Abstract
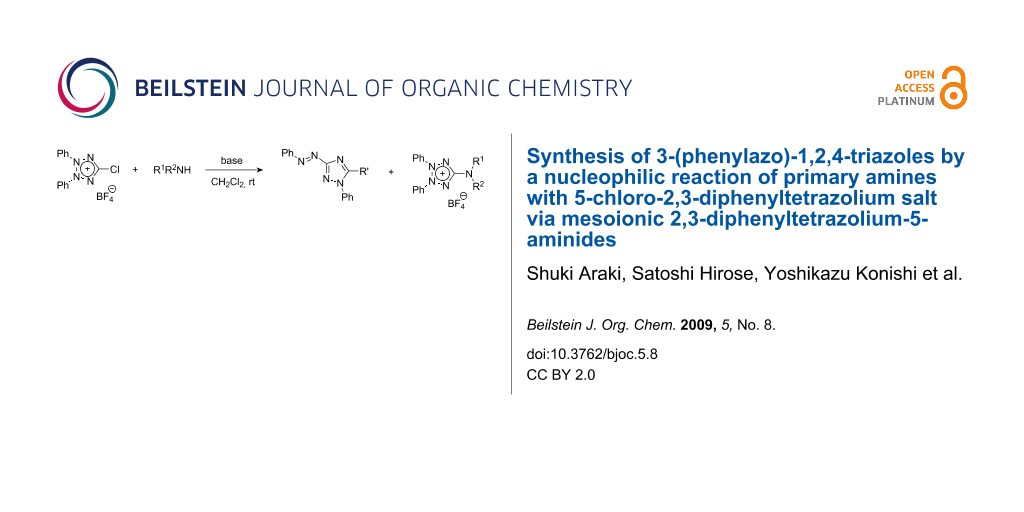
The reactions of a 5-chloro-2,3-diphenyltetrazolium salt with amines have been examined. In the presence of an inorganic base such as NaHCO3, primary and secondary amines undergo a nucleophilic substitution to give the corresponding 5-aminotetrazolium salts. When triethylamine is used as a base, primary amines give 3-phenylazo-1,2,4-triazoles. A plausible dual-path mechanism is proposed for the formation of the triazoles via Type B mesoionic tetrazolium-5-aminides.
Graphical Abstract
Introduction
Mesoionic compounds can be classified into two families, type A and type B mesoions, according to their electronic arrangements [1-5]. Type B mesoionic compounds are less common compared with type A mesoions. The 5-chloro-1,3-diphenyltetrazolium salt (1) has been proved to be a versatile precursor to several type A 1,3-diphenyltetrazolium mesoionic compounds, because the chlorine atom is a good leaving group and easily replaced by a variety of nucleophiles [6,7]. Although the corresponding 5-chloro-2,3-diphenyltetrazolium salt 2 is not yet known, it is expected that 2 is also useful for the synthesis of type B tetrazolium mesoions. In this paper, we disclose the first preparation of this chlorotetrazolium salt 2 and its reactions with amines, which involve the unexpected formation of 3-(phenylazo)-1,2,4-triazole derivatives.
Results and Discussion
The 5-chloro-2,3-diphenyltetrazolium salt 2 was prepared in an analogous manner to the corresponding 1,3-diphenyl isomer 1 [6,7]. Thus, the treatment of 2,3-diphenyltetrazolium-5-olate with phosphorus oxychloride gave 2 in high yield as stable crystals, after an anion exchange to tetrafluoroborate.
Next, the reactions of 2 with various amines were examined. When 2 was treated with benzylamine in dichloromethane in the presence of triethylamine, 1,5-diphenyl-3-(phenylazo)-1H-1,2,4-triazole (3a) was formed in 50% yield. The expected substitution product, 2,3-diphenyl-5-(benzylamino)tetrazolium salt 4a, was not found in the reaction mixture. Interestingly, when the base was changed to solid sodium carbonate or sodium hydrogencarbonate, 4a was obtained exclusively (Scheme 1).
Scheme 1: Reaction of chlorotetrazolium 2 with benzylamine.
Scheme 1: Reaction of chlorotetrazolium 2 with benzylamine.
The results with other amines are summarized in Table 1. The reaction of butylamine showed a similar tendency: with the triethylamine base triazole 3b was obtained, whereas with sodium hydrogencarbonate 5-aminotetrazolium 4b was formed. Other primary amines, such as ethanolamine and ethyl glycinate gave the corresponding triazoles 3c and 3d. Interestingly, sterically bulky primary amines as well as secondary amines gave the respective 5-aminotetrazolium salts 4d–h. It is worthy to note that the reaction of isopropylamine/triethylamine gave 3-(isopropylideneamino)-1,5-diphenylformazan as the major product.
Table 1: Reaction of chlorotetrazolium 2 with various amines.
|
|
|||||||
| Entry | R1R2NH (equiv) | Base (equiv) | Time (h) | Yield (%) | |||
|---|---|---|---|---|---|---|---|
| 3 | 4 | ||||||
| 1a,b | PhCH2NH2 | 1.0 | Et3N | 3.0 | 2.5 | 3a 50 (R' = Ph) | 0 |
| 2c | PhCH2NH2 | 1.0 | NaHCO3 | 1.0 | 22 | 0 | 4a 68 (R1 = CH2Ph, R2 = H) |
| 3 | PhCH2NH2 | 1.0 | Na2CO3 | 1.0 | 19 | 0 | 4a 39 |
| 4 | n-BuNH2 | 1.0 | Et3N | 2.0 | 72 | 3b 23 (R' = n-Pr) | 0 |
| 5 | n-BuNH2 | 1.0 | NaHCO3 | 1.0 | 20 | 0 | 4b 34 (R1 = n-Bu, R2 = H) |
| 6a,b,c | HOCH2CH2NH2 | 1.0 | Et3N | 3.0 | 3.5 | 3c 50 (R' = CH2OH) | 4c 2 (R1 = CH2CH2OH, R2 = H) |
| 7a,b,c | NH2CH2CO2Etd | 1.0 | Et3N | 4.0 | 4 | 3d 39 (R' = CO2Et) | 0 |
| 8b,e | i-PrNH2 | 1.0 | Et3N | 1.0 | 20 | 0 | 4d 38 (R1 = i-Pr, R2 = H) |
| 9 | i-PrNH2 | 1.0 | NaHCO3 | 1.0 | 20 | 0 | 4d 22 |
| 10a,c | t-BuNH2 | 1.4 | Et3N | 1.0 | 2 | 0 | 4e 55 (R1 = t-Bu, R2 = H) |
| 11 | (PhCH2)2NH | 0.83 | Et3N | 2.0 | 3 | 0 | 4f 73 (R1 = R2 = CH2Ph) |
| 12 | n-Bu2NH | 1.0 | Et3N | 1.0 | 4 | 0 | 4g 96 (R1 = R2 = n-Bu) |
| 13 | Et2NH | 1.0 | NaHCO3 | 1.0 | 18 | 0 | 4h 41 (R1 = R2 = Et) |
aThe reaction was conducted under reflux. bUnder air. cMeCN was used as a solvent. dThe hydrochloride salt was used. e3-(Isopropylideneamino)-1,5-diphenylformazan was obtained (48% yield).
As a representative tertiary amine, triethylamine was also subjected to the reaction with 2. The reaction proceeded under reflux in dichloromethane to afford 5-(diethylamino)tetrazolium salt 4h in 60% yield, presumably via an Arbuzov-type reaction (Scheme 2). The reactions with other nucleophiles than amines such as alcohols and thiols did not give the corresponding oxa- and thiadiazole derivatives.
Scheme 2: Reaction of chlorotetrazolium 2 with triethylamine.
Scheme 2: Reaction of chlorotetrazolium 2 with triethylamine.
Reaction mechanism
The reaction mechanism for the formation of triazole 3 was investigated. First, the 5-(benzylamino)tetrazolium salt 4a was treated with triethylamine in dichloromethane at room temperature. Triazole 3a was obtained in 16% yield. Although the yield is not high, this fact shows that 3a is derived, at least partially, from 4a (Scheme 3).
Scheme 3: Conversion of benzylaminotetrazolium 4a to 1,2,4-triazole 3a.
Scheme 3: Conversion of benzylaminotetrazolium 4a to 1,2,4-triazole 3a.
Next, the possibility that 3 is formed by the oxidation of 3-amino-1,5-diphenylformazan 5 was examined. In order to synthesize 3-(benzylamino)formazan 5a, the reduction of 4a with reductants such as NaBH4 and DIBAL was attempted. However, the desired formazan 5a was not obtained at all. Then, a nucleophilic substitution of 3-chloroformazan 6 with benzylamine was undertaken. The chlorotetrazolium salt 2 was easily converted to 6 with NaBH4 (43% yield) or p-(dimethylamino)aniline (73% yield). The reaction of 6 with benzylamine in ethanol, however, did not give the expected 5a, but triazole 3a was formed in 30% yield. In this reaction, 3a is considered to be formed via initially produced formazan 5a, which is spontaneously oxidized in situ. As these results imply that formazan 5a is too unstable to be isolated, the preparation of 3-(butylamino)formazan 5b was next planned. Fortunately, the reaction of 6 with butylamine proceeded and the desired 5b could be isolated in 22% yield. As expected, aerobic oxidation of 5b in ethanol gave triazole 3b in 69% yield, though the reaction took 10 d (Scheme 4).
Scheme 4: Synthesis of chloroformazan 6 and its reaction with benzylamine and butylamine.
Scheme 4: Synthesis of chloroformazan 6 and its reaction with benzylamine and butylamine.
On the basis of these experimental results, the most plausible reaction mechanism is illustrated in Scheme 5. Nucleophilic substitution of the chlorine atom of 2 by amine gives 5-aminotetrazolium salt 4. Deprotonation of 4 affords tetazolium-5-aminide A, which is in the equilibrium with the acyclic tautomer B. Further deprotonation from B followed by an intramolecular ring-closure gives C, which is oxidized by air to furnish triazole 3. The sterically demanding primary amines (isopropylamine and tert-butylamine) are considered to be difficult to give the cyclized intermediate C. The less efficient behaviour of the carbonates in the deprotonation process may be attributed to the heterogeneous reaction system. In the last oxidation stage from C to 3, aminotetrazolium 4 is considered also to act as a hydride acceptor and 4 itself is transformed to 3-aminoformazan 5. Thus, the formation of triazole 3 is considered to be a dual-path process: via oxidation of the intermediate C and formazan 5. It is worthy to note here that a similar 1,5-dipolar ring closure of type B mesoionic thiocarbonyl ylides is known to furnish thiadiazolines [8].
Scheme 5: Plausible reaction mechanism for the formation of 1,2,4-triazole 3.
Scheme 5: Plausible reaction mechanism for the formation of 1,2,4-triazole 3.
Conclusion
It has now been revealed that the 5-chlorotetrazolium salt 2 reacts with primary and secondary amines to give the corresponding 5-aminotetrazolium salts 4. When triethylamine is employed as a base in the reaction with primary amines, deprotonation from 4 proceeds readily to give tetrazolium-5-aminides A. Contrary to the stable type A mesoionic tetrazolium-5-aminides [6,7], type B mesoionic aminide A undergoes further transformations from its acyclic tautomer B to furnish 1,2,4-triazoles 3.
Experimental
General
Melting points were measured with a hot-stage apparatus Yanaco MP 50533 and are uncorrected. Elemental analyses were carried out with a Perkin Elmer 2400 II CHNS/O. IR spectra were taken as KBr discs on a JASCO A-102 spectrometer. Electronic spectra were measured on a Hitachi U-3500 or a Shimadzu UV-2450 spectrophotometer. 1H NMR spectra were obtained using a Varian Mercury 200 (200 MHz) or a Varian Mercury 300 (300 MHz), and 13C NMR spectra were obtained using a Varian Mercury 200 (50 MHz). Chemical shifts are recorded in ppm downfield from tetramethylsilane. J values are given in Hz. Mass spectra were taken with a Hitachi M-2000 spectrometer (EI, 70 eV). For TLC, Merck Silica gel 60 F254 Plate was used. For column chromatography, Merck Silica gel 60 (0.063–0.200 mm) was used.
5-Chloro-2,3-diphenyltetrazolium tetrafluoroborate (2)
A mixture of 2,3-diphenyltetrazolium-5-olate (1.0 g, 4.2 mmol) and phosphorus oxychloride (2.2 ml, 24 mmol) was heated at 110 °C for 16 h. The excess phosphorus oxychloride was removed under reduced pressure and the residue was mixed with 42% fluoroboric acid (4.4 ml) and ultrasonicated for 30 min. The mixture was filtered, and the solid was washed with cold THF to give 2 (1.0 g, 72%). From the filtrate and washings, a further amount (0.27 g, 18%) of 2 was obtained; total 1.27 g, 90%. mp 208–212 °C (EtOH); IR (KBr) νmax 3060, 3045, 1482, 1462, 1388, 1362, 1300, 1158, 1122, 1082, 1036, 1000, 788, 780, 764, 692, 684; 1H NMR (200 MHz, CD3CN) δ 7.67–7.70 (m, 8H), 7.75–7.87 (m, 2H), 13C NMR (50 MHz, CD3CN) δ 126.29 (o-Ph), 131.08 (m-Ph), 133.00 (i-Ph), 135.14 (p-Ph), 158.10 (C+); UV/VIS (MeCN): λmax (log ε) = 243.0 (3.41), 272.0 (3.57). Anal. calcd for C13H10BClF4N4 (344.5): C, 45.32; H, 2.93; N, 16.26. Found: C, 45.32; H, 3.10; N, 16.52.
1,5-Diphenyl-3-(phenylazo)-1H-[1,2,4]triazole (3a)
A mixture of 2 (345 mg, 1.0 mmol), benzylamine (110 μl, 1.0 mmol), and triethylamine (0.42 ml, 3.0 mmol) in dichloromethane (6 ml) was heated at reflux for 2.5 h. The reaction mixture was poured into water, and the product was extracted with dichloromethane. The extract was washed with water and diluted hydrochloric acid (1 N). After being dried over Na2SO4, the solvent was removed and the residue (334 mg) was purified by column chromatography on silica gel (eluent: dichloromethane, then acetonitrile) to give 3a (163 mg, 50%).
Orange needles, mp 146.7–148.0 °C (EtOH); IR (KBr) νmax 3070, 1594, 1496, 1448, 1380, 1362, 1300, 1178, 1150, 1076, 1020, 990, 924, 916, 800, 770, 740, 718, 690; 1H NMR (200 MHz, CDCl3) δ 7.35–7.62 (m, 13H), 8.10–8.15 (m, 2H, o-PhN=N-); 13C NMR (50 MHz, CDCl3) δ 123.5, 125.1, 126.8, 128.2, 128.8, 129.0, 129.0, 129.2, 130.3, 132.1, 137.4, 152.4, 155.0, 168.1; MS (EI, 70 eV) m/z 325 (M+, 3), 220 (4), 180 (100), 117 (24), 77 (53), 31 (13), 27 (54); UV-vis (MeCN) λmax (log ε)/nm: 325 (3.3); Anal. calcd. for C20H15N5 (325.37): C, 73.82; H, 4.65; N, 21.53. Found: C, 73.73; H, 4.44; N, 21.58.
Reaction of 2 with triethylamine
The salt 2 (175 mg, 0.51 mmol) was treated with triethylamine (140 μl, 1.0 mmol) in dichloromethane (6 ml) under reflux for 16 h. The reaction mixture was poured into water, and the product was extracted with dichloromethane. The extract was washed with diluted hydrochloric acid (1 N). After being dried over Na2SO4, the solvent was removed to give 4h (115 mg, 60%).
Conversion of 4a to 3a
A mixture of 4a (167 mg, 0.40 mmol) and triethylamine (112 μl, 0.80 mmol) in dichloromethane (4 ml) was stirred at room temperature for 21 h under argon. The reaction mixture was diluted with ether, and washed with diluted hydrochloric acid (1 N) and water. After being dried over Na2SO4, the solvent was removed and the residue (72 mg) was purified by column chromatography on silica gel (eluent: CH2Cl2→MeCN) to give a crude product. Recrystallization from ethanol gave 3a (21 mg, 16%).
Reduction of chlorotetrazolium salt 2
1. With NaBH4
To a solution of 2 (176 mg, 0.51 mmol) in dichloromethane (5 ml) was added NaBH4 (7 mg, 0.18 mmol) and the mixture was stirred for 4 h. Additional NaBH4 (2 mg, 0.053 mmol) was added and the reaction was continued further for 4 h. Water was added and the product was extracted with dichloromethane. The extract was washed with water and diluted hydrochloric acid (1 N). After being dried over Na2SO4, the solvent was removed to give 6 (56 mg, 43%).
2. With p-(dimethylamino)aniline
A mixture of 2 (345 mg, 1.0 mmol) and p-(dimethylamino)aniline (140 mg, 1.0 mmol) in dichloromethane (5 ml) was stirred overnight at room temperature. The solvent was evaporated and the residue was dissolved in acetone. Hexane was added and the resultant black solid was filtered off. Evaporation of the solvent from the filtrate left 6 (190 mg, 73%) as orange crystals.
Reaction of 3-chloroformazan 6 with benzylamine
A mixture of 3-chloroformazan 6 (130 mg, 0.50 mmol) and benzylamine (0.30 ml, 2.5 mmol) in ethanol (3 ml) was stirred at room temperature for 5 h. The precipitated solid (benzylamine hydrochloride) was filtered off and the filtrate was concentrated. The residue was washed several times with ether. The washings were poured into water and extracted with dichloromethane. The extract was washed with diluted hydrochloric acid (1 N). After being dried over Na2SO4, the solvent was removed and the residue (141 mg) was purified by column chromatography on silica gel (eluent: CH2Cl2→MeCN) to give a brown solid (57 mg). This product was further purified by column chromatography on silica gel (eluent: CH2Cl2) to give triazole 3a (49 mg, 30%).
3-(Butylamino)-1,5-diphenylformazan (5b)
A mixture of 6 (85 mg, 0.33 mmol) and butylamine (100 μl, 1.0 mmol) in dichloromethane (5 ml) was stirred overnight at room temperature. Diluted hydrochloric acid (1 N) was added and the product was extracted with dichloromethane. After being dried over Na2SO4, the solvent was removed and the residue (80 mg) was purified by column chromatography on silica gel (eluent: CH2Cl2→MeOH) to give a mixture of E/Z isomer of 5b (21 mg, 22%) as a green solid. This compound was easily oxidized by air, and satisfactory elemental analysis data and mass spectrum were not obtained.
1H NMR (300 MHz, CDCl3) δ 0.90–1.00 (m, 3H), 1.40–1.69 (m, 4H), 3.12 (q), 3.41 (q), 4.90 (br, NH), 5.26 (br, NH), 6.99 (br, NH), 7.15–7.60 (m), 7.78–7.82 (m), 7.89–7.92 (m), 8.08 (s, NH), 9.42 (s, NH).
13C NMR (50 MHz, CDCl3) δ 14.0, 14.2, 20.1, 20.5, 31.7, 33.1, 41.7, 43.7, 113.9, 117.9, 121.2, 123.0, 125.3, 128.8, 129.0, 130.6, 143.3, 147.6, 148.0, 151.1, 154.1.
Oxidation of 5b to triazole 3b
A solution of 5b (139 mg, 0.47 mmol) in ethanol (20 ml) was allowed to stand at room temperature for 10 d. The solvent was evaporated and the residue was column chromatographed on silica gel (eluent: CH2Cl2) to give 3b (94 mg, 69%).
References
-
Stewart, F. H. C. Chem. Rev. 1964, 64, 129–147. doi:10.1021/cr60228a004
Return to citation in text: [1] -
Ohta, M.; Kato, M. In Nonbenzenoid Aromatics; Snyder, J. P., Ed.; Academic Press: New York, 1969; pp 117–248.
Return to citation in text: [1] -
Ollis, W. D.; Ramsden, C. A. Adv. Heterocycl. Chem. 1976, 19, 1–122. doi:10.1016/S0065-2725(08)60230-5
Return to citation in text: [1] -
Newton, C. G.; Ramsden, C. A. Tetrahedron 1982, 38, 2965–3011. doi:10.1016/0040-4020(82)80186-5
Return to citation in text: [1] -
Simas, A. M.; Miller, J.; de Athayde Filho, P. F. Can. J. Chem. 1998, 76, 869–872.
Return to citation in text: [1] -
Araki, S.; Wanibe, Y.; Uno, F.; Morikawa, K.; Yamamoto, K.; Chiba, K.; Butsugan, Y. Chem. Ber. 1993, 126, 1149–1155. doi:10.1002/cber.19931260514
Return to citation in text: [1] [2] [3] -
Araki, S.; Yamamoto, K.; Yagi, M.; Inoue, T.; Fukagawa, H.; Hattori, H.; Yamamura, H.; Kawai, M.; Butsugan, Y. Eur. J. Org. Chem. 1998, 121–127. doi:10.1002/(SICI)1099-0690(199801)1998:1<121::AID-EJOC121>3.0.CO;2-5
Return to citation in text: [1] [2] [3] -
Araki, S.; Goto, T.; Butsugan, Y. Bull. Chem. Soc. Jpn. 1988, 61, 2979–2980. doi:10.1246/bcsj.61.2979
Return to citation in text: [1]
| 1. | Stewart, F. H. C. Chem. Rev. 1964, 64, 129–147. doi:10.1021/cr60228a004 |
| 2. | Ohta, M.; Kato, M. In Nonbenzenoid Aromatics; Snyder, J. P., Ed.; Academic Press: New York, 1969; pp 117–248. |
| 3. | Ollis, W. D.; Ramsden, C. A. Adv. Heterocycl. Chem. 1976, 19, 1–122. doi:10.1016/S0065-2725(08)60230-5 |
| 4. | Newton, C. G.; Ramsden, C. A. Tetrahedron 1982, 38, 2965–3011. doi:10.1016/0040-4020(82)80186-5 |
| 5. | Simas, A. M.; Miller, J.; de Athayde Filho, P. F. Can. J. Chem. 1998, 76, 869–872. |
| 6. | Araki, S.; Wanibe, Y.; Uno, F.; Morikawa, K.; Yamamoto, K.; Chiba, K.; Butsugan, Y. Chem. Ber. 1993, 126, 1149–1155. doi:10.1002/cber.19931260514 |
| 7. | Araki, S.; Yamamoto, K.; Yagi, M.; Inoue, T.; Fukagawa, H.; Hattori, H.; Yamamura, H.; Kawai, M.; Butsugan, Y. Eur. J. Org. Chem. 1998, 121–127. doi:10.1002/(SICI)1099-0690(199801)1998:1<121::AID-EJOC121>3.0.CO;2-5 |
| 8. | Araki, S.; Goto, T.; Butsugan, Y. Bull. Chem. Soc. Jpn. 1988, 61, 2979–2980. doi:10.1246/bcsj.61.2979 |
| 6. | Araki, S.; Wanibe, Y.; Uno, F.; Morikawa, K.; Yamamoto, K.; Chiba, K.; Butsugan, Y. Chem. Ber. 1993, 126, 1149–1155. doi:10.1002/cber.19931260514 |
| 7. | Araki, S.; Yamamoto, K.; Yagi, M.; Inoue, T.; Fukagawa, H.; Hattori, H.; Yamamura, H.; Kawai, M.; Butsugan, Y. Eur. J. Org. Chem. 1998, 121–127. doi:10.1002/(SICI)1099-0690(199801)1998:1<121::AID-EJOC121>3.0.CO;2-5 |
| 6. | Araki, S.; Wanibe, Y.; Uno, F.; Morikawa, K.; Yamamoto, K.; Chiba, K.; Butsugan, Y. Chem. Ber. 1993, 126, 1149–1155. doi:10.1002/cber.19931260514 |
| 7. | Araki, S.; Yamamoto, K.; Yagi, M.; Inoue, T.; Fukagawa, H.; Hattori, H.; Yamamura, H.; Kawai, M.; Butsugan, Y. Eur. J. Org. Chem. 1998, 121–127. doi:10.1002/(SICI)1099-0690(199801)1998:1<121::AID-EJOC121>3.0.CO;2-5 |
© 2009 Araki et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)