Abstract
A well-defined N-heterocyclic carbene–palladium(II)-1-methylimidazole [NHC-Pd(II)-Im] complex 1 was found to be an effective catalyst for the Mizoroki–Heck reaction of a variety of aryl chlorides with styrenes. Both activated and deactivated aryl chlorides work well to give the corresponding coupling products in good to excellent yields by using tetrabutylammonium bromide (TBAB) as the ionic liquid.
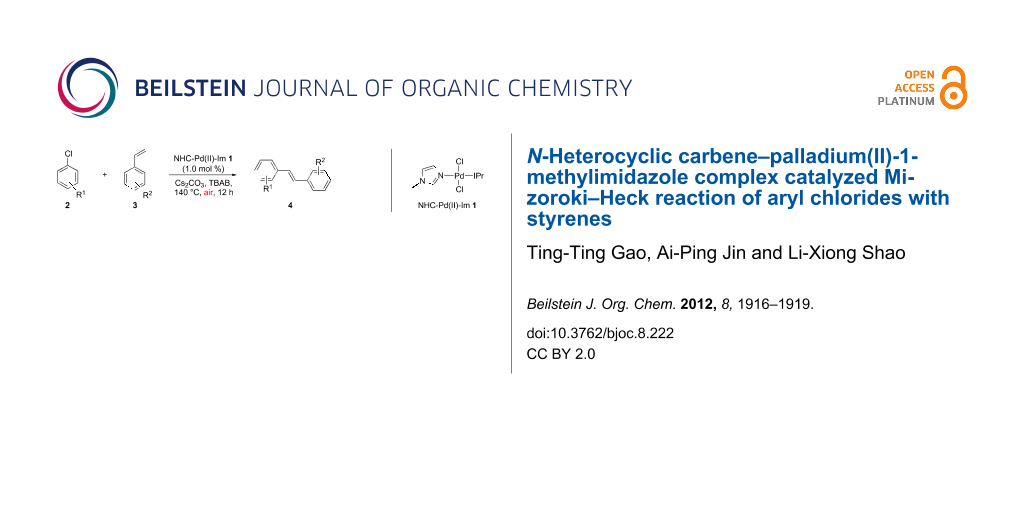
Graphical Abstract
Introduction
The palladium-catalyzed reaction between organic halides and alkenes, the Mizoroki–Heck reaction, is one of the most versatile methods for the formation of carbon–carbon bonds [1-7]. Usually, in order to achieve the highest efficiency of the palladium-catalyzed Mizoroki–Heck reaction, toxic, air-sensitive and expensive phosphine ligands are introduced to facilitate the corresponding transformations [8,9]. In order to overcome the drawbacks associated with the phosphine ligands, the development of alternative stable, inexpensive and easily available phosphine-free ligands is still in great demand.
In the meantime, N-heterocyclic carbenes (NHCs), possessing the advantages of being stronger σ-donors and weaker π-acceptors than traditional phosphine ligands, which can then increase the catalytic activity of the metal centre and the stability of the NHC–metal complexes, have attracted growing attention in the metal-catalyzed carbon–carbon and carbon–heteroatom bond-formation reactions during the past two decades [10-15]. Consequently, since the seminal papers reported by Herrmann and co-workers [16,17], the NHC–Pd complexes have become a strong challenge to the phosphine–metal complexes in the Mizoroki–Heck reaction. Among the electrophiles participating in the NHC–Pd-complex-catalyzed Mizoroki–Heck reaction, organic iodides and bromides, which can be easily activated by the metal centre, are usually used as good partners [18-25]. In contrast to the common electrophiles such as organic iodides and bromides, organic chlorides are the most attractive in the Mizoroki–Heck reaction toward industrial applications due to their lower cost and the widest availability [26-28]. However, organic chlorides, especially the deactivated ones, are generally unreactive in the NHC–Pd-complex-catalyzed Mizoroki-Heck reaction [29-31]. Furthermore, synthetic routes for the above mentioned NHC–Pd complexes that showed good catalytic activity toward activated aryl chlorides, are all lengthy [32-36]. Therefore, although the progress has been achieved for the NHC–Pd-complex-catalyzed Mizoroki–Heck reaction of aryl chlorides, the drive to develop easily available and widely applicable complexes with broad substrate scope, is still a current topic of interest.
Recently, we have demonstrated that the well-defined N-heterocyclic carbene–palladium(II)-1-methylimidazole [NHC-Pd(II)-Im] complex 1 (Figure 1), which can be easily prepared from commercially available PdCl2, IPr.HCl [1,3-bis(2,6-diisopropylphenyl)imidazolium chloride], and 1-methylimidazole in a one-step procedure in high yield, was an effective catalyst in C–C and C–N bond-formation reactions [37-44]. In our continuing investigations on the further applications of this complex in organic synthesis, we found that complex 1 was an active catalyst for the Mizoroki–Heck reaction of aryl chlorides, including both activated and deactivated aryl chlorides, with styrenes performed under air. Herein, we wish to report these results in detail.
Results and Discussion
Initially, the model reaction between chlorobenzene (2a) (0.75 mmol) and styrene (3a) (0.90 mmol) was carried out in common polar solvents, such as N,N-dimethylformamide, N,N-dimethylacetamide and dimethylsulfoxide, in the presence of different inorganic bases and NHC-Pd(II)-Im complex 1 (1.0 mol %) at 150 °C for 24 h. To our disappointment, almost no desired product 4a was observed in all cases. It was reported that tetrabutylammonium bromide (TBAB), as a simple ionic liquid, possessing the advantages of low price and easy availability, can facilitate the NHC–Pd-complex-catalyzed Mizoroki–Heck reaction [29-31,33]. Thus, we then turned our interest to carrying out the reaction between chlorobenzene (2a) and styrene (3a) in the presence of NHC-Pd(II)-Im 1 using TBAB as the solvent at 140 °C (Table 1). As can be seen from Table 1, all reactions took place smoothly under air to give the desired coupling product 4a in low to excellent yields within 12 h in the presence of any of the bases tested. To our pleasure, the best result can be achieved when Cs2CO3 was used as the base (Table 1, entry 1) [45].
Table 1: Optimization for the NHC-Pd(II)-Im complex 1 catalyzed reaction of chlorobenzene (2a) with styrene (3a).
|
|
||
| Entrya | Base | Yield (%)b |
|---|---|---|
| 1 | Cs2CO3 | 94 |
| 2 | NaOH | 54 |
| 3 | KOH | 67 |
| 4 | NaHCO3 | 80 |
| 5 | KHCO3 | 22 |
| 6 | Na2CO3 | 52 |
| 7 | K2CO3 | 34 |
| 8 | KOt-Bu | 15 |
| 9 | NaOt-Bu | 44 |
| 10 | LiOt-Bu | 85 |
aAll reactions were carried out by using 2a (0.75 mmol), 3a (0.9 mmol), base (2.0 equiv), 1 (1.0 mol %), TBAB (2.0 g) at 140 °C for 12 h. bIsolated yields.
The optimal reaction conditions (Table 1, entry 1) were then applied to a variety of aryl chlorides and styrenes to investigate the generality (Table 2). As can be seen from Table 2, most reactions proceeded well to give products 4 in good to high yields. For instance, in the first round, the reactions between various aryl chlorides 2 and styrene (3a) were examined (Table 2, entries 1–7). It seems that all of the activated and deactivated aryl chlorides work well under the optimal conditions to give the products 4b, 4c, 4e, 4f and 4g in good to high yields (Table 2, entries 1, 2, 4, 5 and 6). 2-Methylphenyl chloride (2d) gave product 4d in a slightly lower yield (71%) probably due to its steric hindrance (Table 2, entry 3). The reaction between 2-chlorothiophene (2h) and styrene (3a) also gave product 4h in 63% yield (Table 2, entry 7). In a second round, the reactions between chlorobenzene (2a) and various styrenes 3 were also investigated (Table 2, entries 8–13). It also seems that the electronic effect of the substituents on the styrenes did not affect the reactions, and all reactions took place smoothly to give products 4 in good yields (Table 2, entries 8–12). The reaction between chlorobenzene (2a) and 2,4-dimethylstyrene (3g) only gave product 4l in 67% yield, which may also be due to the steric effect. In addition, acceptable yields can be achieved from the reactions of 2-chlorothiophene (2h) with 4-fluorostyrene (3b) and 4-methylstyrene (3c), respectively (Table 2, entries 19 and 20).
Table 2: NHC-Pd(II)-Im complex 1 catalyzed Mizoroki–Heck reactions of aryl chlorides 2 with styrenes 3.
|
|
|||
| Entrya | 2 (R1) | 3 (R2) | Yield (%)b |
|---|---|---|---|
| 1 | 2b (3-MeO) | 3a (H) | 4b, 85 |
| 2 | 2c (3-Me) | 3a | 4c, 94 |
| 3 | 2d (2-Me) | 3a | 4d, 71 |
| 4 | 2e (4-F) | 3a | 4e, 91 |
| 5 | 2f (4-Me) | 3a | 4f, 80 |
| 6 | 2g (4-NO2) | 3a | 4g, 89 |
| 7c | 2h | 3a | 4h, 63 |
| 8 | 2a (H) | 3b (4-F) | 4e, 81 |
| 9 | 2a | 3c (4-Me) | 4f, 85 |
| 10 | 2a | 3d (4-MeO) | 4i, 83 |
| 11 | 2a | 3e (3-F) | 4j, 81 |
| 12 | 2a | 3f (4-t-Bu) | 4k, 80 |
| 13 | 2a | 3g (2,4-Me2) | 4l, 67 |
| 14 | 2c (3-Me) | 3c | 4m, 85 |
| 15 | 2g | 3b | 4n, 90 |
| 16 | 2g | 3c | 4o, 89 |
| 17 | 2g | 3e | 4p, 95 |
| 18 | 2g | 3f | 4q, 86 |
| 19c | 2h | 3b | 4r, 53 |
| 20c | 2h | 3c | 4s, 51 |
aAll reactions were carried out by using 2 (0.75 mmol), 3 (0.9 mmol), Cs2CO3 (2.0 equiv), 1 (1.0 mol %), TBAB (2.0 g) at 140 °C for 12 h.
bIsolated yields. c
Conclusion
In summary, an easily available NHC-Pd(II)-Im complex 1 showed efficient catalytic activity toward the Mizoroki–Heck reaction between aryl chlorides and styrenes under air at 140 °C within 12 h. Various activated and deactivated aryl chlorides work well under the optimal reaction conditions. In addition, TBAB, as a “green solvent” and one of the most advantageous ionic liquids due to its low price and availability, was used as the solvent to facilitate the reaction [46-48]. Compared to the previously reported NHC–Pd(II) complexes used in the Mizoroki–Heck reaction with aryl chlorides as the substrates, the method reported in this paper possesses the advantages of easy availability of the catalyst and broader substrate applicability.
Supporting Information
| Supporting Information File 1: General procedure for the NHC-Pd(II)-Im complex 1 catalyzed Mizoroki–Heck reaction, characterization data and copies of spectra. | ||
| Format: PDF | Size: 1.3 MB | Download |
References
-
Mizoroki, T.; Mori, K.; Ozaki, A. Bull. Chem. Soc. Jpn. 1971, 44, 581. doi:10.1246/bcsj.44.581
Return to citation in text: [1] -
Heck, R. F.; Nolley, J. P., Jr. J. Org. Chem. 1972, 37, 2320–2322. doi:10.1021/jo00979a024
Return to citation in text: [1] -
Heck, R. F. Palladium Reagents in Organic Synthesis; Academic Press: London, 1985.
Return to citation in text: [1] -
Beletskaya, I. P.; Cheprakov, A. V. Chem. Rev. 2000, 100, 3009–3066. doi:10.1021/cr9903048
Return to citation in text: [1] -
Whitcombe, N. J.; Hii, K. K.; Gilbson, S. E. Tetrahedron 2001, 57, 7449–7476. doi:10.1016/S0040-4020(01)00665-2
Return to citation in text: [1] -
Alonso, F.; Beletskaya, I. P.; Yus, M. Tetrahedron 2005, 61, 11771–11835. doi:10.1016/j.tet.2005.08.054
Return to citation in text: [1] -
Felpin, F.-X.; Nassar-Hardy, L.; Le Callonnec, F.; Fouquet, E. Tetrahedron 2011, 67, 2815–2831. doi:10.1016/j.tet.2011.02.051
Return to citation in text: [1] -
Birkholz, M.-N.; Freixa, Z.; van Leeuwen, P. W. N. M. Chem. Soc. Rev. 2009, 38, 1099–1118. doi:10.1039/b806211k
and references therein.
Return to citation in text: [1] -
Fu, G. C. Acc. Chem. Res. 2008, 41, 1555–1564. doi:10.1021/ar800148f
Return to citation in text: [1] -
Hillier, A. C.; Grasa, G. A.; Viciu, M. S.; Lee, H. M.; Yang, C.; Nolan, S. P. J. Organomet. Chem. 2002, 653, 69–82. doi:10.1016/S0022-328X(02)01154-3
Return to citation in text: [1] -
Kantchev, E. A. B.; O’Brien, C. J.; Organ, M. G. Angew. Chem., Int. Ed. 2007, 46, 2768–2813. doi:10.1002/anie.200601663
Return to citation in text: [1] -
Marion, N.; Nolan, S. P. Acc. Chem. Res. 2008, 41, 1440–1449. doi:10.1021/ar800020y
Return to citation in text: [1] -
Würtz, S.; Glorius, F. Acc. Chem. Res. 2008, 41, 1523–1533. doi:10.1021/ar8000876
Return to citation in text: [1] -
Fortman, G. C.; Nolan, S. P. Chem. Soc. Rev. 2011, 40, 5151–5169. doi:10.1039/c1cs15088j
Return to citation in text: [1] -
Valente, C.; Çalimsiz, S.; Hoi, K. H.; Mallik, D.; Sayah, M.; Organ, M. G. Angew. Chem., Int. Ed. 2012, 51, 3314–3332. doi:10.1002/anie.201106131
Return to citation in text: [1] -
Herrmann, W. A.; Elison, M.; Fischer, J.; Köcher, C.; Artus, G. R. J. Angew. Chem., Int. Ed. Engl. 1995, 34, 2371–2374. doi:10.1002/anie.199523711
Return to citation in text: [1] -
Herrmann, W. A.; Reisinger, C.-P.; Spiegler, M. J. Organomet. Chem. 1998, 557, 93–96. doi:10.1016/S0022-328X(97)00736-5
Return to citation in text: [1] -
Paulose, T. A. P.; Wu, S.-C.; Olson, J. A.; Chau, T.; Theaker, N.; Hassler, M.; Wilson Quail, J.; Foley, S. R. Dalton Trans. 2012, 41, 251–260. doi:10.1039/c1dt10858a
Return to citation in text: [1] -
Liu, Y.-M.; Lin, Y.-C.; Chen, W.-C.; Cheng, J.-H.; Chen, Y.-L.; Yap, G. P. A.; Sun, S.-S.; Ong, T.-G. Dalton Trans. 2012, 41, 7382–7389. doi:10.1039/c2dt30520h
Return to citation in text: [1] -
Dunsford, J. J.; Cavell, K. J. Dalton Trans. 2011, 40, 9131–9135. doi:10.1039/c1dt10596e
Return to citation in text: [1] -
Guo, S.; Huynh, H. V. Organometallics 2012, 31, 4565–4573. doi:10.1021/om3003625
Return to citation in text: [1] -
Tang, Y.-Q.; Chu, C.-Y.; Zhu, L.; Qian, B.; Shao, L.-X. Tetrahedron 2011, 67, 9479–9483. doi:10.1016/j.tet.2011.10.033
Return to citation in text: [1] -
Ma, M.-T.; Lu, J.-M. Appl. Organomet. Chem. 2012, 26, 175–179. doi:10.1002/aoc.2833
Return to citation in text: [1] -
Blakemore, J. D.; Chalkley, M. J.; Farnaby, J. H.; Guard, L. M.; Hazari, N.; Incarvito, C. D.; Luzik, E. D.; Suh, H. W., Jr. Organometallics 2011, 30, 1818–1829. doi:10.1021/om100890q
Return to citation in text: [1] -
Saito, S.; Saika, M.; Yamasaki, R.; Azumaya, I.; Masu, H. Organometallics 2011, 1366–1373. doi:10.1021/om1008583
Return to citation in text: [1] -
Grushin, V. V.; Alper, H. Activation of otherwise unreactive C–Cl bonds. In Activation of unreactive bonds and organic synthesis; Murai, S., Ed.; Topics in Organometallic Chemistry, Vol. 3; Springer: Berlin, 1999; pp 193–226. doi:10.1007/3-540-68525-1_8
Return to citation in text: [1] -
Grushin, V. V.; Alper, H. Chem. Rev. 1994, 94, 1047–1062. doi:10.1021/cr00028a008
Return to citation in text: [1] -
Bedford, R. B.; Cazin, C. S. J.; Holder, D. Coord. Chem. Rev. 2004, 248, 2283–2321. doi:10.1016/j.ccr.2004.06.012
Return to citation in text: [1] -
Selvakumar, K.; Zapf, A.; Beller, M. Org. Lett. 2002, 4, 3031–3033. doi:10.1021/ol020103h
Return to citation in text: [1] [2] -
Lee, J.-Y.; Cheng, P.-Y.; Tsai, Y.-H.; Lin, G.-R.; Liu, S.-P.; Sie, M.-H.; Lee, H. M. Organometallics 2010, 29, 3901–3911. doi:10.1021/om1006402
Return to citation in text: [1] [2] -
Sie, M.-H.; Hsieh, Y.-H.; Tsai, Y.-H.; Wu, J.-R.; Chen, S.-J.; Vijaya Kumar, P.; Lii, J.-H.; Lee, H. M. Organometallics 2010, 29, 6473–6481. doi:10.1021/om100819q
Return to citation in text: [1] [2] -
Huynh, H. V.; Ho, J. H. H.; Neo, T. C.; Koh, L. L. J. Organomet. Chem. 2005, 690, 3854–3860. doi:10.1016/j.jorganchem.2005.04.053
Return to citation in text: [1] -
Frey, G. D.; Schütz, J.; Herdtweck, E.; Herrmann, W. A. Organometallics 2005, 24, 4416–4426. doi:10.1021/om049001g
Return to citation in text: [1] [2] -
Huynh, H. V.; Neo, T. C.; Tan, G. K. Organometallics 2006, 25, 1298–1302. doi:10.1021/om0510369
Return to citation in text: [1] -
Inomata, S.; Hiroki, H.; Terashima, T.; Ogata, K.; Fukuzawa, S.-i. Tetrahedron 2011, 67, 7263–7267. doi:10.1016/j.tet.2011.07.045
Return to citation in text: [1] -
Huynh, H. V.; Jothibasu, R. J. Organomet. Chem. 2011, 696, 3369–3375. doi:10.1016/j.jorganchem.2011.07.018
Return to citation in text: [1] -
Tang, Y.-Q.; Lu, J.-M.; Shao, L.-X. J. Organomet. Chem. 2011, 696, 3741–3744. doi:10.1016/j.jorganchem.2011.08.042
Return to citation in text: [1] -
Zhou, X.-X.; Shao, L.-X. Synthesis 2011, 3138–3142. doi:10.1055/s-0030-1260169
Return to citation in text: [1] -
Zhu, L.; Gao, T.-T.; Shao, L.-X. Tetrahedron 2011, 67, 5150–5155. doi:10.1016/j.tet.2011.05.057
Return to citation in text: [1] -
Gu, Z.-S.; Shao, L.-X.; Lu, J.-M. J. Organomet. Chem. 2012, 700, 132–134. doi:10.1016/j.jorganchem.2011.11.030
Return to citation in text: [1] -
Zhu, L.; Ye, Y.-M.; Shao, L.-X. Tetrahedron 2012, 68, 2414–2420. doi:10.1016/j.tet.2012.01.008
Return to citation in text: [1] -
Xiao, Z.-K.; Shao, L.-X. Synthesis 2012, 711–716. doi:10.1055/s-0031-1289698
Return to citation in text: [1] -
Lin, X.-F.; Li, Y.; Li, S.-Y.; Xiao, Z.-K.; Lu, J.-M. Tetrahedron 2012, 68, 5806–5809. doi:10.1016/j.tet.2012.05.016
Return to citation in text: [1] -
Wang, Z.-Y.; Chen, G.-Q.; Shao, L.-X. J. Org. Chem. 2012, 77, 6608–6614. doi:10.1021/jo301270t
Return to citation in text: [1] -
Initial investigations showed that good yields can also be achieved when 1.5 equiv of bases were used, but further studies revealed that the repeatability is very poor.
Return to citation in text: [1] -
Calò, V.; Nacci, A.; Monopoli, A. Eur. J. Org. Chem. 2006, 3791–3802. doi:10.1002/ejoc.200600045
Return to citation in text: [1] -
Calò, V.; Nacci, A.; Monopoli, A.; Cotugno, P. Angew. Chem., Int. Ed. 2009, 48, 6101–6103. doi:10.1002/anie.200902337
Return to citation in text: [1] -
Wang, L.; Li, H.; Li, P. Tetrahedron 2009, 65, 364–368. doi:10.1016/j.tet.2008.10.042
Return to citation in text: [1]
| 1. | Mizoroki, T.; Mori, K.; Ozaki, A. Bull. Chem. Soc. Jpn. 1971, 44, 581. doi:10.1246/bcsj.44.581 |
| 2. | Heck, R. F.; Nolley, J. P., Jr. J. Org. Chem. 1972, 37, 2320–2322. doi:10.1021/jo00979a024 |
| 3. | Heck, R. F. Palladium Reagents in Organic Synthesis; Academic Press: London, 1985. |
| 4. | Beletskaya, I. P.; Cheprakov, A. V. Chem. Rev. 2000, 100, 3009–3066. doi:10.1021/cr9903048 |
| 5. | Whitcombe, N. J.; Hii, K. K.; Gilbson, S. E. Tetrahedron 2001, 57, 7449–7476. doi:10.1016/S0040-4020(01)00665-2 |
| 6. | Alonso, F.; Beletskaya, I. P.; Yus, M. Tetrahedron 2005, 61, 11771–11835. doi:10.1016/j.tet.2005.08.054 |
| 7. | Felpin, F.-X.; Nassar-Hardy, L.; Le Callonnec, F.; Fouquet, E. Tetrahedron 2011, 67, 2815–2831. doi:10.1016/j.tet.2011.02.051 |
| 18. | Paulose, T. A. P.; Wu, S.-C.; Olson, J. A.; Chau, T.; Theaker, N.; Hassler, M.; Wilson Quail, J.; Foley, S. R. Dalton Trans. 2012, 41, 251–260. doi:10.1039/c1dt10858a |
| 19. | Liu, Y.-M.; Lin, Y.-C.; Chen, W.-C.; Cheng, J.-H.; Chen, Y.-L.; Yap, G. P. A.; Sun, S.-S.; Ong, T.-G. Dalton Trans. 2012, 41, 7382–7389. doi:10.1039/c2dt30520h |
| 20. | Dunsford, J. J.; Cavell, K. J. Dalton Trans. 2011, 40, 9131–9135. doi:10.1039/c1dt10596e |
| 21. | Guo, S.; Huynh, H. V. Organometallics 2012, 31, 4565–4573. doi:10.1021/om3003625 |
| 22. | Tang, Y.-Q.; Chu, C.-Y.; Zhu, L.; Qian, B.; Shao, L.-X. Tetrahedron 2011, 67, 9479–9483. doi:10.1016/j.tet.2011.10.033 |
| 23. | Ma, M.-T.; Lu, J.-M. Appl. Organomet. Chem. 2012, 26, 175–179. doi:10.1002/aoc.2833 |
| 24. | Blakemore, J. D.; Chalkley, M. J.; Farnaby, J. H.; Guard, L. M.; Hazari, N.; Incarvito, C. D.; Luzik, E. D.; Suh, H. W., Jr. Organometallics 2011, 30, 1818–1829. doi:10.1021/om100890q |
| 25. | Saito, S.; Saika, M.; Yamasaki, R.; Azumaya, I.; Masu, H. Organometallics 2011, 1366–1373. doi:10.1021/om1008583 |
| 16. | Herrmann, W. A.; Elison, M.; Fischer, J.; Köcher, C.; Artus, G. R. J. Angew. Chem., Int. Ed. Engl. 1995, 34, 2371–2374. doi:10.1002/anie.199523711 |
| 17. | Herrmann, W. A.; Reisinger, C.-P.; Spiegler, M. J. Organomet. Chem. 1998, 557, 93–96. doi:10.1016/S0022-328X(97)00736-5 |
| 10. | Hillier, A. C.; Grasa, G. A.; Viciu, M. S.; Lee, H. M.; Yang, C.; Nolan, S. P. J. Organomet. Chem. 2002, 653, 69–82. doi:10.1016/S0022-328X(02)01154-3 |
| 11. | Kantchev, E. A. B.; O’Brien, C. J.; Organ, M. G. Angew. Chem., Int. Ed. 2007, 46, 2768–2813. doi:10.1002/anie.200601663 |
| 12. | Marion, N.; Nolan, S. P. Acc. Chem. Res. 2008, 41, 1440–1449. doi:10.1021/ar800020y |
| 13. | Würtz, S.; Glorius, F. Acc. Chem. Res. 2008, 41, 1523–1533. doi:10.1021/ar8000876 |
| 14. | Fortman, G. C.; Nolan, S. P. Chem. Soc. Rev. 2011, 40, 5151–5169. doi:10.1039/c1cs15088j |
| 15. | Valente, C.; Çalimsiz, S.; Hoi, K. H.; Mallik, D.; Sayah, M.; Organ, M. G. Angew. Chem., Int. Ed. 2012, 51, 3314–3332. doi:10.1002/anie.201106131 |
| 8. |
Birkholz, M.-N.; Freixa, Z.; van Leeuwen, P. W. N. M. Chem. Soc. Rev. 2009, 38, 1099–1118. doi:10.1039/b806211k
and references therein. |
| 9. | Fu, G. C. Acc. Chem. Res. 2008, 41, 1555–1564. doi:10.1021/ar800148f |
| 37. | Tang, Y.-Q.; Lu, J.-M.; Shao, L.-X. J. Organomet. Chem. 2011, 696, 3741–3744. doi:10.1016/j.jorganchem.2011.08.042 |
| 38. | Zhou, X.-X.; Shao, L.-X. Synthesis 2011, 3138–3142. doi:10.1055/s-0030-1260169 |
| 39. | Zhu, L.; Gao, T.-T.; Shao, L.-X. Tetrahedron 2011, 67, 5150–5155. doi:10.1016/j.tet.2011.05.057 |
| 40. | Gu, Z.-S.; Shao, L.-X.; Lu, J.-M. J. Organomet. Chem. 2012, 700, 132–134. doi:10.1016/j.jorganchem.2011.11.030 |
| 41. | Zhu, L.; Ye, Y.-M.; Shao, L.-X. Tetrahedron 2012, 68, 2414–2420. doi:10.1016/j.tet.2012.01.008 |
| 42. | Xiao, Z.-K.; Shao, L.-X. Synthesis 2012, 711–716. doi:10.1055/s-0031-1289698 |
| 43. | Lin, X.-F.; Li, Y.; Li, S.-Y.; Xiao, Z.-K.; Lu, J.-M. Tetrahedron 2012, 68, 5806–5809. doi:10.1016/j.tet.2012.05.016 |
| 44. | Wang, Z.-Y.; Chen, G.-Q.; Shao, L.-X. J. Org. Chem. 2012, 77, 6608–6614. doi:10.1021/jo301270t |
| 45. | Initial investigations showed that good yields can also be achieved when 1.5 equiv of bases were used, but further studies revealed that the repeatability is very poor. |
| 32. | Huynh, H. V.; Ho, J. H. H.; Neo, T. C.; Koh, L. L. J. Organomet. Chem. 2005, 690, 3854–3860. doi:10.1016/j.jorganchem.2005.04.053 |
| 33. | Frey, G. D.; Schütz, J.; Herdtweck, E.; Herrmann, W. A. Organometallics 2005, 24, 4416–4426. doi:10.1021/om049001g |
| 34. | Huynh, H. V.; Neo, T. C.; Tan, G. K. Organometallics 2006, 25, 1298–1302. doi:10.1021/om0510369 |
| 35. | Inomata, S.; Hiroki, H.; Terashima, T.; Ogata, K.; Fukuzawa, S.-i. Tetrahedron 2011, 67, 7263–7267. doi:10.1016/j.tet.2011.07.045 |
| 36. | Huynh, H. V.; Jothibasu, R. J. Organomet. Chem. 2011, 696, 3369–3375. doi:10.1016/j.jorganchem.2011.07.018 |
| 46. | Calò, V.; Nacci, A.; Monopoli, A. Eur. J. Org. Chem. 2006, 3791–3802. doi:10.1002/ejoc.200600045 |
| 47. | Calò, V.; Nacci, A.; Monopoli, A.; Cotugno, P. Angew. Chem., Int. Ed. 2009, 48, 6101–6103. doi:10.1002/anie.200902337 |
| 48. | Wang, L.; Li, H.; Li, P. Tetrahedron 2009, 65, 364–368. doi:10.1016/j.tet.2008.10.042 |
| 29. | Selvakumar, K.; Zapf, A.; Beller, M. Org. Lett. 2002, 4, 3031–3033. doi:10.1021/ol020103h |
| 30. | Lee, J.-Y.; Cheng, P.-Y.; Tsai, Y.-H.; Lin, G.-R.; Liu, S.-P.; Sie, M.-H.; Lee, H. M. Organometallics 2010, 29, 3901–3911. doi:10.1021/om1006402 |
| 31. | Sie, M.-H.; Hsieh, Y.-H.; Tsai, Y.-H.; Wu, J.-R.; Chen, S.-J.; Vijaya Kumar, P.; Lii, J.-H.; Lee, H. M. Organometallics 2010, 29, 6473–6481. doi:10.1021/om100819q |
| 26. | Grushin, V. V.; Alper, H. Activation of otherwise unreactive C–Cl bonds. In Activation of unreactive bonds and organic synthesis; Murai, S., Ed.; Topics in Organometallic Chemistry, Vol. 3; Springer: Berlin, 1999; pp 193–226. doi:10.1007/3-540-68525-1_8 |
| 27. | Grushin, V. V.; Alper, H. Chem. Rev. 1994, 94, 1047–1062. doi:10.1021/cr00028a008 |
| 28. | Bedford, R. B.; Cazin, C. S. J.; Holder, D. Coord. Chem. Rev. 2004, 248, 2283–2321. doi:10.1016/j.ccr.2004.06.012 |
| 29. | Selvakumar, K.; Zapf, A.; Beller, M. Org. Lett. 2002, 4, 3031–3033. doi:10.1021/ol020103h |
| 30. | Lee, J.-Y.; Cheng, P.-Y.; Tsai, Y.-H.; Lin, G.-R.; Liu, S.-P.; Sie, M.-H.; Lee, H. M. Organometallics 2010, 29, 3901–3911. doi:10.1021/om1006402 |
| 31. | Sie, M.-H.; Hsieh, Y.-H.; Tsai, Y.-H.; Wu, J.-R.; Chen, S.-J.; Vijaya Kumar, P.; Lii, J.-H.; Lee, H. M. Organometallics 2010, 29, 6473–6481. doi:10.1021/om100819q |
| 33. | Frey, G. D.; Schütz, J.; Herdtweck, E.; Herrmann, W. A. Organometallics 2005, 24, 4416–4426. doi:10.1021/om049001g |
© 2012 Gao et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)