Abstract
Mitomycins are a class of very potent antibacterial and anti-cancer compounds having a broad activity against a range of tumours. They have been used in clinics since the 1960’s, and the challenges represented by their total synthesis have challenged generations of chemists. Despite these chemical and medicinal features, these compounds, in racemic form, have succumbed to total synthesis only four times over the last 30 years.
Graphical Abstract
Review
Introduction
The mitomycins pose unique challenges to the synthetic chemist. As S. Danishefsky noted, “The complexity of the problem arises from the need to accommodate highly interactive functionality in a rather compact matrix and to orchestrate the chemical progression such as to expose and maintain vulnerable structural elements as the synthesis unfolds. The synthesis of a mitomycin is the chemical equivalent of walking on egg shells.”
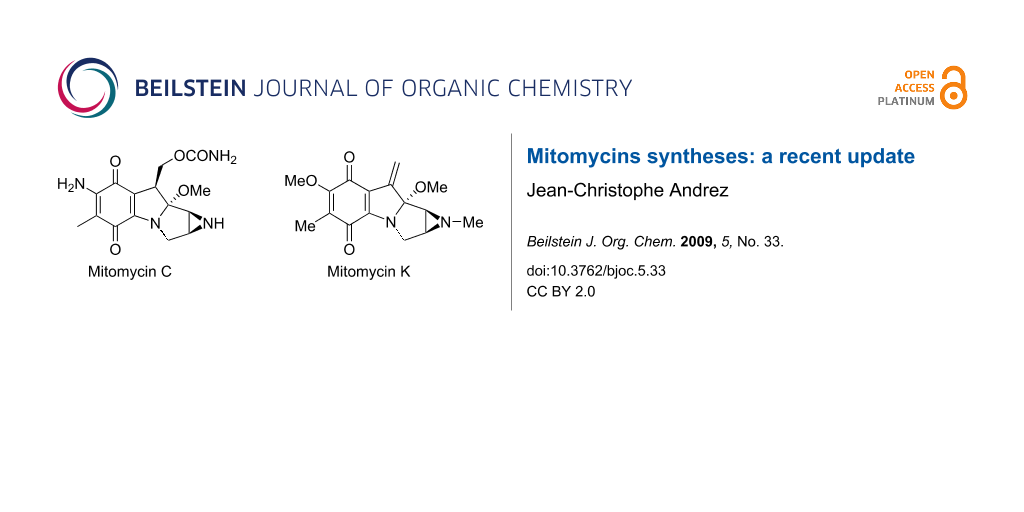
The first discovery of a mitomycin (mitomycin C, Scheme 2, compound 7) dated from 1958 [1]. Its structural elucidation was remarkable at that time considering the presence of 4 contiguous stereogenic carbons in the molecule. The tetracyclic pyrrolo-indole skeleton of a mitomycin is embellished with an aziridine ring, a carbamoyl moiety and a bridged carbinolamine packed in a constrained architecture [2]. The presence of such a concentration of functional groups renders this molecule only moderately stable to bases, acid and nucleophiles but particularly reactive in presence of reducing agents. Notwithstanding their apparent fragility, mitomycins were rapidly identified to act as prodrugs and their unique activity was thought to originate from their ability to transform in vivo to generate the active metabolite. This was followed by decades of investigations to understand in detail their singular mode of action. It was found that the aziridine played a crucial role, allowing an irreversible bis-alkylation of DNA [3]. The decisive role of the aziridine is far from unusual since its presence in a small number of other naturally occurring molecules such as azinomycins [4,5], FR-900482 [6], maduropeptin [7], and azicemicins [8] is accompanied by significant biological properties (Scheme 1) [3,9]. Mitomycin C, 7, the most potent mitomycin, has been used medicinally since the 1970’s for its activity against breast, stomach, oesophagus and bladder tumours [9]. Besides the well-known antibiotic and antitumour properties of these compounds [9-12], other semi-synthetic derivatives were prepared for investigation in clinical trials [13,14].
Altogether, the biological features of the mitomycins and the challenges represented by their total synthesis have continually drawn the attention of numerous brilliant chemists who conceived many different routes for their synthesis. However, only four total syntheses have been achieved [15-20]. Also, this review will summarize the current state of the art concerning the chemistry and biology of mitomycins. It will show and comment on the methodologies that have been successfully employed in total syntheses as well as approaches leading to mitomycin analogs. The review will focus on the synthetic literature of the past 30 years. From time to time earlier references will be provided to give background information. The mitomycin’s close cousins, the FR family (Scheme 1, compounds 3 and 4) will not be discussed, nor will the different strategies that have been employed to improve the efficacy of mitomycins in vivo by structure activity relationship studies. As a matter of fact, the natural products themselves are so sensitive that only minor modifications have been possible in connection with medicinal chemistry studies.
Scheme 1: Aziridine containing natural products.
Scheme 1: Aziridine containing natural products.
Discussion
1. Mitomycin isolation and nomenclature
Mitomycins are natural products isolated from extracts of genus Streptomyces, a filamentous gram-positive soil bacterium that produces a wide array of biologically active compounds, including over two-thirds of the commercially important natural-product metabolites [21]. Mitomycin C is extracted from the bacterium Streptomyces lavendulae and is far from the most known compound of the series. It has become one of the most effective drugs against non-small-cell lung carcinoma, as well as other soft and solid tumours [22]. The seven most abundant mitomycins (A to K) in nature are presented in Scheme 2.
Scheme 2: Mitomycin structures and nomenclature.
Scheme 2: Mitomycin structures and nomenclature.
Since many synthetic attempts did not succeed in providing mitomycins per se but only close relatives of these molecules, a special nomenclature has been elaborated for these compounds: structures of type 15, which do not contain an aziridine ring, but bear the p-quinone are called mitosanes or mitosenes depending whether they are at the oxidation state of an indoline (Y1 or Y2 = H) or an indole (Y1 = Y2 = C=C). Compounds bearing an aziridine at C1 and C2 are described specifically as aziridinomitosanes and aziridinomitosenes respectively. Compounds possessing a hydroquinone (protected or not) in place of the original quinone are identified by inclusion of the prefix leuco in reference to the Greek word leukos (clear, white) and the lack of intense color usually specific of the corresponding quinone ring.
The mitomycins A and C differ only by the substituents on the quinone ring and transformation from 6 to 7 is realized by simple treatment with ammonia [23,24]. The mitomycins F, 8, and porfiromycin, 9, are synthesized by methylation of the aziridine of mitomycin A and mitomycin C, respectively. Mitomycin G, 12, mitomycin H, 13, and mitomycin K, 14, are derivatives of this first series obtained by elimination of the carbamate at position 10 [25,26]. Mitomycin B, 10, and mitomycin D, 11, possess the opposite absolute configuration of the asymmetric carbon C9. Interestingly, Hornemann proved that this carbon could be easily epimerized to give 9-epi-mitomycin B, 19. This compound showed better activity than the non-epimerized one. He proposed a based-catalyzed mechanism wherein the tetracyclic-pyrolido-indole structure 10 opens up reversibly at the bridged carbinolamine junction to give the eight-membered ring 17. The base, in this case DBU, then reversibly deprotonates the activated C9 position to give the more stable isomer 18 (Scheme 3) [27]. The basis of this surmise was the finding that mitomycin B eliminates carbamic acid at the C10 position in basic medium whereas the angular methoxy series (mitomycin C), which can not open to the amino ketone, needs a better leaving group (such as a sulfonate).
Scheme 3: Base catalysed epimerization of mitomycin B.
Scheme 3: Base catalysed epimerization of mitomycin B.
2. Biology
2.1. Biosynthesis
A significant amount of information on the biosynthesis of mitomycin C has been accumulated since 1970 [28]. The mitosane core was shown to be derived from combination of 3-amino-5-hydroxybenzoic acid 20 (AHBA), D-glucosamine 21 and carbamoyl phosphate (Scheme 4) [29-32]. The key intermediate, AHBA, is also a common precursor to other anticancer drugs, such as rifamycin and ansamycin.
Scheme 4: Biosynthesis of mitomycin C (MMC) 7.
Scheme 4: Biosynthesis of mitomycin C (MMC) 7.
2.2. Mode of action
Mitomycins are quinone antitumor antibiotics that exert their biological activity through DNA alkylation and cross-linking. The success of mitomycin C in cancer treatment is due to a great cytotoxic selectivity for hypoxic (O2-deficient) cells characteristic of solid tumors [33,34]. Mitomycin C itself is indeed relatively unreactive toward DNA [35,36] but becomes remarkably reactive upon reduction (enzymatic, electrochemical or chemical) by the mechanism shown in Scheme 5 [37-39]. This mechanism was proposed 40 years ago based purely on structural considerations and has been accepted since [40]. Only the first reductive activation step has been questioned as to whether it proceeds by a one-electron reduction to give the semiquinone [41-44] or by a two-electron reduction to give the hydroquinone [45-47]. Studies have shown that one-electron reduction in an organic solvent can trigger formation of the semiquinone and the subsequent reaction cascade [42]. On the other hand, two-electron reduction led to formation of the stable hydroquinone, which can be oxidized back to the quinone in the presence of oxygen [48]. Nonetheless, different results were observed in water where both one- and two-electron reductions gave the same DNA adducts. Moreover, the disproportion of the semiquinone in aqueous anaerobic medium is also very fast [49] whereas under aqueous aerobic conditions, the semiquinone reoxidizes to the quinone more quickly than it disproportionates [50]. Thus, the conclusion was made that in aqueous medium the same hydroquinone intermediate was responsible for the reaction cascade.
The following section will focus on different synthetic approaches to mitomycins and will be divided into seven sections, each corresponding to a retrosynthetic disconnection involving at least one common bond formation. Part 9 will deal with miscellaneous disconnections.
3. The N–C3–C9a disconnection
3.1. Danishefsky. Mitomycin K (MMK)
The retrosynthetic approach of Danishefsky is based on an intramolecular Diels–Alder reaction between a nitrosoaryl and a suitably functionalized diene (Scheme 7) [19]. Historically, this strategy was designed to synthesize the related natural product FR-900482, 4, but their investigations also led to this significant and concise total synthesis of mitomycin K. In fact, assembly of the tetracyclic structure occured in only four steps, with all the key structures in place, making their synthesis very practical. Another key reaction was the efficient introduction of the N-methyl aziridine in only three steps from an olefin by cycloaddition of methylthiophenyl azide onto the unsaturated amide 29.
Scheme 7: Danishefsky’s Retrosynthesis of mitomycin K.
Scheme 7: Danishefsky’s Retrosynthesis of mitomycin K.
As mentioned before, the construction of FR-900482 was thought to occur by intramolecular hetero Diels–Alder reaction of a compound of type 32 (Scheme 8) [51]. After careful analysis, it was envisioned that the reaction could occur either in the bridged mode to give the FR series (compounds 3 and 4) or in the fused mode to give access to mitomycins. Further investigations confirmed that the intramolecular hetero Diels–Alder reaction favoured the fused mode and did not constitute a viable route for the synthesis of the FR series. Nonetheless, changing the approach by using an intermolecular Diels–Alder reaction gave efficiently the bridged adduct and allowed one of the most elegant total syntheses of FR-900482 [52]. As a result, this astonishing synthesis of mitomycin K can be seen as a “by-product” of the synthesis of FR-900482.
Scheme 8: Hetero Diels–Alder reaction en route to mitomycins.
Scheme 8: Hetero Diels–Alder reaction en route to mitomycins.
The functionalized nitroso-diene 36 was efficiently formed by photochemical rearrangement of the nitro-carbinol 35, which arose from the addition of vinyl lithium 30 into aldehyde 31 (Scheme 9). Further irradiation at 350 nm triggered a cycloaddition to give compound 38, probably through intermediate 37. Deserving of mention is the unusual instability of compound 37 under the reaction conditions, which can be attributed to the high captodative stabilization of the aniline radical formed upon cleavage of the nitrogen-oxygen bond. Functionalization of the olefin in compound 38 was accomplished first by dihydroxylation with osmium tetroxide. The reaction was stereospecific, resulting in formation of the diol 39 derived from attack of the reagent from the concave face of the molecule.
Scheme 9: Nitroso Diels–Alder cycloaddition.
Scheme 9: Nitroso Diels–Alder cycloaddition.
Diol 39 was found to have the undesired stereochemistry for the planned construction of the aziridine by tandem SN2 displacement. Exploiting the facial selectivity of compound 38, the direct introduction of an aziridine equivalent was attempted by 1,3-dipolar cycloaddition of an alkyl azide. As Frank noted, cycloaddition of azides to 3H-pyrrolo [1,2-a]indoles gives rise to complex reaction mixtures due to the possibility of nitrene insertion. Cycloaddition of phenyl azide, however, to the unsaturated carbonyl 41 was readily accomplished to give triazoline 42 in 56% yield (Scheme 10) [53-55].
According to this scheme, the allylic alcohol 38 was oxidized with pyridinium dichromate and reacted with methylthiophenyl azide [56] to give the triazoline 40 derived from attack of the reagent from the concave face of the molecule with high diastereoselectivity (Scheme 11). The electronic effect or the α-methoxy group, as well as shielding of the α-face of the molecule, provides the desired steroselectivity.
An uncommon transformation was then accomplished: the reduction, with L-Selectride, of a lactam in the presence of a ketone. There are several causes for the unusual chemoselectivity of this reduction. The lone pair electrons of the amide nitrogen in compound 40 are conjugated with the ketone through the aromatic ring. Thus, the lactam behaves as an imide whose carbonyls express ketone-like reactivity. Moreover, the upper carbonyl is deactivated by conjugation with a methoxy group in the ortho position of the benzene ring, and by the steric crowding by both this methoxy group and the one at the ring junction. Finally, the nitrogen of the lactam is located in the bridgehead position of a [3.3.0] bicycle resulting in poor delocalization into the adjacent carbonyl.
Scheme 11: Final steps of mitomycin K synthesis. aPDC, DCM; bPhSCH2N3, PhH, 80 °C; cL-selectride, THF, −78 °C; d1,1′-(thiocarbonyl)diimidazole, DMAP, DCM, 35 °C; eBu3SnH, AIBN, PhH, 80 °C; fhυ 254nm, PhH; gRaNi, AcOH, 60 °C; h[(trimethylsilyl)methyl]lithium, THF, −10 °C; isilver (II) pinacolate, NaOAc, CH3CN/H2O; jPPTS, DCM.
Scheme 11: Final steps of mitomycin K synthesis. aPDC, DCM; bPhSCH2N3, PhH, 80 °C; cL-selectride, THF, −78 °C; ...
A Barton deoxygenation gave compound 43, which was irradiated to decompose the triazoline to an aziridine. The thiophenyl component was then removed with Raney nickel, which provided an elegant way to introduce the N-methyl group of the aziridine. Compound 44 was then treated with (trimethylsilyl)methyl lithium to install the exocyclic olefin via Peterson’s method. The p-dimethoxyhydroquinone was then oxidized with silver(II) pinacolate (in poor yield) to give mitomycin K.
3.2. Naruta, Maruyama. Azide cycloaddition
From a synthetic point of view, the intramolecular [1+4] cyclisation of a nitrene with a dienyl moiety gives a pyrrolizidine, the key structure found in mitomycins and many other mammal and vegetal alkaloids. The Naruta–Maruyama group exploited this reaction for the synthesis of a leucoaziridinomitosane based on the retrosynthesis shown in Scheme 12 [57,58].
Scheme 12: Naruta–Maruyama retrosynthesis.
Scheme 12: Naruta–Maruyama retrosynthesis.
The synthesis began with a Claisen rearrangement of the pentadienyl aryl ether 47 under Lewis acidic conditions (Scheme 13). After protection of the resulting phenol with a MOM group, the regioselective introduction of an alkoxymethyl group at the C9 position, the most crowded location on the pentadienyl moiety of compound 50, was the next obstacle. The best results were obtained by forming the pentadienyl anion with butyllithium followed by quenching with benzyloxymethyl chloride. The desired compound was obtained in 55% yield while the other regioisomers were isolated in a combined 22% yield. The more direct approach using the alkoxymethyl substituted pentadienyl aryl ether 49 failed to give the Claisen rearrangement product.
Scheme 13: Synthesis of a leucoaziridinomitosane by nitrene cycloaddition. aAlCl3-Et2O; bNaH, ClCH2OMe; cn-BuLi, ClCH2OBn; dH3O+; eCAN; fNaN3; gZn, AcOH; hBnBr, K2CO3; iOsO4, NMO; jMsCl, Et3N; kn-Bu4N3; lMsCl, Et3N; mP(OMe)3; nNaH; oLiAlH4.
Scheme 13: Synthesis of a leucoaziridinomitosane by nitrene cycloaddition. aAlCl3-Et2O; bNaH, ClCH2OMe; cn-BuL...
The MOM group was then removed, the phenol oxidized to the p-quinone with CAN and the fluorine displaced by azide to give compound 46. Decomposition of azide-containing olefins have been performed under thermal, photolytic, acid-catalyzed or transition metal-catalyzed conditions [59]. With this substrate the subsequent cyclisation of azidodienylquinone 46 was performed with Cu(acac)2 as catalyst and afforded 48 with a high degree of diastereoselectivity. Thermal reaction led to the formation of the ring-contracted cyclopentendione derivative 54. The reaction was thought to proceed by attack of the quinone by an intermediate nitrene via intermediates 52 and 53 (Scheme 14) [60].
Scheme 14: Thermal decomposition of azidoquinone 51.
Scheme 14: Thermal decomposition of azidoquinone 51.
The diastereoselectivity observed during the cycloaddition originated from the most favored staggered conformation in the transition state based on the Houk model, wherein the allylic hydrogen is eclipsed by the olefinic hydrogen (compound 55) to minimize 1,3-allylic strain (Scheme 15) [61].
Scheme 15: Diastereoselectivity during the cycloaddition.
Scheme 15: Diastereoselectivity during the cycloaddition.
Attempts to introduce the aziridine from the olefin 48 using halogeno-azides like BrN3 or IN3 and subsequent reduction failed because the compound oxidized rapidly to the indoloquinone 58 (Scheme 16).
A longer sequence using dihydroxylation with osmium tetroxide, mesylation and displacement with azide was used to produce leucoaziridinomitosane 45, whose spectral data matched those of an authentic sample derived from natural mitomycin C.
3.3. Williams. Mitsunobu reaction
R.M. Williams successfully used this disconnection during the total synthesis of FR-900482 [62,63]. Coupling of nitrotoluene 59 and aldehyde 60 [64] gave the aldol product 61 as a 2:1 mixture of diastereomers (Scheme 17). Manipulation of protecting groups and oxidation states led to compound 62, which cyclised smoothly under Mitsunobu conditions to form the eight-membered ring of 63 [65]. Unfortunately, all attempts to introduce the hydroxymethyl group at C9 by aldol-type strategies met with failure. Electronics seem to play a major role, as very similar substrates en route to FR-900482 underwent analogous reactions [66]. Solving this issue at the stage of the benzazocenol or at an earlier intermediate would constitute a major breakthrough.
Scheme 17: Williams’ approach towards mitomycins.aDEIPSCl, Imidazole, DCM; bPd/C, HCO2NH4, MeOH; cAllocCl, NaHCO3, DCM-H2O; dDDQ, CH2Cl2.
Scheme 17: Williams’ approach towards mitomycins.aDEIPSCl, Imidazole, DCM; bPd/C, HCO2NH4, MeOH; cAllocCl, NaH...
3.4. Danishefsky. Homoconjugate addition
The homoconjugate opening of activated cyclopropanes has been studied by many groups, utilizing all kinds of nucleophiles [67-70]. Although this methodology was known before 1900 [71], it did not draw much attention in total synthesis because of its lack of efficiency. In the middle of 1970’s Danishefsky and co-workers focused on the systematic study of this reaction and made relevant improvements for both the preparation of the cyclopropane and the enhancement of reactivity of the cyclic acylal 64 [72-76]. This methodology has seen application in the synthesis of diverse heterocycles, as exemplified by the reaction of aniline with 64 to form the 1,5-addition adduct 65, and finally the amide 66 by internal acylation and extrusion of acetone (Scheme 18).
Scheme 18: Synthesis of pyrrolidones by homoconjugate addition.
Scheme 18: Synthesis of pyrrolidones by homoconjugate addition.
This methodology provided a new entry for the construction of pyrrolidine-indole structures based on the intramolecular opening of an activated cyclopropane [75-77]. This work culminated in the synthesis of an advanced intermediate en route to the mitosane series [78]. Compound 67 was thermolyzed in refluxing chlorobenzene in the presence of cupric acetonylacetonate to give the cyclopropane adduct in 35% yield (Scheme 19) [79]. The product arose as a 5:1 mixture of diastereoisomers, with the major component 69 having the bulky hexasubstituted phenyl group on the convex face of the bicyclic[4.1.0] ring system. It was necessary to methanolyse the lactone 69 prior to removal of the phthalimide with methyl hydrazine, which occurred with concomitant cyclization to form penultimate intermediate 70. The final ring closure was realized by treatment with camphorsulfonic acid to form lactam 71 in 35% yield over 4 steps.
Scheme 19: Homoconjugate addition on the fully functionalized substrate.
Scheme 19: Homoconjugate addition on the fully functionalized substrate.
The transformation of this compound into an intermediate suitable for the introduction of an aziridine was found to be extremely difficult. A sequence leading to the formation of the olefin 73 was enacted. This compound was thought to be a good candidate for the installation of the aziridine. They exploited the presence of a β-dicarbonyl functionality in 71 to introduce a phenylseleno function α to the lactam centre (Scheme 20). Then, hydrolysis of the methyl ester followed by decarboxylation and reduction of the lactam with borane gave the selenide diastereoisomers 72 in 46% overall yield. Interestingly, oxidation with hydrogen peroxide gave predominantly the N-allylic system rather than the vinylic one. This is in accordance with the precedent established by the laboratory of K.B. Sharpless [80,81].
The overall yield from diazo-ester 67 was only 2% and was deemed insufficient to pursue further synthetic study. However, the idea of a tandem reaction opened the door to more innovative and fascinating syntheses.
3.5. Danishefsky. One pot N–C9a, N–C3 formation
In the early 1980’s the Danishefsky group envisioned the possibility of the diastereoselective introduction of the aziridine via the olefinic portion of a pyrroline such as 74 [82]. The construction of this compound would proceed through a sequence such as that depicted in Scheme 21. Upon electrophilic activation of the olefin 75, the aniline would attack it to form a pyrroline ring, making the terminal leaving group X free for nucleophilic displacement with the nitrogen of the newly formed pyrroline.
Scheme 21: Retrosynthesis of N–C9a, N–C3 bond formation.
Scheme 21: Retrosynthesis of N–C9a, N–C3 bond formation.
The synthesis began by Mitsunobu coupling of phenol 76 with allylic alcohol 77 to give ether 78, which was heated in N,N-dimethylaniline to trigger a Claisen rearrangement (Scheme 22). A sequence of straightforward reactions then led to compound 80. As expected, the trans double bond in 80 prevented intramolecular alkylation of the amino group by the homoallylic bromide, thus explaining the somewhat surprising stability of this compound. The key step cyclisation was then carried out with the Nicolaou’s reagent, N-phenylselenophthalimide (N-PSP) [83,84]. The attack of this reagent upon the double bond led to indoline 81 which underwent a second alkylation to generate the complete pyrroloindole system 82.
Scheme 22: Synthesis of the pyrrolo[1,2]indole 82 using N-PSP activation.aAc2O, Py; bAc2O, Hg(OAc)2, AcOH, 90% HNO3; cZn, HCl, MeOH; dCBr4, PPh3, Et2O. (31% overall yield from 78).
Scheme 22: Synthesis of the pyrrolo[1,2]indole 82 using N-PSP activation.aAc2O, Py; bAc2O, Hg(OAc)2, AcOH, 90%...
It is noteworthy that the cyclization reaction was completely stereospecific. Indeed, one could argue that the addition of N-PSP to the olefin 80 to form a selenonium ion is reversible and that the attack of the nitrogen is favored when the two large groups emerge trans relative to the indoline ring.
Treatment with m-CPBA then created the double bond which was later fashioned into aziridine 84 (Scheme 23). First, the phenol acetate was replaced by a benzyl group. Among the different oxidants screened for dihydroxylation, osmium tetroxide was preferred, since it did not effect aromatization of the indoline ring and gave diol 83 as a single isomer. Dihydroxylation occurred selectively from the concave face of the molecule, anti to the exo-disposed benzyloxymethyl group. The standard methodology involving azide displacement gave the aziridine 84. The benzyl groups were removed using a Birch reduction and subsequent oxidation with DDQ furnished the aziridinomitosane 85 in good overall yield.
Scheme 23: Synthesis of an aziridinomitosane. am-CPBA, DCM then iPr2NH, CCl4 reflux; bK2CO3, MeOH; cBnBr, KH; dOsO4, NMO; eMsCl, Et3N; fn-Bu4NN3, C6H6; gMsCl, Et3N; hP(OMe)3 then NaH; iLAH; jMeLi, MeI.
Scheme 23: Synthesis of an aziridinomitosane. am-CPBA, DCM then iPr2NH, CCl4 reflux; bK2CO3, MeOH; cBnBr, KH; d...
Although this scheme was suitable for the synthesis of aziridinomitosanes, a venture to introduce the C9a heteroatom functionality (essential for activity [85]) was attempted unsuccessfully in this study. The same authors observed that any attempts to oxidize a leucoaziridinomitosane of type 86 via a Polonovski reaction inevitably gave either the corresponding leucoaziridinomitosene 88 or the oxidation product at C3, 89 (Scheme 24) [86]. The N-oxo-acetyl intermediate 87 can eliminate an acetate group upon deprotonation at carbon C9a or C3 leading to two regioisomeric iminium species. The iminium formed by deprotonation at carbon C9a probably rearranged to leucoaziridinomitosene 88 faster than it was trapped by a nucleophile. The putative attack of an nucleophile on the transient iminium species would create an equilibrium with the starting iminium which would eventually convert to the thermodynamically more stable indole 88.
Scheme 24: Oxidation products of a leucoaziridinomitosane obtained from a Polonovski oxidation.
Scheme 24: Oxidation products of a leucoaziridinomitosane obtained from a Polonovski oxidation.
More recently, a suitable substrate for the selective oxygenation of the C9a position of a mitomycin derivative was discovered by F.E. Ziegler and co-workers [87]. The use of a Polonovski reaction [88] on the aziridinomitosane 90 gave the C9a oxygenated compound 92 in 67% yield (Scheme 25). Rewardingly, the selectivity for this reaction was good, giving only minor quantities of the C3 oxidation product 91.
Scheme 25: Polonovski oxidation of an aziridinomitosane. am-CPBA; bPd/C, H2; cDimethoxypropane, PPTS.
Scheme 25: Polonovski oxidation of an aziridinomitosane. am-CPBA; bPd/C, H2; cDimethoxypropane, PPTS.
A possible explanation for this selectivity is the participation of the carbonyl of the carbobenzyloxy protecting group in the elimination process by internally assisting deprotonation at C9a through a seven-membered ring and thereby accelerating the formation of the desired iminium ion. The authors found that changing the carbobenzyloxy protecting group to less basic groups such as a silyloxy ether or a methoxymethyl ether gave virtually no selectivity between C9a and C3 oxidation. In an attempt to conclude the synthesis of FR-900482, substrate 93a was subjected to decarbonylation conditions, using 2.2 equivalents of Wilkinson’s catalyst, (PPh3)3RhCl, to give the corresponding decarbonylated product 93b with retention of configuration of the C10 side chain. However, the reaction proved to be capricious and inconsistent results were routinely observed making the synthesis not very practical.
4. The C1–C9a disconnection
4.1. Ziegler. Aziridinyl radical
Ziegler and Berlin proposed a disconnection for the synthesis of aziridinomitosanes based on a 5-exo-trig cyclisation of an aziridinyl radical (Scheme 27) [89]. This approach allowed stereocontrolled, rapid access to either enantiomer of this type of structure. Compound 94 was obtained by SN2 displacement with chiral aziridinyl triflate 100 followed by decarboxylation. Among the numerous routes to synthesize compound 100 [90-92] the authors opted for an economical way starting from the common food preservative sodium erythorbate 97, the enantiomer of the sodium salt of ascorbic acid (vitamin C) (Scheme 28). It was transformed in three steps into the (2S,3R)-4-hydroxy-2,3-epoxybutyrate 99 [93] which was in turn advanced to the triflate aziridine 100 by the general procedure developed by Blum [94]. The cyclisation compound 94 took place by generating the aziridinyl radical in the presence of tributyltin hydride and a radical initiator, azobisisobutyronitrile, in refluxing toluene. The transient radical that formed at C9 then abstracted a hydrogen atom from n-Bu3SnH to the convex face of the molecule, giving leucoaziridinomitosane 95 with a cis relationship between H9 and H1. The desmethoxymitomycin A 96 was then elaborated using standard protocols.
Scheme 27: Ziegler synthesis of desmethoxymitomycin A.aIm2C=O, THF; bNH3; cTMSOTf, 2,6-di-tert-butylpyridine, DCM; dAc2O, Et3N; eH2, Pd/C, EtOAc; fDDQ, −78 °C→25 °C; gNH3, MeOH.
Scheme 27: Ziegler synthesis of desmethoxymitomycin A.aIm2C=O, THF; bNH3; cTMSOTf, 2,6-di-tert-butylpyridine, ...
Scheme 28: Transformation of sodium erythorbate.aTBDMSCl; bNaN3; cPPh3; d(Boc)2O, DMAP; eTBAF; fTf2O, Pyr.
Scheme 28: Transformation of sodium erythorbate.aTBDMSCl; bNaN3; cPPh3; d(Boc)2O, DMAP; eTBAF; fTf2O, Pyr.
The oxidative introduction of a methoxy group at the C9a position remains challenging. Recently, the Ziegler group disclosed an interesting opportunity to achieve this goal in a related study (Scheme 29) [95]. Keeping in mind that oxidation of the C9a position would be possible if the C9 is quaternized, this group focused on the idea of introducing a protecting group at C9 which, upon liberation, would reveal the C9–C10 double bond found in mitomycin K. Applying the same radical process depicted in Scheme 27 with substrate 101, a tandem cyclization was observed to afford pentacycle 102. The basic pyrrolidine nitrogen was protected as its N-oxide and the exocyclic olefin was then converted to the corresponding ketone through ozonolysis. The ozonide and N-oxide were reduced with dimethyl sulfide at the end of the reaction. Using the Gardner protocol [96] to hydroxylate the alpha position of the resultant ketone failed to give any α-ketol, but instead provided directly the desired styrene 103 in good overall yield.
Scheme 29: Formation of C9,C10-unsaturation in the mitomycins. am-CPBA, DCM; bO3, MeOH; cMe2S; dKHMDS, (EtO)3P, O2, THF.
Scheme 29: Formation of C9,C10-unsaturation in the mitomycins. am-CPBA, DCM; bO3, MeOH; cMe2S; dKHMDS, (EtO)3P...
A possible explanation for this fragmentation is outlined in Scheme 30. The enolate of ketone 104 reacts with oxygen to form the peroxide anion 105, which cleaves to give the α-keto-γ−butyrolactone 106. Treatment of the latter with aqueous base generates the styrene 107 and oxalic acid.
4.2. Vedejs. Anionic Michael addition
This strategy is very similar to Ziegler’s preceding approach since both share the same method for forming the C3–N4 bond and aim at constructing the C1–C9a bond. But while Ziegler uses a tandem radical cyclization, Vedejs employs an anionic Michael addition to form the C1–C9a bond [97].
Interestingly, preliminary attempts to effect metal exchange and internal Michael addition revealed a complex situation. Treating stannane 108 with an excess of methyl lithium followed by quenching with deuterated ethanol provided the monodeuterio derivative 109, the corresponding de-stannylated dideuterio structure 112 and a small amount of the desired tetracyclic 113 (109:112:113 = 55:36:9). This suggested that deprotonation of the indole was faster than the lithium metal exchange and prevented the desired Michael addition. They rationalized that monodeuterio derivative 109 should be a better substrate, having a slower indole deprotonation rate, due to a primary kinetic isotope effect. Accordingly, treatment of the monodeuterio derivative 109 with methyl lithium followed by quenching with phenylselenium chloride yielded directly the mitosene 111 (Scheme 31). As expected, in this case, the lithium-metal exchange occurred much faster and inverted the ratio of tetracyclic:tricyclic product from 1:10 to 4:1.
Removal of the trityl protecting group was then achieved using triethylsilane and methanesulfonic acid to give the parent free aziridine 114 in 65% yield. Although these types of aziridinomitosene are usually very unstable and aziridine solvolysis products are often formed, the presence of a deactivating ester group promoted the stability of the molecule.
4.3. Reissig. Addition of samarium ketyls to alkynes
The direct synthesis of eight membered rings such as benzazocenol 116 is highly desirable since it provides a straightforward synthesis of mitomycins. However, the formation of these ring sizes from acyclic precursors is entropically and enthalpically disfavoured. Therefore, their synthesis became a great challenge in recent years [98]. In this regard, Reissig recently developed an 8-endo-dig cyclisation to give benzazocenols 116 and 117 using samarium iodide in moderate yields (Scheme 32) [99].
In these studies, simplified model compounds unsuitable for the synthesis of mitomycins were used and no further developments have been reported on this series of compounds.
4.4. Jones. Radical cyclization
A radical cyclization was used by the Jones group for the formation of smaller rings via a 5-exo-dig radical cyclization. Bromoalkyne 118 was chosen to construct pyrrolo[1,2-a]indole 119. The reaction was initiated with tributyltin hydride and gave the cyclized product 119 in 37% yield. (Scheme 33) [100].
Scheme 33: Synthesis of pyrrolo[1,2-a]indole by 5-exo-dig radical cyclization.
Scheme 33: Synthesis of pyrrolo[1,2-a]indole by 5-exo-dig radical cyclization.
Although compound 119 was not used in further studies, it might serve as a good forerunner for the synthesis of mitosenes as shown by Remers and discussed in section 6.3 of this review.
5. The C9–C9a disconnection
5.1. Kozikowski. Nitrile oxide cycloaddition, INOC
The Kozikowski group used an intramolecular nitrile oxide cycloaddition (INOC) to form the eight membered ring of a benzazocine system [101]. The hydroxylamine 120 was treated with sodium hypochlorite to generate in situ a nitrile oxide which reacted with the terminal olefin to form the eight membered ring 121 (Scheme 35). Easy cleavage of the nitrogen–oxygen bond was realized using Raney nickel and the unsaturated ketone 122 was obtained in good yield.
Scheme 35: Intramolecular nitrile oxide cycloaddition.
Scheme 35: Intramolecular nitrile oxide cycloaddition.
It is known that when a medium-sized ring is being generated by the INOC process none of the “normal” 5-substituted isoxazoline is formed. Hence, the nitrodecene 123 gives rise to only the nine-membered carbocycle 125 upon reaction with phenyl isocyanate. The matching HOMO-LUMO interactions for such cycloadditions favour the formation of 126 but ring strain and transannular steric effects oppose this orientation (Scheme 36).
This methodology was used later on by the Fukuyama group to achieve the synthesis of mitomycin analogs [102]. Treating hydroxylamine 127 with sodium hypochlorite effected the cycloaddition, but the wrong regioisomer 128 was obtained (Scheme 37). Steric repulsions between the methoxy ether and the olefin were probably responsible for this unexpected outcome. However, introduction of a carboethoxy group on the terminus of the olefin 129 restored the regioselectivity observed by Kozikowski to give oxazoline 130.
5.2. Reinhoudt. Rearrangement of 1-(1-pyrrolidinyl)-1,3-butadienes
Reinhoudt illustrated the stereoselective thermal rearrangement of 1-(1-pyrrolidinyl)-1,3-butadienes for the synthesis of mitosane analogs [103-105]. They proposed that the transformation proceeded through two consecutive pericyclic reactions (Scheme 38). Starting from compound 131, a [1,6] hydrogen shift produced the conjugated 1,5-dipolar species 132 that subsequently underwent concerted disrotatory electrocyclisation of the 6π-electron system to give a mixture of the cis and trans isomers 133a and 133b (ratio 1.8:1). Compound 133b was separated by chromatography and a final dihydroxylation using a stoichiometric quantity of osmium tetroxide gave mitosane 134b, which resulted from attack on the convex face of the molecule. The same stereochemical outcome was observed using the other diastereomer 133a showing that the substituents on C9 had only a minor influence on the stereoselectivity of the dihydroxylation.
Scheme 38: Synthesis of a mitosane core by rearrangement of a 1-(1-pyrrolidinyl)-1,3-butadiene.
Scheme 38: Synthesis of a mitosane core by rearrangement of a 1-(1-pyrrolidinyl)-1,3-butadiene.
Compounds 134a and 134b could be tailored as mitomycin A and mitomycin B derivatives respectively but issues pertaining to the introductions of the C9a oxygenated functionality and the carbamoyl moiety at C10 remain unsolved.
5.3. Sulikowski. Buchwald coupling and carbene insertion
The laboratory of G.A. Sulikowski proposed a synthesis of 1,2-aziridinomitosenes [106,107] using as key transformations a Buchwald–Hartwig cross-coupling [108-110] and a chemoselective intramolecular carbon-hydrogen metal-carbenoid insertion reaction (Scheme 39).
Scheme 39: Sulikowski synthesis of an aziridinomitosene. aPd(Tol3P)2Cl2, Bu3SnF, 140; bH2, Pd/C; cTFAA, Et3N; dTBAF; eMsCl, Et3N; fDBU; gK2CO3, MeOH; hp-TsCl, Et3N; iCl2CO, Pyridine; jp-TSA, MeOH; kNaHMDS then PNBSA (para-nitrobenzenesulfonylazide).
Scheme 39: Sulikowski synthesis of an aziridinomitosene. aPd(Tol3P)2Cl2, Bu3SnF, 140; bH2, Pd/C; cTFAA, Et3N; d...
The chiral pyrolidine 136 was prepared in 94.7% ee by asymmetric ring opening of the corresponding N-Boc protected meso epoxide using the Jacobsen (salen)Cr(III) complex and TMSN3 [111]. The acetate group was installed on the aromatic using a palladium catalyzed reaction with the silylketene acetal 140 in the presence of tributyltin fluoride [112]. After protecting group manipulations and formation of the diazoester 138 with p-nitrobenzenesulfonylazide (PNBSA), the key carbene insertion was achieved using copper(I) in the presence of bis-oxazoline 139. A 9:1 ratio of 141 and 142 was obtained after direct chloranil oxidation of the indoline intermediate to the indole. The regioselectivity is in accord with the results of Adams who showed that the C–H bond with the highest electron density was the most likely to migrate during rhodium(II) mediated C–H insertion [113].
A related study assessed the possibility of circumventing this regioselectivity issue by exploiting the enantioselective intramolecular C–H insertion of diazoester 144 into a meso pyrrolidine using chiral catalyst 145. Unfortunately the reaction displayed low enantio- and diastereoselectivity, with the major isomer 146 having an ee of only 51% (Scheme 40) [114].
Scheme 40: Enantioselective carbene insertion.
Scheme 40: Enantioselective carbene insertion.
5.4. Parson. Radical cyclization
The development of novel cascade (or domino) radical reactions is an active area of current research, and one approach to the mitomycin ring system focused on the application of 1-6-hydrogen atom transfer to create a pyrrolidinone radical, which could then undergo 5-exo cyclisation [115]. Following reaction of 150 with tributyltin hydride and AIBN, the desired 5-5-6-tricycle 151 was isolated in 50% yield as a 7.3:1 mixture of diastereoisomers (Scheme 41). Gratifyingly, the 6-endo product 152 was formed in only 20% yield and no simple reduced product was isolated.
This route allowed a convergent approach to the mitomycins via a tandem radical cyclisation process. It also provided an elegant approach to an intermediate pyrrolidinone radical, which proved impossible to access from a classical halogen-atom transfer route because of the difficulty in preparing the requisite 5-halopyrrolidinone precursor.
5.5. Cha. Dialkoxytitanacyclopropane addition to imides
This methodology provides a very elegant way to install the C9a hydroxyl group, which remains the biggest challenge of mitomycin synthesis. Based on the precedent of ester cyclopropanation in presence of a titanocyclopropane developed by Kulinkovich [116], Cha’s approach to mitomycins involves the intramolecular addition of the same dialkoxytitanacyclopropane to an imide [117]. In contrast to the Kulinkovich’s cyclopropane synthesis, the imide proved to be resistant to cyclopropanation and the titanacycle intermediate 154 reacted further with electrophiles. Application of this methodology to imidostyrene 153 provided the mitomycin B core structure 155 in a reasonable 40% yield (Scheme 42).
Scheme 42: Cha’s mitomycin B core synthesis.
Scheme 42: Cha’s mitomycin B core synthesis.
Although this approach allowed the easy introduction of the C9a hydroxyl functionality, many problems remain unsolved: will the reaction remain efficient with a more functionalized substrate (i.e., an electron rich aromatic, as necessary to introduce the quinone, and substituents at the C1 and C2 position, as needed for the later introduction of the aziridine) and, more importantly, will it be possible to conserve the extremely labile C9a hydroxyl group after removal of the stabilizing carbonyl at C3?
6. The N-aromatic disconnection
6.1. Kishi. Synthesis of mitomycin C
This is the first total synthesis of a mitomycin and it surely constitutes a landmark in the field of organic chemistry. The retrosynthesis is extremely logical in the sense that Kishi noticed that the aminal moiety was the most sensitive part of the molecule [15-17]. Therefore, he introduced it at the very end of the synthesis. To do so, the transannular cyclisation of the methoxy-ketal derivative 156 was very appealing. The requisite eight membered ring was formed by the well-established chemistry of quinones using an intramolecular Michael addition by the primary amine 157 (Scheme 44) [118].
The synthesis began with the known phenol 158 which was reacted with allyl bromide to trigger a Claisen rearrangement (Scheme 45). This installed the three carbon chain on the newly formed para-methoxyphenol which was subsequently oxidized to the quinone then reduced to yield the corresponding para-catechol. This latter compound was protected with benzyl groups. Although this sequence of protecting group interconversion required many steps it was absolutely necessary for the success of the synthesis. Protection with benzyl groups has tremendous advantages in that they are both robust and easily removed under neutral conditions – necessary for removal without damage to the sensitive aziridine and ketal groups later in the synthesis. Moreover, the simultaneous deprotection of both benzyl groups leads to a hydroquinone which is readily oxidized into the quinone by simple exposure to oxygen. On the other hand, the oxidation of a para-methoxyphenol is more perilous since it would involve stronger oxidants that could damage other parts of the molecule. The synthesis followed Scheme 45 with a noteworthy methoxy-ketal formation going through the dithiane 161.
Scheme 45: Kishi synthesis of a starting material. aallyl bromide, K2CO3, acetone, reflux; bN,N-Dimethylaniline, reflux; c70% HNO3/AcOH; dZn, AcOH, 0 °C; eBnBr, K2CO3, DME-DMF, reflux; fH2O2, C6H5CN, K2CO3, MeOH-dioxane; gLDA, MeCN, −30 °C; hCrO3, H2SO4, water-acetone; iMeONa, (CH2O)3, MeOH-THF, 0 °C; jAc2O, Py, 0 °C; kMeSH, BF3 · 2AcOH, −30 °C; lEt3N, MeOH, mNaHCO3, MeOH-DCM; nBnBr, KH, DMF; oHgCl2, MeOH.
Scheme 45: Kishi synthesis of a starting material. aallyl bromide, K2CO3, acetone, reflux; bN,N-Dimethylanilin...
The introduction of the aziridine was elaborated via a diol in the usual way [119]. However, three equivalents of osmium tetroxide and one week of reaction time were needed to get decent yield of the diasteromeric diols 164 and 165 from olefin 163 (Scheme 46). This is probably due to the poor reactivity of the olefin, which is considerably reduced by the inductive effects of the neighbouring ketal and acetate groups [120,121]. The diol 164 was isolated by chromatography and selectively mesylated on hydroxyl 2. Treatment with sodium hydride formed the epoxide. The acetate was removed and the epoxide was opened with lithium azide at 150°C. The two resulting alcohols were mesylated and the primary mesylate displaced with benzylamine. The resulting secondary amine was benzylated to give 166. Reduction of the azide with trimethylphosphite followed by intramolecular SN2 displacement gave aziridine 167. Due to the reactivity of the aziridine in mitomycin C, a special protecting group was used during the synthesis. The aziridine protecting phosphate group in 167 was cleaved using lithium aluminum hydride and replaced by a 3-acetyl propyl group to give 168. This group was removed by hydrolysis, Pfitzner–Moffat oxidation [122] and retro-Michael (steps x, y, z). As discussed before, all the benzyl groups were removed at once to yield compound 169 after exposure to air.
Scheme 46: Kishi synthesis of MMC 7. aLDA, THF, −78 °C then PhSeBr, THF, −78 °C; bH2O2, THF-EtOAc; cDIBAL, DCM-Tol, 0 °C; dNaBH4, MeOH-DCM, 0 °C; eAc2O, Py; fOsO4, Py-THF. gMsCl, Et3N, DCM, 0 °C; hNaH, DMF; iMeONa, MeOH-DCM; jLiN3, DMF, 150 °C; kMs2O, Py; lBnNH2, 150 °C; mBnBr, K2CO3, acetone, reflux; n(MeO)3P, reflux; oNaH, THF; pLAH, Et2O; qCH2=CHCHO; rB2H6, DCM; sAc2O, Pyr; tH2, Pd-C, DCM; uO2, MeOH; vCOCl2, N,N-dimethylaniline, DCM-Tol; wNH3, DCM-Tol, 0 °C; xNaOCH3, MeOH-DCM; yDMSO-DCC, TFA-Py; zHClO4, DCM.
Scheme 46: Kishi synthesis of MMC 7. aLDA, THF, −78 °C then PhSeBr, THF, −78 °C; bH2O2, THF-EtOAc; cDIBAL, DCM...
The key step was the trans-annulation using tetrafluoroboric acid as catalyst. It was expected that the product of this reaction would be fairly stable because of the decreased nucleophilicity of the pyrrole nitrogen in conjugation with the quinone ring. Mitomycin C itself is indeed only moderately reactive with acids before reduction of the quinone [35]. And the other hand, Boruah and Skibo have provided evidence that the loss of the C9a methoxy group to give aziridinomitosene 171 was specifically acid-catalyzed in mitomycin C (Scheme 47) [123], but thankfully the aminal functionality in compound 170 remained stable in the presence of tetrafluoroboric acid.
Scheme 47: Acid catalyzed degradation of MMC 7.
Scheme 47: Acid catalyzed degradation of MMC 7.
The end of the synthesis involved the introduction of the carbamoyl moiety by reaction of the primary alcohol with phosgene then ammonia. The protecting group of the aziridine was then removed to give mitomycin A, which was converted in mytomycin C by treatment with ammonia (Scheme 46). The Kishi synthesis was realized in 44 linear steps with a global yield of 0.16%.
6.2. Kametani
This methodology was investigated by T. Kametani in Japan in the late 1960’s and led to the formation of a derivative of apomitomycin B [124-128]. This compound comes from the metabolic hydrolysis of either mitomycin B, 10, or mitomycin F, 8, by the mechanism shown in Scheme 48 [129].
Scheme 48: In vivo formation of apomitomycin B.
Scheme 48: In vivo formation of apomitomycin B.
This compound has shown anti-tumour and anti-bacterial activities and thus is a valid target for a synthesis [85]. Being completed just after the period culminating in the synthesis of Vitamin B12 by the groups of Woodward and Eschenmoser [130], this synthesis utilized a powerful methodology developed during this period to make vinylogous amides [131,132]. Pyrrolidinethione 176 was reacted with the dibromoester 177 to give the Z isomer of the vinylogous carbamate 178 (Scheme 49). An erosion of the trans relationship between the two substituents of the pyrrolidine was observed giving a 1:1 mixture of cis and trans isomers. An intramolecular Buchwald coupling with copper iodide concluded the formation of the pyrrolo-indole tricycle. During this last step the trans relationship between the substituents of the pyrrolidine was restored to give the more stable isomer 179.
Scheme 49: Advanced intermediate for apomitomycin B synthesis.
Scheme 49: Advanced intermediate for apomitomycin B synthesis.
The quinone ring was then elaborated by aromatic nitration of compound 179, reduction to the aniline and subsequent treatment with Fremy’s salt to give compound 180. No further developments were made on this substrate in part due to the difficulties encountered during reduction of the methyl ester [124]. It is known that the treatment of quinones with mild reducing agents gives hydroquinones while strong reductants modify the quinone in a less specific manner [133,134].
6.3. Remers. Synthesis of a functionalized mitosene
W.A. Remers used a similar approach to build the quinone ring of a 1,2-disubstituted mitosene [135]. The target compound had an arrangement of the C1 and C2 substituents opposite to that found in mitomycin solvolysis products. Unfortunately, these compounds did not show any anti-tumour activity [136]. Starting with the tricyclic ketone 181 [137,138] a low yielding bromination reaction was undertaken followed by an acetate displacement of the resulting α-bromoketone to give 182 (Scheme 50). The amine was introduced by formation of an oxime followed by catalytic hydrogenation in the presence of acetic anhydride to give the cis acetamido-acetate 183.
Scheme 50: Remers synthesis of a functionalized mitosene. aTMSCl, Et3N, ZnCl2 then NBS; bAcOK; cNH2OH; dPd/C, H2, Ac2O; ePOCl3, DMF; f90% HNO3; gFe, AcOH; hFremy’s salt; iNaBH4 then FeCl3; jO=C=NMe, Et3N.
Scheme 50: Remers synthesis of a functionalized mitosene. aTMSCl, Et3N, ZnCl2 then NBS; bAcOK; cNH2OH; dPd/C, H...
The methylene side chain containing the methyl-carbamate was introduced via a Vilsmeier–Haack reaction and the aromatic ring was oxidized with the same method discussed in section 6.2 (vide supra). The synthesis was completed by selective sodium borohydride reduction of the remaining aldehyde, followed by alkylation of the resulting primary alcohol with methyl isocyanate to give 184.
6.4. Coleman. Allylation reaction and 1,4-quinone addition
The Coleman group proposed an elegant synthesis of an enantiomerically pure mitosane. One of the key transformations involved an allylation reaction [139-143] between the allyl stannane 185 and the iminium formed in situ from the enantiomerically pure pyrrolidine 187 (Scheme 51) [144]. The reaction showed good diastereoselectivity, giving a 3:1 ratio of diastereomers favoring the desired isomer. Presumably, allylstannane 185 approached the iminium ion by a synclinal transition state 192 as shown in Scheme 52. The pro-S face of the iminium being less hindered and possessing a lower LUMO energy than the pro-R face, the approach occurred accordingly from the face opposite the alkoxy substituent. When the allylation reaction was done with the iminium derived from pyrrolidine 186, no diastereoselectivity for the formation of 188 was observed. The bulkiness of the benzyl carbamate now proximal to the iminium ion was responsible for this poor result. The pyrrolidine 187 was synthesized from D-ribose 193. Treatment of D-ribose in acetone with allylic alcohol in presence of catalytic amount of sulfuric acid provided the corresponding protected acetonide allyl glycoside. The primary alcohol was transformed into a N-Boc amine by successive Mitsunobu reaction, reduction and acylation to give compound 194. The allyl group was cleaved using nickel chloride and triethylaluminium and fragmentation with iodosobenzene and iodine afforded compound 195. The formyl group was hydrolyzed and the azide was introduced using diphenylphosphoryl azide (DPPA) to give 187.
Scheme 51: Coleman synthesis of desmethoxymitomycin A. aSnCl2, PhSH, Et3N, CH3CN; bClCO2Bn, Et3N; cPPh3, DIAD, THF; dO3; eNaBH4, MeOH; fMe3SiOTf, 2,6-di-tert-butylpyridine, DCM; gClCO2Bn, Et3N; htrichloroacetylisocyanate, DCM; iCAN, CH3CN-H2O.
Scheme 51: Coleman synthesis of desmethoxymitomycin A. aSnCl2, PhSH, Et3N, CH3CN; bClCO2Bn, Et3N; cPPh3, DIAD,...
The azide 189 was then selectively reduced to the corresponding amine with stannous chloride and thiophenol and the aziridine was fashioned by protection of the primary amine followed by an intramolecular Mistunobu reaction. The tetracyclic framework was completed by an intramolecular Michael addition to give desmethoxymitomycin A 191. Although the angular methoxy group at the C9a position is lacking in regards to natural mitomycin A, the authors claim that oxidation of the C9a position might still be possible, in accordance with a precedent in the literature [145].
Scheme 52: Transition state and pyrrolidine synthesis.
Scheme 52: Transition state and pyrrolidine synthesis.
A direct C9a oxidative transformation from desmethoxymitomycin A is an interesting but difficult approach. Previous reports proved that the mitosanes are very sensitive molecules. In his studies directed towards the oxidation of an aziridinomitosane to an aziridinomitosene, Danishefsky found that the mitosane 196 underwent oxidative transformation to mitosene 197 upon simple exposure to silica gel in the presence of air (Scheme 53). On the other hand, attempting the same reaction with the aziridinomitosane 198 met with failure and only non-characterizable products were obtained. The presence of the aziridine was presumed to be responsible for this limitation [146]. The study showed that while mitomycins are stable to these conditions, their aziridinomitosane equivalents are not and proved that the C9a methoxy/hydroxyl group is important for the stability of mitomycins.
Scheme 53: Air oxidation of mitosanes and aziridinomitosanes.
Scheme 53: Air oxidation of mitosanes and aziridinomitosanes.
7. The C9-aromatic disconnection
Scheme 54: The C9-aromatic disconnection.
Scheme 54: The C9-aromatic disconnection.
7.1. Johnston. Enamine conjugate addition
Johnston utilizes the natural negative polarity of the C9 carbon for the construction of an advanced intermediate en route to a mitomycin [147]. A Darzens reaction using the azomethine electrophile 200 provided an easy entry for the construction of the starting cis aziridine 202 which was converted in five steps to the alkynyl amine 203 (Scheme 55).
Scheme 55: Synthesis of the aziridine precursor. aLHMDS, THF; bNaOH; c(s)-α-Me-BnNH2, DCC, HOBT; dDIBAL; eK2CO3, Gilbert’s reagent [148]; fRed-Al, 90 °C.
Scheme 55: Synthesis of the aziridine precursor. aLHMDS, THF; bNaOH; c(s)-α-Me-BnNH2, DCC, HOBT; dDIBAL; eK2CO3...
An aminomercuration of compound 203 with an Hg(II) salt generated the enamine 205 which was reacted in situ with quinone 204. The addition was regioselective for methoxy substitution at the bromomethoxy olefin and gave access to an advanced intermediate lacking only the C10 hydroxymethyl to complete the mitomycin carbon backbone (Scheme 56). However, compound 206 was very unstable with a half-life of 1.5 days at −15 °C.
Scheme 56: Synthesis of 206 via enamine conjugate addition.
Scheme 56: Synthesis of 206 via enamine conjugate addition.
7.2. Rapoport. Intramolecular Heck coupling
In 1983 Rapoport released a fascinating synthesis of aziridinomitosenes based on a photochemical oxidation and an intramolecular Heck reaction [149]. Exposure of the quinone 207 to sunlight triggered the formation of benzoxazole 208, which cleaved to form an intermediate iminium salt. Subsequent proton transfer gave the vinylogous carbamate 209 (Scheme 57). After oxidation of the hydroquinone to the quinone, a palladium catalyzed ring closure afforded the aziridinomitosene 210.
Scheme 57: Rapoport synthesis of an aziridinomitosene.
Scheme 57: Rapoport synthesis of an aziridinomitosene.
A more direct approach involved the addition of unsaturated aziridinopyrrolidine 212 to dibromoquinone 211 followed by cupric bromide catalyzed ring closure (Scheme 58). This one-step strategy unfortunately afforded the unnatural regioisomer 214 as the major product. Because carbon C2 in quinone 211 is more electrophilic than carbon C1, nucleophilic addition-elimination of vinylogous carbamate 212 at the carbon centre gave intermediate 213 which cyclized in situ to give compound 214.
Scheme 58: One pot synthesis of a mitomycin analog.
Scheme 58: One pot synthesis of a mitomycin analog.
7.3. Michael. Intramolecular Heck coupling
Recently the Michael group proposed a formal enantioselective asymmetric synthesis of an aziridinomitosene also based on an intramolecular Heck coupling [150]. They succeeded in incorporating all the reactive functionalities, namely the quinone, the carbamate and the aziridine and thus bypassed the challenging reduction of the robust C9 ester (found, for instance, in the Rapoport synthesis). Coupling of bromo aniline 215 with lactone 220 formed the lactam 216 in three steps with 90% yield (Scheme 59). This latter compound was transformed into the vinylogous carbamate 217 using a Reformatsky addition to the corresponding thiolactam. The crucial intramolecular Heck cyclisation was carried out with palladium acetate, tri-o-tolylphosphine and triethylamine to give 218 in 82% yield.
Scheme 59: Synthesis of compound 218 via intramolecular Heck coupling. aEtMgCl, THF, then 220; bMsCl, Et3N; cNaH, THF-DMF; dLawesson’s reagent, toluene; eBrCH2CO2Et, Zn, I2.
Scheme 59: Synthesis of compound 218 via intramolecular Heck coupling. aEtMgCl, THF, then 220; bMsCl, Et3N; cN...
It was discovered that transformation of compound 218 into an aziridinomitosane was unexpectedly difficult. The most successful approach involved deprotection of the benzyl ether to generate the corresponding phenol 219 followed by reduction of the ester with lithium aluminium hydride and direct oxidation of the phenol to the quinone 221 with molecular oxygen and a catalytic amount of salcomine in an overall 30% yield over the three steps (Scheme 60). However many problems arose during this process, including over-reduction of the ester to the alkane 224 and the oxidation of the alcohol to the aldehyde 225 during the quinone oxidation step.
Scheme 60: Elaboration of indole 223. aEt3N, Ac2O; bAcOH; cSOCl2, Et3N; dNaN3, DMF; eH2SO4, THF; fK2CO3, MeOH; gPhOCOCl, pyridine; hMsCl, Et3N.
Scheme 60: Elaboration of indole 223. aEt3N, Ac2O; bAcOH; cSOCl2, Et3N; dNaN3, DMF; eH2SO4, THF; fK2CO3, MeOH; ...
The synthesis was completed by protection of the primary alcohol with an acetyl group and by deprotection of the ketal with acetic acid. The resultant diol was reacted with thionyl chloride and the corresponding sulfite 222 was opened with sodium azide; oxidation to the more reactive sulfate was not necessary. The final sequence involved deprotection of the acetate followed by acylation of the more reactive primary alcohol with phenyl chloroformate and subsequent mesylation of the secondary alcohol to give 223, whose spectroscopic data agreed with those reported by Jimenez [151].
8. From indoles
Scheme 61: C9-C9a functionalization from indole.
Scheme 61: C9-C9a functionalization from indole.
8.1. Jimenez. Synthesis of mitomycin K
The idea of converting an indole to a mitomycin is appealing since it simplifies considerably the retrosynthetic pathway. Using this idea, Jimenez was able to affect the direct oxidation of indole 226 with (hexamethylphosphoramido)oxodiperoxomolybdenum (VI) (MoO5 • HMPA) to give diastereomers 227a and 227b, which were readily elaborated into mitomycin K (Scheme 62) [20,152].
Scheme 62: Synthesis of mitomycin K. a2 equiv. MoO5.HMPA, MeOH; bPPh3, Et3N, THF-H2O; cMeOTf, Py, DCM; dMe3SiCH2Li, THF; ePCC.
Scheme 62: Synthesis of mitomycin K. a2 equiv. MoO5.HMPA, MeOH; bPPh3, Et3N, THF-H2O; cMeOTf, Py, DCM; dMe3SiCH...
The critical indole oxidation step gave a selectivity of about 2:1 in favor of the undesired isomer 227b (β−OMe) but fortunately this compound could be epimerized at the C9a carbon using 0.2M HCl in MeOH to give a 1:1 ratio of the 2 diastereoisomers 227a and 227b. The Danishefsky group had observed an extraordinary configurational stability of the C9a carbon in epi-mitomycin K [146]. Epi-mitomycin K 229 or its demethylated derivative 228 treated under various acidic or basic conditions were constantly recovered intact (Scheme 63). They rationalized this phenomenon by the incapacity of those compounds to open to the carbinolamine intermediate 230 in contrary to what was observed for the mitomycin B series (Scheme 3) [27].
Scheme 63: Configurational stability of mitomycin K derivatives.
Scheme 63: Configurational stability of mitomycin K derivatives.
For compound 227b, we can infer that the aromatic nitrogen receives significant enhancement of its nucleophilic character compared to compound 229 because of the presence of the TBS protected hydroquinone. Consequently, the formation of the reactive iminium species 232 under acid catalyzed conditions can lead to epimerization of the C9a centre (Scheme 64).
Scheme 64: Epimerization of carbon C9a in compound 227b.
Scheme 64: Epimerization of carbon C9a in compound 227b.
The overall yield for the 13 step synthesis was 1.4%. The elegant synthesis of the starting material 235 used an intramolecular Corey–Chaykovsky reaction. The anion of indole 233 was reacted with dimethylvinylsulfonium iodide to give the transient epoxide 234 which was opened in situ by the addition of sodium azide to give azido-alcohol 235 (Scheme 65).
Scheme 65: Corey–Chaykovsky synthesis of indol 235.
Scheme 65: Corey–Chaykovsky synthesis of indol 235.
Before this work, Cory used a similar strategy involving an intramolecular aza-Darzens reaction to give directly the aziridinomitosene analogue 239 (Scheme 66) [153]. Treatment of 2-(N-phenylformimidoyl)indole 236 with sodium hydride followed by addition of methyl 2-bromopropenoate gave the aziridinomitosene 239. The reaction presumably proceeded through intermediates 237 and 238.
Scheme 66: Cory intramolecular aza-Darzens reaction for the formation of aziridinomitosene 239.
Scheme 66: Cory intramolecular aza-Darzens reaction for the formation of aziridinomitosene 239.
8.2. Jimenez. Synthesis of an aziridinomitosene
A few years later, Jimenez and co-workers were able to transform the azido mesylate indole 226 into a fully functionalized mitosene [151]. Formylation of the mitosene 226 using a Vilsmeyer–Haack reaction followed by oxidative cleavage of the TBS groups with PCC gave the quinone 240 in 80% yield (Scheme 67). Reduction of the aldehyde with sodium borohydride followed by reoxidation of the hydroquinone by bubbling oxygen into the reaction mixture provided the alcohol 241 in 74% yield. This latter material was transformed to the aziridinomitosene 242 in a three step sequence in 53% yield.
Scheme 67: Jimenez synthesis of aziridinomitosene 242.
Scheme 67: Jimenez synthesis of aziridinomitosene 242.
The synthesis of the fully functionalized aziridinomitosene 242 was accomplished in 16 steps from 2,5-dimethylanisole in 3.4% overall yield.
8.3. Kametani. Von Braun opening of indoline
Kametani found another alternative for the elaboration of an advanced intermediate for the synthesis of mitomycins using indoles as starting materials [154,155]. Pyrrolo[1,2-a]indole 243 was reduced with sodium borohydride in acetic acid [156]. The resulting indoline 244 was opened using a Von Braun reaction with cyanogen bromide to give 245. The direct Kornblum oxidation [157] of this compound with dimethylsulfoxide and sodium bicarbonate at 150 °C gave only a few percent yield of the desired ketone. Therefore, the authors turned to a three step synthesis involving elimination, epoxidation and epoxide rearrangement mediated by boron trifluoride to give the desired benzazocin-5-one 246 [158,159] (Scheme 68). The transannular cyclisation of an eight membered ring ketone such as 246 seems a promising approach considering the Kishi’s successful synthesis (see above). The access of such structures is usually difficult, often requiring a long and poor yielding synthesis. The benefit of this method is that the pyrrolo[1,2-a]indoles 243 are easily available [160-162] and thus can be regarded as good precursors.
Scheme 68: Von Braun opening of indoline 244.
Scheme 68: Von Braun opening of indoline 244.
Although the overall yield of this process is not very high, it offers the possibility of accessing the benzazocinone substructures very quickly.
8.4. Danishefsky. Oxidation of a leucoaziridinomitosane
Danishefsky and co-workers were very active in the field of the mitomycins and discovered an effective way to oxidize leucoaziridinomitosane 247 to leucoaziridinomitosene 248 using DDQ (Scheme 69) [146]. The unusual stability of this leucoaziridinomitosene certainly accrues from the C10 aldehyde which attenuates the nucleophilicity of the indolic nitrogen. Based on precedents [163,164], osmylation of the indole with a large excess of osmium tetroxide in pyridine over a few days gave the deformylated product 250 where the hydroxyl group arose cis to the aziridine. This result was surprising since the osmylation was expected to occur on the convex face of the molecule (i.e., anti to the aziridine). The authors claimed that the stereochemical outcome of the reaction was directed by the nitrogen of the aziridine, which, they reasoned, should be a better ligand for the osmium reagent than the vinylogous amide nitrogen of the indole.
Scheme 69: C9a oxidation of an aziridinomitosane with DDQ/OsO4.
Scheme 69: C9a oxidation of an aziridinomitosane with DDQ/OsO4.
The synthesis of epi-mitomycin K was completed by methylation of the tertiary alcohol 250, conversion of the benzyl protecting group on the phenol to a triethylsilyl group, Peterson olefination, deprotection of the triethylsilyl group and oxidation to quinone 229 (Scheme 70). This unnatural mitomycin possessed the opposite C9a configuration compared to mitomycin K and isomerisation by basic or acidic treatment was ineffective (Scheme 63).
Scheme 70: Synthesis of epi-mitomycin K. aNaH, Me2SO4; bH2, Pd/C; cMitscher reagent [165]; d[(trimethylsilyl)methyl]lithium; eTBAF; fDDQ.
Scheme 70: Synthesis of epi-mitomycin K. aNaH, Me2SO4; bH2, Pd/C; cMitscher reagent [165]; d[(trimethylsilyl)methyl...
9. Miscellaneous
9.1. Fukuyama. Synthesis of mitomycin C via the mitomycin rearrangement
Fukuyama’s approach to the mitomycins took into account the fact that these molecules can rearrange to give isomeric compounds called “isomitomycins” [26]. Mitomycin C can therefore equilibrate with “isomitomycin C” 253, going through an isolable species 252 called “albomitomycin C” (Scheme 71). This transformation, called the “mitomycins rearrangement,” seemingly occurrs via a Michael, retro-Michael mechanism. The equilibrium favors the mitomycin form.
This discovery led to an innovative strategy for the synthesis of mitomycin C [18,166]. There are tremendous advantages to using isomitomycin as a target since the elimination of methanol at C9a, the most problematic issue in mitomycin synthesis, is no longer a threat in isomitomycin because of its bridgehead position. Having isomitomycin A as a target, the corresponding tetracyclic structure was constructed by an intramolecular cycloaddition of the azide of compound 255 with the olefin of the five-membered lactone. Compound 255 was easily prepared via a Mukaiyama reaction of the silylenol ether 257 with the readily available chalcone 256 in 95% yield (Scheme 72). Although this reaction was racemic, other asymmetric versions of this reaction were recently studied, [167-171] but further attempts to achieve asymmetric synthesis proved unsuccessful using this route. Also, questions arose as to whether compound 255 was formed through a Mukaiyama reaction or through a Lewis acid (stannyl chloride) promoted Diels–Alder reaction favoring the endo addition. No direct evidence was produced to distinguish between the two reactions but the unusually high stereoselectivity observed might support the Diels–Alder reaction mode.
Heating compound 255 in toluene triggered a [2+3] cycloaddition between the azide and the lactone olefin. The intermediate triazoline was not observed and the aziridine was directly obtained in 86% yield (Scheme 73). A subsequent reduction of the lactone with DIBAL gave the corresponding lactol, which was protected with an acetyl group to give 258.
Scheme 73: [2+3] Cycloaddition en route to isomitomycin A. aToluene, 110 °C; bDIBAL, THF, −78 °C; cAc2O, Py.; dRuO2, NaIO4, EtOAc/H2O (1/1); eNaBH4, MeOH; fCCl3CONCO, DCM.
Scheme 73: [2+3] Cycloaddition en route to isomitomycin A. aToluene, 110 °C; bDIBAL, THF, −78 °C; cAc2O, Py.; d...
The silyl enol ether of 258 was then cleaved using ruthenium tetroxide generated in situ by the Sharpless methodology [172]. Although the aldehyde formed during the course of this reaction is usually over-oxidized into a carboxylic acid, in this specific case it seemed that the steric hindrance surrounding the aldehyde prevented the formation of the gem-diol mandatory for further oxidation. Nonetheless, the thiol was oxidized into the corresponding sulfone.
The aldehyde was then reduced and transformed into the trichloroacetyl carbamate 259 by treatment with trichloroacetyl isocyanate. Subsequent treatment with ammonia triggered a series of transformations that led to compound 261 (Scheme 74). Under these conditions, the trichloroacetyl carbamate was cleaved as well as the acetate, which furnished 260 after loss of a molecule of ethane sulfinic acid. The formation of 261 occured most likely by addition of a molecule of methanol to the corresponding imine. Compound 261 was not isolated because of stability issues but was directly reduced with sodium borohydride to give 262. The bridged hemiaminal of 262 was not reduced because it would involve the formation of an extremely strained bridgehead double bond. Accordingly, the authors used a strong acid (camphorsulfonic acid, CSA) in methanol to enable iminium formation and subsequent transformation to the methoxy aminal 263. The aromatic ring was then oxidized into the corresponding quinone by hydrogenolysis of the benzyl group followed by DDQ oxidation to give isomitomycin A, 254. A final treatment with ammonia gave mitomycin C 7.
Scheme 74: Final steps of Fukuyama’s synthesis.
Scheme 74: Final steps of Fukuyama’s synthesis.
Ten years after the Kishi synthesis, Fukuyama realized the second and last racemic total synthesis of mitomycin C with an overall yield of 10%.
9.2. Ban–Shibasaki. “Crisscross” annulation
The rapid synthesis of medium-sized heterocycles has been the main research focus of Y. Ban since the beginning of the 1980’s. The “crisscross annulation” involves intramolecular nucleophilic addition of the nitrogen in β-diketone 264 followed by a retro-aldol ring opening to give keto-amide 266 before a final transannular cyclisation to give the “crisscrossed” product 267 in a one pot process (Scheme 75) [173,174].
This scheme was successfully employed by the same group for the synthesis of a decarbamoyloxymitomycin derivative [175,176]. Compound 271 was obtained readily from commercially available 2-amino-5-nitrotoluene 269 in 94% overall yield. An electrochemical removal of the tosyl group triggered the crisscross annulation cascade reaction to afford 272 (Scheme 76). A long sequence of reactions then allowed the oxidation of the aromatic ring into the desired quinone: first, a selective deprotection of the benzyl aniline with the Pearlman catalyst left the less reactive benzylamide untouched [177]. The resultant primary aniline was then oxidized using Pb(OAc)4 to give the corresponding o-quinone imide. Hydrolysis of this crude material with perchloric acid followed by hydrogenation provided the corresponding ortho-hydroquinone before 1,3-dioxolane protection of the ketone gave compound 273 in 48% overall yield. Interestingly, this compound appeared as a pair of conformational diastereoisomers, 273a and 273b, that could be separated by chromatography (Scheme 77). The free energy of activation for interconversion was estimated to be as high as 25 kcal · mol−1 at 25 °C.
Scheme 76: Synthesis of 274; the 8-membered ring 274 was made using a crisscross annulation. a20% Pd(OH)2/C, H2, AcOEt; bPb(OAc)4, DCM; cHClO4, THF, DCM; d10% Pd/C, H2, AcOEt; eTMSCl, HOCH2CH2OH, MeOH, DCM; fMe2SO4, K2CO3, CHCl3; gLAH, THF; h20% Pd(OH)2/C, H2, MeOH; ITsCl, Py, DCM; jSalcomine/O2, DMF.
Scheme 76: Synthesis of 274; the 8-membered ring 274 was made using a crisscross annulation. a20% Pd(OH)2/C, H2...
Detailed study by NMR and X-ray crystallography showed that both of the two compounds 273a and 273b adopt a twist-boat-chair conformation (usually more energetic than the boat-boat conformation) in which the C6 methyl group is either in a pseudoequatorial (273a) or pseudoaxial position (273b) [178]. Further studies aimed at converting one isomer into the other showed that 273a was, as expected, the thermodynamically favoured compound (273b converts to 273a after refluxing in benzene for 2 days). More interestingly, when a mixture of di-benzylated derivatives 275a and 275b were left at room temperature, isomer 275a slowly isomerizes to 275b. This special feature was attributed to the severe steric interactions between the C8 benzyloxy and C9 methyl groups in 275a.
Scheme 77: Conformational analysis of compound 273 and 275.
Scheme 77: Conformational analysis of compound 273 and 275.
The catechol 273 was then turned into the desired quinone 274 by selective methylation of the phenol at C7 (the phenol at C8 being hindered by the C9 methyl group), reduction of the lactam with lithium aluminium hydride, and hydrogenolysis to give an unstable aminophenol which was directly tosylated. Finally, oxidation with bis(salicylidene)ethylenediiminocobalt(II) (salcomine) [179,180] provided the p-quinone 274 in 94% yield. The key step towards the elaboration of a mitomycin was the introduction of the aziridine at the benzazocenol stage, namely on the allylic alcohol 276.
To this end, quinone 274 was reduced with sodium thiosulfate to the corresponding hydroquinone and bis-protected with benzyl bromide (Scheme 78). The ketal group was cleaved with concentrated hydrochloric acid before a selenium oxidation gave the corresponding benzazocenone. This sequence proceeded in a good overall yield of 65% and, surprisingly, the addition of PhSeCl in acidic medium occurred selectively on the less hindered alpha carbon of the benzazocin ketone. The authors did not comment on this unusual selectivity but one can imagine that the formation of an enol on the C9 side of the molecule would greatly enhance the steric effects between the C9 methyl group and the C8 benzyloxy group since the carbons C8, C8a, C9 and the methyl would become coplanar. A stereoselective 1,2 reduction of the enone with DIBAL gave the allylic alcohol 276. All attempts to introduce the aziridine via the typical epoxidation or dihydroxylation and displacement with sodium azide failed. Therefore an intramolecular sequence was pursued. The allylic alcohol 276 was converted to the corresponding allylic carbamate by treatment with tosyl isocyanate. Activation of the olefin with iodine provided the cyclic carbamate 277 [181]. Hydrolysis of the carbamate using potassium carbonate in methanol was readily achieved due to the presence of an electron withdrawing tosyl group on the nitrogen. This process released a free sulfonamide that cyclized in situ by SN2 displacement to give the aziridine with complete stereoselectivity. The homobenzylic alcohol was then oxidized using PCC to give compound 278.
Scheme 78: Synthesis of a mitomycin analog. aNa2S2O4, H2O, DCM; bBnBr (10 equiv), K2CO3, 18-crown-6 (cat.), THF, reflux; cconc. HCI, THF, 0 °C; dPhSeCl, 10% HCl (cat.), AcOEt; eNaIO4, H2O, THF; fDIBAL, THF, −70 °C; gTs-N=C=O, THF; hI2, K2CO3, THF; iK2CO3, MeOH-DCM (1/2); jPCC (33 equiv.), DCM; kTBSOTf (2 equiv), NEt3, DCM, −78 °C; l10% Pd/C, H2, NEt3, AcOEt; then O2; mNa-naphthalene, THF, −98 °C; then O2; nTBAF, AcOH, THF.
Scheme 78: Synthesis of a mitomycin analog. aNa2S2O4, H2O, DCM; bBnBr (10 equiv), K2CO3, 18-crown-6 (cat.), TH...
Having the ketone 278 in hand, the authors’ next step was to affect the trans-annular cyclisation to construct the mitomycin skeleton. Whether or not this transformation should occur at the stage of the protected hydroquinone or at the quinone stage frequently arises in the debate about the stability of leucoaziridinomitosenes. Although the more reasonable pathway would be the transformation at the quinone stage following the Kishi procedure, the design of the synthesis and especially the presence of a tosyl group on the aniline prevented such a scheme. The removal of the tosyl group at the quinone stage would indeed most likely interfere with the other functionalities present in the molecule. Therefore, the trans-annular cyclisation was attempted at the stage of the protected hydroquinone.
Ketone 278 displayed a rather low IR absorption at 1700 cm−1, indicating the presence of a transannular effect with the N4 nitrogen. Therefore, an increase of the electrophilic character of the ketone would increase this transannular interaction until it reached a point where a new bond could be formed, causing loss of the tosyl group. The use of TBSOTf effectively activated the ketone towards this cyclisation to give 279 in a highly stereoselective manner. It is noteworthy that the conditions used in this reaction were mild enough for the leucoaziridinomitosane structure to be unaffected. The same reaction using TMSOTf also gave the cyclised product, but led to decomposition during the isolation process. Hydrogenolysis of 279, followed by treatment with oxygen afforded the corresponding quinone, which was subsequently treated with sodium-naphthalene to remove the tosyl group on the aziridine in low yield (16%). The removal of the tosyl group at the quinone stage was probably the biggest limitation of the synthesis since one-electron reducing agents were shown to generate a reactive species that suffered elimination of the C9a heteroatom functionality [43], which could explain the low yield of this reaction. The final compound 280 was obtained by deprotection of the tertiary alcohol by treatment with TBAF and acetic acid.
9.3. Vedejs. Intramolecular [3+2] cycloaddition
Vedejs developed a very innovative way to construct mitosenes, as he departed from the usual practice of forming the quinone by oxidation of an aromatic, as had been done in all previous syntheses [182,183]. This is surely an advantage since it allows manipulation of the labile leucoaziridinomitosenes at advanced synthetic stages to be avoided. His approach uses an intramolecular [3+2] cycloaddition between an azomethine ylide and an alkyne to assemble the tetracyclic aziridinomitosene core. The sequence began with conversion of oxazole 283 into the azomethine ylide 282 (Scheme 79).
The azomethine ylide was prepared in 12 steps starting from the lithiated oxazole 284. After reaction with the protected serinal 285, a Mitsunobu reaction was used to install the aziridine (Scheme 80). The trityl protecting group was replaced with a methyl, and the O-allyl was removed using a low valent zirconium species generated in situ [184]. A few manipulations led to oxazole 283 which cyclized to 288 in the presence of silver triflate. The resulting salt was treated with a soluble source of cyanide (benzyltrimethylammonium cyanide, BnMe3NCN) which triggered the opening of the oxazole and the formation of the azomethine ylide 282, probably through intermediate 289. This compound reacted with the internal alkyne to give 281, the tetracyclic core of the mitomycins.
Scheme 80: Formation of the azomethine ylide.
Scheme 80: Formation of the azomethine ylide.
This route was suitable for the synthesis of aziridinomitosenes lacking the C7 methoxy group. Continuing efforts by the Vedejs group made possible the extension of this methodology to allow access to the fully functionalized quinone core found in mitomycin A [185]. A Negishi coupling of compound 290 with vinyl triflate 291 generated the oxazole 292 which was transformed in four steps into the alkyne 293 (Scheme 81). A similar activation cascade leading to the dipolar cycloaddition produced compound 294 after oxidation of the intermediate with a mixture of HF/pyridine/O2. A fair three-step yield of 25% was obtained, considering the dramatic changes in structure. The main isolated impurity of this process was compound 295, arising from oxidation of the intermediate without elimination of hydrogen cyanide.
Scheme 81: Vedejs second synthesis of an aziridinomitosene. aDIBAL; bTPAP, NMO; c287; dTBSCl, imidazole.
Scheme 81: Vedejs second synthesis of an aziridinomitosene. aDIBAL; bTPAP, NMO; c287; dTBSCl, imidazole.
Having compound 294 in hand, the target aziridinomitosene A could potentially be obtained by installation of the carbamate on the primary alcohol and removal of the trityl protecting group. In the hope that the vinylogous amide in 298 would behave similarly to the vinylogous carbamate in compound 296 that was successfully detritylated using methanesulfonic acid and triethylsilane, the same conditions were employed on compound 298; unfortunately, only solvolysis product 299 was isolated (Scheme 82). Manifestly, the carbonyl of the quinone in 298 does not provide the same deactivating effect on the indole nitrogen as the carbonyl of the ester in 296, making the aziridine of compound 298 more labile. Efforts to find a suitable protecting group for this reactive aziridine that could survive all the previous steps in the synthesis but, more importantly, could easily be removed at a late stage using non-acidic conditions led to the identification of the bulky silyl group 300 [186].
Scheme 82: Trityl deprotection and new aziridine protecting group 300.
Scheme 82: Trityl deprotection and new aziridine protecting group 300.
9.4. Ciufolini. Homo-Brook rearrangement
Recently Ciufolini reported a highly innovative and sophisticated retrosynthetic analysis for the construction of benzazocenols 306 which made possible the synthesis of FR-66979 [187]. Early studies aimed at the rapid synthesis of benzazacinones of type 305 identified the sequence depicted in Scheme 83 as a very efficient strategy. However, benzazocinone 305 could not be converted into the desired benzazocenol 306 which meant a dead end for the synthesis [188,189].
Scheme 83: Ene reaction towards benzazocinones.
Scheme 83: Ene reaction towards benzazocinones.
A thorough analysis of the molecule suggested that an even more concise avenue to benzazocenols 306 might be possible by fragmentation of a silylated aziridine, followed by homo-Brook rearrangement [190]. The synthesis started by the addition of organometallic species 308, developed by Reetz [191], to the azidoaldehyde 307, which was followed by thermal azide cycloaddition and photochemical nitrogen extrusion to form the aziridine 310 (Scheme 84). Treatment of this compound with tetrabutylammonium hydroxide triggered a homo-Brook rearrangement, giving benzazocenol 313 in only four steps from aldehyde 307.
Scheme 84: Benzazocenols via homo-Brook rearrangement.
Scheme 84: Benzazocenols via homo-Brook rearrangement.
This represents one of the most efficient sequences for the assembly of benzazocenols, even compared with those involving olefin metathesis. The synthesis of mitomycins using this methodology is currently being investigated.
9.5. Iwasawa. Pt(II) or Au(II) catalyzed [3+2] cycloaddition of azomethine ylides
In 2006, this group disclosed the use of third row transition metals to catalyze efficiently the one-pot formation of pyrroloindoles starting from monocyclic materials. A number of transition metals have emerged as efficient catalysts for the electrophilic activation of alkynes in the past decades [192], and this group exploited such methodology by activation of the internal alkyne moiety in 314 to induce nucleophilic attack of the imino nitrogen, generating the corresponding metal-containing azomethine ylide 315 via 5-endo cyclisation (Scheme 85). Successive [3+2] cycloaddition and 1,2-alkyl migration gave the mitosene 317, bearing a silyloxymethyl group in the C9 position, in 73% yield.
Scheme 85: Pt-catalyzed [3+2] cycloaddition.
Scheme 85: Pt-catalyzed [3+2] cycloaddition.
This sequence appears to be a very efficient way to generate mitosenes but many questions remain unsolved for the possible synthesis of mitomycins: is it possible to find an alkyl group at the imine of the starting material that can be easily removed at the end of the reaction? How to affect oxidation at the C2 position for the later introduction of the aziridine? Will the reaction work with the electron rich aromatic needed for conversion to the quinone?
9.6. Trost. Pd-catalyzed carbonylative lactamization
Although this study was mainly directed towards the synthesis of FR-900482, it provides an easy entry to enantiopure benzazocenol structures. Based on Carreira’s asymmetric acetylide addition to aldehydes in the presence of zinc (II) triflate and (+)-N-methylephedrine [193], the addition of trimethylsilyl acetylene into aldehyde 318 gave the corresponding propargylic alcohol with very high ee. Subsequent transformations led to the (Z) olefin 319 which cyclised in presence of palladium and carbon monoxide to give the eight membered lactam 320 (Scheme 86). The lactam was reduced with borane-methyl sulfide complex without competing with 1,4-reduction or cleavage of the N–O bond to provide the benzazocenol core found in FR-900482.
Scheme 86: Carbonylative lactamization entry to benzazocenols. aZn(OTf)2, (+)-N-methylephedrine, Et3N, TMS-acetylene; bDIBAL; cK2CO3, MeOH; dNaH, BnBr; eNIS, 10% AgNO3; fdipotassium azodicarboxylate, pyridine, AcOH, MeOH; gSmI2; hTBDPSCl, imidazole.
Scheme 86: Carbonylative lactamization entry to benzazocenols. aZn(OTf)2, (+)-N-methylephedrine, Et3N, TMS-ace...
This synthetic sequence seems suitable for the synthesis of both FR-900482 and the mitomycins, although difficulties may arise from the introduction of the hydroxymethyl side-chain on C9 in the mitomycin series as previously experienced by Williams (see paragraph 3.3).
9.7. Metathesis
Olefin metathesis has been known since the 1960’s, but it was not until the early 1990’s that this transformation became an important tool in synthetic organic chemistry. Since then a number of elegant applications of RCM (ring closure metathesis) in the total synthesis of heterocycles have been recorded [194].
Miller’s approach
The construction of a benzazocenone of type 326 could provide access to mitomycin derivatives. Miller’s group was interested in this approach for the synthesis of mitosene analogs [195]. The alcohol 322 was easily elaborated into benzazocenone 326 through a metathesis reaction using Grubbs II catalyst 324 (Scheme 87). Oxidation to the enone allowed introduction of the aziridine by enantioselective peptide-mediated conjugate addition of azide [196]. However this reaction proved to be inefficient, as enantiomeric excesses of no more than 35% were recorded for compound 327. In general, enone 326 proved to be sluggish towards conjugate additions. This comes from the fact that the carbonyl group and the olefin are twisted 100.4° out of conjugation.
Scheme 87: 8 membered ring formation by RCM. aBOC2O, NaHCO3; bTBSCl, Imidazole, DMF; callyl bromide, NaH, DMF; dPPTS, MeOH; e(COCl)2, DMSO, Et3N, then vinyl magnesium bromide, −78 °C; fTBSCl, imidazole, DMF.
Scheme 87: 8 membered ring formation by RCM. aBOC2O, NaHCO3; bTBSCl, Imidazole, DMF; callyl bromide, NaH, DMF; ...
Regardless, synthesis of the mitosene core could be achieved by transannular cyclisation, formylation/oxidation, nitration, bromination and aziridine formation to give 329 (Scheme 88).
Scheme 88: Aziridinomitosene synthesis. aTMSN3; bTFA; cPOCl3, DMF; dNaClO2, NaH2PO4, 2-methyl-2-butene; eMeI, Hünig’s base; fCu(NO3)2, Ac2O; gNBS; hPPh3, H2O-THF.
Scheme 88: Aziridinomitosene synthesis. aTMSN3; bTFA; cPOCl3, DMF; dNaClO2, NaH2PO4, 2-methyl-2-butene; eMeI, ...
Pérez–Castells’ approach
The syntheses of pyrrolo-indoles has been attempted using many different methodologies, but Castells was the first to report the use of RCM starting from an indole [197]. Readily available aldehyde 330 was subjected to an efficient cascade Wittig-metathesis reaction to give compound 331, where the double bond emerging from the metathesis reaction had shifted towards the indole nitrogen (Scheme 89).
Unfortunately, this migration moderated the utility of this methodology since it compromised efficient introduction of the aziridine at the C1–C2 position.
9.8. Williams biosynthetic approach
In section 2.1 of this review, we described the two building blocks AHBA and D-glucosamine involved in the biosyntheses of mitomycins. Although a large amount of information was gathered on the genes and enzymes involved in the biosynthetic process, speculative assertions have arisen on how those two simple building blocks were assembled into the complex architectures of mitomycins [28]. In a recent paper, the Williams group described the synthesis of potential early biosynthetic intermediates to the mitomycins [198]. The compounds 332a and 332c were obtained as the β-anomer by condensation of AHBA with protected D-glucosamine 21a and 21c respectively (Scheme 90). The N-Cbz protected amine 332a was unmasked using catalytic hydrogenolysis and the carboxylic acid was transformed to the N-acetylcysteamine (SNAC) thioester analog 333a. The SNAC derivative of compound 332c was obtained in a synthetically useful yield using the same procedure. The other putative biosynthetic intermediate equivalent 334b was synthesized by reductive amination of AHBA hydrochloride with 21b, followed by SNAC derivatization and unmasking the N-Boc protected amine with trifluoroacetic acid. Intermediates 332a, 332c, 333c, 334b could give valuable information about the biosynthesis pathway of mitomycins and provide fundamental understanding of biosynthetic enzymes implicated in the pathway. The success of combinatorial biosynthesis often depends on this later factor and the availability of the genetic information [199]. Many of the genes responsible for the biosynthesis of mitomycins being already identified, better drugs could be synthesized in the future through genetic engineering [200].
Scheme 90: Synthesis of early biosynthetic intermediates of mitomycins.
Scheme 90: Synthesis of early biosynthetic intermediates of mitomycins.
Conclusion
Even 50 years after their discovery and 30 years after first submitting to total synthesis, the mitomycins are still regarded as challenging synthetic targets, and a practical synthetic route has yet to be discovered. Because mitomycin C is a valuable anti-cancer drug, thorough medicinal chemistry investigation, which would be abetted by the knowledge and material generated during a total synthesis, is still required. Such knowledge may well lead to new anti-cancer agents with a broad spectrum and potent activity.
References
-
Kudo, S.; Marumo, T.; Tomioka, T.; Kato, H.; Fujimoto, Y. Antibiot. Chemother. 1958, 8, 228.
Return to citation in text: [1] [2] -
Webb, J. S.; Cosalich, D. B.; Mowat, J. H.; Patrick, J. B.; Broschard, R. W.; Meyor, W. E.; Williams, R. P.; Wolf, C. F.; Fulmor, W.; Pidacks, C.; Lancaster, J. E. J. Am. Chem. Soc. 1962, 84, 3185–3186. doi:10.1021/ja00875a032
Return to citation in text: [1] -
Yudin, A. K. Aziridines and Epoxides in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006. doi:10.1002/3527607862
Return to citation in text: [1] [2] -
Hata, T.; Koga, F.; Sano, Y.; Kanamori, K.; Matsumae, A.; Sugawara, R.; Hoshi, T.; Shimi, T.; Ito, S.; Tomizawa, S. J. Antibiot. 1954, 7, 107–112.
Return to citation in text: [1] -
Nagaoka, K.; Matsumoto, M.; Oono, J.; Yokoi, K.; Ishizeki, S.; Nakashima, T. J. Antibiot. 1986, 39, 1527–1532.
Return to citation in text: [1] -
Kiyoto, S.; Shibata, T.; Yamashita, M.; Komori, T.; Okuhara, M.; Terano, H.; Kohsaka, M.; Aoki, H.; Imanaka, H. J. J. Antibiot. 1987, 40, 594–599.
Return to citation in text: [1] [2] -
Hanada, M.; Ohkuma, H.; Yonemoto, T.; Tomita, K.; Ohbayashi, M.; Kamei, H.; Miyaki, T.; Konishi, M.; Kawaguchi, H.; Forenza, S. J. Antibiot. 1991, 44, 403–414.
Return to citation in text: [1] -
Tsuchida, T.; Inuma, H.; Kinoshita, N.; Ikeda, T.; Sawa, T.; Hamada, M.; Takeuchi, T. J. Antibiot. 1995, 48, 217–221.
Return to citation in text: [1] -
Wolkenberg, S. E.; Boger, D. L. Chem. Rev. 2002, 102, 2477–2496. doi:10.1021/cr010046q
Return to citation in text: [1] [2] [3] -
Coleman, R. S.; Burk, C. H.; Navarro, A.; Brueggemeier, R. W.; Diaz-Cruz, E. S. Org. Lett. 2002, 4, 3545–3548. doi:10.1021/ol0267275
Return to citation in text: [1] -
Alcaro, S.; Ortuso, F.; Coleman, R. S. J. Med. Chem. 2002, 45, 861–870. doi:10.1021/jm011040w
Return to citation in text: [1] -
Zein, N.; Solomon, W.; Colson, K. L.; Schroeder, D. R. Biochemistry 1995, 34, 11591–11597. doi:10.1021/bi00036a035
Return to citation in text: [1] -
Casely-Hayford, M. A.; Pors, K.; James, C. H.; Patterson, L. H.; Hartley, J. A.; Searcey, M. Org. Biomol. Chem. 2005, 3, 3585–3589. doi:10.1039/b508908e
Return to citation in text: [1] -
Naoe, Y.; Inami, M.; Matsumoto, S.; Nishigaki, F.; Tsujimoto, S.; Kawamura, I.; Miyayasu, K.; Manda, T.; Shimomura, K. Cancer Chemother. Pharmacol. 1998, 4, 31–36. doi:10.1007/s002800050781
Return to citation in text: [1] -
Kishi, Y. J. Nat. Prod. 1979, 42, 549–568. doi:10.1021/np50006a001
Return to citation in text: [1] [2] -
Nakatsubo, F.; Fukuyama, T.; Cocuzza, A. J.; Kishi, Y. J. Am. Chem. Soc. 1977, 99, 8115–8116. doi:10.1021/ja00466a089
Return to citation in text: [1] [2] -
Fukuyama, T.; Nakatsubo, F.; Cocuzza, A. J.; Kishi, Y. Tetrahedron Lett. 1977, 4295. doi:10.1016/S0040-4039(01)83489-4
Return to citation in text: [1] [2] -
Fukuyama, T.; Yang, L. J. Am. Chem. Soc. 1987, 109, 7881–7882. doi:10.1021/ja00259a046
Return to citation in text: [1] [2] -
Benbow, J. W.; McClure, K. F.; Danishesky, S. J. J. Am. Chem. Soc. 1993, 115, 12305–12314. doi:10.1021/ja00079a010
Return to citation in text: [1] [2] -
Wang, Z.; Jimenez, L. S. Tetrahedron Lett. 1996, 37, 6049–6052. doi:10.1016/0040-4039(96)01312-3
Return to citation in text: [1] [2] -
Mao, Y.; Varoglu, M.; Sherman, D. H. J. Bacteriol. 1999, 181, 2199.
Return to citation in text: [1] -
Bradner, W. T. Cancer Treat. Rev. 2001, 27, 35–50. doi:10.1053/ctrv.2000.0202
and references cited therein.
Return to citation in text: [1] -
Lefemine, D. V.; Dann, M.; Barbatschi, F.; Hausmann, W. K.; Zbinovsky, V.; Monnikendam, P.; Adam, J.; Bohonos, N. J. Am. Chem. Soc. 1962, 84, 3184–3185. doi:10.1021/ja00875a031
Return to citation in text: [1] -
Uzu, K.; Harada, Y.; Wakiki, S. Agric. Biol. Chem. 1964, 28, 388–393.
Return to citation in text: [1] -
Urakawa, C.; Nakano, K. J. Antibiot. 1981, 34, 243–244.
Return to citation in text: [1] -
Kono, M.; Saitoh, Y.; Shirahata, K.; Arai, Y.; Ishii, S. J. Am. Chem. Soc. 1987, 109, 7224–7225. doi:10.1021/ja00257a072
Return to citation in text: [1] [2] -
Hornemann, U.; Heinz, M. J. J. Org. Chem. 1985, 50, 1301–1302. doi:10.1021/jo00208a031
Return to citation in text: [1] [2] -
Grüschow, S.; Chang, L. C.; Mao, Y.; Sherman, D. H. J. Am. Chem. Soc. 2007, 129, 6470–6476. doi:10.1021/ja0700193
and references cited therein.
Return to citation in text: [1] [2] -
Hornemann, Y.; Kehrer, J. P.; Nunez, C. S.; Ranieri, R. L. J. Am. Chem. Soc. 1974, 96, 320–322. doi:10.1021/ja00808a087
Return to citation in text: [1] -
Hornemann, U. Biosynthesis of the mitomycins. In Biosynthesis.; Corcoran, J. W., Ed.; The Chemical Society: London, United Kingdom, 1981; pp 295–312.
Return to citation in text: [1] -
Varoglu, M.; Mao, Y.; Sherman, D. H. J. Am. Chem. Soc. 2001, 123, 6712–6713. doi:10.1021/ja015646l
and references cited therein.
Return to citation in text: [1] -
Anderson, G. M.; Kibby, J. J.; Rickards, R. W.; Rotschild, J. M. J. Chem. Soc., Chem. Commun. 1980, 24, 1277–1278. doi:10.1039/C39800001277
Return to citation in text: [1] -
Kennedy, K. A.; Rockwell, S.; Sartorelli, A. C. Cancer Res. 1980, 40, 2356–2360.
Return to citation in text: [1] -
Sartorelli, A. C. Cancer Res. 1988, 48, 775–778.
Return to citation in text: [1] -
Kumar, G. S.; He, Q. Y.; Behr-Ventura, D.; Tomasz, M. Biochemistry 1995, 34, 2662–2671. doi:10.1021/bi00008a033
Return to citation in text: [1] [2] -
Rodighiero, G.; Marciani Magno, S.; Dell Acqua, F.; Vedaldi, D. Farmaco. Ed. Sci. 1978, 3, 651.
Return to citation in text: [1] -
Penketh, P. G.; Hodnick, W. F.; Belcourt, M. F.; Shyam, K.; Sherman, D. H.; Sartorelli, A. C. J. Biol. Chem. 2001, 276, 34445–34452. doi:10.1074/jbc.M104263200
Return to citation in text: [1] -
Cera, C.; Egbertson, M.; Teng, S. P.; Crothers, D. M.; Danishefsky, S. J. Biochemistry 1989, 28, 5665–5669. doi:10.1021/bi00439a049
Return to citation in text: [1] -
Keyes, S. R.; Fracasso, P. M.; Heimbrook, D. C.; Rockwell, S.; Sligar, S. G.; Sartorelli, A. C. Cancer Res. 1984, 44, 5638–5643.
Return to citation in text: [1] -
Iyer, V. N.; Szybalski, W. Science 1964, 145, 55–58. doi:10.1126/science.145.3627.55
Return to citation in text: [1] -
Andrews, P. A.; Pan, S. S.; Bachur, N. R. J. Am. Chem. Soc. 1986, 108, 4158–4166. doi:10.1021/ja00274a052
Return to citation in text: [1] -
Kohn, H.; Zein, N.; Lin, X. Q.; Ding, J. Q.; Kadish, K. M. J. Am. Chem. Soc. 1987, 109, 1833–1840. doi:10.1021/ja00240a038
Return to citation in text: [1] [2] -
Egbertson, M.; Danishefsky, S. J. J. Am. Chem. Soc. 1987, 109, 2204–2205. doi:10.1021/ja00241a061
Return to citation in text: [1] [2] -
Danishefsky, S. J.; Egbertson, M. J. J. Am. Chem. Soc. 1986, 108, 4648–4650. doi:10.1021/ja00275a065
Return to citation in text: [1] -
Moore, H. W. Science 1977, 197, 527–532. doi:10.1126/science.877572
Return to citation in text: [1] -
Kumar, G. S.; Lipman, R.; Cummings, J.; Tomasz, M. Biochemistry 1997, 36, 14128–14136. doi:10.1021/bi971394i
Return to citation in text: [1] -
Prakash, A. S.; Beall, H.; Ross, D.; Gibson, N. W. Biochemistry 1993, 32, 5518–5525. doi:10.1021/bi00072a005
Return to citation in text: [1] -
Danishefsky, S.; Ciufolini, M. J. Am. Chem. Soc. 1984, 106, 6424–6425. doi:10.1021/ja00333a056
Return to citation in text: [1] -
Hoey, B. M.; Butler, J.; Swallow, A. J. Biochemistry 1988, 27, 2608–2614. doi:10.1021/bi00407a051
Return to citation in text: [1] -
Kalyanaraman, B.; Perez-Reyes, E.; Mason, R. P. Biochim. Biophys. Acta 1980, 630, 119–130.
Return to citation in text: [1] -
Boger, D. L. Heterodiene Additions. In Comp. Org. Syn.; Trost, B. M.; Fleming, I., Eds.; Pergamon Press: Oxford, 1991; Vol. 5, pp 451–550.
Return to citation in text: [1] -
McClure, K. F.; Danishefsky, S. J. J. Am. Chem. Soc. 1993, 115, 6094–6100. doi:10.1021/ja00067a026
Return to citation in text: [1] -
Auerbach, J.; Franck, R. W. Chem. Commun. 1969, 991–992.
Return to citation in text: [1] -
Franck, R. W.; Bernady, K. F. J. Org. Chem. 1968, 33, 3050–3055. doi:10.1021/jo01272a007
Return to citation in text: [1] -
Franck, R. W.; Tomasz, M. In Chemistry of Anti-Tumor Agents;; Wilman, D. E. V., Ed.; Blackie and Son/Chapman and Hall: New York, 1989.
Return to citation in text: [1] -
Trost, B. M.; Pearson, W. H. J. Am. Chem. Soc. 1981, 103, 2483–2485. doi:10.1021/ja00399a089
Return to citation in text: [1] -
Naruta, Y.; Nagai, N.; Arita, Y.; Maruyama, K. J. Org. Chem. 1987, 52, 3956–3967. doi:10.1021/jo00227a003
Return to citation in text: [1] -
Naruta, Y.; Nagai, N.; Arita, Y.; Maruyama, K. J. Chem. Soc., Perkin Trans. 1 1988, 1143–1148. doi:10.1039/P19880001143
Return to citation in text: [1] -
Hudlicky, T.; Frazier, J. O.; Seoane, G.; Tiedje, M.; Seoane, A.; Kwart, L. D.; Beal, C. J. Am. Chem. Soc. 1986, 108, 3755–3762. doi:10.1021/ja00273a033
Return to citation in text: [1] -
Weyler, W.; Pearse, D. S.; Moore, H. W. J. Am. Chem. Soc. 1973, 95, 2603–2610. doi:10.1021/ja00789a032
Return to citation in text: [1] -
Caramella, P.; Rondan, N. G.; Paddon-Row, M. N.; Houk, K. N. J. Am. Chem. Soc. 1981, 103, 2438–2440. doi:10.1021/ja00399a064
Return to citation in text: [1] -
Judd, T. C.; Williams, R. M. Angew. Chem., Int. Ed. 2002, 41, 4683–4685. doi:10.1002/anie.200290015
Return to citation in text: [1] -
Ducept, P.; Gubler, D. A.; Williams, R. M. Heterocycles 2006, 67, 597–619. doi:10.3987/COM-05-S(T)18
Return to citation in text: [1] -
Rollins, S. B.; Williams, R. M. Tetrahedron Lett. 1997, 38, 4033–4036. doi:10.1016/S0040-4039(97)00865-4
Return to citation in text: [1] -
Dembinski, R. Eur. J. Org. Chem. 2004, 13, 2763–2772. doi:10.1002/ejoc.200400003
Return to citation in text: [1] -
Namiki, H.; Chamberland, S.; Gubler, D. A.; Williams, R. M. Org. Lett. 2007, 9, 5341–5344. doi:10.1021/ol701960v
Return to citation in text: [1] -
Fuchs, P. L. J. Am. Chem. Soc. 1974, 96, 1607–1609. doi:10.1021/ja00812a062
Return to citation in text: [1] -
Steward, J. M.; Westberg, H. H. J. Org. Chem. 1965, 30, 1951–1955. doi:10.1021/jo01017a058
Return to citation in text: [1] -
Dolfini, J. E.; Menich, K.; Corliss, P.; Danishefsky, S. J.; Cavanaugh, R.; Chakrabartty, S. Tetrahedron Lett. 1966, 7, 4421–4426. doi:10.1016/S0040-4039(00)70053-0
Return to citation in text: [1] -
Corey, E. J.; Fuchs, P. L. J. Am. Chem. Soc. 1972, 94, 4014–4015. doi:10.1021/ja00766a062
Return to citation in text: [1] -
Bone, T. A.; Perkin, W. H. J. Chem. Soc. 1895, 67, 108–119.
Return to citation in text: [1] -
Danishefsky, S. J.; Rovnyak, G. J. Org. Chem. 1975, 40, 114–115. doi:10.1021/jo00889a026
Return to citation in text: [1] -
Danishefsky, S. J.; Singh, R. K. J. Org. Chem. 1975, 40, 3807–3808. doi:10.1021/jo00913a053
Return to citation in text: [1] -
Danishefsky, S. J.; Singh, R. K. J. Am. Chem. Soc. 1975, 97, 3239–3241. doi:10.1021/ja00844a065
Return to citation in text: [1] -
Danishefsky, S. J. Acc. Chem. Res. 1979, 12, 66–72. doi:10.1021/ar50134a004
Return to citation in text: [1] [2] -
Singh, R. K.; Danishefsky, S. J. In Organic Syntheses; Freeman, J. P., Ed.; Wiley: New York, 1990; Vol. 7, pp 411–414.
Return to citation in text: [1] [2] -
Danishefsky, S.; Doehner, R. Tetrahedron Lett. 1977, 3031–3034. doi:10.1016/S0040-4039(01)83148-8
Return to citation in text: [1] -
Danishefsky, S.; Regan, J.; Doehner, R. J. Org. Chem. 1981, 46, 5255–5261. doi:10.1021/jo00339a001
Return to citation in text: [1] -
Danishefsky, S.; McKee, R.; Singh, R. K. J. Am. Chem. Soc. 1977, 99, 7711–7713. doi:10.1021/ja00465a054
Return to citation in text: [1] -
Sharpless, K. B.; Lauer, R. F. J. Org. Chem. 1974, 39, 429–430. doi:10.1021/jo00917a038
Return to citation in text: [1] -
Sharpless, K. B.; Lauer, R. F. J. Am. Chem. Soc. 1973, 95, 2697–2699. doi:10.1021/ja00789a055
Return to citation in text: [1] -
Danishefsky, S.; Berman, E. M.; Ciufolini, M.; Etheredge, S. J.; Segmuller, B. E. J. Am. Chem. Soc. 1985, 107, 3891–3898. doi:10.1021/ja00299a022
Return to citation in text: [1] -
Nicolaou, K. C.; Clarmon, D. A.; Narnette, W. E.; Sietz, S. P. J. Am. Chem. Soc. 1979, 101, 3704–3706. doi:10.1021/ja00507a069
Return to citation in text: [1] -
Nicolaou, K. C. Tetrahedron 1981, 37, 4097–4109. doi:10.1016/S0040-4020(01)93285-5
Return to citation in text: [1] -
Kinoshita, S.; Uzu, K.; Nakano, K.; Shimizu, M.; Takahashi, T.; Matsui, M. J. Med. Chem. 1971, 14, 103–109. doi:10.1021/jm00284a005
Return to citation in text: [1] [2] -
Danishefsky, S. J.; Feigelson, G. B. Heterocycles 1987, 25, 301–304. doi:10.3987/S-1987-01-0301
Return to citation in text: [1] -
Ziegler, F. E.; Belema, M. J. Org. Chem. 1997, 62, 1083–1094. doi:10.1021/jo961992n
Return to citation in text: [1] -
Grierson, D. Org. React. 1990, 39, 85–295.
Return to citation in text: [1] -
Ziegler, F. E.; Berlin, M. Y. Tetrahedron Lett. 1998, 39, 2455–2458. doi:10.1016/S0040-4039(98)00286-X
Return to citation in text: [1] -
Kende, A. S. Organic Syntheses; John Wiley & Sons: New York, 1986; Vol. 64, pp 104 ff.
Return to citation in text: [1] -
Seebach, D.; Wasmuth, D. Helv. Chim. Acta 1980, 63, 197–200. doi:10.1002/hlca.19800630118
Return to citation in text: [1] -
Mori, K.; Iwasawa, H. Tetrahedron 1980, 36, 87–90. doi:10.1016/0040-4020(80)85029-0
Return to citation in text: [1] -
Dunigan, J.; Weigel, L. O. J. Org. Chem. 1991, 56, 6225–6227. doi:10.1021/jo00021a048
Return to citation in text: [1] -
Ittah, Y.; Sasson, Y.; Shahak, I.; Tsaroom, S.; Blum, J. J. Org. Chem. 1978, 43, 4271–4273. doi:10.1021/jo00416a003
Return to citation in text: [1] -
Ziegler, F. E.; Berlin, M. Y.; Lee, K.; Looker, A. R. Org. Lett. 2001, 2, 3619–3621. doi:10.1021/ol000245g
Return to citation in text: [1] -
Gardner, J. N.; Carlon, F. E.; Gnoj, O. J. Org. Chem. 1968, 33, 3294–3297. doi:10.1021/jo01272a055
Return to citation in text: [1] -
Vedejs, E.; Little, J. J. Am. Chem. Soc. 2001, 124, 748–749. doi:10.1021/ja0120835
Return to citation in text: [1] -
Yet, L. Chem. Rev. 2000, 100, 2963–3007. doi:10.1021/cr990407q
Return to citation in text: [1] -
Holemann, A.; Reissig, H. U. Synlett 2004, 15, 2732–2735. doi:10.1055/s-2004-835657
Return to citation in text: [1] -
Dobbs, A. P.; Jones, K.; Veal, K. T. Tetrahedron Lett. 1995, 36, 4857–4860. doi:10.1016/0040-4039(95)00838-4
Return to citation in text: [1] -
Kozokowski, A. P.; Mugrage, B. B. J. Chem. Soc., Perkin Trans. 1 1988, 198–200.
Return to citation in text: [1] -
Kambe, M.; Arai, E.; Suzuki, M.; Tokuyama, H.; Fukuyama, T. Org. Lett. 2001, 3, 2575–2578. doi:10.1021/ol016243t
Return to citation in text: [1] -
Reinhoudt, D. N.; Visser, G. W.; Verboom, W.; Benders, P. H.; Pennings, M. L. M. J. Am. Chem. Soc. 1983, 105, 4775–4781. doi:10.1021/ja00352a041
Return to citation in text: [1] -
Dijksman, W. C.; Verboom, W.; Egberink, J. M.; Reinhoudt, D. N. J. Org. Chem. 1985, 50, 3791–3797. doi:10.1021/jo00220a023
Return to citation in text: [1] -
Verboom, W.; Lammerink, B. H. M.; Egberink, R. J. M.; Reinhoudt, D. N.; Harkema, S. J. Org. Chem. 1985, 50, 3797–3806. doi:10.1021/jo00220a024
Return to citation in text: [1] -
Lee, S.; Lee, W. M.; Sulikowski, G. A. J. Org. Chem. 1999, 64, 4224–4225. doi:10.1021/jo990469g
Return to citation in text: [1] -
Lim, H. J.; Sulikowski, G. A. Tetrahedron Lett. 1996, 37, 5243–5246. doi:10.1016/0040-4039(96)01109-4
Return to citation in text: [1] -
Baranano, D.; Mann, G.; Hartwig, J. F. Curr. Org. Chem. 1997, 1, 287–305.
Return to citation in text: [1] -
Hartwig, J. F. Synlett 1997, 329–340. doi:10.1055/s-1997-789
Return to citation in text: [1] -
Hartwig, J. F. Angew. Chem., Int. Ed. Engl. 1998, 37, 2046–2067. doi:10.1002/(SICI)1521-3773(19980817)37:15<2046::AID-ANIE2046>3.0.CO;2-L
Return to citation in text: [1] -
Martinez, L. E.; Leighton, J. L.; Carsten, D. H.; Jacobsen, E. N. J. Am. Chem. Soc. 1995, 117, 5897–5898. doi:10.1021/ja00126a048
Return to citation in text: [1] -
Kuwajima, I.; Urabe, H. J. Am. Chem. Soc. 1982, 104, 6831–6833. doi:10.1021/ja00388a083
Return to citation in text: [1] -
Wang, P.; Adams, J. J. Am. Chem. Soc. 1994, 116, 3296–3305. doi:10.1021/ja00087a016
Return to citation in text: [1] -
Lee, S.; Lim, H.; Cha, K. L.; Sulikowski, G. A. Tetrahedron 1997, 53, 16521–16532. doi:10.1016/S0040-4020(97)01033-8
Return to citation in text: [1] -
Allan, G. M.; Parson, A. F.; Pons, J. F. Synlett 2002, 1431–1434. doi:10.1055/s-2002-33522
Return to citation in text: [1] -
Kulinkovich, O. G.; Sviridov, S. V.; Vasilevskii, D. A. Synthesis 1991, 234–235. doi:10.1055/s-1991-26431
Return to citation in text: [1] -
Lee, J.; Ha, J. D.; Cha, J. K. J. Am. Chem. Soc. 1997, 119, 8127–8128. doi:10.1021/ja9716564
Return to citation in text: [1] -
Finley, K. T. The addition and substitution chemistry of quinones. In Chemistry of the Quinonoid Compounds; Patai, S., Ed.; Wiley: New York, 1974; Vol. 2, pp 877–1144.
Return to citation in text: [1] -
Watson, I. D.; Yu, L.; Yudin, A. K. Acc. Chem. Res. 2006, 39, 194–206.
(See for a review on aziridine formation.)
Return to citation in text: [1] -
Balachari, D.; O’Doherty, G. A. Org. Lett. 2000, 6, 863–866.
(See for similar effect.) Similar, results have been found in our laboratory on a similar compound (not published).
Return to citation in text: [1] -
Fujioka, H.; Christ, W. J.; Cha, J. K.; Leder, J.; Kishi, Y.; Uemura, D.; Hirata, Y. J. Am. Chem. Soc. 1982, 25, 7367–7369. doi:10.1021/ja00389a100
Return to citation in text: [1] -
Tidwell, T. T. Organic reactions; John Wiley and Sons, 1990; Vol. 39, pp 297–572.
Return to citation in text: [1] -
Boruah, R. C.; Skibo, E. B. J. Org. Chem. 1995, 60, 2232–2243. doi:10.1021/jo00112a051
Return to citation in text: [1] -
Kametani, T.; Kigawa, Y.; Nemoto, H.; Ihara, M.; Fukumoto, K. J. Chem. Soc., Perkin Trans. 1 1980, 1607–1613.
Return to citation in text: [1] [2] -
Kametani, T.; Takahashi, K.; Ihara, M.; Fukumoto, K. Heterocycles 1978, 9, 435–438. doi:10.3987/R-1978-04-0435
Return to citation in text: [1] -
Kametani, T.; Takahashi, K. Heterocycles 1978, 9, 293–351. doi:10.3987/R-1978-03-0293
Return to citation in text: [1] -
Kametani, T.; Takahashi, K.; Ihara, M.; Fukumoto, K. Heterocycles 1978, 9, 439–442. doi:10.3987/R-1978-04-0439
Return to citation in text: [1] -
Kametani, T.; Osawa, T.; Ihara, M. Heterocycles 1979, 12, 913–916. doi:10.3987/R-1979-07-0913
Return to citation in text: [1] -
Taylor, W. G.; Remers, W. A. J. Med. Chem. 1975, 18, 307–311. doi:10.1021/jm00237a020
Return to citation in text: [1] -
Eschenmoser, A.; Wintner, C. E. Science 1977, 196, 1410–1420. doi:10.1126/science.867037
Return to citation in text: [1] -
Yamada, Y.; Miljkovic, D.; Wehrli, P.; Golding, B.; Loeliger, P.; Keese, R.; Mueller, K.; Eschenmoser, A. Angew. Chem. 1969, 81, 301–306. doi:10.1002/ange.19690810902
Return to citation in text: [1] -
Gotschi, E.; Hunkeler, W.; Wild, H. J.; Fuhrer, W.; Gleason, J.; Eschenmoser, A. Angew. Chem., Int. Ed. Engl. 1973, 12, 910–912. doi:10.1002/anie.197309101
Return to citation in text: [1] -
Boyland, E.; Manson, D. J. Chem. Soc. 1951, 1837–1840. doi:10.1039/jr9510001837
Return to citation in text: [1] -
Luly, J. R.; Rapoport, H. J. Am. Chem. Soc. 1983, 105, 2859–2866. doi:10.1021/ja00347a055
Return to citation in text: [1] -
Remers, W. A.; Roth, R. H.; Weiss, M. J. J. Org. Chem. 1965, 30, 2910–2917. doi:10.1021/jo01020a008
Return to citation in text: [1] -
Taylor, W. G.; Leadbetter, G.; Fost, D. L.; Remers, W. A. J. Med. Chem. 1977, 20, 138–141. doi:10.1021/jm00211a029
Return to citation in text: [1] -
Leadbetter, G.; Fost, D. L.; Ekwuribe, N. N.; Remers, W. A. J. Org. Chem. 1974, 39, 3580–3583. doi:10.1021/jo00938a028
Return to citation in text: [1] -
Poletto, J. F.; Allen, G. R., Jr.; Weiss, M. J. J. Med. Chem. 1968, 11, 882–885. doi:10.1021/jm00310a041
Return to citation in text: [1] -
Yamamoto, Y.; Asao, N. Chem. Rev. 1993, 93, 2207–2293. doi:10.1021/cr00022a010
Return to citation in text: [1] -
Bloch, R. Chem. Rev. 1998, 98, 1407–1438. doi:10.1021/cr940474e
Return to citation in text: [1] -
Kobayashi, S.; Ishitani, H. Chem. Rev. 1999, 99, 1069–1094. doi:10.1021/cr980414z
Return to citation in text: [1] -
Shibata, I.; Nose, K.; Sakamoto, K.; Yasuda, M.; Baba, A. J. Org. Chem. 2004, 69, 2185–2187. doi:10.1021/jo0356659
Return to citation in text: [1] -
Remuson, R. Beilstein J. Org. Chem. 2007, 3, No. 32. doi:10.1186/1860-5397-3-32
Return to citation in text: [1] -
Coleman, R. S.; Felpin, F. X.; Chen, W. J. Org. Chem. 2004, 69, 7309–7316. doi:10.1021/jo048924i
Return to citation in text: [1] -
He, F.; Bo, Y.; Altom, J. D.; Corey, E. J. J. Am. Chem. Soc. 1999, 121, 6771–6772. doi:10.1021/ja9915201
Return to citation in text: [1] -
Feigelson, G. B.; Danishefsky, S. J. J. Org. Chem. 1988, 53, 3391–3393. doi:10.1021/jo00249a061
Return to citation in text: [1] [2] [3] -
Williams, A. L.; Srinivasan, J. M.; Johnston, J. N. Org. Lett. 2006, 8, 6047–6049. doi:10.1021/ol0624676
Return to citation in text: [1] -
Brown, D. G.; Velthuisen, E. J.; Commerford, J. R.; Brisbois, R. G.; Hoye, T. R. J. Org. Chem. 1996, 61, 2540–2541. doi:10.1021/jo951944n
Return to citation in text: [1] -
Shaw, K. J.; Luly, J. R.; Rapoport, H. J. Org. Chem. 1985, 50, 4515–4523. doi:10.1021/jo00223a019
Return to citation in text: [1] -
Michael, J. P.; Koning, C. B.; Mudzunga, T. T.; Petersen, R. L. Synlett 2006, 19, 3284–3288. doi:10.1055/s-2006-951532
Return to citation in text: [1] -
Dong, W.; Jimenez, L. J. Org. Chem. 1999, 64, 2520–2523. doi:10.1021/jo981143v
Return to citation in text: [1] [2] -
Wang, Z.; Jimenez, L. S. J. Am. Chem. Soc. 1994, 116, 4977–4978. doi:10.1021/ja00090a049
Return to citation in text: [1] -
Cory, R. M.; Ritchie, B. M. J. Chem. Soc., Chem. Commun. 1983, 1244–1245. doi:10.1039/C39830001244
Return to citation in text: [1] -
Kametani, T.; Takahashi, K.; Ihara, M.; Fukumoto, K. J. Chem. Soc., Perkin Trans. 1 1978, 662–666. doi:10.1039/P19780000662
Return to citation in text: [1] -
Kametani, T.; Takahashi, K.; Ihara, M.; Fukumoto, K. Heterocycles 1977, 6, 1371. doi:10.3987/R-1977-09-1371
Return to citation in text: [1] -
Gribble, G. W.; Lord, D.; Skotnicki, J.; Dietz, S. E.; Eaton, J. T.; Johnson, J. L. J. Am. Chem. Soc. 1974, 96, 7812–7814. doi:10.1021/ja00832a035
Return to citation in text: [1] -
Kornblum, N.; Jones, W. J.; Anderson, G. J. J. Am. Chem. Soc. 1959, 81, 4113–4114. doi:10.1021/ja01524a080
Return to citation in text: [1] -
Henbest, H. B.; Wrigley, T. I. J. Chem. Soc. 1957, 4596–4604. doi:10.1039/jr9570004596
Return to citation in text: [1] -
Corey, E. J.; Ursprung, J. J. J. Am. Chem. Soc. 1956, 78, 183–188. doi:10.1021/ja01582a052
Return to citation in text: [1] -
Allen, G. R.; Polletto, J. F.; Weiss, M. J. J. Org. Chem. 1965, 30, 2897–2904. doi:10.1021/jo01020a006
Return to citation in text: [1] -
Kametani, T.; Takahashi, K.; Ihara, M.; Fukumoto, K. J. Chem. Soc. Perkin. Trans. 1 1976, 389–390. doi:10.1039/P19760000389
Return to citation in text: [1] -
Jones, G. B.; Guzel, M.; Mathews, J. E. Tetrahedron Lett. 2000, 41, 1123–1126. doi:10.1016/S0040-4039(99)02259-5
Return to citation in text: [1] -
Feigelson, G. B.; Egbertson, M.; Danishefsky, S. J. J. Org. Chem. 1988, 53, 3390–3391. doi:10.1021/jo00249a060
Return to citation in text: [1] -
Ockenden, D. W.; Schofield, K. J. Chem. Soc. 1953, 3440–3443. doi:10.1039/jr9530003440
Return to citation in text: [1] -
Veysoglu, T.; Mitscher, L. A. Tetrahedron Lett. 1981, 22, 1303–1306. doi:10.1016/S0040-4039(01)90302-8
Return to citation in text: [1] -
Fukuyama, T.; Yang, L. J. Am. Chem. Soc. 1989, 111, 8303–8304. doi:10.1021/ja00203a055
Return to citation in text: [1] -
Harada, T.; Iwai, H.; Takatsuki, H.; Fujita, K.; Kubo, M.; Oku, A. Org. Lett. 2001, 13, 2101–2103. doi:10.1021/ol016062r
Return to citation in text: [1] -
Remy, P.; Langner, M.; Bolm, C. Org. Lett. 2006, 6, 1209–1211. doi:10.1021/ol060077d
Return to citation in text: [1] -
Bluet, G.; Campagne, J. M. J. Org. Chem. 2001, 12, 4293–4298. doi:10.1021/jo015567s
Return to citation in text: [1] -
Wadamoto, M.; Ozasa, N.; Yanagisawa, A.; Yamamoto, H. J. Org. Chem. 2003, 14, 5593–5601. doi:10.1021/jo020691c
Return to citation in text: [1] -
Jankowska, J.; Mlynarski, J. J. Org. Chem. 2006, 4, 1317–1321. doi:10.1021/jo0514568
Return to citation in text: [1] -
Carlsen, P. H. J.; Katsuki, T.; Martin, V. S.; Sharpless, K. B. J. Org. Chem. 1981, 46, 3936–3938. doi:10.1021/jo00332a045
Return to citation in text: [1] -
Ohnuma, T.; Sekine, Y.; Ban, Y. Tetrahedron Lett. 1979, 27, 2533–2537. doi:10.1016/S0040-4039(01)86341-3
Return to citation in text: [1] -
Oda, K.; Ohnuma, T.; Ban, Y. J. Org. Chem. 1984, 49, 953–959. doi:10.1021/jo00180a001
Return to citation in text: [1] -
Nakajima, S.; Yoshida, K.; Mori, M.; Ban, Y.; Shibasaki, M. J. Chem. Soc., Chem. Commun. 1990, 468–469.
Return to citation in text: [1] -
Yoshida, K.; Nakajima, S.; Ohnuma, T.; Ban, Y.; Shibasaki, M.; Aoe, K.; Date, T. J. Org. Chem. 1988, 53, 5355–5359. doi:10.1021/jo00257a031
Return to citation in text: [1] -
Greene, T. W.; Wuts, P. G. Protective goups in organic synthesis, 5th ed.; John Wiley & Sons, Inc., 1999; pp 638 ff.
Return to citation in text: [1] -
Oda, K.; Ohnuma, T.; Ban, Y.; Aoe, K. J. Am. Chem. Soc. 1984, 106, 5378–5379. doi:10.1021/ja00330a072
Return to citation in text: [1] -
Wakamatsu, T.; Nishi, T.; Ohnuma, T.; Ban, Y. Synth. Commun. 1984, 14, 1167–1173. doi:10.1080/00397918408059649
Return to citation in text: [1] -
Kubo, I.; Kim, M.; Ganjian, I.; Kamikawa, T.; Yamagiwa, Y. Tetrahedron 1987, 43, 2653–2660. doi:10.1016/S0040-4020(01)86870-8
Return to citation in text: [1] -
Hirama, M.; Iwashita, M.; Yamazaki, Y.; Ito, S. Tetrahedron Lett. 1984, 25, 4963–4964. doi:10.1016/S0040-4039(01)91270-5
Return to citation in text: [1] -
Vedejs, E.; Klapars, A.; Naidu, B. N.; Piotrowski, D. W.; Tucci, F. C. J. Am. Chem. Soc. 2000, 122, 5401–5402. doi:10.1021/ja994504c
Return to citation in text: [1] -
Vedejs, E.; Naidu, B. N.; Klapars, A.; Warner, D. L.; Li, V. S.; Na, Y.; Kohn, H. J. Am. Chem. Soc. 2003, 125, 15796–15806. doi:10.1021/ja030452m
Return to citation in text: [1] -
Ito, H.; Taguchi, T.; Hanzawa, Y. J. J. Org. Chem. 1993, 58, 774–775. doi:10.1021/jo00055a041
Return to citation in text: [1] -
Bobeck, D. R.; Warner, D. L.; Vedejs, E. J. Org. Chem. 2007, 72, 8506–8518. doi:10.1021/jo7013559
Return to citation in text: [1] -
Warner, D. L.; Hibberd, A. M.; Kalman, M.; Klapars, A.; Vedejs, E. J. Org. Chem. 2007, 72, 8519–8522. doi:10.1021/jo7013615
Return to citation in text: [1] -
Ducray, R.; Ciufolini, M. A. Angew. Chem., Int. Ed. 2002, 41, 4688–4691. doi:10.1002/anie.200290017
Return to citation in text: [1] -
Ciufolini, M. A.; Deaton, M. V.; Zhu, S.; Chen, M. Tetrahedron 1997, 57, 16299–16312. doi:10.1016/S0040-4020(97)01016-8
Return to citation in text: [1] -
Ciufolini, M. A. Il Pharmaco 2005, 60, 627–641.
Return to citation in text: [1] -
Ducray, R.; Cramer, N.; Ciufolini, M. A. Tetrahedron Lett. 2001, 42, 9175–9178. doi:10.1016/S0040-4039(01)02018-4
Return to citation in text: [1] -
Reetz, M. T.; Steinbach, R.; Westermann, J.; Peter, R.; Wenderoth, B. Chem. Ber. 1985, 118, 1441–1454. doi:10.1002/cber.19851180413
Return to citation in text: [1] -
Alonso, F.; Beletskaya, I. P.; Yus, M. Chem. Rev. 2004, 104, 3079–3160. doi:10.1021/cr0201068
Return to citation in text: [1] -
Anand, N. K.; Carreira, E. M. J. Am. Chem. Soc. 2001, 123, 9687–9688. doi:10.1021/ja016378u
Return to citation in text: [1] -
Deiters, A.; Martin, S. F. Chem. Rev. 2004, 104, 2199–2238. doi:10.1021/cr0200872
Return to citation in text: [1] -
Tsuboike, K.; Guerin, D. J.; Mennen, S. M.; Miller, S. J. Tetrahedron 2004, 60, 7367–7374. doi:10.1016/j.tet.2004.06.022
Return to citation in text: [1] -
Davie, E. A. C.; Mennen, S. M.; Xu, Y.; Miller, S. J. Chem. Rev. 2007, 107, 5759–5812. doi:10.1021/cr068377w
Return to citation in text: [1] -
González-Pérez, P.; Pérez-Serrano, L.; Casarrubios, L.; Dominguez, G.; Pérez-Castells, J. Tetrahedron Lett. 2002, 43, 4765–4767. doi:10.1016/S0040-4039(02)00909-7
Return to citation in text: [1] -
Chamberland, S.; Grüschow, S.; Sherman, D. H.; Williams, R. M. Org. Lett. 2009, 11, 791–794. doi:10.1021/ol802631c
Return to citation in text: [1] -
Van Lanen, S. G.; Shen, B. Drug Discovery Today: Technologies 2006, 3, 285–292. doi:10.1016/j.ddtec.2006.09.014
Return to citation in text: [1] -
Mao, Y.; Varoglu, M.; Sherman, D. H. Chem. Biol. 1999, 6, 251–263. doi:10.1016/S1074-5521(99)80040-4
Return to citation in text: [1]
| 112. | Kuwajima, I.; Urabe, H. J. Am. Chem. Soc. 1982, 104, 6831–6833. doi:10.1021/ja00388a083 |
| 113. | Wang, P.; Adams, J. J. Am. Chem. Soc. 1994, 116, 3296–3305. doi:10.1021/ja00087a016 |
| 108. | Baranano, D.; Mann, G.; Hartwig, J. F. Curr. Org. Chem. 1997, 1, 287–305. |
| 109. | Hartwig, J. F. Synlett 1997, 329–340. doi:10.1055/s-1997-789 |
| 110. | Hartwig, J. F. Angew. Chem., Int. Ed. Engl. 1998, 37, 2046–2067. doi:10.1002/(SICI)1521-3773(19980817)37:15<2046::AID-ANIE2046>3.0.CO;2-L |
| 111. | Martinez, L. E.; Leighton, J. L.; Carsten, D. H.; Jacobsen, E. N. J. Am. Chem. Soc. 1995, 117, 5897–5898. doi:10.1021/ja00126a048 |
| 106. | Lee, S.; Lee, W. M.; Sulikowski, G. A. J. Org. Chem. 1999, 64, 4224–4225. doi:10.1021/jo990469g |
| 107. | Lim, H. J.; Sulikowski, G. A. Tetrahedron Lett. 1996, 37, 5243–5246. doi:10.1016/0040-4039(96)01109-4 |
| 15. | Kishi, Y. J. Nat. Prod. 1979, 42, 549–568. doi:10.1021/np50006a001 |
| 16. | Nakatsubo, F.; Fukuyama, T.; Cocuzza, A. J.; Kishi, Y. J. Am. Chem. Soc. 1977, 99, 8115–8116. doi:10.1021/ja00466a089 |
| 17. | Fukuyama, T.; Nakatsubo, F.; Cocuzza, A. J.; Kishi, Y. Tetrahedron Lett. 1977, 4295. doi:10.1016/S0040-4039(01)83489-4 |
| 116. | Kulinkovich, O. G.; Sviridov, S. V.; Vasilevskii, D. A. Synthesis 1991, 234–235. doi:10.1055/s-1991-26431 |
| 117. | Lee, J.; Ha, J. D.; Cha, J. K. J. Am. Chem. Soc. 1997, 119, 8127–8128. doi:10.1021/ja9716564 |
| 114. | Lee, S.; Lim, H.; Cha, K. L.; Sulikowski, G. A. Tetrahedron 1997, 53, 16521–16532. doi:10.1016/S0040-4020(97)01033-8 |
| 115. | Allan, G. M.; Parson, A. F.; Pons, J. F. Synlett 2002, 1431–1434. doi:10.1055/s-2002-33522 |
| 35. | Kumar, G. S.; He, Q. Y.; Behr-Ventura, D.; Tomasz, M. Biochemistry 1995, 34, 2662–2671. doi:10.1021/bi00008a033 |
| 146. | Feigelson, G. B.; Danishefsky, S. J. J. Org. Chem. 1988, 53, 3391–3393. doi:10.1021/jo00249a061 |
| 123. | Boruah, R. C.; Skibo, E. B. J. Org. Chem. 1995, 60, 2232–2243. doi:10.1021/jo00112a051 |
| 20. | Wang, Z.; Jimenez, L. S. Tetrahedron Lett. 1996, 37, 6049–6052. doi:10.1016/0040-4039(96)01312-3 |
| 152. | Wang, Z.; Jimenez, L. S. J. Am. Chem. Soc. 1994, 116, 4977–4978. doi:10.1021/ja00090a049 |
| 120. |
Balachari, D.; O’Doherty, G. A. Org. Lett. 2000, 6, 863–866.
(See for similar effect.) Similar, results have been found in our laboratory on a similar compound (not published). |
| 121. | Fujioka, H.; Christ, W. J.; Cha, J. K.; Leder, J.; Kishi, Y.; Uemura, D.; Hirata, Y. J. Am. Chem. Soc. 1982, 25, 7367–7369. doi:10.1021/ja00389a100 |
| 153. | Cory, R. M.; Ritchie, B. M. J. Chem. Soc., Chem. Commun. 1983, 1244–1245. doi:10.1039/C39830001244 |
| 122. | Tidwell, T. T. Organic reactions; John Wiley and Sons, 1990; Vol. 39, pp 297–572. |
| 27. | Hornemann, U.; Heinz, M. J. J. Org. Chem. 1985, 50, 1301–1302. doi:10.1021/jo00208a031 |
| 118. | Finley, K. T. The addition and substitution chemistry of quinones. In Chemistry of the Quinonoid Compounds; Patai, S., Ed.; Wiley: New York, 1974; Vol. 2, pp 877–1144. |
| 149. | Shaw, K. J.; Luly, J. R.; Rapoport, H. J. Org. Chem. 1985, 50, 4515–4523. doi:10.1021/jo00223a019 |
| 119. |
Watson, I. D.; Yu, L.; Yudin, A. K. Acc. Chem. Res. 2006, 39, 194–206.
(See for a review on aziridine formation.) |
| 148. | Brown, D. G.; Velthuisen, E. J.; Commerford, J. R.; Brisbois, R. G.; Hoye, T. R. J. Org. Chem. 1996, 61, 2540–2541. doi:10.1021/jo951944n |
| 151. | Dong, W.; Jimenez, L. J. Org. Chem. 1999, 64, 2520–2523. doi:10.1021/jo981143v |
| 150. | Michael, J. P.; Koning, C. B.; Mudzunga, T. T.; Petersen, R. L. Synlett 2006, 19, 3284–3288. doi:10.1055/s-2006-951532 |
| 147. | Williams, A. L.; Srinivasan, J. M.; Johnston, J. N. Org. Lett. 2006, 8, 6047–6049. doi:10.1021/ol0624676 |
| 85. | Kinoshita, S.; Uzu, K.; Nakano, K.; Shimizu, M.; Takahashi, T.; Matsui, M. J. Med. Chem. 1971, 14, 103–109. doi:10.1021/jm00284a005 |
| 130. | Eschenmoser, A.; Wintner, C. E. Science 1977, 196, 1410–1420. doi:10.1126/science.867037 |
| 124. | Kametani, T.; Kigawa, Y.; Nemoto, H.; Ihara, M.; Fukumoto, K. J. Chem. Soc., Perkin Trans. 1 1980, 1607–1613. |
| 125. | Kametani, T.; Takahashi, K.; Ihara, M.; Fukumoto, K. Heterocycles 1978, 9, 435–438. doi:10.3987/R-1978-04-0435 |
| 126. | Kametani, T.; Takahashi, K. Heterocycles 1978, 9, 293–351. doi:10.3987/R-1978-03-0293 |
| 127. | Kametani, T.; Takahashi, K.; Ihara, M.; Fukumoto, K. Heterocycles 1978, 9, 439–442. doi:10.3987/R-1978-04-0439 |
| 128. | Kametani, T.; Osawa, T.; Ihara, M. Heterocycles 1979, 12, 913–916. doi:10.3987/R-1979-07-0913 |
| 129. | Taylor, W. G.; Remers, W. A. J. Med. Chem. 1975, 18, 307–311. doi:10.1021/jm00237a020 |
| 151. | Dong, W.; Jimenez, L. J. Org. Chem. 1999, 64, 2520–2523. doi:10.1021/jo981143v |
| 83. | Nicolaou, K. C.; Clarmon, D. A.; Narnette, W. E.; Sietz, S. P. J. Am. Chem. Soc. 1979, 101, 3704–3706. doi:10.1021/ja00507a069 |
| 84. | Nicolaou, K. C. Tetrahedron 1981, 37, 4097–4109. doi:10.1016/S0040-4020(01)93285-5 |
| 165. | Veysoglu, T.; Mitscher, L. A. Tetrahedron Lett. 1981, 22, 1303–1306. doi:10.1016/S0040-4039(01)90302-8 |
| 85. | Kinoshita, S.; Uzu, K.; Nakano, K.; Shimizu, M.; Takahashi, T.; Matsui, M. J. Med. Chem. 1971, 14, 103–109. doi:10.1021/jm00284a005 |
| 163. | Feigelson, G. B.; Egbertson, M.; Danishefsky, S. J. J. Org. Chem. 1988, 53, 3390–3391. doi:10.1021/jo00249a060 |
| 164. | Ockenden, D. W.; Schofield, K. J. Chem. Soc. 1953, 3440–3443. doi:10.1039/jr9530003440 |
| 18. | Fukuyama, T.; Yang, L. J. Am. Chem. Soc. 1987, 109, 7881–7882. doi:10.1021/ja00259a046 |
| 166. | Fukuyama, T.; Yang, L. J. Am. Chem. Soc. 1989, 111, 8303–8304. doi:10.1021/ja00203a055 |
| 82. | Danishefsky, S.; Berman, E. M.; Ciufolini, M.; Etheredge, S. J.; Segmuller, B. E. J. Am. Chem. Soc. 1985, 107, 3891–3898. doi:10.1021/ja00299a022 |
| 26. | Kono, M.; Saitoh, Y.; Shirahata, K.; Arai, Y.; Ishii, S. J. Am. Chem. Soc. 1987, 109, 7224–7225. doi:10.1021/ja00257a072 |
| 1. | Kudo, S.; Marumo, T.; Tomioka, T.; Kato, H.; Fujimoto, Y. Antibiot. Chemother. 1958, 8, 228. |
| 158. | Henbest, H. B.; Wrigley, T. I. J. Chem. Soc. 1957, 4596–4604. doi:10.1039/jr9570004596 |
| 159. | Corey, E. J.; Ursprung, J. J. J. Am. Chem. Soc. 1956, 78, 183–188. doi:10.1021/ja01582a052 |
| 157. | Kornblum, N.; Jones, W. J.; Anderson, G. J. J. Am. Chem. Soc. 1959, 81, 4113–4114. doi:10.1021/ja01524a080 |
| 146. | Feigelson, G. B.; Danishefsky, S. J. J. Org. Chem. 1988, 53, 3391–3393. doi:10.1021/jo00249a061 |
| 160. | Allen, G. R.; Polletto, J. F.; Weiss, M. J. J. Org. Chem. 1965, 30, 2897–2904. doi:10.1021/jo01020a006 |
| 161. | Kametani, T.; Takahashi, K.; Ihara, M.; Fukumoto, K. J. Chem. Soc. Perkin. Trans. 1 1976, 389–390. doi:10.1039/P19760000389 |
| 162. | Jones, G. B.; Guzel, M.; Mathews, J. E. Tetrahedron Lett. 2000, 41, 1123–1126. doi:10.1016/S0040-4039(99)02259-5 |
| 6. | Kiyoto, S.; Shibata, T.; Yamashita, M.; Komori, T.; Okuhara, M.; Terano, H.; Kohsaka, M.; Aoki, H.; Imanaka, H. J. J. Antibiot. 1987, 40, 594–599. |
| 94. | Ittah, Y.; Sasson, Y.; Shahak, I.; Tsaroom, S.; Blum, J. J. Org. Chem. 1978, 43, 4271–4273. doi:10.1021/jo00416a003 |
| 4. | Hata, T.; Koga, F.; Sano, Y.; Kanamori, K.; Matsumae, A.; Sugawara, R.; Hoshi, T.; Shimi, T.; Ito, S.; Tomizawa, S. J. Antibiot. 1954, 7, 107–112. |
| 5. | Nagaoka, K.; Matsumoto, M.; Oono, J.; Yokoi, K.; Ishizeki, S.; Nakashima, T. J. Antibiot. 1986, 39, 1527–1532. |
| 3. | Yudin, A. K. Aziridines and Epoxides in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006. doi:10.1002/3527607862 |
| 90. | Kende, A. S. Organic Syntheses; John Wiley & Sons: New York, 1986; Vol. 64, pp 104 ff. |
| 91. | Seebach, D.; Wasmuth, D. Helv. Chim. Acta 1980, 63, 197–200. doi:10.1002/hlca.19800630118 |
| 92. | Mori, K.; Iwasawa, H. Tetrahedron 1980, 36, 87–90. doi:10.1016/0040-4020(80)85029-0 |
| 156. | Gribble, G. W.; Lord, D.; Skotnicki, J.; Dietz, S. E.; Eaton, J. T.; Johnson, J. L. J. Am. Chem. Soc. 1974, 96, 7812–7814. doi:10.1021/ja00832a035 |
| 2. | Webb, J. S.; Cosalich, D. B.; Mowat, J. H.; Patrick, J. B.; Broschard, R. W.; Meyor, W. E.; Williams, R. P.; Wolf, C. F.; Fulmor, W.; Pidacks, C.; Lancaster, J. E. J. Am. Chem. Soc. 1962, 84, 3185–3186. doi:10.1021/ja00875a032 |
| 93. | Dunigan, J.; Weigel, L. O. J. Org. Chem. 1991, 56, 6225–6227. doi:10.1021/jo00021a048 |
| 154. | Kametani, T.; Takahashi, K.; Ihara, M.; Fukumoto, K. J. Chem. Soc., Perkin Trans. 1 1978, 662–666. doi:10.1039/P19780000662 |
| 155. | Kametani, T.; Takahashi, K.; Ihara, M.; Fukumoto, K. Heterocycles 1977, 6, 1371. doi:10.3987/R-1977-09-1371 |
| 9. | Wolkenberg, S. E.; Boger, D. L. Chem. Rev. 2002, 102, 2477–2496. doi:10.1021/cr010046q |
| 3. | Yudin, A. K. Aziridines and Epoxides in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006. doi:10.1002/3527607862 |
| 9. | Wolkenberg, S. E.; Boger, D. L. Chem. Rev. 2002, 102, 2477–2496. doi:10.1021/cr010046q |
| 89. | Ziegler, F. E.; Berlin, M. Y. Tetrahedron Lett. 1998, 39, 2455–2458. doi:10.1016/S0040-4039(98)00286-X |
| 8. | Tsuchida, T.; Inuma, H.; Kinoshita, N.; Ikeda, T.; Sawa, T.; Hamada, M.; Takeuchi, T. J. Antibiot. 1995, 48, 217–221. |
| 86. | Danishefsky, S. J.; Feigelson, G. B. Heterocycles 1987, 25, 301–304. doi:10.3987/S-1987-01-0301 |
| 7. | Hanada, M.; Ohkuma, H.; Yonemoto, T.; Tomita, K.; Ohbayashi, M.; Kamei, H.; Miyaki, T.; Konishi, M.; Kawaguchi, H.; Forenza, S. J. Antibiot. 1991, 44, 403–414. |
| 87. | Ziegler, F. E.; Belema, M. J. Org. Chem. 1997, 62, 1083–1094. doi:10.1021/jo961992n |
| 97. | Vedejs, E.; Little, J. J. Am. Chem. Soc. 2001, 124, 748–749. doi:10.1021/ja0120835 |
| 43. | Egbertson, M.; Danishefsky, S. J. J. Am. Chem. Soc. 1987, 109, 2204–2205. doi:10.1021/ja00241a061 |
| 181. | Hirama, M.; Iwashita, M.; Yamazaki, Y.; Ito, S. Tetrahedron Lett. 1984, 25, 4963–4964. doi:10.1016/S0040-4039(01)91270-5 |
| 95. | Ziegler, F. E.; Berlin, M. Y.; Lee, K.; Looker, A. R. Org. Lett. 2001, 2, 3619–3621. doi:10.1021/ol000245g |
| 96. | Gardner, J. N.; Carlon, F. E.; Gnoj, O. J. Org. Chem. 1968, 33, 3294–3297. doi:10.1021/jo01272a055 |
| 182. | Vedejs, E.; Klapars, A.; Naidu, B. N.; Piotrowski, D. W.; Tucci, F. C. J. Am. Chem. Soc. 2000, 122, 5401–5402. doi:10.1021/ja994504c |
| 183. | Vedejs, E.; Naidu, B. N.; Klapars, A.; Warner, D. L.; Li, V. S.; Na, Y.; Kohn, H. J. Am. Chem. Soc. 2003, 125, 15796–15806. doi:10.1021/ja030452m |
| 177. | Greene, T. W.; Wuts, P. G. Protective goups in organic synthesis, 5th ed.; John Wiley & Sons, Inc., 1999; pp 638 ff. |
| 175. | Nakajima, S.; Yoshida, K.; Mori, M.; Ban, Y.; Shibasaki, M. J. Chem. Soc., Chem. Commun. 1990, 468–469. |
| 176. | Yoshida, K.; Nakajima, S.; Ohnuma, T.; Ban, Y.; Shibasaki, M.; Aoe, K.; Date, T. J. Org. Chem. 1988, 53, 5355–5359. doi:10.1021/jo00257a031 |
| 179. | Wakamatsu, T.; Nishi, T.; Ohnuma, T.; Ban, Y. Synth. Commun. 1984, 14, 1167–1173. doi:10.1080/00397918408059649 |
| 180. | Kubo, I.; Kim, M.; Ganjian, I.; Kamikawa, T.; Yamagiwa, Y. Tetrahedron 1987, 43, 2653–2660. doi:10.1016/S0040-4020(01)86870-8 |
| 178. | Oda, K.; Ohnuma, T.; Ban, Y.; Aoe, K. J. Am. Chem. Soc. 1984, 106, 5378–5379. doi:10.1021/ja00330a072 |
| 167. | Harada, T.; Iwai, H.; Takatsuki, H.; Fujita, K.; Kubo, M.; Oku, A. Org. Lett. 2001, 13, 2101–2103. doi:10.1021/ol016062r |
| 168. | Remy, P.; Langner, M.; Bolm, C. Org. Lett. 2006, 6, 1209–1211. doi:10.1021/ol060077d |
| 169. | Bluet, G.; Campagne, J. M. J. Org. Chem. 2001, 12, 4293–4298. doi:10.1021/jo015567s |
| 170. | Wadamoto, M.; Ozasa, N.; Yanagisawa, A.; Yamamoto, H. J. Org. Chem. 2003, 14, 5593–5601. doi:10.1021/jo020691c |
| 171. | Jankowska, J.; Mlynarski, J. J. Org. Chem. 2006, 4, 1317–1321. doi:10.1021/jo0514568 |
| 103. | Reinhoudt, D. N.; Visser, G. W.; Verboom, W.; Benders, P. H.; Pennings, M. L. M. J. Am. Chem. Soc. 1983, 105, 4775–4781. doi:10.1021/ja00352a041 |
| 104. | Dijksman, W. C.; Verboom, W.; Egberink, J. M.; Reinhoudt, D. N. J. Org. Chem. 1985, 50, 3791–3797. doi:10.1021/jo00220a023 |
| 105. | Verboom, W.; Lammerink, B. H. M.; Egberink, R. J. M.; Reinhoudt, D. N.; Harkema, S. J. Org. Chem. 1985, 50, 3797–3806. doi:10.1021/jo00220a024 |
| 173. | Ohnuma, T.; Sekine, Y.; Ban, Y. Tetrahedron Lett. 1979, 27, 2533–2537. doi:10.1016/S0040-4039(01)86341-3 |
| 174. | Oda, K.; Ohnuma, T.; Ban, Y. J. Org. Chem. 1984, 49, 953–959. doi:10.1021/jo00180a001 |
| 1. | Kudo, S.; Marumo, T.; Tomioka, T.; Kato, H.; Fujimoto, Y. Antibiot. Chemother. 1958, 8, 228. |
| 6. | Kiyoto, S.; Shibata, T.; Yamashita, M.; Komori, T.; Okuhara, M.; Terano, H.; Kohsaka, M.; Aoki, H.; Imanaka, H. J. J. Antibiot. 1987, 40, 594–599. |
| 172. | Carlsen, P. H. J.; Katsuki, T.; Martin, V. S.; Sharpless, K. B. J. Org. Chem. 1981, 46, 3936–3938. doi:10.1021/jo00332a045 |
| 101. | Kozokowski, A. P.; Mugrage, B. B. J. Chem. Soc., Perkin Trans. 1 1988, 198–200. |
| 102. | Kambe, M.; Arai, E.; Suzuki, M.; Tokuyama, H.; Fukuyama, T. Org. Lett. 2001, 3, 2575–2578. doi:10.1021/ol016243t |
| 99. | Holemann, A.; Reissig, H. U. Synlett 2004, 15, 2732–2735. doi:10.1055/s-2004-835657 |
| 100. | Dobbs, A. P.; Jones, K.; Veal, K. T. Tetrahedron Lett. 1995, 36, 4857–4860. doi:10.1016/0040-4039(95)00838-4 |
| 49. | Hoey, B. M.; Butler, J.; Swallow, A. J. Biochemistry 1988, 27, 2608–2614. doi:10.1021/bi00407a051 |
| 50. | Kalyanaraman, B.; Perez-Reyes, E.; Mason, R. P. Biochim. Biophys. Acta 1980, 630, 119–130. |
| 194. | Deiters, A.; Martin, S. F. Chem. Rev. 2004, 104, 2199–2238. doi:10.1021/cr0200872 |
| 19. | Benbow, J. W.; McClure, K. F.; Danishesky, S. J. J. Am. Chem. Soc. 1993, 115, 12305–12314. doi:10.1021/ja00079a010 |
| 193. | Anand, N. K.; Carreira, E. M. J. Am. Chem. Soc. 2001, 123, 9687–9688. doi:10.1021/ja016378u |
| 190. | Ducray, R.; Cramer, N.; Ciufolini, M. A. Tetrahedron Lett. 2001, 42, 9175–9178. doi:10.1016/S0040-4039(01)02018-4 |
| 188. | Ciufolini, M. A.; Deaton, M. V.; Zhu, S.; Chen, M. Tetrahedron 1997, 57, 16299–16312. doi:10.1016/S0040-4020(97)01016-8 |
| 189. | Ciufolini, M. A. Il Pharmaco 2005, 60, 627–641. |
| 192. | Alonso, F.; Beletskaya, I. P.; Yus, M. Chem. Rev. 2004, 104, 3079–3160. doi:10.1021/cr0201068 |
| 191. | Reetz, M. T.; Steinbach, R.; Westermann, J.; Peter, R.; Wenderoth, B. Chem. Ber. 1985, 118, 1441–1454. doi:10.1002/cber.19851180413 |
| 60. | Weyler, W.; Pearse, D. S.; Moore, H. W. J. Am. Chem. Soc. 1973, 95, 2603–2610. doi:10.1021/ja00789a032 |
| 185. | Bobeck, D. R.; Warner, D. L.; Vedejs, E. J. Org. Chem. 2007, 72, 8506–8518. doi:10.1021/jo7013559 |
| 61. | Caramella, P.; Rondan, N. G.; Paddon-Row, M. N.; Houk, K. N. J. Am. Chem. Soc. 1981, 103, 2438–2440. doi:10.1021/ja00399a064 |
| 184. | Ito, H.; Taguchi, T.; Hanzawa, Y. J. J. Org. Chem. 1993, 58, 774–775. doi:10.1021/jo00055a041 |
| 57. | Naruta, Y.; Nagai, N.; Arita, Y.; Maruyama, K. J. Org. Chem. 1987, 52, 3956–3967. doi:10.1021/jo00227a003 |
| 58. | Naruta, Y.; Nagai, N.; Arita, Y.; Maruyama, K. J. Chem. Soc., Perkin Trans. 1 1988, 1143–1148. doi:10.1039/P19880001143 |
| 187. | Ducray, R.; Ciufolini, M. A. Angew. Chem., Int. Ed. 2002, 41, 4688–4691. doi:10.1002/anie.200290017 |
| 59. | Hudlicky, T.; Frazier, J. O.; Seoane, G.; Tiedje, M.; Seoane, A.; Kwart, L. D.; Beal, C. J. Am. Chem. Soc. 1986, 108, 3755–3762. doi:10.1021/ja00273a033 |
| 186. | Warner, D. L.; Hibberd, A. M.; Kalman, M.; Klapars, A.; Vedejs, E. J. Org. Chem. 2007, 72, 8519–8522. doi:10.1021/jo7013615 |
| 53. | Auerbach, J.; Franck, R. W. Chem. Commun. 1969, 991–992. |
| 54. | Franck, R. W.; Bernady, K. F. J. Org. Chem. 1968, 33, 3050–3055. doi:10.1021/jo01272a007 |
| 55. | Franck, R. W.; Tomasz, M. In Chemistry of Anti-Tumor Agents;; Wilman, D. E. V., Ed.; Blackie and Son/Chapman and Hall: New York, 1989. |
| 56. | Trost, B. M.; Pearson, W. H. J. Am. Chem. Soc. 1981, 103, 2483–2485. doi:10.1021/ja00399a089 |
| 51. | Boger, D. L. Heterodiene Additions. In Comp. Org. Syn.; Trost, B. M.; Fleming, I., Eds.; Pergamon Press: Oxford, 1991; Vol. 5, pp 451–550. |
| 52. | McClure, K. F.; Danishefsky, S. J. J. Am. Chem. Soc. 1993, 115, 6094–6100. doi:10.1021/ja00067a026 |
| 62. | Judd, T. C.; Williams, R. M. Angew. Chem., Int. Ed. 2002, 41, 4683–4685. doi:10.1002/anie.200290015 |
| 63. | Ducept, P.; Gubler, D. A.; Williams, R. M. Heterocycles 2006, 67, 597–619. doi:10.3987/COM-05-S(T)18 |
| 64. | Rollins, S. B.; Williams, R. M. Tetrahedron Lett. 1997, 38, 4033–4036. doi:10.1016/S0040-4039(97)00865-4 |
| 65. | Dembinski, R. Eur. J. Org. Chem. 2004, 13, 2763–2772. doi:10.1002/ejoc.200400003 |
| 200. | Mao, Y.; Varoglu, M.; Sherman, D. H. Chem. Biol. 1999, 6, 251–263. doi:10.1016/S1074-5521(99)80040-4 |
| 199. | Van Lanen, S. G.; Shen, B. Drug Discovery Today: Technologies 2006, 3, 285–292. doi:10.1016/j.ddtec.2006.09.014 |
| 79. | Danishefsky, S.; McKee, R.; Singh, R. K. J. Am. Chem. Soc. 1977, 99, 7711–7713. doi:10.1021/ja00465a054 |
| 197. | González-Pérez, P.; Pérez-Serrano, L.; Casarrubios, L.; Dominguez, G.; Pérez-Castells, J. Tetrahedron Lett. 2002, 43, 4765–4767. doi:10.1016/S0040-4039(02)00909-7 |
| 80. | Sharpless, K. B.; Lauer, R. F. J. Org. Chem. 1974, 39, 429–430. doi:10.1021/jo00917a038 |
| 81. | Sharpless, K. B.; Lauer, R. F. J. Am. Chem. Soc. 1973, 95, 2697–2699. doi:10.1021/ja00789a055 |
| 196. | Davie, E. A. C.; Mennen, S. M.; Xu, Y.; Miller, S. J. Chem. Rev. 2007, 107, 5759–5812. doi:10.1021/cr068377w |
| 75. | Danishefsky, S. J. Acc. Chem. Res. 1979, 12, 66–72. doi:10.1021/ar50134a004 |
| 76. | Singh, R. K.; Danishefsky, S. J. In Organic Syntheses; Freeman, J. P., Ed.; Wiley: New York, 1990; Vol. 7, pp 411–414. |
| 77. | Danishefsky, S.; Doehner, R. Tetrahedron Lett. 1977, 3031–3034. doi:10.1016/S0040-4039(01)83148-8 |
| 198. | Chamberland, S.; Grüschow, S.; Sherman, D. H.; Williams, R. M. Org. Lett. 2009, 11, 791–794. doi:10.1021/ol802631c |
| 78. | Danishefsky, S.; Regan, J.; Doehner, R. J. Org. Chem. 1981, 46, 5255–5261. doi:10.1021/jo00339a001 |
| 28. |
Grüschow, S.; Chang, L. C.; Mao, Y.; Sherman, D. H. J. Am. Chem. Soc. 2007, 129, 6470–6476. doi:10.1021/ja0700193
and references cited therein. |
| 72. | Danishefsky, S. J.; Rovnyak, G. J. Org. Chem. 1975, 40, 114–115. doi:10.1021/jo00889a026 |
| 73. | Danishefsky, S. J.; Singh, R. K. J. Org. Chem. 1975, 40, 3807–3808. doi:10.1021/jo00913a053 |
| 74. | Danishefsky, S. J.; Singh, R. K. J. Am. Chem. Soc. 1975, 97, 3239–3241. doi:10.1021/ja00844a065 |
| 75. | Danishefsky, S. J. Acc. Chem. Res. 1979, 12, 66–72. doi:10.1021/ar50134a004 |
| 76. | Singh, R. K.; Danishefsky, S. J. In Organic Syntheses; Freeman, J. P., Ed.; Wiley: New York, 1990; Vol. 7, pp 411–414. |
| 66. | Namiki, H.; Chamberland, S.; Gubler, D. A.; Williams, R. M. Org. Lett. 2007, 9, 5341–5344. doi:10.1021/ol701960v |
| 195. | Tsuboike, K.; Guerin, D. J.; Mennen, S. M.; Miller, S. J. Tetrahedron 2004, 60, 7367–7374. doi:10.1016/j.tet.2004.06.022 |
| 67. | Fuchs, P. L. J. Am. Chem. Soc. 1974, 96, 1607–1609. doi:10.1021/ja00812a062 |
| 68. | Steward, J. M.; Westberg, H. H. J. Org. Chem. 1965, 30, 1951–1955. doi:10.1021/jo01017a058 |
| 69. | Dolfini, J. E.; Menich, K.; Corliss, P.; Danishefsky, S. J.; Cavanaugh, R.; Chakrabartty, S. Tetrahedron Lett. 1966, 7, 4421–4426. doi:10.1016/S0040-4039(00)70053-0 |
| 70. | Corey, E. J.; Fuchs, P. L. J. Am. Chem. Soc. 1972, 94, 4014–4015. doi:10.1021/ja00766a062 |
| 137. | Leadbetter, G.; Fost, D. L.; Ekwuribe, N. N.; Remers, W. A. J. Org. Chem. 1974, 39, 3580–3583. doi:10.1021/jo00938a028 |
| 138. | Poletto, J. F.; Allen, G. R., Jr.; Weiss, M. J. J. Med. Chem. 1968, 11, 882–885. doi:10.1021/jm00310a041 |
| 139. | Yamamoto, Y.; Asao, N. Chem. Rev. 1993, 93, 2207–2293. doi:10.1021/cr00022a010 |
| 140. | Bloch, R. Chem. Rev. 1998, 98, 1407–1438. doi:10.1021/cr940474e |
| 141. | Kobayashi, S.; Ishitani, H. Chem. Rev. 1999, 99, 1069–1094. doi:10.1021/cr980414z |
| 142. | Shibata, I.; Nose, K.; Sakamoto, K.; Yasuda, M.; Baba, A. J. Org. Chem. 2004, 69, 2185–2187. doi:10.1021/jo0356659 |
| 143. | Remuson, R. Beilstein J. Org. Chem. 2007, 3, No. 32. doi:10.1186/1860-5397-3-32 |
| 135. | Remers, W. A.; Roth, R. H.; Weiss, M. J. J. Org. Chem. 1965, 30, 2910–2917. doi:10.1021/jo01020a008 |
| 136. | Taylor, W. G.; Leadbetter, G.; Fost, D. L.; Remers, W. A. J. Med. Chem. 1977, 20, 138–141. doi:10.1021/jm00211a029 |
| 124. | Kametani, T.; Kigawa, Y.; Nemoto, H.; Ihara, M.; Fukumoto, K. J. Chem. Soc., Perkin Trans. 1 1980, 1607–1613. |
| 133. | Boyland, E.; Manson, D. J. Chem. Soc. 1951, 1837–1840. doi:10.1039/jr9510001837 |
| 134. | Luly, J. R.; Rapoport, H. J. Am. Chem. Soc. 1983, 105, 2859–2866. doi:10.1021/ja00347a055 |
| 131. | Yamada, Y.; Miljkovic, D.; Wehrli, P.; Golding, B.; Loeliger, P.; Keese, R.; Mueller, K.; Eschenmoser, A. Angew. Chem. 1969, 81, 301–306. doi:10.1002/ange.19690810902 |
| 132. | Gotschi, E.; Hunkeler, W.; Wild, H. J.; Fuhrer, W.; Gleason, J.; Eschenmoser, A. Angew. Chem., Int. Ed. Engl. 1973, 12, 910–912. doi:10.1002/anie.197309101 |
| 23. | Lefemine, D. V.; Dann, M.; Barbatschi, F.; Hausmann, W. K.; Zbinovsky, V.; Monnikendam, P.; Adam, J.; Bohonos, N. J. Am. Chem. Soc. 1962, 84, 3184–3185. doi:10.1021/ja00875a031 |
| 24. | Uzu, K.; Harada, Y.; Wakiki, S. Agric. Biol. Chem. 1964, 28, 388–393. |
| 25. | Urakawa, C.; Nakano, K. J. Antibiot. 1981, 34, 243–244. |
| 26. | Kono, M.; Saitoh, Y.; Shirahata, K.; Arai, Y.; Ishii, S. J. Am. Chem. Soc. 1987, 109, 7224–7225. doi:10.1021/ja00257a072 |
| 22. |
Bradner, W. T. Cancer Treat. Rev. 2001, 27, 35–50. doi:10.1053/ctrv.2000.0202
and references cited therein. |
| 13. | Casely-Hayford, M. A.; Pors, K.; James, C. H.; Patterson, L. H.; Hartley, J. A.; Searcey, M. Org. Biomol. Chem. 2005, 3, 3585–3589. doi:10.1039/b508908e |
| 14. | Naoe, Y.; Inami, M.; Matsumoto, S.; Nishigaki, F.; Tsujimoto, S.; Kawamura, I.; Miyayasu, K.; Manda, T.; Shimomura, K. Cancer Chemother. Pharmacol. 1998, 4, 31–36. doi:10.1007/s002800050781 |
| 146. | Feigelson, G. B.; Danishefsky, S. J. J. Org. Chem. 1988, 53, 3391–3393. doi:10.1021/jo00249a061 |
| 15. | Kishi, Y. J. Nat. Prod. 1979, 42, 549–568. doi:10.1021/np50006a001 |
| 16. | Nakatsubo, F.; Fukuyama, T.; Cocuzza, A. J.; Kishi, Y. J. Am. Chem. Soc. 1977, 99, 8115–8116. doi:10.1021/ja00466a089 |
| 17. | Fukuyama, T.; Nakatsubo, F.; Cocuzza, A. J.; Kishi, Y. Tetrahedron Lett. 1977, 4295. doi:10.1016/S0040-4039(01)83489-4 |
| 18. | Fukuyama, T.; Yang, L. J. Am. Chem. Soc. 1987, 109, 7881–7882. doi:10.1021/ja00259a046 |
| 19. | Benbow, J. W.; McClure, K. F.; Danishesky, S. J. J. Am. Chem. Soc. 1993, 115, 12305–12314. doi:10.1021/ja00079a010 |
| 20. | Wang, Z.; Jimenez, L. S. Tetrahedron Lett. 1996, 37, 6049–6052. doi:10.1016/0040-4039(96)01312-3 |
| 144. | Coleman, R. S.; Felpin, F. X.; Chen, W. J. Org. Chem. 2004, 69, 7309–7316. doi:10.1021/jo048924i |
| 9. | Wolkenberg, S. E.; Boger, D. L. Chem. Rev. 2002, 102, 2477–2496. doi:10.1021/cr010046q |
| 10. | Coleman, R. S.; Burk, C. H.; Navarro, A.; Brueggemeier, R. W.; Diaz-Cruz, E. S. Org. Lett. 2002, 4, 3545–3548. doi:10.1021/ol0267275 |
| 11. | Alcaro, S.; Ortuso, F.; Coleman, R. S. J. Med. Chem. 2002, 45, 861–870. doi:10.1021/jm011040w |
| 12. | Zein, N.; Solomon, W.; Colson, K. L.; Schroeder, D. R. Biochemistry 1995, 34, 11591–11597. doi:10.1021/bi00036a035 |
| 145. | He, F.; Bo, Y.; Altom, J. D.; Corey, E. J. J. Am. Chem. Soc. 1999, 121, 6771–6772. doi:10.1021/ja9915201 |
| 29. | Hornemann, Y.; Kehrer, J. P.; Nunez, C. S.; Ranieri, R. L. J. Am. Chem. Soc. 1974, 96, 320–322. doi:10.1021/ja00808a087 |
| 30. | Hornemann, U. Biosynthesis of the mitomycins. In Biosynthesis.; Corcoran, J. W., Ed.; The Chemical Society: London, United Kingdom, 1981; pp 295–312. |
| 31. |
Varoglu, M.; Mao, Y.; Sherman, D. H. J. Am. Chem. Soc. 2001, 123, 6712–6713. doi:10.1021/ja015646l
and references cited therein. |
| 32. | Anderson, G. M.; Kibby, J. J.; Rickards, R. W.; Rotschild, J. M. J. Chem. Soc., Chem. Commun. 1980, 24, 1277–1278. doi:10.1039/C39800001277 |
| 27. | Hornemann, U.; Heinz, M. J. J. Org. Chem. 1985, 50, 1301–1302. doi:10.1021/jo00208a031 |
| 28. |
Grüschow, S.; Chang, L. C.; Mao, Y.; Sherman, D. H. J. Am. Chem. Soc. 2007, 129, 6470–6476. doi:10.1021/ja0700193
and references cited therein. |
| 42. | Kohn, H.; Zein, N.; Lin, X. Q.; Ding, J. Q.; Kadish, K. M. J. Am. Chem. Soc. 1987, 109, 1833–1840. doi:10.1021/ja00240a038 |
| 48. | Danishefsky, S.; Ciufolini, M. J. Am. Chem. Soc. 1984, 106, 6424–6425. doi:10.1021/ja00333a056 |
| 41. | Andrews, P. A.; Pan, S. S.; Bachur, N. R. J. Am. Chem. Soc. 1986, 108, 4158–4166. doi:10.1021/ja00274a052 |
| 42. | Kohn, H.; Zein, N.; Lin, X. Q.; Ding, J. Q.; Kadish, K. M. J. Am. Chem. Soc. 1987, 109, 1833–1840. doi:10.1021/ja00240a038 |
| 43. | Egbertson, M.; Danishefsky, S. J. J. Am. Chem. Soc. 1987, 109, 2204–2205. doi:10.1021/ja00241a061 |
| 44. | Danishefsky, S. J.; Egbertson, M. J. J. Am. Chem. Soc. 1986, 108, 4648–4650. doi:10.1021/ja00275a065 |
| 45. | Moore, H. W. Science 1977, 197, 527–532. doi:10.1126/science.877572 |
| 46. | Kumar, G. S.; Lipman, R.; Cummings, J.; Tomasz, M. Biochemistry 1997, 36, 14128–14136. doi:10.1021/bi971394i |
| 47. | Prakash, A. S.; Beall, H.; Ross, D.; Gibson, N. W. Biochemistry 1993, 32, 5518–5525. doi:10.1021/bi00072a005 |
| 37. | Penketh, P. G.; Hodnick, W. F.; Belcourt, M. F.; Shyam, K.; Sherman, D. H.; Sartorelli, A. C. J. Biol. Chem. 2001, 276, 34445–34452. doi:10.1074/jbc.M104263200 |
| 38. | Cera, C.; Egbertson, M.; Teng, S. P.; Crothers, D. M.; Danishefsky, S. J. Biochemistry 1989, 28, 5665–5669. doi:10.1021/bi00439a049 |
| 39. | Keyes, S. R.; Fracasso, P. M.; Heimbrook, D. C.; Rockwell, S.; Sligar, S. G.; Sartorelli, A. C. Cancer Res. 1984, 44, 5638–5643. |
| 40. | Iyer, V. N.; Szybalski, W. Science 1964, 145, 55–58. doi:10.1126/science.145.3627.55 |
| 33. | Kennedy, K. A.; Rockwell, S.; Sartorelli, A. C. Cancer Res. 1980, 40, 2356–2360. |
| 34. | Sartorelli, A. C. Cancer Res. 1988, 48, 775–778. |
| 35. | Kumar, G. S.; He, Q. Y.; Behr-Ventura, D.; Tomasz, M. Biochemistry 1995, 34, 2662–2671. doi:10.1021/bi00008a033 |
| 36. | Rodighiero, G.; Marciani Magno, S.; Dell Acqua, F.; Vedaldi, D. Farmaco. Ed. Sci. 1978, 3, 651. |
© 2009 Andrez; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)