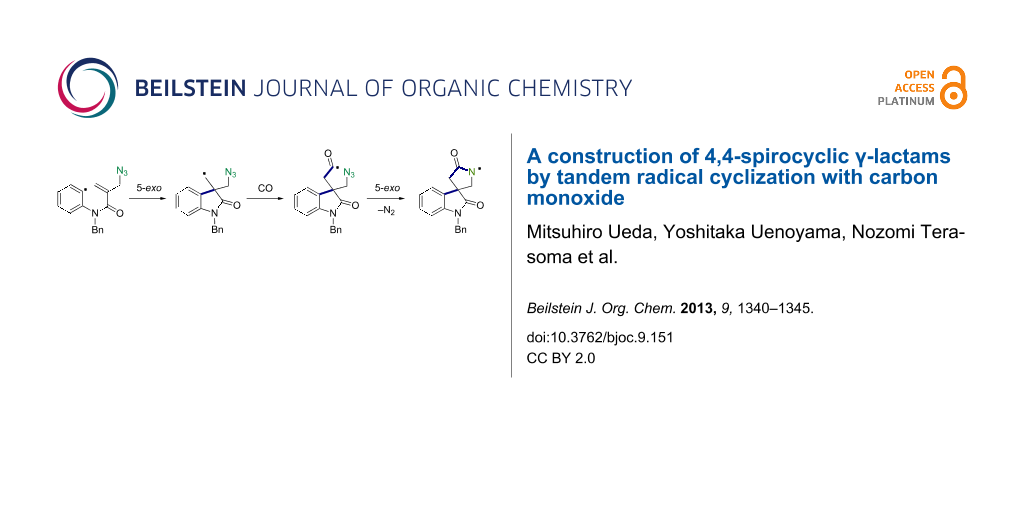
Abstract
A straightforward synthesis of 4,4-spirocyclic indol γ-lactams by tandem radical cyclization of iodoaryl allyl azides with CO was achieved. The reaction of iodoaryl allyl azides, TTMSS and AIBN under CO pressure (80 atm) in THF at 80 °C gave the desired 4,4-spirocyclic indoline, benzofuran, and oxindole γ-lactams in moderate to good yields.
Graphical Abstract
Introduction
4,4-Spirocyclic oxindole γ-lactams containing a quaternary carbon center are key structures for the synthesis of biologically active natural products and the related analogues [1-4]. Therefore, the development of an efficient synthesis of this spiro structure is of continued interest for synthetic chemists. Recently, Comesse and Daïch reported the synthesis of 4,4-spirocyclic oxindole γ-lactams by tandem spirocyclization via nucleophilic halide displacement and amide coupling [4]. Shaw and co-workers reported the synthesis of 4,4-spirocyclic oxindole γ-lactams by the cycloaddition of imines and succinic anhydrides [5]. Tandem radical cyclization can also provide a powerful tool for the construction of heterocycles [6-12]. One of us previously reported on the construction of spirocyclic pyrrolidinyl oxindoles by the tandem reaction of iodoaryl alkenyl azides under radical conditions (Scheme 1) [13,14]. Curran et al. reported the synthesis of spirocyclic pyrrolidinyl dihydroquinolinones by tandem radical cyclization [15,16].
Scheme 1: A construction of spirocyclic pyrrolidinyl oxindole by tandem radical cyclization with azide [14].
Scheme 1: A construction of spirocyclic pyrrolidinyl oxindole by tandem radical cyclization with azide [14].
In this study we report a radical cyclization/annulation approach to 4.4-spirocyclic γ-lactams in which CO was introduced as the lactam carbonyl moiety [17-23]. Our approach consists of a sequence of aryl radical cyclization, radical carbonylation [24-27], and spirocyclization of the resulting acyl radical onto an azide group, which can give 4,4-spirocyclic γ-lactams (Scheme 2).
Scheme 2: A tandem radical cyclization/annulation strategy for the synthesis of 4,4-spirocyclic γ-lactams with the incorporation of CO.
Scheme 2: A tandem radical cyclization/annulation strategy for the synthesis of 4,4-spirocyclic γ-lactams wit...
Results and Discussion
For the first model reaction in our investigation of the development of a novel tandem radical cyclization/annulation strategy, we prepared N-(2-(azidomethyl)allyl)-N-(2-iodophenyl)-4-methylbenzenesulfonamide (1a) according to the methods shown in Scheme 3. The reaction of 1a with Bu3SnH (2.0 equiv) and AIBN (2,2’- azobisisobutyronitrile, 0.3 equiv) was carried out under CO pressure (80 atm) in THF (0.02 M) at 80 °C for 12 h, which gave the desired 4,4-spirocyclic indoline γ-lactam 2a in 48% yield (Scheme 4). We found that the modest improvement in the yield of 2a to 53% was achieved by changing the mediator from Bu3SnH to TTMSS [tris(trimethylsilyl)silane].
Scheme 4: The tandem radical spirocyclization reaction of N-(2-(azidomethyl)allyl)-N-(2-iodophenyl)-4-methylbenzenesulfonamide (1a) with CO.
Scheme 4: The tandem radical spirocyclization reaction of N-(2-(azidomethyl)allyl)-N-(2-iodophenyl)-4-methylb...
The tandem spirocyclization with CO was investigated with several 2-iodoaryl compounds having an allyl azide moiety. Results are summarized in Table 1. The reaction of N-(2-(azidomethyl)allyl)-N-(2-iodo-5-methoxyphenyl)-4-methylbenzenesulfonamide (1b) with CO gave the corresponding spiro lactam 2b in 53% yield (Table 1, entry 2). N-(2-(Azidomethyl)allyl)-N-(2-iodophenyl)methanesulfonamide (1c) showed a comparable reactivity with 1a and 1b (Table 1, entry 3). The reaction of 1-(2-(azidomethyl)allyloxy)-2-iodobenzene (1d) also gave the spiro benzofuran lactam 2d in 58% yield (Table 1, entry 4). On the other hand, 2-(azidomethyl)allyl(2-iodophenyl)sulfane (1e) gave a low yield of the corresponding spiro thiobenzofuran lactam (19%, Table 1, entry 5), which may be rationalized by the less effective cyclization due to the longer C–S bonds.
Table 1: Synthesis of 4,4-spirocyclic γ-lactams 2 by tandem radical spirocyclization of 1 with CO.a
|
|
|||
| Entry | Substrate (1) | Product (2) | Yield (%) |
|---|---|---|---|
| 1b |
1a |
2a |
53 |
| 2b |
1b |
2b |
53 |
| 3 |
1c |
2c |
55 |
| 4 |
1d |
2d |
58 |
| 5c |
1e |
2e |
19 |
| 6 |
1f |
2f |
62 |
| 7 |
1g |
3 |
60d |
aReaction conditions: 1 (1.0 equiv), CO (80 atm), AIBN (0.3 equiv), TTMSS (2.0 equiv), THF (0.02 M), bath temperature 80 °C, 12 h. bReaction time: 24 h. cThe reaction was carried out at a bath temperature of 110 °C. dYield of 3.
We then tried to extend the tandem spirocyclization approach to obtain 4,4-spirocyclic oxindole γ-lactam and tested two substrates, 2-(azidomethyl)-N-benzyl-N-(2-iodophenyl)acrylamide (1f) and the nitrogen-unprotected analogue 1g. The reaction of 1f was smooth to give the desired 4,4-spirocyclic oxindole γ-lactam 2f in 62% yield (Table 1, entry 6). On the other hand, the reaction of 1g gave the cyclized product in only a trace amount, and instead THF-incorporating 6-endo cyclization product 3 was obtained in 60% yield (Table 1, entry 7) [28].
Based on the known chemistry of radical cyclization and carbonylation reactions, a possible mechanism for the spirocyclization of 1f with CO is shown in Scheme 5. The iodoaryl allyl azide 1f is converted to an aryl radical A via the iodine atom abstraction by the (TMS)3Si radical. The subsequent 5-exo cyclization of aryl radical A gives an alkyl radical B, which adds to CO to give an acyl radical C. Finally, the 5-exo addition of acyl radical C onto an azide group takes place with the liberation of dinitrogen to give a cyclized amidyl radical D [29,30], which abstracts hydrogen from TTMSS, affording the 4,4-spirocyclic indoline γ-lactam 2f and a (TMS)3Si radical, thus creating a radical chain.
Scheme 5: Proposed mechanism for a construction of 4,4-spirocyclic indoline γ-lactam 2f by the tandem radical cyclization of 1f with CO.
Scheme 5: Proposed mechanism for a construction of 4,4-spirocyclic indoline γ-lactam 2f by the tandem radical...
On the other hand, the unusual formation of THF-incorporating lactam 3 from 1g may be rationalized by the consecutive 6-endo cyclization of E and β-elimination of an azidyl radical from the resulting F, to give 2-methylene lactam G (Scheme 6). Then, the THF radical is formed via the α-hydrogen abstraction by the azidyl radical [31-34], which is attached to G to give α-carbonyl radical H. Finally, H abstracts hydrogen from TTMSS, affording the THF-incorporating product 3 and the (TMS)3Si radical, which participates in the next chain reaction.
Scheme 6: Proposed mechanism for the formation of THF-incorporating product 3 from 1g.
Scheme 6: Proposed mechanism for the formation of THF-incorporating product 3 from 1g.
Conclusion
We have examined a TTMSS-mediated 5-exo radical cyclization/carbonylation/spirocyclization sequence to synthesize 4,4-spirocyclic rings. By using this protocol, indoline, benzofuran and oxindole γ-lactams can be conveniently prepared in moderate to good yields. As shown in the contrasting results of acrylic amides 1f and 1g, to cause the requisite 5-exo cyclization of aryl radicals onto allylic azide in preference to the 6-endo cyclization, the angle compression caused by the substitution on the nitrogen has to be considered carefully. Nevertheless, our method can provide a steady tool for the ring formation of 4,4-spirocyclic γ-lactams with the incorporation of CO as a carbonyl group.
Experimental
Typical procedure for a construction of 4,4-spirocyclic γ-lactams by tandem radical cyclization with CO: A magnetic stirring bar, 2-(azidomethyl)-N-benzyl-N-(2-iodophenyl)acrylamide (1f) (150.0 mg, 0.36 mmol), AIBN (2,2’-azobisisobutyronitrile, 17.7 mg, 0.11 mmol), TTMSS ([tris(trimethylsilyl)silane], 178.3 mg, 0.72 mmol) and THF (17.9 mL; 0.02 M) were placed in a 50 mL stainless steel autoclave. The autoclave was closed, purged three times with CO, pressurized with 80 atm of CO, and then heated at 80 °C (bath temperature) for 12 h. Excess CO was discharged after the reaction. The reaction mixture was concentrated in vacuo. The resulting residue was purified by column chromatography on silica gel (hexane/EtOAc 2:1) to give the desired 4,4-spirocyclic oxindole γ-lactam product 2f as a colorless oil in 62% yield (65.3 mg, 0.22 mmol). ¹H NMR (400 MHz, CDCl3) δ 7.39–7.16 (m, 7H), 7.07 (t, J = 7.6 Hz, 1H), 6.79 (d, J = 7.6 Hz, 1H), 5.89 (s, 1H), 4.93 (s, 2H), 3.91 (d, J = 9.2 Hz, 1H), 3.50 (d, J = 9.2 Hz, 1H) 3.02 (d, J = 16.8 Hz, 1H), 2.51 (d, J = 16.8 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 177.5, 175.4, 141.9, 135.5, 133.2, 129.1, 129.0, 128.0, 127.4, 123.6, 122.3, 109.7, 51.1, 49.7, 44.3, 40.4; IR (neat): 3418, 3061, 2927, 1696, 1613, 1488, 1467, 1455, 1380, 1368, 1177 cm−1; HRMS–FAB (m/z): [M + H]+ calcd for C18H17N2O2, 293.1290; found, 293.1299.
References
-
Edmondson, S.; Danishefsky, S. J.; Sepp-Lorenzino, L.; Rosen, N. J. Am. Chem. Soc. 1999, 121, 2147–2155. doi:10.1021/ja983788i
Return to citation in text: [1] -
Cravotto, G.; Giovenzana, G. B.; Pilati, T.; Sisti, M.; Palmisano, G. J. Org. Chem. 2001, 66, 8447–8453. doi:10.1021/jo015854w
Return to citation in text: [1] -
Marti, C.; Carreira, E. M. Eur. J. Org. Chem. 2003, 2209–2219. doi:10.1002/ejoc.200300050
Return to citation in text: [1] -
Allous, I.; Comesse, S.; Berkeš, D.; Alkyat, A.; Daïch, A. Tetrahedron Lett. 2009, 50, 4411–4415. doi:10.1016/j.tetlet.2009.02.114
Return to citation in text: [1] [2] -
Tan, D. Q.; Atherton, A. L.; Smith, A. J.; Soldi, C.; Hurley, K. A.; Fettinger, J. C.; Shaw, J. T. ACS Comb. Sci. 2012, 14, 218–223. doi:10.1021/co2001873
Return to citation in text: [1] -
Beckwith, A. L. J. Chem. Soc. Rev. 1993, 22, 143–151. doi:10.1039/cs9932200143
Return to citation in text: [1] -
Ishibashi, H.; Sato, T.; Ikeda, M. Synthesis 2002, 695–713. doi:10.1055/s-2002-25759
Return to citation in text: [1] -
Srikanth, G. S. C.; Castle, S. L. Tetrahedron 2005, 61, 10377–10441. doi:10.1016/j.tet.2005.07.077
Return to citation in text: [1] -
Fallis, A. G.; Brinza, I. M. Tetrahedron 1997, 53, 17543–17594. doi:10.1016/S0040-4020(97)10060-6
Return to citation in text: [1] -
Friestad, G. K. Tetrahedron 2001, 57, 5461–5496. doi:10.1016/S0040-4020(01)00384-2
Return to citation in text: [1] -
Bowman, W. R.; Fletcher, A. J.; Potts, G. B. S. J. Chem. Soc., Perkin Trans. 1 2002, 2747–2762. doi:10.1039/B108582B
Return to citation in text: [1] -
Minozzi, M.; Nanni, D.; Spagnolo, P. Chem.–Eur. J. 2009, 15, 7830–7840. doi:10.1002/chem.200802710
Return to citation in text: [1] -
Patro, B.; Murphy, J. A. Org. Lett. 2000, 2, 3599–3601. doi:10.1021/ol006477x
Return to citation in text: [1] -
Lizos, D. E.; Murphy, J. A. Org. Biomol. Chem. 2003, 1, 117–122. doi:10.1039/b208114h
Return to citation in text: [1] [2] -
González-López de Turiso, F.; Curran, D. P. Org. Lett. 2005, 7, 151–154. doi:10.1021/ol0477226
Return to citation in text: [1] -
Zhang, H.; Curran, D. P. J. Am. Chem. Soc. 2011, 133, 10376–10378. doi:10.1021/ja2042854
Return to citation in text: [1] -
Ryu, I.; Matsu, K.; Minakata, S.; Komatsu, M. J. Am. Chem. Soc. 1998, 120, 5838–5839. doi:10.1021/ja980731n
Return to citation in text: [1] -
Ryu, I.; Miyazato, H.; Kuriyama, H.; Matsu, K.; Tojino, M.; Fukuyama, T.; Minakata, S.; Komatsu, M. J. Am. Chem. Soc. 2003, 125, 5632–5633. doi:10.1021/ja034896u
Return to citation in text: [1] -
Tojino, M.; Otsuka, N.; Fukuyama, T.; Matsubara, H.; Schiesser, C. H.; Kuriyama, H.; Miyazato, H.; Minakata, S.; Komatsu, M.; Ryu, I. Org. Biomol. Chem. 2003, 1, 4262–4267. doi:10.1039/b309944j
Return to citation in text: [1] -
Tojino, M.; Otsuka, N.; Fukuyama, T.; Matsubara, H.; Ryu, I. J. Am. Chem. Soc. 2006, 128, 7712–7713. doi:10.1021/ja0623865
Return to citation in text: [1] -
Kyne, S. H.; Lin, C. Y.; Ryu, I.; Coote, M. L.; Schiesser, C. H. Chem. Commun. 2010, 46, 6521–6523. doi:10.1039/c0cc01262a
Return to citation in text: [1] -
Ryu, I.; Fukuyama, T.; Tojino, M.; Uenoyama, Y.; Yonamine, Y.; Terasoma, N.; Matsubara, H. Org. Biomol. Chem. 2011, 9, 3780–3786. doi:10.1039/c1ob05145h
Return to citation in text: [1] -
Fukuyama, T.; Nakashima, N.; Okada, T.; Ryu, I. J. Am. Chem. Soc. 2013, 135, 1006–1008. doi:10.1021/ja312654q
Return to citation in text: [1] -
Ryu, I.; Sonoda, N. Angew. Chem., Int. Ed. Engl. 1996, 35, 1050–1066. doi:10.1002/anie.199610501
Return to citation in text: [1] -
Ryu, I.; Sonoda, N.; Curran, D. P. Chem. Rev. 1996, 96, 177–194. doi:10.1021/cr9400626
Return to citation in text: [1] -
Ryu, I. Chem. Soc. Rev. 2001, 30, 16–25. doi:10.1039/a904591k
Return to citation in text: [1] -
Chatgilialogu, C.; Crich, D.; Komatsu, M.; Ryu, I. Chem. Rev. 1999, 99, 1991–2070. doi:10.1021/cr9601425
Return to citation in text: [1] -
Jones, K.; Wilkinson, J. J. Chem. Soc., Chem. Commun. 1992, 1767–1769. doi:10.1039/C39920001767
Similar 6-endo cyclization was reported previously.
Return to citation in text: [1] -
Kim, S.; Joe, G. H.; Do, J. Y. J. Am. Chem. Soc. 1994, 116, 5521–5522. doi:10.1021/ja00091a087
Return to citation in text: [1] -
Benati, L.; Leardini, R.; Minozzi, M.; Nanni, D.; Spagnolo, P.; Strazzari, S.; Zanardi, G. Org. Lett. 2002, 4, 3079–3081. doi:10.1021/ol026366t
Return to citation in text: [1] -
Viuf, C.; Bols, M. Angew. Chem., Int. Ed. 2001, 40, 623–625. doi:10.1002/1521-3773(20010202)40:3<623::AID-ANIE623>3.0.CO;2-G
Return to citation in text: [1] -
Marinescu, L. G.; Pedersen, C. M.; Bols, M. Tetrahedron 2005, 61, 123–127. doi:10.1016/j.tet.2004.10.040
Return to citation in text: [1] -
Pedersen, C. M.; Marinescu, L. G.; Bols, M. Org. Biomol. Chem. 2005, 3, 816–822. doi:10.1039/b500037h
Return to citation in text: [1] -
Klima, R. F.; Jadhav, A. V.; Singh, P. N. D.; Chang, M.; Vanos, C.; Sankaranarayanan, J.; Vu, M.; Ibrahim, N.; Ross, E.; McCloskey, S.; Murthy, R. S.; Krause, J. A.; Ault, B. S.; Gudmundsdóttir, A. D. J. Org. Chem. 2007, 72, 6372–6381. doi:10.1021/jo070558q
Return to citation in text: [1]
| 1. | Edmondson, S.; Danishefsky, S. J.; Sepp-Lorenzino, L.; Rosen, N. J. Am. Chem. Soc. 1999, 121, 2147–2155. doi:10.1021/ja983788i |
| 2. | Cravotto, G.; Giovenzana, G. B.; Pilati, T.; Sisti, M.; Palmisano, G. J. Org. Chem. 2001, 66, 8447–8453. doi:10.1021/jo015854w |
| 3. | Marti, C.; Carreira, E. M. Eur. J. Org. Chem. 2003, 2209–2219. doi:10.1002/ejoc.200300050 |
| 4. | Allous, I.; Comesse, S.; Berkeš, D.; Alkyat, A.; Daïch, A. Tetrahedron Lett. 2009, 50, 4411–4415. doi:10.1016/j.tetlet.2009.02.114 |
| 13. | Patro, B.; Murphy, J. A. Org. Lett. 2000, 2, 3599–3601. doi:10.1021/ol006477x |
| 14. | Lizos, D. E.; Murphy, J. A. Org. Biomol. Chem. 2003, 1, 117–122. doi:10.1039/b208114h |
| 6. | Beckwith, A. L. J. Chem. Soc. Rev. 1993, 22, 143–151. doi:10.1039/cs9932200143 |
| 7. | Ishibashi, H.; Sato, T.; Ikeda, M. Synthesis 2002, 695–713. doi:10.1055/s-2002-25759 |
| 8. | Srikanth, G. S. C.; Castle, S. L. Tetrahedron 2005, 61, 10377–10441. doi:10.1016/j.tet.2005.07.077 |
| 9. | Fallis, A. G.; Brinza, I. M. Tetrahedron 1997, 53, 17543–17594. doi:10.1016/S0040-4020(97)10060-6 |
| 10. | Friestad, G. K. Tetrahedron 2001, 57, 5461–5496. doi:10.1016/S0040-4020(01)00384-2 |
| 11. | Bowman, W. R.; Fletcher, A. J.; Potts, G. B. S. J. Chem. Soc., Perkin Trans. 1 2002, 2747–2762. doi:10.1039/B108582B |
| 12. | Minozzi, M.; Nanni, D.; Spagnolo, P. Chem.–Eur. J. 2009, 15, 7830–7840. doi:10.1002/chem.200802710 |
| 5. | Tan, D. Q.; Atherton, A. L.; Smith, A. J.; Soldi, C.; Hurley, K. A.; Fettinger, J. C.; Shaw, J. T. ACS Comb. Sci. 2012, 14, 218–223. doi:10.1021/co2001873 |
| 4. | Allous, I.; Comesse, S.; Berkeš, D.; Alkyat, A.; Daïch, A. Tetrahedron Lett. 2009, 50, 4411–4415. doi:10.1016/j.tetlet.2009.02.114 |
| 24. | Ryu, I.; Sonoda, N. Angew. Chem., Int. Ed. Engl. 1996, 35, 1050–1066. doi:10.1002/anie.199610501 |
| 25. | Ryu, I.; Sonoda, N.; Curran, D. P. Chem. Rev. 1996, 96, 177–194. doi:10.1021/cr9400626 |
| 26. | Ryu, I. Chem. Soc. Rev. 2001, 30, 16–25. doi:10.1039/a904591k |
| 27. | Chatgilialogu, C.; Crich, D.; Komatsu, M.; Ryu, I. Chem. Rev. 1999, 99, 1991–2070. doi:10.1021/cr9601425 |
| 29. | Kim, S.; Joe, G. H.; Do, J. Y. J. Am. Chem. Soc. 1994, 116, 5521–5522. doi:10.1021/ja00091a087 |
| 30. | Benati, L.; Leardini, R.; Minozzi, M.; Nanni, D.; Spagnolo, P.; Strazzari, S.; Zanardi, G. Org. Lett. 2002, 4, 3079–3081. doi:10.1021/ol026366t |
| 17. | Ryu, I.; Matsu, K.; Minakata, S.; Komatsu, M. J. Am. Chem. Soc. 1998, 120, 5838–5839. doi:10.1021/ja980731n |
| 18. | Ryu, I.; Miyazato, H.; Kuriyama, H.; Matsu, K.; Tojino, M.; Fukuyama, T.; Minakata, S.; Komatsu, M. J. Am. Chem. Soc. 2003, 125, 5632–5633. doi:10.1021/ja034896u |
| 19. | Tojino, M.; Otsuka, N.; Fukuyama, T.; Matsubara, H.; Schiesser, C. H.; Kuriyama, H.; Miyazato, H.; Minakata, S.; Komatsu, M.; Ryu, I. Org. Biomol. Chem. 2003, 1, 4262–4267. doi:10.1039/b309944j |
| 20. | Tojino, M.; Otsuka, N.; Fukuyama, T.; Matsubara, H.; Ryu, I. J. Am. Chem. Soc. 2006, 128, 7712–7713. doi:10.1021/ja0623865 |
| 21. | Kyne, S. H.; Lin, C. Y.; Ryu, I.; Coote, M. L.; Schiesser, C. H. Chem. Commun. 2010, 46, 6521–6523. doi:10.1039/c0cc01262a |
| 22. | Ryu, I.; Fukuyama, T.; Tojino, M.; Uenoyama, Y.; Yonamine, Y.; Terasoma, N.; Matsubara, H. Org. Biomol. Chem. 2011, 9, 3780–3786. doi:10.1039/c1ob05145h |
| 23. | Fukuyama, T.; Nakashima, N.; Okada, T.; Ryu, I. J. Am. Chem. Soc. 2013, 135, 1006–1008. doi:10.1021/ja312654q |
| 31. | Viuf, C.; Bols, M. Angew. Chem., Int. Ed. 2001, 40, 623–625. doi:10.1002/1521-3773(20010202)40:3<623::AID-ANIE623>3.0.CO;2-G |
| 32. | Marinescu, L. G.; Pedersen, C. M.; Bols, M. Tetrahedron 2005, 61, 123–127. doi:10.1016/j.tet.2004.10.040 |
| 33. | Pedersen, C. M.; Marinescu, L. G.; Bols, M. Org. Biomol. Chem. 2005, 3, 816–822. doi:10.1039/b500037h |
| 34. | Klima, R. F.; Jadhav, A. V.; Singh, P. N. D.; Chang, M.; Vanos, C.; Sankaranarayanan, J.; Vu, M.; Ibrahim, N.; Ross, E.; McCloskey, S.; Murthy, R. S.; Krause, J. A.; Ault, B. S.; Gudmundsdóttir, A. D. J. Org. Chem. 2007, 72, 6372–6381. doi:10.1021/jo070558q |
| 14. | Lizos, D. E.; Murphy, J. A. Org. Biomol. Chem. 2003, 1, 117–122. doi:10.1039/b208114h |
| 15. | González-López de Turiso, F.; Curran, D. P. Org. Lett. 2005, 7, 151–154. doi:10.1021/ol0477226 |
| 16. | Zhang, H.; Curran, D. P. J. Am. Chem. Soc. 2011, 133, 10376–10378. doi:10.1021/ja2042854 |
| 28. |
Jones, K.; Wilkinson, J. J. Chem. Soc., Chem. Commun. 1992, 1767–1769. doi:10.1039/C39920001767
Similar 6-endo cyclization was reported previously. |
© 2013 Ueda et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)