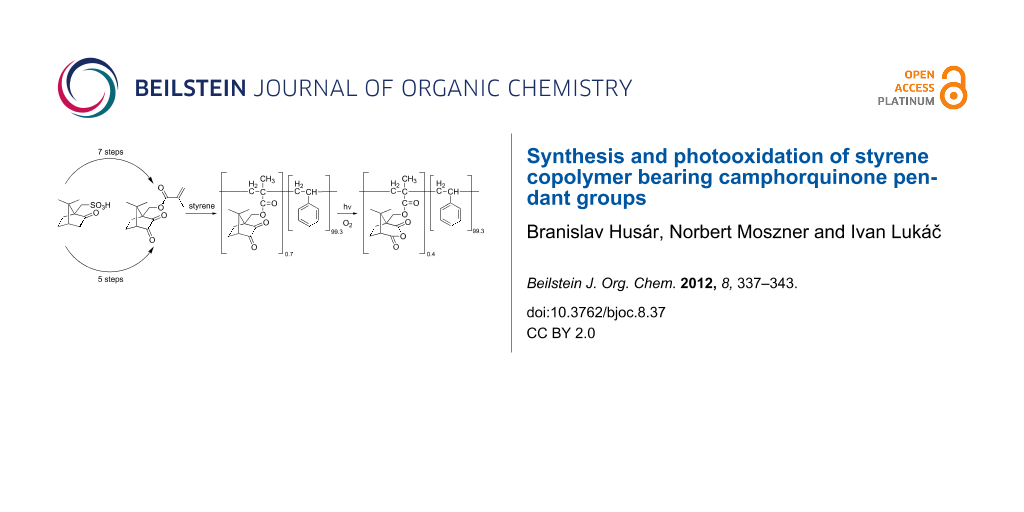
Abstract
(±)-10-Methacryloyloxycamphorquinone (MCQ) was synthesized from (±)-10-camphorsulfonic acid either by a known seven-step synthetic route or by a novel, shorter five-step synthetic route. MCQ was copolymerized with styrene (S) and the photochemical behavior of the copolymer MCQ/S was compared with that of a formerly studied copolymer of styrene with monomers containing the benzil (BZ) moiety (another 1,2-dicarbonyl). Irradiation (λ > 380 nm) of aerated films of styrene copolymers with monomers containing the BZ moiety leads to the insertion of two oxygen atoms between the carbonyl groups of BZ and to the formation of benzoyl peroxide (BP) as pendant groups on the polymer backbone. An equivalent irradiation of MCQ/S led mainly to the insertion of only one oxygen atom between the carbonyl groups of camphorquinone (CQ) and to the formation of camphoric anhydride (11) covalently bound to the polymer backbone. While the decomposition of pendant BP groups formed in irradiated films of styrene copolymers with pendant BZ groups leads to crosslinking, only small molecular-weight changes in irradiated MCQ/S were observed.
Graphical Abstract
Introduction
Camphorquinone (CQ) in the presence of H-atom donors such as ethers (H abstraction), or more efficiently tertiary amines (electron/proton transfer), is known to be an effective photoinitiator for curing methacrylate-based dental restorative resins [1-9]. CQ photochemistry in solution in the absence of oxygen [10-16] and in the presence of oxygen [10,13,17-20] has been studied extensively. In an inert atmosphere, the excited n→π* triplet state of the carbonyl group of CQ abstracts an H-atom from a hydrogen donor. The two primarily formed radicals undergo subsequent reactions leading to photoproducts [21]. The rate-determining step in photoinitiation by CQ/amine is hydrogen transfer by the excited n→π* triplet state of the carbonyl group of CQ from the alkylamino group [8,9]. The photochemistry of the low molecular CQ in the polystyrene (PS) film was the subject of previous studies [21,22]. It is reasonable to compare the photochemical properties of polymer-bound CQ with a polymer matrix containing another well-studied 1,2-dicarbonyl compound, namely benzil (BZ). BZ can be converted almost quantitatively to benzoyl peroxide (BP) in an aerated polymer matrix by irradiation at λ > 400 nm (Scheme 1) [23,24]
Scheme 1: Photoperoxidation of BZ in an aerated glassy polymer matrix.
Scheme 1: Photoperoxidation of BZ in an aerated glassy polymer matrix.
The pendant BZ groups of the styrene copolymers may be transformed into pendant BP groups [25,26]. Subsequent decomposition of BP pendant groups results in the formation of highly crosslinked films [27-30].
Owing to the interesting properties of styrene copolymers formed from BZ-containing monomers, a CQ-bearing monomer (±)-10-methacryloyloxycamphorquinone (MCQ) (another monomer with a 1,2-dicarbonyl moiety) was prepared and copolymerized with styrene to give MCQ/S copolymer bearing CQ pendant groups. Enantiopure MCQ is known from the literature [31]. The goal of this work was to prepare a more easily accessible racemic MCQ and to compare the photochemistry of MCQ/S with that of low molecular CQ in PS films in the presence of oxygen.
Results and Discussion
Synthesis of MCQ
Racemic MCQ was synthesized in seven steps from (±)-10-camphorsulfonic acid (1) as a starting material in 18% overall yield (Scheme 2, see Supporting Information File 1 and Supporting Information File 2 for full experimental data). Total synthesis of (1R)-10-methacryloyloxycamphorquinone from (1S)-10-camphorsulfonic acid [31] as well as of enantiopure stable intermediates 2–7 [8,32-34] are known. Though the physiological activity of optically active compounds depends on their configuration, the photochemical activity of CQ does not depend on the configuration. Without the need for an enantiopure product, the reaction economics could be improved both by the use of a cheaper racemic starting material and by a simplified multistep synthesis.
Scheme 2: Synthesis of MCQ from (±)-10-camphorsulfonic acid (1). Only one enantiomer of each compound is depicted.
Scheme 2: Synthesis of MCQ from (±)-10-camphorsulfonic acid (1). Only one enantiomer of each compound is depi...
A shorter alternative synthetic route to 6 from 1 was proposed (Scheme 2, see Supporting Information File 1 and Supporting Information File 2 for full experimental data). (±)-10-Iodocamphor (8) was prepared in one step from 1 with iodine and PPh3 in toluene under reflux in 39% yield (lit. [35] 85% yield). Compound 8 was selectively oxidized with SeO2 in bromobenzene under reflux to (±)-10-iodocamphorquinone (9) in 84% yield. A suspension of 9 in anhydrous acetic acid with freshly molten potassium acetate was heated to 170 °C for 8 h to provide 6 in 37% yield.
The advantage of this synthetic pathway lies in the reduction of synthetic steps as well as avoiding the use of rather expensive PBr5. Although we obtained MCQ in overall yield of only 5%, optimization of conditions will likely provide a higher value. For example, iodocamphor 8 was previously obtained in 85% yield (much better than the 39% yield expressed here). Low yield in this step is blamed on the poor quality of reactants. The yield of 6 from 9 was lower due to the formation of side products. Thus, iodocamphor 8 should be transformed first to 5 and afterwards to 6.
All attempts to transform 9 directly to MCQ were unsuccessful. For example, such a transformation was tested by stirring compound 9 at ambient temperature with the following reagents: Methacrylic acid/Cs2CO3/DMF, methacrylic acid/DBN/benzene, methacrylic acid/NaH/n-hexane, methacrylic acid/NaHCO3/DMF, potassium methacrylate/acetone, and silver methacrylate/toluene.
From the last step of the MCQ synthesis, a side-product 10 was isolated and identified. It was previously reported that a commercial sample of methacryloyl chloride contains its oxa-Diels–Alder dimer, responsible for the formation of 10 [36]. This side-product could be avoided by using freshly distilled methacryloyl chloride. A significant difference in the determined melting points of the racemates and enantiomers of 6, 7, and MCQ is caused by the configuration of these compounds (Table 1). The decrease of melting point of the racemate is 10–12 °C in the case of 6 and 43–45 °C in the case of MCQ compared to the pure enantiomer. Racemic 7 melts over a broad range of temperatures in contrast to enantiomeric 7, which has a sharp melting point. The compounds in this work are racemates, but the corresponding compounds in the literature are pure enantiomers. The racemates can crystallize as a racemic mixture (lower mp), as a racemic compound (lower or higher mp), or rarely as a racemic solid solution (slightly lower or higher mp).
Table 1: Values of melting points of synthesized racemic camphor derivatives and corresponding pure enantiomers (R or S) from the literature.
| Compound |
Melting point of racemate
(°C) |
Melting point of enantiomer
(°C) |
Configuration of enantiomer
(R or S) |
Reference |
|---|---|---|---|---|
| 4 | 77–78 | 78 | S | [35] |
| 4 | 77–78 | 78 | R | [32] |
| 4 | 77–78 | 76–78 | R | [8] |
| 6 | 76–78 | 88 | R | [33] |
| 6 | 76–78 | 87–90 | R | [8] |
| 7 | ≈100–255 | 201–203 | R | [8] |
| 7 | ≈100–255 | 205 | R | [34] |
| 8 | 67–72 | 71 | S | [35] |
| MCQ | 46–49 | 91–92 | R | [31] |
Polymerization
As introduced in Supporting Information File 1, the copolymer MCQ/S was synthesized by copolymerization of styrene (99.54 mol %) and MCQ (0.46 mol %), initiated by AIBN at 60 °C and resulting in 10% conversion. FTIR spectroscopy was used to estimate the content of CQ units in the MCQ/S copolymer by interpolation of the peak area of the carbonyl band (1740–1790 cm−1) using a calibration curve consisting of five different concentrations of MCQ in CCl4 solution. UV–vis spectroscopy was used in a similar way by interpolation of the n→π* peak area (390–510 nm) using a calibration curve. The content of MCQ units in MCQ/S copolymer was determined to be 0.72 mol % from FTIR and 0.62 mol % from UV–vis. MCQ is therefore more reactive than styrene, which is in agreement with the copolymerization parameters of structurally similar monomers.
Photooxidation of MCQ/S
Since it is difficult to follow the structural changes of the CQ structures of the MCQ/S copolymer during photochemical transformation, an analogous study with low molecular CQ doped in a PS matrix was first performed. Elucidation of the structures of low molecular photoproducts was conveniently followed by spectral methods [22] and by isolation from the polymer matrix followed by spectral identification [21]. The results of the CQ photooxidation in PS are summarized in Scheme 3 [21].
Scheme 3: Photooxidation of CQ in aerated glassy PS matrix.
Scheme 3: Photooxidation of CQ in aerated glassy PS matrix.
The addition of molecular oxygen to the excited n→π* triplet state of ketones and 1,2-diketones to form 1,4-biradicals is a generally accepted mechanism, which has been theoretically treated and reviewed [37]. The oxygen atom released during the formation of 11 can oxidize another molecule of CQ to 11. It is likely that common biradical intermediates are responsible for the formation of lactones 12 and 13 in solution [10] and acids 14a and 14b formed in the PS matrix [21]. The intramolecular recombination of biradical intermediates is favored in benzene solution. However, in glassy PS matrix the intramolecular abstraction of a hydrogen atom and formation of a double C=C bond occurs. The glassy polymer matrix should retard the intramolecular recombination of biradicals.
During the irradiation of MCQ/S film at λ > 380 nm in air, the changes were followed by FTIR (Figure 1) and UV–vis (Figure 2) spectroscopy. The evolution of both spectra of low molecular CQ doped in the PS matrix and that of copolymer MCQ/S during irradiation (beside ester carbonyl absorption in FTIR spectra) are similar. Absorption bands of the CQ 1,2-dicarbonyl group vibrations (1776, 1759 cm−1) decreased quantitatively. This decrease is accompanied by the formation of bands at 1815 and 1770 cm−1 assigned to anhydride 11 (Figure 1). Increased absorption near 1700 cm−1 was assigned to acids 14. After thermal treatment of the irradiated MCQ/S film at 90 °C for 2 h, no change was observed by FTIR spectroscopy. This signifies that no thermally unstable eight-member ring peroxide (in analogy with BP formed from BZ as shown in Scheme 1) was present in the PS matrix after irradiation. FTIR vibration bands for such cyclic diacylperoxide would be expected to be found near 1800 cm−1. Also in UV–vis spectra of the MCQ/S film after irradiation (Figure 2), complete reduction of the n→π* absorption band of the 1,2-dicarbonyl group of the CQ structure is seen.
![[1860-5397-8-37-1]](/bjoc/content/figures/1860-5397-8-37-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: FTIR spectra of MCQ/S film after irradiation in a carousel for the indicated periods. Spectrum of PS film was subtracted.
Figure 1: FTIR spectra of MCQ/S film after irradiation in a carousel for the indicated periods. Spectrum of PS...
![[1860-5397-8-37-2]](/bjoc/content/figures/1860-5397-8-37-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: UV–vis spectra of MCQ/S film after irradiation in a carousel apparatus for the indicated periods.
Figure 2: UV–vis spectra of MCQ/S film after irradiation in a carousel apparatus for the indicated periods.
The GC–MS results [21] allow the rough estimation of the distribution of CQ photooxidation products in PS from the GC record. About 54% of the whole area corresponds to 11 and its secondary products formed during isolation (secondary products are not formed in the MCQ/S film during irradiation). 16% corresponds to 14a–c and the remaining 30% are unidentified compounds. A similar distribution of products is most probably also present in the photooxidized MCQ/S copolymer.
Whereas the previously published irradiation of styrene copolymers with the monomers containing BZ (Mn = 84000, PD = 1.8) with 0.54 mol % of the BZ-containing component led to extensive crosslinking with 69% gel fraction [21], MCQ/S with 0.72 mol % of CQ moieties did not reach the gel point under the same conditions of irradiation; MCQ/S film retained its solubility in organic solvents. PS equivalent molar masses were then determined by GPC before and after irradiation. The molecular weights and polydispersities of the irradiated copolymer (Mn = 190000, PD = 2.7) were higher than those of the initial copolymer (Mn = 170000, PD = 2.0). Therefore, some recombination of macroradicals occurred during irradiation. However, the crosslinking of styrene copolymers with the monomers containing BZ is significantly more efficient than those of MCQ/S.
While in the case of styrene copolymers with the monomers containing BZ decomposition of pendant BP groups produces only one polymeric benzoyloxy radical that is efficient in crosslinking, in the MCQ/S copolymer two acyloxy radicals could be produced from the postulated covalently bound cyclic diacylperoxide and could cause extensive crosslinking. As the changes in the chemical and molecular weights show, the mechanism of MCQ/S crosslinking (increase of molecular weight) is clearly different from that in the presence of BZ structures. Hence, the covalently bound cyclic diacylperoxide as an intermediate is most probably not formed. Similar to BZ, the n→π* triplet state of the CQ structure may also add molecular oxygen to form a 1,4-biradical. In the case of the BZ structures, formation of the 1,4-biradical is followed by BP formation. In comparison, CQ structures react with oxygen forming 1,4-biradicals, which decompose to camphoric anhydride 11 structures covalently bound to the polymer backbone (Scheme 4a). Liberated atomic oxygen during the formation of covalently bound 11 may oxidize another pendant CQ unit to the covalently bound 11. Cyclopentenecarboxylic acid 14 structures shown in Scheme 3 according to the low molecular study [21] were formed to a much lesser extent. Since the phototransformation of CQ to 11 is not quantitative, n + m = 99.7; the remaining 0.3 consists of cyclopentenoic acids 14 and other unknown photoproducts.
Scheme 4: Proposed mechanism of MCQ/S photochemistry.
Scheme 4: Proposed mechanism of MCQ/S photochemistry.
The increase of the molecular weight of MCQ/S during irradiation under low oxygen conditions is induced primarily by an abstraction of hydrogen from the polymer backbone by the CQ structure in the triplet state under the formation of ketyl and alkyl radicals. The formed macroradicals can recombine and/or disproportionate (Scheme 4b). Recombination of macroradicals leads to an increase of the molecular weight and polydispersity. As previously shown, oxygen increases the consumption rate of CQ added to PS and lowers the rate of decrease of the molecular weight of PS [22]. Therefore in simultaneous photooxidation and photoreduction (Scheme 4) in the presence of oxygen, the intramolecular photooxidation of CQ has almost no effect on the molecular weight [21]. In the case of MCQ/S, photoreduction forms macroradicals that recombine causing an increase in the molecular weight.
Conclusion
Upon irradiation of MCQ/S copolymer film by light with λ > 380 nm in air, the CQ structure in the copolymer was transformed mainly to pendant camphoric anhydride 11 structures. Also cyclopentenecarboxylic acid 14 structures covalently bound to copolymer backbone were identified to a minor extent. No cyclic camphordiacyl peroxide as an intermediate of the CQ photooxidation was found. Crosslinking of MCQ/S is inefficient compared to the case of styrene copolymers with monomers containing the BZ moiety.
Monomer MCQ (racemate) was synthesized from camphorsulfonic acid in seven steps by a known synthetic pathway for optically active MCQ. As an improvement, an alternative five-step synthesis of MCQ was proposed as well.
Supporting Information
Supporting information contains detailed experimental data for the synthesis of the compounds 2–10, MCQ, and copolymer MCQ/S, irradiation conditions, and NMR spectra of 5–10 and MCQ.
| Supporting Information File 1: Experimental part. | ||
| Format: PDF | Size: 168.7 KB | Download |
| Supporting Information File 2: NMR spectra of compounds 5–10 and MCQ. | ||
| Format: PDF | Size: 1.3 MB | Download |
References
-
Mateo, J. L.; Bosch, P.; Lozano, A. E. Macromolecules 1994, 27, 7794–7799. doi:10.1021/ma00104a038
Return to citation in text: [1] -
Munksgaard, E. C.; Irie, M.; Asmussen, E. J. Dent. Res. 1985, 64, 1409–1411. doi:10.1177/00220345850640121801
Return to citation in text: [1] -
Cook, W. D. Polymer 1992, 33, 600–609. doi:10.1016/0032-3861(92)90738-I
Return to citation in text: [1] -
Taira, M.; Urabe, H.; Hirose, T.; Wakasa, K.; Yamaki, M. J. Dent. Res. 1988, 67, 24–28. doi:10.1177/00220345880670010401
Return to citation in text: [1] -
Fujimori, Y.; Kaneko, T.; Kaku, T.; Yoshioka, N.; Nishide, H.; Tsuchida, E. Polym. Adv. Technol. 1992, 3, 437–441. doi:10.1002/pat.1992.220030804
Return to citation in text: [1] -
Inomata, K.; Minoshima, I.; Matsumoto, T.; Tokumaru, K. Polym. J. 1993, 25, 1199–1202. doi:10.1295/polymj.25.1199
Return to citation in text: [1] -
Jakubiak, J.; Allonas, X.; Fouassier, J. P.; Sionkowska, A.; Andrzejewska, A.; Lindén, L. Å.; Rabek, J. F. Polymer 2003, 44, 5219–5226. doi:10.1016/S0032-3861(03)00568-8
Return to citation in text: [1] -
Ullrich, G.; Herzog, D.; Liska, R.; Burtscher, P.; Moszner, N. J. Polym. Sci., Part A: Polym. Chem. 2004, 42, 4948–4963. doi:10.1002/pola.20318
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Andrzejewska, E.; Lindén, L.-Å.; Rabek, J. F. Macromol. Chem. Phys. 1998, 199, 441–449. doi:10.1002/(SICI)1521-3935(19980301)199:3<441::AID-MACP441>3.0.CO;2-N
Return to citation in text: [1] [2] -
Meinwald, J.; Klingele, H. O. J. Am. Chem. Soc. 1966, 88, 2071–2073. doi:10.1021/ja00961a056
Return to citation in text: [1] [2] [3] -
Monroe, B. M.; Weiner, S. A.; Hammond, G. S. J. Am. Chem. Soc. 1968, 90, 1913–1914. doi:10.1021/ja01009a049
Return to citation in text: [1] -
Monroe, B. M.; Weiner, S. A. J. Am. Chem. Soc. 1969, 91, 450–456. doi:10.1021/ja01030a041
Return to citation in text: [1] -
Rubin, M. B.; LaBarge, R. G. J. Org. Chem. 1966, 31, 3283–3289. doi:10.1021/jo01348a041
Return to citation in text: [1] [2] -
Rubin, M. B. Tetrahedron Lett. 1969, 45, 3931–3934. doi:10.1016/S0040-4039(01)88579-8
Return to citation in text: [1] -
Rubin, M. B.; Ben-Bassat, J. M. Tetrahedron 1970, 26, 3579–3589. doi:10.1016/S0040-4020(01)92937-0
Return to citation in text: [1] -
Rubin, M. B.; Gutman, A. L. J. Org. Chem. 1986, 51, 2511–2515. doi:10.1021/jo00363a020
Return to citation in text: [1] -
Gream, G. E.; Paice, J. C.; Ramsay, C. C. R. Aust. J. Chem. 1969, 22, 1229–1247. doi:10.1071/CH9691229
Return to citation in text: [1] -
Bredt-Savelsberg, M.; Zaunbrecher, K.; Knieke, L. Chem. Ber. 1927, 60, 1801–1808. doi:10.1002/cber.19270600813
Return to citation in text: [1] -
Bredt-Savelsberg, M. Chem. Ber. 1932, 65, 1–11. doi:10.1002/cber.19320650102
Return to citation in text: [1] -
Ji, S.-J.; Lu, J.; Lang, J.-P.; Horiuchi, C. A. Synth. Commun. 2002, 32, 1659–1663. doi:10.1081/SCC-120004256
Return to citation in text: [1] -
Husár, B.; Lukáč, I. J. Photochem. Photobiol., A 2011, 223, 189–193. doi:10.1016/j.jphotochem.2011.08.017
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] [9] -
Mosnáček, J.; Lukáč, I. J. Photochem. Photobiol., A 2002, 151, 95–104. doi:10.1016/S1010-6030(02)00024-2
Return to citation in text: [1] [2] [3] -
Lukáč, I.; Kósa, C. Macromol. Rapid Commun. 1994, 15, 929–934. doi:10.1002/marc.1994.030151204
Return to citation in text: [1] -
Kósa, C.; Lukáč, I.; Weiss, R. G. Macromol. Chem. Phys. 1999, 200, 1080–1085. doi:10.1002/(SICI)1521-3935(19990501)200:5<1080::AID-MACP1080>3.0.CO;2-7
Return to citation in text: [1] -
Husár, B.; Lukáč, I. J. Photochem. Photobiol., A 2008, 195, 191–197. doi:10.1016/j.jphotochem.2007.10.001
Return to citation in text: [1] -
Husár, B.; Commereuc, S.; Chmela, Š.; Verney, V. Polym. Int. 2010, 59, 1563–1570. doi:10.1002/pi.2901
Return to citation in text: [1] -
Kósa, C.; Lukáč, I.; Weiss, R. G. Macromolecules 2000, 33, 4015–4022. doi:10.1021/ma991493z
Return to citation in text: [1] -
Mosnáček, J.; Weiss, R. G.; Lukáč, I. Macromolecules 2002, 35, 3870–3875. doi:10.1021/ma0117458
Return to citation in text: [1] -
Mosnáček, J.; Weiss, R. G.; Lukáč, I. Macromolecules 2004, 37, 1304–1311. doi:10.1021/ma030213j
Return to citation in text: [1] -
Mosnáček, J.; Lukáč, I.; Chromik, Š.; Kostič, I.; Hrdlovič, P. J. Polym. Sci., Part A: Polym. Chem. 2004, 42, 765–771. doi:10.1002/pola.10860
Return to citation in text: [1] -
Angiolini, L.; Caretti, D.; Salatelli, E. Macromol. Chem. Phys. 2000, 201, 2646–2653. doi:10.1002/1521-3935(20001201)201:18<2646::AID-MACP2646>3.0.CO;2-D
Return to citation in text: [1] [2] [3] -
Dallacker, F.; Alroggen, I.; Krings, H.; Laurs, B.; Lipp, M. Justus Liebigs Ann. Chem. 1961, 647, 23–36. doi:10.1002/jlac.19616470105
Return to citation in text: [1] [2] -
Dallacker, F.; Ulrichs, K.; Lipp, M. Justus Liebigs Ann. Chem. 1963, 667, 50–55. doi:10.1002/jlac.19636670108
Return to citation in text: [1] [2] -
Połoński, T.; Dauter, Z. J. Chem. Soc., Perkin Trans. 1 1986, 1781–1788. doi:10.1039/P19860001781
Return to citation in text: [1] [2] -
Sell, T.; Laschat, S.; Dix, I.; Jones, P. G. Eur. J. Org. Chem. 2000, 4119–4124. doi:10.1002/1099-0690(200012)2000:24<4119::AID-EJOC4119>3.0.CO;2-X
Return to citation in text: [1] [2] [3] -
Fischer, W.; Belluš, D.; Alder, A.; Francotte, E.; Roloff, A. Chimia 1985, 39, 19–20.
Return to citation in text: [1] -
Nau, W. M. J. Inf. Recording 1998, 24, 105–114.
Return to citation in text: [1]
| 10. | Meinwald, J.; Klingele, H. O. J. Am. Chem. Soc. 1966, 88, 2071–2073. doi:10.1021/ja00961a056 |
| 21. | Husár, B.; Lukáč, I. J. Photochem. Photobiol., A 2011, 223, 189–193. doi:10.1016/j.jphotochem.2011.08.017 |
| 21. | Husár, B.; Lukáč, I. J. Photochem. Photobiol., A 2011, 223, 189–193. doi:10.1016/j.jphotochem.2011.08.017 |
| 1. | Mateo, J. L.; Bosch, P.; Lozano, A. E. Macromolecules 1994, 27, 7794–7799. doi:10.1021/ma00104a038 |
| 2. | Munksgaard, E. C.; Irie, M.; Asmussen, E. J. Dent. Res. 1985, 64, 1409–1411. doi:10.1177/00220345850640121801 |
| 3. | Cook, W. D. Polymer 1992, 33, 600–609. doi:10.1016/0032-3861(92)90738-I |
| 4. | Taira, M.; Urabe, H.; Hirose, T.; Wakasa, K.; Yamaki, M. J. Dent. Res. 1988, 67, 24–28. doi:10.1177/00220345880670010401 |
| 5. | Fujimori, Y.; Kaneko, T.; Kaku, T.; Yoshioka, N.; Nishide, H.; Tsuchida, E. Polym. Adv. Technol. 1992, 3, 437–441. doi:10.1002/pat.1992.220030804 |
| 6. | Inomata, K.; Minoshima, I.; Matsumoto, T.; Tokumaru, K. Polym. J. 1993, 25, 1199–1202. doi:10.1295/polymj.25.1199 |
| 7. | Jakubiak, J.; Allonas, X.; Fouassier, J. P.; Sionkowska, A.; Andrzejewska, A.; Lindén, L. Å.; Rabek, J. F. Polymer 2003, 44, 5219–5226. doi:10.1016/S0032-3861(03)00568-8 |
| 8. | Ullrich, G.; Herzog, D.; Liska, R.; Burtscher, P.; Moszner, N. J. Polym. Sci., Part A: Polym. Chem. 2004, 42, 4948–4963. doi:10.1002/pola.20318 |
| 9. | Andrzejewska, E.; Lindén, L.-Å.; Rabek, J. F. Macromol. Chem. Phys. 1998, 199, 441–449. doi:10.1002/(SICI)1521-3935(19980301)199:3<441::AID-MACP441>3.0.CO;2-N |
| 8. | Ullrich, G.; Herzog, D.; Liska, R.; Burtscher, P.; Moszner, N. J. Polym. Sci., Part A: Polym. Chem. 2004, 42, 4948–4963. doi:10.1002/pola.20318 |
| 9. | Andrzejewska, E.; Lindén, L.-Å.; Rabek, J. F. Macromol. Chem. Phys. 1998, 199, 441–449. doi:10.1002/(SICI)1521-3935(19980301)199:3<441::AID-MACP441>3.0.CO;2-N |
| 35. | Sell, T.; Laschat, S.; Dix, I.; Jones, P. G. Eur. J. Org. Chem. 2000, 4119–4124. doi:10.1002/1099-0690(200012)2000:24<4119::AID-EJOC4119>3.0.CO;2-X |
| 21. | Husár, B.; Lukáč, I. J. Photochem. Photobiol., A 2011, 223, 189–193. doi:10.1016/j.jphotochem.2011.08.017 |
| 32. | Dallacker, F.; Alroggen, I.; Krings, H.; Laurs, B.; Lipp, M. Justus Liebigs Ann. Chem. 1961, 647, 23–36. doi:10.1002/jlac.19616470105 |
| 10. | Meinwald, J.; Klingele, H. O. J. Am. Chem. Soc. 1966, 88, 2071–2073. doi:10.1021/ja00961a056 |
| 13. | Rubin, M. B.; LaBarge, R. G. J. Org. Chem. 1966, 31, 3283–3289. doi:10.1021/jo01348a041 |
| 17. | Gream, G. E.; Paice, J. C.; Ramsay, C. C. R. Aust. J. Chem. 1969, 22, 1229–1247. doi:10.1071/CH9691229 |
| 18. | Bredt-Savelsberg, M.; Zaunbrecher, K.; Knieke, L. Chem. Ber. 1927, 60, 1801–1808. doi:10.1002/cber.19270600813 |
| 19. | Bredt-Savelsberg, M. Chem. Ber. 1932, 65, 1–11. doi:10.1002/cber.19320650102 |
| 20. | Ji, S.-J.; Lu, J.; Lang, J.-P.; Horiuchi, C. A. Synth. Commun. 2002, 32, 1659–1663. doi:10.1081/SCC-120004256 |
| 35. | Sell, T.; Laschat, S.; Dix, I.; Jones, P. G. Eur. J. Org. Chem. 2000, 4119–4124. doi:10.1002/1099-0690(200012)2000:24<4119::AID-EJOC4119>3.0.CO;2-X |
| 10. | Meinwald, J.; Klingele, H. O. J. Am. Chem. Soc. 1966, 88, 2071–2073. doi:10.1021/ja00961a056 |
| 11. | Monroe, B. M.; Weiner, S. A.; Hammond, G. S. J. Am. Chem. Soc. 1968, 90, 1913–1914. doi:10.1021/ja01009a049 |
| 12. | Monroe, B. M.; Weiner, S. A. J. Am. Chem. Soc. 1969, 91, 450–456. doi:10.1021/ja01030a041 |
| 13. | Rubin, M. B.; LaBarge, R. G. J. Org. Chem. 1966, 31, 3283–3289. doi:10.1021/jo01348a041 |
| 14. | Rubin, M. B. Tetrahedron Lett. 1969, 45, 3931–3934. doi:10.1016/S0040-4039(01)88579-8 |
| 15. | Rubin, M. B.; Ben-Bassat, J. M. Tetrahedron 1970, 26, 3579–3589. doi:10.1016/S0040-4020(01)92937-0 |
| 16. | Rubin, M. B.; Gutman, A. L. J. Org. Chem. 1986, 51, 2511–2515. doi:10.1021/jo00363a020 |
| 36. | Fischer, W.; Belluš, D.; Alder, A.; Francotte, E.; Roloff, A. Chimia 1985, 39, 19–20. |
| 27. | Kósa, C.; Lukáč, I.; Weiss, R. G. Macromolecules 2000, 33, 4015–4022. doi:10.1021/ma991493z |
| 28. | Mosnáček, J.; Weiss, R. G.; Lukáč, I. Macromolecules 2002, 35, 3870–3875. doi:10.1021/ma0117458 |
| 29. | Mosnáček, J.; Weiss, R. G.; Lukáč, I. Macromolecules 2004, 37, 1304–1311. doi:10.1021/ma030213j |
| 30. | Mosnáček, J.; Lukáč, I.; Chromik, Š.; Kostič, I.; Hrdlovič, P. J. Polym. Sci., Part A: Polym. Chem. 2004, 42, 765–771. doi:10.1002/pola.10860 |
| 31. | Angiolini, L.; Caretti, D.; Salatelli, E. Macromol. Chem. Phys. 2000, 201, 2646–2653. doi:10.1002/1521-3935(20001201)201:18<2646::AID-MACP2646>3.0.CO;2-D |
| 22. | Mosnáček, J.; Lukáč, I. J. Photochem. Photobiol., A 2002, 151, 95–104. doi:10.1016/S1010-6030(02)00024-2 |
| 25. | Husár, B.; Lukáč, I. J. Photochem. Photobiol., A 2008, 195, 191–197. doi:10.1016/j.jphotochem.2007.10.001 |
| 26. | Husár, B.; Commereuc, S.; Chmela, Š.; Verney, V. Polym. Int. 2010, 59, 1563–1570. doi:10.1002/pi.2901 |
| 8. | Ullrich, G.; Herzog, D.; Liska, R.; Burtscher, P.; Moszner, N. J. Polym. Sci., Part A: Polym. Chem. 2004, 42, 4948–4963. doi:10.1002/pola.20318 |
| 32. | Dallacker, F.; Alroggen, I.; Krings, H.; Laurs, B.; Lipp, M. Justus Liebigs Ann. Chem. 1961, 647, 23–36. doi:10.1002/jlac.19616470105 |
| 33. | Dallacker, F.; Ulrichs, K.; Lipp, M. Justus Liebigs Ann. Chem. 1963, 667, 50–55. doi:10.1002/jlac.19636670108 |
| 34. | Połoński, T.; Dauter, Z. J. Chem. Soc., Perkin Trans. 1 1986, 1781–1788. doi:10.1039/P19860001781 |
| 21. | Husár, B.; Lukáč, I. J. Photochem. Photobiol., A 2011, 223, 189–193. doi:10.1016/j.jphotochem.2011.08.017 |
| 23. | Lukáč, I.; Kósa, C. Macromol. Rapid Commun. 1994, 15, 929–934. doi:10.1002/marc.1994.030151204 |
| 24. | Kósa, C.; Lukáč, I.; Weiss, R. G. Macromol. Chem. Phys. 1999, 200, 1080–1085. doi:10.1002/(SICI)1521-3935(19990501)200:5<1080::AID-MACP1080>3.0.CO;2-7 |
| 21. | Husár, B.; Lukáč, I. J. Photochem. Photobiol., A 2011, 223, 189–193. doi:10.1016/j.jphotochem.2011.08.017 |
| 21. | Husár, B.; Lukáč, I. J. Photochem. Photobiol., A 2011, 223, 189–193. doi:10.1016/j.jphotochem.2011.08.017 |
| 22. | Mosnáček, J.; Lukáč, I. J. Photochem. Photobiol., A 2002, 151, 95–104. doi:10.1016/S1010-6030(02)00024-2 |
| 31. | Angiolini, L.; Caretti, D.; Salatelli, E. Macromol. Chem. Phys. 2000, 201, 2646–2653. doi:10.1002/1521-3935(20001201)201:18<2646::AID-MACP2646>3.0.CO;2-D |
| 21. | Husár, B.; Lukáč, I. J. Photochem. Photobiol., A 2011, 223, 189–193. doi:10.1016/j.jphotochem.2011.08.017 |
| 8. | Ullrich, G.; Herzog, D.; Liska, R.; Burtscher, P.; Moszner, N. J. Polym. Sci., Part A: Polym. Chem. 2004, 42, 4948–4963. doi:10.1002/pola.20318 |
| 8. | Ullrich, G.; Herzog, D.; Liska, R.; Burtscher, P.; Moszner, N. J. Polym. Sci., Part A: Polym. Chem. 2004, 42, 4948–4963. doi:10.1002/pola.20318 |
| 33. | Dallacker, F.; Ulrichs, K.; Lipp, M. Justus Liebigs Ann. Chem. 1963, 667, 50–55. doi:10.1002/jlac.19636670108 |
| 21. | Husár, B.; Lukáč, I. J. Photochem. Photobiol., A 2011, 223, 189–193. doi:10.1016/j.jphotochem.2011.08.017 |
| 22. | Mosnáček, J.; Lukáč, I. J. Photochem. Photobiol., A 2002, 151, 95–104. doi:10.1016/S1010-6030(02)00024-2 |
| 21. | Husár, B.; Lukáč, I. J. Photochem. Photobiol., A 2011, 223, 189–193. doi:10.1016/j.jphotochem.2011.08.017 |
| 35. | Sell, T.; Laschat, S.; Dix, I.; Jones, P. G. Eur. J. Org. Chem. 2000, 4119–4124. doi:10.1002/1099-0690(200012)2000:24<4119::AID-EJOC4119>3.0.CO;2-X |
| 31. | Angiolini, L.; Caretti, D.; Salatelli, E. Macromol. Chem. Phys. 2000, 201, 2646–2653. doi:10.1002/1521-3935(20001201)201:18<2646::AID-MACP2646>3.0.CO;2-D |
| 8. | Ullrich, G.; Herzog, D.; Liska, R.; Burtscher, P.; Moszner, N. J. Polym. Sci., Part A: Polym. Chem. 2004, 42, 4948–4963. doi:10.1002/pola.20318 |
| 34. | Połoński, T.; Dauter, Z. J. Chem. Soc., Perkin Trans. 1 1986, 1781–1788. doi:10.1039/P19860001781 |
© 2012 Husár et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)