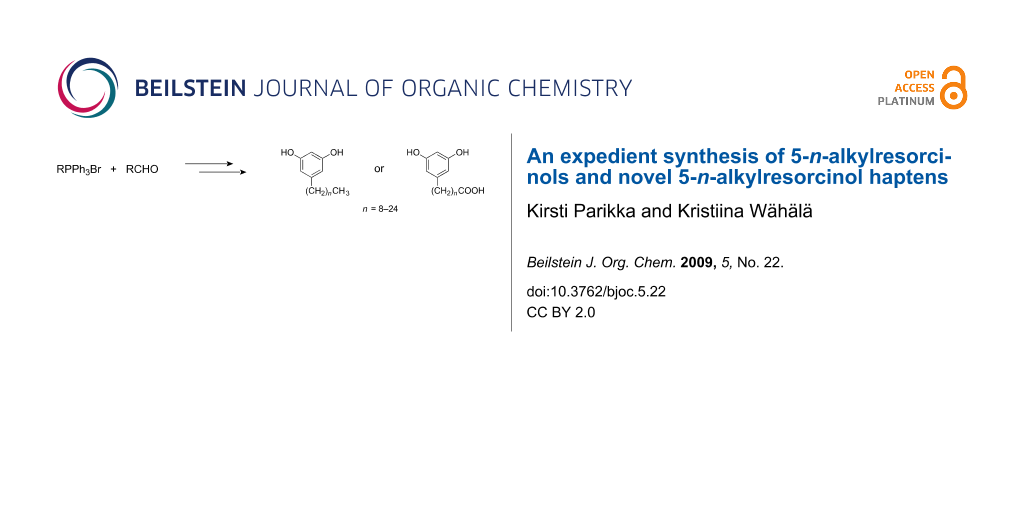
Abstract
The first synthesis of bioactive long alkyl chain 5-n-alkylresorcinols, present in whole grain products, by a novel modification of the Wittig reaction is described. All the main long chain 5-n-alkylresorcinols present in rye and wheat, including C23 and C25 analogues and haptens, which have not been previously prepared, were synthesised. Microwave-promoted reactions of a semi-stabilized ylid and alkanals in water gave good yields in both pressurized and open systems. An alternative microwave-promoted synthesis starting from non-stabilized alkyltriphenylphosphonium salts and 3,5-dimethoxybenzaldehyde worked as well. Aqueous media were suitable for the reactions even if the starting materials were not soluble in water. The 5-n-alkylresorcinols are potential biomarkers of whole grain intake, and the new hapten derivatives of 5-n-alkylresorcinols will open the way for the immunochemical detection techniques of alkylresorcinols.
Graphical Abstract
Introduction
5-Alk(en)ylresorcinols and related compounds are phenolic lipids present in several families of plants (e.g. Gramineae, Anacardiaceae, Proteaceae) and in some families of bacteria [1]. We have recently shown that 5-n-alkylresorcinols (AR, 1, see Figure 1) act as antioxidants protecting LDL from oxidative damage in in vitro experiments using synthesized pure analogues with varying chain lengths [2]. In addition, AR have various biological effects including antimutagenic activity [3-6], antibacterial properties [7], inhibition of enzymes [8-12] and interaction with biological membranes by incorporation to the membrane structure [13,14]. Whole grain rye and wheat products, linked to a healthy diet, are the most important dietary source of AR [1]. According to animal and human studies, these compounds are absorbed and at least partially metabolised, and due to their presence in significant amounts in whole grain products they are currently investigated as highly potential biomarkers of whole grain intake [15-21].
Figure 1: 5-n-Alkylresorcinols 1 and new hapten derivatives 2.
Figure 1: 5-n-Alkylresorcinols 1 and new hapten derivatives 2.
However, AR are not generally nor readily available. Thus an efficient preparation method is needed for various analytical, metabolic or bioactivity investigations. The critical step in the synthesis of long alkyl chain (≥C15) AR is the formation of C–C bond between the aromatic ring and the alkyl chain. The previously reported multistep syntheses utilize techniques that are time-consuming, require an inert atmosphere and give highly variable overall yields. Undoubtedly, the most common approach has been Grignard or alkyllithium techniques starting from 3,5-dimethoxybenzaldehyde (18–48% yields whenever reported) [22-26]. Additional synthetic methods have been developed for shorter chain AR (<C15), such as the aromatization of cyclohexane derivatives in 61–66% yield [27]. A recent Wittig approach utilises the ozonolysis product of a pentadecylresorcinol (C15) and odd carbon chain ylids, but its use is limited because of the poor availability of odd carbon chain alkyl bromides [28]. The synthesis of hapten derivatives of alkylresorcinols 2, potential compounds in the development of immunochemical analysis techniques, has not been reported previously.
Only few papers have reported Wittig reactions in water without an organic solvent, although the related Horner-Wadsworth-Emmons reactions, using ester enolate type stabilized phosphonate ylids, have often been conducted in aqueous solutions [29]. The existing cases have mostly been targeted for the preparation of stilbenes from benzyltriphenylphosphonium salts and aryl aldehydes [30,31] and also include reactions of stabilized ylids with aryl aldehydes or short alkyl chain alkanals [32-36] and the preparation of o- and p-nitrostyrenes from the highly reactive formaldehyde [37]. To provide water solubility more generally, aryl modified phosphonium salts, carrying a –COOH group [38] or PEG attachments [39], have been developed but require extensive synthetic work. Previously, non-stabilized alkylphosphonium salts have appeared much less amenable than the benzyl analogues. A single paper describes the synthesis of two 1-phenylalkenes in 20–30% yield using benzaldehyde and a CH2Cl2/H2O solvent [31].
We report here a fast and efficient synthesis of the long chain 5-n-alkylresorcinols 1 and the new potential hapten derivatives of 5-n-alkylresorcinols 2, ready for the development of antibodies. We also report that the use of microwave (MW) irradiation brings major benefits as regards reaction times and yields in aqueous Wittig reactions. Both semi-stabilized and non-stabilized ylids provide an expedient entry to 5-(1-alkenyl)resorcinols, readily converted to AR and AR haptens.
Results and Discussion
We approached the Wittig synthesis of the precursors of AR and AR haptens from two aspects choosing semi-stabilized and non-stabilized benzylphosphonium or alkylphosphonium ylids (from salts 3 and 4,) as starting materials with 5 or 6, respectively. Water or a mixture of water and an organic solvent were used as a reaction medium with K2CO3. MW irradiation was used to speed up reaction rates after the preliminary experiments under conventional heating were found to require very long reaction times.
For the reactions of 3 and 5, 0.1 M K2CO3 was an optimal solvent. The reactions were performed in 3 or 10 min yielding 66–89% of 7a–d (Scheme 1, Table 1). The methyl group at C-2 of 5d hindered the reaction and required the use of an increased amount of 3 to give a yield of 66%.
Scheme 1: Synthesis of AR derivatives and haptens. a) sealed vessel: MW, 0.1M K2CO3, 100–150°C, 100–150 W, 4–9 bar, 66–81%; b) open vessel: MW, 0.1M K2CO3, 80 °C, 50–150W, 89%; c) 9-BBN, K3PO4, Pd(PPh3)4, I−(CH2)10COOMe, 26%; d) H2, Pd/C, CH2Cl2, 90–91%; e) HBr, reflux, 77–79%.
Scheme 1: Synthesis of AR derivatives and haptens. a) sealed vessel: MW, 0.1M K2CO3, 100–150°C, 100–150 W, 4–...
Table 1: Wittig reactions performed in 0.1M K2CO3 or DMSO/H2O.
| Phosphonium salt | Aldehyde | Product | Time (min) | Yield % (Open vessel) | Yield % (Pressure vessel) | Solvent |
|---|---|---|---|---|---|---|
| 3 | 5a | 7a | 3 | 89 | 81a | 0.1 M K2CO3 |
| 3 | 5b | 7b | 3 | – | 78 | 0.1 M K2CO3 |
| 3 | 5c | 7c | 3 | – | 77 | 0.1 M K2CO3 |
| 3 | 5d | 7d | 10 | – | 66b | 0.1 M K2CO3 |
| 4a | 6 | 8a | 5 | 81 | – | DMSO/H2O |
| 4a | 6 | 8a | 5 | – | 48 | sat. K2CO3 |
| 4b | 6 | 8b | 5 | 78 | – | DMSO/H2O |
| 4c | 6 | 8c | 5 | 75 | – | DMSO/H2O |
| 4d | 6 | 8d | 5 | 76 | – | DMSO/H2O |
| 4e | 6 | 8e | 5 | 70 | – | DMSO/H2O |
| 4f | 6 | 8f | 5 | 68 | – | DMSO/H2O |
| 4g | 6 | 8g | 5 | 68 | – | DMSO/H2O |
| 4h | 6 | 8h | 5 | 65 | – | DMSO/H2O |
a4 bar pressure, otherwise 9 bar.
b3 equiv of the phosphonium salt was used in the reaction.
The reactions of 4 and 6 required the presence of an organic solvent (Scheme 2, Table 1), as the yield was not satisfactory in the different K2CO3 solutions tested (0.1 M/1 M/5 M/saturated). Of those, the saturated solution gave less than 50% at best. A DMSO/H2O solution was found to be optimal for these reactions giving the products 8 in 65–81% yield. In both approaches, MW heating shortened the reaction time to minutes and increased the yield (e.g. products 7a and 8e) in comparison with the reactions performed under conventional conditions (refluxing several hours in e.g. dioxane/H2O/K2CO3).
Scheme 2: Synthesis of 5-n-AR and haptens. a) PPh3, toluene, reflux, 79–87%; b) open vessel: MW, DMSO/H2O 10:1, K2CO3, 130–150 °C, 150 W, 65–81%; c) H2, Pd/C, CH2Cl2, 90–97%; d) BBr3, 80–84%.
Scheme 2: Synthesis of 5-n-AR and haptens. a) PPh3, toluene, reflux, 79–87%; b) open vessel: MW, DMSO/H2O 10:...
The reactions were performed in either open vessels or sealed pressure vessels. The yields of the reactions of the alkylphosphonium salts 4 in sealed vessels were not good due to degradation of the ylids under high pressure (ca. 9 bar). In contrast, reaction of the semi-stabilized benzylic ylid from 3 gave good yield under pressurized conditions, as well as in the open vessel (product 5, Table 1). A one-pot procedure was investigated using alkyl bromide, PPh3 and 6 in various organic solvents but remained unsuccessful.
Mixtures of cis and trans isomers 7a–d and 8 had Z/E ratios varying from ca. 40:60 (8) to ca. 80:20 (7a, both in open and sealed vessel) according to 1H NMR and GC-MS. As the ultimate targets were the C=C reduced AR, the lack of stereochemical control was of no consequence. Following the Wittig reaction, catalytic hydrogenation and demethylation gave AR and AR haptens in ca. 40% overall yield. The Wittig product 7b was a practical starting material for the C23 hapten (2d), for which commercial alkanal or alkyl bromide precursors are not available (Scheme 1). 5a was readily synthesised by Swern-type oxidation (see Supporting Information File 1).
The Wittig reagents were poorly soluble in water. Nevertheless, functional groups enhancing their solubility, such as previously reported –COOH or PEG attachments [38,39], were not necessary. Both the yields of the preliminary experiments without MW irradiation and the yields of the MW promoted reactions were superior to those reported previously (20–30%) for Wittig reactions of non-stabilized ylids [31].
The monitoring of AR from biological samples, including human plasma [15], human and animal ileostomy fluids [16,17] and e.g. perirenal adipose tissue [18], has mainly been performed by gas chromatography-mass spectrometry (GC-MS) requiring time-consuming sample preparation such as extraction, derivatisation, chromatography, and special equipment. Compared with this, immunochemical techniques would be relatively straightforward being rapid and suitable for screening purposes in large populations. In the development of immunoanalytical methods, haptens are needed for the preparation of immunogens, which produce specific antisera. Due to the lack of the required haptens, immunoassay has not yet been used in the analysis of AR.
For the haptens prepared, four different alkyl chain lengths were chosen to represent AR with a very short, medium length and long alkyl chain, of which C17 and C23 are equivalent to the AR present in whole grains and whole grain products. The shorter chain length AR are present, for example, in the plant families Proteaceae (chain lengths C9, C11) or Anacardiaceae and Primulaceae (C13) [1]. The non-polar and hydrophobic character of AR increases as the alkyl chain length grows, which may lead to differences in reactivity.
Conclusion
We have shown that the MW catalyzed reactions of semi-stabilized ylids and alkanals give good yield in pressurized or open systems and without organic solvent, even if they are not soluble in water, being thus practical starting materials for alkylresorcinols and related compounds. An alternative MW catalyzed synthesis route where 3,5-dimethoxy benzaldehyde and unstabilized alkyltriphenyl phosphonium ylids react in DMSO/H2O works as well and alkylresorcinol precursors that do not have a commercially available alkanal starting material can be synthesized rapidly and efficiently by way of this approach. The procedure is suitable for all the long chain 5-n-alkylresorcinols, including the C23:0 and C25:0 analogues, for which synthesis has not been reported previously. Functional groups enhancing the solubility in water are not necessary in the MW promoted Wittig reactions of the long alkyl chain reactants. Thus an efficient preparation method of 5-n-alkylresorcinols and their hapten derivatives was developed. There was no need for dry solvents or inert atmosphere.
Supporting Information
| Supporting Information File 1: Experimental and data | ||
| Format: DOC | Size: 50.0 KB | Download |
References
-
Kozubek, A.; Tyman, J. H. P. Chem. Rev. 1999, 99, 1–25. doi:10.1021/cr970464o
Return to citation in text: [1] [2] [3] -
Parikka, K.; Rowland, I. R.; Welch, R.; Wähälä, K. J. Agric. Food Chem. 2006, 54, 1646–1650. doi:10.1021/jf052486e
Return to citation in text: [1] -
Gasiorowski, K.; Brokos, B. Cell. Mol. Biol. Lett. 2001, 6, 897–911.
Return to citation in text: [1] -
Starck, S. R.; Deng, J.-Z.; Hecht, S. M. Biochemistry 2000, 39, 2413–2419. doi:10.1021/bi991509d
Return to citation in text: [1] -
Gasiorowski, K.; Brokos, B.; Kozubek, A.; Oszmianski, J. Cell. Mol. Biol. Lett. 2000, 5, 171–190.
Return to citation in text: [1] -
Gasiorowski, K.; Szyba, K.; Brokos, B.; Kozubek, A. Cancer Lett. 1996, 106, 109–115. doi:10.1016/0304-3835(96)04294-2
Return to citation in text: [1] -
Himejima, M.; Kubo, I. J. Agric. Food Chem. 1991, 39, 418–421. doi:10.1021/jf00002a039
Return to citation in text: [1] -
Kumagai, M.; Suhara, Y.; Aoyagi, T.; Umezava, H. J. Antibiot. 1971, 24, 870–875.
Return to citation in text: [1] -
Yamada, H.; Shiomi, K.; Xu, Q.; Nagai, T.; Shibata, M.; Oya, I.; Takahashi, Y.; Omura, S. J. Antibiot. 1995, 48, 205–210.
Return to citation in text: [1] -
Deszcz, L.; Kozubek, A. Cell. Mol. Biol. Lett. 1997, 2, 213–222.
Return to citation in text: [1] -
Roufogalis, B.; Li, Q.; Tran, V. H.; Kable, E. P. W.; Duke, C. C. Drug Dev. Res. 1999, 46, 235–249. doi:10.1002/(SICI)1098-2299(199903/04)46:3/4<239::AID-DDR8>3.0.CO;2-N
Return to citation in text: [1] -
Rejman, J.; Kozubek, A. J. Agric. Food Chem. 2004, 52, 246–250. doi:10.1021/jf034745a
Return to citation in text: [1] -
Kozubek, A.; Jezierski, A.; Sikorski, A. F. Biochim. Biophys. Acta, Biomembr. 1988, 944, 465–472. doi:10.1016/0005-2736(88)90517-2
Return to citation in text: [1] -
Linko, A.-M.; Adlercreutz, H. Br. J. Nutr. 2005, 93, 11–13. doi:10.1079/BJN20041281
Return to citation in text: [1] -
Linko, A.-M.; Parikka, K.; Wähälä, K.; Adlercreutz, H. Anal. Biochem. 2002, 308, 307–313. doi:10.1016/S0003-2697(02)00226-9
Return to citation in text: [1] [2] -
Ross, A. B.; Shepherd, M. J.; Knudsen, K. E. B.; Glitso, E. B.; Philips, J.; Rowland, I.; Guo, Z.-X.; Massy, D. J. R.; Åman, P.; Kamal-Eldin, A. Br. J. Nutr. 2003, 90, 787–794. doi:10.1079/BJN2003965
Return to citation in text: [1] [2] -
Ross, A. B.; Kamal-Eldin, A.; Lundin, E. A.; Zhang, J.-X.; Hallmans, G.; Åman, P. J. Nutr. 2003, 133, 2222–2224.
Return to citation in text: [1] [2] -
Ross, A. B.; Chen, Y.; Frank, J.; Swanson, J. E.; Parker, R. S.; Kozubek, A.; Lundh, T.; Vessby, B. P.; Åman, P.; Kamal-Eldin, A. J. Nutr. 2004, 134, 506–510.
Return to citation in text: [1] [2] -
Aubertin-Leheudre, M.; Koskela, A.; Marjamaa, A.; Adlercreutz, H. Cancer Epidemiol., Biomarkers Prev. 2008, 17, 2244–2248. doi:10.1158/1055-9965.EPI-08-0215
Return to citation in text: [1] -
Landberg, R.; Kamal-Eldin, A.; Andersson, A.; Vessby, B.; Åman, P. Am. J. Clin. Nutr. 2008, 87, No. 4832–838.
Return to citation in text: [1] -
van Dam, R. M.; Hu, F. B. Am. J. Clin. Nutr. 2008, 87, No. 4797–798.
Return to citation in text: [1] -
Singh, U. S.; Scannell, R. T.; An, H.; Carter, B. J.; Hecht, S. M. J. Am. Chem. Soc. 1995, 117, 12691–12699. doi:10.1021/ja00156a005
Return to citation in text: [1] -
Kozubek, A.; Tyman, J. H. P. Chem. Phys. Lipids 1995, 78, 29–35. doi:10.1016/0009-3084(95)02480-7
Return to citation in text: [1] -
Brown, G. D. J. Nat. Prod. 1992, 55, 1756–1760. doi:10.1021/np50090a006
Return to citation in text: [1] -
Cirigottis, K. A.; Cleaver, L.; Corrie, J. E. T.; Grasby, R. G.; Green, G. H.; Mock, J.; Nimgirawath, S.; Read, R. W.; Ritchie, E.; Taylor, W. C.; Vadasz, A.; Webb, W. R. G. Aust. J. Chem. 1974, 27, 345–355.
Return to citation in text: [1] -
Wenkert, E.; Loeser, E.-M.; Mahapatra, S. N.; Schenker, F.; Wilson, E. M. J. Org. Chem. 1964, 29, 435–439. doi:10.1021/jo01025a046
Return to citation in text: [1] -
Marmor, R. S. J. Org. Chem. 1972, 37, 2901–2904. doi:10.1021/jo00983a025
Return to citation in text: [1] -
Tyman, J. H. P. Synthesis of 5-(C15-25-alkyl/alkenyl)resorcinols via 8-(3,5-dihydroxyphenyl)octanal, the ozonolysis product of cardol, using Wittig and Grignard methodology. GB 2429455, Feb 28, 2007.
Return to citation in text: [1] -
Li, C.-J. Chem. Rev. 2005, 105, 3095–3165. doi:10.1021/cr030009u
Return to citation in text: [1] -
Hwang, J.-J.; Lin, R.-L.; Shieh, R.-L.; Jwo, J.-J. J. Mol. Catal. A: Chem. 1999, 142, 125–139. doi:10.1016/S1381-1169(98)00274-X
Return to citation in text: [1] -
Märkl, G.; Merz, A. Synthesis 1973, 295–297. doi:10.1055/s-1973-22193
Return to citation in text: [1] [2] [3] -
Matikainen, J.; Kaltia, S.; Hase, T. Synlett 1994, 817–818. doi:10.1055/s-1994-23014
Return to citation in text: [1] -
Wu, J.; Zhang, D.; Wei, S. Synth. Commun. 2005, 35, 1213–1222. doi:10.1081/SCC-200054816
Return to citation in text: [1] -
Yamamoto, K.; Watanabe, M.; Ideta, K.; Mataka, S.; Thiemann, T. Z. Naturforsch. 2005, 60b, 1299–1307.
Return to citation in text: [1] -
Orsini, F.; Sello, G.; Fumagalli, T. Synlett 2006, 1717–1718. doi:10.1055/s-2006-947323
Return to citation in text: [1] -
El-Batta, A.; Jiang, C.; Zhao, W.; Anness, R.; Cooksy, A. L.; Bergdahl, M. J. Org. Chem. 2007, 72, 5244–5259. doi:10.1021/jo070665k
Return to citation in text: [1] -
Butcher, M.; Mathews, R. J.; Middleton, S. Aust. J. Chem. 1973, 26, 2067–2069.
Return to citation in text: [1] -
Russell, M. G.; Warren, S. J. J. Chem. Soc., Perkin Trans. 1 2000, 4, 505–513.
Return to citation in text: [1] [2] -
Sieber, F.; Wentworth, P., Jr.; Toker, J. D.; Wentworth, A. D.; Metz, W. A.; Reed, N. N.; Janda, K. D. J. Org. Chem. 1999, 64, 5188–5192. doi:10.1021/jo9903712
Return to citation in text: [1] [2]
| 38. | Russell, M. G.; Warren, S. J. J. Chem. Soc., Perkin Trans. 1 2000, 4, 505–513. |
| 39. | Sieber, F.; Wentworth, P., Jr.; Toker, J. D.; Wentworth, A. D.; Metz, W. A.; Reed, N. N.; Janda, K. D. J. Org. Chem. 1999, 64, 5188–5192. doi:10.1021/jo9903712 |
| 39. | Sieber, F.; Wentworth, P., Jr.; Toker, J. D.; Wentworth, A. D.; Metz, W. A.; Reed, N. N.; Janda, K. D. J. Org. Chem. 1999, 64, 5188–5192. doi:10.1021/jo9903712 |
| 8. | Kumagai, M.; Suhara, Y.; Aoyagi, T.; Umezava, H. J. Antibiot. 1971, 24, 870–875. |
| 9. | Yamada, H.; Shiomi, K.; Xu, Q.; Nagai, T.; Shibata, M.; Oya, I.; Takahashi, Y.; Omura, S. J. Antibiot. 1995, 48, 205–210. |
| 10. | Deszcz, L.; Kozubek, A. Cell. Mol. Biol. Lett. 1997, 2, 213–222. |
| 11. | Roufogalis, B.; Li, Q.; Tran, V. H.; Kable, E. P. W.; Duke, C. C. Drug Dev. Res. 1999, 46, 235–249. doi:10.1002/(SICI)1098-2299(199903/04)46:3/4<239::AID-DDR8>3.0.CO;2-N |
| 12. | Rejman, J.; Kozubek, A. J. Agric. Food Chem. 2004, 52, 246–250. doi:10.1021/jf034745a |
| 37. | Butcher, M.; Mathews, R. J.; Middleton, S. Aust. J. Chem. 1973, 26, 2067–2069. |
| 7. | Himejima, M.; Kubo, I. J. Agric. Food Chem. 1991, 39, 418–421. doi:10.1021/jf00002a039 |
| 38. | Russell, M. G.; Warren, S. J. J. Chem. Soc., Perkin Trans. 1 2000, 4, 505–513. |
| 3. | Gasiorowski, K.; Brokos, B. Cell. Mol. Biol. Lett. 2001, 6, 897–911. |
| 4. | Starck, S. R.; Deng, J.-Z.; Hecht, S. M. Biochemistry 2000, 39, 2413–2419. doi:10.1021/bi991509d |
| 5. | Gasiorowski, K.; Brokos, B.; Kozubek, A.; Oszmianski, J. Cell. Mol. Biol. Lett. 2000, 5, 171–190. |
| 6. | Gasiorowski, K.; Szyba, K.; Brokos, B.; Kozubek, A. Cancer Lett. 1996, 106, 109–115. doi:10.1016/0304-3835(96)04294-2 |
| 30. | Hwang, J.-J.; Lin, R.-L.; Shieh, R.-L.; Jwo, J.-J. J. Mol. Catal. A: Chem. 1999, 142, 125–139. doi:10.1016/S1381-1169(98)00274-X |
| 31. | Märkl, G.; Merz, A. Synthesis 1973, 295–297. doi:10.1055/s-1973-22193 |
| 2. | Parikka, K.; Rowland, I. R.; Welch, R.; Wähälä, K. J. Agric. Food Chem. 2006, 54, 1646–1650. doi:10.1021/jf052486e |
| 32. | Matikainen, J.; Kaltia, S.; Hase, T. Synlett 1994, 817–818. doi:10.1055/s-1994-23014 |
| 33. | Wu, J.; Zhang, D.; Wei, S. Synth. Commun. 2005, 35, 1213–1222. doi:10.1081/SCC-200054816 |
| 34. | Yamamoto, K.; Watanabe, M.; Ideta, K.; Mataka, S.; Thiemann, T. Z. Naturforsch. 2005, 60b, 1299–1307. |
| 35. | Orsini, F.; Sello, G.; Fumagalli, T. Synlett 2006, 1717–1718. doi:10.1055/s-2006-947323 |
| 36. | El-Batta, A.; Jiang, C.; Zhao, W.; Anness, R.; Cooksy, A. L.; Bergdahl, M. J. Org. Chem. 2007, 72, 5244–5259. doi:10.1021/jo070665k |
| 22. | Singh, U. S.; Scannell, R. T.; An, H.; Carter, B. J.; Hecht, S. M. J. Am. Chem. Soc. 1995, 117, 12691–12699. doi:10.1021/ja00156a005 |
| 23. | Kozubek, A.; Tyman, J. H. P. Chem. Phys. Lipids 1995, 78, 29–35. doi:10.1016/0009-3084(95)02480-7 |
| 24. | Brown, G. D. J. Nat. Prod. 1992, 55, 1756–1760. doi:10.1021/np50090a006 |
| 25. | Cirigottis, K. A.; Cleaver, L.; Corrie, J. E. T.; Grasby, R. G.; Green, G. H.; Mock, J.; Nimgirawath, S.; Read, R. W.; Ritchie, E.; Taylor, W. C.; Vadasz, A.; Webb, W. R. G. Aust. J. Chem. 1974, 27, 345–355. |
| 26. | Wenkert, E.; Loeser, E.-M.; Mahapatra, S. N.; Schenker, F.; Wilson, E. M. J. Org. Chem. 1964, 29, 435–439. doi:10.1021/jo01025a046 |
| 28. | Tyman, J. H. P. Synthesis of 5-(C15-25-alkyl/alkenyl)resorcinols via 8-(3,5-dihydroxyphenyl)octanal, the ozonolysis product of cardol, using Wittig and Grignard methodology. GB 2429455, Feb 28, 2007. |
| 16. | Ross, A. B.; Shepherd, M. J.; Knudsen, K. E. B.; Glitso, E. B.; Philips, J.; Rowland, I.; Guo, Z.-X.; Massy, D. J. R.; Åman, P.; Kamal-Eldin, A. Br. J. Nutr. 2003, 90, 787–794. doi:10.1079/BJN2003965 |
| 17. | Ross, A. B.; Kamal-Eldin, A.; Lundin, E. A.; Zhang, J.-X.; Hallmans, G.; Åman, P. J. Nutr. 2003, 133, 2222–2224. |
| 15. | Linko, A.-M.; Parikka, K.; Wähälä, K.; Adlercreutz, H. Anal. Biochem. 2002, 308, 307–313. doi:10.1016/S0003-2697(02)00226-9 |
| 16. | Ross, A. B.; Shepherd, M. J.; Knudsen, K. E. B.; Glitso, E. B.; Philips, J.; Rowland, I.; Guo, Z.-X.; Massy, D. J. R.; Åman, P.; Kamal-Eldin, A. Br. J. Nutr. 2003, 90, 787–794. doi:10.1079/BJN2003965 |
| 17. | Ross, A. B.; Kamal-Eldin, A.; Lundin, E. A.; Zhang, J.-X.; Hallmans, G.; Åman, P. J. Nutr. 2003, 133, 2222–2224. |
| 18. | Ross, A. B.; Chen, Y.; Frank, J.; Swanson, J. E.; Parker, R. S.; Kozubek, A.; Lundh, T.; Vessby, B. P.; Åman, P.; Kamal-Eldin, A. J. Nutr. 2004, 134, 506–510. |
| 19. | Aubertin-Leheudre, M.; Koskela, A.; Marjamaa, A.; Adlercreutz, H. Cancer Epidemiol., Biomarkers Prev. 2008, 17, 2244–2248. doi:10.1158/1055-9965.EPI-08-0215 |
| 20. | Landberg, R.; Kamal-Eldin, A.; Andersson, A.; Vessby, B.; Åman, P. Am. J. Clin. Nutr. 2008, 87, No. 4832–838. |
| 21. | van Dam, R. M.; Hu, F. B. Am. J. Clin. Nutr. 2008, 87, No. 4797–798. |
| 18. | Ross, A. B.; Chen, Y.; Frank, J.; Swanson, J. E.; Parker, R. S.; Kozubek, A.; Lundh, T.; Vessby, B. P.; Åman, P.; Kamal-Eldin, A. J. Nutr. 2004, 134, 506–510. |
| 13. | Kozubek, A.; Jezierski, A.; Sikorski, A. F. Biochim. Biophys. Acta, Biomembr. 1988, 944, 465–472. doi:10.1016/0005-2736(88)90517-2 |
| 14. | Linko, A.-M.; Adlercreutz, H. Br. J. Nutr. 2005, 93, 11–13. doi:10.1079/BJN20041281 |
| 15. | Linko, A.-M.; Parikka, K.; Wähälä, K.; Adlercreutz, H. Anal. Biochem. 2002, 308, 307–313. doi:10.1016/S0003-2697(02)00226-9 |
© 2009 Parikka and Wähälä; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)