Abstract
A practical and high yielding synthesis of α,β-unsaturated esters from aldehydes and 1,1-diethoxyethylene was developed.
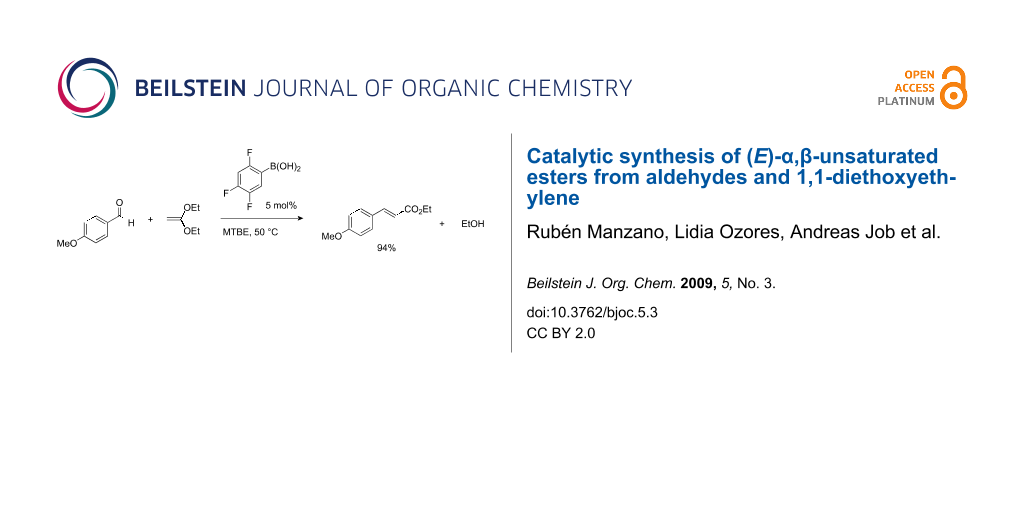
Graphical Abstract
Introduction
A number of reactions transforming aldehydes into α,β-unsaturated esters have been developed and especially Wittig reaction variants are widely used [1]. A general problem with these approaches, however, is their unsatisfactory atom economy resulting in significant by-product formation (Scheme 1, eq 1–2). Alternatives have been suggested but a solution that is both as general and efficient as the Wittig procedures and satisfactory with respect to the demand for atom economy is still needed [2-22]. We have recently developed a new approach based on the Knoevenagel condensation of readily available and inexpensive malonate half esters with aldehydes which leads to the formation of water and CO2 as the only by-products (Scheme 1, eq 3) [23,24]. Here we describe as a new approach, the boronic acid-catalyzed condensation of ketene dialkyl acetal with aldehydes to furnish α,β-unsaturated esters in good yields and reliably high (E)-stereoselectivities (Scheme 1, eq 4). Although previously attempted under thermal conditions with poor success, our catalytic reaction is entirely new [25].
Scheme 1: Conversions of aldehydes into α,β-unsaturated esters.
Scheme 1: Conversions of aldehydes into α,β-unsaturated esters.
We reasoned that the reaction of readily available and inexpensive ketene diethyl acetal [26] or ketene dimethyl acetal (available from Aldrich) with aldehydes upon treatment with a suitable reagent or catalyst could readily lead to the corresponding unsaturated ester and ethanol or methanol as the only by-product. The use of ketene dialkyl acetals for organic transformations is infrequent [27]. To the best of our knowledge, they have been employed in [2+2] cycloadditions [28-30], Diels-Alder processes [31] and reactions with alcohols [32] and N-formyl imines [33], to afford orthoesters and N-formyl-β-aminoesters respectively.
Results and Discussion
Initial experiments revealed that Brønsted acids such as acetic acid indeed catalyze the newly designed transformation. After screening various catalysts and different reaction conditions, we found that boronic acids [34], especially 2,4,5-trifluorophenylboronic acid, proved to be quite active catalysts of the condensation of benzaldehyde with ketene diethyl acetal (Table 1). Brønsted acids are also effective but this particular boronic acid, which possesses both Lewis acidity and Brønsted acidity, was the most active catalyst and gave the highest yields of the corresponding α,β-unsaturated ester. The optimized procedure involved the slow addition of 1,1-diethoxyethylene to benzaldehyde in the presence of a catalytic amount (5 mol%) of 2,4,5-trifluorophenylboronic acid at 50 °C in MTBE, affording the corresponding α,β-unsaturated ester in 98% yield as the pure (E)-isomer.
Table 1: Selected results for catalyst screening.
|
|
||
| Entry | Catalyst | Yielda |
|---|---|---|
| 1 | 2,4,5-C6H2F3-B(OH)2 | 98 |
| 2 | B(OH)3 | 71 |
| 3 | p-TsOH·H2O | 86 |
| 4 | C6F5-B(OH)2 | 86 |
| 5 | CF3CO2H | 82 |
aYield of isolated product
The procedure proved general and both aromatic as well as aliphatic aldehydes can be utilized (Table 2). For aromatic aldehydes the yields are typically very high (93–98%, entries 1–5). Cinnamaldehyde, as an example of an unsaturated aldehyde gave the desired product in 83% yield (entry 6). The unsaturated esters derived from branched aliphatic aldehydes were isolated in 80–89% yield (entries 7–8). Even α-unbranched, linear aldehydes furnished the expected product (entries 9–10). However, in these cases, the yields are slightly lower (61–64%). In addition, 1,1-dimethoxyethylene has also been tested and was found to give the corresponding methyl esters analogously.
Table 2: Olefination of different aldehydes.
|
|
||
| Entry | R | Yielda |
|---|---|---|
| 1 | p-MeO-Ph | 94 |
| 2 | Ph | 98 |
| 3 | p-Br-Ph | 97 |
| 4 | 1-naphthyl | 93 |
| 5 | p-MePh | 97 |
| 6 | PhCH=CH | 83 |
| 7 | Cy | 89 |
| 8 | iPr | 80 |
| 9 | n-C4H9 | 64 |
| 10 | n-C6H13 | 61 |
aYield of isolated product
Despite the absence of any supporting evidence, we envision a plausible mechanism that explains the peculiar effectiveness of boronic acids as catalysts (Scheme 2). Accordingly, the boronic acid functions as a Lewis acid activating the aldehyde but also as a hydroxide donor facilitating the departure of ethanol from an activated intermediate. Clearly, alternative mechanisms may be proposed.
Experimental
A solution of benzaldehyde (500 mg, 4.7 mmol) and 2,4,5-trifluorophenylboronic acid (0.05 equiv) in MTBE (2.5 mL) was prepared. After heating the reaction mixture to 50 °C, a solution of ketene diethyl acetal (4 equiv) in MTBE (2.5 mL) was added dropwise over 2 h, and the reaction was stirred at the same temperature for 12 h. The solvent was removed under vacuum, and the crude material was purified by flash chromatography to afford the pure product (silica gel, hexane/EtOAc 95/5).
References
-
Maryanoff, B. E.; Reitz, A. B. Chem. Rev. 1989, 89, 863–927. doi:10.1021/cr00094a007
Return to citation in text: [1] -
Hatsuda, M.; Kuroda, T.; Seki, M. Synth. Commun. 2003, 33, 427–434. doi:10.1081/SCC-120015773
Return to citation in text: [1] -
Kisanga, P.; D’Sa, B.; Verkade, J. Tetrahedron 2001, 57, 8047–8052. doi:10.1016/S0040-4020(01)00782-7
Return to citation in text: [1] -
Herrmann, W. A.; Wang, M. Angew. Chem. 1991, 103, 1709–1711. doi:10.1002/ange.19911031235
Return to citation in text: [1] -
Ledford, B. E.; Carreira, E. M. Tetrahedron Lett. 1997, 38, 8125–8128. doi:10.1016/S0040-4039(97)10182-4
Return to citation in text: [1] -
Lee, M.-Y.; Chen, Y.; Zhang, X. P. Organometallics 2003, 22, 4905–4909. doi:10.1021/om034115y
Return to citation in text: [1] -
Barma, D. K.; Kundu, A.; Bandyopadhyay, A.; Kundu, A.; Sangras, B.; Briot, A.; Mioskowski, C.; Falck, J. R. Tetrahedron Lett. 2004, 45, 5917–5920. doi:10.1016/j.tetlet.2004.05.113
Return to citation in text: [1] -
Taguchi, H.; Shimoji, K.; Yamamoto, H.; Nozaki, H. Bull. Chem. Soc. Jpn. 1974, 47, 2529–2531. doi:10.1246/bcsj.47.2529
Return to citation in text: [1] -
Matui, S.; Tanaka, K.; Kaji, A. Synthesis 1983, 127–128. doi:10.1055/s-1983-30249
Return to citation in text: [1] -
Huang, Y.; Shen, Y.; Chen, C. Tetrahedron Lett. 1986, 27, 2903–2904. doi:10.1016/S0040-4039(00)84675-4
Return to citation in text: [1] -
Huang, X.; Xie, L.; Wu, H. Tetrahedron Lett. 1987, 28, 801–802. doi:10.1016/S0040-4039(01)80993-X
Return to citation in text: [1] -
Huang, Z.-Z.; Ye, S.; Xia, W.; Tang, Y. Chem. Commun. 2001, 1384–1385. doi:10.1039/b104100m
Return to citation in text: [1] -
Olah, G. A.; Wu, A.-h.; Farooq, O.; Prakash, G. K. S. Synthesis 1988, 537–538. doi:10.1055/s-1988-27628
Return to citation in text: [1] -
Sampath Kumar, H. M.; Shesha Rao, M.; Joyasawal, S.; Yadav, J. S. Tetrahedron Lett. 2003, 44, 4287–4289. doi:10.1016/S0040-4039(03)00815-3
Return to citation in text: [1] -
Kagabu, S.; Shimizu, C.; Takahashi, J.; Hara, K.; Koketsu, M.; Ishida, M. Bull. Soc. Chim. Fr. 1992, 129, 435–439.
Return to citation in text: [1] -
Blakemore, P. R.; Ho, D. K. H.; Nap, W. M. Org. Biomol. Chem. 2005, 3, 1365–1368. doi:10.1039/b500713e
Return to citation in text: [1] -
Engel, D. A.; Lopez, S. S.; Dudley, G. B. Tetrahedron 2008, 64, 6988–6996. doi:10.1016/j.tet.2008.02.030
Return to citation in text: [1] -
Concellón, J. M.; Rodríguez-Solla, H.; Díaz, P.; Llavona, R. J. Org. Chem. 2007, 72, 4396–4400. doi:10.1021/jo070209w
Return to citation in text: [1] -
Smith, J. M.; Greaney, M. F. Tetrahedron Lett. 2007, 48, 8687–8690. doi:10.1016/j.tetlet.2007.10.029
Return to citation in text: [1] -
Beletskaya, I. P.; Cheprakov, A. V. Chem. Rev. 2000, 100, 3009–3066. doi:10.1021/cr9903048
Return to citation in text: [1] -
Chatterjee, A. K.; Toste, F. D.; Choi, T.-L.; Grubbs, R. H. Adv. Synth. Catal. 2002, 344, 634–637. doi:10.1002/1615-4169(200208)344:6/7<634::AID-ADSC634>3.0.CO;2-K
Return to citation in text: [1] -
Zeitler, K. Org. Lett. 2006, 8, 637–640. doi:10.1021/ol052826h
Return to citation in text: [1] -
List, B.; Doehring, A.; Hechavarria Fonseca, M. T.; Wobser, K.; van Thienen, H.; Rios Torres, R.; Llamas Galilea, P. Adv. Synth. Catal. 2005, 347, 1558–1560. doi:10.1002/adsc.200505196
Return to citation in text: [1] -
List, B.; Doehring, A.; Hechavarria Fonseca, M. T.; Job, A.; Rios Torres, R. Tetrahedron 2006, 62, 476–482. doi:10.1016/j.tet.2005.09.081
Return to citation in text: [1] -
McElvain, S. M.; Degginger, E. R.; Behun, J. D. J. Am. Chem. Soc. 1954, 76, 5736–5739. doi:10.1021/ja01651a034
Return to citation in text: [1] -
McElvain, S. M.; Kundiger, D. Ketene Diethylacetal. In Organic Syntheses, Collective Volume 3; Horning, E. C., Ed.; John Wiley & Sons: New York, 1955; pp 506–508.
Return to citation in text: [1] -
McElvain, S. M. Chem. Rev. 1949, 45, 453–492. doi:10.1021/cr60142a003
Return to citation in text: [1] -
Avenoza, A.; Busto, J. H.; Canal, N.; Peregrina, J. M. Chem. Commun. 2003, 1376–1377. doi:10.1039/b302000b
Return to citation in text: [1] -
Avenoza, A.; Busto, J. H.; Canal, N.; Peregrina, J. M. J. Org. Chem. 2005, 70, 330–333. doi:10.1021/jo048943s
Return to citation in text: [1] -
Rustullet, A.; Alibés, R.; de March, P.; Figueredo, M.; Font, J. Org. Lett. 2007, 9, 2827–2830. doi:10.1021/ol0710616
Return to citation in text: [1] -
Balázs, L.; Kádas, I.; Tõke, L. Tetrahedron Lett. 2000, 41, 7583–7587. doi:10.1016/S0040-4039(00)01302-2
Return to citation in text: [1] -
Cosgrove, K. L.; McGeary, R. P. Synlett 2008, 2425–2428. doi:10.1055/s-2008-1078215
Return to citation in text: [1] -
Rossen, K.; Jakubec, P.; Kiesel, M.; Janik, M. Tetrahedron Lett. 2005, 46, 1819–1821. doi:10.1016/j.tetlet.2005.01.114
Return to citation in text: [1] -
Ishihara, K.; Ohara, S.; Yamamoto, H. J. Org. Chem. 1996, 61, 4196–4197. doi:10.1021/jo9606564
Return to citation in text: [1]
| 1. | Maryanoff, B. E.; Reitz, A. B. Chem. Rev. 1989, 89, 863–927. doi:10.1021/cr00094a007 |
| 26. | McElvain, S. M.; Kundiger, D. Ketene Diethylacetal. In Organic Syntheses, Collective Volume 3; Horning, E. C., Ed.; John Wiley & Sons: New York, 1955; pp 506–508. |
| 25. | McElvain, S. M.; Degginger, E. R.; Behun, J. D. J. Am. Chem. Soc. 1954, 76, 5736–5739. doi:10.1021/ja01651a034 |
| 23. | List, B.; Doehring, A.; Hechavarria Fonseca, M. T.; Wobser, K.; van Thienen, H.; Rios Torres, R.; Llamas Galilea, P. Adv. Synth. Catal. 2005, 347, 1558–1560. doi:10.1002/adsc.200505196 |
| 24. | List, B.; Doehring, A.; Hechavarria Fonseca, M. T.; Job, A.; Rios Torres, R. Tetrahedron 2006, 62, 476–482. doi:10.1016/j.tet.2005.09.081 |
| 2. | Hatsuda, M.; Kuroda, T.; Seki, M. Synth. Commun. 2003, 33, 427–434. doi:10.1081/SCC-120015773 |
| 3. | Kisanga, P.; D’Sa, B.; Verkade, J. Tetrahedron 2001, 57, 8047–8052. doi:10.1016/S0040-4020(01)00782-7 |
| 4. | Herrmann, W. A.; Wang, M. Angew. Chem. 1991, 103, 1709–1711. doi:10.1002/ange.19911031235 |
| 5. | Ledford, B. E.; Carreira, E. M. Tetrahedron Lett. 1997, 38, 8125–8128. doi:10.1016/S0040-4039(97)10182-4 |
| 6. | Lee, M.-Y.; Chen, Y.; Zhang, X. P. Organometallics 2003, 22, 4905–4909. doi:10.1021/om034115y |
| 7. | Barma, D. K.; Kundu, A.; Bandyopadhyay, A.; Kundu, A.; Sangras, B.; Briot, A.; Mioskowski, C.; Falck, J. R. Tetrahedron Lett. 2004, 45, 5917–5920. doi:10.1016/j.tetlet.2004.05.113 |
| 8. | Taguchi, H.; Shimoji, K.; Yamamoto, H.; Nozaki, H. Bull. Chem. Soc. Jpn. 1974, 47, 2529–2531. doi:10.1246/bcsj.47.2529 |
| 9. | Matui, S.; Tanaka, K.; Kaji, A. Synthesis 1983, 127–128. doi:10.1055/s-1983-30249 |
| 10. | Huang, Y.; Shen, Y.; Chen, C. Tetrahedron Lett. 1986, 27, 2903–2904. doi:10.1016/S0040-4039(00)84675-4 |
| 11. | Huang, X.; Xie, L.; Wu, H. Tetrahedron Lett. 1987, 28, 801–802. doi:10.1016/S0040-4039(01)80993-X |
| 12. | Huang, Z.-Z.; Ye, S.; Xia, W.; Tang, Y. Chem. Commun. 2001, 1384–1385. doi:10.1039/b104100m |
| 13. | Olah, G. A.; Wu, A.-h.; Farooq, O.; Prakash, G. K. S. Synthesis 1988, 537–538. doi:10.1055/s-1988-27628 |
| 14. | Sampath Kumar, H. M.; Shesha Rao, M.; Joyasawal, S.; Yadav, J. S. Tetrahedron Lett. 2003, 44, 4287–4289. doi:10.1016/S0040-4039(03)00815-3 |
| 15. | Kagabu, S.; Shimizu, C.; Takahashi, J.; Hara, K.; Koketsu, M.; Ishida, M. Bull. Soc. Chim. Fr. 1992, 129, 435–439. |
| 16. | Blakemore, P. R.; Ho, D. K. H.; Nap, W. M. Org. Biomol. Chem. 2005, 3, 1365–1368. doi:10.1039/b500713e |
| 17. | Engel, D. A.; Lopez, S. S.; Dudley, G. B. Tetrahedron 2008, 64, 6988–6996. doi:10.1016/j.tet.2008.02.030 |
| 18. | Concellón, J. M.; Rodríguez-Solla, H.; Díaz, P.; Llavona, R. J. Org. Chem. 2007, 72, 4396–4400. doi:10.1021/jo070209w |
| 19. | Smith, J. M.; Greaney, M. F. Tetrahedron Lett. 2007, 48, 8687–8690. doi:10.1016/j.tetlet.2007.10.029 |
| 20. | Beletskaya, I. P.; Cheprakov, A. V. Chem. Rev. 2000, 100, 3009–3066. doi:10.1021/cr9903048 |
| 21. | Chatterjee, A. K.; Toste, F. D.; Choi, T.-L.; Grubbs, R. H. Adv. Synth. Catal. 2002, 344, 634–637. doi:10.1002/1615-4169(200208)344:6/7<634::AID-ADSC634>3.0.CO;2-K |
| 22. | Zeitler, K. Org. Lett. 2006, 8, 637–640. doi:10.1021/ol052826h |
| 32. | Cosgrove, K. L.; McGeary, R. P. Synlett 2008, 2425–2428. doi:10.1055/s-2008-1078215 |
| 34. | Ishihara, K.; Ohara, S.; Yamamoto, H. J. Org. Chem. 1996, 61, 4196–4197. doi:10.1021/jo9606564 |
| 31. | Balázs, L.; Kádas, I.; Tõke, L. Tetrahedron Lett. 2000, 41, 7583–7587. doi:10.1016/S0040-4039(00)01302-2 |
| 28. | Avenoza, A.; Busto, J. H.; Canal, N.; Peregrina, J. M. Chem. Commun. 2003, 1376–1377. doi:10.1039/b302000b |
| 29. | Avenoza, A.; Busto, J. H.; Canal, N.; Peregrina, J. M. J. Org. Chem. 2005, 70, 330–333. doi:10.1021/jo048943s |
| 30. | Rustullet, A.; Alibés, R.; de March, P.; Figueredo, M.; Font, J. Org. Lett. 2007, 9, 2827–2830. doi:10.1021/ol0710616 |
| 33. | Rossen, K.; Jakubec, P.; Kiesel, M.; Janik, M. Tetrahedron Lett. 2005, 46, 1819–1821. doi:10.1016/j.tetlet.2005.01.114 |
© 2009 Manzano et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)