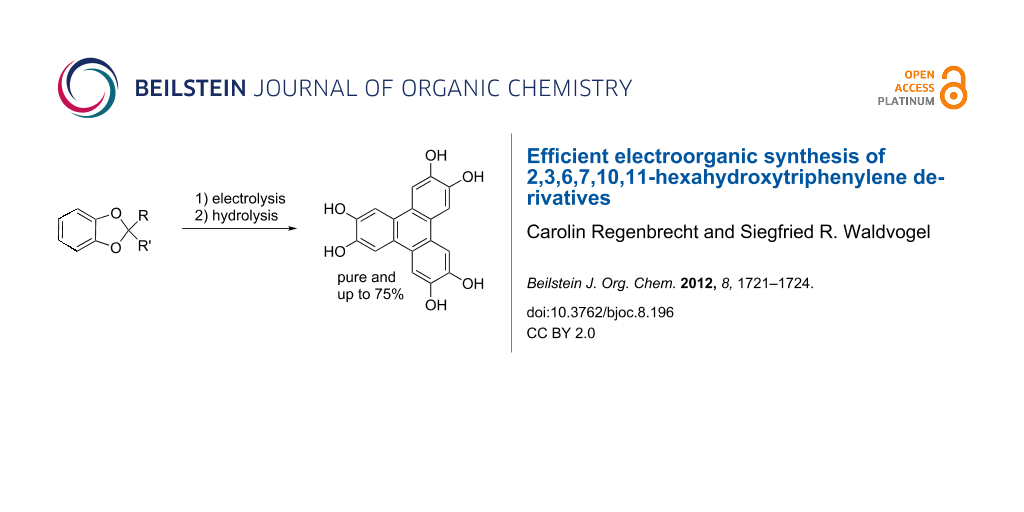
Abstract
2,3,6,7,10,11-Hexahydroxytriphenylene of good quality and purity can be obtained via anodic treatment of catechol ketals and subsequent acidic hydrolysis. The electrolysis is conducted in propylene carbonate circumventing toxic and expensive acetonitrile. The protocol is simple to perform and superior to other chemical or electrochemical methods. The key of the method is based on the low solubility of the anodically trimerized product. The shift of potentials is supported by cyclic voltammetry studies.
Graphical Abstract
Introduction
The unique spectroscopic and geometric features of triphenylenes give rise to a variety of applications for this very common structural motif. The use of triphenylenes in discotic liquid crystals [1,2] as building blocks in supramolecular chemistry [3,4] as well as in solid-state chemistry [5] is well documented. Furthermore, triphenylenes are applied as components of functional polymers [6,7] and fluorescent labels [8,9]. Typically, the oxidative trimerization of catechol derivatives can be induced by metal salts in high oxidation states, for example by molybdenum pentachloride [4,10]. Electrochemical methods can be applied to realize the oxidative trimerization since the formation of metal waste is avoided [11,12]. Furthermore, no transition metal cations, which promote the cleavage of the ketal moiety [11], are involved.
The anodic oxidation of catechol derivatives was first demonstrated by Parker et al. [13]. Applying this methodology in a specific electrolysis cell, Simonet et al. established a trimerization protocol [14-16]. Usually, yields are in a moderate range (≤35%) [15] when the electrolysis is performed on platinum or graphite anodes in anhydrous and non-nucleophilic electrolytes [14]. Poor yields are caused by the low oxidation potential of the products and the preference for over-oxidation followed by decomposition of the generated radical cations [11,16].
Since we have a specific interest in a large-scale access to derivatives of 2,3,6,7,10,11-hexahydroxytriphenylene, we were prompted to develop a sustainable protocol providing the target compound by electroorganic methods. Precipitation during electrolysis avoided over-oxidation of the electron-rich triphenylene derivatives [12]. Yields up to 62% were obtained when tetrabutylammonium tetrafluoroborate (TBABF4) in acetonitrile (ACN) was used as electrolyte [11]. To our delight, we found that the electrolysis is accomplished under practical galvanostatic conditions using platinum sheets as electrode materials. Due to the large potential window and outstanding solubility for common conducting salts, ACN is among the standard solvents for electrochemical purposes [17]. However, the application of ACN in electrochemical processes is adversely affected by its severe toxicity and significant costs. Therefore, switching to an environmentally benign and inexpensive solvent is highly desired. This work demonstrates that propylene carbonate (PC) successfully replaces ACN by fine-tuning of the supporting electrolytes.
Results and Discussion
Anodic oxidation of catechol ketals
We report a simple protocol for the anodic oxidation of catechol ketals forming triphenylene ketals (Scheme 1).
Scheme 1: Experimental conditions for the anodic oxidation of catechol ketals.
Scheme 1: Experimental conditions for the anodic oxidation of catechol ketals.
Best results were obtained in PC and TMABF4 (method A) or TBABF4 (method B) as electrolytes. Performing the reaction in PC is beneficial for several reasons: PC is hazard-free, inexpensive and suitable for large-scale reaction. Moreover, triphenylene ketals have lower oxidation potentials than the corresponding catechol ketals. Since the non-polar products exhibit a relative poor solubility in PC, precipitation is achieved during the electrolysis. Usually, the precipitate floats as fine particles in the cell and is prevented from over-oxidation. The best results for the individual catechol ketals are listed in Table 1.
Table 1: Anodic trimerization of catechol ketals.a
| Entry | Catechol ketal | Method | Triphenylene ketal [%] | CE [%] | |
|---|---|---|---|---|---|
| 1 |
1a
1a |
A
B |
2a
2a |
80
70 |
52
45 |
| 2 |
1b
1b |
A
B |
2b
2b |
61
37 |
39
24 |
| 3 |
1c
1c |
A
B |
2c
2c |
61
50 |
39
32 |
| 4 | 1d | B | 2d | 29 | 25 |
| 5 | 1e | B | 2e | 39 | 19 |
aConditions: Pt electrodes, T = 20 °C, J = 16 mA cm−2, 3.1 F/catechol ketal, argon.
Yields up to 80% are obtained for the dehydrotrimers 2a–c in PC and TMABF4 as electrolyte. The solvation effects and polarity of the solvent greatly influence the precipitation of the desired products. Since the solvation increases with a decreasing size of the tetraalkylammonium cations, the polarity of the electrolyte increases as well [18,19]. The precipitation of the non-polar triphenylenes is promoted in the strongly polar electrolyte. Triphenylene derivatives 2d and 2e were obtained with 29 and 39% yield, respectively, using TBABF4 in PC. The yields are higher compared to that of reported electrochemical methods [15]. The simple reaction setup using an undivided cell and galvanostatic conditions applying this moderate current density allows a scale-up of this electroorganic synthesis.
Cyclic voltammetry
Cyclic voltammetry studies show that triphenylene ketal 2a gets more easily oxidized at lower potentials than subunit 1a (Figure 1).
Figure 1: Cyclic voltammograms of catechol ketal 1a in ACN and PC and triphenylene ketal 2a in ACN, for magnification of 2a see inner rectangle (1a: 5∙10−3 M in 0.1 M TBAClO4/ACN or 0.1 M TBAClO4/PC vs Ag/AgCl, 2a: 5∙10−3 M in 0.1 M TBAClO4/ACN vs Ag/AgCl; sweep rates: 50 mV/s; third cycles).
Figure 1: Cyclic voltammograms of catechol ketal 1a in ACN and PC and triphenylene ketal 2a in ACN, for magni...
The first reversible process at Eox = 1.22 V corresponds to the oxidation of 2a to a radical cation [13]. This SET is followed by the irreversible oxidation at Eox = 1.70 V. According to the Nernst equation, oxidation potentials should be increased due to the insolubility of 2a in PC. Because of the insufficient solubility in this solvent, cyclic voltammograms were measured solely in ACN. The oxidation of 1a was analyzed in PC and ACN. Both cyclic voltammograms show an irreversible oxidation at Eox = 1.61 and 1.58 V, respectively [15]. The increase of the current densities beginning at 2.14 V is caused by the decomposition of electrolyte which was confirmed by blank experiments. The obtained data clearly indicate that triphenylene derivatives should precipitate during electrolysis to avoid over-oxidation.
Preparation of 2,3,6,7,10,11-hexahydroxytriphenylene
2,3,6,7,10,11-Hexahydroxytriphenylene (3) was obtained almost quantitatively by acidic cleavage of the ketal moieties of 2b (Scheme 2).
Scheme 2: Acid-catalyzed cleavage of ketal moieties.
Scheme 2: Acid-catalyzed cleavage of ketal moieties.
The hydrolysis is initiated by the water which is present in the acetic acid. Commonly, 3 is prepared by dealkylation of 2,3,6,7,10,11-hexamethoxytriphenylene [20,21]. The resulting product is usually a black solid being colorized by chinoide byproducts [20,22] which are not present in our method. However, our method provides easy access to pure 3 which can easily be separated and purified by simple filtration.
Conclusion
Triphenylene ketals are easily available by anodic oxidation using a simple galvanostatic protocol. Best results are obtained when the synthesized dehydrotrimers are almost insoluble in the electrolyte. Precipitation occurs during the electrolysis when employing tetraalkylammonium salts in PC. This effectively prevents the desired products from over-oxidation. Since PC is environmentally benign and inexpensive, the procedure significantly improved the access to the triphenylene derivatives. Moreover, acid-catalyzed cleavage of triphenylene ketals provides 2,3,6,7,10,11-hexahydroxytriphenylene almost quantitatively in very good quality.
Experimental
General protocol for anodic oxidation
The catechol ketal (10 mmol) was mixed with the electrolyte (method A: 2.012 g TMABF4 and 23.3 mL PC; method B: 4.116 g TBABF4 and 20.7 mL PC) in an undivided standard electrolysis cell and stirred under argon for 5 min. At 20 °C a galvanostatic electrolysis with a current density of 16 mA cm−2 was performed on platinum foil as electrodes (2.2 cm × 3.2 cm). The polarity of electrodes was reversed every 15 min. During the electrolysis, vigorous stirring was necessary and the formation of triphenylene products as light brown precipitates could be observed. After application of 3000 C (3.1 F) the electrolyte and precipitate were removed from the cell by dissolving in dichloromethane. After removal of the solvent under reduced pressure, the crude product and PC were dissolved in 40 mL of hot methanol. Water (17 mL) was slowly added to the hot solution and the mixture was allowed to cool to room temperature. Subsequently, the mixture was kept at 5 °C for several hours. The precipitate was filtered off and washed with methanol:water (7:3, 25 mL) and dried (45 °C, 8·10−3 mbar). Triphenylene derivatives 2a–e were unequivocally identified.
Supporting Information
| Supporting Information File 1: Characterization data and spectra of synthesized compounds. | ||
| Format: PDF | Size: 828.0 KB | Download |
References
-
Barón, M. Pure Appl. Chem. 2001, 73, 845–895. doi:10.1351/pac200173050845
Return to citation in text: [1] -
Boden, N.; Bushby, R. J.; Cooke, G.; Lozman, O. R.; Lu, Z. J. Am. Chem. Soc. 2001, 123, 7915–7916. doi:10.1021/ja003443b
Return to citation in text: [1] -
Schopohl, M. C.; Siering, C.; Kataeva, O.; Waldvogel, S. R. Angew. Chem., Int. Ed. 2003, 42, 2620–2623. doi:10.1002/anie.200351102
Return to citation in text: [1] -
Waldvogel, S. R.; Fröhlich, R.; Schalley, C. A. Angew. Chem., Int. Ed. 2000, 39, 2472–2475. doi:10.1002/1521-3773(20000717)39:14<2472::AID-ANIE2472>3.0.CO;2-F
Return to citation in text: [1] [2] -
Côté, A. P.; Benin, A. I.; Ockwig, N. W.; O'Keeffe, M.; Matzger, A. J.; Yaghi, O. M. Science 2005, 310, 1166–1170. doi:10.1126/science.1120411
Return to citation in text: [1] -
Ringsdorf, H.; Schlarb, B.; Venzmer, J. Angew. Chem., Int. Ed. Engl. 1988, 27, 113–158. doi:10.1002/anie.198801131
Return to citation in text: [1] -
Rose, A.; Zhu, Z.; Madigan, C. F.; Swager, T. M.; Bulović, V. Nature 2005, 434, 876–879. doi:10.1038/nature03438
Return to citation in text: [1] -
Siering, C.; Kerschbaumer, H.; Nieger, M.; Waldvogel, S. R. Org. Lett. 2006, 8, 1471–1474. doi:10.1021/ol0603110
Return to citation in text: [1] -
Berlman, I. B. Handbook of Fluorescence Spectra of Aromatic Molecules; Academic Press: New York, 1971.
Return to citation in text: [1] -
Waldvogel, S. R.; Wartini, A. R.; Rasmussen, P. H.; Rebek, J., Jr. Tetrahedron Lett. 1999, 40, 3515–3517. doi:10.1016/S0040-4039(99)00545-6
Return to citation in text: [1] -
Waldvogel, S. R.; Mirk, D. Tetrahedron Lett. 2000, 41, 4769–4772. doi:10.1016/S0040-4039(00)00722-X
Return to citation in text: [1] [2] [3] [4] -
Waldvogel, S. R.; Mirk, D.; Herbrüggen, J. GDCh-Monographie 2001, 23, 233–239.
Return to citation in text: [1] [2] -
Berchgaard, K.; Parker, V. D. J. Am. Chem. Soc. 1974, 94, 4749–4750. doi:10.1021/ja00768a063
Return to citation in text: [1] [2] -
Le Berre, V.; Carlier, R.; Tallec, A.; Simonet, J. J. Electroanal. Chem. 1983, 143, 425–432. doi:10.1016/S0022-0728(83)80279-4
Return to citation in text: [1] [2] -
Chapuzet, J.-M.; Simonet, J. Tetrahedron 1991, 47, 791–798. doi:10.1016/S0040-4020(01)87068-X
Return to citation in text: [1] [2] [3] [4] -
Chapuzet, J.-M.; Simonet-Guégen, N.; Taillepied, I.; Simonet, J. Tetrahedron Lett. 1991, 32, 7405–7408. doi:10.1016/0040-4039(91)80118-P
Return to citation in text: [1] [2] -
Beck, F. Elektroorganische Chemie; Akademie-Verlag: Berlin, 1974.
Return to citation in text: [1] -
Fry, A. J. Electrochem. Commun. 2005, 7, 602–606. doi:10.1016/j.elecom.2005.04.007
Return to citation in text: [1] -
Fry, A. J. Tetrahedron 2006, 62, 6558–6565. doi:10.1016/j.tet.2006.03.057
Return to citation in text: [1] -
Naarmann, H.; Hanack, M.; Mattmer, R. Synthesis 1994, 477–478. doi:10.1055/s-1994-25505
Return to citation in text: [1] [2] -
Krebs, F. C.; Schiødt, N. C.; Batsberg, W.; Bechgaard, K. Synthesis 1997, 1285–1290. doi:10.1055/s-1997-3188
Return to citation in text: [1] -
Voisin, E.; Williams, V. E. Macromolecules 2008, 41, 2994–2997. doi:10.1021/ma800320j
Return to citation in text: [1]
| 13. | Berchgaard, K.; Parker, V. D. J. Am. Chem. Soc. 1974, 94, 4749–4750. doi:10.1021/ja00768a063 |
| 18. | Fry, A. J. Electrochem. Commun. 2005, 7, 602–606. doi:10.1016/j.elecom.2005.04.007 |
| 19. | Fry, A. J. Tetrahedron 2006, 62, 6558–6565. doi:10.1016/j.tet.2006.03.057 |
| 15. | Chapuzet, J.-M.; Simonet, J. Tetrahedron 1991, 47, 791–798. doi:10.1016/S0040-4020(01)87068-X |
| 1. | Barón, M. Pure Appl. Chem. 2001, 73, 845–895. doi:10.1351/pac200173050845 |
| 2. | Boden, N.; Bushby, R. J.; Cooke, G.; Lozman, O. R.; Lu, Z. J. Am. Chem. Soc. 2001, 123, 7915–7916. doi:10.1021/ja003443b |
| 8. | Siering, C.; Kerschbaumer, H.; Nieger, M.; Waldvogel, S. R. Org. Lett. 2006, 8, 1471–1474. doi:10.1021/ol0603110 |
| 9. | Berlman, I. B. Handbook of Fluorescence Spectra of Aromatic Molecules; Academic Press: New York, 1971. |
| 11. | Waldvogel, S. R.; Mirk, D. Tetrahedron Lett. 2000, 41, 4769–4772. doi:10.1016/S0040-4039(00)00722-X |
| 6. | Ringsdorf, H.; Schlarb, B.; Venzmer, J. Angew. Chem., Int. Ed. Engl. 1988, 27, 113–158. doi:10.1002/anie.198801131 |
| 7. | Rose, A.; Zhu, Z.; Madigan, C. F.; Swager, T. M.; Bulović, V. Nature 2005, 434, 876–879. doi:10.1038/nature03438 |
| 5. | Côté, A. P.; Benin, A. I.; Ockwig, N. W.; O'Keeffe, M.; Matzger, A. J.; Yaghi, O. M. Science 2005, 310, 1166–1170. doi:10.1126/science.1120411 |
| 11. | Waldvogel, S. R.; Mirk, D. Tetrahedron Lett. 2000, 41, 4769–4772. doi:10.1016/S0040-4039(00)00722-X |
| 16. | Chapuzet, J.-M.; Simonet-Guégen, N.; Taillepied, I.; Simonet, J. Tetrahedron Lett. 1991, 32, 7405–7408. doi:10.1016/0040-4039(91)80118-P |
| 3. | Schopohl, M. C.; Siering, C.; Kataeva, O.; Waldvogel, S. R. Angew. Chem., Int. Ed. 2003, 42, 2620–2623. doi:10.1002/anie.200351102 |
| 4. | Waldvogel, S. R.; Fröhlich, R.; Schalley, C. A. Angew. Chem., Int. Ed. 2000, 39, 2472–2475. doi:10.1002/1521-3773(20000717)39:14<2472::AID-ANIE2472>3.0.CO;2-F |
| 12. | Waldvogel, S. R.; Mirk, D.; Herbrüggen, J. GDCh-Monographie 2001, 23, 233–239. |
| 13. | Berchgaard, K.; Parker, V. D. J. Am. Chem. Soc. 1974, 94, 4749–4750. doi:10.1021/ja00768a063 |
| 15. | Chapuzet, J.-M.; Simonet, J. Tetrahedron 1991, 47, 791–798. doi:10.1016/S0040-4020(01)87068-X |
| 20. | Naarmann, H.; Hanack, M.; Mattmer, R. Synthesis 1994, 477–478. doi:10.1055/s-1994-25505 |
| 22. | Voisin, E.; Williams, V. E. Macromolecules 2008, 41, 2994–2997. doi:10.1021/ma800320j |
| 11. | Waldvogel, S. R.; Mirk, D. Tetrahedron Lett. 2000, 41, 4769–4772. doi:10.1016/S0040-4039(00)00722-X |
| 14. | Le Berre, V.; Carlier, R.; Tallec, A.; Simonet, J. J. Electroanal. Chem. 1983, 143, 425–432. doi:10.1016/S0022-0728(83)80279-4 |
| 11. | Waldvogel, S. R.; Mirk, D. Tetrahedron Lett. 2000, 41, 4769–4772. doi:10.1016/S0040-4039(00)00722-X |
| 12. | Waldvogel, S. R.; Mirk, D.; Herbrüggen, J. GDCh-Monographie 2001, 23, 233–239. |
| 15. | Chapuzet, J.-M.; Simonet, J. Tetrahedron 1991, 47, 791–798. doi:10.1016/S0040-4020(01)87068-X |
| 4. | Waldvogel, S. R.; Fröhlich, R.; Schalley, C. A. Angew. Chem., Int. Ed. 2000, 39, 2472–2475. doi:10.1002/1521-3773(20000717)39:14<2472::AID-ANIE2472>3.0.CO;2-F |
| 10. | Waldvogel, S. R.; Wartini, A. R.; Rasmussen, P. H.; Rebek, J., Jr. Tetrahedron Lett. 1999, 40, 3515–3517. doi:10.1016/S0040-4039(99)00545-6 |
| 14. | Le Berre, V.; Carlier, R.; Tallec, A.; Simonet, J. J. Electroanal. Chem. 1983, 143, 425–432. doi:10.1016/S0022-0728(83)80279-4 |
| 15. | Chapuzet, J.-M.; Simonet, J. Tetrahedron 1991, 47, 791–798. doi:10.1016/S0040-4020(01)87068-X |
| 16. | Chapuzet, J.-M.; Simonet-Guégen, N.; Taillepied, I.; Simonet, J. Tetrahedron Lett. 1991, 32, 7405–7408. doi:10.1016/0040-4039(91)80118-P |
| 20. | Naarmann, H.; Hanack, M.; Mattmer, R. Synthesis 1994, 477–478. doi:10.1055/s-1994-25505 |
| 21. | Krebs, F. C.; Schiødt, N. C.; Batsberg, W.; Bechgaard, K. Synthesis 1997, 1285–1290. doi:10.1055/s-1997-3188 |
© 2012 Regenbrecht and Waldvogel; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)