Abstract
Titanium-based Lewis acids catalyze the α-fluorination of β-ketoesters by electrophilic N–F-fluorinating reagents. Asymmetric catalysis with TADDOLato–titanium(IV) dichloride (TADDOL = α,α,α',α'-tetraaryl-(1,3-dioxolane-4,5-diyl)-dimethanol) Lewis acids produces enantiomerically enriched α-fluorinated β-ketoesters in up to 91% enantiomeric excess, with either F–TEDA (1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate)) in acetonitrile solution or NFSI (N-fluorobenzenesulfonimide) in dichloromethane solution as fluorinating reagents. The effects of various reaction parameters and of the TADDOL ligand structure on the catalytic activity and enantioselectivity were investigated. The absolute configuration of several fluorination products was assigned through correlation. Evidence for ionization of the catalyst complex by chloride dissociation, followed by generation of titanium β-ketoenolates as key reaction intermediates, was obtained. Based on the experimental findings, a general mechanistic sketch and a steric model of induction are proposed.
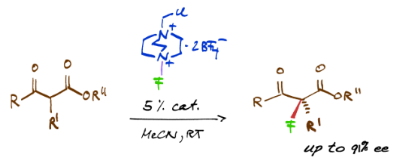
Graphical Abstract
Introduction
Fluoroorganic compounds have peculiar properties, rendering them interesting for a variety of specific applications [1-13]. Single substitutions of hydrogen by fluorine can change the biological effects of low-molecular-weight organic compounds tremendously, for example, unlike acetic acid, fluoroacetic acid (Figure 1a) is highly toxic [14]. Modulation of drug action by inclusion of fluorine, described already in 1954 for steroids (Figure 1b) [15], is now regularly explored in medicinal chemistry. An example of interest for the present work is the modification of the antibiotic erythromycine by α-fluorination of a β-ketoester substructure (Figure 1c) [16]. Fluorinated agrochemicals and drugs are now produced industrially on a large scale by a range of methods, including reactions with notoriously reactive fluorine gas in the production of the anticancer drug fluorouracil (Figure 1d) [17].
Figure 1: Fluorinated substances of biomedical relevance.
Figure 1: Fluorinated substances of biomedical relevance.
However, many specifically fluorinated compounds remain difficult to access because of limitations of the synthetic methodology or unpredictable reaction outcomes in the case of fluorinated starting materials. Seebach coined the term “flustrates” for “fluorinated substrates”; a term that also hints at the frustrations experienced by synthetic chemists in their struggle to prepare a desired fluoroorganic target [18]. A major synthetic challenge of fluoroorganic chemistry is the enantioselective generation of fluorinated stereogenic carbon centers [2,19,20]. Initially, chiral auxiliary approaches and diastereoselective reactions were developed, before Differding and Lang found the first stoichiometric asymmetric fluorination of β-ketoester enolates with a chiral N–F (N-fluoroamine) reagent in 1988 [21]. Later work by Davis [22,23], Takeuchi [24] and their respective coworkers extended this chemistry, while Haufe and coworkers were able to open meso-epoxides asymmetrically with HF equivalents and chiral chromium–salen complexes [25,26]. In the year 2000, two conceptually different applications of Banks’ electrophilic fluorinating reagent F–TEDA (1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate); TEDA = triethylenediamine) [27-29] marked some important discoveries: First, a new generation of highly enantioselective chiral fluorinating reagents, derived by fluorine transfer [30] from F–TEDA to the quinuclidine portion of cinchona-alkaloids, was introduced by the groups of Cahard [31-33] and Shibata [34,35]. Second, the research efforts of our group towards realizing metal-catalyzed fluorinations [36-38] and asymmetric catalytic fluorination reactions [39], successfully channeled into the discovery of a catalytic asymmetric α-fluorination of β-ketoesters (Scheme 1) by means of the reagent F–TEDA and chiral titanium Lewis acid catalysts of the TiCl2(TADDOLate) type [40-42]. The same catalytic reaction principle has also allowed the performance of asymmetric chlorinations and brominations of β-ketoesters [43-45].
Scheme 1: Enantioselective electrophilic fluorination catalyzed by TADDOLates K1, K2. TADDOL = α,α,α',α'-tetraaryl-(2,2-dimethyl-1,3-dioxolane-4,5-diyl)dimethanol. Phenyl-TADDOL (T1) = tetraphenyl-(2,2-dimethyl-1,3-dioxolane) derivative; 1-naphthyl-TADDOL (T2) = tetra-1-naphthyl-(2,2-dimethyl-1,3-dioxolane) derivative.
Scheme 1: Enantioselective electrophilic fluorination catalyzed by TADDOLates K1, K2. TADDOL = α,α,α',α'-tetr...
After the initial report [40], many metal-catalyzed asymmetric fluorinations were discovered by others and ourselves, based on complexes of Pd(II) [46], Cu(II) [47-49], Ni(II) [48,50] and Ru(II) [51] as catalysts [4,51]. In parallel, organocatalytic asymmetric electrophilic fluorination of carbonyl compounds by means of phase-transfer catalysts [52] and small-molecule chiral amine catalysts were introduced and explored with great success [53-56]. Here we present the full range of observations on titanium-catalyzed fluorinations of β-ketoesters. The focus is on the development of the reaction and the study of factors influencing its stereoselectivity depending on reaction parameters and catalyst-ligand effects. We also present stereochemical correlations, to assign the absolute configuration of the fluorination products, and observations relevant to the mechanism of the catalytic reaction. A subsequent paper will cover the substrate range of the catalytic fluorination and its extension to other activated carbonyl compounds [57].
Results and Discussion
Lewis acid catalyzed halogenation of activated carbonyl compounds
Overview
Nonfunctionalized ketones are halogenated in the α-position by halogens and other electrophilic halogenating agents [58-62]. These reactions can be slow due to rate-limiting enolization of the substrate, thus enolates or enolate equivalents are often preferred as substrates [58]. If activated methylene derivatives are mixed with a strong halogenating agent, the enol portion of the tautomeric equilibirum mixture is halogenated quickly, leaving behind the keto tautomer [63]. Further halogenation is then limited by the rate of enolization. This effect forms the basis of Meyer’s famous enol-titration experiments for determining the degree of enolization in activated carbonyl compounds (Scheme 2a) [63-65]. Preparative halogenations of 1,3-dicarbonyl substrates at ambient temperatures are relatively slow and it is more common to halogenate their alkali enolates instead [58]. From studies on the electrophilic fluorination with N–F reagents, it is known that some β-carbonyl compounds are α-fluorinated by simple combination of the reactants in solution at room temperature (Scheme 2b) [66-71]. The ease of this “neutral fluorination” protocol is directly connected to the ability of a substrate to spontaneously enolize. We hypothesized that catalytic acceleration of the enolization should also result in catalysis of the overall electrophilic halogenation. As a working hypothesis, it was assumed that coordination of β-ketocarbonyl compounds to Lewis acidic metal centers should facilitate their transformation to a covalently coordinated enolate, which may be fluorinated at carbon with asymmetric induction exerted by chiral steering ligands X* at the metal (Scheme 2c).
Scheme 2: Halogenation of β-ketocarbonyl compounds: Importance of enolization and the potential role of a metal catalyst.
Scheme 2: Halogenation of β-ketocarbonyl compounds: Importance of enolization and the potential role of a met...
Incidentally, Meyer had already observed acceleration of the keto-enol equilibration of acetoacetic ester in the presence of iron(III) chloride in ethanol solution [64]. Scattered reports on polyhalogenations of ketones in the presence of antimony chloride have appeared [59], while Umemoto used Lewis acids in substoichiometric amounts (0.4 equiv of ZnCl2 or AlCl3) to accelerate fluorinations of 1,3-dicarbonyl compounds with N-fluoropyridinium salts [72]. In 1998, Chambers and Hutchinson reported the reaction of malonic esters with elemental fluorine in the presence of hydrated copper(II) nitrate at the 10 mol % level [73]. Still, the generality and synthetic potential of Lewis acid catalyzed α-halogenations of carbonyl compounds were not established.
Reactivity screening: Fluorination of β-ketoesters in the presence of Lewis acids
As a starting point for our investigation, we chose to study the fluorinations of the readily enolizable β-ketoesters 1 and 2 and of α-cyanoester 3 as model compounds with the reagent F–TEDA under either “neutral” conditions [66-71] or in the presence of Lewis acids (Figure 2).
Figure 2: Model substrates for catalytic fluorinations, with the degree of enolization determined by 1H NMR measurements.
Figure 2: Model substrates for catalytic fluorinations, with the degree of enolization determined by 1H NMR m...
Freshly dissolved ethyl benzoylpropionate 1 contained some enol tautomer in CDCl3. In CD3CN, the value decreased to below 0.5% on standing (Figure 2). Fluorination of 1 in a saturated solution of F–TEDA in acetonitrile was negligible (<1%) after two weeks at ambient temperature, assuring us that a background reaction would not interfere when searching for catalytic reactions. After the addition of 5 to 10 mol % of Lewis acids to solutions of 1 and F–TEDA in acetonitrile, the reaction course (1→1-F) was monitored by 1H NMR analysis. Figure 3 shows representative 1H NMR spectra of reaction samples removed after 28 h reaction time at ambient temperature. The catalysts and the corresponding conversions induced by them for the fluorination of substrates 1 and 2 are listed in Table 1.
Figure 3: 1H NMR (250 MHz) spectra of fluorination reaction mixtures diluted with CDCl3 and filtered. a) Full range spectrum with signals from substrate 1 and product 1-F. The catalyst was Cp2Ti(OTf)2. Signals resulting from F–TEDA or H–TEDA are missing because of the insolubility of those species in CDCl3. The signals for MeCN and its 13C-satellites have been cut for clarity. b) Reaction progress monitoring after 28 h (region 3.9–4.3 ppm) in catalytic reactions with the Lewis acids indicated, along with the results from a blank experiment.
Figure 3: 1H NMR (250 MHz) spectra of fluorination reaction mixtures diluted with CDCl3 and filtered. a) Full...
Table 1: Fluorination of 1 and 2 in the presence of different Lewis acids.
|
|
||||
| Entry | Catalyst (mol %) | Conversion after 28 h (%) | ||
|---|---|---|---|---|
| 1→1-F | 2→2-F | |||
| neata | equilibratedb | |||
| 1 | none (“blank”) | 0 | 20 | 5 |
| 2 | Cp2Ti(OTf)2 (5) | 38 | >95 | 95 |
| 3 | Cp2Zr(OTf)2(thf) (5) | ≤2 | n.d.c | 8 |
| 4 | HBF4·H2O (10) | 24 | 47 | 47 |
| 5 | BF3·Et2O (10) | 11 | 31 | 22 |
| 6 | ZnF2 (5) | n.d. | 25 | n.d. |
aneat = substrate added in substance. bequilibrated = substrate added as stock solution in MeCN. cn.d. = not determined.
The titanium complex Cp2Ti(OTf)2 (Cp = η5-cyclopentadienyl) catalyzed the fluorination of both 1 and 2 (Table 1, entry 2), whereas the corresponding zirconium complex was inactive (Table 1, entry 3). Both the Brønsted acid HBF4 and the Lewis acid BF3 accelerated the reaction. Substrate 2 with a higher tendency towards enolization (Figure 2) gave higher conversions, but a similar trend. Interestingly, the conversions were different when substrate 2 was added to the reaction mixture in neat form compared to when it was added as a stock solution in MeCN (Table 1). This appears to reflect the enol content of 2 (Figure 2), which is much higher in a freshly dissolved sample than in an aged solution of a polar solvent. Presumably, the enol tautomer reacts uncatalyzed with F–TEDA, followed by a slow, Lewis acid catalyzed fluorination of the remaining ketone. The catalytic effects of other Lewis acids in the fluorination of 1 were semiquantitatively studied by TLC experiments and found as presented in Scheme 3.
Scheme 3: Qualitative ordering of catalytic activity of several Lewis acids in the fluorination 1→1-F.
Scheme 3: Qualitative ordering of catalytic activity of several Lewis acids in the fluorination 1→1-F.
Strong catalytic activity was observed for complexes of titanium and aluminum. Activity increases with the number of strongly σ-acidic ligands (TiCl4 > CpTiCl3 > Cp2Ti(OTf)2) and with the σ-acidity of the ligands themselves (Cp2Ti(OTf)2 > Cp2TiCl2). Fluorides as strong π-donors are not suitable ligands for Lewis acidic centers in the present reaction, as implied by the inactivity of TiF4. Organometallic Cp-complexes (Cp = η5-cyclopentadienyl) are suitable halogenation catalysts, even though halogenolysis of the metal–carbon bonds might have been expected [27]. For metals other than Ti and Al, no significant catalytic activity was observed in this specific model reaction (Scheme 3). The fluorination of cyanoester 3 with F–TEDA was not accelerated by any of the potential catalysts investigated (TiCl4, BF3·Et2O, SnCl4, Sn(OTf)2, AlCl3, Mg(OTf)2, ZnF2 or SbF3). The conversion 3→3-F in a blank reaction amounted to 20% (3 d, MeCN, rt) or 40% (4.5 h, MeCN, 40 °C), but the addition of any of the aforementioned substances rather suppressed the reaction. This may be explained by the inability of 3 to bind in a chelating mode to metal complexes. In fact, when ketoesters 1 or 2 were mixed with TiCl4 in MeCN, they produced yellow to red colors characteristic for β-dicarbonylate complexes, whereas solutions of 3 remained colorless.
Catalytic halogenation under “neutral” conditions
While the remainder of this paper is concerned with the development of asymmetric catalytic halogenations, the Lewis acid catalyzed fluorination under neutral conditions (i.e., without stoichiometric base) is an attractive method also for carrying out nonstereoselective halogenations, which can profit from catalyst optimization for controlling reaction selectivity. Reactions were readily performed on preparative scale as shown for α-methyl-β-ketoester 4, which was fluorinated in high yield with the aid of TiCl4 as a catalyst (Scheme 4a). With the same catalyst, β-ketoester 5 suffered partial cleavage of the ester group, but the milder Lewis acid CpTiCl3 induced a clean fluorination with high selectivity towards monofluorination (Scheme 4b) [74,75]. We have further explored aspects of selective monofluorination and sequential mixed dihalogenation elsewhere [74]. This example illustrates the potential of catalyst tuning for specific substrates in catalytic fluorination reactions. TiCl4 as well as CpTiCl3 (and other Lewis acids) [76-78] also catalyzed the chlorination of β-ketoesters with N-chlorosuccinimide or N-chlorosulfonamides as halogenating agents [43]. The Lewis acid catalyzed halogenations of β-ketoesters present an interesting alternative to either radical or neutral noncatalyzed reaction modes.
Scheme 4: Catalysis of the “neutral” fluorination of β-ketoesters with F–TEDA by Lewis acidic titanium complexes.
Scheme 4: Catalysis of the “neutral” fluorination of β-ketoesters with F–TEDA by Lewis acidic titanium comple...
Catalytic asymmetric fluorination
Having shown the viability of Lewis acid catalyzed fluorination, we focused on the development of an asymmetric catalytic fluorination reaction using enantiopure Lewis acid catalysts. Based on the screening results (Scheme 3), titanium or aluminum catalysts were logical choices. An enantiopure sample of the ansa-titanocene [(EBTHI)Ti(OTf)2] (Figure 4) [79-81] indeed catalyzed the slow fluorination of 1 (14% conversion after 18 h, 25% after 36 h, 30 d to completion) or 2 (85% conversion after 18 h), but gave racemic products 1-F and 2-F.
Figure 4: Structure of the chiral ansa-metallocene [(EBTHI)Ti(OTf)2].
Figure 4: Structure of the chiral ansa-metallocene [(EBTHI)Ti(OTf)2].
Dialkoxyaluminium chlorides, which were prepared in situ from Me2AlCl and several chiral diols, were not catalytically active. However, the well-established Lewis acid [(R,R-TADDOLato)TiCl2] [82] (Scheme 1) displayed good catalytic activity (5 mol %, 1→1-F in 4 h) and gave (S)-1-F with an enantiomeric excess of 28% (for a complex incorporating the ligand (R,R)-phenyl-TADDOL (L1)) (Table 2). Consistent catalysis results were obtained when the crystalline Lewis acid complexes K1 and K2 were applied instead of the complexes generated in situ. The synthesis, characterization and X-ray structures of these complexes, which are now commercially available, have been described elsewhere [83].
Table 2: Effect of catalyst loading, temperature, and water content on the fluorination of 1 with catalyst K1.a
|
|
|||||
| Entry | K1 (mol %) | T (°C) | Additives (mol %) | t (h) | ee (%) |
|---|---|---|---|---|---|
| 1 | 1.2 | rt | 45b | 25.2 | |
| 2 | 5 | rt | 6 | 26.6 | |
| 3 | 10 | rt | 2 | 26.5 | |
| 4 | 20 | rt | <2 | 25.5 | |
| 5 | 50 | rt | <0.3 | 23.4 | |
| 6 | 100 | rt | <0.3 | 22.4 | |
| 7 | 5 | rt | MS 3 Å | 6 | 27 |
| 8 | 5 | rt | 6 | 28.5c | |
| 9 | 5 | rt | H2O (5) | 10 | 28.5 |
| 10 | 5 | rt | H2O (50) | >12d | 28 |
| 11 | 5 | rt | H2O (1000) | – | –e |
| 12 | 5 | 0 | 16 | 32 | |
| 13 | 5 | rt | <8 | 28 | |
| 14 | 5 | 40 | <2 | 23 | |
| 15 | 5 | 60 | 0.1f | 18 | |
aAll reactions were performed in reagent grade acetonitrile stored over 3 Å molecular sieves and run to complete conversion (TLC), unless otherwise mentioned. bReaction stopped before complete conversion. cEntries 1–6 vs 7–11 vs 12–15 were run with different batches of F–TEDA, solvent and catalyst, leading to small selectivity deviations under seemingly identical conditions. d50% conversion. eNo reaction. fConversion stopped at 80%.
Effects of catalyst loading, moisture, and temperature
The reaction rate of catalytic fluorination increased with the catalyst loading, but there was little change in the enantioselectivity (Table 2, entries 1–6). A minor decrease of selectivity at higher catalyst loadings is attributed to the temperature effect of the fast exothermic reactions. At low catalyst loadings (<2 mol %) catalyst poisoning occurs, presumably, by moisture and/or fluoride impurities from the reagent F–TEDA. The negative effect of fluoride anions on the catalytic activity is suggested by the inactivity of TiF4 as opposed to TiCl4 (Scheme 3), and by the finding that an in situ catalyst prepared from Ti(OiPr)4, TiF4 and TADDOL (1:1:2) (in situ [TiF2(TADDOLate)], but not characterized) was inactive. The controlled addition of water to catalytic runs did not affect the selectivity, but the activity decreased at high moisture levels (Table 2, entries 7–11). Use of reagent-grade acetonitrile, stored over molecular sieves (3 Å) in air, was satisfactory for most purposes. Performing the reaction in the presence of powdered molecular sieves had no beneficial effects (Table 2, entry 7). All catalytic runs were conveniently carried out in closed vessels in air.
Temperature effect
The catalysis proceeded faster at higher temperature, with a concomitant decrease of enantioselectivity (Table 3, entries 12–15). At 60 °C, catalyst decomposition became notable by incomplete conversions (Table 3, entry 15). At temperatures below 0 °C, the catalytic reaction was slow not only because of the thermal effect, but because of the limited solubility of F–TEDA. Additional temperature variation experiments were carried out with the more selective catalyst K2 and phenyl ester 6 as substrate (Table 3). Results for either fluorination (→6-F) with F–TEDA or the more soluble reagent NFSI (N-fluorobenzenesulfonimide) and NCS (N-chlorosuccinimide) for chlorination [43] (→6-Cl) are displayed in Table 3.
Table 3: Effect of the temperature on the halogenation of 6 with catalyst K2.a
|
|
||||||
| Entry | Reagent | Solvent | T (°C) | t (h) | Product | ee (%) |
|---|---|---|---|---|---|---|
| 1 | F–TEDA | MeCN | 0 | 0.3 | 6-F | 86 |
| 2 | F–TEDA | MeCN | rt | 0.15 | 6-F | 88 |
| 3 | F–TEDA | MeCN | 50 | <0.3 | 6-F | 79 |
| 4 | NFSI | CH2Cl2 | −25 | –b | 6-F | 89b |
| 5 | NFSI | CH2Cl2 | 0 | 19 | 6-F | 91 |
| 6 | NFSI | CH2Cl2 | rt | 10 | 6-F | 87.5 |
| 7 | NCS | MeCN | rt | 0.5 | 6-Cl | 82 |
| 8 | NCS | CH2Cl2 | 0 | 7 | 6-Cl | 90 |
aReactions were run to complete conversion (TLC), unless otherwise mentioned. bAnalysis at 15% conversion. Complete conversion occurred after warming to rt.
Above ambient temperature, increasing temperatures give lower selectivity (Table 3, entries 1–3). Cooling to 0 °C in some cases increased enantioselectivity at the cost of longer reaction times (Table 3, entries 1–3, 4–6, compare also Table 2, entry 12). The optimum temperature depends on substrate, halogenation agent and solvent, but satisfactory results were often achieved at ambient temperature.
Solvent effects
The choice of solvent for the catalytic fluorinations with F–TEDA is limited by the solubility of this reagent. Solvents must dissolve the ionic reagent, but not deactivate the Lewis acidic catalyst. Suspensions of F–TEDA in MeCN gave results inferior to saturated F–TEDA solutions in MeCN (c = 0.15 mol·L−1). Nitromethane dissolved less of the reagent, and the reaction was slower. Aside from water, one of the best solvents for F–TEDA was N,N-dimethylformamide, however, it deactivated the titanium catalyst. It was possible to study a wider range of solvents in the asymmetric catalytic fluorination by choosing NFSI (N-fluorobenzenesulfonimide) as the reagent with better solubility. Reaction with NFSI in dichloroethane gave 1-F with 24% ee (5 mol % K1, 40 °C, 3 d, 66% conversion), which is close to the enantiomeric excess observed with F–TEDA in acetonitrile at the same temperature (Table 2, entry 14). The fluorination of 6 with NFSI in several solvents led to similar results in all solvents that supported the reaction (Table 4). In conclusion, MeCN is the preferred solvent for catalytic fluorinations with F–TEDA, whereas CH2Cl2 is the preferred solvent for fluorinations with NFSI [46].
Table 4: Solvent effects on selectivity of fluorination of 6 with NFSI and K2.
|
|
|||
| Entry | Solvent | t (h) | ee (%) |
|---|---|---|---|
| 1 | MeCN | 40 | 84.5 |
| 2 | CH2Cl2 | 10 | 87.5 |
| 3 | THF | 18a | 77.5 |
| 4 | toluene | 24 | 85.6 |
a28% conversion.
Variation of the electrophilic fluorinating agent
A selection of commercially available electrophilic fluorinating reagents (Figure 5) was tested in the catalytic reaction in MeCN (Table 5). The dicationic reagents F–TEDA and NFTh [84] (Table 5, entries 1 and 2) were the most reactive. Neutral N-fluoro-sulfonamides supported the reaction, but electron-withdrawing groups on nitrogen were needed to induce a fast reaction (Table 5, entries 3, 7 and 8). N-fluoropyridinium salts (Table 5, entries 4–6) gave rise to slow catalyses. With the exception of the dicationic N,N'-difluorobipyridinium salt (Table 5, entry 6), the reagents induced similar enantioselectivity.
Figure 5: Electrophilic fluorinating reagents of the N–F-type. F–TEDA [27]; NFTh = 1-fluoro-4-hydroxy-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) [84]; NFSI = N-fluorobenzosulfonimide [85]; SaSu–NF = saccharine-sultam derived NF reagent (3,3-dimethyl-2,3-dihydro-1,2-benzothiazole-1,1-dioxide) [86,87]; p-TsN(F)Me [88]; NFColl = N-fluorocollidinium tetrafluoroborate and NFPy = N-fluoropyridinium tetrafluoroborate [72,89]; F2-bipy = 1,1'-difluoro-bipyridinium bis(tetrafluoroborate) [90].
Figure 5: Electrophilic fluorinating reagents of the N–F-type. F–TEDA [27]; NFTh = 1-fluoro-4-hydroxy-1,4-diazoni...
Table 5: Effect of the fluorinating agent on the catalytic fluorination of F1 with 10 mol % K1.
|
|
||||
| Entry | Reagent | Time | Conversion (%) | ee (%) |
|---|---|---|---|---|
| 1 | F–TEDAa | <5 h | >90 | 28 |
| 2 | NFTh | <30 h | >90 | 32.5 |
| 3 | NFSI | 5 d | 90 | 28.0 |
| 4 | NFPy | 5 d | 30 | 28.8 |
| 5 | NFColl | 5 d | <5 | – |
| 6 | F2-bipy | 7 weeks | 80 | ≤3 |
| 7 | p-TsN(F)Me | >1 week | 0 | – |
| 8 | SaSu–NF | >1 week | 0 | – |
a5 mol % of catalyst.
Ligand variations in the asymmetric titanium-catalyzed fluorination reaction
We tested several chiral diols in the titanium-catalyzed asymmetric fluorination, but notable success was only achieved with TADDOL ligands [91]. We obtained several TADDOLs from commercial sources (T1–T3) or courtesy of A. K. Beck from the Seebach group (T4, T8–T10). In addition, we prepared trifluoromethylated TADDOLs (Scheme 5), because introduction of fluorine into the ligands was expected to increase the Lewis acidity of the catalyst complex. The syntheses started from dioxolane-diester 7 by reaction with 3-trifluoromethylphenyl or 3,5-bis(trifluormethylphenyl) Grignard reagents in the usual way to give TADDOLs T6 and T7 [92] in high yield (Scheme 5a). Interestingly, the reaction of o-trifluoromethylphenyl Grignard reagent with 7 gave hydroxyester 8 (Scheme 5b); the latter, with an excess of phenylmagnesium chloride, gave a mixed aryl TADDOL T5. For another approach to mixed aryl TADDOLs [93]. The more reactive o-trifluoromethylphenyllithium failed to give a TADDOL as well, but instead furnished the hydroxyketone 9 in low yield (Scheme 5b).
Scheme 5: Synthesis of trifluoromethyl-substituted TADDOL ligands.
Scheme 5: Synthesis of trifluoromethyl-substituted TADDOL ligands.
The performance of several TADDOLs as ligands in the titanium-catalyzed asymmetric fluorination of the substrates 1, 10 and 11 to the respective products 1-F, 10-F and 11-F is shown in Table 6. The catalysts of the type [TiCl2(TADDOLato)] [94] were all prepared in situ from the ligands and TiCl2(OiPr)2 in MeCN. To start with, the (R)-BINOL-derived complex displayed low activity and selectivity (Table 6, entry 1). The phenyl-TADDOL ligand T1 induced enantiomeric excesses that increased with the steric requirements of the substrate ester group (Table 6, entry 2). The effect was more pronounced for 1-naphthyl-TADDOL T2 (Table 6, entry 3), which was the ligand with the best overall performance, displaying both high selectivity and fast kinetics in the catalysis. On the contrary, T3 with a 2-naphthyl group behaved very much like T1 towards substrate 1 and was not studied further (entry 4). A further increase in steric requirement and aromatic stacking area was realized with 9-phenanthrenyl-TADDOL T4; this gave a maximal enantioselectivity with benzyl ester 10, rather than with the bulky ester 11. Presumably, the combined steric effects of substrate and ligand cannot exceed certain optimal limits. In the series of trifluoromethylated TADDOLs T5, T6, T7 there was a notable decrease of enantioselectivity and finally an inversion (with substrate 1) of the sense of induction that roughly correlates with the number of CF3 groups in the ligand (Table 6, entries 6–8). Variations of the TADDOLs in the dioxolane backbone had little influence on stereoselectivity (Table 6, entry 2 and 3 versus entry 9 and 10). The presence of an ortho-methoxy group in the TADDOL aryl group was not tolerated (Table 6, entry 11).
Table 6: Effects of the variation of the ligand structure and substrate stereochemistry.a,b
|
|
|||||
| Entry | Diol ligand | ee (%) | |||
|---|---|---|---|---|---|
|
|
1-F | 10-F | 11-F | ||
| 1 | (R)-BINOL | −1.7c,d | n.d. | n.d. | |
| 2 | T1 | R,R' = Me; Ar,Ar' = Ph | 28.2 | 30–37 | 53–55 |
| 3 | T2 | R,R' = Me; Ar,Ar' = 1-Nap | 57.5 | 55 | 85e |
| 4 | T3 | R,R' = Me; Ar,Ar' = 2-Nap | 26 | n. d. | n. d. |
| 5 | T4 | R,R' = Me; Ar,Ar' = 9-Phen | 36f | 74 | 35f |
| 6 | T5 | R,R' = Me; Ar = Ph; Ar' = 2-CF3C6H4 | 11.5 | 19 | 12 |
| 7 | T6 | R,R' = Me; Ar,Ar' = 3-CF3C6H4 | −5d | 28 | 13 |
| 8 | T7 | R,R' = Me; Ar,Ar' = 3,5-(CF3)2C6H3 | −32d | 11 | −12d |
| 9 | T8 | R = Me; R' = Ph; Ar,Ar' = Ph | 25 | 32 | 45 |
| 10 | T9 | R = Me; R' = t-Bu; Ar,Ar' = 1-Nap | 56 | 56 | 84 |
| 11 | T10 | R,R' = Me; Ar,Ar' = 2-MeOC6H4 | 0f | 0 | 0 |
aTADDOLs in this table all have (R,R)-configuration. bAbbreviations: 1-Nap = 1-naphthyl; 2-Nap = 2-naphthyl; 9-Phen = 9-phenanthrenyl; n.d. = not determined. cReaction for 10 h at 0 °C, conversion not complete. dA negative sign denotes inversion of the excess configuration for the fluorination product (R), relative to the excess configuration obtained with T1 (which is (S)). eWith pure K2 instead of the in situ catalyst from T2, selectivity is 90% ee. fConversion stops before completion of the reaction.
Assignment of absolute configurations of selected reaction products
The excess configuration of (+)-1-F was directly assigned as (2S) by comparison with literature data. Kitazume et al. reported [α]D = +85.4 (c 1.97, MeOH) for (S)-1-F with an enantiomeric excess of >98% [95]. Our sample ([α]D = +53.8, (c 0.545, MeOH)) had an optical purity of 63%, which is in good agreement with the 62% ee from the HPLC measurement. A sample of the benzyl ester (+)-10-F (68% ee by HPLC) was transesterified (EtOH, cat. Na, <5 min at rt) to give the known [95] ethyl ester (+)-(S)-12-F (67.9% ee by GC). By comparison with the literature optical rotation value, the material had an optical purity of 73%, which is sufficiently close to the enantioselectivity determined by GC, considering the difficulties of purifying this volatile compound on a small scale (Scheme 6). A sample of (+)-11-F of 85.6% ee (by HPLC) was treated with BCl3 and the resulting carboxylic acid esterified in situ with cyanuric chloride, N,N-dimethylaniline and benzyl alcohol yielding (+)-10-F in 77% yield (84.9% ee by HPLC), whose configuration has already been established by correlation with 12-F. Comparison of the elution order on a chiral HPLC column with the (+)-sign of the optical rotation implied an (S)-configuration of this sample, and therefore likewise for (+)-11-F. In addition, a sample of (+)-6-F was transesterified in acidic ethanol to 12-F. Assignment of the retention time of the major enantiomer in GC allowed the assignment of the (S)-configuration to (+)-(S)-6-F (Scheme 6). So far, samples emerging from our catalytic fluorination with a catalyst incorporating (R,R)-TADDOL ligands have displayed positive optical rotation and a (2S)-configuration in the excess enantiomer.
Scheme 6: Correlation experiments for the assignment of absolute configuration to fluorination products 11-F, 10-F and 6-F. The absolute configuration of 1-F, 13-F and 12-F in relation to the sign of their optical rotation was established by Kitazume et al. [95] a) 1. BCl3, CH2Cl2, −20 °C, 15 min; 2. cyanuric chloride, PhNMe2, BnOH. b) EtOH, cat. Na, rt, 15 min. c) p-TsOH, EtOH, rt, 1 d.
Scheme 6: Correlation experiments for the assignment of absolute configuration to fluorination products 11-F, ...
Mechanistic considerations
The addition of TiCl4 to acetyl acetone (2,4-pentanedione) gives rise to the red-colored chelate complex TiCl2(acac)2 (acac = acetylacetonate, κO, κO'-2,4-pentanedionate) [96,97]. The complex [TiCl(κO,κO'-1,3-diphenyl-1,3-propanedionate)(TADDOLate)(THF)], derived from dibenzoylmethane, was described as being yellow [98]. When the complex K1 was mixed with β-ketoester substrates (S) in acetonitrile solution, yellow- or red-colored solutions were formed within few minutes, which implies that a β-diketonate complex was formed. We therefore assume that the catalytic acceleration of the electrophilic fluorination reaction with F–TEDA by Lewis acidic titanium complexes is due to the intermediacy of chelating titanium–enolate complexes B (Scheme 7).
Scheme 7: Mechanistic scheme proposed, based on visual and spectroscopic observations. L = solvent, counterions.
Scheme 7: Mechanistic scheme proposed, based on visual and spectroscopic observations. L = solvent, counterio...
The intermediacy of cationic species A is postulated based on NMR experiments: A solution of K1 in CDCl3 did not show any signs of ionization and kept the spectral properties expected for a C2 symmetric species down to 230 K. The limited solubility of K1 in CD3CN prevented low temperature NMR experiments in that solvent. However, the reaction of K1 with an equivalent of AgBF4 in CD3CN produced the signals of species A (Scheme 7) corresponding to the formula [TiCl(TADDOLato)(DME)(MeCN)]BF4, which is characterized by two signals for diastereotopic geminal methyl groups (δ = 0.48 and 0.93 ppm), and two doublets for the diastereotopic methyne hydrogens (δ = 5.08 and 5.20 ppm, J = 7.2 Hz) of the TADDOLate backbone (Figure 6a). This monocationic species could not be isolated; after evaporation and redissolution in CDCl3, the sample had lost DME (1,2-dimethoxyethane) and displayed signals corresponding to the free TADDOL ligand, implying decomposition of the complex. Still we were able to show that a silver cation is not needed for ionizing the catalyst under these reaction conditions: When a suspension of K1 was stirred with excess solid F–TEDA in CD3CN, and the suspension filtered, the 1H NMR spectrum of the filtrate displayed signals for a similar cationic species corresponding to A, in addition to signals from the F–TEDA cation (Figure 6b). The spectrum of this C1-symmetric species displayed signals for the diastereotopic methyl groups (δ = 0.64 and 1.01 ppm), and two broadened signals for the diastereotopic methine hydrogens (δ = 5.25 and 5.65 ppm) of the TADDOLate backbone. The signals for ligated DME showed considerable broadening. The chemical shift difference observed may either be explained by a fast equilibrium between [TiCl(TADDOLato)(DME)(MeCN)]BF4 and [TiCl(BF4)(TADDOLato)(DME)], or through the effects of ion pairing and variable concentrations.
Figure 6: 1H NMR spectra of a species of the type A, generated in CD3CN solution from K1 by ionization in the presence of: a) AgBF4, or: b) excess F–TEDA reagent. Assignments: α-CH = dioxolane H-C(4) and H-C(5). F = signals due to F–TEDA (and some H–TEDA). DME = coordinated 1,2-dimethoxyethane. Me = dioxolane methyl-C(2) groups.
Figure 6: 1H NMR spectra of a species of the type A, generated in CD3CN solution from K1 by ionization in the...
These experiments show that ionization of K1 to generate A, under the conditions of the catalytic reaction, readily takes place. In a second step, we assume that A undergoes ligand-exchange reactions with coordination of a substrate S for DME, followed by loss of the α-carbonyl hydrogen as H+ to give a mono-ketonate complex B. The proton may combine with chloride counterion to give HCl, which is only weakly dissociated in acetonitrile (the pKa of HCl in MeCN has recently been measured as 10.6) [99]. Complex B would then be attacked by external F–TEDA reagent at the central carbon of the enolate, diastereoselectively, from the face opposite to a shielding TADDOLate aryl group (Scheme 7, B→C). In order to arrive at a simple structural model of B, we used the coordinates from the X-ray crystal structure of the 1-naphthyl-TADDOL complex K2 [83] for the [TiCl(TADDOLate)(MeCN)] fragment and coordinates for the enol of benzyl ester substrate 10, generated with the simple model-building software ALCOGEN [100], for the ketoenolate fragment. The two substructures were joined manually, such that the enolate oxygen occupies the axial position trans to chlorine at titanium, and the carbonyl oxygen occupies the position of a dissociated acetonitrile ligand. The resulting model is shown in Figure 7. The model nicely illustrates the shielding function of a face-on 1-naphthyl group, which may direct the attack of the incoming fluorinating reagent.
Figure 7: Steric model explaining the face selectivity observed in the titanium–TADDOLate complex catalyzed fluorination. The structural model of this complex [TiCl(TADDOLate)(β-ketoenolate)(MeCN)] of the type B (Scheme 7) was obtained by combining X-ray data of K2 with a modeled structure of the enol of substrate 10. The arrow symbolizes the external attack of the fluorinating reagent N–F.
Figure 7: Steric model explaining the face selectivity observed in the titanium–TADDOLate complex catalyzed f...
This hypothesis was used as a starting point for more detailed studies; QM/MM calculations on the postulated intermediary isomeric β-ketoenolate complexes and their attack by the N–F reagent were carried out, as described elsewhere [101]. Among the eight diastereomeric forms of B, the most stable complex is indeed the one predicted by the simple steric model (Figure 7), which is expected to produce the fluorination product F with (S)-configuration, as experimentally observed [101]. More recent experimental studies led to the isolation of complexes [Ti(1-naphthyl-TADDOLato)(β-ketoenolate)2], which were characterized by X-ray crystallography in the solid state and by NMR spectroscopy in solution [102]. In either mono- or bis(ketonato) complex, the shielding of one diastereomorphic face of the coordinated enolate by a 1-naphthyl group of the ligand was found. Either a C1-symmetric mono-ketonato or a C2-symmetric bis(ketonato) complex will react with F–TEDA to give the fluorinated β-ketoester product with the same sense of induction as experimentally found in the catalytic reaction [102]. In the X-ray structure of the bis(enolate) of benzyl 2-methyl-3-oxopentanoate [102] (Figure 8), the face-to-face alignment of the substrate enolate, which directs the attack of the incoming electrophile to the other face, is clearly an important element. However, closer inspection also reveals an attractive edge-to-face CH-to-arene-π interaction between the substrate benzyl ester and the ligand naphthyl group, which was not predicted by the simplistic steric models in Figure 6. This attractive interaction may be responsible for the generally higher level of enantioselectivity observed in the fluorination of benzyl and aryl esters [57] when compared to substrates containing aliphatic alkyl ester groups [40].
Figure 8: Excerpt from the X-ray structure of a catalyst/substrate complex [Ti(1-naphthyl-TADDOLato)(β-ketoenolate)2] [102]. Two important attractive interactions between substrate and TADDOL ligand fragments can be identified: 1. Face-to-face arrangement of the ligand 1-naphthyl group and the substrate ketoenolate plane; 2. edge-to-face stacking interaction between the ligand naphthyl and the substrate benzyl ester groups.
Figure 8: Excerpt from the X-ray structure of a catalyst/substrate complex [Ti(1-naphthyl-TADDOLato)(β-ketoen...
A subsequent paper will concentrate on the substrate scope of the titanium–TADDOLate-catalyzed asymmetric fluorination of β-ketoesters and its extension to other activated carbonyl compounds as substrates [57].
Supporting Information
| Supporting Information File 1: Experimental procedures and characterization data. | ||
| Format: PDF | Size: 823.2 KB | Download |
References
-
Shimizu, M.; Hiyama, T. Angew. Chem., Int. Ed. 2005, 44, 214–231. doi:10.1002/anie.200460441
Return to citation in text: [1] -
Kirsch, P. Modern Fluoroorganic Chemistry; Wiley-VCH: Weinheim, Germany, 2004.
Return to citation in text: [1] [2] -
Hiyama, T. Organofluorine Compounds; Springer: Berlin, Germany, 2000.
Return to citation in text: [1] -
Ma, J.; Cahard, D. Chem. Rev. 2004, 104, 6119–6146. doi:10.1021/cr030143e
Return to citation in text: [1] [2] -
Ramachandran, P. V., Ed.; Asymmetric Fluoroorganic Chemistry; American Chemical Society: Washington DC, 2000.
Return to citation in text: [1] -
Baasner, B.; Hagemann, H.; Tatlow, J. C., Eds.; Methoden der Organischen Chemie, Houben-Weyl; Vol. E10a; Thieme Verlag: Stuttgart, Germany, 1999.
Return to citation in text: [1] -
Soloshonok, V. A. Enantiocontrolled Synthesis of Fluoro-Organic Compounds; Wiley: Chichester, U.K., 1999.
Return to citation in text: [1] -
Hudlicky, M.; Pavlath, A. E. Chemistry of Organic Fluorine Comounds II; American Chemical Society: Washington DC, 1995.
Return to citation in text: [1] -
Welch, J. T. Selective Fluorination in Organic and Bioinorganic Chemistry; American Chemical Society: Washington DC, 1991.
Return to citation in text: [1] -
Lowe, K. C.; Powell, R. L. J. Fluorine Chem. 2001, 109, 1. doi:10.1016/S0022-1139(01)00371-2
Return to citation in text: [1] -
Ojima, I.; McCarthy, J. R.; Welch, J. T., Eds. Biomedical frontiers of fluorine chemistry; American Chemical Society: Washington DC, 1996.
Return to citation in text: [1] -
Filler, R.; Kobayashi, Y.; Yagupolskii, L. M., Eds. Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications; Elsevier: Amsterdam, 1993.
Return to citation in text: [1] -
Banks, R. E., Ed. Fluorine in Agriculture; Fluorine Technology Ltd.: Chesire, 1994.
Return to citation in text: [1] -
O’Hagan, D.; Harper, D. B. J. Fluorine Chem. 1999, 100, 127–133. doi:10.1016/S0022-1139(99)00201-8
Return to citation in text: [1] -
Fried, J.; Sabo, E. F. J. Am. Chem. Soc. 1954, 76, 1455–1456. doi:10.1021/ja01634a101
Return to citation in text: [1] -
Phan, L. T.; Clark, R. F.; Rupp, M.; Or, Y. S.; Chu, D. T. W.; Ma, Z. Org. Lett. 2000, 2, 2951–2954. doi:10.1021/ol006226o
Return to citation in text: [1] -
Hesse, R.; Barton, D. Preparation of 5-fluoropyrimidines and intermediate therefrom. GB 1’362’839, 1972.
Return to citation in text: [1] -
Seebach, D. Angew. Chem., Int. Ed. Engl. 1990, 29, 1320–1367. doi:10.1002/anie.199013201
Return to citation in text: [1] -
Davis, F. A.; Yi, H.; Sundarababu, G. In Enantiocontrolled Synthesis of Fluoro-Organic Compounds; Soloshonok, V. A., Ed.; Wiley: Chichester, U.K., 1999; pp 1–32.
Return to citation in text: [1] -
Taylor, S. D.; Kotoris, C. C.; Hum, G. Tetrahedron 1999, 55, 12431–12477. doi:10.1016/S0040-4020(99)00748-6
Return to citation in text: [1] -
Differding, E.; Lang, R. W. Tetrahedron Lett. 1988, 29, 6087–6090. doi:10.1016/S0040-4039(00)82271-6
Return to citation in text: [1] -
Davis, F. A.; Zhou, P.; Murphy, C. K. Tetrahedron Lett. 1993, 34, 3971–3974. doi:10.1016/S0040-4039(00)60592-0
Return to citation in text: [1] -
Davis, F. A.; Zhou, P.; Murphy, C. K.; Sundarababu, G.; Qi, H.; Han, W.; Przeslawski, R. M.; Chen, B.; Carroll, P. J. J. Org. Chem. 1998, 63, 2273–2280. doi:10.1021/jo972045x
Return to citation in text: [1] -
Takeuchi, Y.; Suzuki, T.; Satoh, A.; Shiragami, T.; Shibata, N. J. Org. Chem. 1999, 64, 5708–5711. doi:10.1021/jo9904011
Return to citation in text: [1] -
Bruns, S.; Haufe, G. J. Fluorine Chem. 2000, 104, 247–254. doi:10.1016/S0022-1139(00)00249-9
Return to citation in text: [1] -
Haufe, G.; Bruns, S. Adv. Synth. Catal. 2002, 344, 165–171. doi:10.1002/1615-4169(200202)344:2<165::AID-ADSC165>3.0.CO;2-1
Return to citation in text: [1] -
Banks, R. E. J. Fluorine Chem. 1998, 87, 1–17. doi:10.1016/S0022-1139(97)00127-9
Return to citation in text: [1] [2] [3] -
Nyffeler, P. T.; Gonzalez Durón, S.; Burkart, M. D.; Vincent, S. P.; Wong, C.-H. Angew. Chem., Int. Ed. 2005, 44, 192–212. doi:10.1002/anie.200400648
Return to citation in text: [1] -
Singh, R. P.; Shreeve, J. M. Acc. Chem. Res. 2004, 37, 31–44. doi:10.1021/ar030043v
Return to citation in text: [1] -
Abdul-Ghani, M.; Banks, E. R.; Besheesh, M. K.; Sharif, I.; Syvret, R. G. J. Fluorine Chem. 1995, 73, 255–257. doi:10.1016/0022-1139(94)03225-O
Return to citation in text: [1] -
Cahard, D.; Audouard, C.; Plaquevent, J.-C.; Roques, N. Org. Lett. 2000, 2, 3699–3701. doi:10.1021/ol006610l
Return to citation in text: [1] -
Mohar, B.; Baudoux, J.; Plaquevent, J.-C.; Cahard, D. Angew. Chem., Int. Ed. 2001, 40, 4214–4216. doi:10.1002/1521-3773(20011119)40:22<4214::AID-ANIE4214>3.0.CO;2-B
Return to citation in text: [1] -
Cahard, D.; Audouard, C.; Plaquevent, J.-C.; Toupet, L.; Roques, N. Tetrahedron Lett. 2001, 42, 1867–1869. doi:10.1016/S0040-4039(01)00017-X
Return to citation in text: [1] -
Shibata, N.; Suzuki, E.; Takeuchi, Y. J. Am. Chem. Soc. 2000, 122, 10728–10729. doi:10.1021/ja002732x
Return to citation in text: [1] -
Shibata, N.; Suzuki, E.; Asahi, T.; Shiro, M. J. Am. Chem. Soc. 2001, 123, 7001–7009. doi:10.1021/ja010789t
Return to citation in text: [1] -
Hintermann, L.; Läng, F.; Maire, P.; Togni, A. Eur. J. Inorg. Chem. 2006, 1397–1412. doi:10.1002/ejic.200500795
Return to citation in text: [1] -
Barthazy, P.; Hintermann, L.; Stoop, R. M.; Wörle, M.; Mezzetti, A.; Togni, A. Helv. Chim. Acta 1999, 82, 2448–2453. doi:10.1002/(SICI)1522-2675(19991215)82:12<2448::AID-HLCA2448>3.0.CO;2-G
Return to citation in text: [1] -
Barthazy, P.; Togni, A.; Mezzetti, A. Organometallics 2001, 20, 3472–3477. doi:10.1021/om010288g
Return to citation in text: [1] -
Togni, A.; Mezzetti, A.; Barthazy, P.; Becker, C.; Devillers, I.; Frantz, R.; Hintermann, L.; Perseghini, M.; Sanna, M. Chimia 2001, 55, 801–805.
Return to citation in text: [1] -
Hintermann, L.; Togni, A. Angew. Chem., Int. Ed. 2000, 39, 4359–4362. doi:10.1002/1521-3773(20001201)39:23<4359::AID-ANIE4359>3.0.CO;2-P
Return to citation in text: [1] [2] [3] -
Hintermann, L.; Togni, A. Catalytic halogenation of activated methylene and methine compound. EP 1151980 A1, Nov 7, 2001.
Return to citation in text: [1] -
Muñiz, K. Angew. Chem., Int. Ed. 2001, 40, 1653–1656. doi:10.1002/1521-3773(20010504)40:9<1653::AID-ANIE16530>3.0.CO;2-W
Return to citation in text: [1] -
Hintermann, L.; Togni, A. Helv. Chim. Acta 2000, 83, 2425–2435. doi:10.1002/1522-2675(20000906)83:9<2425::AID-HLCA2425>3.0.CO;2-V
Return to citation in text: [1] [2] [3] -
Ibrahim, H.; Kleinbeck, F.; Togni, A. Helv. Chim. Acta 2004, 87, 605–610. doi:10.1002/hlca.200490058
Return to citation in text: [1] -
Oestreich, M. Angew. Chem., Int. Ed. 2005, 44, 2324–2327. doi:10.1002/anie.200500478
Return to citation in text: [1] -
Hamashima, Y.; Yagi, K.; Takano, H.; Tamás, L.; Sodeoka, M. J. Am. Chem. Soc. 2002, 124, 14530–14531. doi:10.1021/ja028464f
Return to citation in text: [1] [2] -
Ma, J.-A.; Cahard, D. Tetrahedron: Asymmetry 2004, 15, 1007–1011. doi:10.1016/j.tetasy.2004.01.014
Return to citation in text: [1] -
Shibata, N.; Ishimaru, T.; Nagai, T.; Kohno, J.; Toru, T. Synlett 2004, 1703–1706. doi:10.1055/s-2004-829571
Return to citation in text: [1] [2] -
Sanna, M. Recenti sviluppi fluorurazione catalitica enantioselettiva. Ph.D. Thesis, ETH Zürich, Switzerland, 2003. doi:10.3929/ethz-a-004636088
Return to citation in text: [1] -
Shibata, N.; Kohno, J.; Takai, K.; Ishimaru, T.; Nakamura, S.; Toru, T.; Kanemasa, S. Angew. Chem., Int. Ed. 2005, 44, 4204–4207. doi:10.1002/anie.200501041
Return to citation in text: [1] -
Ibrahim, H.; Togni, A. Chem. Commun. 2004, 1147–1155. doi:10.1039/b317004g
Return to citation in text: [1] [2] -
Kim, D. Y.; Park, E. J. Org. Lett. 2002, 4, 545–547. doi:10.1021/ol010281v
Return to citation in text: [1] -
Enders, D.; Hüttl, M. R. M. Synlett 2005, 991–993. doi:10.1055/s-2005-864813
Return to citation in text: [1] -
Marigo, M.; Fielenbach, D.; Braunton, A.; Kjærsgaard, A.; Jørgensen, K. A. Angew. Chem., Int. Ed. 2005, 44, 3703–3706. doi:10.1002/anie.200500395
Return to citation in text: [1] -
Steiner, D. D.; Mase, N.; Barbas, C. F., III. Angew. Chem., Int. Ed. 2005, 44, 3706–3710. doi:10.1002/anie.200500571
Return to citation in text: [1] -
Beeson, T. D.; MacMillan, D. W. C. J. Am. Chem. Soc. 2005, 127, 8826–8828. doi:10.1021/ja051805f
Return to citation in text: [1] -
Bertogg, A.; Hintermann, L.; Huber, D. P.; Perseghini, M.; Sanna, M.; Togni, A. Helv. Chim. Acta 2011, in press. doi:10.1002/hlca.201100375
Return to citation in text: [1] [2] [3] -
Smith, M. B.; March, J. Advanced Organic Chemistry; John Wiley & Sons: New York, 2001; pp 775–777.
Return to citation in text: [1] [2] [3] -
De Kimpe, N.; Verhé, R. The chemistry of α-haloketones, α-haloaldehydes and α-haloimines; John Wiley & Sons: Chichester, 1988; pp 1–38.
Return to citation in text: [1] [2] -
Verhé, R.; De Kimpe, N. Synthesis and reactivity of α-halogenated ketones. In Halides, Pseudo-Halides and Azides; Patai, S.; Rappoport, Z., Eds.; John Wiley & Sons: Chichester, U.K., 1983; pp 813–931. doi:10.1002/9780470771716.ch19
Return to citation in text: [1] -
Stavber, S.; Zupan, M. Tetrahedron Lett. 1996, 37, 3591–3594. doi:10.1016/0040-4039(96)00630-2
Return to citation in text: [1] -
Rozen, S.; Filler, R. Tetrahedron 1985, 41, 1111–1153. doi:10.1016/S0040-4020(01)96514-7
Return to citation in text: [1] -
Toullec, J. Keto-enol equilibrium constants. In The chemistry of enols; Rappoport, Z., Ed.; John Wiley & Sons: Chichester , 1990; pp 323–398.
Return to citation in text: [1] [2] -
Meyer, K. H. Justus Liebigs Ann. Chem. 1911, 380, 212–220. doi:10.1002/jlac.19113800206
Return to citation in text: [1] [2] -
Meyer, K. H.; Kappelmeier, P. Ber. Dtsch. Chem. Ges. 1911, 44, 2718–2724. doi:10.1002/cber.191104403105
Return to citation in text: [1] -
Banks, R. E.; Lawrence, N. J.; Popplewell, A. L. J. Chem. Soc., Chem. Commun. 1994, 343–344. doi:10.1039/C39940000343
Return to citation in text: [1] [2] -
Davis, F. A.; Han, W.; Murphy, C. K. J. Org. Chem. 1995, 60, 4730–4737. doi:10.1021/jo00120a014
Return to citation in text: [1] [2] -
Umemoto, T.; Tomizawa, G. J. Org. Chem. 1995, 60, 6563–6570. doi:10.1021/jo00125a049
Return to citation in text: [1] [2] -
Resnati, G.; DesMarteau, D. D. J. Org. Chem. 1991, 56, 4925–4929. doi:10.1021/jo00016a022
Return to citation in text: [1] [2] -
Yoshida, M.; Fujikawa, K.; Sato, S.; Hara, S. ARKIVOC 2003, 36–42.
Return to citation in text: [1] [2] -
Xiao, J.-C.; Shreeve, J. M. J. Fluorine Chem. 2005, 126, 473–476. doi:10.1016/j.jfluchem.2004.10.043
Return to citation in text: [1] [2] -
Umemoto, T.; Fukami, S.; Tomizawa, G.; Harasawa, K.; Kawada, K.; Tomita, K. J. Am. Chem. Soc. 1990, 112, 8563–8575. doi:10.1021/ja00179a047
Return to citation in text: [1] [2] -
Chambers, R. D.; Hutchinson, J. J. Fluorine Chem. 1998, 92, 45–52. doi:10.1016/S0022-1139(98)00254-1
Return to citation in text: [1] -
Frantz, R.; Hintermann, L.; Perseghini, M.; Broggini, D.; Togni, A. Org. Lett. 2003, 5, 1709–1712. doi:10.1021/ol0343459
Return to citation in text: [1] [2] -
Huber, D. P.; Stanek, K.; Togni, A. Tetrahedron: Asymmetry 2006, 17, 658–664. doi:10.1016/j.tetasy.2006.01.035
Return to citation in text: [1] -
Marigo, M.; Kumaragurubaran, N.; Jørgensen, K. A. Chem.–Eur. J. 2004, 10, 2133–2137. doi:10.1002/chem.200305759
Return to citation in text: [1] -
Wang, C.; Tunge, J. Chem. Commun. 2004, 2694–2695. doi:10.1039/b410576a
Return to citation in text: [1] -
Yang, D.; Yan, Y.-L.; Lui, B. J. Org. Chem. 2002, 67, 7429–7431. doi:10.1021/jo026025t
Return to citation in text: [1] -
Togni, A.; Halterman, R., Eds.; Metallocenes; Wiley-VCH: Weinheim, Germany, 1998.
Return to citation in text: [1] -
Halterman, R. L. Chem. Rev. 1992, 92, 965–994. doi:10.1021/cr00013a011
Return to citation in text: [1] -
Jaquith, J. B.; Guan, J.; Wang, S.; Collins, S. Organometallics 1995, 14, 1079–1081. doi:10.1021/om00003a001
Return to citation in text: [1] -
Seebach, D.; Beck, A. K.; Heckel, A. Angew. Chem., Int. Ed. 2001, 40, 92–138. doi:10.1002/1521-3773(20010105)40:1<92::AID-ANIE92>3.0.CO;2-K
Return to citation in text: [1] -
Hintermann, L.; Broggini, D.; Togni, A. Helv. Chim. Acta 2002, 85, 1597–1612. doi:10.1002/1522-2675(200206)85:6<1597::AID-HLCA1597>3.0.CO;2-N
Return to citation in text: [1] [2] -
Poss, A. J.; Shia, G. A. Tetrahedron Lett. 1999, 40, 2673–2676. doi:10.1016/S0040-4039(99)00303-2
Return to citation in text: [1] [2] -
Differding, E.; Ofner, H. Synlett 1991, 187–189. doi:10.1055/s-1991-20673
Return to citation in text: [1] -
Differding, E.; Lang, R. W. Helv. Chim. Acta 1989, 72, 1248–1252. doi:10.1002/hlca.19890720609
Return to citation in text: [1] -
Differding, E.; Bersier, P. M. Tetrahedron 1992, 48, 1595–1604. doi:10.1016/S0040-4020(01)88718-4
Return to citation in text: [1] -
Barnette, W. E. J. Am. Chem. Soc. 1984, 106, 452–454. doi:10.1021/ja00314a050
Return to citation in text: [1] -
Umemoto, T.; Harasawa, K.; Tomizawa, G.; Kawada, K.; Tomita, K. Bull. Chem. Soc. Jpn. 1991, 64, 1081–1092. doi:10.1246/bcsj.64.1081
Return to citation in text: [1] -
Umemoto, T.; Nagayoshi, M.; Adachi, K.; Tomizawa, G. J. Org. Chem. 1998, 63, 3379–3385. doi:10.1021/jo972338q
Return to citation in text: [1] -
Beck, A. K.; Bastani, B.; Plattner, D. A.; Petter, W.; Seebach, D.; Braunschweiger, H.; Gysi, P.; La Vecchia, L. Chimia 1991, 45, 238–244.
Return to citation in text: [1] -
Seebach, D.; Beck, A. K.; Dahinden, R.; Hoffmann, M.; Kuehnle, F. N. M. Croat. Chem. Acta 1996, 69, 459–484.
Return to citation in text: [1] -
Seebach, D.; Marti, R. E.; Hintermann, T. Helv. Chim. Acta 1996, 79, 1710–1740. doi:10.1002/hlca.19960790621
Return to citation in text: [1] -
Narasaka, K.; Iwasawa, N.; Inoue, M.; Yamada, T.; Nakashima, M.; Sugimori, J. J. Am. Chem. Soc. 1989, 111, 5340–5345. doi:10.1021/ja00196a045
Return to citation in text: [1] -
Kitazume, T.; Kobayashi, T. J. Fluorine Chem. 1986, 31, 357–361. doi:10.1016/S0022-1139(00)81437-2
Return to citation in text: [1] [2] [3] -
Dilthey, W. Ber. Dtsch. Chem. Ges. 1904, 37, 588–592. doi:10.1002/cber.19040370195
Return to citation in text: [1] -
Doron, V.; Shriver, D. Bis[Tris(Acetylacetonato)Titanium(IV)] Hexachlorotitanate(IV): (Bis[Tris(2,4-Pentanedionato)Titanium(IV)] Hexachlorotitanate(IV)). In Inorganic Syntheses; Kleinberg, J., Ed.; John Wiley & Sons: Hoboken, NJ, 1963; Vol. 7, pp 50–51. doi:10.1002/9780470132388.ch12
Return to citation in text: [1] -
Shao, M.-Y.; Sheen, W.-S.; Gau, H.-M. Inorg. Chim. Acta 2001, 314, 105–110. doi:10.1016/S0020-1693(01)00291-2
Return to citation in text: [1] -
Kütt, A.; Rodima, T.; Saame, J.; Raamat, E.; Mäemets, V.; Kaljurand, I.; Koppel, I. A.; Garlyauskayte, R. Y.; Yagupolskii, Y. L.; Yagupolskii, L. M.; Bernhardt, E.; Willner, H.; Leito, I. J. Org. Chem. 2011, 76, 391–395. doi:10.1021/jo101409p
Return to citation in text: [1] -
Sadowski, J.; Gasteiger, J.; Klebe, G. J. Chem. Inf. Comput. Sci. 1994, 34, 1000–1008. doi:10.1021/ci00020a039
Return to citation in text: [1] -
Piana, S.; Devillers, I.; Togni, A.; Rothlisberger, U. Angew. Chem., Int. Ed. 2002, 41, 979–982. doi:10.1002/1521-3773(20020315)41:6<979::AID-ANIE979>3.0.CO;2-E
Return to citation in text: [1] [2] -
Perseghini, M.; Massaccesi, M.; Liu, Y.; Togni, A. Tetrahedron 2006, 62, 7180–7190. doi:10.1016/j.tet.2005.12.071
Return to citation in text: [1] [2] [3] [4]
| 57. | Bertogg, A.; Hintermann, L.; Huber, D. P.; Perseghini, M.; Sanna, M.; Togni, A. Helv. Chim. Acta 2011, in press. doi:10.1002/hlca.201100375 |
| 58. | Smith, M. B.; March, J. Advanced Organic Chemistry; John Wiley & Sons: New York, 2001; pp 775–777. |
| 59. | De Kimpe, N.; Verhé, R. The chemistry of α-haloketones, α-haloaldehydes and α-haloimines; John Wiley & Sons: Chichester, 1988; pp 1–38. |
| 60. | Verhé, R.; De Kimpe, N. Synthesis and reactivity of α-halogenated ketones. In Halides, Pseudo-Halides and Azides; Patai, S.; Rappoport, Z., Eds.; John Wiley & Sons: Chichester, U.K., 1983; pp 813–931. doi:10.1002/9780470771716.ch19 |
| 61. | Stavber, S.; Zupan, M. Tetrahedron Lett. 1996, 37, 3591–3594. doi:10.1016/0040-4039(96)00630-2 |
| 62. | Rozen, S.; Filler, R. Tetrahedron 1985, 41, 1111–1153. doi:10.1016/S0040-4020(01)96514-7 |
| 102. | Perseghini, M.; Massaccesi, M.; Liu, Y.; Togni, A. Tetrahedron 2006, 62, 7180–7190. doi:10.1016/j.tet.2005.12.071 |
| 58. | Smith, M. B.; March, J. Advanced Organic Chemistry; John Wiley & Sons: New York, 2001; pp 775–777. |
| 102. | Perseghini, M.; Massaccesi, M.; Liu, Y.; Togni, A. Tetrahedron 2006, 62, 7180–7190. doi:10.1016/j.tet.2005.12.071 |
| 101. | Piana, S.; Devillers, I.; Togni, A.; Rothlisberger, U. Angew. Chem., Int. Ed. 2002, 41, 979–982. doi:10.1002/1521-3773(20020315)41:6<979::AID-ANIE979>3.0.CO;2-E |
| 102. | Perseghini, M.; Massaccesi, M.; Liu, Y.; Togni, A. Tetrahedron 2006, 62, 7180–7190. doi:10.1016/j.tet.2005.12.071 |
| 101. | Piana, S.; Devillers, I.; Togni, A.; Rothlisberger, U. Angew. Chem., Int. Ed. 2002, 41, 979–982. doi:10.1002/1521-3773(20020315)41:6<979::AID-ANIE979>3.0.CO;2-E |
| 72. | Umemoto, T.; Fukami, S.; Tomizawa, G.; Harasawa, K.; Kawada, K.; Tomita, K. J. Am. Chem. Soc. 1990, 112, 8563–8575. doi:10.1021/ja00179a047 |
| 73. | Chambers, R. D.; Hutchinson, J. J. Fluorine Chem. 1998, 92, 45–52. doi:10.1016/S0022-1139(98)00254-1 |
| 64. | Meyer, K. H. Justus Liebigs Ann. Chem. 1911, 380, 212–220. doi:10.1002/jlac.19113800206 |
| 59. | De Kimpe, N.; Verhé, R. The chemistry of α-haloketones, α-haloaldehydes and α-haloimines; John Wiley & Sons: Chichester, 1988; pp 1–38. |
| 58. | Smith, M. B.; March, J. Advanced Organic Chemistry; John Wiley & Sons: New York, 2001; pp 775–777. |
| 102. | Perseghini, M.; Massaccesi, M.; Liu, Y.; Togni, A. Tetrahedron 2006, 62, 7180–7190. doi:10.1016/j.tet.2005.12.071 |
| 66. | Banks, R. E.; Lawrence, N. J.; Popplewell, A. L. J. Chem. Soc., Chem. Commun. 1994, 343–344. doi:10.1039/C39940000343 |
| 67. | Davis, F. A.; Han, W.; Murphy, C. K. J. Org. Chem. 1995, 60, 4730–4737. doi:10.1021/jo00120a014 |
| 68. | Umemoto, T.; Tomizawa, G. J. Org. Chem. 1995, 60, 6563–6570. doi:10.1021/jo00125a049 |
| 69. | Resnati, G.; DesMarteau, D. D. J. Org. Chem. 1991, 56, 4925–4929. doi:10.1021/jo00016a022 |
| 70. | Yoshida, M.; Fujikawa, K.; Sato, S.; Hara, S. ARKIVOC 2003, 36–42. |
| 71. | Xiao, J.-C.; Shreeve, J. M. J. Fluorine Chem. 2005, 126, 473–476. doi:10.1016/j.jfluchem.2004.10.043 |
| 57. | Bertogg, A.; Hintermann, L.; Huber, D. P.; Perseghini, M.; Sanna, M.; Togni, A. Helv. Chim. Acta 2011, in press. doi:10.1002/hlca.201100375 |
| 63. | Toullec, J. Keto-enol equilibrium constants. In The chemistry of enols; Rappoport, Z., Ed.; John Wiley & Sons: Chichester , 1990; pp 323–398. |
| 57. | Bertogg, A.; Hintermann, L.; Huber, D. P.; Perseghini, M.; Sanna, M.; Togni, A. Helv. Chim. Acta 2011, in press. doi:10.1002/hlca.201100375 |
| 63. | Toullec, J. Keto-enol equilibrium constants. In The chemistry of enols; Rappoport, Z., Ed.; John Wiley & Sons: Chichester , 1990; pp 323–398. |
| 64. | Meyer, K. H. Justus Liebigs Ann. Chem. 1911, 380, 212–220. doi:10.1002/jlac.19113800206 |
| 65. | Meyer, K. H.; Kappelmeier, P. Ber. Dtsch. Chem. Ges. 1911, 44, 2718–2724. doi:10.1002/cber.191104403105 |
| 40. | Hintermann, L.; Togni, A. Angew. Chem., Int. Ed. 2000, 39, 4359–4362. doi:10.1002/1521-3773(20001201)39:23<4359::AID-ANIE4359>3.0.CO;2-P |
| 66. | Banks, R. E.; Lawrence, N. J.; Popplewell, A. L. J. Chem. Soc., Chem. Commun. 1994, 343–344. doi:10.1039/C39940000343 |
| 67. | Davis, F. A.; Han, W.; Murphy, C. K. J. Org. Chem. 1995, 60, 4730–4737. doi:10.1021/jo00120a014 |
| 68. | Umemoto, T.; Tomizawa, G. J. Org. Chem. 1995, 60, 6563–6570. doi:10.1021/jo00125a049 |
| 69. | Resnati, G.; DesMarteau, D. D. J. Org. Chem. 1991, 56, 4925–4929. doi:10.1021/jo00016a022 |
| 70. | Yoshida, M.; Fujikawa, K.; Sato, S.; Hara, S. ARKIVOC 2003, 36–42. |
| 71. | Xiao, J.-C.; Shreeve, J. M. J. Fluorine Chem. 2005, 126, 473–476. doi:10.1016/j.jfluchem.2004.10.043 |
| 27. | Banks, R. E. J. Fluorine Chem. 1998, 87, 1–17. doi:10.1016/S0022-1139(97)00127-9 |
| 74. | Frantz, R.; Hintermann, L.; Perseghini, M.; Broggini, D.; Togni, A. Org. Lett. 2003, 5, 1709–1712. doi:10.1021/ol0343459 |
| 75. | Huber, D. P.; Stanek, K.; Togni, A. Tetrahedron: Asymmetry 2006, 17, 658–664. doi:10.1016/j.tetasy.2006.01.035 |
| 43. | Hintermann, L.; Togni, A. Helv. Chim. Acta 2000, 83, 2425–2435. doi:10.1002/1522-2675(20000906)83:9<2425::AID-HLCA2425>3.0.CO;2-V |
| 46. | Hamashima, Y.; Yagi, K.; Takano, H.; Tamás, L.; Sodeoka, M. J. Am. Chem. Soc. 2002, 124, 14530–14531. doi:10.1021/ja028464f |
| 82. | Seebach, D.; Beck, A. K.; Heckel, A. Angew. Chem., Int. Ed. 2001, 40, 92–138. doi:10.1002/1521-3773(20010105)40:1<92::AID-ANIE92>3.0.CO;2-K |
| 83. | Hintermann, L.; Broggini, D.; Togni, A. Helv. Chim. Acta 2002, 85, 1597–1612. doi:10.1002/1522-2675(200206)85:6<1597::AID-HLCA1597>3.0.CO;2-N |
| 43. | Hintermann, L.; Togni, A. Helv. Chim. Acta 2000, 83, 2425–2435. doi:10.1002/1522-2675(20000906)83:9<2425::AID-HLCA2425>3.0.CO;2-V |
| 79. | Togni, A.; Halterman, R., Eds.; Metallocenes; Wiley-VCH: Weinheim, Germany, 1998. |
| 80. | Halterman, R. L. Chem. Rev. 1992, 92, 965–994. doi:10.1021/cr00013a011 |
| 81. | Jaquith, J. B.; Guan, J.; Wang, S.; Collins, S. Organometallics 1995, 14, 1079–1081. doi:10.1021/om00003a001 |
| 74. | Frantz, R.; Hintermann, L.; Perseghini, M.; Broggini, D.; Togni, A. Org. Lett. 2003, 5, 1709–1712. doi:10.1021/ol0343459 |
| 76. | Marigo, M.; Kumaragurubaran, N.; Jørgensen, K. A. Chem.–Eur. J. 2004, 10, 2133–2137. doi:10.1002/chem.200305759 |
| 77. | Wang, C.; Tunge, J. Chem. Commun. 2004, 2694–2695. doi:10.1039/b410576a |
| 78. | Yang, D.; Yan, Y.-L.; Lui, B. J. Org. Chem. 2002, 67, 7429–7431. doi:10.1021/jo026025t |
| 27. | Banks, R. E. J. Fluorine Chem. 1998, 87, 1–17. doi:10.1016/S0022-1139(97)00127-9 |
| 84. | Poss, A. J.; Shia, G. A. Tetrahedron Lett. 1999, 40, 2673–2676. doi:10.1016/S0040-4039(99)00303-2 |
| 84. | Poss, A. J.; Shia, G. A. Tetrahedron Lett. 1999, 40, 2673–2676. doi:10.1016/S0040-4039(99)00303-2 |
| 1. | Shimizu, M.; Hiyama, T. Angew. Chem., Int. Ed. 2005, 44, 214–231. doi:10.1002/anie.200460441 |
| 2. | Kirsch, P. Modern Fluoroorganic Chemistry; Wiley-VCH: Weinheim, Germany, 2004. |
| 3. | Hiyama, T. Organofluorine Compounds; Springer: Berlin, Germany, 2000. |
| 4. | Ma, J.; Cahard, D. Chem. Rev. 2004, 104, 6119–6146. doi:10.1021/cr030143e |
| 5. | Ramachandran, P. V., Ed.; Asymmetric Fluoroorganic Chemistry; American Chemical Society: Washington DC, 2000. |
| 6. | Baasner, B.; Hagemann, H.; Tatlow, J. C., Eds.; Methoden der Organischen Chemie, Houben-Weyl; Vol. E10a; Thieme Verlag: Stuttgart, Germany, 1999. |
| 7. | Soloshonok, V. A. Enantiocontrolled Synthesis of Fluoro-Organic Compounds; Wiley: Chichester, U.K., 1999. |
| 8. | Hudlicky, M.; Pavlath, A. E. Chemistry of Organic Fluorine Comounds II; American Chemical Society: Washington DC, 1995. |
| 9. | Welch, J. T. Selective Fluorination in Organic and Bioinorganic Chemistry; American Chemical Society: Washington DC, 1991. |
| 10. | Lowe, K. C.; Powell, R. L. J. Fluorine Chem. 2001, 109, 1. doi:10.1016/S0022-1139(01)00371-2 |
| 11. | Ojima, I.; McCarthy, J. R.; Welch, J. T., Eds. Biomedical frontiers of fluorine chemistry; American Chemical Society: Washington DC, 1996. |
| 12. | Filler, R.; Kobayashi, Y.; Yagupolskii, L. M., Eds. Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications; Elsevier: Amsterdam, 1993. |
| 13. | Banks, R. E., Ed. Fluorine in Agriculture; Fluorine Technology Ltd.: Chesire, 1994. |
| 17. | Hesse, R.; Barton, D. Preparation of 5-fluoropyrimidines and intermediate therefrom. GB 1’362’839, 1972. |
| 34. | Shibata, N.; Suzuki, E.; Takeuchi, Y. J. Am. Chem. Soc. 2000, 122, 10728–10729. doi:10.1021/ja002732x |
| 35. | Shibata, N.; Suzuki, E.; Asahi, T.; Shiro, M. J. Am. Chem. Soc. 2001, 123, 7001–7009. doi:10.1021/ja010789t |
| 92. | Seebach, D.; Beck, A. K.; Dahinden, R.; Hoffmann, M.; Kuehnle, F. N. M. Croat. Chem. Acta 1996, 69, 459–484. |
| 16. | Phan, L. T.; Clark, R. F.; Rupp, M.; Or, Y. S.; Chu, D. T. W.; Ma, Z. Org. Lett. 2000, 2, 2951–2954. doi:10.1021/ol006226o |
| 36. | Hintermann, L.; Läng, F.; Maire, P.; Togni, A. Eur. J. Inorg. Chem. 2006, 1397–1412. doi:10.1002/ejic.200500795 |
| 37. | Barthazy, P.; Hintermann, L.; Stoop, R. M.; Wörle, M.; Mezzetti, A.; Togni, A. Helv. Chim. Acta 1999, 82, 2448–2453. doi:10.1002/(SICI)1522-2675(19991215)82:12<2448::AID-HLCA2448>3.0.CO;2-G |
| 38. | Barthazy, P.; Togni, A.; Mezzetti, A. Organometallics 2001, 20, 3472–3477. doi:10.1021/om010288g |
| 15. | Fried, J.; Sabo, E. F. J. Am. Chem. Soc. 1954, 76, 1455–1456. doi:10.1021/ja01634a101 |
| 30. | Abdul-Ghani, M.; Banks, E. R.; Besheesh, M. K.; Sharif, I.; Syvret, R. G. J. Fluorine Chem. 1995, 73, 255–257. doi:10.1016/0022-1139(94)03225-O |
| 90. | Umemoto, T.; Nagayoshi, M.; Adachi, K.; Tomizawa, G. J. Org. Chem. 1998, 63, 3379–3385. doi:10.1021/jo972338q |
| 14. | O’Hagan, D.; Harper, D. B. J. Fluorine Chem. 1999, 100, 127–133. doi:10.1016/S0022-1139(99)00201-8 |
| 31. | Cahard, D.; Audouard, C.; Plaquevent, J.-C.; Roques, N. Org. Lett. 2000, 2, 3699–3701. doi:10.1021/ol006610l |
| 32. | Mohar, B.; Baudoux, J.; Plaquevent, J.-C.; Cahard, D. Angew. Chem., Int. Ed. 2001, 40, 4214–4216. doi:10.1002/1521-3773(20011119)40:22<4214::AID-ANIE4214>3.0.CO;2-B |
| 33. | Cahard, D.; Audouard, C.; Plaquevent, J.-C.; Toupet, L.; Roques, N. Tetrahedron Lett. 2001, 42, 1867–1869. doi:10.1016/S0040-4039(01)00017-X |
| 91. | Beck, A. K.; Bastani, B.; Plattner, D. A.; Petter, W.; Seebach, D.; Braunschweiger, H.; Gysi, P.; La Vecchia, L. Chimia 1991, 45, 238–244. |
| 22. | Davis, F. A.; Zhou, P.; Murphy, C. K. Tetrahedron Lett. 1993, 34, 3971–3974. doi:10.1016/S0040-4039(00)60592-0 |
| 23. | Davis, F. A.; Zhou, P.; Murphy, C. K.; Sundarababu, G.; Qi, H.; Han, W.; Przeslawski, R. M.; Chen, B.; Carroll, P. J. J. Org. Chem. 1998, 63, 2273–2280. doi:10.1021/jo972045x |
| 25. | Bruns, S.; Haufe, G. J. Fluorine Chem. 2000, 104, 247–254. doi:10.1016/S0022-1139(00)00249-9 |
| 26. | Haufe, G.; Bruns, S. Adv. Synth. Catal. 2002, 344, 165–171. doi:10.1002/1615-4169(200202)344:2<165::AID-ADSC165>3.0.CO;2-1 |
| 88. | Barnette, W. E. J. Am. Chem. Soc. 1984, 106, 452–454. doi:10.1021/ja00314a050 |
| 21. | Differding, E.; Lang, R. W. Tetrahedron Lett. 1988, 29, 6087–6090. doi:10.1016/S0040-4039(00)82271-6 |
| 27. | Banks, R. E. J. Fluorine Chem. 1998, 87, 1–17. doi:10.1016/S0022-1139(97)00127-9 |
| 28. | Nyffeler, P. T.; Gonzalez Durón, S.; Burkart, M. D.; Vincent, S. P.; Wong, C.-H. Angew. Chem., Int. Ed. 2005, 44, 192–212. doi:10.1002/anie.200400648 |
| 29. | Singh, R. P.; Shreeve, J. M. Acc. Chem. Res. 2004, 37, 31–44. doi:10.1021/ar030043v |
| 72. | Umemoto, T.; Fukami, S.; Tomizawa, G.; Harasawa, K.; Kawada, K.; Tomita, K. J. Am. Chem. Soc. 1990, 112, 8563–8575. doi:10.1021/ja00179a047 |
| 89. | Umemoto, T.; Harasawa, K.; Tomizawa, G.; Kawada, K.; Tomita, K. Bull. Chem. Soc. Jpn. 1991, 64, 1081–1092. doi:10.1246/bcsj.64.1081 |
| 2. | Kirsch, P. Modern Fluoroorganic Chemistry; Wiley-VCH: Weinheim, Germany, 2004. |
| 19. | Davis, F. A.; Yi, H.; Sundarababu, G. In Enantiocontrolled Synthesis of Fluoro-Organic Compounds; Soloshonok, V. A., Ed.; Wiley: Chichester, U.K., 1999; pp 1–32. |
| 20. | Taylor, S. D.; Kotoris, C. C.; Hum, G. Tetrahedron 1999, 55, 12431–12477. doi:10.1016/S0040-4020(99)00748-6 |
| 18. | Seebach, D. Angew. Chem., Int. Ed. Engl. 1990, 29, 1320–1367. doi:10.1002/anie.199013201 |
| 24. | Takeuchi, Y.; Suzuki, T.; Satoh, A.; Shiragami, T.; Shibata, N. J. Org. Chem. 1999, 64, 5708–5711. doi:10.1021/jo9904011 |
| 86. | Differding, E.; Lang, R. W. Helv. Chim. Acta 1989, 72, 1248–1252. doi:10.1002/hlca.19890720609 |
| 87. | Differding, E.; Bersier, P. M. Tetrahedron 1992, 48, 1595–1604. doi:10.1016/S0040-4020(01)88718-4 |
| 43. | Hintermann, L.; Togni, A. Helv. Chim. Acta 2000, 83, 2425–2435. doi:10.1002/1522-2675(20000906)83:9<2425::AID-HLCA2425>3.0.CO;2-V |
| 44. | Ibrahim, H.; Kleinbeck, F.; Togni, A. Helv. Chim. Acta 2004, 87, 605–610. doi:10.1002/hlca.200490058 |
| 45. | Oestreich, M. Angew. Chem., Int. Ed. 2005, 44, 2324–2327. doi:10.1002/anie.200500478 |
| 39. | Togni, A.; Mezzetti, A.; Barthazy, P.; Becker, C.; Devillers, I.; Frantz, R.; Hintermann, L.; Perseghini, M.; Sanna, M. Chimia 2001, 55, 801–805. |
| 40. | Hintermann, L.; Togni, A. Angew. Chem., Int. Ed. 2000, 39, 4359–4362. doi:10.1002/1521-3773(20001201)39:23<4359::AID-ANIE4359>3.0.CO;2-P |
| 41. | Hintermann, L.; Togni, A. Catalytic halogenation of activated methylene and methine compound. EP 1151980 A1, Nov 7, 2001. |
| 42. | Muñiz, K. Angew. Chem., Int. Ed. 2001, 40, 1653–1656. doi:10.1002/1521-3773(20010504)40:9<1653::AID-ANIE16530>3.0.CO;2-W |
| 95. | Kitazume, T.; Kobayashi, T. J. Fluorine Chem. 1986, 31, 357–361. doi:10.1016/S0022-1139(00)81437-2 |
| 95. | Kitazume, T.; Kobayashi, T. J. Fluorine Chem. 1986, 31, 357–361. doi:10.1016/S0022-1139(00)81437-2 |
| 93. | Seebach, D.; Marti, R. E.; Hintermann, T. Helv. Chim. Acta 1996, 79, 1710–1740. doi:10.1002/hlca.19960790621 |
| 94. | Narasaka, K.; Iwasawa, N.; Inoue, M.; Yamada, T.; Nakashima, M.; Sugimori, J. J. Am. Chem. Soc. 1989, 111, 5340–5345. doi:10.1021/ja00196a045 |
| 53. | Enders, D.; Hüttl, M. R. M. Synlett 2005, 991–993. doi:10.1055/s-2005-864813 |
| 54. | Marigo, M.; Fielenbach, D.; Braunton, A.; Kjærsgaard, A.; Jørgensen, K. A. Angew. Chem., Int. Ed. 2005, 44, 3703–3706. doi:10.1002/anie.200500395 |
| 55. | Steiner, D. D.; Mase, N.; Barbas, C. F., III. Angew. Chem., Int. Ed. 2005, 44, 3706–3710. doi:10.1002/anie.200500571 |
| 56. | Beeson, T. D.; MacMillan, D. W. C. J. Am. Chem. Soc. 2005, 127, 8826–8828. doi:10.1021/ja051805f |
| 83. | Hintermann, L.; Broggini, D.; Togni, A. Helv. Chim. Acta 2002, 85, 1597–1612. doi:10.1002/1522-2675(200206)85:6<1597::AID-HLCA1597>3.0.CO;2-N |
| 4. | Ma, J.; Cahard, D. Chem. Rev. 2004, 104, 6119–6146. doi:10.1021/cr030143e |
| 51. | Ibrahim, H.; Togni, A. Chem. Commun. 2004, 1147–1155. doi:10.1039/b317004g |
| 100. | Sadowski, J.; Gasteiger, J.; Klebe, G. J. Chem. Inf. Comput. Sci. 1994, 34, 1000–1008. doi:10.1021/ci00020a039 |
| 47. | Ma, J.-A.; Cahard, D. Tetrahedron: Asymmetry 2004, 15, 1007–1011. doi:10.1016/j.tetasy.2004.01.014 |
| 48. | Shibata, N.; Ishimaru, T.; Nagai, T.; Kohno, J.; Toru, T. Synlett 2004, 1703–1706. doi:10.1055/s-2004-829571 |
| 49. | Sanna, M. Recenti sviluppi fluorurazione catalitica enantioselettiva. Ph.D. Thesis, ETH Zürich, Switzerland, 2003. doi:10.3929/ethz-a-004636088 |
| 98. | Shao, M.-Y.; Sheen, W.-S.; Gau, H.-M. Inorg. Chim. Acta 2001, 314, 105–110. doi:10.1016/S0020-1693(01)00291-2 |
| 48. | Shibata, N.; Ishimaru, T.; Nagai, T.; Kohno, J.; Toru, T. Synlett 2004, 1703–1706. doi:10.1055/s-2004-829571 |
| 50. | Shibata, N.; Kohno, J.; Takai, K.; Ishimaru, T.; Nakamura, S.; Toru, T.; Kanemasa, S. Angew. Chem., Int. Ed. 2005, 44, 4204–4207. doi:10.1002/anie.200501041 |
| 99. | Kütt, A.; Rodima, T.; Saame, J.; Raamat, E.; Mäemets, V.; Kaljurand, I.; Koppel, I. A.; Garlyauskayte, R. Y.; Yagupolskii, Y. L.; Yagupolskii, L. M.; Bernhardt, E.; Willner, H.; Leito, I. J. Org. Chem. 2011, 76, 391–395. doi:10.1021/jo101409p |
| 40. | Hintermann, L.; Togni, A. Angew. Chem., Int. Ed. 2000, 39, 4359–4362. doi:10.1002/1521-3773(20001201)39:23<4359::AID-ANIE4359>3.0.CO;2-P |
| 95. | Kitazume, T.; Kobayashi, T. J. Fluorine Chem. 1986, 31, 357–361. doi:10.1016/S0022-1139(00)81437-2 |
| 46. | Hamashima, Y.; Yagi, K.; Takano, H.; Tamás, L.; Sodeoka, M. J. Am. Chem. Soc. 2002, 124, 14530–14531. doi:10.1021/ja028464f |
| 96. | Dilthey, W. Ber. Dtsch. Chem. Ges. 1904, 37, 588–592. doi:10.1002/cber.19040370195 |
| 97. | Doron, V.; Shriver, D. Bis[Tris(Acetylacetonato)Titanium(IV)] Hexachlorotitanate(IV): (Bis[Tris(2,4-Pentanedionato)Titanium(IV)] Hexachlorotitanate(IV)). In Inorganic Syntheses; Kleinberg, J., Ed.; John Wiley & Sons: Hoboken, NJ, 1963; Vol. 7, pp 50–51. doi:10.1002/9780470132388.ch12 |
© 2011 Hintermann et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)