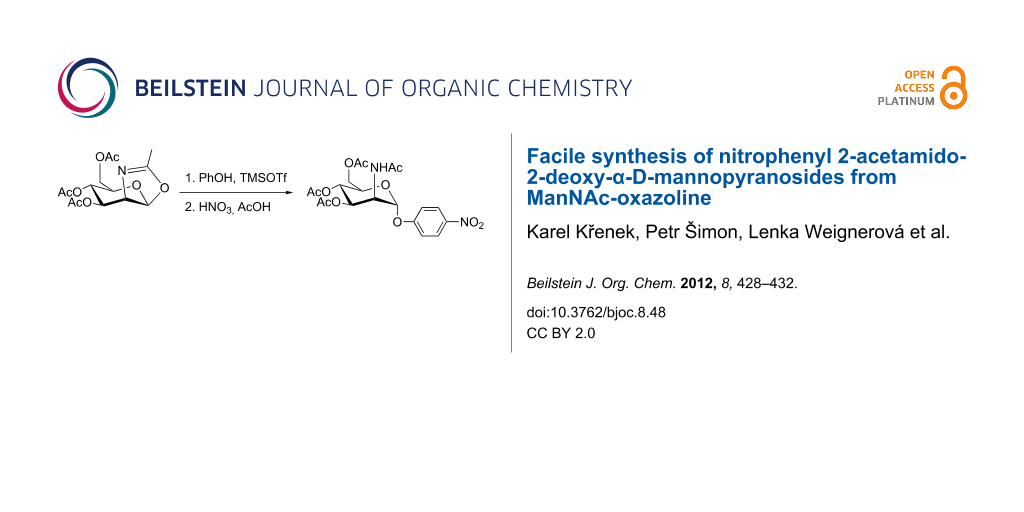
Abstract
The synthetic procedures for a large-scale preparation of o- and p-nitrophenyl 2-acetamido-2-deoxy-α-D-mannopyranoside are described. The synthetic pathway employs the glycosylation of phenol with ManNAc oxazoline, followed by nitration of the aromatic moiety yielding a separable mixture of the o- and p-nitrophenyl derivative in a 2:3 ratio.
Graphical Abstract
Introduction
Hexosamines are fundamental structural elements and precursors of the peptidoglycan and membrane lipopolysaccharide layer as well as of capsular polysaccharides in Gram-negative bacteria. N-Acetyl-D-mannosamine (ManNAc) has been found to be, presumably, the strongest monosaccharidic ligand for the natural killer cells (NK-cells) activating protein NKR-P1 [1], and some ManNAc-containing oligosaccharides (e.g., GlcpNAc-β-(1→4)ManpNAc) have been identified to be strong immunoactivators [2]. Detection of ManNAc by the immune system is probably very important for recognizing bacterial infection, as this carbohydrate is an unambiguous signal of a microbial invader [3]. In the somatic cells (vertebrates) there are no structures composed of ManNAc. This carbohydrate is a precursor of sialic acid(s). The pathogenicity of some bacterial strains occurring in their R-forms (R stands for rough: virulent, containing ManNAc in the capsular structures) and S-forms (S stands for smooth: nonvirulent, lower level of ManNAc in the capsules) is related partly to the content of ManNAc. Thus, ManNAc units play a significant role in bacterial pathogenicity and virulence (e.g., Streptococcus pneumoniae) [4,5]. Surprisingly, glycosidases active upon β-ManpNAc and α-ManpNAc glycosides are not known so far. Therefore, the building blocks for the chemical synthesis of ManNAc-containing compounds, as well as the substrates for the hypothetical α-N-acetylmannopyranosidase, are required.
This paper describes new robust and effective methods for the synthesis of both o-nitrophenyl 2-acetamido-2-deoxy-α-D-mannopyranoside (7) and p-nitrophenyl 2-acetamido-2-deoxy-α-D-mannopyranoside (8) usable as practical chromogenic substrates for the screening of such enzymes.
Results and Discussion
The first reported preparation of p-nitrophenyl 2-acetamido-2-deoxy-α-D-mannopyranoside (pNP-α-ManNAc, 8) was described in [6], starting from 2-acetamido-2-deoxy-D-glucose and providing the product in 2% overall yield. We have previously published [7] a 9-step synthesis of pNP-α-ManNAc (8) from commercially available methyl α-D-glucopyranoside with an overall yield of 1.5%. Later, Popelová et al. [8] published a concise synthesis starting either from D-glucose (5 steps, 1.5% yield) or from methyl 4,6-O-benzylidene-α-D-glucopyranoside (5 steps, 23%). Unfortunately, in our hands this synthetic procedure did not afford the published yields, namely in the triflate and consequent azide substitution steps.
Oxazolines, such as 3, have been used as glycosylation agents in the preparation of glycoproteins [9,10], various alcohol glycosides [11,12], phosphates [13] and oligosaccharides [12]. To the best of our knowledge, glycosylation of phenolic OH with oxazoline has not been accomplished so far. Therefore, we decided to test oxazoline 3 [14] glycosylation in our synthetic approach.
Synthetic pathway
The synthesis (see Scheme 1) was started from commercially available 2-acetamido-2-deoxy-D-mannopyranose (1, ManNAc), which was converted to its peracetate 2 (Ac2O/py/DMAP (cat.), 90%). A crude mixture of peracetylated ManNAc 2 was treated with trifluoromethanesulfonic acid [14] affording oxazoline 3 in 75% yield.
The glycosylation of phenol and p-nitrophenol with oxazoline 3 was extensively tested. We tested a large array of reaction conditions, including variation of catalyst (copper(II) chloride [12,15], 2,2-diphenyl-1-picrylhydrazyl, zinc chloride, tin(IV) chloride) and solvent (CH2Cl2, THF, toluene, benzene), all of which were not successful. Finally, we found that trimethylsilyl trifluoromethanesulfonate in dichloromethane [11] was capable of catalyzing the oxazoline ring opening with phenol to afford α-phenyl glycoside 4 in a reasonable 56% yield. This reaction was also tested with p-nitrophenol; however, the required p-nitrophenyl glycoside 6 was produced under these conditions only in trace amounts (observed by TLC). Our and previously published results [11] indicate that the deactivation of phenol by the electron-withdrawing nitro group substantially decreases its reactivity in the glycosylation reaction with oxazoline and, on the other hand, the presence of electron-donating groups increases the yields.
The resulting phenyl glycoside 4 was treated with a solution of red fuming nitric acid in acetic acid [16], producing a mixture of o-nitrophenyl glycoside 5 (22% yield) and p-nitrophenyl glycoside 6 (34% yield) in approximately 2:3 ratio, which was separated by flash chromatography. Zemplén deacetylation of 4 and 5 afforded the title compounds 7 and 8 in almost quantitative yields.
Conclusion
In conclusion, a simple and robust procedure for the synthesis of o- and p-nitrophenyl 2-acetamido-2-deoxy-α-D-mannopyranoside (7 and 8) from commercially available ManNAc, via its oxazoline, is described affording an overall total yield of both 7 and 8 of over 21%, including the purification steps.
Experimental
General methods
All chemicals were purchased from Sigma-Aldrich, except for 2-acetamido-2-deoxy-D-mannose, which was purchased from BIOSYNTH AG, Staad, CH. The reactions were monitored by TLC with precoated silica gel 60 F254 aluminium sheets from Merck, detected with UV light and/or charred with sulfuric acid (5% in EtOH). The compounds were purified either by column flash chromatography with silica gel 60 (230–240 mesh, Merck) or by gel permeation chromatography with Sephadex LH20 from Sigma Aldrich. The solvents were distilled and dried according to the standard procedures before use.
NMR spectroscopy
NMR spectra were recorded on a Bruker Avance III 400 MHz spectrometer (400.13 MHz for 1H, 100.55 MHz for 13C at 30 °C) and a Bruker Avance III 600 MHz spectrometer (600.23 MHz for 1H, 150.93 MHz for 13C at 30 °C) in CD3OD or CDCl3 (Sigma-Aldrich). Residual signals of the deuterated solvents were used as internal standards (for CD3OD δH 3.330 ppm, δC 49.30 ppm, for CDCl3 δH 7.265 ppm, δC 77.23 ppm). NMR experiments 1H NMR, 13C NMR, gCOSY, gHSQC, and gHMBC were performed using the manufacturer’s software. 1H NMR and 13C NMR spectra were zero filled to fourfold data points and multiplied by a window function before Fourier transformation. A two-parameter double-exponential Lorentz–Gauss function was applied for 1H to improve resolution, and line broadening (1 Hz) was applied to get a better 13C signal-to-noise ratio. Chemical shifts are given on a δ-scale with the digital resolution justifying the reported values to three (δH) or two (δC) decimal places.
The anomeric configuration of manno-structures was determined based on the value of 1J (C-1, H-1) [17], which was measured by using gHMQC or gHSQC with retained direct coupling constants between directly bonded carbon and hydrogen.
Mass spectrometry
Mass spectra were measured on MALDI–TOF/TOF ultraFLEX III mass spectrometers (Bruker-Daltonics). Positive spectra were calibrated externally by using the monoisotopic [M + H]+ ions of PepMixII calibrant (Bruker-Daltonics). For the MALDI experiment 0.4 μL of sample dissolved in 50% acetonitrile was allowed to dry at ambient temperature on the target and overlaid with matrix solution (either 2,5-dihydroxybenzoic acid, DHB or α-cyano-4-hydroxycinnamic acid, CCA). The spectra were collected in reflectron mode.
Phenyl 2-acetamido-2-deoxy-3,4,6-tri-O-acetyl-α-D-mannopyranoside (4): 2-Methyl-(3,4,6-tri-O-acetyl-1,2-dideoxy-β-D-mannopyrano)-[2,1:4’,5’]-2-oxazoline [18] 3 (280 mg, 0.85 mmol) was dissolved in 10 mL of dry dichloromethane. To this solution, phenol (250 mg, 2.66 mmol, 3 equiv) and trimethylsilyl trifluoromethanesulfonate (0.2 mL, 0.73 mmol, 1 equiv) were added. The reaction mixture was stirred at room temperature for 3 days (monitored by TLC on silica gel, chloroform/acetone 8:1). The reaction mixture was evaporated to dryness in vacuo. The product was isolated by column chromatography (silica gel, chloroform/acetone 20:1) as a white solid (200 mg, 56%). 1H NMR (400 MHz, CDCl3) δ 2.015 (s, 3H, 6-Ac), 2.022 (s, 3H, 3-Ac), 2.048 (s, 3H, 4-Ac), 2.080 (s, 3H, 2-Ac), 4.022 (dd, J = 2.4, 12.2 Hz, 1H, H-6u), 4.104 (ddd, J = 2.4, 5.6, 10.2 Hz, 1H, H-5), 4.265 (dd, J = 5.6, 12.2 Hz, 1H, H-6d), 4.821 (ddd, J = 1.6, 4.7, 8.9 Hz, 1H, H-2), 5.168 (dd, J = 10.2, 10.2 Hz, 1H, H-4), 5.475 (d, J = 1.6 Hz, 1H, H-1), 5.564 (dd, J = 4.7, 10.2 Hz, 1H, H-3), 5.919 (d, J = 8.9 Hz, 1H, 2-NH), 7.047 (m, 1H, H-para), 7.075 (m, 2H, H-ortho), 7.291 (m, 2H, H-meta); 13C NMR (100 MHz, CDCl3) δ 20.60, 20.70 (3 x q, 3-Ac, 4-Ac, 6-Ac), 23.29 (q, 2-Ac), 50.51 (d, C-2), 62.14 (t, C-6), 65.99 (d, C-4), 68.67 (d, C-5), 68.89 (d, C-3), 97.12 (d, C-1), 116.49 (d, C-ortho), 122.99 (d, C-para), 129.56 (d, C-meta), 155.60 (s, C-ipso), 169.82 (s, 3-CO), 169.94 (s, 4-CO), 170.15 (s, 2-CO), 170.40 (s, 6-CO).
o-Nitrophenyl 2-acetamido-2-deoxy-3,4,6-tri-O-acetyl-α-D-mannopyranoside (5) and p-nitrophenyl 2-acetamido-2-deoxy-3,4,6-tri-O-acetyl-α-D-mannopyranoside (6): Compound 4 (1.1 g, 2.59 mmol) was dissolved in a mixture of acetic anhydride (10 mL) and glacial acetic acid (2 mL). The solution was cooled to 0 °C in an ice bath, and red fuming nitric acid (≥99.5%, 0.5 mL) was added in one portion under stirring. The reaction mixture was stirred overnight, allowing the ice bath to melt and warm up to ambient temperature. The reaction mixture was diluted by an ice–water mixture (50 mL), stirred for an additional 30 min, and neutralized by the saturated aqueous solution of NaHCO3. After extraction with methylene dichloride (3 × 50 mL), the combined organic layers were washed with 50 mL of sodium bicarbonate solution and 50 mL of water. After drying (Na2SO4) the organic phase was evaporated and chromatographed (silica gel, chloroform/acetone 5:1). o-Nitrophenyl derivative 5 was isolated as a white solid (270 mg, 22%) and p-nitrophenyl derivative 6 was isolated as a white solid (410 mg, 34%).
(5) 1H NMR (400 MHz, CDCl3) δ 2.032 (s, 3H, 3-Ac), 2.039 (s, 3H, 6-Ac), 2.080 (s, 3H, 4-Ac), 2.104 (s, 3H, 2-Ac), 4.042 (dd, J = 2.2, 12.3 Hz, 1H, H-6u), 4.212 (ddd, J = 2.2, 5.2, 10.2 Hz, 1H, H-5), 4.286 (dd, J = 5.2, 12.3 Hz, 1H, H-6d), 4.812 (ddd, J = 1.8, 4.8, 7,7 Hz, 1H, H-2), 5.213 (dd, J = 10.2, 10.2 Hz, 1H, H-4), 5.593 (dd, J = 4.8, 10.2 Hz, 1H, H-3), 5.750 (d, J = 1.8 Hz, 1H, H-1), 5.909 (d, J = 7.7 Hz, 1H, 2-NH), 7.170 (ddd, J = 1.2, 7.4, 8.2 Hz, 1H, H-4´), 7.329 (dd, J = 1.2, 8.5 Hz, 1H, H-6´), 7.544 (ddd, J = 1.7, 7.4, 8.5 Hz, 1H, H-5´), 7.905 (dd, J = 1.7, 8.2 Hz, 1H, H-3´); 13C NMR (100 MHz, CDCl3) δ 20.62, 20.63, 20.65 (3 x q, 3-Ac, 4-Ac, 6-Ac), 23.28 (q, 2-Ac), 50.68 (d, C-2), 61.97 (t, C-6), 65.52 (d, C-4), 68.33 (d, C-3), 69.68 (d, C-5), 97.34 (d, C-1), 117.24 (d, C-6´), 122.82 (d, C-4´), 125.88 (d, C-3´), 134.09 (d, C-5´), 140.58 (s, C-2´), 148.66 (s, C-1´), 169.20 (s, 3-CO), 170.07 (s, 4-CO), 170.32 (s, 6-CO), 170.47 (s, 2-CO).
(6) 1H NMR (400 MHz, CDCl3) δ 2.028 (s, 3H, 6-Ac), 2.047 (s, 3H, 3-Ac), 2.065 (s, 3H, 4-Ac), 2.109 (s, 3H, 2-Ac), 4.02* (m, 1H, H-5), 4.03* (m, 1H, H-6u), 4.273 (dd, J = 5.6, 12.4 Hz, 1H, H-6d), 4.818 (ddd, J = 1.6, 4.8, 8.4 Hz, 1H, H-2), 5.196 (dd, J = 10.2, 10.3 Hz, 1H, H-4), 5.555 (dd, J = 4.8, 10.3 Hz, 1H, H-3), 5.629 (d, J = 1.6 Hz, 1H, H-1), 5.905 (d, J = 8.4 Hz, 1H, 2-NH), 7.205 (m, 2H, H-ortho), 8.225 (m, 2H, H-meta) (* = HSQC readout); 13C NMR (100 MHz, CDCl3) δ 20.59, 20.62, 20.67 (3 x q, 3-Ac, 4-Ac, 6-Ac), 23.28 (q, 2-Ac), 50.42 (d, C-2), 61.94 (t, C-6), 65.58 (d, C-4), 68.35 (d, C-3), 69.31 (d, C-5), 96.89 (d, C-1), 116.47 (d, C-ortho), 125.82 (d, C-meta), 143.19 (s, C-para), 160.22 (s, C-ipso), 169.68 (s, 3-CO), 169.86 (s, 4-CO), 170.26 (s, 6-CO), 170.36 (s, 2-CO).
o-Nitrophenyl 2-acetamido-2-deoxy-α-D-mannopyranoside (7): Compound 5 (270 mg, 0.58 mmol) was dissolved in dry methanol (2 mL) and three drops of NaOMe in MeOH (35%, w/w) were added. The reaction mixture was stirred at ambient temperature for 20 minutes. The solution was directly loaded onto the gel permeation chromatography column (Sephadex LH-20) with methanol as a mobile phase (2 mL/min, UV detection 254 nm). The product was isolated as a white solid (197 mg, 99%). 1H NMR (400 MHz, CD3OD) δ 2.070 (s, 3H, 2-Ac), 3.660 (ddd, J = 2.3, 4.5, 10.0 Hz, 1H, H-5), 3.761 (dd, J = 2.5, 12.0 Hz, 1H, H-6u), 3.756 (dd, J = 9.7, 9.9 Hz, 1H, H-4), 3.824 (dd, J = 4.5, 12.0 Hz, 1H, H-6d), 4.183 (dd, J = 4.9, 9.7 Hz, 1H, H-3), 4.541 (dd, J = 1.7, 4.9 Hz, 1H, H-2), 5.689 (d, J = 1.7 Hz, 1H, H-1), 7.188 (ddd, J = 1.2, 7.4, 8.1 Hz, 1H, H-4´), 7.515 (dd, J = 1.2, 8.5 Hz, 1H, H-6´), 7.612 (ddd, J = 1.7, 7.4, 8.5 Hz, 1H, H-5´), 7.854 (dd, J = 1.7, 8.1 Hz, 1H, H-3´); 13C NMR (100 MHz, CD3OD) δ 22.93 (q, 2-Ac), 54.36 (d, C-2), 62.23 (t, C-6), 68.14 (d, C-4), 70.43 (d, C-3), 76.04 (d, C-5), 99.65 (d, C-1), 118.74 (d, C-6´), 123.58 (d, C-4´), 126.51 (d, C-3´), 135.52 (d, C-5´), 142.17 (s, C-2´), 150.50 (s, C-1´), 174.61 (s, 2-CO); MS–MALDI–TOF (m/z): 365.1 [M + Na]+ (DHB), 365.1 [M + Na]+ (CCA).
p-Nitrophenyl 2-acetamido-2-deoxy-α-D-mannopyranoside (8): Compound 6 (400 mg, 0.85 mmol) was deacetylated and purified as described for compound 7 yielding 8 as a white solid (290 mg, 99%). 1H NMR (400 MHz, CD3OD) δ 2.078 (s, 2-Ac3H, ), 3.562 (ddd, J = 2.4, 4.7, 9.9 Hz, 1H, H-5), 3.746 (dd, J = 2.4, 12.0 Hz, 1H, H-6u), 3.752 (dd, J = 9.7, 9.9 Hz, 1H, H-4), 3.812 (dd, J = 4.7, 12.0 Hz, 1H, H-6d), 4.164 (dd, J = 4.9, 9.7 Hz, 1H, H-3), 4.544 (dd, J = 1.7, 4.9 Hz, 1H, H-2), 5.657 (d, J = 1.7 Hz, 1H, H-1), 7.300 (m, 2H, H-ortho), 8.230 (m, 2H, H-meta). 13C NMR (100 MHz, CD3OD) δ 22.93 (q, 2-Ac), 54.28 (d, C-2), 62.23 (t, C-6), 68.20 (d, C-4), 70.51 (d, C-3), 75.79 (d, C-5), 99.09 (d, C-1), 118.05 (d, C-ortho), 127.00 (d, C-meta), 144.21 (s, C-para), 162.80 (s, C-ipso), 174.66 (s, 2-CO); MS–MALDI–TOF (m/z): 365.1 [M + Na]+ (DHB), 365.1 [M + Na]+ (CCA).
Phenyl 2-acetamido-2-deoxy-α-D-mannopyranoside (9): Compound 4 (50 mg, 0.12 mmol) was deacetylated and purified as described for compound 7, yielding 9 as a white solid (35 mg, 99%). 1H NMR (400 MHz, CD3OD) δ 2.067 (s, 3H, 2-Ac), 3.654 (m, 1H, H-5), 3.73* (m, 1H, H-6u), 3.75* (m, 1H, H-4), 3.838 (dd, J = 4.2, 11.9 Hz, 1H, H-6d), 4.178 (dd, J = 4.8, 9.6 Hz, 1H, H-3), 4.525 (dd, J = 1.7, 4.8 Hz, 1H, H-2), 5.466 (d, J = 1.7 Hz, 1H, H-1), 7.023 (m, 1H, H-para), 7.110 (m, 2H, H-ortho), 7.298 (m, 2H, H-meta) (* - HSQC readouts); 13C NMR (100 MHz, CD3OD) δ 22.95 (q, 2-Ac), 54.64 (d, C-2), 62.22 (t, C-6), 68.35 (d, C-4), 70.81 (d, C-3), 75.06 (d, C-5), 99.22 (d, C-1), 118.00 (d, C-ortho), 123.79 (d, C-para), 130.83 (d, C-meta), 157.95 (s, C-ipso), 174.53 (s, 2-CO); MS–MALDI–TOF (m/z): 320.1 [M + Na]+ (DHB), 320.1 [M + Na]+ (CCA).
References
-
Krist, P.; Herkommerová-Rajnochová, E.; Rauvolfová, J.; Semeňuk, T.; Vavrušková, P.; Pavlíček, J.; Bezouška, K.; Petruš, L.; Křen, V. Biochem. Biophys. Res. Commun. 2001, 287, 11–20. doi:10.1006/bbrc.2001.5537
Return to citation in text: [1] -
Sedmera, P.; Přikrylová, V.; Bezouška, K.; Rajnochová, E.; Thiem, J.; Křen, V. J. Carbohydr. Chem. 1998, 17, 1351–1357. doi:10.1080/07328309808002358
Return to citation in text: [1] -
Attolino, E.; Bonaccorsi, F.; Catelani, G.; D'Andrea, F.; Křenek, K.; Bezouška, K.; Křen, V. J. Carbohydr. Chem. 2008, 27, 156–171. doi:10.1080/07328300802030845
Return to citation in text: [1] -
Lee, C. J.; Fraser, B. A. J. Biol. Chem. 1980, 255, 6847–6853.
Return to citation in text: [1] -
Lee, C. J.; Banks, S. D.; Li, J. P. Crit. Rev. Microbiol. 1991, 18, 89–114. doi:10.3109/10408419109113510
Return to citation in text: [1] -
Yoshimura, J.; Sakai, H.; Oda, N.; Hashimoto, H. Bull. Chem. Soc. Jpn. 1972, 45, 2027–2031. doi:10.1246/bcsj.45.2027
Return to citation in text: [1] -
Krist, P.; Kuzma, M.; Pelyvás, I. F.; Simerská, P.; Křen, V. Collect. Czech. Chem. Commun. 2003, 68, 801–811. doi:10.1135/cccc20030801
Return to citation in text: [1] -
Popelová, A.; Kefurt, K.; Hlaváčková, M.; Moravcová, J. Carbohydr. Res. 2005, 340, 161–166. doi:10.1016/j.carres.2004.11.002
Return to citation in text: [1] -
Rich, J. R.; Withers, S. G. Nat. Chem. Biol. 2009, 5, 206–215. doi:10.1038/nchembio.148
Return to citation in text: [1] -
Hollósi, M.; Kollát, E.; Laczkó, I.; Medzihradsky, K. F.; Thurin, J.; Otvös, L., Jr. Tetrahedron Lett. 1991, 32, 1531–1534. doi:10.1016/S0040-4039(00)74264-X
Return to citation in text: [1] -
Lopin, C.; Jacquinet, J.-C. Angew. Chem., Int. Ed. 2006, 45, 2574–2578. doi:10.1002/anie.200503551
Return to citation in text: [1] [2] [3] -
Wittmann, V.; Lennartz, D. Eur. J. Org. Chem. 2002, 1363–1367. doi:10.1002/1099-0690(200204)2002:8<1363::AID-EJOC1363>3.0.CO;2-#
Return to citation in text: [1] [2] [3] -
Heidlas, J. E.; Lees, W. J.; Pale, P.; Whitesides, G. M. J. Org. Chem. 1992, 57, 146–151. doi:10.1021/jo00027a028
Return to citation in text: [1] -
Freese, S. J.; Vann, W. F. Carbohydr. Res. 1996, 281, 313–319. doi:10.1016/0008-6215(95)00345-2
Return to citation in text: [1] [2] -
Subramanian, V.; Moume-Pymbock, M.; Hu, T.; Crich, D. J. Org. Chem. 2011, 76, 3691–3709. doi:10.1021/jo102411j
Return to citation in text: [1] -
Weissmann, B. J. Org. Chem. 1966, 31, 2505–2509. doi:10.1021/jo01346a018
Return to citation in text: [1] -
Bock, K.; Pedersen, C. J. Chem. Soc., Perkin Trans. 2 1974, 293–297. doi:10.1039/P29740000293
Return to citation in text: [1] -
Yamayaki, T.; Warden, C. H.; Herscovics, A.; Jeanloz, R. W. Carbohydr. Res. 1980, 79, C9–C12. doi:10.1016/S0008-6215(00)85143-5
Return to citation in text: [1]
| 18. | Yamayaki, T.; Warden, C. H.; Herscovics, A.; Jeanloz, R. W. Carbohydr. Res. 1980, 79, C9–C12. doi:10.1016/S0008-6215(00)85143-5 |
| 17. | Bock, K.; Pedersen, C. J. Chem. Soc., Perkin Trans. 2 1974, 293–297. doi:10.1039/P29740000293 |
| 1. | Krist, P.; Herkommerová-Rajnochová, E.; Rauvolfová, J.; Semeňuk, T.; Vavrušková, P.; Pavlíček, J.; Bezouška, K.; Petruš, L.; Křen, V. Biochem. Biophys. Res. Commun. 2001, 287, 11–20. doi:10.1006/bbrc.2001.5537 |
| 6. | Yoshimura, J.; Sakai, H.; Oda, N.; Hashimoto, H. Bull. Chem. Soc. Jpn. 1972, 45, 2027–2031. doi:10.1246/bcsj.45.2027 |
| 11. | Lopin, C.; Jacquinet, J.-C. Angew. Chem., Int. Ed. 2006, 45, 2574–2578. doi:10.1002/anie.200503551 |
| 4. | Lee, C. J.; Fraser, B. A. J. Biol. Chem. 1980, 255, 6847–6853. |
| 5. | Lee, C. J.; Banks, S. D.; Li, J. P. Crit. Rev. Microbiol. 1991, 18, 89–114. doi:10.3109/10408419109113510 |
| 11. | Lopin, C.; Jacquinet, J.-C. Angew. Chem., Int. Ed. 2006, 45, 2574–2578. doi:10.1002/anie.200503551 |
| 3. | Attolino, E.; Bonaccorsi, F.; Catelani, G.; D'Andrea, F.; Křenek, K.; Bezouška, K.; Křen, V. J. Carbohydr. Chem. 2008, 27, 156–171. doi:10.1080/07328300802030845 |
| 14. | Freese, S. J.; Vann, W. F. Carbohydr. Res. 1996, 281, 313–319. doi:10.1016/0008-6215(95)00345-2 |
| 2. | Sedmera, P.; Přikrylová, V.; Bezouška, K.; Rajnochová, E.; Thiem, J.; Křen, V. J. Carbohydr. Chem. 1998, 17, 1351–1357. doi:10.1080/07328309808002358 |
| 12. | Wittmann, V.; Lennartz, D. Eur. J. Org. Chem. 2002, 1363–1367. doi:10.1002/1099-0690(200204)2002:8<1363::AID-EJOC1363>3.0.CO;2-# |
| 15. | Subramanian, V.; Moume-Pymbock, M.; Hu, T.; Crich, D. J. Org. Chem. 2011, 76, 3691–3709. doi:10.1021/jo102411j |
| 11. | Lopin, C.; Jacquinet, J.-C. Angew. Chem., Int. Ed. 2006, 45, 2574–2578. doi:10.1002/anie.200503551 |
| 12. | Wittmann, V.; Lennartz, D. Eur. J. Org. Chem. 2002, 1363–1367. doi:10.1002/1099-0690(200204)2002:8<1363::AID-EJOC1363>3.0.CO;2-# |
| 12. | Wittmann, V.; Lennartz, D. Eur. J. Org. Chem. 2002, 1363–1367. doi:10.1002/1099-0690(200204)2002:8<1363::AID-EJOC1363>3.0.CO;2-# |
| 9. | Rich, J. R.; Withers, S. G. Nat. Chem. Biol. 2009, 5, 206–215. doi:10.1038/nchembio.148 |
| 10. | Hollósi, M.; Kollát, E.; Laczkó, I.; Medzihradsky, K. F.; Thurin, J.; Otvös, L., Jr. Tetrahedron Lett. 1991, 32, 1531–1534. doi:10.1016/S0040-4039(00)74264-X |
| 14. | Freese, S. J.; Vann, W. F. Carbohydr. Res. 1996, 281, 313–319. doi:10.1016/0008-6215(95)00345-2 |
| 8. | Popelová, A.; Kefurt, K.; Hlaváčková, M.; Moravcová, J. Carbohydr. Res. 2005, 340, 161–166. doi:10.1016/j.carres.2004.11.002 |
| 7. | Krist, P.; Kuzma, M.; Pelyvás, I. F.; Simerská, P.; Křen, V. Collect. Czech. Chem. Commun. 2003, 68, 801–811. doi:10.1135/cccc20030801 |
| 13. | Heidlas, J. E.; Lees, W. J.; Pale, P.; Whitesides, G. M. J. Org. Chem. 1992, 57, 146–151. doi:10.1021/jo00027a028 |
© 2012 Křenek et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)