Abstract
m-Iodosylbenzoic acid performs iodinations of arenes in the presence of iodine at room temperature in acetonitrile. Separation of pure products is conveniently achieved by scavenging any aryl iodide by ion exchange with IRA-900 (hydroxide form). The reduced form of the reagent, m-iodobenzoic acid, can be easily recovered from the ion exchange resin or from the basic aqueous solution by simple acidification with HCl.
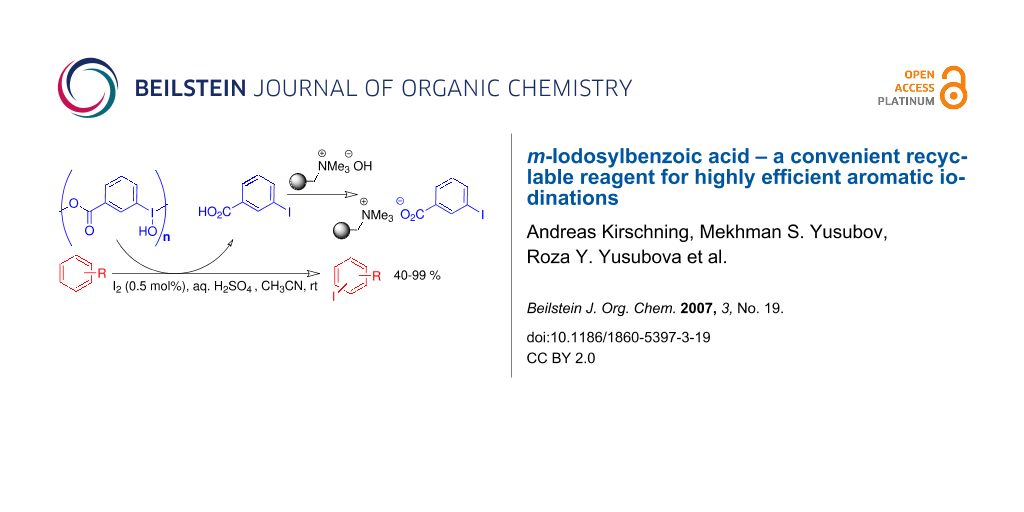
Graphical Abstract
Background
In recent years, iodoarenes have gained increasing importance because they are widely used as building blocks in organic synthesis. They are particularly important as indispensable substrates for numerous methods of N-N bond formation, [1,2] for the chemistry of heterocyclic [3] and organometallic compounds, [4-8] and for the synthesis of polyvalent iodine organic compounds. [9,10] In addition, polyvalent organoiodine compounds have served as cooxidants in the iodination of arenes. [11-36] Typical polyvalent iodine sources for these iodination reactions are reagents 1–4 (Figure 1). Iodosylbenzene 5 is not suitable for iodinations because of its low activity. [37]
Figure 1: Hypervalent iodine reagents 1 – 6.
Figure 1: Hypervalent iodine reagents 1 – 6.
In this report we describe a practical improvement for these iodinations as far as purification of the products and recycling of the iodine reagent is concerned. The broad use of hypervalent iodine reagents is still hampered by tedious purification and recycling protocols. Commonly, purification relies on chromatography. Recently, tagging strategies for reagents and catalysts have widely been investigated that allow easy purification by means of specific phase separation or scavenging. [39-41]
Results and discussion
In this context, we recently described an improved procedure for the preparation of the hardly known m-iodosylbenzoic acid 6 and showed that it is a recyclable reagent for the highly efficient RuCl3-catalyzed oxidation of alcohols to aldehydes and ketones. [42] In the present work we demonstrate the utility of m-iodosylbenzoic acid 6 as a recyclable reagent for the iodination of arenes. In fact reagent 6 can be regarded as a tagged version of iodoso benzene 5 which, if used in access, can be conveniently removed at the end of the reaction by filtration after addition of IRA 900 (hydroxide form) (Scheme 1). This scavenging concept can also be applied to reduction products such as m-iodobenzoic acid 9. Importantly, 9 which also serves as the starting material for the preparation of 6 can easily be regenerated (> 95%) from polymer 10 in pure form by treatment with aqueous HCl.
Scheme 1: Iodine(III)-promoted iodination of arenes and concept of purification.
Scheme 1: Iodine(III)-promoted iodination of arenes and concept of purification.
We found that the reaction of aromatic substrates 7a-o with I2 and 6 in CH3CN (commonly in the presence of 50% aqueous H2SO4) led to the corresponding iodinated arenes in 40 – 99% yield under mild conditions (Scheme 1 and Table 1 and Table 2). Addition of aqueous H2SO4 accelerated the iodination of benzenes. For heteroarenes 7j and 7o this additive was not required and if an additional alcohol group was present (see 7n), addition of aqueous H2SO4 resulted in its oxidation. Compared to diacetoxyiodobenzene (DIB) 1a and its polymeric analog 1b, the use of m-iodosylbenzoic acid 6 for mono- and diiodination requires the use of smaller amounts of iodine as well as of the polyvalent iodine reagent. [15] For example, the preparation of 2,4-diiodoanisole 8k from anisole 7k in the presence of 1b was achieved using 4.8 equiv. of both iodine and 1b while our iodination protocol required only 2.4 equiv. of iodine and 1.2 equiv. of m-iodosylbenzoic acid 6.
Table 1: Monoiodination of arenes with m-iodosylbenzoic acid 6 (see Supporting Information File 1 for full experimental data).
| Arene | Iodoarene | Conditions | Yield (%)a | mp or bp °C (lit. mp) |
|---|---|---|---|---|
|
|
|
5 h, 60°C | 91 | 250–254 (249 – 254; [43]) |
|
|
|
24 h, rt | 76b | Determined by GC-analysis |
|
|
|
|||
| 7c R = Br | 8c R = Br | 0.5 h, rt | 92 | 8c 62–64 (oil; [10]) |
| 7d R = -C(O)Ph | 8d R = -C(O)Ph | 0.2 h, rtc | 90 | 8d; ref. 44) 70–72 (71–72[44]) |
| 7e R = -C(O)CH3 | 8e R = -C(O)CH3 | 0.1 h, rtc | 90 | 8e 101–103 (103.6; [45]) |
| 7f R = -CH2C(O)CH3 | 8f R = -CH2C(O)CH3 | 0.1 h, rtc | 79 | 8f oil (oil; [46]) |
| 7g R = -CHO | 8g R = -CHO | 2.0, rtc | 85 | 8g 103–105 (105–10; [47]) |
|
|
|
16 h, rt | 40 | 95–96 (96; [48]) |
|
|
|
3.0 h, rt | 60 | 8i : 8i' = 1.0 : 0.8 |
|
|
|
1.0 h, rtd | 97e | 134–135 (134–136; [49]) |
a Molar ratio ArH/6/iodine 0.2/0.24/0.12 (in mmol) and 0.05 mL aq. (50%) H2SO4; isolated yields. b Determined by GC-analysis. c Instead of 0.05 mL only 0.02 mL aq. (50%) H2SO4 was added. d No aq. (50%) H2SO4 was added. eNaHCO3 was used instead of IRA 900 (hydroxide form).
Table 2: Diiodination of Arenes with m-Iodosylbenzoic acid 6 (see Supporting Information File 1 for full experimental data).
a Isolated yields. b Molar ratio ArH/6/iodine 0.2/0.48/0.24 (in mmol) and 0.05 mL aq. (50%) H2SO4. c No aq. (50%) H2SO4 was added. d NaHCO3 was used instead of IRA 900 (hydroxide form).
Likewise, for the preparation of aryliodide 8c a 2.4 molar excess of both iodine and reagent 1a had to be employed while in our case 1.2 equiv. of iodine and 1.2 equiv. of reagent 6 were required for full conversion.
As is evident from the tables, iodination of arenes that are acylated like 7d,e,g and 7i commonly led to excellent yields of selectively iodinated arenes 8d,e,g and 8i. Increasing the nucleophilicity of the aromatic ring such as in 3,5-dimethoxybenzyl alcohol 7n led to diiodinated benzyl alcohol 8n in good yield. Oxidation of the alcohol group was not observed.
Based on related iodine(III)-mediated iodinations of arenas [12-37] we suggest that the hydrated form of 6 oxidizes iodine to HOI which serves as the reactive electrophilic intermediate (Scheme 2).
From the results collected it can be concluded that m-iodosylbenzoic acid 6 shows a similar reactivity as 1-(arenesulfonyloxy)benziodoxolones 4a-f [36,37]. However, reagent 6 is cheaper and exerts better selectivity in the iodination reactions.
Conclusion
In conclusion, we disclose that the rarely employed m-iodosylbenzoic acid is an ideal tagged iodine(III) reagent which in our view allows the easiest purification protocol for aryliodine reagents known so far. This tagging concept was utilized in the mild iodination of arenes but could potentially be applied to most other iodine(III)-mediated reactions.
Supporting Information
| Supporting Information File 1: Experimental details. The data provide general experimental details as well as an improved procedure for the preparation of m-iodosylbenzoic acid (6), a typical iodination procedure and spectroscopic and analytic data for 8n. | ||
| Format: DOC | Size: 30.0 KB | Download |
Acknowledgements
M. S. Y. thanks the Russian Ministry of Education and the Deutsche Akademische Austauschdienst (DAAD) for a scholarship and RFBR (Grant 07-03-12141-ofi). Additionally, the work was funded by the Fonds der Chemischen Industrie. Ki-Whan Chi thanks the University of Ulsan Research Fund 2006. We are grateful to Prof. Viktor Filimonov from Tomsk Polytechnic University for helpful discussions.
References
-
Hiyama, T. Organosilicon Compounds in Cross-Coupling Reactions. In Metal-catalyzed cross-coupling reactions; Diederich, F.; Stang, P. J., Eds.; Wiley-VCH: Weinheim, 1998; pp 421–453.
Return to citation in text: [1] -
Comprehensive Organic Synthesis. Trost, B. M., Ed.; Pergamon: Oxford, UK, 1991; Vol. 3, pp 521–549.
Return to citation in text: [1] -
Cacchi, S.; Fabrizi, G. Chem. Rev. 2005, 105, 2873–2920. doi:10.1021/cr040639b
Return to citation in text: [1] -
Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem. Rev. 2002, 102, 1359–1470. doi:10.1021/cr000664r
Return to citation in text: [1] -
Beletskaya, I. P.; Cheprakov, A. V. Chem. Rev. 2000, 100, 3009–3066. doi:10.1021/cr9903048
Return to citation in text: [1] -
Kotha, S.; Lahiri, K.; Kashinath, D. Tetrahedron 2002, 58, 9633–9695. doi:10.1016/S0040-4020(02)01188-2
Return to citation in text: [1] -
Bellina, F.; Carpita, A.; Rossi, R. Synthesis 2004, 2419–2440. doi:10.1055/s-2004-831223
Return to citation in text: [1] -
Knochel, P.; Dohle, W.; Gommermann, N.; Kneisel, F. F.; Kopp, F.; Korn, T.; Sapountzis, I.; Vu, V. A. Angew. Chem. 2003, 115, 4438–4456. doi:10.1002/ange.200300579
Angew. Chem., Int. Ed. 2003, 42, 4302–4320. doi:10.1002/anie.200300579
Return to citation in text: [1] -
Moriarty, R. M. J. Org. Chem. 2005, 70, 2893–2903. doi:10.1021/jo050117b
Return to citation in text: [1] -
Wirth, T. Angew. Chem. 2005, 117, 3722–3731. doi:10.1002/ange.200500115
Angew. Chem., Int. Ed. 2005, 44, 3656–3665. doi:10.1002/anie.200500115
Return to citation in text: [1] [2] [3] [4] -
Ogata, Y.; Aoki, K. J. Am. Chem. Soc. 1968, 90, 6187–6191. doi:10.1021/ja01024a043
Return to citation in text: [1] -
Kryska, A.; Skulski, L. J. Chem. Res., Synop. 1999, 590–591. doi:10.1039/a904053f
Return to citation in text: [1] [2] -
Giri, R.; Chen, X.; Yu, J.-Q. Angew. Chem. 2005, 117, 2150–2153. doi:10.1002/ange.200462884
Angew. Chem., Int. Ed. 2005, 44, 2112–2115. doi:10.1002/anie.200462884
Return to citation in text: [1] [2] -
Krasnokutskaya, E. A.; Trusova, M. E.; Filimonov, V. D. Zh. Org. Khim. 2005, 41, 1788–1789.
Russ. J. Org. Chem. 2005, 41, 1750–1751.
Return to citation in text: [1] [2] -
Togo, H.; Nogami, G.; Yokoyama, M. Synlett 1998, 534–536. doi:10.1055/s-1998-1713
Return to citation in text: [1] [2] [3] -
Togo, H.; Nabana, T.; Yamaguchi, K. J. Org. Chem. 2000, 65, 8391–8394. doi:10.1021/jo001186n
Return to citation in text: [1] [2] -
Boyer, J. H.; Natesh, A. Synthesis 1988, 980–981. doi:10.1055/s-1988-27774
Return to citation in text: [1] [2] -
Yudina, N. D.; Raida, V. S.; Vasil'eva, O. L.; Deniskin, V. V.; Stepanets, M. P.; Sitnikov, A. S. Polym. Sci. USSR 1989, 31, 1318–1321. doi:10.1016/0032-3950(89)90159-7
Return to citation in text: [1] [2] -
Vasil'eva, O. L.; Raida, V. S.; Sirotkina, E. E. Vysokomol. Soedin., Ser. A 1992, 34, 131–134.
Return to citation in text: [1] [2] -
D'Auria, M.; Mauriello, G. Tetrahedron Lett. 1995, 36, 4883–4884.
Return to citation in text: [1] [2] -
Boyle, R. W.; Johnson, C. K.; Dolphin, D. J. Chem. Soc., Chem. Commun. 1995, 527–528.
Return to citation in text: [1] [2] -
D'Auria, M.; de Luca, E.; Mauriello, G.; Racioppi, R.; Sleiter, G. J. Chem. Soc., Perkin Trans. 1 1997, 2369–2374. doi:10.1039/a701674c
Return to citation in text: [1] [2] -
Lambert, C.; Nöll, G. Angew. Chem. 1998, 110, 2239–2242. doi:10.1002/(SICI)1521-3757(19980803)110:15<2239::AID-ANGE2239>3.0.CO;2-H
Angew. Chem., Int. Ed. 1998, 37, 2107–2110. doi:10.1002/(SICI)1521-3773(19980817)37:15<2107::AID-ANIE2107>3.0.CO;2-H
Return to citation in text: [1] [2] -
Benhida, R.; Blanchard, P.; Fourrey, J.-L. Tetrahedron Lett. 1998, 39, 6849–6852. doi:10.1016/S0040-4039(98)01494-4
Return to citation in text: [1] [2] -
Shanmugathasan, S.; Johnson, C. K.; Edwards, C.; Matthews, E. K.; Dolphin, D.; Boyle, R. W. J. Porphyrins Phthalocyanines 2000, 4, 228–232. doi:10.1002/(SICI)1099-1409(200004/05)4:3<228::AID-JPP199>3.0.CO;2-7
Return to citation in text: [1] [2] -
Miyaji, H.; Sato, W.; Sessler, J. L.; Lynch, V. M. Tetrahedron Lett. 2000, 41, 1369–1373. doi:10.1016/S0040-4039(99)02295-9
Return to citation in text: [1] [2] -
Anzenbacher, P.; Jursikova, K.; Shriver, J. A.; Miyaji, H.; Lynch, V. M.; Sessler, J. L.; Gale, P. A. J. Org. Chem. 2000, 65, 7641–7645. doi:10.1021/jo005610w
Return to citation in text: [1] [2] -
Yu, L.; Lindsey, J. S. Tetrahedron 2001, 57, 9285–9298. doi:10.1016/S0040-4020(01)00928-0
Return to citation in text: [1] [2] -
Füchtner, F.; Angelberger, P.; Kvaternik, H.; Hammerschmidt, F.; Simovc, P.; Steinbach, J. Nucl. Med. Biol. 2002, 29, 477–481. doi:10.1016/S0969-8051(02)00298-6
Return to citation in text: [1] [2] -
Tomizaki, K.-y.; Lysenko, A. B.; Taniguchi, M.; Lindsey, J. S. Tetrahedron 2004, 60, 2011–2023. doi:10.1016/j.tet.2004.01.003
Return to citation in text: [1] [2] -
Taniguchi, M.; Kim, M. N.; Ra, D.; Lindsey, J. S. J. Org. Chem. 2005, 70, 275–285. doi:10.1021/jo048440m
Return to citation in text: [1] [2] -
Jin, L.-M.; Chen, L.; Yin, J.-J.; Zhou, J.-M.; Giu, C.-C.; Chen, Q.-Y. J. Org. Chem. 2006, 71, 527–536. doi:10.1021/jo051672g
Return to citation in text: [1] [2] -
Hashimoto, M.; Kato, Y.; Hatanaka, Y. Tetrahedron Lett. 2006, 47, 3391–3394. doi:10.1016/j.tetlet.2006.03.068
Return to citation in text: [1] [2] -
Bovonsombat, P.; Angara, G. J.; McNelis, E. Synlett 1992, 131–132. doi:10.1055/s-1992-21290
Return to citation in text: [1] [2] -
Bovonsombat, P.; Djuardi, E.; McNelis, E. Tetrahedron Lett. 1994, 35, 2841–2844. doi:10.1016/S0040-4039(00)76639-1
Return to citation in text: [1] [2] -
Muraki, T.; Togo, H.; Yokoyama, M. Synlett 1998, 286–288. doi:10.1055/s-1998-1639
Return to citation in text: [1] [2] [3] -
Muraki, T.; Togo, H.; Yokoyama, M. J. Org. Chem. 1999, 64, 2883–2889. doi:10.1021/jo9825207
Return to citation in text: [1] [2] [3] -
Sohmiya, H.; Kimura, T.; Fujita, M.; Ando, T. Tetrahedron 1998, 54, 13737–13750. doi:10.1016/S0040-4020(98)00844-8
-
Barrett, A. G. M.; Hopkins, B. T.; Köbberling, J. Chem. Rev. 2002, 102, 3301–3324. doi:10.1021/cr0103423
Return to citation in text: [1] -
Yoshida, J.-i.; Itami, K. Chem. Rev. 2002, 102, 3693–3716. doi:10.1021/cr0103524
Return to citation in text: [1] -
Bhattacharyya, S. Curr. Opin. Drug Discovery Dev. 2004, 7, 752–764.
Return to citation in text: [1] -
Yusubov, M. S.; Gilmkhanova, M. P.; Zhdankin, V. V.; Kirschning, A. Synlett 2007, 563–566. doi:10.1055/s-2007-970742
Return to citation in text: [1] -
Fields, E. K.; Meyerson, S. J. Org. Chem. 1978, 43, 4705–4708. doi:10.1021/jo00419a005
Return to citation in text: [1] -
Bachki, A.; Foubelo, F.; Yus, M. Tetrahedron 1994, 50, 5139–5146. doi:10.1016/S0040-4020(01)90424-7
Return to citation in text: [1] -
Bogert, M. T.; Curtin, L. P. J. Am. Chem. Soc. 1923, 45, 2161–2167. doi:10.1021/ja01662a020
Return to citation in text: [1] -
Pavlinac, J.; Zupan, M.; Stavber, S. J. Org. Chem. 2006, 71, 1027–1032. doi:10.1021/jo052021n
Return to citation in text: [1] -
Hart, D. J.; Hong, W.-P. J. Org. Chem. 1985, 50, 3670–3672. doi:10.1021/jo00219a061
Return to citation in text: [1] -
Butler, A. R.; Sanderson, A. P. J. Chem. Soc., Perkin Trans. 2 1972, 989–992. doi:10.1039/p29720000989
Return to citation in text: [1] -
Cheng, D.-P.; Chen, Z.-C.; Zheng, Q.-G. Synth. Commun. 2003, 33, 2671–2676. doi:10.1081/SCC-120021987
Return to citation in text: [1] -
Orito, K.; Hatakeyama, T.; Takeo, M.; Suginome, H. Synthesis 1995, 1273–1277. doi:10.1055/s-1995-4089
Return to citation in text: [1] -
Dickens, J. P.; Dyer, R. L.; Hamill, B. J.; Harrow, T. A.; Bible, R. H., Jr.; Finnegan, P. M.; Henrick, K.; Owston, P. G. J. Org. Chem. 1981, 46, 1781–1786. doi:10.1021/jo00322a005
Return to citation in text: [1]
| 50. | Orito, K.; Hatakeyama, T.; Takeo, M.; Suginome, H. Synthesis 1995, 1273–1277. doi:10.1055/s-1995-4089 |
| 49. | Cheng, D.-P.; Chen, Z.-C.; Zheng, Q.-G. Synth. Commun. 2003, 33, 2671–2676. doi:10.1081/SCC-120021987 |
| 10. |
Wirth, T. Angew. Chem. 2005, 117, 3722–3731. doi:10.1002/ange.200500115
Angew. Chem., Int. Ed. 2005, 44, 3656–3665. doi:10.1002/anie.200500115 |
| 1. | Hiyama, T. Organosilicon Compounds in Cross-Coupling Reactions. In Metal-catalyzed cross-coupling reactions; Diederich, F.; Stang, P. J., Eds.; Wiley-VCH: Weinheim, 1998; pp 421–453. |
| 2. | Comprehensive Organic Synthesis. Trost, B. M., Ed.; Pergamon: Oxford, UK, 1991; Vol. 3, pp 521–549. |
| 11. | Ogata, Y.; Aoki, K. J. Am. Chem. Soc. 1968, 90, 6187–6191. doi:10.1021/ja01024a043 |
| 12. | Kryska, A.; Skulski, L. J. Chem. Res., Synop. 1999, 590–591. doi:10.1039/a904053f |
| 13. |
Giri, R.; Chen, X.; Yu, J.-Q. Angew. Chem. 2005, 117, 2150–2153. doi:10.1002/ange.200462884
Angew. Chem., Int. Ed. 2005, 44, 2112–2115. doi:10.1002/anie.200462884 |
| 14. |
Krasnokutskaya, E. A.; Trusova, M. E.; Filimonov, V. D. Zh. Org. Khim. 2005, 41, 1788–1789.
Russ. J. Org. Chem. 2005, 41, 1750–1751. |
| 15. | Togo, H.; Nogami, G.; Yokoyama, M. Synlett 1998, 534–536. doi:10.1055/s-1998-1713 |
| 16. | Togo, H.; Nabana, T.; Yamaguchi, K. J. Org. Chem. 2000, 65, 8391–8394. doi:10.1021/jo001186n |
| 17. | Boyer, J. H.; Natesh, A. Synthesis 1988, 980–981. doi:10.1055/s-1988-27774 |
| 18. | Yudina, N. D.; Raida, V. S.; Vasil'eva, O. L.; Deniskin, V. V.; Stepanets, M. P.; Sitnikov, A. S. Polym. Sci. USSR 1989, 31, 1318–1321. doi:10.1016/0032-3950(89)90159-7 |
| 19. | Vasil'eva, O. L.; Raida, V. S.; Sirotkina, E. E. Vysokomol. Soedin., Ser. A 1992, 34, 131–134. |
| 20. | D'Auria, M.; Mauriello, G. Tetrahedron Lett. 1995, 36, 4883–4884. |
| 21. | Boyle, R. W.; Johnson, C. K.; Dolphin, D. J. Chem. Soc., Chem. Commun. 1995, 527–528. |
| 22. | D'Auria, M.; de Luca, E.; Mauriello, G.; Racioppi, R.; Sleiter, G. J. Chem. Soc., Perkin Trans. 1 1997, 2369–2374. doi:10.1039/a701674c |
| 23. |
Lambert, C.; Nöll, G. Angew. Chem. 1998, 110, 2239–2242. doi:10.1002/(SICI)1521-3757(19980803)110:15<2239::AID-ANGE2239>3.0.CO;2-H
Angew. Chem., Int. Ed. 1998, 37, 2107–2110. doi:10.1002/(SICI)1521-3773(19980817)37:15<2107::AID-ANIE2107>3.0.CO;2-H |
| 24. | Benhida, R.; Blanchard, P.; Fourrey, J.-L. Tetrahedron Lett. 1998, 39, 6849–6852. doi:10.1016/S0040-4039(98)01494-4 |
| 25. | Shanmugathasan, S.; Johnson, C. K.; Edwards, C.; Matthews, E. K.; Dolphin, D.; Boyle, R. W. J. Porphyrins Phthalocyanines 2000, 4, 228–232. doi:10.1002/(SICI)1099-1409(200004/05)4:3<228::AID-JPP199>3.0.CO;2-7 |
| 26. | Miyaji, H.; Sato, W.; Sessler, J. L.; Lynch, V. M. Tetrahedron Lett. 2000, 41, 1369–1373. doi:10.1016/S0040-4039(99)02295-9 |
| 27. | Anzenbacher, P.; Jursikova, K.; Shriver, J. A.; Miyaji, H.; Lynch, V. M.; Sessler, J. L.; Gale, P. A. J. Org. Chem. 2000, 65, 7641–7645. doi:10.1021/jo005610w |
| 28. | Yu, L.; Lindsey, J. S. Tetrahedron 2001, 57, 9285–9298. doi:10.1016/S0040-4020(01)00928-0 |
| 29. | Füchtner, F.; Angelberger, P.; Kvaternik, H.; Hammerschmidt, F.; Simovc, P.; Steinbach, J. Nucl. Med. Biol. 2002, 29, 477–481. doi:10.1016/S0969-8051(02)00298-6 |
| 30. | Tomizaki, K.-y.; Lysenko, A. B.; Taniguchi, M.; Lindsey, J. S. Tetrahedron 2004, 60, 2011–2023. doi:10.1016/j.tet.2004.01.003 |
| 31. | Taniguchi, M.; Kim, M. N.; Ra, D.; Lindsey, J. S. J. Org. Chem. 2005, 70, 275–285. doi:10.1021/jo048440m |
| 32. | Jin, L.-M.; Chen, L.; Yin, J.-J.; Zhou, J.-M.; Giu, C.-C.; Chen, Q.-Y. J. Org. Chem. 2006, 71, 527–536. doi:10.1021/jo051672g |
| 33. | Hashimoto, M.; Kato, Y.; Hatanaka, Y. Tetrahedron Lett. 2006, 47, 3391–3394. doi:10.1016/j.tetlet.2006.03.068 |
| 34. | Bovonsombat, P.; Angara, G. J.; McNelis, E. Synlett 1992, 131–132. doi:10.1055/s-1992-21290 |
| 35. | Bovonsombat, P.; Djuardi, E.; McNelis, E. Tetrahedron Lett. 1994, 35, 2841–2844. doi:10.1016/S0040-4039(00)76639-1 |
| 36. | Muraki, T.; Togo, H.; Yokoyama, M. Synlett 1998, 286–288. doi:10.1055/s-1998-1639 |
| 47. | Hart, D. J.; Hong, W.-P. J. Org. Chem. 1985, 50, 3670–3672. doi:10.1021/jo00219a061 |
| 9. | Moriarty, R. M. J. Org. Chem. 2005, 70, 2893–2903. doi:10.1021/jo050117b |
| 10. |
Wirth, T. Angew. Chem. 2005, 117, 3722–3731. doi:10.1002/ange.200500115
Angew. Chem., Int. Ed. 2005, 44, 3656–3665. doi:10.1002/anie.200500115 |
| 48. | Butler, A. R.; Sanderson, A. P. J. Chem. Soc., Perkin Trans. 2 1972, 989–992. doi:10.1039/p29720000989 |
| 4. | Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Chem. Rev. 2002, 102, 1359–1470. doi:10.1021/cr000664r |
| 5. | Beletskaya, I. P.; Cheprakov, A. V. Chem. Rev. 2000, 100, 3009–3066. doi:10.1021/cr9903048 |
| 6. | Kotha, S.; Lahiri, K.; Kashinath, D. Tetrahedron 2002, 58, 9633–9695. doi:10.1016/S0040-4020(02)01188-2 |
| 7. | Bellina, F.; Carpita, A.; Rossi, R. Synthesis 2004, 2419–2440. doi:10.1055/s-2004-831223 |
| 8. |
Knochel, P.; Dohle, W.; Gommermann, N.; Kneisel, F. F.; Kopp, F.; Korn, T.; Sapountzis, I.; Vu, V. A. Angew. Chem. 2003, 115, 4438–4456. doi:10.1002/ange.200300579
Angew. Chem., Int. Ed. 2003, 42, 4302–4320. doi:10.1002/anie.200300579 |
| 45. | Bogert, M. T.; Curtin, L. P. J. Am. Chem. Soc. 1923, 45, 2161–2167. doi:10.1021/ja01662a020 |
| 46. | Pavlinac, J.; Zupan, M.; Stavber, S. J. Org. Chem. 2006, 71, 1027–1032. doi:10.1021/jo052021n |
| 15. | Togo, H.; Nogami, G.; Yokoyama, M. Synlett 1998, 534–536. doi:10.1055/s-1998-1713 |
| 10. |
Wirth, T. Angew. Chem. 2005, 117, 3722–3731. doi:10.1002/ange.200500115
Angew. Chem., Int. Ed. 2005, 44, 3656–3665. doi:10.1002/anie.200500115 |
| 12. | Kryska, A.; Skulski, L. J. Chem. Res., Synop. 1999, 590–591. doi:10.1039/a904053f |
| 13. |
Giri, R.; Chen, X.; Yu, J.-Q. Angew. Chem. 2005, 117, 2150–2153. doi:10.1002/ange.200462884
Angew. Chem., Int. Ed. 2005, 44, 2112–2115. doi:10.1002/anie.200462884 |
| 14. |
Krasnokutskaya, E. A.; Trusova, M. E.; Filimonov, V. D. Zh. Org. Khim. 2005, 41, 1788–1789.
Russ. J. Org. Chem. 2005, 41, 1750–1751. |
| 15. | Togo, H.; Nogami, G.; Yokoyama, M. Synlett 1998, 534–536. doi:10.1055/s-1998-1713 |
| 16. | Togo, H.; Nabana, T.; Yamaguchi, K. J. Org. Chem. 2000, 65, 8391–8394. doi:10.1021/jo001186n |
| 17. | Boyer, J. H.; Natesh, A. Synthesis 1988, 980–981. doi:10.1055/s-1988-27774 |
| 18. | Yudina, N. D.; Raida, V. S.; Vasil'eva, O. L.; Deniskin, V. V.; Stepanets, M. P.; Sitnikov, A. S. Polym. Sci. USSR 1989, 31, 1318–1321. doi:10.1016/0032-3950(89)90159-7 |
| 19. | Vasil'eva, O. L.; Raida, V. S.; Sirotkina, E. E. Vysokomol. Soedin., Ser. A 1992, 34, 131–134. |
| 20. | D'Auria, M.; Mauriello, G. Tetrahedron Lett. 1995, 36, 4883–4884. |
| 21. | Boyle, R. W.; Johnson, C. K.; Dolphin, D. J. Chem. Soc., Chem. Commun. 1995, 527–528. |
| 22. | D'Auria, M.; de Luca, E.; Mauriello, G.; Racioppi, R.; Sleiter, G. J. Chem. Soc., Perkin Trans. 1 1997, 2369–2374. doi:10.1039/a701674c |
| 23. |
Lambert, C.; Nöll, G. Angew. Chem. 1998, 110, 2239–2242. doi:10.1002/(SICI)1521-3757(19980803)110:15<2239::AID-ANGE2239>3.0.CO;2-H
Angew. Chem., Int. Ed. 1998, 37, 2107–2110. doi:10.1002/(SICI)1521-3773(19980817)37:15<2107::AID-ANIE2107>3.0.CO;2-H |
| 24. | Benhida, R.; Blanchard, P.; Fourrey, J.-L. Tetrahedron Lett. 1998, 39, 6849–6852. doi:10.1016/S0040-4039(98)01494-4 |
| 25. | Shanmugathasan, S.; Johnson, C. K.; Edwards, C.; Matthews, E. K.; Dolphin, D.; Boyle, R. W. J. Porphyrins Phthalocyanines 2000, 4, 228–232. doi:10.1002/(SICI)1099-1409(200004/05)4:3<228::AID-JPP199>3.0.CO;2-7 |
| 26. | Miyaji, H.; Sato, W.; Sessler, J. L.; Lynch, V. M. Tetrahedron Lett. 2000, 41, 1369–1373. doi:10.1016/S0040-4039(99)02295-9 |
| 27. | Anzenbacher, P.; Jursikova, K.; Shriver, J. A.; Miyaji, H.; Lynch, V. M.; Sessler, J. L.; Gale, P. A. J. Org. Chem. 2000, 65, 7641–7645. doi:10.1021/jo005610w |
| 28. | Yu, L.; Lindsey, J. S. Tetrahedron 2001, 57, 9285–9298. doi:10.1016/S0040-4020(01)00928-0 |
| 29. | Füchtner, F.; Angelberger, P.; Kvaternik, H.; Hammerschmidt, F.; Simovc, P.; Steinbach, J. Nucl. Med. Biol. 2002, 29, 477–481. doi:10.1016/S0969-8051(02)00298-6 |
| 30. | Tomizaki, K.-y.; Lysenko, A. B.; Taniguchi, M.; Lindsey, J. S. Tetrahedron 2004, 60, 2011–2023. doi:10.1016/j.tet.2004.01.003 |
| 31. | Taniguchi, M.; Kim, M. N.; Ra, D.; Lindsey, J. S. J. Org. Chem. 2005, 70, 275–285. doi:10.1021/jo048440m |
| 32. | Jin, L.-M.; Chen, L.; Yin, J.-J.; Zhou, J.-M.; Giu, C.-C.; Chen, Q.-Y. J. Org. Chem. 2006, 71, 527–536. doi:10.1021/jo051672g |
| 33. | Hashimoto, M.; Kato, Y.; Hatanaka, Y. Tetrahedron Lett. 2006, 47, 3391–3394. doi:10.1016/j.tetlet.2006.03.068 |
| 34. | Bovonsombat, P.; Angara, G. J.; McNelis, E. Synlett 1992, 131–132. doi:10.1055/s-1992-21290 |
| 35. | Bovonsombat, P.; Djuardi, E.; McNelis, E. Tetrahedron Lett. 1994, 35, 2841–2844. doi:10.1016/S0040-4039(00)76639-1 |
| 36. | Muraki, T.; Togo, H.; Yokoyama, M. Synlett 1998, 286–288. doi:10.1055/s-1998-1639 |
| 37. | Muraki, T.; Togo, H.; Yokoyama, M. J. Org. Chem. 1999, 64, 2883–2889. doi:10.1021/jo9825207 |
| 42. | Yusubov, M. S.; Gilmkhanova, M. P.; Zhdankin, V. V.; Kirschning, A. Synlett 2007, 563–566. doi:10.1055/s-2007-970742 |
| 44. | Bachki, A.; Foubelo, F.; Yus, M. Tetrahedron 1994, 50, 5139–5146. doi:10.1016/S0040-4020(01)90424-7 |
| 36. | Muraki, T.; Togo, H.; Yokoyama, M. Synlett 1998, 286–288. doi:10.1055/s-1998-1639 |
| 37. | Muraki, T.; Togo, H.; Yokoyama, M. J. Org. Chem. 1999, 64, 2883–2889. doi:10.1021/jo9825207 |
| 39. | Barrett, A. G. M.; Hopkins, B. T.; Köbberling, J. Chem. Rev. 2002, 102, 3301–3324. doi:10.1021/cr0103423 |
| 40. | Yoshida, J.-i.; Itami, K. Chem. Rev. 2002, 102, 3693–3716. doi:10.1021/cr0103524 |
| 41. | Bhattacharyya, S. Curr. Opin. Drug Discovery Dev. 2004, 7, 752–764. |
| 10. |
Wirth, T. Angew. Chem. 2005, 117, 3722–3731. doi:10.1002/ange.200500115
Angew. Chem., Int. Ed. 2005, 44, 3656–3665. doi:10.1002/anie.200500115 |
| 37. | Muraki, T.; Togo, H.; Yokoyama, M. J. Org. Chem. 1999, 64, 2883–2889. doi:10.1021/jo9825207 |
| 43. | Fields, E. K.; Meyerson, S. J. Org. Chem. 1978, 43, 4705–4708. doi:10.1021/jo00419a005 |
| 51. | Dickens, J. P.; Dyer, R. L.; Hamill, B. J.; Harrow, T. A.; Bible, R. H., Jr.; Finnegan, P. M.; Henrick, K.; Owston, P. G. J. Org. Chem. 1981, 46, 1781–1786. doi:10.1021/jo00322a005 |
© 2007 Kirschning et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)