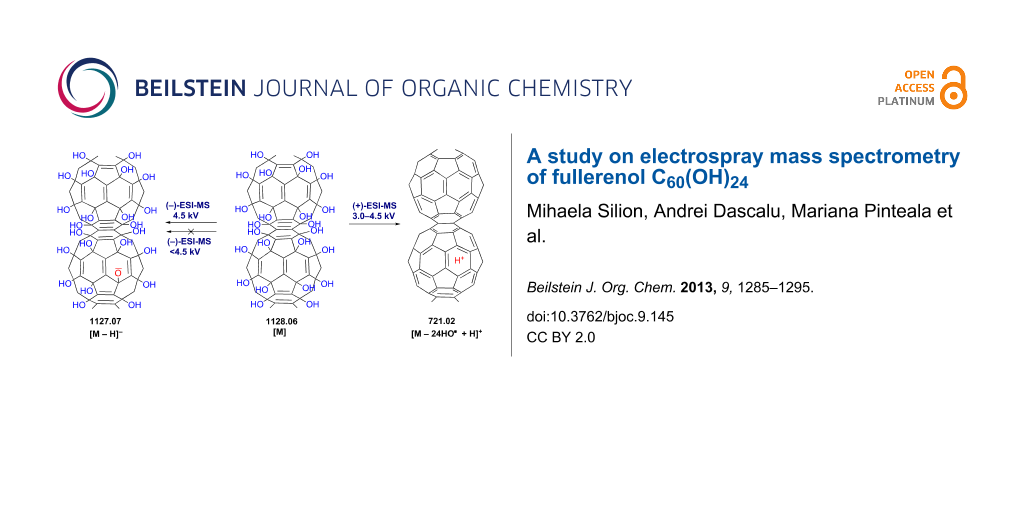
Abstract
Full characterization of fullerenol C60(OH)24 by HPLC ESI-MS in negative and positive ionization modes was achieved. Fragmentor voltage and capillary voltage were optimized in order to obtain a good signal stability and the best peak intensity distribution for the fullerenol C60(OH)24 in both negative and positive modes. While the predominant base peak observed for C60(OH)24 in the negative ionization mode was [M − H]− at m/z 1127, those observed in the positive mode were multiply charged [M − H2O + 4H]4+ at m/z 279 and [M − 12H2O + 2NH3 + 6H]6+ at m/z 158.
Graphical Abstract
Introduction
Because of their potential for chemical tunability and exciting range of biological activities as glutamate-receptor antagonists [1] and antiproliferative [2,3], neuroprotective [4-7], and anticancer agents [8-13], polyhydroxylated [C60]fullerenes, C60(OH)x, have received much attention in recent years. However, to the best of our knowledge, except for the compositionally and structurally well characterized C60(OH)24, prepared by alkaline hydrolysis of C60Br24 [14,15], most of these fullerenols are not pure C60(OH)x, but a complex mixture of products with an average composition of C60(OH)x–y, C60Ox(OH)y [16-19] or C60(OH)x(ONa)y [20].
Therefore, the HPLC separation and accurate measurement of the molecular weight for structure characterization by electrospray ionization mass spectrometry (ESI-MS) have become essential for fullerenol research. Fullerenols C60(OH)18–44 are very small neutral molecules with the highest density of hydroxy groups on a given particle surface (up to 10.7 OH/nm2) [21] achieved so far, complicating spectra acquisition if the correct conditions are not used. Initial attempts made by Isaacson et al [22] in 1994 to develop mass-spectrometric methods for fullerenols using ESI-MS with commercially available C60(OH)24 and C60(OH)22–24 dissolved in acidic, basic, and neutral solutions did not produced ions diagnostic of C60(OH)x. Afterwards, very few isolated attempts have been made on the identification of fullerenols [23] and/or their derivatives [24-28] by ESI-MS analysis, and very little detail was provided. We carried out an experimental investigation on ESI-MS of fullerenol C60(OH)24 over a wide range of total capillary and fragmentor voltages.
Herein, we are happy to report the first experimental evidences that fullerenols are ESI-MS-inactive compounds in aqueous media at low and medium capillary and fragmentor voltage under negative ionization conditions, but they are ESI-MS-active compounds in aqueous and ammonia media, in both negative and positive ionization mode at a capillary voltage of 4.5 kV and a fragmentor voltage of 400 V with no C60-cage fragmentation.
Results and Discussion
Nomenclature and ionization mechanisms
In the discussion, we will use the expressions protonated [M + H]+ and deprotonated [M − H]− molecules, and distonic deprotonated molecules for those identified from a deprotonated molecule plus loss of HO• and/or H• radicals [M − HO• − H• − H]−. However, while only radical-ion pairs have been identified by ESI-MS as distonic ions, the distonic deprotonated molecules observed in (−)ESI-MS spectra of C60(OH)24 contain a different number of charges and radicals on the C60 cage. Consequently, these distonic species should be described as quasi-distonic radical anions. The proposed mechanisms for the formation of fullerenol C60(OH)24 anions and distonic radical anions are shown in Scheme 1.
Scheme 1: Proposed mechanisms for the formation of fullerenol anions and distonic radical anions observed by (−)ESI-MS spectra of C60(OH)24 in pure water.
Scheme 1: Proposed mechanisms for the formation of fullerenol anions and distonic radical anions observed by ...
For naming these individual radical species using “yl” and “oxyl” suffixes the terms fullerenyl for radicals generated by the loss of HO• (M1 in Scheme 1) and fullerenoxyl for radicals generated by the loss of H• (M2 in Scheme 1) will be used (IUPAC 2002) [29]. Radical anions formed by electron addition to a fullerenyl radical shall be described as fullerene carbanions and referred to as fullerenide anions (M3 in Scheme 1) and [mM + ne]m–n∙ shall be described as molecular anions. As regards to the observation of distonic fullerenyl-fullerenate M7 ions, such distonic dehydroalcoxide radical anions, namely dehydrophenoxide radical anions, were first observed by Bowie et al [30] and recently investigated by ESI-MS by Mariappandar et al [31].
Anions formed by the deprotonation of OH (M4 in Scheme 1) shall be named as fullerenate anions. Consequently, the ions M6 shall be named fullerenide-fullerenate anions, and ions M5 and M7 shall be named fullerenoxyl-fullerenate and fullerenyl-fullerenate distonic anions, respectively. To avoid any confusion with dissociative electron attachment (DEA) (addition of a free electron to a gas-phase molecule to give an anion and a neutral molecule via a superexcited anion), the term quasi-electron-capture will be used for the addition of an electron to a fullerenyl radical M7 to generate a carbanion M6.
Negative-ionization-mode mass spectra
The negative-ion ESI-MS spectra and ionization data of C60(OH)24 in ultrapure water and in the presence of small amounts of aqueous ammonia will be discussed here.
Fullerenol in pure water
Three distinct regions can be identified in the mass spectra: m/z 100–600, m/z 600–1500 (Figure 1b) and above m/z 1500. Between m/z 600 and 1500, the most abundant ion is centered at m/z 1127 and corresponds to the singly charged base molecular ion [M − H]− generated through the deprotonation of fullerenol C60(OH)24.
![[1860-5397-9-145-1]](/bjoc/content/figures/1860-5397-9-145-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Negative-ion mass spectra for a 0.5 × 10−5 M solution of C60(OH)24 in ultrapure water: (a) full scan spectrum (b) m/z range 600–1500. (●[M(n) − nH2O − xH]x-, ▲[M(n) + nH2O(m) − xH]x−, ♦[M(n) − H2O(m) − yHO• + ye − xH](x+y)−, ○[M(n) − mH2O − yH• − xH]x−y•−, ∆[M(n) + H2O(m) − yH• − xH]x−y•−, ◊[M − mH2O − yHO• − zH• − xH]x−(y+z)•−).
Figure 1: Negative-ion mass spectra for a 0.5 × 10−5 M solution of C60(OH)24 in ultrapure water: (a) full sca...
As shown in Table 1 (entries 1–3) and Scheme 2, consecutive weight loss of 18 mass units from the deprotonated molecule [M − H]− at m/z 1127 was clearly observed through the presence of singly charged ions centered at m/z 1055 [M − 4H2O − H]− and m/z 983 [M − 8H2O − H]−.
Table 1: Summary of the dominant ESI-MS negative ionic species of fullerenol C60(OH)24 in ultrapure water (relative abundance, assignments, and m/z values) at 4.5 kV capillary voltage and 400 V fragmentor voltage.
| Entry | Relative intensity (%)a | Assignments | m/z | ||
|---|---|---|---|---|---|
| Calculated | Found | Deviation | |||
| 1 | 100 | [M − 1H]− | 1127.0570 | 1127.0719 | −0.0149 |
| 2 | 83 | [M − 4H2O − 1H]− | 1055.0146 | 1055.0172 | −0.0026 |
| 3 | 63 | [M − 8H2O − 1H]− | 982.9722 | 982.9495 | 0.0227 |
| 4 | 55 | [M + 7H2O −1H• − 1H]−1• | 1252.1234 | 1252.1617 | −0.0383 |
| 5 | 54 | [2M + 12H2O − 1H• − 1H]−1• | 2470.2432 | 2470.4280 | −0.1848 |
| 6 | 54 | [3M − 12H2O − 3H• − 2H]2−3• | 1581.5141 | 1581.6201 | −0.1060 |
| 7 | 63 | [3M + 20H2O − 3H• −2H]2−3• | 1869.6852 | 1869.6562 | 0.0290 |
| 8 | 20 | [M − 8H2O − 3OH• − 3H]3−3• | 309.9961 | 309.9241 | 0.0720 |
| 9 | 51 | [2M + 19H2O − 3H]3− | 865.1027 | 865.1792 | −0.0765 |
| 10 | 52 | [3M + 18H2O − 12H• − 3H]3−12• | 1231.0894 | 1231.0091 | 0.0803 |
| 11 | 49 | [4M + 9H2O − 9H• − 3H]3−9• | 1554.0869 | 1554.0754 | 0.0115 |
| 12 | 65 | [6M + 20H2O − 3H]3− | 2375.1924 | 2375.2462 | −0.0538 |
| 13 | 71 | [M − 4H2O − 4H• − 8HO• − 4H]4−12• | 227.9846 | 227.9089 | 0.0757 |
| 14 | 55 | [M − 1H• − 6H2O − 4H]4−1• | 253.7405 | 253.7368 | 0.0037 |
| 15 | 70 | [M − 2H2O − 4OH• + 4e]4− | 256.0082 | 256.0519 | −0.0437 |
| 16 | 32 | [M + 62H2O − 4H]4− | 560.1727 | 560.1382 | 0.0345 |
| 17 | 43 | [2M + 26H2O − 4H]4− | 680.0935 | 680.0391 | 0.0544 |
| 18 | 62 | [4M + 10H2O − 4H]4− | 1172.0835 | 1172.0648 | 0.0187 |
| 19 | 65 | [4M + 66H2O − 4H]4− | 1424.2319 | 1424.2480 | −0.0161 |
| 20 | 71 | [6M − 3H• + 16H2O − 4H]4−3• | 1762.3759 | 1762.4616 | −0.0857 |
| 21 | 55 | [4M − 6H2O − 5H]5− | 879.8313 | 879.8669 | −0.0356 |
| 22 | 51 | [5M − 2H• − 19H2O − 5H]5−• | 1058.3645 | 1058.3025 | 0.0620 |
| 23 | 86 | [M − 9H2O − OH• − 6H]6−1• | 157.1533 | 157.2115 | −0.0582 |
| 24 | 84 | [M − 2H2O − 3OH• + 3e − 3H]6− | 173.0020 | 173.0518 | −0.0498 |
| 25 | 51 | [M − 1H• − H2O − 6H]6−1• | 183.8332 | 183.8562 | −0.0149 |
aThe intensity relative to the base peak at m/z 1127.0719 in the spectrum with tallest peak set to 100%.
Scheme 2: Examples of proposed structures for the main deprotonated molecules and final distonic molecular ion observed by (−)ESI-MS of C60(OH)24 in pure water (a) and aqueous ammonia solution (b).
Scheme 2: Examples of proposed structures for the main deprotonated molecules and final distonic molecular io...
Along with the singly charged [M − nH2O − H]− ions, six types of singly and multiply charged simple and distonic ion clusters and solvent adducts were detected within m/z 1000 and m/z 3000 (Figure 1 and Table 1): [M(n) − nH2O − xH]x−, [M(n) + H2O(m) − xH]x−y•, [M(n) + mH2O(m) − yH• − xH]x−y•, [M(n) − mH2O − yH• − xH]x−y•, [M(n) − mH2O − yHO• + ye − xH](x+y)− and [M − mH2O − yHO• − zH• − xH]x−(y+z)•.
Between m/z 100 and 600 (Table 1 and Scheme 3) the most intense peaks are designated to highly charged anions and distonic radical anions generated by ionization of the parent fullerenol C60(OH)24 as in the following examples: [M − 9H2O − HO• + 5H]6−1• at m/z 157 (Table 1, entry 23; B in Scheme 3); [M − 2H2O − 3HO• + 3e − 3H]6− at m/z 173 (Table 1, entry 24; D in Scheme 3); [M − 1H• − 6H2O − 4H]4−1• at m/z 253 (Table 1, entry 14; E in Scheme 3); and [M − 4H• − 4H2O − 8HO• − 4H]4−4• at m/z 227 (Table 1, entry 13; G in Scheme 3). Above m/z 1500 the most intense peaks are assigned to the highly charged protonated and distonic deprotonated molecules containing 2–6 fullerenol and up to 66 H2O clusters.
Scheme 3: Proposed (−)ESI-MS ionization mechanisms for fullerenol C60(OH)24 in pure water.
Scheme 3: Proposed (−)ESI-MS ionization mechanisms for fullerenol C60(OH)24 in pure water.
Formation of these ions can be rationalized by the mechanisms shown in Scheme 1 and graphically depicted in Scheme 3. One can suppose that, in the electrospray source, M1-fullerenyl and M2-fullerenoxyl radicals are formed by neutral cleavage of C–OH and O–H bonds, respectively. As soon as fullerenyl radicals M1 containing a total of less than eight odd electrons with random distribution are generated, they readily capture free electrons present in the gas (due to cosmic rays or background radiation) to form fullerenide carbanion M3, while the fullerenoxyl radicals M2 do not capture free electrons, thus generating fullerenate anions M4. While only a few M1-fullerenyl radicals were detected as distonic anions [M(m) − nH2O − yHO• − 3H]3−y• at m/z 309, 335 and 781, fullerenoxyl radicals are much more resistant against free electrons and can be identified as abundant singly and multiply charged molecular anions containing up to 12 fullerenoxyl odd electrons.
A particular case is the abundant distonic anion [M − 4H2O − 8HO• − 4H• −4H]4−12• at m/z 227 (entry 13 in Table 1, and C in Scheme 3), which appears to contain four fullerenoxyl radicals and eight fullerenyl radicals. In fact, the partial π-bond reconstruction by rehybridization and redistribution of odd fullerenyl electrons of intermediate C should account for the distonic anion G. The structure of the species [M − xH2O − yHO• + ye − zH](z+y)− depicted as a high-range of relative abundance in the mass spectra, due to a loss of protons from fullerenyl-carbanion-containing species M3, could be assigned to fullerenide-fullerenate carbanion species M6 (B and D in Scheme 3).
Fullerenol in ammonia aqueous solution
In order to enhance the ionization process of the fullerenol, small amounts of aqueous ammonia were used. Very abundant doubly charged ions with good intensity corresponding to dimeric species were observed for fullerenol C60(OH)24 in aqueous ammonia solution recorded at 4.5 kV capillary voltage and 400 V fragmentor voltage (Figure 2).
![[1860-5397-9-145-2]](/bjoc/content/figures/1860-5397-9-145-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Negative-ion mass spectra of a 0.5 × 10−5 M aqueous solution of C60(OH)24 in ammonia solution: (a) full scan spectrum; (b) m/z ranges of 700–1200.
Figure 2: Negative-ion mass spectra of a 0.5 × 10−5 M aqueous solution of C60(OH)24 in ammonia solution: (a) ...
Consecutive losses of m/z 17 units are attributed to the neutral loss of nOH radicals (n = 2–48), followed by the rehybridization and redistribution of odd electrons of fullerenyl radical anion, accounting for the formation of a final molecular anion [2M − 48OH + 2e]2− as observed at m/z 720 and depicted in Scheme 2. Taking into account the moderate electron affinity of fullerenols [32,33] and the fact that earlier extensive studies focused on pristine fullerene anion radicals [34-37] unquestionably confirmed their generation by electron transfer from various electron donors (aliphatic and aromatic amines especially) [38,39], one can postulate that, under ESI conditions, a distonic radical carbanion [(C60(OH)24−•)2]2−• is first generated by electron transfer from H2N− produced by ammonia ionization and then undergoes consecutive OH radical losses and electron redistribution until the entire reconstruction of the electronic structure of pristine fullerene is achieved.
Positive-ionization-mode mass spectra
In Figure 3 are shown positive-ion-mode ESI mass spectra of C60(OH)24 with the addition of (a) 10 μL 3 × 10−1 M and (b) 10 μL 2 × 10−2 M aqueous ammonia solution. All of the observed ion species were obtained under 4.5 kV capillary voltage and 400 V fragmentor voltage.
![[1860-5397-9-145-3]](/bjoc/content/figures/1860-5397-9-145-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Positive ionization ESI mass spectrum of C60(OH)24 in (a) 3 × 10−1 M (b) 2 × 10−2 M aqueous ammonia solution. (●[M(n) + H2O(m) + xH]x+, ▲[M(n) − OH(m) + xH]x+, ♦[M(n) − H2O(m) + nNH3 + xH]x+, ○[M(n) − H2O(m) + xH]x+, ∆[M(n) − OH(m) + yNH4+ + xH](x+y)+, ◊[M(n) + H2O(m) + nNH3 + xH]x+).
Figure 3: Positive ionization ESI mass spectrum of C60(OH)24 in (a) 3 × 10−1 M (b) 2 × 10−2 M aqueous ammonia...
With regard to molecular species and their stability, the positive-ion spectra of fullerenol in the presence of aqueous ammonia were significantly different from the negative-ion spectra. While the relative intensities of 2- to 6-protonated fullerenol with dilute 2 × 10−2 M ammonia aqueous solution were about threefold smaller than those observed under negative ionization conditions, the relative intensities recorded in the presence of 10 μL 3 × 10−1 M ammonia solution were about tenfold smaller than in the negative mode.
While the predominant base peak observed for C60(OH)24 in the negative mode was [M − H]− those observed in the positive mode were [M − 12H2O + 2NH3 + 6H]6+ (96%) and [M − H2O + 4H]4+ (100%) (see Supporting Information File 1, Table S1, entries 9 and 7; D and E in Scheme 4) with 10 μL 2 × 10−2 M aqueous ammonia solution and [M − 24HO + 3NH4+ + 3H]6+ (100%) (see Supporting Information File 1, Table S1, entry 9; F in Scheme 4) with 10 μL 3 × 10−1 M aqueous ammonia solution.
Scheme 4: Proposed (+)ESI-MS ionization mechanisms for fullerenol C60(OH)24 in ammonia solution.
Scheme 4: Proposed (+)ESI-MS ionization mechanisms for fullerenol C60(OH)24 in ammonia solution.
The relative intensity of singly protonated fullerenol [M + H]+ (9%) at m/z 1129 was very low (Supporting Information File 1, entry 5 in Table S1 and C in Scheme 4). On the other hand, while under negative-ionization-mode conditions abundant [M(n) + H2O(m) − xH]x− clustered ions with medium and high intensity are formed up to n = 6 and m = 66, only very few dimers, one tetramer and one pentamer with very low intensity were observed under positive-mode conditions.
Conclusion
It is demonstrated here that electrospray mass spectrometry is a perfectly suitable method to study fullerenol C60(OH)24 in pure water and in the presence of aqueous ammonia solution in the negative and positive ionization modes, under the optimal capillary and fragmentor voltage. While the predominant base peak observed for C60(OH)24 in the negative-ionization mode was [M − H]− at m/z 1127, those observed in the positive mode were multiply charged [M − H2O + 4H]4− at m/z 279 (100%) and [M − 12H2O + 2NH3 + 6H]6+ at m/z 158 (96%).
We show that in the negative ionization mode, fullerenol C60(OH)24 readily lose not only H2O but also lose OH• and H• radicals giving rise to ion species that contain long-living fullerenoxyl radicals and/or short-living fullerenyl radicals, respectively. Affinity of short-lived fullerenyl radicals for free electrons to form carbanion-like fullerenyde charges on the C60 cage revealed an atypical pattern, characterized by neutral cleavage of C−OH bonds to form fullerenyl radicals, which can capture free electrons to generate negative charges, while fullerenoxyl radicals produced by O−H bond cleavage are much more stable against the free radicals.
In addition to simple bond cleavages, fullerenol ions containing at least eight fullerenyl radicals undergo an unanticipated gas-phase rearrangement that involves the partial (in the negative mode from pure aqueous media) or total (in the positive mode and negative mode from ammonia aqueous solutions) π-bond reconstruction of the C60 cage by rehybridization and redistribution of odd electrons of fullerenyl radicals to form C60 anions or cations, respectively.
Experimental
Materials
All chemicals and reagents used for fullerenol preparation and HPLC/ESI-MS analyses were commercially available (Sigma-Aldrich, St. Louis, MO, USA). The high purity C60(OH)24 sample was prepared as previously described [14,15] (see Supporting Information File 1). Freshly produced ultrapure water (TKA Lab TowerEDI 0.067–0.080 µS/cm) was used for sample preparation and as the mobile phase. The Tuning Mix solution for Q-TOF parameter optimization was purchased from Agilent Technologies.
Sample preparation
A stock solution containing 0.5 × 10−5 M fullerenol C60(OH)24 was prepared by dissolution of dry fullerenol in ultrapure water, filtered and injected directly into the HPLC system. In order to prepare the samples with the ammonia medium, to each 1 mL of the 0.5 × 10−5 M fullerenol stock solution, 10 µL of 2 × 10−2 M or 3 × 10−1 M aqueous ammonia was added for two series of assays.
HPLC separation conditions
HPLC separation was performed on a chromatographic system Agilent 1200 series separation module equipped with a binary pump, heated column compartment, autosampler and diode array detector. Separations were achieved using a new Agilent Zorbax SB-C18 reverse phase column (4.6 mm × 150 mm, 5 μm particle size) with a column temperature kept at 35 °C. Using a 20 μL injection volume, the fullerenol C60(OH)24 was monitored through UV detection at 280 nm for a total runtime of 30 minutes at a flow rate of 1 mL/min. Because the fullerenol C60(OH)24 is sparingly soluble in alcohols and acetonitrile, ultrapure water was used as the mobile phase for the HPLC separation and ESI-MS measurements, in negative- and positive-ionization mode. The mobile phase was filtered through a 0.45 μm filter and degassed for 30 minutes by sonication.
A typical chromatogram of the aqueous solution of C60(OH)24 (see Supporting Information File 1, Figure S1a) indicates a very small peak at approximately 1.5 min retention time, corresponding to contaminants from the sample, and a major peak at 2.6 min, corresponding to the analyte. The blank chromatogram (see Supporting Information File 1, Figure S1b) showed no peaks in the HPLC beside the solvent front. Hence, it is a clear evidence that the used column allows separation of C60(OH)24, and it is clear that fullerenol is essentially an individual pure compound.
ESI-MS analysis
Mass spectrometry results were obtained using an Agilent 6520 Series Accurate-Mass Quadrupole Time-of-Flight (Q-TOF) LC/MS. The solutions were introduced into the electrospray ion source (ESI) after HPLC separation via a 4:1 splitter at a flow-rate of 0.2 mL/min. During the experiments, the following Q/TOF MS parameters were optimized: electrospray ionization (positive- and negative-ion mode), drying gas (N2) flow rate, drying-gas temperature, nebulizer pressure, capillary voltage, and fragmentor voltage. The mass scale was calibrated by the standard calibration procedure with compounds provided by the manufacturer. Data were collected and processed by using MassHunter workstation data acquisition software for the 6200/6500 series, version B.01.03. The calculated m/z values are based on the weight of the most abundant isotopes (monoisotopic mass).
The key experimental observations
The first experiments studied the effect of ESI source parameters and mobile phase composition on the formation of fullerenol C60(OH)24 ions under negative and positive ionization mode. The fragmentor voltage and capillary voltage were the first parameters to be optimized because they have the greatest impact on the sensitivity and fragmentation. Consequently, the first objective was to find the optimum values for the fragmentor and capillary voltage that provides a strong molecular ion and a good relative abundance. The use of high voltages generates a greater signal, but this needs to be balanced with an increased background-to-noise ratio at the higher corona-current settings.
To optimize the conditions for obtaining maximum intensity, the capillary voltage was varied between 2.0 and 4.5 kV and the fragmentor voltage was varied between 100 and 400 V. Typical values for the other source parameters were: drying gas (N2) flow rate 8.0 L/min; drying-gas temperature 325 °C; nebulizer pressure 35 psig, skimmer 58 V and the octopol RF 750 V. The full-scan mass spectra of the investigated compounds were acquired in the range m/z 100–3000. The results are summarized in Figure S2 in Supporting Information File 1 showing the ESI-MS reference grid for C60(OH)24 as a function of fragmentor and capillary voltage in negative- and positive-ionization modes.
The experiments that were carried out at capillary voltage values of 2.0–4.0 kV and fragmentor voltage of 100–300 V (Supporting Information File 1, Figure S3a–c) did not affect any ionization and only peaks associated with unknown contaminants were observed at m/z 113, 294 and 420, detected in ultrapure water (Supporting Information File 1, Figure S1c) under negative-ionization-mode conditions. However, the fullerenol ionization takes place with good peak intensity distribution in the electrospray source at 4.5 kV capillary voltage and 400 V fragmentor voltage in negative-ionization mode (Figure 1 and Supporting Information File 1, Figure S3d) and at capillary voltage range of 3.0–4.5 eV in positive-ionization mode. Although a capillary voltage of 4.5 kV was recently reported to effect positive ionization of the MER commercial fullerenol C60(OH)16(ONa)8 [23] the reported results based only on a full scan spectrum of MER fullerenol sample were completely different from the current reported results.
Supporting Information
| Supporting Information File 1: Additional material. | ||
| Format: PDF | Size: 316.3 KB | Download |
Acknowledgements
This work was supported by a grant of the Romanian National Authority for Scientific Research, CNCS – UEFISCDI, project number PN-II-ID-PCCE-2011-2-0028 and the European Social Fund “Cristofor I. Simionescu” Postdoctoral Fellowship Program (ID POSDRU/89/1.5/S/55216), Sectoral Operational Program Human Resources Development 2007–2013.
References
-
Jin, H.; Chen, W. Q.; Tang, X. W.; Chiang, L. Y.; Yang, C. Y.; Schloss, J. V.; Wu, J. Y. Neurosci. Res. 2000, 62, 600–607. doi:10.1002/1097-4547(20001115)62:4<600::AID-JNR15>3.0.CO;2-F
Return to citation in text: [1] -
Gelderman, M. P.; Simakova, O.; Clogston, J. D.; Patri, A. K.; Siddiqui, S. F.; Vostal, A. C.; Simak, J. Int. J. Nanomed. 2008, 3, 59–68. doi:10.2147/IJN.S1680
Return to citation in text: [1] -
Lu, L.-H.; Lee, Y.-T.; Chen, H.-W.; Chiang, L. Y.; Huang, H.-C. Br. J. Pharmacol. 1998, 123, 1097–1102. doi:10.1038/sj.bjp.0701722
Return to citation in text: [1] -
Silva, G. A. Nat. Rev. Neurosci. 2006, 65–74. doi:10.1038/nrn1827
Return to citation in text: [1] -
Silva, G. A. Surg. Neurol. 2005, 63, 301–306. doi:10.1016/j.surneu.2004.06.008
Return to citation in text: [1] -
Dugan, L. L.; Lovett, E. G.; Quick, K. L.; Lotharius, J.; Lin, T. T.; O’Malley, K. L. Parkinsonism Rel. Disord. 2001, 7, 243–246. doi:10.1016/S1353-8020(00)00064-X
Return to citation in text: [1] -
Dugan, L. L.; Gabrielsen, J. K.; Yu, S. P.; Lin, T.-S.; Choi, D. W. Neurobiology of Diseases. 1996, 3, 129–135. doi:10.1006/nbdi.1996.0013
Return to citation in text: [1] -
Injac, R.; Radic, N.; Govedarica, B.; Perse, M.; Cerar, A.; Djordjevic, A.; Strukelj, B. Pharmacol. Rep. 2009, 61, 335–342.
Return to citation in text: [1] -
Injac, R.; Perse, M.; Cerne, M.; Potocnik, N.; Radic, N.; Govedarica, B.; Djordjevic, A.; Cerar, A.; Strukelj, B. Biomaterials 2009, 30, 1184–1196. doi:10.1016/j.biomaterials.2008.10.060
Return to citation in text: [1] -
Injac, R.; Boskovic, M.; Perse, M.; Koprivec-Furlan, E.; Cerar, A.; Djordjevic, A.; Strukelj, B. Pharmacol. Rep. 2008, 60, 742–749.
Return to citation in text: [1] -
Chaudhuri, P.; Paraskar, A.; Sonii, S.; Mashelkar, R. A.; Sengupta, S. ACS Nano 2009, 3, 2505–2514. doi:10.1021/nn900318y
Return to citation in text: [1] -
Yin, J.-J.; Lao, F.; Meng, J.; Fu, P. P.; Zhao, Y.; Xing, G.; Gao, X.; Sun, B.; Wang, P. C.; Chen, C.; Liang, X.-J. Mol. Pharmacol. 2008, 74, 1132–1140. doi:10.1124/mol.108.048348
Return to citation in text: [1] -
Zhu, J.; Ji, Z.; Wang, J.; Sun, R.; Zhang, X.; Gao, Y.; Sun, H.; Liu, Y.; Wang, Z.; Li, A. Q.; Ma, J.; Wang, T.; Jia, G.; Gu, Y. Small 2008, 4, 1168–1175. doi:10.1002/smll.200701219
Return to citation in text: [1] -
Pinteala, M.; Dascalu, A.; Ungurenasu, C. Int. J. Nanomed. 2009, 4, 193–199. doi:10.2147/IJN.S6630
Return to citation in text: [1] [2] -
Mrdanovic, J. Z.; Solajic, S. V.; Bogdanovic, V. V.; Djordjevic, A. N.; Bogdanovic, G. M.; Ijac, R. D.; Rakocevic, Z. L. J. Dig. J. Nanomater. Biostruct. 2012, 7, 673–686.
Return to citation in text: [1] [2] -
Bogdanović, G.; Kojić, V.; Ðordević, A.; Canadanović-Brunet, J.; Vojinović-Miloradov, M.; Baltić, V. V. Toxicol. in Vitro 2004, 18, 629–637. doi:10.1016/j.tiv.2004.02.010
Return to citation in text: [1] -
Chiang, L. Y.; Upasani, R. B.; Swirczewski, J. W.; Soled, S. J. Am. Chem. Soc. 1993, 115, 5453–5457. doi:10.1021/ja00066a014
Return to citation in text: [1] -
Schneider, N. S.; Darwish, A. D.; Kroto, H. W.; Taylor, R.; Walton, D. R. M. J. Chem. Soc., Chem. Commun. 1994, 463–464. doi:10.1039/C39940000463
Return to citation in text: [1] -
Chiang, L. Y.; Upasani, R. B.; Swirczewski, J. W. J. Am. Chem. Soc. 1992, 114, 10154–10157. doi:10.1021/ja00052a010
Return to citation in text: [1] -
Husebo, L. O.; Sitharaman, B.; Furukawa, K.; Kato, T.; Wilson, L. J. J. Am. Chem. Soc. 2004, 126, 12055–12064. doi:10.1021/ja047593o
Return to citation in text: [1] -
Singh, R.; Goswami, T. J. Phys. Org. Chem. 2008, 21, 225–236. doi:10.1002/poc.1304
Return to citation in text: [1] -
Isaacson, C. W.; Kleber, M.; Field, J. A. Environ. Sci. Technol. 2009, 43, 6463–6474. doi:10.1021/es900692e
Return to citation in text: [1] -
Chao, T.-C.; Guixue, S.; Hansmeier, N.; Westerhoff, P.; Herckes, P.; Halden, R. U. Anal. Chem. 2011, 83, 1777–1783. doi:10.1021/ac1031379
Return to citation in text: [1] [2] -
Singh, R.; Goswami, T. Thermochim. Acta 2011, 513, 60–67. doi:10.1016/j.tca.2010.11.012
Return to citation in text: [1] -
Bobylev, A. G.; Kornev, A. B.; Bobyleva, L. G.; Shpagina, M. D.; Fadeeva, I. S.; Fadeev, R. S.; Deryabin, D. G.; Balzarini, J.; Troshin, P. A.; Podlubnay, Z. A. Org. Biomol. Chem. 2011, 9, 5714–5719. doi:10.1039/c1ob05067b
Return to citation in text: [1] -
Kokubo, K.; Matsubayashi, K.; Tategaki, H.; Takada, H.; Oshima, T. ACS Nano 2008, 2, 327–333. doi:10.1021/nn700151z
Return to citation in text: [1] -
Kokubo, K.; Shirakawa, S.; Kobayashi, N.; Aoshima, H.; Oshima, T. Nano Res. 2011, 4, 204–215. doi:10.1007/s12274-010-0071-z
Return to citation in text: [1] -
Semenov, K. N.; Letenko, D. G.; Charykov, N. A.; Nikitin, V. A.; Matuzenko, M. Y.; Keskinov, V. A.; Postnov, V. N.; Kopyrin, A. A. Russ. J. Appl. Chem. 2010, 83, 2076–2080. doi:10.1134/S1070427210120025
Return to citation in text: [1] -
Powell, W. H.; Cozzi, F.; Moss, G. P.; Thilgen, C.; Hwu, R. J.-R.; Yeryn, A. Pure Appl. Chem. 2002, 74, 629–695. doi:10.1351/pac200274040629
Return to citation in text: [1] -
Reeks, L. P.; Eichinger, P. C. H.; Bowie, J. H. Rapid Commun. Mass Spectrom. 1993, 7, 286–287. doi:10.1002/rcm.1290070405
Return to citation in text: [1] -
Morishetti, K. M.; Sripadi, P.; Mariappandar, V.; Ren, J. Int. J. Mass Spectrom. 2011, 299, 169–177. doi:10.1016/j.ijms.2010.10.025
Return to citation in text: [1] -
Mohan, H.; Palit, D. K.; Mittal, J. P.; Chiang, L. Y.; Asmus, K.-D.; Guldi, D. M. J. Chem. Soc., Faraday Trans. 1998, 94, 359–363. doi:10.1039/a705293f
Return to citation in text: [1] -
Mohan, H.; Chiang, L. Y.; Mittal, J. P. Res. Chem. Intermed. 1997, 23, 403–414. doi:10.1163/156856797X00150
Return to citation in text: [1] -
Arbogast, J. W.; Foote, C. S.; Kao, M. J. Am. Chem. Soc. 1992, 114, 2277–2279. doi:10.1021/ja00032a063
Return to citation in text: [1] -
Biczok, L.; Linschitz, H.; Walter, R. I. Chem. Phys. Lett. 1992, 195, 339–346. doi:10.1016/0009-2614(92)85613-F
Return to citation in text: [1] -
Stasko, A.; Brezova, V.; Biskupic, S.; Dinse, K.-P.; Schweitzer, P; Baumgarten, M. J. Phys. Chem. 1995, 99, 8782–8789. doi:10.1021/j100021a052
Return to citation in text: [1] -
Dimitrijevic, N. M.; Kamat, P. V. J. Phys. Chem. 1993, 97, 7623–7626. doi:10.1021/j100131a035
Return to citation in text: [1] -
Brezova, V.; Stasko, A.; Rapta, P.; Domschke, G.; Bartl, A.; Dunsch, L. J. Phys. Chem. 1995, 99, 16234–16241. doi:10.1021/j100044a006
Return to citation in text: [1] -
Janaki, J.; Premila, M.; Goplan, P.; Sastry, V. S.; Sundar, C. S. Thermochim. Acta 2000, 356, 109–116. doi:10.1016/S0040-6031(00)00477-9
Return to citation in text: [1]
| 23. | Chao, T.-C.; Guixue, S.; Hansmeier, N.; Westerhoff, P.; Herckes, P.; Halden, R. U. Anal. Chem. 2011, 83, 1777–1783. doi:10.1021/ac1031379 |
| 38. | Brezova, V.; Stasko, A.; Rapta, P.; Domschke, G.; Bartl, A.; Dunsch, L. J. Phys. Chem. 1995, 99, 16234–16241. doi:10.1021/j100044a006 |
| 39. | Janaki, J.; Premila, M.; Goplan, P.; Sastry, V. S.; Sundar, C. S. Thermochim. Acta 2000, 356, 109–116. doi:10.1016/S0040-6031(00)00477-9 |
| 14. | Pinteala, M.; Dascalu, A.; Ungurenasu, C. Int. J. Nanomed. 2009, 4, 193–199. doi:10.2147/IJN.S6630 |
| 15. | Mrdanovic, J. Z.; Solajic, S. V.; Bogdanovic, V. V.; Djordjevic, A. N.; Bogdanovic, G. M.; Ijac, R. D.; Rakocevic, Z. L. J. Dig. J. Nanomater. Biostruct. 2012, 7, 673–686. |
| 1. | Jin, H.; Chen, W. Q.; Tang, X. W.; Chiang, L. Y.; Yang, C. Y.; Schloss, J. V.; Wu, J. Y. Neurosci. Res. 2000, 62, 600–607. doi:10.1002/1097-4547(20001115)62:4<600::AID-JNR15>3.0.CO;2-F |
| 14. | Pinteala, M.; Dascalu, A.; Ungurenasu, C. Int. J. Nanomed. 2009, 4, 193–199. doi:10.2147/IJN.S6630 |
| 15. | Mrdanovic, J. Z.; Solajic, S. V.; Bogdanovic, V. V.; Djordjevic, A. N.; Bogdanovic, G. M.; Ijac, R. D.; Rakocevic, Z. L. J. Dig. J. Nanomater. Biostruct. 2012, 7, 673–686. |
| 32. | Mohan, H.; Palit, D. K.; Mittal, J. P.; Chiang, L. Y.; Asmus, K.-D.; Guldi, D. M. J. Chem. Soc., Faraday Trans. 1998, 94, 359–363. doi:10.1039/a705293f |
| 33. | Mohan, H.; Chiang, L. Y.; Mittal, J. P. Res. Chem. Intermed. 1997, 23, 403–414. doi:10.1163/156856797X00150 |
| 8. | Injac, R.; Radic, N.; Govedarica, B.; Perse, M.; Cerar, A.; Djordjevic, A.; Strukelj, B. Pharmacol. Rep. 2009, 61, 335–342. |
| 9. | Injac, R.; Perse, M.; Cerne, M.; Potocnik, N.; Radic, N.; Govedarica, B.; Djordjevic, A.; Cerar, A.; Strukelj, B. Biomaterials 2009, 30, 1184–1196. doi:10.1016/j.biomaterials.2008.10.060 |
| 10. | Injac, R.; Boskovic, M.; Perse, M.; Koprivec-Furlan, E.; Cerar, A.; Djordjevic, A.; Strukelj, B. Pharmacol. Rep. 2008, 60, 742–749. |
| 11. | Chaudhuri, P.; Paraskar, A.; Sonii, S.; Mashelkar, R. A.; Sengupta, S. ACS Nano 2009, 3, 2505–2514. doi:10.1021/nn900318y |
| 12. | Yin, J.-J.; Lao, F.; Meng, J.; Fu, P. P.; Zhao, Y.; Xing, G.; Gao, X.; Sun, B.; Wang, P. C.; Chen, C.; Liang, X.-J. Mol. Pharmacol. 2008, 74, 1132–1140. doi:10.1124/mol.108.048348 |
| 13. | Zhu, J.; Ji, Z.; Wang, J.; Sun, R.; Zhang, X.; Gao, Y.; Sun, H.; Liu, Y.; Wang, Z.; Li, A. Q.; Ma, J.; Wang, T.; Jia, G.; Gu, Y. Small 2008, 4, 1168–1175. doi:10.1002/smll.200701219 |
| 34. | Arbogast, J. W.; Foote, C. S.; Kao, M. J. Am. Chem. Soc. 1992, 114, 2277–2279. doi:10.1021/ja00032a063 |
| 35. | Biczok, L.; Linschitz, H.; Walter, R. I. Chem. Phys. Lett. 1992, 195, 339–346. doi:10.1016/0009-2614(92)85613-F |
| 36. | Stasko, A.; Brezova, V.; Biskupic, S.; Dinse, K.-P.; Schweitzer, P; Baumgarten, M. J. Phys. Chem. 1995, 99, 8782–8789. doi:10.1021/j100021a052 |
| 37. | Dimitrijevic, N. M.; Kamat, P. V. J. Phys. Chem. 1993, 97, 7623–7626. doi:10.1021/j100131a035 |
| 4. | Silva, G. A. Nat. Rev. Neurosci. 2006, 65–74. doi:10.1038/nrn1827 |
| 5. | Silva, G. A. Surg. Neurol. 2005, 63, 301–306. doi:10.1016/j.surneu.2004.06.008 |
| 6. | Dugan, L. L.; Lovett, E. G.; Quick, K. L.; Lotharius, J.; Lin, T. T.; O’Malley, K. L. Parkinsonism Rel. Disord. 2001, 7, 243–246. doi:10.1016/S1353-8020(00)00064-X |
| 7. | Dugan, L. L.; Gabrielsen, J. K.; Yu, S. P.; Lin, T.-S.; Choi, D. W. Neurobiology of Diseases. 1996, 3, 129–135. doi:10.1006/nbdi.1996.0013 |
| 30. | Reeks, L. P.; Eichinger, P. C. H.; Bowie, J. H. Rapid Commun. Mass Spectrom. 1993, 7, 286–287. doi:10.1002/rcm.1290070405 |
| 2. | Gelderman, M. P.; Simakova, O.; Clogston, J. D.; Patri, A. K.; Siddiqui, S. F.; Vostal, A. C.; Simak, J. Int. J. Nanomed. 2008, 3, 59–68. doi:10.2147/IJN.S1680 |
| 3. | Lu, L.-H.; Lee, Y.-T.; Chen, H.-W.; Chiang, L. Y.; Huang, H.-C. Br. J. Pharmacol. 1998, 123, 1097–1102. doi:10.1038/sj.bjp.0701722 |
| 31. | Morishetti, K. M.; Sripadi, P.; Mariappandar, V.; Ren, J. Int. J. Mass Spectrom. 2011, 299, 169–177. doi:10.1016/j.ijms.2010.10.025 |
| 22. | Isaacson, C. W.; Kleber, M.; Field, J. A. Environ. Sci. Technol. 2009, 43, 6463–6474. doi:10.1021/es900692e |
| 24. | Singh, R.; Goswami, T. Thermochim. Acta 2011, 513, 60–67. doi:10.1016/j.tca.2010.11.012 |
| 25. | Bobylev, A. G.; Kornev, A. B.; Bobyleva, L. G.; Shpagina, M. D.; Fadeeva, I. S.; Fadeev, R. S.; Deryabin, D. G.; Balzarini, J.; Troshin, P. A.; Podlubnay, Z. A. Org. Biomol. Chem. 2011, 9, 5714–5719. doi:10.1039/c1ob05067b |
| 26. | Kokubo, K.; Matsubayashi, K.; Tategaki, H.; Takada, H.; Oshima, T. ACS Nano 2008, 2, 327–333. doi:10.1021/nn700151z |
| 27. | Kokubo, K.; Shirakawa, S.; Kobayashi, N.; Aoshima, H.; Oshima, T. Nano Res. 2011, 4, 204–215. doi:10.1007/s12274-010-0071-z |
| 28. | Semenov, K. N.; Letenko, D. G.; Charykov, N. A.; Nikitin, V. A.; Matuzenko, M. Y.; Keskinov, V. A.; Postnov, V. N.; Kopyrin, A. A. Russ. J. Appl. Chem. 2010, 83, 2076–2080. doi:10.1134/S1070427210120025 |
| 21. | Singh, R.; Goswami, T. J. Phys. Org. Chem. 2008, 21, 225–236. doi:10.1002/poc.1304 |
| 29. | Powell, W. H.; Cozzi, F.; Moss, G. P.; Thilgen, C.; Hwu, R. J.-R.; Yeryn, A. Pure Appl. Chem. 2002, 74, 629–695. doi:10.1351/pac200274040629 |
| 20. | Husebo, L. O.; Sitharaman, B.; Furukawa, K.; Kato, T.; Wilson, L. J. J. Am. Chem. Soc. 2004, 126, 12055–12064. doi:10.1021/ja047593o |
| 16. | Bogdanović, G.; Kojić, V.; Ðordević, A.; Canadanović-Brunet, J.; Vojinović-Miloradov, M.; Baltić, V. V. Toxicol. in Vitro 2004, 18, 629–637. doi:10.1016/j.tiv.2004.02.010 |
| 17. | Chiang, L. Y.; Upasani, R. B.; Swirczewski, J. W.; Soled, S. J. Am. Chem. Soc. 1993, 115, 5453–5457. doi:10.1021/ja00066a014 |
| 18. | Schneider, N. S.; Darwish, A. D.; Kroto, H. W.; Taylor, R.; Walton, D. R. M. J. Chem. Soc., Chem. Commun. 1994, 463–464. doi:10.1039/C39940000463 |
| 19. | Chiang, L. Y.; Upasani, R. B.; Swirczewski, J. W. J. Am. Chem. Soc. 1992, 114, 10154–10157. doi:10.1021/ja00052a010 |
| 23. | Chao, T.-C.; Guixue, S.; Hansmeier, N.; Westerhoff, P.; Herckes, P.; Halden, R. U. Anal. Chem. 2011, 83, 1777–1783. doi:10.1021/ac1031379 |
© 2013 Silion et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)