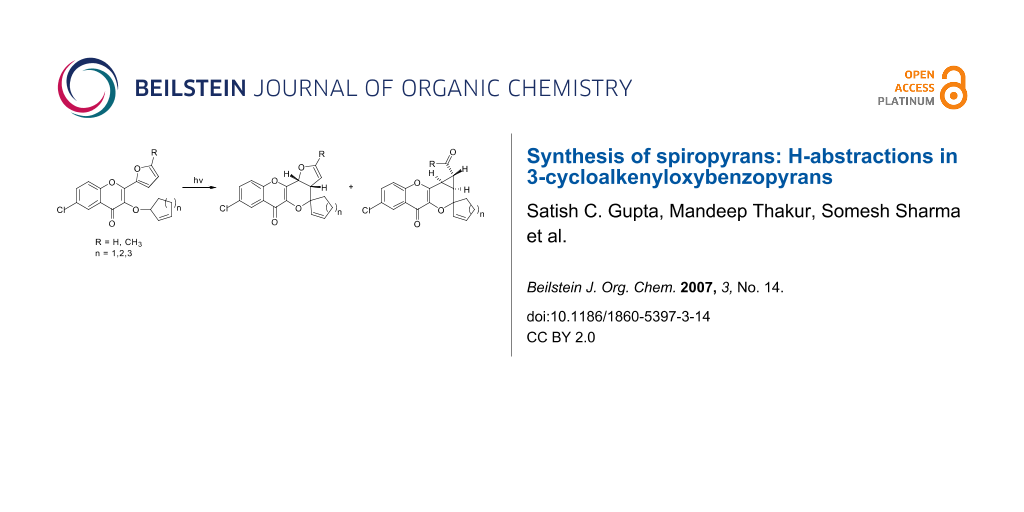
Abstract
A photochemical route for the synthesis of some benzopyronospiropyrans from 2-furyl-3-cycloalkenyloxybenzopyrones involving H-abstraction is reported. How a methyl group on the furyl ring affects the product formation is also investigated.
Graphical Abstract
1. Background
Spiropyrans exhibit photochromism due to the photoequilibrium with their open chain analogues merocyanins – a property that makes them a material of choice in digital storage technology. [1-5] Some spiropyrans have been found to have antiphlogistic, spasmolytic, antidiabetic and antifeedant activities amongst others. [6-10] The methods available for the synthesis of spiropyrans include enamine eliminations, reductive/thermal cyclisations and [4+2] cycloadditions. [11-16] These methods being specific in nature for a particular spirocyclic compound, offer limited synthetic utility. In the past, we have investigated the use of photochemical methods for the synthesis of organic molecules like vinyl ethers, [17] pyranopyrones [18,19] etc. and in continuation of that work we published a preliminary report on photochemical synthesis of spiropyrans. [20] To extend and examine the scope of this methodology, in this communication, we report the synthesis of spiropyrans bearing dihydrofuryls and acylcyclopropanes.
2. Results
The substrates 1 and 2 required for this study were obtained through the alkylation of 6-chloro-2-(2'-furyl)-3-hydroxy-4-oxo-4H-1-benzopyrans [21]3 (R = H) and 4 (R = CH3) with an appropriate bromocycloalkene (R' Br).
Scheme 1: Synthesis of 3-Cycloalkenyloxybenzopyrans.
Scheme 1: Synthesis of 3-Cycloalkenyloxybenzopyrans.
The irradiation of 1c in methanol with pyrex filtered light from a 125W Hg lamp produced two products 5c and 6c along with some 3.
Scheme 2: Photolysis of 6-chloro-3-(1"-cyclohept-2"-enyloxy)-2-(2'-furyl)-4-oxo-4H-1-benzopyran.
Scheme 2: Photolysis of 6-chloro-3-(1"-cyclohept-2"-enyloxy)-2-(2'-furyl)-4-oxo-4H-1-benzopyran.
3. Discussion
The structures of the photoproducts 5c and 6c were consistent with their spectral parameters [see Supporting Information File 1]; both the spiropyrans exhibited the presence of ions at m/z 155 and m/z 202 values in their mass spectra; these ions could very well be the result of rDA fragmentation of 5c and 6c. In the mass spectrum of 6c, the base peak was at m/z 327 (M+ -CHO).
The other chromones 1a, 1b and 1d on photoirradiation rearranged to spirocyclic pyranopyrones 5a, 5b, 5d and 6a, 6b, 6d. The photoirradiation of 2a, 2b and 2c yielded 7a, 7b, 7c only and no photoproducts similar to 5 (R = CH3) could be isolated although the 1H NMR of the crude photolysate of 2b did show the presence of 5b (R = CH3) in small proportions.
Scheme 3: Photolysis of 6-chloro-3-(1"-cycloalk-2"-enyloxy)-2-[2'-(5-methylfuryl)]-4-oxo-4H-1-benzopyrans.
Scheme 3: Photolysis of 6-chloro-3-(1"-cycloalk-2"-enyloxy)-2-[2'-(5-methylfuryl)]-4-oxo-4H-1-benzopyrans.
These photoconversions of the cycloalkenyloxy chromones (1a, 1b, 1c, 1d, 2a, 2b and 2c) to pyronospiropyrans can be visualized as having occurred through an initial abstraction of the O-methine proton of cycloalkenyl group by the excited carbonyl of the pyrone moiety to produce 1,4-biradical 8. The photoproduct 5 (a, b, c, d), is then formed through bond formation between the alkoxy radical and furan (8), followed by 1,5-H migration in 8a. [21]
Scheme 4: Mechanism of product formation from 3-Cycloalkenyloxybenzopyrans by photolysis.
Scheme 4: Mechanism of product formation from 3-Cycloalkenyloxybenzopyrans by photolysis.
The spiropyrans 6 (R = H) bearing cyclopropanes are the secondary photoproducts formed as a result of the further reorganization of 5 (R = H), through a ring contraction-ring expansion mechanism. [22,23] The formation of acetylcyclopropane compound 7 (R = CH3) from 2 (R = CH3) can be explained likewise. However, it is pertinent to mention here that when a large amount of 2b was photolysed and the photolysate was carefully chromatographed, a very small amount (<3%) of photoproduct 5 (R = CH3) could be isolated (vide experimental). The reason for such behaviour of 2 (R = CH3) could be that the presence of -CH3 group on dihydrofuryl moiety in the photoproduct 5 (R = CH3) makes the C-O bond easier to cleave. Thus as soon as 5 (R = CH3) is formed, it rearranges to 7 (R = CH3) or in other words the conversion of 5 (R = CH3) into 7 (R = CH3) is much faster than that of the formation of 5 (R = CH3) from 2 (R = CH3).
Regarding the stereochemical disposition of H-11b and H-3a in 5, both of them are cis placed (J = 9.6–9.9 Hz, Ø = 19.2°); in photoproducts bearing cyclopropanes [21]6 (R = H) or 7 (R = CH3), H-1 is trans to H-2 and H-3 (J1,2 = 4.8 Hz, J1,3 = 3.6 Hz) and both H-2 and H-3 are cis (J2,3 = 9.0 Hz).
4. Conclusion
In conclusion this photochemical method can be of utility for synthesizing a variety of pyronospiropyrans bearing both dihydrofuryls and acylcyclopropanes. The substitution on the furan ring makes the primary photoproducts more amenable to cleavage.
Supporting Information
| Supporting Information File 1: Experimental Data. The data provided represents the yield, melting point, IR, 1H NMR and elemental analysis of the compounds. | ||
| Format: DOC | Size: 50.0 KB | Download |
References
-
Newell, A. K.; Utley, J. H. P. J. Chem. Soc., Chem. Commun. 1992, 800–801. doi:10.1039/c39920000800
Return to citation in text: [1] -
Liu, Z. F.; Hashimoto, K.; Fujishima, A. Nature 1990, 347, 658–660. doi:10.1038/347658a0
Return to citation in text: [1] -
Saika, T.; Iyoda, T.; Honda, K.; Shimidzu, T. J. Chem. Soc., Perkin Trans. 2 1993, 1181–1186.
Return to citation in text: [1] -
Crano, J. C.; Guglielmetti, R. J., Eds. Organic Photochromic and Thermodynamic Compounds; Plenum: New York, 1998; Vol. 1.
Return to citation in text: [1] -
Crano, J. C.; Guglielmetti, R. J., Eds. Organic Photochromic and Thermodynamic Compounds; Plenum: New York, 1999; Vol. 2.
Return to citation in text: [1] -
Hegnauer, R. Chemotaxonomie der Pflanzen; Birkhäuser Verlag: Basel, 1964; Vol. 3, pp 447 ff.
Return to citation in text: [1] -
Zdero, C.; Bohlmann, F. Plant Syst. Evol. 1990, 171, 1–14. doi:10.1007/BF00940593
Return to citation in text: [1] -
Martínez, V.; Barberá, O.; Sánchez-Parareda, J.; Marco, J. A. Phytochemistry 1987, 26, 2619–2624. doi:10.1016/S0031-9422(00)83891-1
Return to citation in text: [1] -
Breinlich, J.; Scharnagel, K. Arzneim. Forsch. 1968, 18, 429–431.
Return to citation in text: [1] -
Eggler, J. F.; Larson, E. R. Heterocyclic compounds as aldose reductase inhibitors. US Patent 5,039,672, Aug 13, 1991.
Chem. Abstr. 1992, 116, 59214q.
Return to citation in text: [1] -
Chauhan, M. S.; Dean, F. M.; Matkin, D.; Robinson, M. L. J. Chem. Soc., Perkin Trans. 1 1973, 120–125.
Return to citation in text: [1] -
Cacioli, P.; Mackay, M. F.; Reiss, J. A. Tetrahedron Lett. 1980, 21, 4973–4976. doi:10.1016/S0040-4039(00)71169-5
Return to citation in text: [1] -
Chauhan, M. S.; McKinnon, D. M. Can. J. Chem. 1981, 59, 2223–2227. doi:10.1139/v81-321
Return to citation in text: [1] -
Clark-Lewis, J. W.; Jemison, R. W. Aust. J. Chem. 1968, 21, 2247–2254.
Return to citation in text: [1] -
Mora, P. T.; Szeki, T. J. Am. Chem. Soc. 1950, 22, 3009–3013. doi:10.1021/ja01163a059
Return to citation in text: [1] -
Kabbe, H. J. Synthesis 1979, 886–887.
Return to citation in text: [1] -
Gupta, S. C.; Yusuf, M.; Sharma, S.; Arora, S. Tetrahedron Lett. 2002, 43, 6875–6877. doi:10.1016/S0040-4039(02)01366-7
Return to citation in text: [1] -
Gupta, S. C.; Yadav, N. S.; Dhawan, S. N. Indian J. Chem., Sect. B 1991, 30, 790–792.
Return to citation in text: [1] -
Gupta, S. C.; Yusuf, M.; Sharma, S.; Saini, A.; Arora, S.; Kamboj, R. C. Tetrahedron 2004, 60, 8445–8454. doi:10.1016/j.tet.2004.07.003
Return to citation in text: [1] -
Gupta, S. C.; Saini, A.; Sharma, S.; Kapoor, M.; Dhawan, S. N. Tetrahedron Lett. 1996, 37, 8913–8916. doi:10.1016/S0040-4039(96)02046-1
Return to citation in text: [1] -
Gupta, S. C.; Saini, A.; Kumar, D.; Yadav, N. S.; Chand, K.; Mor, S.; Dhawan, S. N. J. Chem. Soc., Perkin Trans. 1 1995, 177–181. doi:10.1039/p19950000177
Return to citation in text: [1] [2] [3] -
van Tamelen, E. E.; Whitesides, T. H. J. Am. Chem. Soc. 1971, 93, 6129–6140. doi:10.1021/ja00752a025
Return to citation in text: [1] -
van Tamelen, E. E.; Whitesides, T. H. J. Am. Chem. Soc. 1968, 90, 3894–3896. doi:10.1021/ja01016a071
Return to citation in text: [1]
| 1. | Newell, A. K.; Utley, J. H. P. J. Chem. Soc., Chem. Commun. 1992, 800–801. doi:10.1039/c39920000800 |
| 2. | Liu, Z. F.; Hashimoto, K.; Fujishima, A. Nature 1990, 347, 658–660. doi:10.1038/347658a0 |
| 3. | Saika, T.; Iyoda, T.; Honda, K.; Shimidzu, T. J. Chem. Soc., Perkin Trans. 2 1993, 1181–1186. |
| 4. | Crano, J. C.; Guglielmetti, R. J., Eds. Organic Photochromic and Thermodynamic Compounds; Plenum: New York, 1998; Vol. 1. |
| 5. | Crano, J. C.; Guglielmetti, R. J., Eds. Organic Photochromic and Thermodynamic Compounds; Plenum: New York, 1999; Vol. 2. |
| 18. | Gupta, S. C.; Yadav, N. S.; Dhawan, S. N. Indian J. Chem., Sect. B 1991, 30, 790–792. |
| 19. | Gupta, S. C.; Yusuf, M.; Sharma, S.; Saini, A.; Arora, S.; Kamboj, R. C. Tetrahedron 2004, 60, 8445–8454. doi:10.1016/j.tet.2004.07.003 |
| 17. | Gupta, S. C.; Yusuf, M.; Sharma, S.; Arora, S. Tetrahedron Lett. 2002, 43, 6875–6877. doi:10.1016/S0040-4039(02)01366-7 |
| 11. | Chauhan, M. S.; Dean, F. M.; Matkin, D.; Robinson, M. L. J. Chem. Soc., Perkin Trans. 1 1973, 120–125. |
| 12. | Cacioli, P.; Mackay, M. F.; Reiss, J. A. Tetrahedron Lett. 1980, 21, 4973–4976. doi:10.1016/S0040-4039(00)71169-5 |
| 13. | Chauhan, M. S.; McKinnon, D. M. Can. J. Chem. 1981, 59, 2223–2227. doi:10.1139/v81-321 |
| 14. | Clark-Lewis, J. W.; Jemison, R. W. Aust. J. Chem. 1968, 21, 2247–2254. |
| 15. | Mora, P. T.; Szeki, T. J. Am. Chem. Soc. 1950, 22, 3009–3013. doi:10.1021/ja01163a059 |
| 16. | Kabbe, H. J. Synthesis 1979, 886–887. |
| 6. | Hegnauer, R. Chemotaxonomie der Pflanzen; Birkhäuser Verlag: Basel, 1964; Vol. 3, pp 447 ff. |
| 7. | Zdero, C.; Bohlmann, F. Plant Syst. Evol. 1990, 171, 1–14. doi:10.1007/BF00940593 |
| 8. | Martínez, V.; Barberá, O.; Sánchez-Parareda, J.; Marco, J. A. Phytochemistry 1987, 26, 2619–2624. doi:10.1016/S0031-9422(00)83891-1 |
| 9. | Breinlich, J.; Scharnagel, K. Arzneim. Forsch. 1968, 18, 429–431. |
| 10. |
Eggler, J. F.; Larson, E. R. Heterocyclic compounds as aldose reductase inhibitors. US Patent 5,039,672, Aug 13, 1991.
Chem. Abstr. 1992, 116, 59214q. |
| 22. | van Tamelen, E. E.; Whitesides, T. H. J. Am. Chem. Soc. 1971, 93, 6129–6140. doi:10.1021/ja00752a025 |
| 23. | van Tamelen, E. E.; Whitesides, T. H. J. Am. Chem. Soc. 1968, 90, 3894–3896. doi:10.1021/ja01016a071 |
| 21. | Gupta, S. C.; Saini, A.; Kumar, D.; Yadav, N. S.; Chand, K.; Mor, S.; Dhawan, S. N. J. Chem. Soc., Perkin Trans. 1 1995, 177–181. doi:10.1039/p19950000177 |
| 21. | Gupta, S. C.; Saini, A.; Kumar, D.; Yadav, N. S.; Chand, K.; Mor, S.; Dhawan, S. N. J. Chem. Soc., Perkin Trans. 1 1995, 177–181. doi:10.1039/p19950000177 |
| 20. | Gupta, S. C.; Saini, A.; Sharma, S.; Kapoor, M.; Dhawan, S. N. Tetrahedron Lett. 1996, 37, 8913–8916. doi:10.1016/S0040-4039(96)02046-1 |
| 21. | Gupta, S. C.; Saini, A.; Kumar, D.; Yadav, N. S.; Chand, K.; Mor, S.; Dhawan, S. N. J. Chem. Soc., Perkin Trans. 1 1995, 177–181. doi:10.1039/p19950000177 |
© 2007 Gupta et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)