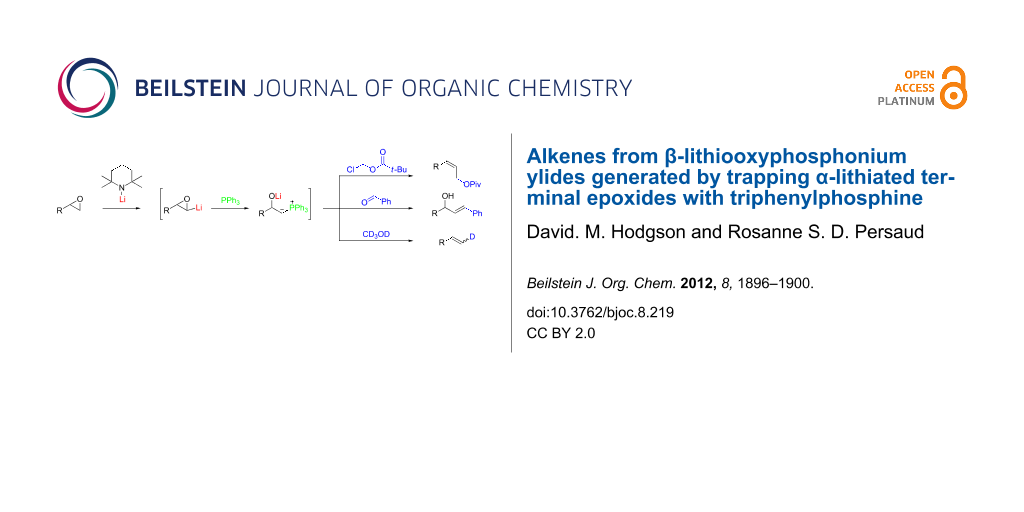
Abstract
Terminal epoxides undergo lithium 2,2,6,6-tetramethylpiperidide-induced α-lithiation and subsequent interception with Ph3P to provide a new and direct entry to β-lithiooxyphosphonium ylides. The intermediacy of such an ylide is demonstrated by representative alkene-forming reactions with chloromethyl pivalate, benzaldehyde and CD3OD, giving a Z-allylic pivalate, a conjugated E-allylic alcohol and a partially deuterated terminal alkene, respectively, in modest yields.
Graphical Abstract
Introduction
β-Lithiooxyphosphonium ylides 4 are useful intermediates in synthesis as they react with a variety of electrophiles to provide a convergent entry to alkenes, often with high regio- and stereocontrol (Scheme 1) [1-9]. These ylide intermediates can be generated by initiating a Wittig reaction between an aldehyde 1 and a phosphorane 2 at low temperature in the presence of lithium salts, which promote ring opening of the initially formed oxaphosphetane 3, followed by deprotonation typically using PhLi [5].
Scheme 1: Typical generation of ylide 4 and reaction examples.
Scheme 1: Typical generation of ylide 4 and reaction examples.
We recently reported the use of methylenetriphenylphosphorane (2) (R2 = H) in this chemistry for the synthesis of Z-allylic esters such as 6 [8] and conjugated E-allylic alcohols such as 7 [9]. β-Lithiooxyphosphonium ylides 4 (R2 = H) can also be generated by double deprotonation of β-hydroxy primary phosphonium salts [10-19], where the latter are obtained from Ph3P and 1,2-halohydrins [10-16,19] or (in the presence of acid) from terminal epoxides [17,18]. In seeking a more concise way than the above approaches to β-lithiooxyphosphonium ylides 4 (R2 = H), we were attracted to the possibility of phosphines intercepting α-lithiated terminal epoxides 10 (Scheme 2) and report here the results of that study. Such carbenoids 10 are unstable, but they can be easily formed from terminal epoxides 8 by using hindered lithium amides, such as lithium tetramethylpiperidide (9, LTMP) [20], and have shown synthetically useful carbene reactivity (e.g., cyclopropanation [21,22], dimerization [23-25]). The reaction of carbenes and carbenoids with heteroatom lone pairs is a popular strategy to access ylides [26], although phosphonium ylides for carbonyl-olefination chemistry are usually prepared by deprotonation of phosphonium salts [1-4]. In fact, phosphine trapping of lithium carbenoids followed by carbonyl olefination has been little studied since Seyferth and Wittig independently reported the synthesis of chloro alkenes in modest yields (20–30%) by this route (using CH2Cl2 and BuLi in the presence of Ph3P) over half a century ago [27-31].
Scheme 2: Proposed ylide 4 formation from α-lithiated epoxide 10.
Scheme 2: Proposed ylide 4 formation from α-lithiated epoxide 10.
Results and Discussion
The feasibility of generating and reacting β-lithiooxyphosphonium ylides 4 (R2 = H) derived directly from epoxides began with studies to produce allylic ester 6 under LTMP-based conditions for α-lithiation of terminal epoxides [20-22] but with Ph3P also present (Scheme 3). Encouragingly, a red-orange colour, which is characteristic of a β-lithiooxyphosphonium ylide [8,9], gradually developed (mixing only LTMP and PPh3 in THF at 0 °C for 24 h, gave no colour change from an initial yellow solution), becoming very intense after 3 h, although some epoxide 11 was still present after 24 h (TLC monitoring); the reduced activity of LTMP may be due to phosphine coordination [32]. At this point, following cooling to −78 °C [8], chloromethyl pivalate (5) was added, resulting in the isolation of allylic ester 6 (23%). Only the Z-isomer of 6 was observed, indicating that stereoselectivity is not altered by this method of β-lithiooxyphosphonium ylide formation. The presence of LiBr (1 equiv) from the start of an otherwise identical reaction made no significant difference to the yield of Z-allylic ester 6 (26%), although the presence of such a salt is considered essential for efficient generation of 4 from carbonyl compounds (Scheme 1) [5]; this observation lends support to the notion that the principal role of LiBr is to facilitate oxaphosphetane ring opening to enable subsequent lithiation, and its presence does not significantly influence subsequent reaction steps, at least with this electrophile. While simple phosphoranes (Ph3PCH2 and Ph3PCHMe) are known to react with epoxides (32–68% yields) in the presence of soluble lithium halides [33,34], the homoallylic alcohol, which would arise [35] from any reaction of β-lithiooxyphosphonium ylide and terminal epoxide, was not observed in the present studies; this suggests that the latter ylides are not capable of reacting with terminal epoxides [35], or the presence of LTMP and/or PPh3 prevents this reaction from occurring.
Scheme 3: Z-Allylic ester 6 from epoxide 11.
Scheme 3: Z-Allylic ester 6 from epoxide 11.
The original study on the reaction between LTMP and terminal epoxides in THF showed this to be an efficient way to prepare the corresponding isomerized aldehydes [20] (later established as proceeding through an intermediate TMP enamine) [36,37]. In the present work, neither decanal nor its corresponding TMP enamine were not detected as side-products, and we also established that the presence of LiBr (1 equiv) did not interfere in this isomerization process, giving decanal from epoxide 11 in 65% yield (67% without LiBr) and with no unreacted epoxide observed. The use of shorter reaction times (2–4 h) for the generation of the epoxide-derived ylide 4 (R2 = H), including increasing the quantities of LTMP and Ph3P (to 3 equiv), or the use of t-BuOMe as solvent [21,22], did not lead to improved yields of ester 6.
As terminal epoxides are readily available as single enantiomers [38,39], it was considered important to study the possibility of using an aldehyde electrophile with the epoxide-derived ylide. This would provide an entry into allylic alcohols [40], where the epoxide stereocentre is preserved in the product [17,18]. In the event, benzaldehyde was successfully trapped to give E-allylic alcohol 7 in up to 33% yield (Scheme 4) by using LTMP (1 equiv), Ph3P (5 equiv) and LiBr (2 equiv; 24% yield in the absence of LiBr). Essentially the same yields (31% and 30%) were obtained under otherwise identical conditions but with 2 equiv of Ph3P, or with excess LTMP (3 equiv) and Ph3P (9 equiv). Other experimental variations (use of sub-stoichiometric TMP (0.25 equiv) [22] or substitution of LiBr by LiCl) did not improve the yield of alcohol 7 (20% and 10%, respectively), whereas substitution of Ph3P by Bu3P or Cy3P did not lead to the orange–red colouration suggestive of ylide formation, and only starting epoxide 11 was observed.
Scheme 4: E-allylic alcohol 7 from epoxide 11.
Scheme 4: E-allylic alcohol 7 from epoxide 11.
We also studied the possibility of generating alcohol 7 from terminal epoxide 11 using an organolithium instead of a hindered lithium amide as the base (Scheme 5). Organolithiums, in particular secondary and tertiary organolithiums, are known to react with terminal epoxides by α-lithiation, although this is typically followed by trapping of the α-lithiated epoxide with a second equivalent of the organolithium and elimination of Li2O to give an E-alkene (e.g., 12): a process referred to as reductive alkylation [41]. Also, while PPh3 is itself capable of being lithiated–carboxylated (at a meta-position, 6% yield) by using BuLi in Et2O [42], this requires significantly higher temperatures (reflux, 46 h) than those applied here. In the event, the use of either s-BuLi or t-BuLi with epoxide 11 in the presence of Ph3P in a variety of solvents (THF, Et2O, t-BuOMe, toluene) followed by the addition of benzaldehyde was found to give allylic alcohol 7, albeit in low yields with reductive alkylation always being the dominant reaction pathway, and typically ~30% of epoxide 11 and ~60% Ph3P being recovered. The highest yield of allylic alcohol 7 (18%) was obtained by using s-BuLi in Et2O at −78 °C with a 24 h lithiation time (Scheme 5); lithiation by using other organolithiums (t-BuLi, PhLi, BuLi, MeLi), or at higher or lower temperatures (−90 °C or −40 °C), for a longer period (48 h) or in the presence of increased Ph3P (2 equiv), or TMEDA (1 equiv) or LiBr (2 equiv) as additives were all less effective.
Scheme 5: E-allylic alcohol 7 and alkene 12 from epoxide 11 by using s-BuLi.
Scheme 5: E-allylic alcohol 7 and alkene 12 from epoxide 11 by using s-BuLi.
The use of a proton (deuterium) source as the electrophile to trap an epoxide-derived ylide prepared by using LTMP was next examined. This was anticipated to provide a base-induced method to deoxygenate epoxides [43], which in the case of deuteration would provide a regiospecific and potentially stereoselective entry to 1-deuterated terminal alkenes [44,45]. Use of a slightly higher molecular weight epoxide, 1,2-epoxydodecane (13) to facilitate product isolation, gave dodecene (14) (41%, 50% D [46]) after reaction with CD3OD (Scheme 6), where the deuterium incorporation was nonstereoselective [44]. Modest deuterium incorporation suggests partial collapse of the intermediate β-lithiooxy ylide occurs under the conditions of its generation, by elimination of Ph3PO after or before protonation (e.g., from solvent) and before electrophile addition. Dodecene was also observed as a byproduct in the corresponding reaction of epoxide 13 with benzaldehyde, supporting this hypothesis.
Scheme 6: Terminal alkene 14 from epoxide 13.
Scheme 6: Terminal alkene 14 from epoxide 13.
Conclusion
Among phosphoranes, β-lithiooxyphosphonium ylides occupy a special place, because of their utility in Wittig–Schlosser and SCOOPY-type stereoselective olefination reactions [1-19]. Here we have shown a new and concise method to such valuable intermediates, directly from readily available terminal epoxides. Significantly, the work validates the compatibility of lithium amide and phosphine to generate such ylides, whose intermediacy is demonstrated by representative alkene-forming reactions with chloromethyl pivalate, benzaldehyde and CD3OD, giving a Z-allylic pivalate, a conjugated E-allylic alcohol and a partially deuterated terminal alkene, respectively. High stereochemical control is retained in the Z-allylic pivalate and E-allylic alcohol syntheses. While the overall yields for the transformations are modest, they stand up to comparison with the earlier methods, given the experimental simplicity and brevity of the current approach.
Supporting Information
| Supporting Information File 1: Preparative details of 6, 7, 12 and 14 are reported, together with their spectroscopic data. | ||
| Format: PDF | Size: 576.1 KB | Download |
References
-
Gosney, I.; Rowley, A. G. Transformations via phosphorus-stabilized anions. 1. Stereoselective synthesis of alkenes. In Organophosphorus Reagents in Organic Synthesis; Cadogan, J. I., Ed.; Academic Press: New York, 1979; pp 17–153.
Return to citation in text: [1] [2] [3] -
Maryanoff, B. E.; Reitz, A. B. Chem. Rev. 1989, 89, 863–927. doi:10.1021/cr00094a007
Return to citation in text: [1] [2] [3] -
Vedejs, E.; Peterson, M. J. Top. Stereochem. 1994, 21, 1–157. doi:10.1002/9780470147306.ch1
Return to citation in text: [1] [2] [3] -
Schobert, R.; Hözel, C.; Barnickel, B. Wittig and related phosphorus-based alkenations. In Science of Synthesis; de Meijere, A., Ed.; Thieme: Stuttgart, Germany, 2010; Vol. 47a, pp 9–84.
Return to citation in text: [1] [2] [3] -
Wang, Q.; Deredas, D.; Huynh, C.; Schlosser, M. Chem.–Eur. J. 2003, 9, 570–574. doi:10.1002/chem.200390061
Return to citation in text: [1] [2] [3] [4] -
Hodgson, D. M.; Arif, T. J. Am. Chem. Soc. 2008, 130, 16500–16501. doi:10.1021/ja8076999
Return to citation in text: [1] [2] -
Hodgson, D. M.; Arif, T. Org. Lett. 2010, 12, 4204–4207. doi:10.1021/ol101843q
Return to citation in text: [1] [2] -
Hodgson, D. M.; Arif, T. Chem. Commun. 2011, 47, 2685–2687. doi:10.1039/c0cc04429f
Return to citation in text: [1] [2] [3] [4] [5] -
Hodgson, D. M.; Persaud, R. S. D. Org. Biomol. Chem. 2012, 10, 7949–7951. doi:10.1039/c2ob26346g
Return to citation in text: [1] [2] [3] [4] -
Corey, E. J.; Shirahama, H.; Yamamoto, H.; Terashima, S.; Venkateswarlu, A.; Schaaf, T. K. J. Am. Chem. Soc. 1971, 93, 1490–1491. doi:10.1021/ja00735a032
Return to citation in text: [1] [2] [3] -
Corey, E. J.; Niwa, H.; Knolle, J. J. Am. Chem. Soc. 1978, 100, 1942–1943. doi:10.1021/ja00474a058
Return to citation in text: [1] [2] [3] -
Corey, E. J.; Marfat, A.; Hoover, D. J. Tetrahedron Lett. 1981, 22, 1587–1590. doi:10.1016/S0040-4039(01)90384-3
Return to citation in text: [1] [2] [3] -
Schwarz, S.; Weber, G.; Depner, J.; Schaumann, J. Tetrahedron 1982, 38, 1261–1268. doi:10.1016/0040-4020(82)85112-0
Return to citation in text: [1] [2] [3] -
Russell, S. W.; Pabon, H. J. J. J. Chem. Soc., Perkin Trans. 1 1982, 545–552. doi:10.1039/P19820000545
Return to citation in text: [1] [2] [3] -
Johnson, F.; Paul, K. G.; Favara, D.; Ciabatti, R.; Guzzi, U. J. Am. Chem. Soc. 1982, 104, 2190–2198. doi:10.1021/ja00372a015
Return to citation in text: [1] [2] [3] -
Yadagiri, P.; Shin, D.-S.; Falck, J. R. Tetrahedron Lett. 1988, 29, 5497–5500. doi:10.1016/S0040-4039(00)80796-0
Return to citation in text: [1] [2] [3] -
Kubota, T.; Yamamoto, M. Tetrahedron Lett. 1992, 33, 2603–2606. doi:10.1016/S0040-4039(00)92255-X
Return to citation in text: [1] [2] [3] [4] -
Okuma, K.; Tanaka, Y.; Ohta, H.; Matsuyama, H. Bull. Chem. Soc. Jpn. 1993, 66, 2623–2632. doi:10.1246/bcsj.66.2623
Return to citation in text: [1] [2] [3] [4] -
El Fangour, S.; Guy, A.; Vidal, J.-P.; Rossi, J.-C.; Durand, T. J. Org. Chem. 2005, 70, 989–997. doi:10.1021/jo048179+
Return to citation in text: [1] [2] [3] -
Yanagisawa, A.; Yasue, K.; Yamamoto, H. J. Chem. Soc., Chem. Commun. 1994, 2103–2104. doi:10.1039/C39940002103
Return to citation in text: [1] [2] [3] -
Hodgson, D. M.; Chung, Y. K.; Paris, J.-M. J. Am. Chem. Soc. 2004, 126, 8664–8665. doi:10.1021/ja047346k
Return to citation in text: [1] [2] [3] -
Hodgson, D. M.; Chung, Y. K.; Nuzzo, I.; Freixas, G.; Kulikiewicz, K. K.; Cleator, E.; Paris, J.-M. J. Am. Chem. Soc. 2007, 129, 4456–4462. doi:10.1021/ja0672932
Return to citation in text: [1] [2] [3] [4] -
Hodgson, D. M.; Bray, C. D.; Kindon, N. D. Org. Lett. 2005, 7, 2305–2308. doi:10.1021/ol050402h
Return to citation in text: [1] -
Hodgson, D. M.; Bray, C. D.; Humphreys, P. G. Synlett 2006, 1–22. doi:10.1055/s-2005-921925
Return to citation in text: [1] -
Hodgson, D. M.; Humphreys, P. G.; Hughes, S. P. Pure Appl. Chem. 2007, 79, 269–279. doi:10.1351/pac200779020269
Return to citation in text: [1] -
Padwa, A.; Hornbuckle, S. F. Chem. Rev. 1991, 91, 263–309. doi:10.1021/cr00003a001
Return to citation in text: [1] -
Seyferth, D.; Grim, S. O.; Read, T. O. J. Am. Chem. Soc. 1960, 82, 1510–1511. doi:10.1021/ja01491a066
Return to citation in text: [1] -
Seyferth, D.; Grim, S. O.; Read, T. O. J. Am. Chem. Soc. 1961, 83, 1617–1620. doi:10.1021/ja01468a017
Return to citation in text: [1] -
Wittig, G.; Schlosser, M. Angew. Chem. 1960, 72, 324. doi:10.1002/ange.19600720913
Return to citation in text: [1] -
Wittig, G.; Schlosser, M. Chem. Ber. 1961, 94, 1373–1383. doi:10.1002/cber.19610940532
Return to citation in text: [1] -
Khaskin, B. A.; Molodova, O. D.; Torgasheva, N. A. Russ. Chem. Rev. 1992, 61, 306–334. doi:10.1070/RC1992v061n03ABEH000947
Return to citation in text: [1] -
Rönnholm, P.; Hilmersson, G. ARKIVOC 2011, No. v, 200–210.
http://www.arkat-usa.org/get-file/38691
Return to citation in text: [1] -
Schlosser, M.; Tuong, H. B.; Respondek, J.; Schaub, B. Chimia 1983, 37, 10–11.
Return to citation in text: [1] -
Materal-Saadi, M. S. Phosphorus, Sulfur Silicon Relat. Elem. 2000, 164, 269–275. doi:10.1080/10426500008045252
Return to citation in text: [1] -
Heath, R. R.; Doolittle, R. E.; Sonnet, P. E.; Tumlinson, J. H. J. Org. Chem. 1980, 45, 2910–2912. doi:10.1021/jo01302a032
Return to citation in text: [1] [2] -
Hodgson, D. M.; Bray, C. D.; Kindon, N. D. J. Am. Chem. Soc. 2004, 126, 6870–6871. doi:10.1021/ja031770o
Return to citation in text: [1] -
Hodgson, D. M.; Bray, C. D.; Kindon, N. D.; Reynolds, N. J.; Coote, S. J.; Um, J. M.; Houk, K. N. J. Org. Chem. 2009, 74, 1019–1028. doi:10.1021/jo802016t
Return to citation in text: [1] -
Schaus, S. E.; Brandes, B. D.; Larrow, J. F.; Tokunaga, M.; Hansen, K. B.; Gould, A. E.; Furrow, M. E.; Jacobsen, E. N. J. Am. Chem. Soc. 2002, 124, 1307–1315. doi:10.1021/ja016737l
Return to citation in text: [1] -
Keith, J. M.; Larrow, J. F.; Jacobsen, E. N. Adv. Synth. Catal. 2001, 343, 5–26. doi:10.1002/1615-4169(20010129)343:1<5::AID-ADSC5>3.0.CO;2-I
Return to citation in text: [1] -
Hodgson, D. M.; Humphreys, P. G. Allylic alcohols. In Science of Synthesis; Clayden, J., Ed.; Thieme: Stuttgart, Germany, 2007; Vol. 32, pp 583–665.
Return to citation in text: [1] -
Doris, E.; Dechoux, L.; Mioskowski, C. Tetrahedron Lett. 1994, 35, 7943–7946. doi:10.1016/0040-4039(94)80017-0
Return to citation in text: [1] -
Gilman, H.; Brown, G. E. J. Am. Chem. Soc. 1945, 67, 824–826. doi:10.1021/ja01221a039
Return to citation in text: [1] -
Murai, S.; Murai, T.; Kato, S. Reduction of epoxides. In Comprehensive Organic Synthesis; Trost, B. M.; Fleming, I., Eds.; Pergamon: Oxford, U.K., 1991; Vol. 8, pp 871–893. doi:10.1016/B978-0-08-052349-1.00249-3
Return to citation in text: [1] -
Schlosser, M.; Christmann, K.-F. Synthesis 1969, 38–39.
Return to citation in text: [1] [2] -
Schlosser, M.; Christmann, K.-F.; Piskala, A. Chem. Ber. 1970, 103, 2814–2820. doi:10.1002/cber.19701030915
Return to citation in text: [1] -
Oda, H.; Sato, M.; Morizawa, Y.; Oshima, K.; Nozaki, H. Tetrahedron Lett. 1983, 24, 2877–2880. doi:10.1016/S0040-4039(00)88048-X
Return to citation in text: [1]
| 22. | Hodgson, D. M.; Chung, Y. K.; Nuzzo, I.; Freixas, G.; Kulikiewicz, K. K.; Cleator, E.; Paris, J.-M. J. Am. Chem. Soc. 2007, 129, 4456–4462. doi:10.1021/ja0672932 |
| 41. | Doris, E.; Dechoux, L.; Mioskowski, C. Tetrahedron Lett. 1994, 35, 7943–7946. doi:10.1016/0040-4039(94)80017-0 |
| 42. | Gilman, H.; Brown, G. E. J. Am. Chem. Soc. 1945, 67, 824–826. doi:10.1021/ja01221a039 |
| 1. | Gosney, I.; Rowley, A. G. Transformations via phosphorus-stabilized anions. 1. Stereoselective synthesis of alkenes. In Organophosphorus Reagents in Organic Synthesis; Cadogan, J. I., Ed.; Academic Press: New York, 1979; pp 17–153. |
| 2. | Maryanoff, B. E.; Reitz, A. B. Chem. Rev. 1989, 89, 863–927. doi:10.1021/cr00094a007 |
| 3. | Vedejs, E.; Peterson, M. J. Top. Stereochem. 1994, 21, 1–157. doi:10.1002/9780470147306.ch1 |
| 4. | Schobert, R.; Hözel, C.; Barnickel, B. Wittig and related phosphorus-based alkenations. In Science of Synthesis; de Meijere, A., Ed.; Thieme: Stuttgart, Germany, 2010; Vol. 47a, pp 9–84. |
| 5. | Wang, Q.; Deredas, D.; Huynh, C.; Schlosser, M. Chem.–Eur. J. 2003, 9, 570–574. doi:10.1002/chem.200390061 |
| 6. | Hodgson, D. M.; Arif, T. J. Am. Chem. Soc. 2008, 130, 16500–16501. doi:10.1021/ja8076999 |
| 7. | Hodgson, D. M.; Arif, T. Org. Lett. 2010, 12, 4204–4207. doi:10.1021/ol101843q |
| 8. | Hodgson, D. M.; Arif, T. Chem. Commun. 2011, 47, 2685–2687. doi:10.1039/c0cc04429f |
| 9. | Hodgson, D. M.; Persaud, R. S. D. Org. Biomol. Chem. 2012, 10, 7949–7951. doi:10.1039/c2ob26346g |
| 10. | Corey, E. J.; Shirahama, H.; Yamamoto, H.; Terashima, S.; Venkateswarlu, A.; Schaaf, T. K. J. Am. Chem. Soc. 1971, 93, 1490–1491. doi:10.1021/ja00735a032 |
| 11. | Corey, E. J.; Niwa, H.; Knolle, J. J. Am. Chem. Soc. 1978, 100, 1942–1943. doi:10.1021/ja00474a058 |
| 12. | Corey, E. J.; Marfat, A.; Hoover, D. J. Tetrahedron Lett. 1981, 22, 1587–1590. doi:10.1016/S0040-4039(01)90384-3 |
| 13. | Schwarz, S.; Weber, G.; Depner, J.; Schaumann, J. Tetrahedron 1982, 38, 1261–1268. doi:10.1016/0040-4020(82)85112-0 |
| 14. | Russell, S. W.; Pabon, H. J. J. J. Chem. Soc., Perkin Trans. 1 1982, 545–552. doi:10.1039/P19820000545 |
| 15. | Johnson, F.; Paul, K. G.; Favara, D.; Ciabatti, R.; Guzzi, U. J. Am. Chem. Soc. 1982, 104, 2190–2198. doi:10.1021/ja00372a015 |
| 16. | Yadagiri, P.; Shin, D.-S.; Falck, J. R. Tetrahedron Lett. 1988, 29, 5497–5500. doi:10.1016/S0040-4039(00)80796-0 |
| 17. | Kubota, T.; Yamamoto, M. Tetrahedron Lett. 1992, 33, 2603–2606. doi:10.1016/S0040-4039(00)92255-X |
| 18. | Okuma, K.; Tanaka, Y.; Ohta, H.; Matsuyama, H. Bull. Chem. Soc. Jpn. 1993, 66, 2623–2632. doi:10.1246/bcsj.66.2623 |
| 19. | El Fangour, S.; Guy, A.; Vidal, J.-P.; Rossi, J.-C.; Durand, T. J. Org. Chem. 2005, 70, 989–997. doi:10.1021/jo048179+ |
| 8. | Hodgson, D. M.; Arif, T. Chem. Commun. 2011, 47, 2685–2687. doi:10.1039/c0cc04429f |
| 9. | Hodgson, D. M.; Persaud, R. S. D. Org. Biomol. Chem. 2012, 10, 7949–7951. doi:10.1039/c2ob26346g |
| 9. | Hodgson, D. M.; Persaud, R. S. D. Org. Biomol. Chem. 2012, 10, 7949–7951. doi:10.1039/c2ob26346g |
| 32. |
Rönnholm, P.; Hilmersson, G. ARKIVOC 2011, No. v, 200–210.
http://www.arkat-usa.org/get-file/38691 |
| 8. | Hodgson, D. M.; Arif, T. Chem. Commun. 2011, 47, 2685–2687. doi:10.1039/c0cc04429f |
| 27. | Seyferth, D.; Grim, S. O.; Read, T. O. J. Am. Chem. Soc. 1960, 82, 1510–1511. doi:10.1021/ja01491a066 |
| 28. | Seyferth, D.; Grim, S. O.; Read, T. O. J. Am. Chem. Soc. 1961, 83, 1617–1620. doi:10.1021/ja01468a017 |
| 29. | Wittig, G.; Schlosser, M. Angew. Chem. 1960, 72, 324. doi:10.1002/ange.19600720913 |
| 30. | Wittig, G.; Schlosser, M. Chem. Ber. 1961, 94, 1373–1383. doi:10.1002/cber.19610940532 |
| 31. | Khaskin, B. A.; Molodova, O. D.; Torgasheva, N. A. Russ. Chem. Rev. 1992, 61, 306–334. doi:10.1070/RC1992v061n03ABEH000947 |
| 1. | Gosney, I.; Rowley, A. G. Transformations via phosphorus-stabilized anions. 1. Stereoselective synthesis of alkenes. In Organophosphorus Reagents in Organic Synthesis; Cadogan, J. I., Ed.; Academic Press: New York, 1979; pp 17–153. |
| 2. | Maryanoff, B. E.; Reitz, A. B. Chem. Rev. 1989, 89, 863–927. doi:10.1021/cr00094a007 |
| 3. | Vedejs, E.; Peterson, M. J. Top. Stereochem. 1994, 21, 1–157. doi:10.1002/9780470147306.ch1 |
| 4. | Schobert, R.; Hözel, C.; Barnickel, B. Wittig and related phosphorus-based alkenations. In Science of Synthesis; de Meijere, A., Ed.; Thieme: Stuttgart, Germany, 2010; Vol. 47a, pp 9–84. |
| 5. | Wang, Q.; Deredas, D.; Huynh, C.; Schlosser, M. Chem.–Eur. J. 2003, 9, 570–574. doi:10.1002/chem.200390061 |
| 6. | Hodgson, D. M.; Arif, T. J. Am. Chem. Soc. 2008, 130, 16500–16501. doi:10.1021/ja8076999 |
| 7. | Hodgson, D. M.; Arif, T. Org. Lett. 2010, 12, 4204–4207. doi:10.1021/ol101843q |
| 8. | Hodgson, D. M.; Arif, T. Chem. Commun. 2011, 47, 2685–2687. doi:10.1039/c0cc04429f |
| 9. | Hodgson, D. M.; Persaud, R. S. D. Org. Biomol. Chem. 2012, 10, 7949–7951. doi:10.1039/c2ob26346g |
| 10. | Corey, E. J.; Shirahama, H.; Yamamoto, H.; Terashima, S.; Venkateswarlu, A.; Schaaf, T. K. J. Am. Chem. Soc. 1971, 93, 1490–1491. doi:10.1021/ja00735a032 |
| 11. | Corey, E. J.; Niwa, H.; Knolle, J. J. Am. Chem. Soc. 1978, 100, 1942–1943. doi:10.1021/ja00474a058 |
| 12. | Corey, E. J.; Marfat, A.; Hoover, D. J. Tetrahedron Lett. 1981, 22, 1587–1590. doi:10.1016/S0040-4039(01)90384-3 |
| 13. | Schwarz, S.; Weber, G.; Depner, J.; Schaumann, J. Tetrahedron 1982, 38, 1261–1268. doi:10.1016/0040-4020(82)85112-0 |
| 14. | Russell, S. W.; Pabon, H. J. J. J. Chem. Soc., Perkin Trans. 1 1982, 545–552. doi:10.1039/P19820000545 |
| 15. | Johnson, F.; Paul, K. G.; Favara, D.; Ciabatti, R.; Guzzi, U. J. Am. Chem. Soc. 1982, 104, 2190–2198. doi:10.1021/ja00372a015 |
| 16. | Yadagiri, P.; Shin, D.-S.; Falck, J. R. Tetrahedron Lett. 1988, 29, 5497–5500. doi:10.1016/S0040-4039(00)80796-0 |
| 17. | Kubota, T.; Yamamoto, M. Tetrahedron Lett. 1992, 33, 2603–2606. doi:10.1016/S0040-4039(00)92255-X |
| 18. | Okuma, K.; Tanaka, Y.; Ohta, H.; Matsuyama, H. Bull. Chem. Soc. Jpn. 1993, 66, 2623–2632. doi:10.1246/bcsj.66.2623 |
| 19. | El Fangour, S.; Guy, A.; Vidal, J.-P.; Rossi, J.-C.; Durand, T. J. Org. Chem. 2005, 70, 989–997. doi:10.1021/jo048179+ |
| 5. | Wang, Q.; Deredas, D.; Huynh, C.; Schlosser, M. Chem.–Eur. J. 2003, 9, 570–574. doi:10.1002/chem.200390061 |
| 20. | Yanagisawa, A.; Yasue, K.; Yamamoto, H. J. Chem. Soc., Chem. Commun. 1994, 2103–2104. doi:10.1039/C39940002103 |
| 21. | Hodgson, D. M.; Chung, Y. K.; Paris, J.-M. J. Am. Chem. Soc. 2004, 126, 8664–8665. doi:10.1021/ja047346k |
| 22. | Hodgson, D. M.; Chung, Y. K.; Nuzzo, I.; Freixas, G.; Kulikiewicz, K. K.; Cleator, E.; Paris, J.-M. J. Am. Chem. Soc. 2007, 129, 4456–4462. doi:10.1021/ja0672932 |
| 21. | Hodgson, D. M.; Chung, Y. K.; Paris, J.-M. J. Am. Chem. Soc. 2004, 126, 8664–8665. doi:10.1021/ja047346k |
| 22. | Hodgson, D. M.; Chung, Y. K.; Nuzzo, I.; Freixas, G.; Kulikiewicz, K. K.; Cleator, E.; Paris, J.-M. J. Am. Chem. Soc. 2007, 129, 4456–4462. doi:10.1021/ja0672932 |
| 26. | Padwa, A.; Hornbuckle, S. F. Chem. Rev. 1991, 91, 263–309. doi:10.1021/cr00003a001 |
| 46. | Oda, H.; Sato, M.; Morizawa, Y.; Oshima, K.; Nozaki, H. Tetrahedron Lett. 1983, 24, 2877–2880. doi:10.1016/S0040-4039(00)88048-X |
| 20. | Yanagisawa, A.; Yasue, K.; Yamamoto, H. J. Chem. Soc., Chem. Commun. 1994, 2103–2104. doi:10.1039/C39940002103 |
| 1. | Gosney, I.; Rowley, A. G. Transformations via phosphorus-stabilized anions. 1. Stereoselective synthesis of alkenes. In Organophosphorus Reagents in Organic Synthesis; Cadogan, J. I., Ed.; Academic Press: New York, 1979; pp 17–153. |
| 2. | Maryanoff, B. E.; Reitz, A. B. Chem. Rev. 1989, 89, 863–927. doi:10.1021/cr00094a007 |
| 3. | Vedejs, E.; Peterson, M. J. Top. Stereochem. 1994, 21, 1–157. doi:10.1002/9780470147306.ch1 |
| 4. | Schobert, R.; Hözel, C.; Barnickel, B. Wittig and related phosphorus-based alkenations. In Science of Synthesis; de Meijere, A., Ed.; Thieme: Stuttgart, Germany, 2010; Vol. 47a, pp 9–84. |
| 17. | Kubota, T.; Yamamoto, M. Tetrahedron Lett. 1992, 33, 2603–2606. doi:10.1016/S0040-4039(00)92255-X |
| 18. | Okuma, K.; Tanaka, Y.; Ohta, H.; Matsuyama, H. Bull. Chem. Soc. Jpn. 1993, 66, 2623–2632. doi:10.1246/bcsj.66.2623 |
| 43. | Murai, S.; Murai, T.; Kato, S. Reduction of epoxides. In Comprehensive Organic Synthesis; Trost, B. M.; Fleming, I., Eds.; Pergamon: Oxford, U.K., 1991; Vol. 8, pp 871–893. doi:10.1016/B978-0-08-052349-1.00249-3 |
| 10. | Corey, E. J.; Shirahama, H.; Yamamoto, H.; Terashima, S.; Venkateswarlu, A.; Schaaf, T. K. J. Am. Chem. Soc. 1971, 93, 1490–1491. doi:10.1021/ja00735a032 |
| 11. | Corey, E. J.; Niwa, H.; Knolle, J. J. Am. Chem. Soc. 1978, 100, 1942–1943. doi:10.1021/ja00474a058 |
| 12. | Corey, E. J.; Marfat, A.; Hoover, D. J. Tetrahedron Lett. 1981, 22, 1587–1590. doi:10.1016/S0040-4039(01)90384-3 |
| 13. | Schwarz, S.; Weber, G.; Depner, J.; Schaumann, J. Tetrahedron 1982, 38, 1261–1268. doi:10.1016/0040-4020(82)85112-0 |
| 14. | Russell, S. W.; Pabon, H. J. J. J. Chem. Soc., Perkin Trans. 1 1982, 545–552. doi:10.1039/P19820000545 |
| 15. | Johnson, F.; Paul, K. G.; Favara, D.; Ciabatti, R.; Guzzi, U. J. Am. Chem. Soc. 1982, 104, 2190–2198. doi:10.1021/ja00372a015 |
| 16. | Yadagiri, P.; Shin, D.-S.; Falck, J. R. Tetrahedron Lett. 1988, 29, 5497–5500. doi:10.1016/S0040-4039(00)80796-0 |
| 19. | El Fangour, S.; Guy, A.; Vidal, J.-P.; Rossi, J.-C.; Durand, T. J. Org. Chem. 2005, 70, 989–997. doi:10.1021/jo048179+ |
| 23. | Hodgson, D. M.; Bray, C. D.; Kindon, N. D. Org. Lett. 2005, 7, 2305–2308. doi:10.1021/ol050402h |
| 24. | Hodgson, D. M.; Bray, C. D.; Humphreys, P. G. Synlett 2006, 1–22. doi:10.1055/s-2005-921925 |
| 25. | Hodgson, D. M.; Humphreys, P. G.; Hughes, S. P. Pure Appl. Chem. 2007, 79, 269–279. doi:10.1351/pac200779020269 |
| 44. | Schlosser, M.; Christmann, K.-F. Synthesis 1969, 38–39. |
| 45. | Schlosser, M.; Christmann, K.-F.; Piskala, A. Chem. Ber. 1970, 103, 2814–2820. doi:10.1002/cber.19701030915 |
| 33. | Schlosser, M.; Tuong, H. B.; Respondek, J.; Schaub, B. Chimia 1983, 37, 10–11. |
| 34. | Materal-Saadi, M. S. Phosphorus, Sulfur Silicon Relat. Elem. 2000, 164, 269–275. doi:10.1080/10426500008045252 |
| 8. | Hodgson, D. M.; Arif, T. Chem. Commun. 2011, 47, 2685–2687. doi:10.1039/c0cc04429f |
| 5. | Wang, Q.; Deredas, D.; Huynh, C.; Schlosser, M. Chem.–Eur. J. 2003, 9, 570–574. doi:10.1002/chem.200390061 |
| 40. | Hodgson, D. M.; Humphreys, P. G. Allylic alcohols. In Science of Synthesis; Clayden, J., Ed.; Thieme: Stuttgart, Germany, 2007; Vol. 32, pp 583–665. |
| 17. | Kubota, T.; Yamamoto, M. Tetrahedron Lett. 1992, 33, 2603–2606. doi:10.1016/S0040-4039(00)92255-X |
| 18. | Okuma, K.; Tanaka, Y.; Ohta, H.; Matsuyama, H. Bull. Chem. Soc. Jpn. 1993, 66, 2623–2632. doi:10.1246/bcsj.66.2623 |
| 21. | Hodgson, D. M.; Chung, Y. K.; Paris, J.-M. J. Am. Chem. Soc. 2004, 126, 8664–8665. doi:10.1021/ja047346k |
| 22. | Hodgson, D. M.; Chung, Y. K.; Nuzzo, I.; Freixas, G.; Kulikiewicz, K. K.; Cleator, E.; Paris, J.-M. J. Am. Chem. Soc. 2007, 129, 4456–4462. doi:10.1021/ja0672932 |
| 38. | Schaus, S. E.; Brandes, B. D.; Larrow, J. F.; Tokunaga, M.; Hansen, K. B.; Gould, A. E.; Furrow, M. E.; Jacobsen, E. N. J. Am. Chem. Soc. 2002, 124, 1307–1315. doi:10.1021/ja016737l |
| 39. | Keith, J. M.; Larrow, J. F.; Jacobsen, E. N. Adv. Synth. Catal. 2001, 343, 5–26. doi:10.1002/1615-4169(20010129)343:1<5::AID-ADSC5>3.0.CO;2-I |
| 20. | Yanagisawa, A.; Yasue, K.; Yamamoto, H. J. Chem. Soc., Chem. Commun. 1994, 2103–2104. doi:10.1039/C39940002103 |
| 36. | Hodgson, D. M.; Bray, C. D.; Kindon, N. D. J. Am. Chem. Soc. 2004, 126, 6870–6871. doi:10.1021/ja031770o |
| 37. | Hodgson, D. M.; Bray, C. D.; Kindon, N. D.; Reynolds, N. J.; Coote, S. J.; Um, J. M.; Houk, K. N. J. Org. Chem. 2009, 74, 1019–1028. doi:10.1021/jo802016t |
| 35. | Heath, R. R.; Doolittle, R. E.; Sonnet, P. E.; Tumlinson, J. H. J. Org. Chem. 1980, 45, 2910–2912. doi:10.1021/jo01302a032 |
| 35. | Heath, R. R.; Doolittle, R. E.; Sonnet, P. E.; Tumlinson, J. H. J. Org. Chem. 1980, 45, 2910–2912. doi:10.1021/jo01302a032 |
© 2012 Hodgson and Persaud; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)