Abstract
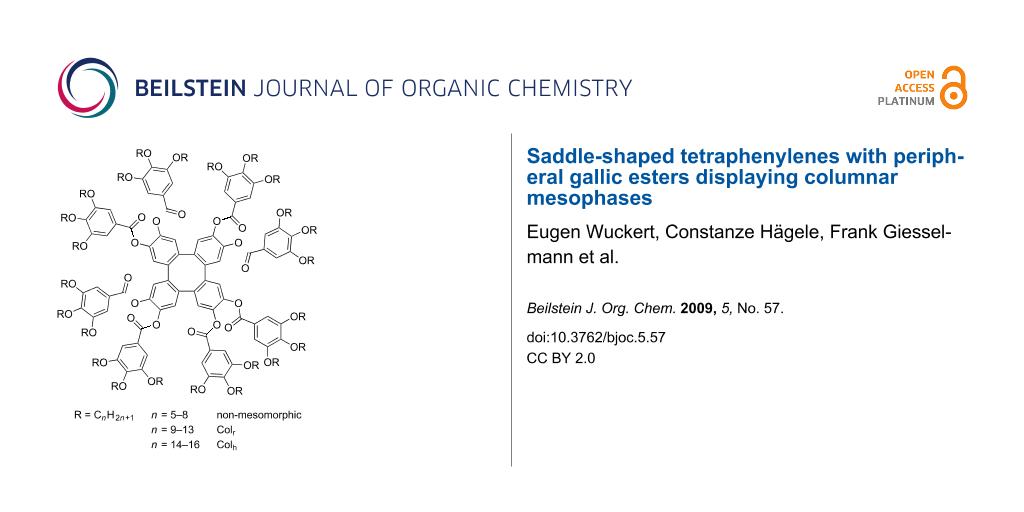
Tetraphenylenes 2 with eight peripheral gallic esters were prepared in two steps from octamethoxytetraphenylene 1 in 19–72% yield. Investigation of the mesomorphic properties of 2 by DSC, POM and X-ray diffraction revealed that derivatives 2a–d with short alkoxy chain lengths (C5–C8) did not show any mesomorphic properties, whereas compounds 2e–i with C9–C13 chains displayed rectangular columnar mesophases and compounds 2j–l with C14–C16 chains displayed hexagonal columnar mesophases. Furthermore an anomalous odd-even effect of the clearing points of compounds 2e–l versus chain length was detected.
Graphical Abstract
Introduction
Columnar liquid crystals have received increasing interest during the last decade due to their 1D charge transport and self-healing properties, which make them particularly promising candidates for organic field effect transistors, organic photovoltaic devices and light emitting diodes [1-3]. Tetraphenylenes, whose saddle-shaped conformation is caused by the anti-aromatic character of the corresponding central flat 8-membered ring [4-6], are suitable building blocks for supramolecular chemistry, asymmetric catalysis and formation of inclusion complexes [3-28]. We have shown that tetraphenylenes with eight peripheral alkoxy or alkanoate chains display thermotropic columnar and smectic mesophases [29,30]. Furthermore, anomalous odd-even effects were discovered for these discotic tetraphenylenes, i.e. the ascending and descending transition temperatures with increasing numbers of methylene groups in the side chain exhibit an inversion of this alternation between n = 12 and n = 14 homologues [31]. In order to explore whether this anomalous odd-even effect is a more general phenomenon, the corresponding gallic ester-substituted tetraphenylenes were prepared and their liquid crystalline properties were investigated. In addition, we were curious about the mesophase types, because tetraphenylenes with peripheral alkoxy or alkanoate chains displayed smectic mesophases in addition to columnar phases, whereas the corresponding tetraphenylenes with p-alkoxybenzoate substituents displayed only columnar mesophases even with long chain lengths [29-31]. Thus, we anticipated that the presence of the sterically demanding gallic esters in the periphery of the tetraphenylene can be accommodated much better by columnar packing as compared to a smectic layer structure. The results are discussed below.
Results and Discussion
The synthesis started from the known octamethoxytetraphenylene 1 [4,19,20,29-31], which was demethylated with boron tribromide in CH2Cl2 at −50 °C to room temp. followed by hydrolysis with MeOH (Scheme 1). Subsequent treatment with gallic acid chlorides in the presence of catalytic amounts of DMAP in pyridine/CH2Cl2 yielded after aqueous workup and chromatographic purification the desired gallic ester-substituted tetraphenylenes 2a–l in 19–72%. In some cases purification turned out to be rather tedious resulting in decreased yields.
Scheme 1: Synthesis of tetraphenylenes 2.
Scheme 1: Synthesis of tetraphenylenes 2.
Mesomorphic properties of compounds 2 were studied by differential scanning calorimetry (DSC), polarizing optical microscopy (POM) and X-ray diffraction (WAXS, SAXS). The DSC results are summarized in Table 1.
Table 1: Phase transition temperatures [°C] and (enthalpies [kJ/mol])a.
| 2 | n | Cr1 | Cr2 | Cr3 | Col | I | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | 5 | • | 51 (10.1) | • | 79 (4.4) | • | 191 (15.3) | -- | • | 2. heating | |
| b | 6 | • | 46 (8.3) | • | 69 (7.4) | • | 142 (11.2) | -- | • | 2. heating | |
| c | 7 | • | 10 (1.7) | • | 41 (0.6) | • | 55 (0.6) | -- | • | 2. heating | |
| d | 8 | • | 4 (15.0) | • | 41 (27.7) | • | 62 (7.2) | -- | • | 2. heating | |
| e | 9 | • | −6 (11.6) | • | 35 (4.2) | -- | • | 37 (3.8) | • | 2. heating | |
| e | 9 | • | 1 (−2.7) | • | 11 (−0.8) | -- | • | 26 (−5.3) | • | 2. cooling | |
| f | 10 | • | 7 (4.1) | • | 40 (12.5) | -- | • | 46 (5.6) | • | 2. heating | |
| f | 10 | • | 24 (−7.9) | -- | -- | • | 39 (−5.7) | • | 2. cooling | ||
| g | 11 | • | 29 (7.4) | -- | -- | • | 43 (1.0) | • | 2. heating | ||
| g | 11 | • | 20 (−7.6) | -- | -- | • | 40 (−1.1) | • | 2. cooling | ||
| h | 12 | • | 3 (19.4) | -- | -- | • | 36 (10.3) | • | 2. heating | ||
| h | 12 | • | 0 (−18.6) | -- | -- | • | 33 (−9.7) | • | 2. cooling | ||
| i | 13 | • | 16 (18.5) | -- | -- | • | 33 (11.9) | • | 2. heating | ||
| i | 13 | • | 13 (−18.9) | -- | -- | • | 28 (−13.4) | • | 2. cooling | ||
| j | 14 | • | 16 (9.6) | -- | -- | • | 41 (2.5) | • | 2. heating | ||
| j | 14 | • | 16 (−5.8) | -- | -- | • | 29 (−1.8) | • | 2. cooling | ||
| k | 15 | • | 25 (8.0) | -- | -- | • | 36 (20.1) | • | 2. heating | ||
| k | 15 | • | 20 (−10.3) | -- | -- | • | 32 (−16.2) | • | 2. cooling | ||
| l | 16 | -- | -- | -- | • | 41 (36.2) | • | 2. heating | |||
| l | 16 | • | 22 (−5.2) | -- | -- | • | 36 (−17.3) | • | 2. cooling | ||
aCr crystalline, Col columnar, I isotropic; • phase was observed, -- phase was not observed; heating/cooling rate 10 K/min for 2a–e,i,j, 5 K/min for 2f–h,k,l.
While compounds 2a–d with chain lengths up to C8 showed only crystal to crystal transitions and isotropic melting, tetraphenylenes 2e–l with chain lengths between C9 and C16 displayed enantiotropic mesomorphism. For compounds 2e,f additional crystal to crystal transitions were detected. A typical DSC curve is shown in Figure 1. Thus tetraphenylene 2h with dodecyl chains displays a melting transition at 3 °C and a clearing transition at 36 °C upon second heating. Upon cooling an isotropic to mesophase transition at 33 °C and a crystallization peak at 0 °C were detected. The hysteresis phenomena observed for some compounds are probably due to supercooling, which is common for such highly viscous materials.
Figure 1: DSC traces of compound 2h during (a) second cooling and (b) second heating (heating/cooling rate 5 K/min).
Figure 1: DSC traces of compound 2h during (a) second cooling and (b) second heating (heating/cooling rate 5 ...
POM investigations revealed focal conic and fan-shaped textures typical for columnar mesophases (Figure 2).
Figure 2: Texture of 2h under the POM at 25 °C upon cooling from the isotropic liquid (heating/cooling rate 5 K/min; magnification 100×).
Figure 2: Texture of 2h under the POM at 25 °C upon cooling from the isotropic liquid (heating/cooling rate 5...
However, clear evidence was possible only by XRD data, which are summarized in Table 2. While rectangular columnar mesophases were observed for tetraphenylenes 2e–i with shorter chains (n = 9–13) (shown for 2f in Figure S1 in the Supporting Information), hexagonal columnar mesophases were found for the long chain derivatives 2j–l (n = 14–16) (shown for 2j in Figure S2 in the Supporting Information). Indeed, as expected the columnar mesophase seems to accommodate the eight bulky gallic esters much better than the smectic layer structure.
Table 2: X-Ray diffraction data for compounds 2e–la.
| 2 | T [°C] | θ [°] | dobs [Å] | hk | dcalc [Å] | Mesophase parameters |
|---|---|---|---|---|---|---|
| 2e | 20 | 1.71 | 25.9 | (20) | 25.9 | Colr |
| 2.93 | 15.1 | (11) | 15.1 | a = 51.8 Å | ||
| 3.33 | 13.3 | (21) | 13.5 | b = 15.8 Å | ||
| 2f | 32 | 1.73 | 25.6 | (20) | 18.4 | Colr |
| 2.32 | 19.0 | (11) | 19.0 | a = 51.2 Å | ||
| 3.34 | 13.2 | (31) | 13.1 | b = 20.5 Å | ||
| 2g | 15 | 1.60 | 27.7 | (20) | 27.7 | Colr |
| 2.17 | 20.4 | (11) | 20.4 | a = 55.4 Å | ||
| 3.14 | 14.1 | (31) | 14.1 | b = 21.9 Å | ||
| 2h | 20 | 1.51 | 29.3 | (20) | 29.3 | Colr |
| 2.25 | 19.6 | (11) | 19.6 | a = 58.6 Å | ||
| b = 20.8 Å | ||||||
| 2i | 25 | 1.47 | 30.1 | (20) | 30.1 | Colr |
| 2.28 | 19.4 | (11) | 19.4 | a = 60.3 Å | ||
| b = 20.5 Å | ||||||
| 2j | 30 | 1.46 | 30.3 | (10) | 30.6 | Colh |
| 2.47 | 17.9 | (11) | 17.7 | a = 35.3 Å | ||
| 2k | 20 | 1.40 | 31.6 | (10) | 31.9 | Colh |
| 2.39 | 18.5 | (11) | 18.4 | a = 36.9 Å | ||
| 2.75 | 16.1 | (20) | 16.0 | |||
| 3.65 | 12.1 | (21) | 12.1 | |||
| 2l | 20 | 1.35 | 32.8 | (10) | 33.1 | Colh |
| 2.32 | 19.0 | (11) | 19.1 | a = 38.2 Å | ||
| 3.50 | 12.6 | (21) | 12.5 | |||
aDiffraction angle θ; observed and calculated diffraction spacings dobs and dcalc; Miller indices hk.
The crossover from rectangular columnar to hexagonal columnar mesophases with increasing chain lengths has been also observed in other columnar systems [32,33] and has been attributed to the enhanced core–core interaction necessary for the formation of the Colr phases [34]. According to molecular modelling [35] and comparison with the XRD data each disk within the hexagonal and rectangular columnar pattern is occupied by one tetraphenylene molecule. For better visibility only the modelled tetraphenylene (octakis)acyl core unit is shown in Figure 3, which reveals the saddle shape.
Figure 3: Molecular modelling of the saddle-shaped tetraphenylene (octakis)acyl core unit of 2 [35].
Figure 3: Molecular modelling of the saddle-shaped tetraphenylene (octakis)acyl core unit of 2 [35].
Next, the clearing points of mesogenic compounds 2e–l were plotted against the chain lengths n (Figure 4). An anomalous odd-even effect can be seen, which inverts at n = 11.
Figure 4: Clearing temperatures Tcl [°C] of tetraphenylenes 2e–l as a function of the chain lengths n.
Figure 4: Clearing temperatures Tcl [°C] of tetraphenylenes 2e–l as a function of the chain lengths n.
For the previously studied tetraphenylene derivatives with alkoxy, alkanoate and p-alkoxybenzoate chains the inversion was observed at n = 12–13 [31]. Although in all four cases an anomalous odd-even effect is present, the chain length dependence of the clearing temperatures differs somewhat. For derivatives with alkoxy or alkanoate chains directly attached to the tetraphenylene unit, the oxygen atom is part of the chain and thus odd carbon chains are actually even-numbered. They should thus have an elongated shape which should lead to a higher degree of orientational order and hence a higher clearing temperature than the odd-numbered chains (including oxygen), i.e. those with an even-number of carbon atoms. The data in Figure 4 suggest that for 3,4,5-trialkoxygallic esters 2 this argument does not hold and the orientational order and hence the clearing temperature is determined both by the alkoxy chain lengths as well as the rigid gallic acid moiety. In order to eliminate the influence of the alkyl side chain on the odd-even effect of the tetraphenylenes the melting temperatures Tmalk of n-alkanes were subtracted from the clearing points Tcl of the respective tetraphenylenes 2 (Figure 5). An almost regular effect could be seen. Thus it seems that transition temperatures are also governed by the influence of the gallic ester moiety on the dynamic properties of the alkyl tails.
Figure 5: The differences between the clearing temperatures Tcl [°C] of tetraphenylenes 2 and the melting points Tmalk of the n-alkanes exhibit an almost normal odd-even effect without any inversion of the alteration.
Figure 5: The differences between the clearing temperatures Tcl [°C] of tetraphenylenes 2 and the melting poi...
Conclusion
In conclusion, only columnar mesophases have been found for gallic ester-substituted tetraphenylenes 2e–l with a minimum chain length of n = 9. An anomalous odd-even effect was detected, in which the alternation of the melting transition inverses at n = 11. The results agree with previous findings, and suggest that the anomalous odd-even effect is a more general phenomenon than previously thought. Investigations to extend this concept to other classes of liquid crystals are currently in progress.
Experimental
General
Melting points were measured on a Mettler Toledo DSC822 and are uncorrected. NMR spectra were recorded on a Bruker Avance 300 and Avance 500 spectrometer. FT-IR spectra were recorded on a Bruker Vektor22 spectrometer with MKII Golden Gate Single Reflection Diamant ATR system. Mass spectra were recorded on a Finnigan MAT 95 and a Varian MAT 711 apparatus. Small-angle scattering data from unaligned samples (filled into Mark capillary tubes of 0.7 mm diameter) were obtained using a Kratky compact camera (A. Paar) provided with a temperature controller (A. Paar) and a one-dimensional electronic detector (M. Braun). Aligned samples were exposed in a home-made flat-film camera and the 2D diffraction patterns recorded with an imaging plate detector (Fuji BAS SR). In the flat-film camera, the sample was placed in a small hole of a brass block, the temperature of which was controlled by a Lakeshore controller and kept in a 1.5 T magnetic field for alignment.
Differential scanning calorimetry (DSC) was performed using a Mettler Toledo DSC822, and polarizing optical microscopy (POM) using an Olympus BX50 polarizing microscope combined with a Linkam LTS350 hot stage and a Linkam TP93 central processor. Flash chromatography was performed using Kieselgel 60, 40–63 μm (Fluka). All solvents were dried, and reactions were performed in dried glassware. The used petroleum ether (PE) had a boiling range of 30–75 °C. Octamethoxytetraphenylene 1 was prepared as described in ref. [31].
General procedure for the preparation of heptakis[(3,4,5-trialkoxybenzoyl)oxy]tetra-phenylen-2-yl 3,4,5-trialkoxybenzoates (2)
To a solution of octamethoxytetraphenylene 1 (0.14 g, 0.25 mmol) in CH2Cl2 (2 mL) were added BBr3 (2.2 mL, 2.2 mmol, 1 M solution in CH2Cl2) at −50 °C and the mixture was stirred for 1 h at room temp. The solvent was removed in vacuo and the residue was dissolved in degassed MeOH (5 mL) for 1 h and evaporated. The residue was dissolved in CH2Cl2 (2 mL), treated with DMAP (4 mg, 0.03 mmol) and pyridine (1 mL) and gallic acid chloride (5 mmol) were added dropwise. After stirring overnight at 30 °C, the mixture was diluted with CH2Cl2 (10 mL), hydrolyzed with 2 M HCl and the layers separated. The aqueous layer was extracted with CH2Cl2 (2 × 10 mL), the organic layers were washed with sat. NaHCO3 (10 mL), H2O (10 mL), dried over MgSO4 and concentrated in vacuo. The crude product was purified by column chromatography on SiO2 (hexanes/ethyl acetate 20 : 1) to yield colorless waxy solids.
3,6,7,10,11,14,15-Heptakis[(3,4,5-dodecyloxybenzoyl)oxy]tetraphenylen-2-yl 3,4,5-tridodecyloxybenzoate (2h)
270 mg (19%) of a colorless solid. 1H NMR (500 MHz, CDCl3): δ = 0.85–0.89 (m, 72H), 1.25–1.49 (m, 432H), 1.70–1.75 (m, 48H), 3.79–3.86 (m, 32H), 3.98 (t, J = 6.5 Hz, 16H), 7.24 (s, 16H), 7.39 (s, 8H) ppm. 13C NMR (125 MHz, CDCl3): δ = 14.1, 22.7, 26.1, 26.2, 29.4, 29.4, 29.5, 29.6, 29.7, 29.7, 29.8, 29.8, 30.4, 32.0, 69.0, 73.5, 108.3, 123.0, 124.5, 138.1, 142.0, 143.0, 152.9, 163.8 ppm. FT-IR (ATR): ν = 2921 (vs), 2852 (s), 1976 (w), 1743 (m), 1585 (m), 1499 (w), 1466 (w), 1430 (m), 1390 (w), 1335 (s), 1291 (w), 1240 (w), 1190 (s), 1114 (s), 947 (w), 802 (w), 748 (w), 623 (m), 546 (w) cm−1. UV/VIS (n-hexane): λmax (lg ε max) = 275 (5.15), 214 (5.60) nm. C368H624O40 (5688.9) calcd. C 77.69, H 11.06; found: C 77.74, H 10.95.
Supporting Information
Supporting information includes experimental and spectroscopic data for compounds 2a–f, 2g–l, and X-ray diffraction measurements of derivatives 2f,j.
| Supporting Information File 1: Analytical data of compounds 2a–f, 2g–l. | ||
| Format: PDF | Size: 115.3 KB | Download |
Acknowledgments
Generous financial support by the Deutsche Forschungsgemeinschaft, the Fonds der Chemischen Industrie, the Ministerium für Wissenschaft, Forschung und Kunst des Landes Baden-Württemberg and the Bundesministerium für Bildung und Forschung (grant # 01 RI 05177) is gratefully acknowledged.
References
-
Laschat, S.; Baro, A.; Steinke, N.; Giesselmann, F.; Hägele, C.; Scalia, G.; Judele, R.; Kapatsina, E.; Sauer, S.; Schreivogel, A.; Tosoni, M. Angew. Chem. 2007, 119, 4916–4973. doi:10.1002/ange.200604203
Angew. Chem., Int. Ed. 2007, 46, 4832–4887. doi:10.1002/anie.200604203 (Review).
Return to citation in text: [1] -
Sergeyev, S.; Pisula, W.; Geerts, Y. H. Chem. Soc. Rev. 2007, 36, 1902–1929. doi:10.1039/b417320c
(Review).
Return to citation in text: [1] -
Tschierske, C. Annu. Rep. Prog. Chem., Sect. C: Phys. Chem. 2001, 97, 191–267. doi:10.1039/b101114f
(Review).
Return to citation in text: [1] [2] -
Rathore, R.; Le Magueres, P.; Lindeman, S. V.; Kochi, J. K. Angew. Chem. 2000, 112, 818–821. doi:10.1002/(SICI)1521-3757(20000218)112:4<818::AID-ANGE818>3.0.CO;2-#
Angew. Chem., Int. Ed. 2000, 39, 809–812. doi:10.1002/(SICI)1521-3773(20000218)39:4<809::AID-ANIE809>3.0.CO;2-6
Return to citation in text: [1] [2] [3] -
Iyoda, M.; Kabir, S. M. H.; Vorasingha, A.; Kuwatani, Y.; Yoshida, M. Tetrahedron Lett. 1998, 39, 5393–5396. doi:10.1016/S0040-4039(98)01082-X
Return to citation in text: [1] [2] -
Irngartinger, H.; Reibel, W. R. K. Acta Crystallogr., Sect. B 1981, 37, 1724–1728. doi:10.1107/S0567740881006985
Return to citation in text: [1] [2] -
Huang, H.; Hau, C.-K.; Law, C. C. M.; Wong, H. N. C. Org. Biomol. Chem. 2009, 7, 1249–1257. doi:10.1039/b818029f
Return to citation in text: [1] -
Rajca, A.; Rajca, S.; Pink, M.; Miyasaka, M. Synlett 2007, 1799–1822. doi:10.1055/s-2007-984538
(Review).
Return to citation in text: [1] -
Hou, X.-L.; Huang, H.; Wong, H. N. C. Synlett 2005, 1073–1089. doi:10.1055/s-2005-865213
(Review).
Return to citation in text: [1] -
Mak, T. C. W.; Wong, H. N. C. In Comprehensive Supramolecular Chemistry; MacNicol, D. D.; Toda, F.; Bishop, R., Eds.; Elsevier: Oxford, U.K., 1996; Vol. 6, pp 351–369.
(Review).
Return to citation in text: [1] -
Mak, T. C. W.; Wong, H. N. C. Top. Curr. Chem. 1987, 140, 141–164. doi:10.1007/BFb0003839
(Review).
Return to citation in text: [1] -
Huang, H.; Stewart, T.; Gutmann, M.; Ohhara, T.; Niimura, N.; Li, Y.-X.; Wen, J.-F.; Bau, R.; Wong, H. N. C. J. Org. Chem. 2009, 74, 359–369. doi:10.1021/jo802061p
Return to citation in text: [1] -
Wu, A.-H.; Hau, C.-K.; Wong, H. N. C. Adv. Synth. Catal. 2007, 349, 601–608. doi:10.1002/adsc.200600499
Return to citation in text: [1] -
Peng, H.-Y.; Lam, C.-K.; Mak, T. C. W.; Cai, Z.; Ma, W.-T.; Li, Y.-X.; Wong, H. N. C. J. Am. Chem. Soc. 2005, 127, 9603–9611. doi:10.1021/ja051013l
Return to citation in text: [1] -
Hui, C. W.; Mak, T. C. W.; Wong, H. N. C. Tetrahedron 2004, 60, 3523–3531. doi:10.1016/j.tet.2004.02.022
Return to citation in text: [1] -
Wen, J.-F.; Hong, W.; Yuan, K.; Mak, T. C. W.; Wong, H. N. C. J. Org. Chem. 2003, 68, 8918–8931. doi:10.1021/jo0302408
Return to citation in text: [1] -
Lai, C. W.; Lam, C. K.; Lee, H. K.; Mak, T. C. W.; Wong, H. N. C. Org. Lett. 2003, 5, 823–826. doi:10.1021/ol020253s
Return to citation in text: [1] -
Rajca, A.; Wang, H.; Bolshov, P.; Rajca, S. Tetrahedron 2001, 57, 3725–3735. doi:10.1016/S0040-4020(01)00241-1
Return to citation in text: [1] -
Kabir, S. M. H.; Hasegawa, M.; Kuwatani, Y.; Yoshida, M.; Matsuyama, H.; Iyoda, M. J. Chem. Soc., Perkin Trans. 1 2001, 159–165. doi:10.1039/b006375o
Return to citation in text: [1] [2] -
Kabir, S. M. H.; Iyoda, M. Synthesis 2000, 1839–1842. doi:10.1055/s-2000-8239
Return to citation in text: [1] [2] -
Yang, X.-P.; Du, D.-M.; Li, Q.; Mak, T. C. W.; Wong, H. N. C. Chem. Commun. 1999, 1607–1608. doi:10.1039/a904144c
Return to citation in text: [1] -
Rajca, A.; Safronov, A.; Rajca, S.; Shoemaker, R. Angew. Chem. 1997, 109, 504–507. doi:10.1002/ange.19971090510
Angew. Chem., Int. Ed. Engl. 1997, 36, 488–491. doi:10.1002/anie.199704881
Return to citation in text: [1] -
Wang, X. M.; Hou, X.; Zhou, Z.; Mak, T. C. W.; Wong, H. N. C. J. Org. Chem. 1993, 58, 7498–7506. doi:10.1021/jo00078a031
Return to citation in text: [1] -
Man, Y. M.; Mak, T. C. W.; Wong, H. N. C. J. Org. Chem. 1990, 55, 3214–3221. doi:10.1021/jo00297a043
Return to citation in text: [1] -
Rashidi-Ranjbar, P.; Man, Y. M.; Sandstroem, J.; Wong, H. N. C. J. Org. Chem. 1989, 54, 4888–4892. doi:10.1021/jo00281a034
Return to citation in text: [1] -
Wong, H. N. C.; Man, Y.-M.; Mak, T. C. W. Tetrahedron Lett. 1987, 28, 6359–6362. doi:10.1016/S0040-4039(01)91373-5
Return to citation in text: [1] -
Wong, H. N. C.; Luh, T. Y.; Mak, T. C. W. Acta Crystallogr., Sect. C 1984, 40, 1721–1723. doi:10.1107/S0108270184009288
Return to citation in text: [1] -
Wong, H. N. C.; Sondheimer, F. Tetrahedron 1981, 37, 99–109. doi:10.1016/0040-4020(81)85045-4
Return to citation in text: [1] -
Wuckert, E.; Laschat, S.; Baro, A.; Hägele, C.; Giesselmann, F.; Luftmann, H. Liq. Cryst. 2006, 33, 103–107. doi:10.1080/02678290500277953
Return to citation in text: [1] [2] [3] -
Wuckert, E.; Dix, M.; Laschat, S.; Baro, A.; Schulte, J. L.; Hägele, C.; Giesselmann, F. Liq. Cryst. 2004, 31, 1305–1309. doi:10.1080/02678290412331312051
Return to citation in text: [1] [2] [3] -
Hägele, C.; Wuckert, E.; Laschat, S.; Giesselmann, F. ChemPhysChem 2009, 10, 1291–1298. doi:10.1002/cphc.200900090
Return to citation in text: [1] [2] [3] [4] [5] -
Lai, C. K.; Tsai, C.-H.; Pang, Y.-S. J. Mater. Chem. 1998, 8, 1355–1360. doi:10.1039/a800657a
Return to citation in text: [1] -
Zheng, H.; Lai, C. K.; Swager, T. M. Chem. Mater. 1995, 7, 2067–2077. doi:10.1021/cm00059a013
Return to citation in text: [1] -
Tinh, N. H.; Levelut, A. M.; Malthête, J. Mol. Cryst. Liq. Cryst. 1984, 106, 121–146. doi:10.1080/00268948408080183
Return to citation in text: [1] -
Chem3D Pro 7.0 Software was used for molecular modelling.
Return to citation in text: [1] [2]
| 1. |
Laschat, S.; Baro, A.; Steinke, N.; Giesselmann, F.; Hägele, C.; Scalia, G.; Judele, R.; Kapatsina, E.; Sauer, S.; Schreivogel, A.; Tosoni, M. Angew. Chem. 2007, 119, 4916–4973. doi:10.1002/ange.200604203
Angew. Chem., Int. Ed. 2007, 46, 4832–4887. doi:10.1002/anie.200604203 (Review). |
| 2. |
Sergeyev, S.; Pisula, W.; Geerts, Y. H. Chem. Soc. Rev. 2007, 36, 1902–1929. doi:10.1039/b417320c
(Review). |
| 3. |
Tschierske, C. Annu. Rep. Prog. Chem., Sect. C: Phys. Chem. 2001, 97, 191–267. doi:10.1039/b101114f
(Review). |
| 31. | Hägele, C.; Wuckert, E.; Laschat, S.; Giesselmann, F. ChemPhysChem 2009, 10, 1291–1298. doi:10.1002/cphc.200900090 |
| 29. | Wuckert, E.; Laschat, S.; Baro, A.; Hägele, C.; Giesselmann, F.; Luftmann, H. Liq. Cryst. 2006, 33, 103–107. doi:10.1080/02678290500277953 |
| 30. | Wuckert, E.; Dix, M.; Laschat, S.; Baro, A.; Schulte, J. L.; Hägele, C.; Giesselmann, F. Liq. Cryst. 2004, 31, 1305–1309. doi:10.1080/02678290412331312051 |
| 3. |
Tschierske, C. Annu. Rep. Prog. Chem., Sect. C: Phys. Chem. 2001, 97, 191–267. doi:10.1039/b101114f
(Review). |
| 4. |
Rathore, R.; Le Magueres, P.; Lindeman, S. V.; Kochi, J. K. Angew. Chem. 2000, 112, 818–821. doi:10.1002/(SICI)1521-3757(20000218)112:4<818::AID-ANGE818>3.0.CO;2-#
Angew. Chem., Int. Ed. 2000, 39, 809–812. doi:10.1002/(SICI)1521-3773(20000218)39:4<809::AID-ANIE809>3.0.CO;2-6 |
| 5. | Iyoda, M.; Kabir, S. M. H.; Vorasingha, A.; Kuwatani, Y.; Yoshida, M. Tetrahedron Lett. 1998, 39, 5393–5396. doi:10.1016/S0040-4039(98)01082-X |
| 6. | Irngartinger, H.; Reibel, W. R. K. Acta Crystallogr., Sect. B 1981, 37, 1724–1728. doi:10.1107/S0567740881006985 |
| 7. | Huang, H.; Hau, C.-K.; Law, C. C. M.; Wong, H. N. C. Org. Biomol. Chem. 2009, 7, 1249–1257. doi:10.1039/b818029f |
| 8. |
Rajca, A.; Rajca, S.; Pink, M.; Miyasaka, M. Synlett 2007, 1799–1822. doi:10.1055/s-2007-984538
(Review). |
| 9. |
Hou, X.-L.; Huang, H.; Wong, H. N. C. Synlett 2005, 1073–1089. doi:10.1055/s-2005-865213
(Review). |
| 10. |
Mak, T. C. W.; Wong, H. N. C. In Comprehensive Supramolecular Chemistry; MacNicol, D. D.; Toda, F.; Bishop, R., Eds.; Elsevier: Oxford, U.K., 1996; Vol. 6, pp 351–369.
(Review). |
| 11. |
Mak, T. C. W.; Wong, H. N. C. Top. Curr. Chem. 1987, 140, 141–164. doi:10.1007/BFb0003839
(Review). |
| 12. | Huang, H.; Stewart, T.; Gutmann, M.; Ohhara, T.; Niimura, N.; Li, Y.-X.; Wen, J.-F.; Bau, R.; Wong, H. N. C. J. Org. Chem. 2009, 74, 359–369. doi:10.1021/jo802061p |
| 13. | Wu, A.-H.; Hau, C.-K.; Wong, H. N. C. Adv. Synth. Catal. 2007, 349, 601–608. doi:10.1002/adsc.200600499 |
| 14. | Peng, H.-Y.; Lam, C.-K.; Mak, T. C. W.; Cai, Z.; Ma, W.-T.; Li, Y.-X.; Wong, H. N. C. J. Am. Chem. Soc. 2005, 127, 9603–9611. doi:10.1021/ja051013l |
| 15. | Hui, C. W.; Mak, T. C. W.; Wong, H. N. C. Tetrahedron 2004, 60, 3523–3531. doi:10.1016/j.tet.2004.02.022 |
| 16. | Wen, J.-F.; Hong, W.; Yuan, K.; Mak, T. C. W.; Wong, H. N. C. J. Org. Chem. 2003, 68, 8918–8931. doi:10.1021/jo0302408 |
| 17. | Lai, C. W.; Lam, C. K.; Lee, H. K.; Mak, T. C. W.; Wong, H. N. C. Org. Lett. 2003, 5, 823–826. doi:10.1021/ol020253s |
| 18. | Rajca, A.; Wang, H.; Bolshov, P.; Rajca, S. Tetrahedron 2001, 57, 3725–3735. doi:10.1016/S0040-4020(01)00241-1 |
| 19. | Kabir, S. M. H.; Hasegawa, M.; Kuwatani, Y.; Yoshida, M.; Matsuyama, H.; Iyoda, M. J. Chem. Soc., Perkin Trans. 1 2001, 159–165. doi:10.1039/b006375o |
| 20. | Kabir, S. M. H.; Iyoda, M. Synthesis 2000, 1839–1842. doi:10.1055/s-2000-8239 |
| 21. | Yang, X.-P.; Du, D.-M.; Li, Q.; Mak, T. C. W.; Wong, H. N. C. Chem. Commun. 1999, 1607–1608. doi:10.1039/a904144c |
| 22. |
Rajca, A.; Safronov, A.; Rajca, S.; Shoemaker, R. Angew. Chem. 1997, 109, 504–507. doi:10.1002/ange.19971090510
Angew. Chem., Int. Ed. Engl. 1997, 36, 488–491. doi:10.1002/anie.199704881 |
| 23. | Wang, X. M.; Hou, X.; Zhou, Z.; Mak, T. C. W.; Wong, H. N. C. J. Org. Chem. 1993, 58, 7498–7506. doi:10.1021/jo00078a031 |
| 24. | Man, Y. M.; Mak, T. C. W.; Wong, H. N. C. J. Org. Chem. 1990, 55, 3214–3221. doi:10.1021/jo00297a043 |
| 25. | Rashidi-Ranjbar, P.; Man, Y. M.; Sandstroem, J.; Wong, H. N. C. J. Org. Chem. 1989, 54, 4888–4892. doi:10.1021/jo00281a034 |
| 26. | Wong, H. N. C.; Man, Y.-M.; Mak, T. C. W. Tetrahedron Lett. 1987, 28, 6359–6362. doi:10.1016/S0040-4039(01)91373-5 |
| 27. | Wong, H. N. C.; Luh, T. Y.; Mak, T. C. W. Acta Crystallogr., Sect. C 1984, 40, 1721–1723. doi:10.1107/S0108270184009288 |
| 28. | Wong, H. N. C.; Sondheimer, F. Tetrahedron 1981, 37, 99–109. doi:10.1016/0040-4020(81)85045-4 |
| 31. | Hägele, C.; Wuckert, E.; Laschat, S.; Giesselmann, F. ChemPhysChem 2009, 10, 1291–1298. doi:10.1002/cphc.200900090 |
| 4. |
Rathore, R.; Le Magueres, P.; Lindeman, S. V.; Kochi, J. K. Angew. Chem. 2000, 112, 818–821. doi:10.1002/(SICI)1521-3757(20000218)112:4<818::AID-ANGE818>3.0.CO;2-#
Angew. Chem., Int. Ed. 2000, 39, 809–812. doi:10.1002/(SICI)1521-3773(20000218)39:4<809::AID-ANIE809>3.0.CO;2-6 |
| 5. | Iyoda, M.; Kabir, S. M. H.; Vorasingha, A.; Kuwatani, Y.; Yoshida, M. Tetrahedron Lett. 1998, 39, 5393–5396. doi:10.1016/S0040-4039(98)01082-X |
| 6. | Irngartinger, H.; Reibel, W. R. K. Acta Crystallogr., Sect. B 1981, 37, 1724–1728. doi:10.1107/S0567740881006985 |
| 34. | Tinh, N. H.; Levelut, A. M.; Malthête, J. Mol. Cryst. Liq. Cryst. 1984, 106, 121–146. doi:10.1080/00268948408080183 |
| 32. | Lai, C. K.; Tsai, C.-H.; Pang, Y.-S. J. Mater. Chem. 1998, 8, 1355–1360. doi:10.1039/a800657a |
| 33. | Zheng, H.; Lai, C. K.; Swager, T. M. Chem. Mater. 1995, 7, 2067–2077. doi:10.1021/cm00059a013 |
| 31. | Hägele, C.; Wuckert, E.; Laschat, S.; Giesselmann, F. ChemPhysChem 2009, 10, 1291–1298. doi:10.1002/cphc.200900090 |
| 4. |
Rathore, R.; Le Magueres, P.; Lindeman, S. V.; Kochi, J. K. Angew. Chem. 2000, 112, 818–821. doi:10.1002/(SICI)1521-3757(20000218)112:4<818::AID-ANGE818>3.0.CO;2-#
Angew. Chem., Int. Ed. 2000, 39, 809–812. doi:10.1002/(SICI)1521-3773(20000218)39:4<809::AID-ANIE809>3.0.CO;2-6 |
| 19. | Kabir, S. M. H.; Hasegawa, M.; Kuwatani, Y.; Yoshida, M.; Matsuyama, H.; Iyoda, M. J. Chem. Soc., Perkin Trans. 1 2001, 159–165. doi:10.1039/b006375o |
| 20. | Kabir, S. M. H.; Iyoda, M. Synthesis 2000, 1839–1842. doi:10.1055/s-2000-8239 |
| 29. | Wuckert, E.; Laschat, S.; Baro, A.; Hägele, C.; Giesselmann, F.; Luftmann, H. Liq. Cryst. 2006, 33, 103–107. doi:10.1080/02678290500277953 |
| 30. | Wuckert, E.; Dix, M.; Laschat, S.; Baro, A.; Schulte, J. L.; Hägele, C.; Giesselmann, F. Liq. Cryst. 2004, 31, 1305–1309. doi:10.1080/02678290412331312051 |
| 31. | Hägele, C.; Wuckert, E.; Laschat, S.; Giesselmann, F. ChemPhysChem 2009, 10, 1291–1298. doi:10.1002/cphc.200900090 |
| 29. | Wuckert, E.; Laschat, S.; Baro, A.; Hägele, C.; Giesselmann, F.; Luftmann, H. Liq. Cryst. 2006, 33, 103–107. doi:10.1080/02678290500277953 |
| 30. | Wuckert, E.; Dix, M.; Laschat, S.; Baro, A.; Schulte, J. L.; Hägele, C.; Giesselmann, F. Liq. Cryst. 2004, 31, 1305–1309. doi:10.1080/02678290412331312051 |
| 31. | Hägele, C.; Wuckert, E.; Laschat, S.; Giesselmann, F. ChemPhysChem 2009, 10, 1291–1298. doi:10.1002/cphc.200900090 |
© 2009 Wuckert et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)