has been established that for electron-deficient π-systems containing cyano groups, the addition–elimination pathway will dominate over hydrolysis. This preference was crucial to prevent the formation of undesired carboxylic acid products [28,30,31]. These results also align with the observations previously reported by Takeda and co-workers [24]. The reaction was carried out using a large excess (10 equiv) of KOH in a 1:1 THF/H2O mixture to improve the solubility of the corresponding DCPQs (Scheme 4) at room temperature. A small amount of 1,4-dioxane (THF/H2O/1,4-dioxane 4:5:1) was also added to improve the solubility of 3a. The solution was rendered neutral by the addition of conc. HCl until precipitation occurred and precipitates were subsequently collected via vacuum filtration with no exception for any DPQDs 1b–6b. The yields obtained in this step were moderate to excellent (61–99%). Target 7b was synthesized from 12 and 7e via the in situ generation of 7a and subsequent SNAr with KOH given the high reactivity of intermediate 7a (Scheme 4).
Scheme 4: Synthesis of DPQDs 1b–7b from their corresponding DCPQs 1a–7a. *THF/H2O/1,4-dioxane (4:5:1). **in situ formation.
Scheme 4: Synthesis of DPQDs 1b–7b from their corresponding DCPQs 1a–7a. *THF/H2O/1,4-dioxane (4:5:1). **in s...
After optimizing the successful synthesis of alkoxylated and diketone derivatives of 7a, we were intrigued to investigate whether other dicyanopyrazinoquinoxaline derivatives could undergo a similar transformation. To assess the efficacy of the reaction, we subjected 6a to the conditions outlined in Scheme 2. Remarkably, under the same conditions, an isostructural bis-ethoxy derivative, 16e, was obtained in an excellent yield (87%). In addition, 10 equivalents of ammonium hydroxide yielded mono-aminated derivative 16f (Scheme S4, Supporting Information File 1). The double substitution did not proceed presumably due to the strong electron-donating effect of -NH2, diminishing the electrophilicity of the π-system. However, the mono-aminated product 16f has the potential to serve as a useful building block for condensation with carbonyl functionality as well as Buchwald–Hartwig amination involving aryl halides.
Thermal studies
Thermal stabilities of DCPQs 1a–6a and DPQDs 1b–7b were evaluated using thermogravimetric analysis (TGA; Figure 2 and Table S4 in Supporting Information File 1). The thermal stability of the DCPQs is attributed to their favorable π–π stacking in the solid state. The 5% weight loss values range from 232 °C to 353 °C. Compounds with larger π-surfaces exhibited greater thermal stability (353 °C for 3a, 304 °C for 4a, 312 °C for 6a) while others displayed 5% weight loss values under 250 °C (232 °C for 1a, 244 °C for 2a and 238 °C for 5a). It was interesting to observe that there was a large difference in the thermal stabilities of non-planar 5a and fully planar 6a (5% weight loss of 238 °C vs 312 °C).
![[1860-5397-20-92-2]](/bjoc/content/figures/1860-5397-20-92-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: TGA of 1a–6a (a) and 1b–7b (b) obtained at 10 °C/min under nitrogen.
Figure 2: TGA of 1a–6a (a) and 1b–7b (b) obtained at 10 °C/min under nitrogen.
The TGA data for H-bonding DPQDs showed thermal stability with a 5% weight loss ranging between 288 °C and 361 °C. The 5% weight loss value is increased by about 100 °C, particularly in the case of 1b as compared to the corresponding DCPQ 1a. This enhancement can be attributed to the synergistic effect of π–π and H-bonding interactions on solid-state cohesive energy. However, 3b and 7b exhibited a decrease in 5% weight loss temperatures of 21 and 7 °C, respectively, from their corresponding DCPQ comparators.
To complement the TGA studies, the thermal stability of compound 4b was evaluated through its exposure to air inside a 200 °C oven for one week. Structural evaluation by 2D NMR and mass spectrometry confirmed no decomposition and its high thermal stability.
Optical properties
The electronic properties of the DCPQs 1a–6a and DPQDs 1b–7b were explored by UV–vis absorption spectroscopy in dimethyl sulfoxide (DMSO) at 25 °C (Figure 3). The electronic absorption spectra of DCPQs exhibited structureless bands with λmax values from 388 to 423 nm, indicative of π–π* transitions. On the contrary, DPQDs exhibited more structured, blue-shifted bands with λmax from 357–400 nm. At the same time, DCPQs exhibit intramolecular charge transfer (ICT) bands at lower energy due to the dicyanopyrazinopyrazine moiety as a strong acceptor and aromatic groups as strong donors in the backbone; the dione groups in the case of DPQDs diminish the acceptor properties of the aza π-system. It is worth mentioning that ICT is significantly influenced by solvent polarity and the comprehensive study of solvent effects is not within the scope of this investigation [32].
![[1860-5397-20-92-3]](/bjoc/content/figures/1860-5397-20-92-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Absorption spectra (20 μM) for a) DCPQs 1a–6a and b) DPQDs 1b–7b in dimethyl sulfoxide.
Figure 3: Absorption spectra (20 μM) for a) DCPQs 1a–6a and b) DPQDs 1b–7b in dimethyl sulfoxide.
Also, DPQDs displayed higher molar extinction coefficients (ε) than the corresponding DCPQs by a factor of approximately 1.4 to 5.3 in DMSO (Table 1). Furthermore, in all cases, the compounds from both families adhere to the Beer–Lambert law at the observed concentrations, as illustrated in Figures S37–S41 (Supporting Information File 1). This serves as good evidence that the synthesized compounds exhibit no signs of aggregation in DMSO within the assessed concentration range (i.e., 2.5–45 μM).
Electronic structure calculations
Gas-phase computational investigations were conducted to estimate energies of the frontier molecular orbitals (FMOs), specifically the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), for DCPQs 1a–7a as well as 1b–7b. The density functional theory (DFT) calculations were performed using the B3LYP/6-31+G* level of theory (depicted in Figure 4 and Figure 5). Figure 4 reveals that the HOMOs of 1a–7a are predominantly localized on the π-donor units, such as benzene and naphthalene, with an illustration in the case of 6a. On the contrary, the LUMOs are delocalized across the entire π-systems, while also displaying some localization on the dicyanopyrazinopyrazine acceptor. Notably, compound 3a displays the highest orbital density separation between donors and acceptors – an attribute relevant to efficient intramolecular charge transfer processes. This aligns with the observed lower optical HOMO–LUMO gap for 3a in comparison to other compounds within the DCPQ family of compounds. As shown in Figure 5 and Table 1, the donor–acceptor character of the H-bonding counterparts is considerably muted and the HOMO and LUMO in these cases are both relatively delocalized. The HOMO energies of DPQD 1b–7b are also deep (<−6.0 eV).
![[1860-5397-20-92-4]](/bjoc/content/figures/1860-5397-20-92-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Calculated HOMO (below) and LUMO (above) energies by DFT analysis (B3LYP/6-31+G* level of theory), and corresponding molecular orbital plots for 1a–7a.
Figure 4: Calculated HOMO (below) and LUMO (above) energies by DFT analysis (B3LYP/6-31+G* level of theory), ...
![[1860-5397-20-92-5]](/bjoc/content/figures/1860-5397-20-92-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: Calculated HOMO (below) and LUMO (above) energies by DFT analysis (B3LYP/6-31+G* level of theory), and corresponding molecular orbital plots for 1b–7b.
Figure 5: Calculated HOMO (below) and LUMO (above) energies by DFT analysis (B3LYP/6-31+G* level of theory), ...
Table 1: Absorbance properties of 1a–6a and 1b–7b in DMSO.a
| Compound |
λmax
(nm) |
λonset
(nm) |
ε
(104 M−1 cm−1) |
EHOMODFT
(eV)b |
ELUMODFT
(eV)b |
EgapDFT
(eV)b |
Egapopt
(eV)a |
| 1a | 400 | 447 | 1.4 | −7.72 | −4.42 | 3.30 | 2.85 |
| 2a | 400 | 455 | 2.1 | −7.45 | −4.21 | 3.24 | 2.71 |
| 3a | 388 | 546 | 0.73 | −6.53 | −3.73 | 2.80 | 2.26 |
| 4a | 394 | 436 | 1.3 | −7.24 | −3.82 | 3.42 | 2.85 |
| 5a | 407 | 466 | 1.7 | −7.16 | −3.92 | 3.24 | 2.67 |
| 6a | 423 | 492 | 2.2 | −7.09 | −4.08 | 3.01 | 2.52 |
| 1b | 368 | 377 | 2.6 | −6.99 | −2.89 | 4.10 | 3.23 |
| 2b | 377 | 388 | 2.8 | −6.71 | −2.72 | 3.98 | 3.20 |
| 3b | 399 | 451 | 3.9 | −6.06 | −3.05 | 3.01 | 2.75 |
| 4b | 391 | 443 | 1.8 | −6.38 | −2.75 | 3.63 | 2.80 |
| 5b | 357 | 391 | 2.4 | −6.39 | −2.49 | 3.89 | 3.17 |
| 6b | 400 | 432 | 4.6 | −6.39 | −2.72 | 3.67 | 2.86 |
| 7b | 396 | 398 | 3.0 | −6.75 | −2.95 | 3.80 | 3.11 |
aAll measurements performed at room temperature and 20 μM concentration; bEnergy values obtained from gas-phase computations using Gaussian 09 at the DFT B3LYP-6-31+G* level.
FMO energies are of great importance not only for the stability but also for device optimization. Raising the HOMO energy level can facilitate hole injection when using Pt electrodes [33], consequently enabling the fabrication of ambipolar devices supporting both hole and electron transport. On the other hand, unipolar electron transport could be facilitated in the case of DCPQs 1a–6a given their extremely deep HOMO energy levels. Deep HOMO levels are important for optimal device performance and resistance to oxidation [34]. Compounds with deep LUMO energy levels are essential for efficient electron transport. However, compounds with LUMO levels ≈−4.0 eV are prone to undergo oxidation with O2 or reduction with H2O [35]. This could be the reason why electron transport properties in the OFETs previously reported by Yamashita are not very good [25]. Therefore, in the case of DPQD derivatives 1b–7b, a balance is achieved, characterized by a HOMO with an energy level below –6.0 eV and moderately low LUMO with an energy level below −2.7 eV. The notable computed HOMO–LUMO difference between DCPQs and DPQDs amounting to more than 1.0 eV in certain cases, correlates well with optical gaps obtained from absorbance spectra, taken as the photon energy corresponding to the cut-off wavelength (Table 1). Subsequently, preliminary electrochemical studies were performed with selected DCPQs and corresponding DPQD counterparts using cyclic voltammetry (Figure S1, Supporting Information File 1). Cyclic voltammetric measurements of DCPQs, specifically 4a and 6a, and DPQDs 4b and 6b, support the overall trends observed through the electronic structure calculations.
X-ray crystallography
Single crystal X-ray diffraction data could be obtained for 2b, 5b, and 6b. Figure 6 illustrates the single-crystal X-ray analysis of 2b, and its important structural parameters were compared with that of 2a (#CCDC 227464) reported in the literature [25]. An improved 2D nanostructure through highly ordered organization facilitated by H-bonding is revealed. Notably, the C–C distance between two carbonyl groups measures 1.531 Å, comparatively larger than that of aromatic systems and on the order of Csp3–Csp3 bonds (Figure 6a). This represents the first evidence that the keto form is largely favored. Next, the molecule does not adopt a fully planar conformation, instead exhibiting a torsional angle of 4.33° with respect to the carbonyl groups (Figure 6b) and 5.25° concerning peripheral rings 1 and 3 (Figure 6c). The unit cell is composed of a dimer of 2b held together by intermolecular N–H∙∙∙N and C–H∙∙∙O=C H-bonds with average distances of 2.30 Å and 2.28 Å, respectively (Figure 6d and 6e). The herringbone packing of 2b is expected to be favorable for charge mobility within stacked arrangements [36,37]. Additionally, the intermolecular π–π stacking distance is reduced to 3.285 Å in the staggered form (Figure 6f and 6g), 0.27 Å shorter than its non-hydrogen bonding dicyano comparator 2a. The single-crystal X-ray analysis of other DPQDs 5b and 6b did not reveal a herringbone lattice pattern, as shown in Tables S5 and S6 and Figures S42–S45 (Supporting Information File 1). In the case of 5b, this is presumably due to the twisted phenyl rings that dictate the packing arrangement. Nonetheless, close π-stacking distances (≈3.2 Å) are observed between the 5b units. For 6b, this is likely due to the included solvent (ethylene glycol) that hydrogen bonds to the quinoxalinedione. Moving forward, 2b stands out as a promising candidate for future solid-state characterization and device fabrication studies.
![[1860-5397-20-92-6]](/bjoc/content/figures/1860-5397-20-92-6.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 6: Asymmetric unit of DPQD 2b with important bond lengths highlighted (a). Torsion angles of 4.33° and 5.25° are associated with the carbonyl groups and rings 1 and 3, respectively (b) and (c). Packing diagram showing the average intermolecular N–H∙∙∙N and C–H∙∙∙O=C distances (d). Unit cell comprised of four molecules of 2b (e). Herringbone packing of 2b showing the distance between adjacent ring centroids (f). Herringbone backing of 2b shown as a spacefilling model (g). Atom color code: blue N, gray C, red O, and white H.
Figure 6: Asymmetric unit of DPQD 2b with important bond lengths highlighted (a). Torsion angles of 4.33° and...
Conclusion
To conclude, we have used an efficient synthetic method to produce a structurally diverse class of H-bonding capable electron-deficient N-heteroacenes DPQDs 1b–7b. The DPQDs were accessed from their respective N-heteroacenes DCPQs 1a–7a in one step. Structure–property relationships for the DCPQs and DPQDs were established using gas-phase computational DFT analysis (B3LYP/6-31+G* level of theory), UV–visible spectroscopy, thermal analysis, and X-ray analysis in the solid state for 2b, 5b, and 6b.
The primary takeaways from the work concern the attractive functional consequences of efficiently converting the DCPQs into hydrogen-bonding capable DPQDs. These include modulation of frontier MO energies, higher molar extinction coefficients, and on-average higher thermal stability (where in some cases the 5% weight loss temperature increased by up to 100 °C). Single-crystal X-ray analysis of DPQD derivative 2b further revealed that it adopts a favorable herringbone motif characterized by short π–π distances and extensive intermolecular hydrogen bonding. These structural features are anticipated to promote efficient charge transport. Future work will involve device fabrication and comparison of the DCPQs and DPQDs in this context. Overall, this work contributes to our overarching objective to investigate the influence of hydrogen-bonding functionality on optoelectronic material organization and next-generation organic semiconductor device performance.
Experimental
General methods
Reagents and solvents were purchased from commercial sources and used without further purification unless otherwise specified. THF, ether, CH2Cl2, and DMF were degassed in 20 L drums and passed through two sequential purification columns (activated alumina; molecular sieves for DMF) under a positive argon atmosphere. Palladium(II) acetate and anhydrous 1,4-dioxane were purchased from Strem Chemicals or Sigma-Aldrich and used as received. Thin-layer chromatography (TLC) was performed on SiO2-60 F254 aluminum plates with visualization by UV light or staining. Flash column chromatography was performed using Purasil SiO2-60, 230−400 mesh from Whatman. Routine 300(75) MHz 1H(13C) NMR spectra were recorded on Varian Mercury 300 or Gemini 300 spectrometers. Routine 500(125) MHz 1H(13C) NMR were recorded on an INOVA 500 spectrometer. 2D NMR spectra were recorded using a 600 MHz Varian instrument. Chemical shifts (δ) are given in parts per million (ppm) relative to TMS and referenced to residual protonated solvent (CDCl3: δH 7.26 ppm, δC 77.23 ppm; DMSO-d6: δH 2.50 ppm, δC 39.50 ppm). Abbreviations used are s (singlet), d (doublet), t (triplet), q (quartet), quin (quintet), hp (heptet), b (broad), and m (multiplet). ESI- and ESI-TOF-MS spectra were recorded on a Bruker APEX II FTICR and Agilent 6210 TOF spectrometer, respectively. EI-, CI-, and DIP-CI-MS spectra were recorded on a Thermo Trace GC DSQ (single quadrupole) spectrometer.
Absorbance measurements
Absorbance spectra were measured for 2.5, 5, 10, 15, 20, and 40 μM solutions of the DCPQs and DPQDs on a Cary 100 Bio spectrophotometer using 1 cm quartz cells. All solvents were HPLC grade (purchased from Fisher) and stored over 4 Å molecular sieves. The absorption intensity at λmax was then plotted against the concentration in all cases to confirm, by linearity, that the compounds followed Beer’s law. Molar extinction coefficients (ε) were determined from the linear plot for each compound (where A = εbc).
Computational analysis
Starting geometries were obtained from semi-empirical calculations using the MM2 method as implemented in Chem3D Pro v. 13.0.0.3015 for Windows. The ground state geometries, energies and orbital energies were then obtained from DFT calculations at the B3LYP/6-31+G* level as implemented in Gaussian 09 [38], accessed through the UF High-Performance Computing Center. Frequency calculations were performed at the same computational level, and no imaginary frequencies were found. Molecular orbital plots were made using GaussView v. 5.0.898 from the Gaussian output files.
Thermogravimetric analysis
Thermal gravimetric analysis (TGA) was performed on a TA Instruments TGA Q5000-0121 V3.8 Build 256 at a heating rate of 10 °C/min using 1–3 mg of sample in a 100 μL platinum pan (under nitrogen). The data was analyzed on Universal Analysis 2000 4.4A software.
Synthetic procedures to access dicyanopyrazinoquinoxalines (DCPQs) 1a–6a
Pyrazino[2,3-b]quinoxaline-2,3-dicarbonitrile (1a)
To a 50 mL round-bottom flask was added DDQ (0.581 g, 2.56 mmol) and CH2Cl2 (20 mL). To the suspension was added 5,10-dihydropyrazino[2,3-b]quinoxaline-2,3-dicarbonitrile (8, 0.300 g, 1.28 mmol) in small portions. The reaction was allowed to proceed overnight at room temperature, after which a pale orange reaction mixture was filtered, washed with copious amounts of CH2Cl2 and THF (10 mL), and dried in vacuo to afford a bright orange solid of 1a (0.264 g, 1.14 mmol, 89%). 1H NMR (600 MHz, DMSO-d6) δ 8.30–8.31 (dd, J = 3 Hz, J = 6.6 Hz, 2H), 8.49–8.51 (dd, J = 3 Hz, J = 6.6 Hz, 2H); 13C NMR (150 MHz, DMSO-d6) δ 114.5, 130.4, 136.1, 136.2, 142.7, 147.4; HRMS (DART): [M + H]+ calcd for C12H4N6, 233.0570; found, 233.0574; [M + NH4]+, 250.0836; found, 250.0839.
7,8-Dimethylpyrazino[2,3-b]quinoxaline-2,3-dicarbonitrile (2a)
Compound 2a was synthesized the same way as 1a using DDQ (0.581 g, 2.56 mmol), 7,8-dimethyl-5,10-dihydropyrazino[2,3-b]quinoxaline-2,3-dicarbonitrile (9, 0.300 g, 1.28 mmol) and CH2Cl2 (20 mL). The reaction mixture was filtered, washed with copious amounts of CH2Cl2, THF (20 mL), and dried in vacuo to give a bright orange solid of 2a (0.26 g, 1.0 mmol, 87%). 1H NMR (500 MHz, CDCl3) δ 2.70 (s, 6H), 8.21 (s, 2H); 1H NMR (500 MHz, DMSO-d6) δ 2.66 (s, 6H), 8.27 (s, 2H); 13C NMR (125 MHz, DMSO-d6) δ 20.1, 114.2, 127.8, 134.6, 142.3, 146.6, 148.7. The proton NMR in chloroform matches the literature value [25]; HRMS (DART): [M + H]+ calcd for C14H8N6, 261.0883; found, 261.0889; [M + NH4]+, 278.1149; found, 278.1154.
Aceanthryleno[1,2-b]pyrazino[2,3-e]pyrazine-11,12-dicarbonitrile (3a)
An oven dried sealed tube was charged with 5,6-diaminopyrazine-2,3-dicarbonitrile (12) (56.6 mg, 0.353 mmol), 1,2-aceanthrylenedione (56.6 mg, 0.244 mmol) and dry pyridine (3 mL). The resulting orange suspension was heated to 80 °C for 72 hours. After this time, brown solids precipitated out. The tube was adequately cooled, and the solid residue was filtered and washed with copious amounts of methanol, ethyl acetate and dichloromethane, to afford pure red-brown colored amorphous material (36 mg, 0.10 mmol, 41%). The material could be further purified by recrystallization from boiling DMSO to obtain highly insoluble red-brown microcrystalline material. 1H and 13C NMR could not be recorded for the compound 3a given its extremely poor solubility in common organic solvents. Rf (CH2Cl2): 0.75, fluorescent orange spot; HRMS (DART): [M + NH4]+ calcd for C22H8N6, 374.1154; found, 374.1155.
Acenaphtho[1,2-b]pyrazino[2,3-e]pyrazine-9,10-dicarbonitrile (4a)
An oven dried sealed tube was charged with 5,6-diaminopyrazine-2,3-dicarbonitrile (12) (0.15 g, 0.92 mmol), acenaphthylene-1,2-dione (0.15 g, 0.84 mmol) and glacial acetic acid (20 mL). The resulting pale-yellow suspension was heated to 115 °C for 20 hours with vigorous stirring. After 20 hours, the reaction was cooled, and yellow-gold shimmering particles began to settle out. The mixture was filtered, and the resulting gold-colored solid was recrystallized from hot DMF to obtain pure compound 4a (0.12 g, 0.39 mmol, 47%). 1H NMR (500 MHz, CDCl3) δ 8.04–8.06 (t, J = 7.5 Hz, 2H), 8.40–8.42 (d, J = 8.5 Hz, 2H), 8.71–8.73 (d, J = 7 Hz, 2H); 13C NMR could not be obtained due to the poor solubility of the compound; HRMS (DART): [M + NH4]+ calcd for C18H6N6, 324.0997; found, 324.0978.
6,7-Diphenylpyrazino[2,3-b]pyrazine-2,3-dicarbonitrile (5a)
Compound 5a was synthesized by a modified literature method [31]. To an oven dried sealed tube was added 5,6-diaminopyrazine-2,3-dicarbonitrile (12, 306 mg, 1.91 mmol), diphenylethanedione (benzil) (365 mg, 1.74 mmol), glacial acetic acid (7 mL), trifluoroacetic acid (1 mL), and 1,4-dioxane (2 mL). The resulting orange suspension was heated at 110 °C for 48 hours. After completion, the tube was allowed to cool to room temperature and placed in a freezer overnight. Gold-colored crystals of 5a were collected by filtration and dried in vacuo (430 mg, 1.28 mmol, 74%). 1H NMR (500 MHz, CDCl3) δ 7.41–7.44 (t, J = 7.5 Hz, 2H), 7.53–7.56 (t, J = 7.5 Hz, 1H), 7.73–7.75 (d, J = 8 Hz, 2H); 13C NMR (125 MHz, CDCl3) δ 113.0, 129.2, 130.9, 132.4, 133.8, 136.5, 143.9, 162.8; HRMS (DART): [M + H]+ calcd for C20H10N6, 335.1040; found, 335.1094; [M + NH4]+, 352.1305; found, 352.1358.
Dibenzo[f,h]pyrazino[2,3-b]quinoxaline-11,12-dicarbonitrile (6a)
An oven dried sealed tube was charged with 5,6-diaminopyrazine-2,3-dicarbonitrile (12, 0.73 g, 4.6 mmol), phenanthrene-9,10-dione (0.86 g, 4.1 mmol), glacial acetic acid (24 mL), TFA (6 mL) and 1,4-dioxane (12 mL). The resulting suspension was heated to 100 °C for 35 hours. By the end of the reaction, a red shimmering precipitate was observed. The reaction mixture was allowed to cool to room temperature, filtered and washed with copious amounts of dichloromethane and dried to obtain red-brick-colored solid 6a (1.10 g, 3.30 mmol, 80%). 1H NMR (500 MHz, DMSO-d6) δ 7.88–7.91 (t, J = 7 Hz, 2H), 8.02–8.05 (t, J = 7.5 Hz, 2H), 8.83–8.84 (d, J = 8 Hz, 2H), 9.21–9.23 (d, J = 7 Hz, 2H). The compound was too insoluble for 13C NMR spectroscopic analysis.
Synthesis of dihydropyrazinoquinoxaline diones 1b–7b (DPQD) from dicyanopyrazinoquinoxalines (DCPQs) 1a–6a
1,4-Dihydropyrazino[2,3-b]quinoxaline-2,3-dione (1b)
To a 20 mL round-bottom flask was added pyrazino[2,3-b]quinoxaline-2,3-dicarbonitrile (1a, 146 mg, 0.628 mmol) and THF (10 mL). To the resulting orange suspension was dropwise added a solution of KOH (353 mg, 6.280 mmol) in water (10 mL) and reacted at room temperature for 3 hours. After this time the light orange solution was diluted with water (50 mL) and acidified with 1 N HCl until a white solid precipitated out. The solids were collected via filtration and dried in an oven (120 °C) for 24 hours. An off-white to pale yellow solid of 1b was obtained (123 mg, 0.572 mmol, 91%). 1H NMR (600 MHz, DMSO-d6) δ 7.65–7.67 (dd, J = 3.6 Hz, J = 6.6 Hz, 2H), 7.84–7.86 (dd, J = 3.6 Hz, J = 6.6 Hz, 2H), 12.71 (s, 1H); 13C NMR (150 MHz, DMSO-d6) δ 126.7, 128.0, 137.4, 137.7, 155.6; HRESIMS: [M + Na]+ calcd for C10H6N4O2, 237.0388; found, 237.0394.
7,8-Dimethyl-1,4-dihydropyrazino[2,3-b]quinoxaline-2,3-dione (2b)
Compound 2b was synthesized with a method similar to that of 1b using 2a (97.7 mg, 0.378 mmol), THF (8 mL), and KOH (64 mg, 1.13 mmol) in water (8 mL). After reaction, the red-orange solution was diluted with water (50 mL) and acidified with 1 N HCl until a pink-colored solid precipitated out. The solids were collected via filtration using a fine sintered funnel and dried in an oven to afford off-white solid of 2b (90 mg, 0.37 mmol, 99%). 1H NMR (500 MHz, DMSO-d6) δ 2.41 (s, 6H), 7.61 (s, 2H), 12.61 (s, 2H); 13C NMR (150 MHz, DMSO-d6) δ 19.7, 126.0, 136.1, 136.8, 137.9, 155.6; HRESIMS: [M + Na]+ calcd for C12H10N4O2, 265.0701; found, 265.0692.
10,13-Dihydroaceanthryleno[1,2-b]pyrazino[2,3-e]pyrazine-11,12-dione (3b)
Compound 3b was synthesized in the same way as 1b using compound 3a (10 mg, 0.03 mmol), THF (4 mL), 1,4-dioxane (1 mL), and KOH (10 equiv) in water (5 mL). The product 3b was obtained as an orange precipitate after the reaction mixture was poured into 30 mL of ice and neutralized with 1 N HCl. Yield: 6 mg, 0.02 mmol, 67%. 1H NMR (500 MHz, DMSO-d6) δ 7.56–7.60 (t, J = 7.5 Hz, 1H), 7.71–7.74 (t, J = 7.5 Hz, 2H), 8.04–8.05 (d, J = 7 Hz, 1H), 8.21–8.23 (d, J = 8.5 Hz, 2H), 8.76 (s, 1H), 9.00–9.02 (d, J = 8.5 Hz, 1H), 12.72 (brs, 2H); 13C NMR (125 MHz, DMSO-d6 + TFA) δ 123.0, 124.3, 124.9, 126.0, 126.8, 127.9, 128.2, 128.8, 128.9, 129.8, 130.1, 130.4, 130.9, 133.5, 133.8, 134.4, 144.7, 147.0, 155.5, 155.6; HRESIMS: [M + K]+ calcd for C20H10N4O2, 377.0441; found, 377.0423.
8,11-Dihydroacenaphtho[1,2-b]pyrazino[2,3-e]pyrazine-9,10-dione (4b)
Compound 4b was synthesized using a method similar to that of 1b using compound 4a (102 mg, 0.333 mmol), THF (5 mL), and KOH (187 mg, 3.33 mmol) in water (5 mL). After the addition of KOH, the reaction mixture turned red-brown. After completion, the reaction mixture was diluted with water (10 mL) and acidified with concentrated HCl until a precipitate became apparent. The yellow-orange precipitate was filtered and washed with copious amounts of water and dried in vacuo (95 mg, 0.300 mmol, 99%). 1H NMR (500 MHz, DMSO-d6) δ 7.68–7.71 (t, J = 7.5 Hz, 2H), 7.96–7.98 (d, J = 7 Hz, 2H), 7.99–8.01 (d, J = 8.5 Hz, 2H), 11.57 (brs, 1H); 1H NMR (500 MHz, C6D6) δ 7.36–7.39 (t, J = 7 Hz, 2H), 7.64–7.65 (d, J = 8.3 Hz, 2H), 7.99–8.00 (d, J = 6.9 Hz, 2H), 12.94 (brs, 1H); 13C NMR (125 MHz, DMSO-d6) δ 121.9, 128.8, 129.1, 129.2, 130.7 131.3, 134.8, 145.3, 155.5; HRESIMS: [M + K]+ calcd for C16H8N4O2, 327.0284; found, 327.0279.
6,7-Diphenyl-1,4-dihydropyrazino[2,3-b]pyrazine-2,3-dione (5b)
Compound 5b was synthesized using a method similar to that of 1b using compound 5a (236 mg, 0.706 mmol), THF (9 mL), and KOH (400 mg, 7.06 mmol) in water (9 mL). The red solution was diluted with water (10 mL) and acidified with 1 N HCl until an orange precipitate of 5b was obtained (198 mg, 0.628 mmol, 89%). 1H NMR (500 MHz, DMSO-d6) δ 7.32 (s, 10H), 12.70 (brs, 2H); 13C NMR (125 MHz, DMSO-d6) δ 128.5, 128.6, 129.9, 135.2, 138.4, 144.1, 156.1; HRESIMS: [M + H]+ calcd for C18H12N4O2, 317.1038; found, 317.1021.
10,13-Dihydrodibenzo[f,h]pyrazino[2,3-b]quinoxaline-11,12-dione (6b)
Compound 6b was synthesized similarly to 1b using compound 6a (1.11 g, 3.34 mmol, THF (50 mL), and KOH (1.87 g, 33.3 mmol) in water (50 mL). After completion, a yellow precipitate was observed which was filtered and washed with copious amounts of 1 N HCl and diethyl ether. The product 6b was then dried in vacuo to obtain pale yellow amorphous solid (1.15 g, 3.31 mmol, 99%). 1H NMR (500 MHz, DMSO-d6) δ 7.60–7.63 (t, J = 7.5 Hz, 2H), 7.66–7.69 (t, J = 8.5 Hz, 2H), 8.71–8.73 (d, J = 8.5 Hz, 2H), 8.98–9.00 (d, J = 8 Hz, 2H); 13C NMR (125 MHz, DMSO-d6) δ 123.0, 123.8, 127.1, 129.1, 130.0, 132.6, 143.1, 160.4; 13C NMR (150 MHz, DMF-d7) δ 123.3, 124.0, 127.7, 128.6, 128.8, 129.9, 133.6, 136.5, 155.7; HRESIMS: [M + H]+ calcd for C18H10N4O2, 315.0881; found, 315.0877.
10,13-Dihydropyrazino[2',3':5,6]pyrazino[2,3-f][1,10]phenanthroline-11,12-dione (7b)
In a 100 mL three-neck round-bottom flask, compounds 12 (100 mg, 0.624 mmol) and 7e (150 mg, 0.713 mmol) were added to 15 mL of tetrahydrofuran (THF). Subsequently, a solution of potassium hydroxide in water (15 mL) was added dropwise at room temperature, resulting in a color change from orange to red. The reaction mixture stirred for 72 hours, resulted in the formation of an orange-brown precipitate. The reaction mixture was then filtered, and 1 N HCl was added until orange-red colored solids appeared. The solids were collected via filtration and washed with copious amounts of water and dried in an oven to obtain red-orange product 7b (120 mg, 0.381 mmol, 61%). 1H NMR (500 MHz, DMSO-d6) δ 7.78–7.81 (dd, J = 4.5 Hz, 8.5 Hz, 2H), 8.96–8.98 (d, J = 7.5 Hz, 2H), 9.34–9.36 (d, J = 8 Hz, 2H); 13C NMR (125 MHz, DMSO-d6 + TFA) δ 126.8, 127.8, 136.8, 138.3, 139.0, 147.9, 155.8; HRESIMS: [M + H]+ calcd for C16H8N6O2, 317.0787; found, 317.0776.
Supporting Information
| Supporting Information File 1: General experimental methods, 1H and 13C NMR, and HRMS spectra of the compounds as well as photophysical, computational and X-ray data. | ||
| Format: PDF | Size: 6.7 MB | Download |
Acknowledgements
This work is based on Tural N. Akhmedov’s doctoral thesis (“Design, Synthesis, and Characterization of Novel N-Heteroacenes for Organic Electronics”, University of Florida, 2017). We acknowledge the University of Florida Research Computing facilities for providing computational resources and support that have contributed to the research results reported herein (https://www.rc.ufl.edu). We are thankful to the UF Center for Nuclear Magnetic Resonance Spectroscopy for providing necessary instrumentation, equipment, and support that have contributed to these results.
Funding
R.K.C. and J.X. are grateful to the National Science Foundation (NSF) for supporting this research (R.K.C.: CHE-1057411; R.K.C./J.X.: CHE-1507561). K.A.A. wishes to acknowledge the NSF and the University of Florida for funding of the purchase of the X-ray equipment. The mass spectrometry data has been generated by the Mass Spectrometry Research and Education Center at the University of Florida which is grateful for funding from the National Institutes of Health (NIH S10 OD021758-01A1 and S10 OD030250-01A1). N.G.A. acknowledges funding from the NSF for NMR instrumentation (CHE-1228336).
Data Availability Statement
All data that supports the findings of this study is available in the published article and/or the supporting information to this article.
References
-
Gospodinova, N.; Tomšík, E. Prog. Polym. Sci. 2015, 43, 33–47. doi:10.1016/j.progpolymsci.2014.10.010
Return to citation in text: [1] -
Chen, J.; Wang, Z.; Deng, Z.; Chen, L.; Wu, X.; Gao, Y.; Hu, Y.; Li, M.; Wang, H. Front. Chem. (Lausanne, Switz.) 2023, 11, 1200644. doi:10.3389/fchem.2023.1200644
Return to citation in text: [1] -
Gómez, P.; Georgakopoulos, S.; Más-Montoya, M.; Cerdá, J.; Pérez, J.; Ortí, E.; Aragó, J.; Curiel, D. ACS Appl. Mater. Interfaces 2021, 13, 8620–8630. doi:10.1021/acsami.0c18928
Return to citation in text: [1] -
Głowacki, E. D.; Coskun, H.; Blood-Forsythe, M. A.; Monkowius, U.; Leonat, L.; Grzybowski, M.; Gryko, D.; White, M. S.; Aspuru-Guzik, A.; Sariciftci, N. S. Org. Electron. 2014, 15, 3521–3528. doi:10.1016/j.orgel.2014.09.038
Return to citation in text: [1] -
Gsänger, M.; Oh, J. H.; Könemann, M.; Höffken, H. W.; Krause, A.-M.; Bao, Z.; Würthner, F. Angew. Chem., Int. Ed. 2010, 49, 740–743. doi:10.1002/anie.200904215
Return to citation in text: [1] -
Watanabe, Y.; Yokoyama, D.; Koganezawa, T.; Katagiri, H.; Ito, T.; Ohisa, S.; Chiba, T.; Sasabe, H.; Kido, J. Adv. Mater. (Weinheim, Ger.) 2019, 31, 1808300. doi:10.1002/adma.201808300
Return to citation in text: [1] -
Fukagawa, H.; Hasegawa, M.; Morii, K.; Suzuki, K.; Sasaki, T.; Shimizu, T. Adv. Mater. (Weinheim, Ger.) 2019, 31, 1904201. doi:10.1002/adma.201904201
Return to citation in text: [1] -
Trinh, C. K.; Abdo, N. I. J. Mol. Struct. 2022, 1269, 133764. doi:10.1016/j.molstruc.2022.133764
Return to citation in text: [1] -
Anthony, J. E. Angew. Chem., Int. Ed. 2008, 47, 452–483. doi:10.1002/anie.200604045
Return to citation in text: [1] -
Bunz, U. H. F. Acc. Chem. Res. 2015, 48, 1676–1686. doi:10.1021/acs.accounts.5b00118
Return to citation in text: [1] -
Bunz, U. H. F.; Engelhart, J. U.; Lindner, B. D.; Schaffroth, M. Angew. Chem., Int. Ed. 2013, 52, 3810–3821. doi:10.1002/anie.201209479
Return to citation in text: [1] -
Wang, J.; Chu, M.; Fan, J.-X.; Lau, T.-K.; Ren, A.-M.; Lu, X.; Miao, Q. J. Am. Chem. Soc. 2019, 141, 3589–3596. doi:10.1021/jacs.8b12671
Return to citation in text: [1] -
Jonkheijm, P.; Stutzmann, N.; Chen, Z.; de Leeuw, D. M.; Meijer, E. W.; Schenning, A. P. H. J.; Würthner, F. J. Am. Chem. Soc. 2006, 128, 9535–9540. doi:10.1021/ja061966z
Return to citation in text: [1] -
Schulze, B. M.; Shewmon, N. T.; Zhang, J.; Watkins, D. L.; Mudrick, J. P.; Cao, W.; Bou Zerdan, R.; Quartararo, A. J.; Ghiviriga, I.; Xue, J.; Castellano, R. K. J. Mater. Chem. A 2014, 2, 1541–1549. doi:10.1039/c3ta13529b
Return to citation in text: [1] -
Shewmon, N. T.; Watkins, D. L.; Galindo, J. F.; Zerdan, R. B.; Chen, J.; Keum, J.; Roitberg, A. E.; Xue, J.; Castellano, R. K. Adv. Funct. Mater. 2015, 25, 5166–5177. doi:10.1002/adfm.201501815
Return to citation in text: [1] -
Weldeab, A. O.; Kornman, C. T.; Li, L.; Starkenburg, D. J.; Zhao, X.; Fagnani, D. E.; Sadovy, S. J.; Perry, S. S.; Xue, J.; Castellano, R. K. Org. Mater. 2021, 03, 390–404. doi:10.1055/a-1534-1508
Return to citation in text: [1] -
Berg, D.; Nielinger, C.; Mader, W.; Sokolowski, M. Synth. Met. 2009, 159, 2599–2602. doi:10.1016/j.synthmet.2009.09.017
Return to citation in text: [1] -
Głowacki, E. D.; Leonat, L.; Voss, G.; Bodea, M.-A.; Bozkurt, Z.; Ramil, A. M.; Irimia-Vladu, M.; Bauer, S.; Sariciftci, N. S. AIP Adv. 2011, 1, 042132. doi:10.1063/1.3660358
Return to citation in text: [1] -
Irimia‐Vladu, M.; Głowacki, E. D.; Troshin, P. A.; Schwabegger, G.; Leonat, L.; Susarova, D. K.; Krystal, O.; Ullah, M.; Kanbur, Y.; Bodea, M. A.; Razumov, V. F.; Sitter, H.; Bauer, S.; Sariciftci, N. S. Adv. Mater. (Weinheim, Ger.) 2012, 24, 375–380. doi:10.1002/adma.201102619
Return to citation in text: [1] -
Irimia-Vladu, M.; Kanbur, Y.; Camaioni, F.; Coppola, M. E.; Yumusak, C.; Irimia, C. V.; Vlad, A.; Operamolla, A.; Farinola, G. M.; Suranna, G. P.; González-Benitez, N.; Molina, M. C.; Bautista, L. F.; Langhals, H.; Stadlober, B.; Głowacki, E. D.; Sariciftci, N. S. Chem. Mater. 2019, 31, 6315–6346. doi:10.1021/acs.chemmater.9b01405
Return to citation in text: [1] -
Głowacki, E. D.; Irimia‐Vladu, M.; Kaltenbrunner, M.; Gsiorowski, J.; White, M. S.; Monkowius, U.; Romanazzi, G.; Suranna, G. P.; Mastrorilli, P.; Sekitani, T.; Bauer, S.; Someya, T.; Torsi, L.; Sariciftci, N. S. Adv. Mater. (Weinheim, Ger.) 2013, 25, 1563–1569. doi:10.1002/adma.201204039
Return to citation in text: [1] -
He, Z.; Liu, D.; Mao, R.; Tang, Q.; Miao, Q. Org. Lett. 2012, 14, 1050–1053. doi:10.1021/ol203404q
Return to citation in text: [1] [2] -
Lindner, B. D.; Engelhart, J. U.; Märken, M.; Tverskoy, O.; Appleton, A. L.; Rominger, F.; Hardcastle, K. I.; Enders, M.; Bunz, U. H. F. Chem. – Eur. J. 2012, 18, 4627–4633. doi:10.1002/chem.201103227
Return to citation in text: [1] [2] -
Takeda, T.; Ikemoto, T.; Yamamoto, S.; Matsuda, W.; Seki, S.; Mitsuishi, M.; Akutagawa, T. ACS Omega 2018, 3, 13694–13703. doi:10.1021/acsomega.8b01943
Return to citation in text: [1] [2] [3] -
Nishida, J.-i.; Naraso; Murai, S.; Fujiwara, E.; Tada, H.; Tomura, M.; Yamashita, Y. Org. Lett. 2004, 6, 2007–2010. doi:10.1021/ol0494010
Return to citation in text: [1] [2] [3] [4] [5] -
Akhmedov, T. Design, Synthesis, and Characterization of Novel N-Heteroacenes for Organic Electronics. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2017.
Return to citation in text: [1] -
Suzuki, T.; Nagae, Y.; Mitsuhashi, K. J. Heterocycl. Chem. 1986, 23, 1419–1421. doi:10.1002/jhet.5570230533
Return to citation in text: [1] -
Richards, G. J.; Hill, J. P.; Okamoto, K.; Shundo, A.; Akada, M.; Elsegood, M. R. J.; Mori, T.; Ariga, K. Langmuir 2009, 25, 8408–8413. doi:10.1021/la8041633
Return to citation in text: [1] [2] -
Li, B.-N.; Liu, Y.-Y.; Wang, Y.-P.; Pan, M. Molecules 2021, 26, 3244. doi:10.3390/molecules26113244
Return to citation in text: [1] -
He, Z.; Mao, R.; Liu, D.; Miao, Q. Org. Lett. 2012, 14, 4190–4193. doi:10.1021/ol301854z
Return to citation in text: [1] -
Richards, G. J.; Hill, J. P.; Subbaiyan, N. K.; D’Souza, F.; Karr, P. A.; Elsegood, M. R. J.; Teat, S. J.; Mori, T.; Ariga, K. J. Org. Chem. 2009, 74, 8914–8923. doi:10.1021/jo901832n
Return to citation in text: [1] [2] -
Li, Y.; Liu, T.; Liu, H.; Tian, M.-Z.; Li, Y. Acc. Chem. Res. 2014, 47, 1186–1198. doi:10.1021/ar400264e
Return to citation in text: [1] -
Lee, J.-H.; Kim, J.-J. Phys. Status Solidi A 2012, 209, 1399–1413. doi:10.1002/pssa.201228199
Return to citation in text: [1] -
Nakayama, K.; Hirose, Y.; Soeda, J.; Yoshizumi, M.; Uemura, T.; Uno, M.; Li, W.; Kang, M. J.; Yamagishi, M.; Okada, Y.; Miyazaki, E.; Nakazawa, Y.; Nakao, A.; Takimiya, K.; Takeya, J. Adv. Mater. (Weinheim, Ger.) 2011, 23, 1626–1629. doi:10.1002/adma.201004387
Return to citation in text: [1] -
Sirringhaus, H. Adv. Mater. (Weinheim, Ger.) 2009, 21, 3859–3873. doi:10.1002/adma.200901136
Return to citation in text: [1] -
Coropceanu, V.; Cornil, J.; da Silva Filho, D. A.; Olivier, Y.; Silbey, R.; Brédas, J.-L. Chem. Rev. 2007, 107, 926–952. doi:10.1021/cr050140x
Return to citation in text: [1] -
Mei, J.; Diao, Y.; Appleton, A. L.; Fang, L.; Bao, Z. J. Am. Chem. Soc. 2013, 135, 6724–6746. doi:10.1021/ja400881n
Return to citation in text: [1] -
Gaussian 09, Revision D. 01; Gaussian Inc.: Wallingford, CT, 2009.
Return to citation in text: [1]
| 35. | Sirringhaus, H. Adv. Mater. (Weinheim, Ger.) 2009, 21, 3859–3873. doi:10.1002/adma.200901136 |
| 25. | Nishida, J.-i.; Naraso; Murai, S.; Fujiwara, E.; Tada, H.; Tomura, M.; Yamashita, Y. Org. Lett. 2004, 6, 2007–2010. doi:10.1021/ol0494010 |
| 25. | Nishida, J.-i.; Naraso; Murai, S.; Fujiwara, E.; Tada, H.; Tomura, M.; Yamashita, Y. Org. Lett. 2004, 6, 2007–2010. doi:10.1021/ol0494010 |
| 1. | Gospodinova, N.; Tomšík, E. Prog. Polym. Sci. 2015, 43, 33–47. doi:10.1016/j.progpolymsci.2014.10.010 |
| 8. | Trinh, C. K.; Abdo, N. I. J. Mol. Struct. 2022, 1269, 133764. doi:10.1016/j.molstruc.2022.133764 |
| 22. | He, Z.; Liu, D.; Mao, R.; Tang, Q.; Miao, Q. Org. Lett. 2012, 14, 1050–1053. doi:10.1021/ol203404q |
| 23. | Lindner, B. D.; Engelhart, J. U.; Märken, M.; Tverskoy, O.; Appleton, A. L.; Rominger, F.; Hardcastle, K. I.; Enders, M.; Bunz, U. H. F. Chem. – Eur. J. 2012, 18, 4627–4633. doi:10.1002/chem.201103227 |
| 7. | Fukagawa, H.; Hasegawa, M.; Morii, K.; Suzuki, K.; Sasaki, T.; Shimizu, T. Adv. Mater. (Weinheim, Ger.) 2019, 31, 1904201. doi:10.1002/adma.201904201 |
| 24. | Takeda, T.; Ikemoto, T.; Yamamoto, S.; Matsuda, W.; Seki, S.; Mitsuishi, M.; Akutagawa, T. ACS Omega 2018, 3, 13694–13703. doi:10.1021/acsomega.8b01943 |
| 4. | Głowacki, E. D.; Coskun, H.; Blood-Forsythe, M. A.; Monkowius, U.; Leonat, L.; Grzybowski, M.; Gryko, D.; White, M. S.; Aspuru-Guzik, A.; Sariciftci, N. S. Org. Electron. 2014, 15, 3521–3528. doi:10.1016/j.orgel.2014.09.038 |
| 5. | Gsänger, M.; Oh, J. H.; Könemann, M.; Höffken, H. W.; Krause, A.-M.; Bao, Z.; Würthner, F. Angew. Chem., Int. Ed. 2010, 49, 740–743. doi:10.1002/anie.200904215 |
| 6. | Watanabe, Y.; Yokoyama, D.; Koganezawa, T.; Katagiri, H.; Ito, T.; Ohisa, S.; Chiba, T.; Sasabe, H.; Kido, J. Adv. Mater. (Weinheim, Ger.) 2019, 31, 1808300. doi:10.1002/adma.201808300 |
| 23. | Lindner, B. D.; Engelhart, J. U.; Märken, M.; Tverskoy, O.; Appleton, A. L.; Rominger, F.; Hardcastle, K. I.; Enders, M.; Bunz, U. H. F. Chem. – Eur. J. 2012, 18, 4627–4633. doi:10.1002/chem.201103227 |
| 2. | Chen, J.; Wang, Z.; Deng, Z.; Chen, L.; Wu, X.; Gao, Y.; Hu, Y.; Li, M.; Wang, H. Front. Chem. (Lausanne, Switz.) 2023, 11, 1200644. doi:10.3389/fchem.2023.1200644 |
| 3. | Gómez, P.; Georgakopoulos, S.; Más-Montoya, M.; Cerdá, J.; Pérez, J.; Ortí, E.; Aragó, J.; Curiel, D. ACS Appl. Mater. Interfaces 2021, 13, 8620–8630. doi:10.1021/acsami.0c18928 |
| 24. | Takeda, T.; Ikemoto, T.; Yamamoto, S.; Matsuda, W.; Seki, S.; Mitsuishi, M.; Akutagawa, T. ACS Omega 2018, 3, 13694–13703. doi:10.1021/acsomega.8b01943 |
| 16. | Weldeab, A. O.; Kornman, C. T.; Li, L.; Starkenburg, D. J.; Zhao, X.; Fagnani, D. E.; Sadovy, S. J.; Perry, S. S.; Xue, J.; Castellano, R. K. Org. Mater. 2021, 03, 390–404. doi:10.1055/a-1534-1508 |
| 21. | Głowacki, E. D.; Irimia‐Vladu, M.; Kaltenbrunner, M.; Gsiorowski, J.; White, M. S.; Monkowius, U.; Romanazzi, G.; Suranna, G. P.; Mastrorilli, P.; Sekitani, T.; Bauer, S.; Someya, T.; Torsi, L.; Sariciftci, N. S. Adv. Mater. (Weinheim, Ger.) 2013, 25, 1563–1569. doi:10.1002/adma.201204039 |
| 25. | Nishida, J.-i.; Naraso; Murai, S.; Fujiwara, E.; Tada, H.; Tomura, M.; Yamashita, Y. Org. Lett. 2004, 6, 2007–2010. doi:10.1021/ol0494010 |
| 14. | Schulze, B. M.; Shewmon, N. T.; Zhang, J.; Watkins, D. L.; Mudrick, J. P.; Cao, W.; Bou Zerdan, R.; Quartararo, A. J.; Ghiviriga, I.; Xue, J.; Castellano, R. K. J. Mater. Chem. A 2014, 2, 1541–1549. doi:10.1039/c3ta13529b |
| 15. | Shewmon, N. T.; Watkins, D. L.; Galindo, J. F.; Zerdan, R. B.; Chen, J.; Keum, J.; Roitberg, A. E.; Xue, J.; Castellano, R. K. Adv. Funct. Mater. 2015, 25, 5166–5177. doi:10.1002/adfm.201501815 |
| 22. | He, Z.; Liu, D.; Mao, R.; Tang, Q.; Miao, Q. Org. Lett. 2012, 14, 1050–1053. doi:10.1021/ol203404q |
| 31. | Richards, G. J.; Hill, J. P.; Subbaiyan, N. K.; D’Souza, F.; Karr, P. A.; Elsegood, M. R. J.; Teat, S. J.; Mori, T.; Ariga, K. J. Org. Chem. 2009, 74, 8914–8923. doi:10.1021/jo901832n |
| 13. | Jonkheijm, P.; Stutzmann, N.; Chen, Z.; de Leeuw, D. M.; Meijer, E. W.; Schenning, A. P. H. J.; Würthner, F. J. Am. Chem. Soc. 2006, 128, 9535–9540. doi:10.1021/ja061966z |
| 36. | Coropceanu, V.; Cornil, J.; da Silva Filho, D. A.; Olivier, Y.; Silbey, R.; Brédas, J.-L. Chem. Rev. 2007, 107, 926–952. doi:10.1021/cr050140x |
| 37. | Mei, J.; Diao, Y.; Appleton, A. L.; Fang, L.; Bao, Z. J. Am. Chem. Soc. 2013, 135, 6724–6746. doi:10.1021/ja400881n |
| 9. | Anthony, J. E. Angew. Chem., Int. Ed. 2008, 47, 452–483. doi:10.1002/anie.200604045 |
| 10. | Bunz, U. H. F. Acc. Chem. Res. 2015, 48, 1676–1686. doi:10.1021/acs.accounts.5b00118 |
| 11. | Bunz, U. H. F.; Engelhart, J. U.; Lindner, B. D.; Schaffroth, M. Angew. Chem., Int. Ed. 2013, 52, 3810–3821. doi:10.1002/anie.201209479 |
| 12. | Wang, J.; Chu, M.; Fan, J.-X.; Lau, T.-K.; Ren, A.-M.; Lu, X.; Miao, Q. J. Am. Chem. Soc. 2019, 141, 3589–3596. doi:10.1021/jacs.8b12671 |
| 17. | Berg, D.; Nielinger, C.; Mader, W.; Sokolowski, M. Synth. Met. 2009, 159, 2599–2602. doi:10.1016/j.synthmet.2009.09.017 |
| 18. | Głowacki, E. D.; Leonat, L.; Voss, G.; Bodea, M.-A.; Bozkurt, Z.; Ramil, A. M.; Irimia-Vladu, M.; Bauer, S.; Sariciftci, N. S. AIP Adv. 2011, 1, 042132. doi:10.1063/1.3660358 |
| 19. | Irimia‐Vladu, M.; Głowacki, E. D.; Troshin, P. A.; Schwabegger, G.; Leonat, L.; Susarova, D. K.; Krystal, O.; Ullah, M.; Kanbur, Y.; Bodea, M. A.; Razumov, V. F.; Sitter, H.; Bauer, S.; Sariciftci, N. S. Adv. Mater. (Weinheim, Ger.) 2012, 24, 375–380. doi:10.1002/adma.201102619 |
| 20. | Irimia-Vladu, M.; Kanbur, Y.; Camaioni, F.; Coppola, M. E.; Yumusak, C.; Irimia, C. V.; Vlad, A.; Operamolla, A.; Farinola, G. M.; Suranna, G. P.; González-Benitez, N.; Molina, M. C.; Bautista, L. F.; Langhals, H.; Stadlober, B.; Głowacki, E. D.; Sariciftci, N. S. Chem. Mater. 2019, 31, 6315–6346. doi:10.1021/acs.chemmater.9b01405 |
| 26. | Akhmedov, T. Design, Synthesis, and Characterization of Novel N-Heteroacenes for Organic Electronics. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2017. |
| 25. | Nishida, J.-i.; Naraso; Murai, S.; Fujiwara, E.; Tada, H.; Tomura, M.; Yamashita, Y. Org. Lett. 2004, 6, 2007–2010. doi:10.1021/ol0494010 |
| 25. | Nishida, J.-i.; Naraso; Murai, S.; Fujiwara, E.; Tada, H.; Tomura, M.; Yamashita, Y. Org. Lett. 2004, 6, 2007–2010. doi:10.1021/ol0494010 |
| 33. | Lee, J.-H.; Kim, J.-J. Phys. Status Solidi A 2012, 209, 1399–1413. doi:10.1002/pssa.201228199 |
| 34. | Nakayama, K.; Hirose, Y.; Soeda, J.; Yoshizumi, M.; Uemura, T.; Uno, M.; Li, W.; Kang, M. J.; Yamagishi, M.; Okada, Y.; Miyazaki, E.; Nakazawa, Y.; Nakao, A.; Takimiya, K.; Takeya, J. Adv. Mater. (Weinheim, Ger.) 2011, 23, 1626–1629. doi:10.1002/adma.201004387 |
| 24. | Takeda, T.; Ikemoto, T.; Yamamoto, S.; Matsuda, W.; Seki, S.; Mitsuishi, M.; Akutagawa, T. ACS Omega 2018, 3, 13694–13703. doi:10.1021/acsomega.8b01943 |
| 32. | Li, Y.; Liu, T.; Liu, H.; Tian, M.-Z.; Li, Y. Acc. Chem. Res. 2014, 47, 1186–1198. doi:10.1021/ar400264e |
| 29. | Li, B.-N.; Liu, Y.-Y.; Wang, Y.-P.; Pan, M. Molecules 2021, 26, 3244. doi:10.3390/molecules26113244 |
| 28. | Richards, G. J.; Hill, J. P.; Okamoto, K.; Shundo, A.; Akada, M.; Elsegood, M. R. J.; Mori, T.; Ariga, K. Langmuir 2009, 25, 8408–8413. doi:10.1021/la8041633 |
| 30. | He, Z.; Mao, R.; Liu, D.; Miao, Q. Org. Lett. 2012, 14, 4190–4193. doi:10.1021/ol301854z |
| 31. | Richards, G. J.; Hill, J. P.; Subbaiyan, N. K.; D’Souza, F.; Karr, P. A.; Elsegood, M. R. J.; Teat, S. J.; Mori, T.; Ariga, K. J. Org. Chem. 2009, 74, 8914–8923. doi:10.1021/jo901832n |
| 27. | Suzuki, T.; Nagae, Y.; Mitsuhashi, K. J. Heterocycl. Chem. 1986, 23, 1419–1421. doi:10.1002/jhet.5570230533 |
| 28. | Richards, G. J.; Hill, J. P.; Okamoto, K.; Shundo, A.; Akada, M.; Elsegood, M. R. J.; Mori, T.; Ariga, K. Langmuir 2009, 25, 8408–8413. doi:10.1021/la8041633 |
© 2024 Akhmedov et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.