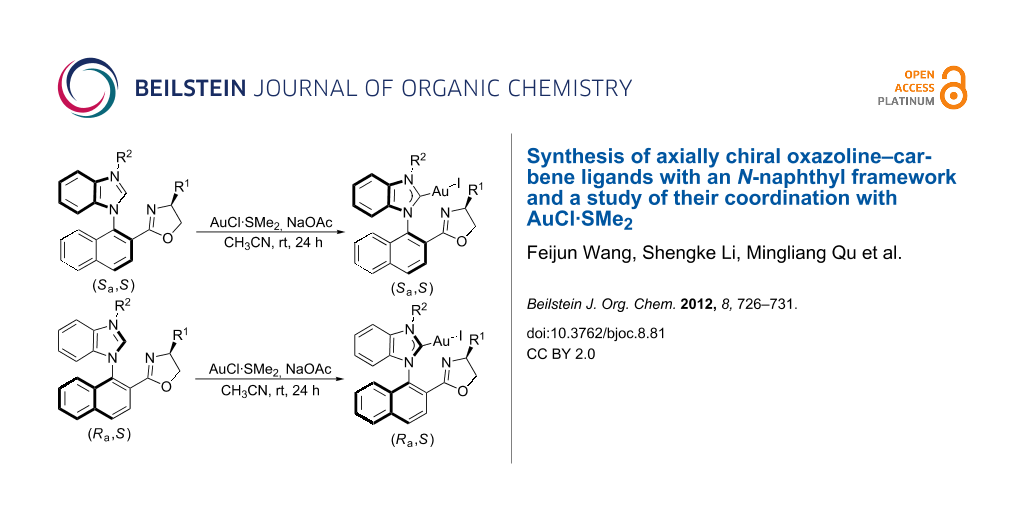
Abstract
Axially chiral oxazoline–carbene ligands with an N-naphthyl framework were successfully prepared, and their coordination behavior with AuCl·SMe2 was also investigated, affording the corresponding Au(I) complexes in moderate to high yields.
Graphical Abstract
Introduction
During the past decade, with an explosive growth of asymmetric homogeneous gold catalysis in C–C, C–O, or C–N bond formations, the design and synthesis of chiral gold complexes has received wide attention. Compared with the more commonly used air-sensitive phosphine ligand, N-heterocyclic carbenes (NHCs), with intrinsic characteristics such as strong δ-donor but poor π-acceptor abilities, ease of preparation, air and thermal stability of their metal complexes, and the convenient introduction of chiral elements, have also emerged as effective ligands for a number of homogeneous gold catalyzes [1-8]. However, during our ongoing survey of chiral NHC–Au(I) complexes in the literature, we only found a few unique papers of relevance. Tomioka and co-workers disclosed the first chiral NHC–Au(I) complex 1 (Figure 1), which was applied to catalyze the asymmetric cyclization of 1,6-enynes giving the corresponding cyclopentane derivatives with moderate enantioselectivity up to 59% [9,10]. Iglesias and co-workers reported a type of NHC–Au(I) complexes 2 containing C2-symmetric bis(NHC)-ligands with two imidazolin-2-ylidene moieties on a chiral dioxolane backbone, produced in up to 95% ee by hydrogenation of a prochiral alkene [11]. Recently, Toste and co-workers reported a novel family of axially chiral (acyclic diaminocarbene) gold(I) complexes 3 derived from 3,3′-substituted 1,1′-binaphthalenyl-2,2′-diamine, and their application in the dynamic kinetic asymmetric transformation of propargyl esters, giving the corresponding substituted chromenes in up to 99% ee [12]. Our group also developed a new family of axially chiral NHC–Au(I) complexes (4–6) with a binaphthyl or biphenyl framework [13,14]. These Au(I) complexes were applied to catalyze the asymmetric cyclization of 1,6-enynes or allene in up to 70% ee, and the asymmetric intramolecular hydroamination of allene in up to 44% ee.
We previously reported a novel type of axially chiral ligand 7 with an N-naphthyl framework (Figure 2) instead of traditional binaphthyl framework [15]. Their palladium complexes 8 showed high stereoselectivities in asymmetric allylic arylations to achieve the kinetic resolution of Morita–Baylis–Hillman adducts, affording up to 99% ee of the (E)-allylation products and 92% ee of the recovered Morita–Baylis–Hillman adducts. These intriguing results stimulated us to further develop the axially chiral oxazoline–carbene ligands 7 with an N-naphthyl framework and to evaluate their coordination with AuCl·SMe2.
Figure 2: Axially chiral oxazoline–carbene ligands and their palladium complexes.
Figure 2: Axially chiral oxazoline–carbene ligands and their palladium complexes.
Results and Discussion
Synthesis of axially chiral ligands
Initially, we attempted to synthesize the desired axially chiral ligands 7, and the synthetic route is shown in Scheme 1. Using methyl 1-hydroxy-2-naphthoate (9) as the starting material, trifluoromethylation with Tf2O in the presence of pyridine afforded its trifluoromethanesulfonate 10 in 95% yield, which was made to react with 2-nitroaniline in the presence of Pd(OAc)2/DPE-phos catalytic system to give the corresponding coupling compound 11 in 98% yield (Scheme 1). Subsequent reduction of compound 11 with Pd/C under a hydrogen atmosphere produced the desired compound 12 in 99% yield. Cyclization of 12 in the presence of triethyl orthoformate and a catalytic amount of TsOH at 80 °C gave the corresponding benzimidazole derivative 13 in 76% yield, which was further treated with (S)-2-amino-2-phenylethanol in the presence of Cs2CO3 in toluene to afford the corresponding amide 14a as a mixture of diastereomeric isomers successfully in 91% yield. To our delight, the two diastereomeric isomers with a chiral N-naphthyl axis, (Sa,S)-15a and (Ra,S)-15a, were synthesized from amide 14a according to the classical synthetic method for the preparation of an oxazoline ring, and were easily isolated by silica gel column chromatography in 37% yield and 44% yield, respectively. Similarly, using L-valinol, (Sa,S)-15b and (Ra,S)-15b could also be synthesized. Quaternization of the benzimidazole ring of (Sa,S)-15 and (Ra,S)-15 with R2I (R2 = Me, Et) gave the corresponding benzimidazolium salts 7, respectively.
Scheme 1: Synthesis of axially chiral benzimidazole derivatives.
Scheme 1: Synthesis of axially chiral benzimidazole derivatives.
Coordination study with AuCl·SMe2
The coordination behavior of ligand 7 with Pd(OAc)2 has been disclosed in previous work. However, only (Sa,S)-7 could give the corresponding Pd-complex (Sa,S)-8, while the Pd(II)-complex with R-geometry of the chiral N-naphthyl axis could not be obtained from (Ra,S)-7. With the NHC precursors 7 in hand, their coordination with AuCl·SMe2 in the presence of NaOAc in acetonitrile was further examined. Au(I)-complexes were isolated by flash column chromatography. Comparing the chemical shifts of protons on the oxazoline ring of the Au(I)-complex (Sa,S)-16aa (R1 = Ph, R2 = Me) in the 1H NMR spectrum (Figure 3B) with those of the NHC precursor (Sa,S)-7aa (Figure 3A), we found that the chemical shifts of these protons did not change much, suggesting that the chiral oxazoline group may not coordinate with the Au atom. However, according to the analysis of the 1H NMR spectrum of the Pd(II)-complex (Sa,S)-8 (R2 = Me) (Figure 3C), the chemical shifts of the protons of the coordinated oxazoline group changed significantly. In order to confirm this hypothesis, complex (Sa,S)-16aa was recrystallized from CH2Cl2/petroleum ether (1/3, v/v), and its structure was established by single-crystal X-ray diffraction studies (Figure 4; Supporting Information File 2). The crystal data of Au(1)–C(7) (1.991(5) Å) is a typical Au–CNHC bond length, in-line with those of other reported examples [16-21]. Furthermore, the angle of I(1)–Au(1)–C(7) = 173.12(16)° suggests a nearly linear coordination geometry around the gold(I) center, which is also a typical feature for known gold(I) complexes. Moreover, no coordinated oxazoline group in the complexes (Sa,S)-16 and (Ra,S)-16 could be further coordinated with other metal atoms such as Pd and Cu to furnish dual-metal catalysts, which is a hot research field in asymmetric catalysis [22-24].
Figure 3: The coordination study of the NHC precursor (Sa,S)-7aa with metal salts by 1H NMR analysis of the chemical shifts of protons on the oxazoline ring. (A) The 1H NMR spectrum of NHC precursor (Sa,S)-7aa. (B) The 1H NMR spectrum of Au(I)-complex (Sa,S)-16aa. (C) The 1H NMR spectrum of Pd(II)-complex (Sa,S)-8 (R2 = Me).
Figure 3: The coordination study of the NHC precursor (Sa,S)-7aa with metal salts by 1H NMR analysis of the c...
![[1860-5397-8-81-4]](/bjoc/content/figures/1860-5397-8-81-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Solid-state molecular structure of (Sa,S)-16aa with thermal ellipsoids at the 30% probability level. Selected bond distances (Å) and angles (°): Au1–I1 2.5260(5), Au1–C7 1.991(5), C7–N1 1.334(7), C7–N2 1.357(7), N2–C14 1.427(6), I1–Au1–C7 173.12(16), Au1–C7–N2 124.5(4), C7–N2–C14–C15 105.8(6).
Figure 4: Solid-state molecular structure of (Sa,S)-16aa with thermal ellipsoids at the 30% probability level...
Au(I)-complexes (Sa,S)-16 with different N-substituents or groups on the oxazoline ring were synthesized from the corresponding NHC precursors 7. The yields are summarized in Table 1. It was found that the geometry the of chiral N-naphthyl axis had a significant influence on the yields of the Au(I)-complexes. The salts (Sa,S)-7 with an S-geometry of the chiral N-naphthyl axis gave higher yields of the corresponding Au(I)-complexes than did the salts (Ra,S)-7, presumably due to the steric repulsion of the phenyl group on the oxazoline ring [25-29]. For example, salt (Sa,S)-7aa gave the corresponding complex (Sa,S)-16aa in up to 95% yield (Table 1, entry 1), while salt (Ra,S)-7aa gave the corresponding complex (Ra,S)-16aa in only 50% yield (Table 1, entry 2).
Table 1: The coordination study with AuCl·SMe2.
|
|
|||||
| entry | compound 15 | R2I | salt 7 | Au(I)-complex | yield (%)a |
|---|---|---|---|---|---|
| 1 | (Sa,S)-15a (R1 = Ph) | MeI | (Sa,S)-7aa | (Sa,S)-16aa | 95 |
| 2 | (Ra,S)-15a (R1 = Ph) | MeI | (Ra,S)-7aa | (Ra,S)-16aa | 50 |
| 3 | (Sa,S)-15a (R1 = Ph) | EtI | (Sa,S)-7ab | (Sa,S)-16ab | 89 |
| 4 | (Ra,S)-15a (R1 = Ph) | EtI | (Ra,S)-7ab | (Ra,S)-16ab | 48 |
| 5 | (Sa,S)-15b (R1 = iPr) | MeI | (Sa,S)-7ba | (Sa,S)-16ba | 90 |
| 6 | (Ra,S)-15b (R1 = iPr) | MeI | (Ra,S)-7ba | (Ra,S)-16ba | 56 |
aisolated yield.
Conclusion
NHC-oxazoline bidentate ligands with an axially chiral N-naphthyl framework were synthesized, and their coordination manners with AuCl·SMe2 were investigated. Diastereomeric NHC precursors 7, (Sa,S)-7 and (Ra,S)-7, gave the corresponding axially chiral NHC–Au complexes bearing a noncoordinated oxazoline group. However, while the ligands (Sa,S)-7 gave their corresponding Au complexes (Sa,S)-16 in excellent yields, only modest yields of complexes (Ra,S)-16 were obtained for ligands (Ra,S)-7. Further studies focusing on the preparation of conformationally stable and enantiomerically pure transition-metal catalysts with an axially chiral N-aryl framework are currently in progress, and their applications in asymmetric catalysis are also undergoing.
Supporting Information
| Supporting Information File 1: Experimental procedures and characterization data of compounds. | ||
| Format: PDF | Size: 845.5 KB | Download |
| Supporting Information File 2:
Crystallographic data of (Sa,S)-16aa. These data (CCDC 785035) can be obtained free of charge from the Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif.
Crystal structure data for NHC–Au(I) complex 16aa. |
||
| Format: CIF | Size: 31.3 KB | Download |
Acknowledgements
Financial support from the National Natural Science Foundation of China (21072206, 20902019, 20472096, 20872162, 20672127, 20821002 and 20732008), the National Basic Research Program of China (973)-2010CB833302, and the Fundamental Research Funds for the Central Universities (WJ1014034 and WK1013004) is gratefully acknowledged.
References
-
Bonati, F.; Burini, A.; Pietroni, B. R.; Bovio, B. J. J. Organomet. Chem. 1989, 375, 147–160. doi:10.1016/0022-328X(89)85094-6
Return to citation in text: [1] -
Nieto-Oberhuber, C.; López, S.; Echavarren, A. M. J. Am. Chem. Soc. 2005, 127, 6178–6179. doi:10.1021/ja042257t
Return to citation in text: [1] -
Gourlaouen, C.; Marion, N.; Nolan, S. P.; Maseras, F. Org. Lett. 2009, 11, 81–84. doi:10.1021/ol802430m
Return to citation in text: [1] -
Marion, N.; Gealageas, R.; Nolan, S. P. Org. Lett. 2007, 9, 2653–2656. doi:10.1021/ol070843w
Return to citation in text: [1] -
Fructos, M. R.; Belderrain, T. R.; de Frémont, P.; Scott, N. M.; Nolan, S. P.; Díaz-Requejo, M. M.; Pérez, P. J. Angew. Chem., Int. Ed. 2005, 44, 5284–5288. doi:10.1002/anie.200501056
Return to citation in text: [1] -
Marion, N.; Carlqvist, P.; Gealageas, R.; de Frémont, P.; Maseras, F.; Nolan, S. P. Chem.–Eur. J. 2007, 13, 6437–6451. doi:10.1002/chem.200700134
Return to citation in text: [1] -
Gorske, B. C.; Mbofana, C. T.; Miller, S. J. Org. Lett. 2009, 11, 4318–4321. doi:10.1021/ol9016782
Return to citation in text: [1] -
Wang, F.; Liu, L.; Wang, W.; Li, S.; Shi, M. Coord. Chem. Rev. 2011, 256, 804–853. doi:10.1016/j.ccr.2011.11.013
Return to citation in text: [1] -
Matsumoto, Y.; Selim, K. B.; Nakanishi, H.; Yamada, K.; Yamamoto, Y.; Tomioka, K. Tetrahedron Lett. 2010, 51, 404–406. doi:10.1016/j.tetlet.2009.11.039
Return to citation in text: [1] -
Matsumoto, Y.; Yamada, K.; Tomioka, K. J. Org. Chem. 2008, 73, 4578–4581. doi:10.1021/jo800613h
Return to citation in text: [1] -
Arnanz, A.; González-Arellano, C.; Juan, A.; Villaverde, G.; Corma, A.; Iglesias, M.; Sánchez, F. Chem. Commun. 2010, 46, 3001–3003. doi:10.1039/b922534j
Return to citation in text: [1] -
Wang, Y.-M.; Kuzniewski, C. N.; Rauniyar, V.; Hoong, C.; Toste, F. D. J. Am. Chem. Soc. 2011, 133, 12972–12975. doi:10.1021/ja205068j
Return to citation in text: [1] -
Wang, W.; Yang, J.; Wang, F.; Shi, M. Organometallics 2011, 30, 3859–3869. doi:10.1021/om2004404
Return to citation in text: [1] -
Liu, L.; Wang, F.; Wang, W.; Zhao, M.; Shi, M. Beilstein J. Org. Chem. 2011, 7, 555–564. doi:10.3762/bjoc.7.64
Return to citation in text: [1] -
Wang, F.; Li, S.; Qu, M.; Zhao, M. X.; Liu, L.; Shi, M. Chem. Commun. 2011, 47, 12813–12815. doi:10.1039/c1cc15543a
Return to citation in text: [1] -
Marion, N.; Nolan, S. P. Chem. Soc. Rev. 2008, 37, 1776–1782. doi:10.1039/b711132k
Return to citation in text: [1] -
Raubenheimer, H. G.; Cronje, S. Chem. Soc. Rev. 2008, 37, 1998–2011. doi:10.1039/b708636a
Return to citation in text: [1] -
Lin, J. C. Y.; Huang, R. T. W.; Lee, C. S.; Bhattacharyya, A.; Hwang, W. S.; Lin, I. J. B. Chem. Rev. 2009, 109, 3561–3598. doi:10.1021/cr8005153
Return to citation in text: [1] -
Khan, M.; Oldham, C.; Tuck, D. G. Can. J. Chem. 1981, 59, 2714–2718. doi:10.1139/v81-391
Return to citation in text: [1] -
Bowmaker, G. A.; Brown, C. L.; Hart, R. D.; Healy, P. C.; Rickard, C. E. F.; White, A. H. J. Chem. Soc., Dalton Trans. 1999, 881–889. doi:10.1039/A808928K
Return to citation in text: [1] -
Böhler, C.; Stein, D.; Donati, N.; Grützmacher, H. New J. Chem. 2002, 26, 1291–1295. doi:10.1039/b203670c
Return to citation in text: [1] -
van den Beuken, E. K.; Feringa, B. L. Tetrahedron 1998, 54, 12985–13011. doi:10.1016/S0040-4020(98)00319-6
Return to citation in text: [1] -
Jacobsen, E. N. Acc. Chem. Res. 2000, 33, 421–431. doi:10.1021/ar960061v
Return to citation in text: [1] -
Hirner, J. J.; Shi, Y.; Blum, S. A. Acc. Chem. Res. 2011, 44, 603–613. doi:10.1021/ar200055y
Return to citation in text: [1] -
Imai, Y.; Zhang, W.; Kida, T.; Nakatsuji, Y.; Ikeda, I. Tetrahedron Lett. 1997, 38, 2681–2684. doi:10.1016/S0040-4039(97)00428-0
Return to citation in text: [1] -
Imai, Y.; Zhang, W.; Kida, T.; Nakatsuji, Y.; Ikeda, I. J. Org. Chem. 2000, 65, 3326–3333. doi:10.1021/jo9915978
Return to citation in text: [1] -
Wang, F.; Zhang, Y. J.; Wei, H.; Zhang, J.; Zhang, W. Tetrahedron Lett. 2007, 48, 4083–4086. doi:10.1016/j.tetlet.2007.04.007
Return to citation in text: [1] -
Wang, F.; Zhang, Y. J.; Yang, G.; Zhang, W. Tetrahedron Lett. 2007, 48, 4179–4182. doi:10.1016/j.tetlet.2007.04.064
Return to citation in text: [1] -
Zhang, Y. J.; Wang, F.; Zhang, W. J. Org. Chem. 2007, 72, 9208–9213. doi:10.1021/jo701469y
Return to citation in text: [1]
| 1. | Bonati, F.; Burini, A.; Pietroni, B. R.; Bovio, B. J. J. Organomet. Chem. 1989, 375, 147–160. doi:10.1016/0022-328X(89)85094-6 |
| 2. | Nieto-Oberhuber, C.; López, S.; Echavarren, A. M. J. Am. Chem. Soc. 2005, 127, 6178–6179. doi:10.1021/ja042257t |
| 3. | Gourlaouen, C.; Marion, N.; Nolan, S. P.; Maseras, F. Org. Lett. 2009, 11, 81–84. doi:10.1021/ol802430m |
| 4. | Marion, N.; Gealageas, R.; Nolan, S. P. Org. Lett. 2007, 9, 2653–2656. doi:10.1021/ol070843w |
| 5. | Fructos, M. R.; Belderrain, T. R.; de Frémont, P.; Scott, N. M.; Nolan, S. P.; Díaz-Requejo, M. M.; Pérez, P. J. Angew. Chem., Int. Ed. 2005, 44, 5284–5288. doi:10.1002/anie.200501056 |
| 6. | Marion, N.; Carlqvist, P.; Gealageas, R.; de Frémont, P.; Maseras, F.; Nolan, S. P. Chem.–Eur. J. 2007, 13, 6437–6451. doi:10.1002/chem.200700134 |
| 7. | Gorske, B. C.; Mbofana, C. T.; Miller, S. J. Org. Lett. 2009, 11, 4318–4321. doi:10.1021/ol9016782 |
| 8. | Wang, F.; Liu, L.; Wang, W.; Li, S.; Shi, M. Coord. Chem. Rev. 2011, 256, 804–853. doi:10.1016/j.ccr.2011.11.013 |
| 13. | Wang, W.; Yang, J.; Wang, F.; Shi, M. Organometallics 2011, 30, 3859–3869. doi:10.1021/om2004404 |
| 14. | Liu, L.; Wang, F.; Wang, W.; Zhao, M.; Shi, M. Beilstein J. Org. Chem. 2011, 7, 555–564. doi:10.3762/bjoc.7.64 |
| 12. | Wang, Y.-M.; Kuzniewski, C. N.; Rauniyar, V.; Hoong, C.; Toste, F. D. J. Am. Chem. Soc. 2011, 133, 12972–12975. doi:10.1021/ja205068j |
| 11. | Arnanz, A.; González-Arellano, C.; Juan, A.; Villaverde, G.; Corma, A.; Iglesias, M.; Sánchez, F. Chem. Commun. 2010, 46, 3001–3003. doi:10.1039/b922534j |
| 9. | Matsumoto, Y.; Selim, K. B.; Nakanishi, H.; Yamada, K.; Yamamoto, Y.; Tomioka, K. Tetrahedron Lett. 2010, 51, 404–406. doi:10.1016/j.tetlet.2009.11.039 |
| 10. | Matsumoto, Y.; Yamada, K.; Tomioka, K. J. Org. Chem. 2008, 73, 4578–4581. doi:10.1021/jo800613h |
| 25. | Imai, Y.; Zhang, W.; Kida, T.; Nakatsuji, Y.; Ikeda, I. Tetrahedron Lett. 1997, 38, 2681–2684. doi:10.1016/S0040-4039(97)00428-0 |
| 26. | Imai, Y.; Zhang, W.; Kida, T.; Nakatsuji, Y.; Ikeda, I. J. Org. Chem. 2000, 65, 3326–3333. doi:10.1021/jo9915978 |
| 27. | Wang, F.; Zhang, Y. J.; Wei, H.; Zhang, J.; Zhang, W. Tetrahedron Lett. 2007, 48, 4083–4086. doi:10.1016/j.tetlet.2007.04.007 |
| 28. | Wang, F.; Zhang, Y. J.; Yang, G.; Zhang, W. Tetrahedron Lett. 2007, 48, 4179–4182. doi:10.1016/j.tetlet.2007.04.064 |
| 29. | Zhang, Y. J.; Wang, F.; Zhang, W. J. Org. Chem. 2007, 72, 9208–9213. doi:10.1021/jo701469y |
| 22. | van den Beuken, E. K.; Feringa, B. L. Tetrahedron 1998, 54, 12985–13011. doi:10.1016/S0040-4020(98)00319-6 |
| 23. | Jacobsen, E. N. Acc. Chem. Res. 2000, 33, 421–431. doi:10.1021/ar960061v |
| 24. | Hirner, J. J.; Shi, Y.; Blum, S. A. Acc. Chem. Res. 2011, 44, 603–613. doi:10.1021/ar200055y |
| 16. | Marion, N.; Nolan, S. P. Chem. Soc. Rev. 2008, 37, 1776–1782. doi:10.1039/b711132k |
| 17. | Raubenheimer, H. G.; Cronje, S. Chem. Soc. Rev. 2008, 37, 1998–2011. doi:10.1039/b708636a |
| 18. | Lin, J. C. Y.; Huang, R. T. W.; Lee, C. S.; Bhattacharyya, A.; Hwang, W. S.; Lin, I. J. B. Chem. Rev. 2009, 109, 3561–3598. doi:10.1021/cr8005153 |
| 19. | Khan, M.; Oldham, C.; Tuck, D. G. Can. J. Chem. 1981, 59, 2714–2718. doi:10.1139/v81-391 |
| 20. | Bowmaker, G. A.; Brown, C. L.; Hart, R. D.; Healy, P. C.; Rickard, C. E. F.; White, A. H. J. Chem. Soc., Dalton Trans. 1999, 881–889. doi:10.1039/A808928K |
| 21. | Böhler, C.; Stein, D.; Donati, N.; Grützmacher, H. New J. Chem. 2002, 26, 1291–1295. doi:10.1039/b203670c |
| 15. | Wang, F.; Li, S.; Qu, M.; Zhao, M. X.; Liu, L.; Shi, M. Chem. Commun. 2011, 47, 12813–12815. doi:10.1039/c1cc15543a |
© 2012 Wang et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)