Abstract
Cycloalkanones were utilized in the Lewis acid catalyzed peroxyacetalization of ß-hydroperoxy homoallylic alcohols (prepared by the ene reaction of the allylic alcohols mesitylol and methyl 4-hydroxytiglate, respectively, with singlet oxygen) to give spiroannulated 1,2,4-trioxanes 5a–5e and 9a–9e, respectively. A second series of 3-arylated trioxanes 10a–10h, that are available from the hydroperoxy alcohol 4 and benzaldehyde derivatives, was investigated by X-ray crystallography.
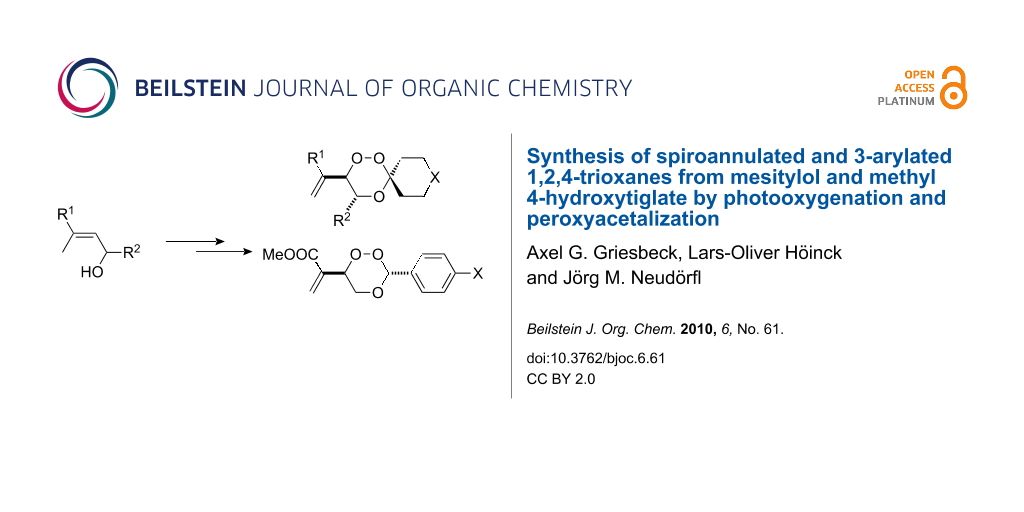
Graphical Abstract
Introduction
The antimalaria-active molecule artemisinin (1) is a naturally occurring sesquiterpene peroxide with remarkable pharmacological properties. Hydrophilic as well as lipophilic derivatives have been prepared from artemisinin and show improved antimalarial properties and better bioavailabilities [1-5]. In recent years, additional medicinal properties of artemisinin and the water soluble artesunates have been discovered such as activities against several cancer cell lines, schistosomiasis and antiviral properties [6,7]. The introduction of substituents into the central peroxide ring system as well as further ring annulation are straightforward approaches for the preparation of other active derivatives which might show promise in overcoming the forthcoming problem of artemisinin resistance [8]. From a synthetic point of view, the preparation of the pharmacophore, the central 1,2,4-trioxane ring system, is possible by a number of strategies [9,10]. We, for example, have previously reported the use of the singlet oxygen ene reaction of allylic alcohols as a route to ß-hydroperoxy alcohols that can be transformed into 1,2,4-trioxanes by reaction with carbonyl compounds in the presence of Lewis acids [11]. This approach leads to simple cyclic peroxides (e.g. 2) which in some cases show similar antimalarial effects as the natural compound (Figure 1) [12]. An apparently useful structural feature is a large 3,3-spirofused hydrophobic group. The adamantane skeleton is a unique motif in other cyclic peroxides with antimalarial activities [13,14] which additionally exhibit other remarkable pharmaceutical properties [15-17]. In this publication we report the use of the alcohols 3 and 6 to explore further the synthetic approach to spirocyclic fused 1,2,4-trioxanes with a series of other spirofused ring structures.
Figure 1: Antimalaria active natural artemisinin 1 and the spirobicyclic 1,2,4-trioxane derivative 2 show the same in vitro activity.
Figure 1: Antimalaria active natural artemisinin 1 and the spirobicyclic 1,2,4-trioxane derivative 2 show the...
Results and Discussion
3,3-Spiroannulated 1,2,4-trioxanes
The photooxygenation reactions via sensitization of triplet oxygen with meso-tetraphenylporphyrin (TPP) were performed in polystyrene beads under solvent-free conditions (Scheme 1) [18,19]. Numerous applications of the hydroperoxides 4 and 7, that result from the singlet oxygen ene reactions, have already been reported [20,21]. In context with our work on bis-peroxide synthesis from bifunctional ketones [22], we have also studied the peroxyacetalization of the allylic hydroperoxide 7 with the bifunctional cyclohexane-1,4-dione (CHD, Scheme 2). In this case, one equivalent of the diketone gave the monoadduct 9c in 20% yield.
Scheme 1: Singlet oxygen ene reaction of methyl 4-hydroxytiglate (3) and mesitylol (6) under solid-phase conditions.
Scheme 1: Singlet oxygen ene reaction of methyl 4-hydroxytiglate (3) and mesitylol (6) under solid-phase cond...
Scheme 2: 1,2,4-trioxane 9c and bis-trioxane 8a,b formation from the bifunctional cyclohexa-1,4-dione.
Scheme 2: 1,2,4-trioxane 9c and bis-trioxane 8a,b formation from the bifunctional cyclohexa-1,4-dione.
The products from the reaction of monofunctional ketones with ß-hydroperoxy alcohols 4 and 7 are collected in Table 1. All trioxanes 5a–e derived from 4 were crystalline and could be analyzed by X-ray structure analysis (Figure 2). The bond lengths of the crucial O-O bond were similar in all cases with the exception of the adamantane derivative 5d which has a remarkably shorter O-O bond distance.
Table 1: 3,3-Spiroannulated 1,2,4-trioxanes by photooxygenation and peroxyacetalization.a
| tiglate-derived trioxanes |
Yield [%]b
O-O [Å]c |
mesitylol-derived trioxanes |
Yield [%]b
O-O [Å]c |
||
|---|---|---|---|---|---|
|
|
5a |
86
1.465d |
|
9a | 73e |
|
|
5b |
12
1.480 |
|
9b | 14 |
|
|
5c |
20
1.466 |
|
9c | 20 |
|
|
5d |
30
1.427d |
|
9d |
40
1.482f |
|
|
5e |
5
1.480 |
|
9e |
19
1.464 |
aStandard reaction conditions: substrate (2 mmol, 4 × 10−2 M), CCl4 (50 mL), meso-tetraphenylporphyrin (0.01 mmol, 2 × 10−4 M), r.t., 10 h; then addition of a solution of the carbonyl compound (2.5 mmol) in CH2Cl2 (10 mL), 0 °C, 3 h. bYields of per-oxyacetalization. cFrom X-ray analysis, CCDC deposited [23]. d[19]. e[20]. f[12].
![[1860-5397-6-61-2]](/bjoc/content/figures/1860-5397-6-61-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Structure of the spirobicyclic trioxane 5c in the crystal.
Figure 2: Structure of the spirobicyclic trioxane 5c in the crystal.
4-Arylated 1,2,4-trioxanes
The 1,2,4-trioxanes 10 were formed in moderate to good yields, with the Hock-type cleavage product from the ß-hydroperdiol as the only side-product, from 4 and substituted benzaldehydes under BF3-catalysis in CH2Cl2 solution (Scheme 3). In all cases the trans products were formed in high (>98:2) diastereoselectivities. All compounds could be crystallized from acetone or from the neat liquid. In the crystal the central 1,2,4-trioxane ring is almost undistorted in a cyclohexane chair conformation with the acrylate and the aryl substituents in equatorial positions (Figure 3). In the crystal lattice the compounds, especially the 4-halophenyl-substituted trioxanes, tend to form π-stacked stabilized chain structures with channels that are filled with water molecules (Figure 4). In the elementary cell of the 4-chloro derivative 10c, an average of 320 Å3 of channel space corresponds to one water molecules per trioxane molecule. By contrast, the 4-trifluormethyl derivative 10f crystallized in a compact chain-like package of anti-parallel arranged pairs of trioxanes.
Scheme 3: BF3-catalyzed acetalization of hydroperoxide 4 with benzaldehyde derivatives.
Scheme 3: BF3-catalyzed acetalization of hydroperoxide 4 with benzaldehyde derivatives.
![[1860-5397-6-61-3]](/bjoc/content/figures/1860-5397-6-61-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Structure of the 3-arylated trioxane 10b in the crystal.
Figure 3: Structure of the 3-arylated trioxane 10b in the crystal.
![[1860-5397-6-61-4]](/bjoc/content/figures/1860-5397-6-61-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Structure of the p-bromophenyl derivative 10d in the crystal lattice (disordered water molecules in the cannels are not shown) viewed along the a axis.
Figure 4: Structure of the p-bromophenyl derivative 10d in the crystal lattice (disordered water molecules in...
The orientation of the aryl groups relative to the 1,2,4-trioxane equator depends largely on the nature of the para-substituent: in the phenyl-substituted trioxane 10a and in the para-halogenated analogs 10b–10d, the aryl group is nearly coplanar with the C(3)-H bond, whereas in the 4-nitro-, 4-trifluoromethyl-, and 4-cyano compounds 10e–10f coplanarity of the aryl substituent with the O(4)-C(3) bond of the trioxane chair was observed (Table 2 and for numbering Figure 5).
Table 2: Structural features of the 1,2,4-trioxanes 10a–h.a
| 10 | R = | Θ4-3-C(ar/q)-C(ar) (°) | Θ2-3-C(ar/q)-C(ar) (°) | ΘH(C3)-3-C(ar/q)-C(ar) (°) |
|---|---|---|---|---|
| 10a | H | 127 | 115 | 3 |
| 10b | F | 142 | 100 | 19 |
| 10c | Cl | 141 | 101 | 18 |
| 10d | Br | 140 | 100 | 18 |
| 10e | NO2 | 179 | 59 | 59 |
| 10f | CF3 | 154 | 90 | 26 |
| 10g | CN | 153 | 89 | 29 |
| 10h | OMe | 139 | 103 | 17 |
aSee [24] for CCDC submission.
Figure 5: Numbering of 3-aryl-1,2,4-trioxanes 10 and relevant bonds; structure of artemether (AM).
Figure 5: Numbering of 3-aryl-1,2,4-trioxanes 10 and relevant bonds; structure of artemether (AM).
In the artemisinin-derived arthemether (AM), the central trioxane ring has a twist-boat conformation resulting from the additional propylene bridge connecting C-3 and C-6. In Table 3 the yields of the peroxyacetalization reactions, the characteristic 13C NMR shifts of the peracetal carbon C-3 and two significant bond lengths are listed. It is clear that the electronic nature of the substituent on the aryl group does not significantly change the bond length of the central peroxide bond (mean value: 1.479 Å). The mean value of the characteristic 13C NMR shift of the peroxyacetal carbon C-3 is 103.4 ppm. The bond length of the central oxygen-oxygen bond in arthemether as determined by an independent structure analysis is 1.472(1) Å.
Table 3: Yields, structural and 13C-NMR properties of 1,2,4-trioxanes 10a–h, and arthemether (AM).
| 10 | R = | yield (%)a | δ(C-3) (ppm)b | O1-O2 (Å) | O2-C3 (Å) |
|---|---|---|---|---|---|
| 10ac | H | 61 | 104.2 | 1.485(7) | 1.451(8) |
| 10b | F | 40 | 103.5 | 1.472(3) | 1.432(3) |
| 10c | Cl | 35 | 103.4 | 1.474(3) | 1.425(4) |
| 10d | Br | 29 | 103.4 | 1.469(9) | 1.415(11) |
| 10ec | NO2 | 31 | 102.6 | 1.471(9) | 1.398(11) |
| 10f | CF3 | 44 | 103.1 | 1.474(2) | 1.432(2) |
| 10g | CN | 38 | 102.7 | 1.4823(14) | 1.436(2) |
| 10h | OMe | 23 | 104.0 | 1.4806(19) | 1.438(2) |
| AM | – | – | 102.9 | 1.472(1) | 1.416(3) |
aIsolated yield after purification by column chromatography. bIn ppm, 75 MHz in CDCl3. cMedium quality crystals, data not deposited.
More pronounced bond lengths effects were observed for the O2-C3 ring bonds that range from 1.39 to 1.45 Å. Analysis of the Cambridge crystallographic data file revealed that the mean oxygen-oxygen (O1-O2) bond distance for 1,2,4-trioxanes (108 compounds) is 1.472 Å with a narrow distribution ranging from the extremes 1.460 (3 compounds) to 1.482 (4 compounds). All compounds 10a–h investigated by us fall into this range, 10a,g,h showing the longest O1-O2 bond distances. With regards to antimalarial activity, all 4-arylated 1,2,4-trioxanes exhibited low in vitro activities (EC50/plasmodium falciparum > 50 μM) with the nitro-substituted compound 10e as the most active derivative (EC50 = 48 μM) [25]. Thus, the peroxide bond lengths do not correlate with biological activity, cf. the highly active AM and the fluoro compound 10b.
Conclusion
In summary, we have reported the synthesis of a series of six-membered ring 3,3-spiroannulated 1,2,4-trioxanes from methyl 4-hydroxytiglate and from mesitylol, respectively, by the singlet oxygen ene reaction and subsequent peroxyacetalization. A series of 4-arylated 1,2,4-trioxanes from methyl 4-hydroxytiglate was obtained by the same protocol. These compounds were fully characterized by spectroscopic methods and by X-ray structure determination.
Experimental
Synthesis of the 4-fluorophenyl derivative 10b: A solution of 290 mg (2.0 mmol) of the hydroperoxide 4 (prepared from methyl 4-hydroxytiglate (3) by the method described in [10]) and 220 mg (2.0 mmol) of 4-fluorobenzaldehyde in 40 ml of dichloromethane was treated at 0 °C with 0.2 ml of boron trifluoride in diethyl ether. After stirring overnight at room temperature, the solution was diluted to 100 ml with dichloromethane, washed successively with 20 ml of saturated aqueous sodium bicarbonate solution, brine and water. The organic phase was separated and dried. After evaporation and column chromatography (silica, EtOAc), 200 mg (40%) of 10b was obrained as a colorless viscous oil that crystallized as thin plates on standing: C13H15FO6 (corresponds to C13H13FO5 × H2O: colorless thin needles from aqueous acetone), M = 286.25, a = 6.1264(3), b = 16.8514(9), c = 26.2519(14), α, β, γ = 90°, orthorhombic, space group Pnaa, Mo-Kα, 15276 reflections measured, 2948 reflections with I > 2σ(I), R1 (all data) = 0.0573, wR2 = 0.1811.
References
-
Klayman, D. L. Science 1985, 228, 1049–1055. doi:10.1126/science.3887571
Return to citation in text: [1] -
O′Neill, P. M.; Posner, G. H. J. Med. Chem. 2004, 47, 2945–2964. doi:10.1021/jm030571c
Return to citation in text: [1] -
Zhou, W. S.; Xu, X. X. Acc. Chem. Res. 1994, 27, 211–216. doi:10.1021/ar00043a005
Return to citation in text: [1] -
Robert, A.; Dechy-Cabaret, O.; Cazelles, J.; Meunier, B. Acc. Chem. Res. 2002, 35, 167–174. doi:10.1021/ar990164o
Return to citation in text: [1] -
Meunier, B. Acc. Chem. Res. 2008, 41, 69–77. doi:10.1021/ar7000843
Return to citation in text: [1] -
Efferth, T.; Romero, M. R.; Wolf, D. G.; Marin, J. J. G.; Marschall, M. Clin. Infect. Dis. 2008, 47, 804–811. doi:10.1086/591195
Return to citation in text: [1] -
Efferth, T. Curr. Drug Targets 2006, 7, 407–421. doi:10.2174/138945006776359412
Return to citation in text: [1] -
Maude, R. J.; Pontavornpinyo, W.; Saralamba, S.; Aguas, R.; Yeung, S.; Dondorp, A. M.; Day, N. P. J.; White, N. J.; White, L. J. Malaria J. 2009, 8, No. 31. doi:10.1186/1475-2875-8-31
Return to citation in text: [1] -
Tang, Y.; Dong, Y.; Vennerstrom, J. L. Med. Res. Rev. 2004, 24, 425–448. doi:10.1002/med.10066
Return to citation in text: [1] -
Rydén, A.-M.; Kayser, O. Chemistry, Biosynthesis and Biological Activity of Artemisinin and Related Natural Peroxides. In Bioactive Heterocycles III; Gupta, R. R., Ed.; Topics in Heterocyclic Chemistry, Vol. 9; Springer: Berlin, 2007; pp 1–31. doi:10.1007/7081_2007_085
Return to citation in text: [1] [2] -
Bartoschek, A.; El-Idreesy, T. T.; Griesbeck, A. G.; Höinck, L.-O.; Lex, J.; Miara, C.; Neudörfl, J. M. Synthesis 2005, 2433–2444.
Return to citation in text: [1] -
Griesbeck, A. G.; El-Idreesy, T. T.; Höinck, L.-O.; Lex, J.; Brun, R. Bioorg. Med. Chem. Lett. 2005, 15, 595–597. doi:10.1016/j.bmcl.2004.11.043
Return to citation in text: [1] [2] -
Vennerstrom, J. L.; Arbe-Barnes, S.; Brun, R.; Charman, S. A.; Chiu, F. C. K.; Chollet, J.; Dong, Y. X.; Dorn, A.; Hunziker, D.; Matile, H.; McIntosh, K.; Padmanilayam, M.; Tomas, J. S.; Scheurer, C.; Scorneaux, B.; Tang, Y. Q.; Urwyler, H.; Wittlin, S.; Charman, W. N. Nature 2004, 430, 900–904. doi:10.1038/nature02779
Return to citation in text: [1] -
Ellis, G. L.; Amewu, R.; Sabbani, S.; Stocks, P. A.; Shone, A.; Stanford, D.; Gibbons, P.; Davies, J.; Vivas, L.; Charnaud, S.; Bongard, E.; Hall, C.; Rimmer, K.; Lozanom, S.; Jesús, M.; Gargallo, D.; Ward, S. A.; O′Neill, P. M. J. Med. Chem. 2008, 51, 2170–2177. doi:10.1021/jm701435h
Return to citation in text: [1] -
Miyazaki, A.; Tsuda, Y.; Fukushima, S.; Yokoi, T.; Vántus, T.; Bökönyi, G.; Szabó, E.; Horváth, A.; Kéri, G.; Okada, Y. J. Med. Chem. 2008, 51, 5121–5124. doi:10.1021/jm701599w
Return to citation in text: [1] -
Furber, M.; Alcaraz, L.; Bent, J. E.; Beyerbach, A.; Bowers, K.; Braddock, M.; Caffrey, M. V.; Cladingboel, D.; Collington, J.; Donald, D. K.; Fagura, M.; Ince, F.; Kinchin, E. C.; Laurent, C.; Lawson, M.; Luker, T. J.; Mortimore, M. M. P.; Pimm, A. D.; Riley, R. J.; Roberts, N.; Robertson, M.; Theaker, J.; Thorne, P. V.; Weaver, R.; Webborn, P.; Willis, P. J. Med. Chem. 2007, 50, 5882–5885. doi:10.1021/jm700949w
Return to citation in text: [1] -
Singh, C.; Kanchan, R.; Sharma, U.; Puri, S. K. J. Med. Chem. 2007, 50, 521–527. doi:10.1021/jm0610043
Return to citation in text: [1] -
Griesbeck, A. G.; Bartoschek, A. Chem. Commun. 2002, 1594–1595. doi:10.1039/b204017d
Return to citation in text: [1] -
Griesbeck, A. G.; El-Idreesy, T. T.; Bartoschek, A. Adv. Synth. Catal. 2004, 346, 245–251. doi:10.1002/adsc.200303181
Return to citation in text: [1] [2] -
Griesbeck, A. G.; Höinck, L.-O.; Lex, J. Lett. Org. Chem. 2006, 3, 247–249. doi:10.2174/157017806775789903
Return to citation in text: [1] [2] -
Griesbeck, A. G.; El-Idreesy, T.; Fiege, M.; Brun, R. Org. Lett. 2002, 4, 4193–4195. doi:10.1021/ol026916n
Return to citation in text: [1] -
Griesbeck, A. G.; Höinck, L.-O.; Lex, J.; Neudörfl, J.; Blunk, D.; El-Idreesy, T. T. Molecules 2008, 13, 1743–1758. doi:10.3390/molecules13081743
Return to citation in text: [1] -
The crystallographic data for the 3,3-spiroannulated 1,2,4-trioxanes 5b, 5c, and 5e have been deposited with the Cambridge Crystallographic Data Centre as supplementary publications no. CCDC-776052 (5b), CCDC-776053 (5c), CCDC-775054 (5e).
Return to citation in text: [1] -
The crystallographic data for the 3-arylated trioxanes 10b–d and 10f–h have been deposited with the Cambridge Crystallographic Data Centre as supplementary publications no. CCDC-762733 (10b), CCDC-762734 (10c), CCDC-762735 (10d), CCDC-762736 (10f), CCDC-762737 (10g), CCDC-762738 (10h).
Return to citation in text: [1] -
Griesbeck, A. G.; Brodwolf, A.; Höinck, L.-O.; El-Idreesy, T. T.; Kim, H.-S. unpublished results.
Return to citation in text: [1]
| 25. | Griesbeck, A. G.; Brodwolf, A.; Höinck, L.-O.; El-Idreesy, T. T.; Kim, H.-S. unpublished results. |
| 10. | Rydén, A.-M.; Kayser, O. Chemistry, Biosynthesis and Biological Activity of Artemisinin and Related Natural Peroxides. In Bioactive Heterocycles III; Gupta, R. R., Ed.; Topics in Heterocyclic Chemistry, Vol. 9; Springer: Berlin, 2007; pp 1–31. doi:10.1007/7081_2007_085 |
| 1. | Klayman, D. L. Science 1985, 228, 1049–1055. doi:10.1126/science.3887571 |
| 2. | O′Neill, P. M.; Posner, G. H. J. Med. Chem. 2004, 47, 2945–2964. doi:10.1021/jm030571c |
| 3. | Zhou, W. S.; Xu, X. X. Acc. Chem. Res. 1994, 27, 211–216. doi:10.1021/ar00043a005 |
| 4. | Robert, A.; Dechy-Cabaret, O.; Cazelles, J.; Meunier, B. Acc. Chem. Res. 2002, 35, 167–174. doi:10.1021/ar990164o |
| 5. | Meunier, B. Acc. Chem. Res. 2008, 41, 69–77. doi:10.1021/ar7000843 |
| 11. | Bartoschek, A.; El-Idreesy, T. T.; Griesbeck, A. G.; Höinck, L.-O.; Lex, J.; Miara, C.; Neudörfl, J. M. Synthesis 2005, 2433–2444. |
| 12. | Griesbeck, A. G.; El-Idreesy, T. T.; Höinck, L.-O.; Lex, J.; Brun, R. Bioorg. Med. Chem. Lett. 2005, 15, 595–597. doi:10.1016/j.bmcl.2004.11.043 |
| 9. | Tang, Y.; Dong, Y.; Vennerstrom, J. L. Med. Res. Rev. 2004, 24, 425–448. doi:10.1002/med.10066 |
| 10. | Rydén, A.-M.; Kayser, O. Chemistry, Biosynthesis and Biological Activity of Artemisinin and Related Natural Peroxides. In Bioactive Heterocycles III; Gupta, R. R., Ed.; Topics in Heterocyclic Chemistry, Vol. 9; Springer: Berlin, 2007; pp 1–31. doi:10.1007/7081_2007_085 |
| 24. | The crystallographic data for the 3-arylated trioxanes 10b–d and 10f–h have been deposited with the Cambridge Crystallographic Data Centre as supplementary publications no. CCDC-762733 (10b), CCDC-762734 (10c), CCDC-762735 (10d), CCDC-762736 (10f), CCDC-762737 (10g), CCDC-762738 (10h). |
| 8. | Maude, R. J.; Pontavornpinyo, W.; Saralamba, S.; Aguas, R.; Yeung, S.; Dondorp, A. M.; Day, N. P. J.; White, N. J.; White, L. J. Malaria J. 2009, 8, No. 31. doi:10.1186/1475-2875-8-31 |
| 19. | Griesbeck, A. G.; El-Idreesy, T. T.; Bartoschek, A. Adv. Synth. Catal. 2004, 346, 245–251. doi:10.1002/adsc.200303181 |
| 6. | Efferth, T.; Romero, M. R.; Wolf, D. G.; Marin, J. J. G.; Marschall, M. Clin. Infect. Dis. 2008, 47, 804–811. doi:10.1086/591195 |
| 7. | Efferth, T. Curr. Drug Targets 2006, 7, 407–421. doi:10.2174/138945006776359412 |
| 20. | Griesbeck, A. G.; Höinck, L.-O.; Lex, J. Lett. Org. Chem. 2006, 3, 247–249. doi:10.2174/157017806775789903 |
| 18. | Griesbeck, A. G.; Bartoschek, A. Chem. Commun. 2002, 1594–1595. doi:10.1039/b204017d |
| 19. | Griesbeck, A. G.; El-Idreesy, T. T.; Bartoschek, A. Adv. Synth. Catal. 2004, 346, 245–251. doi:10.1002/adsc.200303181 |
| 22. | Griesbeck, A. G.; Höinck, L.-O.; Lex, J.; Neudörfl, J.; Blunk, D.; El-Idreesy, T. T. Molecules 2008, 13, 1743–1758. doi:10.3390/molecules13081743 |
| 15. | Miyazaki, A.; Tsuda, Y.; Fukushima, S.; Yokoi, T.; Vántus, T.; Bökönyi, G.; Szabó, E.; Horváth, A.; Kéri, G.; Okada, Y. J. Med. Chem. 2008, 51, 5121–5124. doi:10.1021/jm701599w |
| 16. | Furber, M.; Alcaraz, L.; Bent, J. E.; Beyerbach, A.; Bowers, K.; Braddock, M.; Caffrey, M. V.; Cladingboel, D.; Collington, J.; Donald, D. K.; Fagura, M.; Ince, F.; Kinchin, E. C.; Laurent, C.; Lawson, M.; Luker, T. J.; Mortimore, M. M. P.; Pimm, A. D.; Riley, R. J.; Roberts, N.; Robertson, M.; Theaker, J.; Thorne, P. V.; Weaver, R.; Webborn, P.; Willis, P. J. Med. Chem. 2007, 50, 5882–5885. doi:10.1021/jm700949w |
| 17. | Singh, C.; Kanchan, R.; Sharma, U.; Puri, S. K. J. Med. Chem. 2007, 50, 521–527. doi:10.1021/jm0610043 |
| 23. | The crystallographic data for the 3,3-spiroannulated 1,2,4-trioxanes 5b, 5c, and 5e have been deposited with the Cambridge Crystallographic Data Centre as supplementary publications no. CCDC-776052 (5b), CCDC-776053 (5c), CCDC-775054 (5e). |
| 13. | Vennerstrom, J. L.; Arbe-Barnes, S.; Brun, R.; Charman, S. A.; Chiu, F. C. K.; Chollet, J.; Dong, Y. X.; Dorn, A.; Hunziker, D.; Matile, H.; McIntosh, K.; Padmanilayam, M.; Tomas, J. S.; Scheurer, C.; Scorneaux, B.; Tang, Y. Q.; Urwyler, H.; Wittlin, S.; Charman, W. N. Nature 2004, 430, 900–904. doi:10.1038/nature02779 |
| 14. | Ellis, G. L.; Amewu, R.; Sabbani, S.; Stocks, P. A.; Shone, A.; Stanford, D.; Gibbons, P.; Davies, J.; Vivas, L.; Charnaud, S.; Bongard, E.; Hall, C.; Rimmer, K.; Lozanom, S.; Jesús, M.; Gargallo, D.; Ward, S. A.; O′Neill, P. M. J. Med. Chem. 2008, 51, 2170–2177. doi:10.1021/jm701435h |
| 12. | Griesbeck, A. G.; El-Idreesy, T. T.; Höinck, L.-O.; Lex, J.; Brun, R. Bioorg. Med. Chem. Lett. 2005, 15, 595–597. doi:10.1016/j.bmcl.2004.11.043 |
| 20. | Griesbeck, A. G.; Höinck, L.-O.; Lex, J. Lett. Org. Chem. 2006, 3, 247–249. doi:10.2174/157017806775789903 |
| 21. | Griesbeck, A. G.; El-Idreesy, T.; Fiege, M.; Brun, R. Org. Lett. 2002, 4, 4193–4195. doi:10.1021/ol026916n |
© 2010 Griesbeck et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)