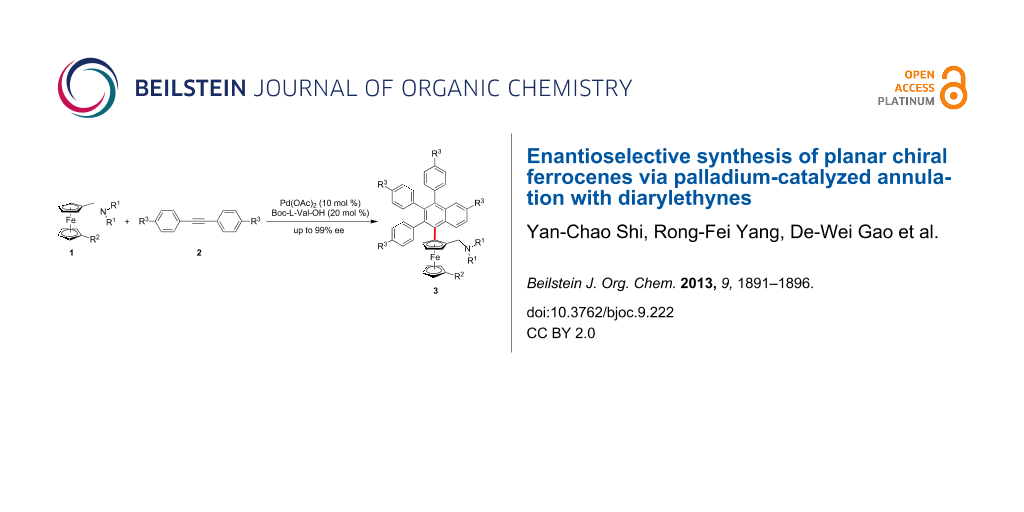
Abstract
When Boc-L-Val-OH was used as a ligand for the enantioselective Pd(II)-catalyzed annulation of N,N-substituted aminomethyl ferrocene derivatives with diarylethynes, ferrocenes with planar chirality could be achieved with excellent enantioselectivity (up to 99% ee).
Graphical Abstract
Introduction
Chiral ferrocene derivatives have been widely applied to asymmetric catalysis, materials science, biomedical research, etc. [1-4]. Particularly, ferrocenes with planar chirality are applied as efficient ligands or catalysts in asymmetric catalysis [5-15]. However, the typical method for introduction of planar chirality in the ferrrocene backbone is still utilizing the chiral auxiliaries strategy [16-21]. Snieckus and co-workers reported the synthesis of planar chiral ferrocenes by utilizing an external chiral base such as (−)-sparteine [22,23]. Ogasawara and co-workers used the ring closing metathesis reaction to provide a novel and efficient route to synthesize the planar chiral ferrocenes [24-28]. Despite these pioneering studies, the catalytic asymmetric methods to introduce ferrocenyl planar chirality are rather limited.
Recently, a monoprotected amino acid was introduced as an efficient ligand in Pd-catalyzed enantioselective C–H activation by Yu and co-workers [29-50]. Inspired by their works, we reported a direct arylation of ferrrocene with arylboronic acid to introduce planar chirality into the ferrocene backbone using N,N-dimethylaminomethyl as the directing group and Boc-L-Val-OH as the ligand [51-57]. The product could be transformed into a planar chiral P,N-ligand, which was found to be efficient for Pd-catalyzed allylic alkylation reaction albeit with low enantioselectivity.
We envisaged that by introducing a larger substituent R in the ferrocene Cp ring would enhance the enantiocontrol of the planar chiral P,N-ligand (Scheme 1). Cui, Wu and their co-workers recently reported a Pd-catalyzed dehydrogenative annulation of N,N-dimethylaminomethylferrocene in a racemic form [58-66]. To test our hypothesis, we decided to turn such a Pd-catalyzed direct coupling of N,N-disubstituted aminomethylferrocenes with diarylethyne into an enantioselective reaction. Then planar chiral ferrocenyl P,N-ligands with a large substituent could be readily synthesized. In this paper, we report the results from this study.
Scheme 1: Design of the planar chiral P,N-ligand.
Scheme 1: Design of the planar chiral P,N-ligand.
Results and Discussion
We initiated the study by testing the reaction of ferrrocene 1a in a palladium-catalyzed direct coupling with diphenylethyne in the presence of 10 mol % Pd(OAc)2, 20 mol % Boc-L-Phe-OH, 25 mol % TBAB, and 100 mol % K2CO3 in DMA at 110 °C under air. To our great delight, the reaction furnished the desired product 3aa in 28% yield and 84% ee (entry 1, Table 1). When the temperature was decreased to 80 °C, the reaction was sluggish (the ferrocene starting material was consumed in 48 h) and the enantioselectivity was improved to 93% ee (33% yield, 93% ee, entry 2, Table 1). When Fmoc-L-Phe-OH was used as the ligand, the enantioselectivity decreased dramatically (37% ee, entry 3, Table 1). Next, an array of N-Boc protected L-amino acids was investigated. The results are summarized in Table 1. In general, all N-Boc protected L-amino acids gave excellent enantioselectivity (>90% ee, entries 4–9, Table 1). Boc-L-Val-OH and Boc-L-Tle-OH were found to be the optimal chiral ligands, providing the desired product in 98% ee (entries 6 and 7, Table 1). Boc-L-Val-OH was chosen as the ligand for further studies because of the higher yield (42% yield) obtained compared with Boc-L-Tle-OH (31% yield).
Table 1: Examination of ligands, temperature and additivesa.
|
|
||||||
| Entry | Ligand | Additive | Temp (°C) | t (h) | Yield (%)b | ee (%)c |
|---|---|---|---|---|---|---|
| 1 | Boc-L-Phe-OH | TBAB | 110 | 24 | 28 | 84 |
| 2 | Boc-L-Phe-OH | TBAB | 80 | 48 | 33 | 93 |
| 3 | Fmoc-L-Phe-OH | TBAB | 80 | 48 | 32 | 37 |
| 4 | Boc-L-Ala-OH | TBAB | 80 | 48 | 29 | 90 |
| 5 | Boc-L-Abu-OH | TBAB | 80 | 48 | 37 | 90 |
| 6 | Boc-L-Val-OH | TBAB | 80 | 48 | 42 | 98 |
| 7 | Boc-L-Tle-OH | TBAB | 80 | 48 | 31 | 98 |
| 8 | Boc-L-Ile-OH·0.5H2O | TBAB | 80 | 48 | 47 | 92 |
| 9 | Boc-L-Leu-OH | TBAB | 80 | 48 | 45 | 90 |
| 10 | Ac-L-Val-OH | TBAB | 80 | 48 | 33 | 94 |
| 11 | Cbz-L-Val-OH | TBAB | 80 | 48 | 18 | 94 |
| 12 | Boc-L-Val-OH | TBAB | 60 | 48 | 29 | 97 |
| 13 | Boc-L-Val-OH | TBAB | 110 | 24 | 31 | 85 |
| 14 | Boc-L-Val-OH | TBACl | 80 | 48 | 28 | 96 |
| 15 | Boc-L-Val-OH | TBAI | 80 | 48 | 39 | 95 |
| 16 | Boc-L-Val-OH | – | 80 | 48 | 29 | 76 |
aReaction conditions: 1a (0.2 mmol), 2a (2.3 equiv), Pd(OAc)2 (10 mol %), ligand (20 mol %), K2CO3 (100 mol %), additive (25 mol %) in 1.5 mL DMA under air. bIsolated yield. cDetermined by HPLC analysis. TBAB = tetrabutylammonium bromide. TBACl = tetrabutylammonium chloride. TBAI = tetrabutylammonium iodide. DMA = dimethylacetamide.
When the protecting group on the nitrogen of L-Val-OH was changed to Ac or Cbz, the enantioselectivity slightly decreased (94% ee, entries 10 and 11, Table 1). The oxidants such as Cu(OAc)2, Cu(OTf)2, Ag2CO3, Ag2O, AgOAc and benzoquinone (BQ) were examined but none of them could improve the yield efficiently (for details, see Supporting Information File 1). Lowering the reaction temperature to 60 °C, excellent enantioselectivity (97% ee) could be obtained, but with a decreased yield as the starting material was not fully consumed (entry 12, Table 1). When TBACl (entry 14, Table 1) or TBAI (entry 15, Table 1) was used instead of TBAB as the additive, excellent enantioselectivity was maintained. The enantioselectivity decreased dramatically when no additive was used (entry 16, Table 1). The optimized conditions were obtained as the following: 10 mol % Pd(OAc)2, 2.3 equiv of diarylethyne, 20 mol % Boc-L-Val-OH, 100 mol % K2CO3 and 25 mol % TBAB in DMA at 80 °C under air (entry 6, Table 1). To be noted, the yields reported in the corresponding racemic study [58] in general are higher; however, these results are not reproduced in our hands. In our studies, although the ferrocene starting material was fully consumed in most of the cases, the sensitivity of ferrocene derivatives toward oxidation conditions might lead to the low yields.
With the above mentioned optimized conditions, various aminomethylferrocene derivatives and diarylethynes were tested to evaluate the scope of this reaction. The results are given in Table 2. Various substituted diarylethynes with either an electron-donating group or an electron-withdrawing group were tolerated providing the corresponding products in 28–45% yields with 92–99% ee. All the reactions gave excellent enantioselectivity but moderate yields. When a diarylethyne bearing a 4-methoxy group was used, the yield was relatively higher (3ac, 45% yield; 3bc, 41% yield; 3cc, 40% yield). To broaden the scope of this methodology, alkyl groups on nitrogen atom were also varied (3ba, 35% yield, 95% ee; 3bb, 30% yield, 97% ee; 3bc, 41% yield, 97% ee). Interestingly, when 1-[(N,N-dimethylamino)methyl]-1’-bromoferrocene (1c), with a bromine atom at the second Cp ring, was used, the annulation reaction could proceed smoothly (28–42% yields, 92–96% ee).
Table 2: Enantioselective synthesis of planar chiral ferrocenes via C–H activationa.
|
|
|||||
| Entry | 1 | 2 | 3 | Yield (%)b | ee (%)c |
|---|---|---|---|---|---|
| 1 | 1a | 2a | 3aa | 42 | 98 |
| 2 | 1a | 2b | 3ab | 35 | 97 |
| 3 | 1a | 2c | 3ac | 45 | 99 |
| 4 | 1a | 2d | 3ad | 31 | 97 |
| 5 | 1b | 2a | 3ba | 35 | 95 |
| 6 | 1b | 2b | 3bb | 30 | 97 |
| 7 | 1b | 2c | 3bc | 41 | 97 |
| 8 | 1c | 2a | 3ca | 42 | 96 |
| 9 | 1c | 2b | 3cb | 28 | 96 |
| 10 | 1c | 2c | 3cc | 40 | 96 |
| 11 | 1c | 2d | 3cd | 30 | 92 |
aReaction conditions: 1 (0.2 mmol or 0.3 mmol), 2 (2.3 equiv), Pd(OAc)2 (10 mol %), Boc-L-Val-OH (20 mol %), K2CO3 (100 mol %), TBAB (25 mol %) in 1.5 mL DMA at 80 °C under air. bIsolated yield. cDetermined by HPLC analysis.
The absolute configuration of the products was assigned as Sp from the cyclopalladated complex described in the literature and our previous study [51,67,68]. Next, to test our original hypothesis, planar chiral P,N-ligand (Sp)-L1 was prepared from (Sp)-3ca. Starting from (Sp)-3ca (96% ee), lithiation with n-BuLi followed by quenching with Ph2PCl gave the planar chiral P,N-ligand (Sp)-L1 in 43% yield and 97% ee (Scheme 2). The allylic substitution reactions of (rac)-4 had been carried out. The allylic alkylation reaction proceeded in 95% yield and 44% ee (Scheme 3, reaction 1) and the allylic amination reaction proceeded in 32% yield and 43% ee (Scheme 3, reaction 2) [69-71]. Although only moderate enantioselectivity was obtained, significant increase of enantioselectivity was obtained comparing with 15% ee obtained by (Sp)-L2 in allylic alkylation reaction [51]. The results indicated that the planar P,N-ligand with a larger R group could improve the enantioselectivity.
Scheme 2: Synthesis of planar chiral P,N-ligand (Sp)-L1.
Scheme 2: Synthesis of planar chiral P,N-ligand (Sp)-L1.
Scheme 3: Pd-catalyzed asymmetric allylic alkylation and amination reactions with (Sp)-L1.
Scheme 3: Pd-catalyzed asymmetric allylic alkylation and amination reactions with (Sp)-L1.
Conclusion
In summary, we reported a highly enantioselective synthesis of planar chiral ferrocenes via palladium-catalyzed direct annulation of N,N-disubstituted aminomethylferrocene derivatives with diarylethynes. The commercially available N-Boc-L-Val-OH is an efficient ligand with air as a suitable oxidant. The planar chiral ferrocenes could be transformed readily into a P,N-ligand, which was found to be suitable for Pd-catalyzed allylic substitution reactions.
Experimental
General procedure for the enantioselective synthesis of planar chiral ferrocenes
To a solution of alkyne 2 (0.46 mmol) in DMA (1.5 mL) was added Boc-L-Val-OH (8.7 mg, 0.04 mmol), Pd(OAc)2 (4.5 mg, 0.02 mmol), K2CO3 (27.6 mg, 0.2 mmol), TBAB (tetrabutyl ammonium bromide) (16.1 mg, 0.05 mmol) and ferrocene 1 (0.02 mmol) successively. The mixture was stirred at 80 °C under air (open flask) for 48 h. After the reaction was complete, it was quenched with saturated aqueous NaHCO3 solution and extracted with EtOAc for three times. The combined organic layers were washed with H2O and brine successively, then dried over anhydrous Na2SO4 and filtrated. After the solvent was removed under reduced pressure, the residue was purified by silica gel column chromatography (ethyl acetate/petroleum ether 1:10, v/v, 3% Et3N) to afford the desired product 3.
Supporting Information
| Supporting Information File 1: Experimental, characterization data and spectra. | ||
| Format: PDF | Size: 2.0 MB | Download |
References
-
Hayashi, T.; Togni, A., Eds. Ferrocenes; VCH: Weinheim, Germany, 1995.
Return to citation in text: [1] -
Dai, L.-X.; Hou, X.-L., Eds. Chiral Ferrocenes in Asymmetric Catalysis; Wiley-VCH: Weinheim, Germany, 2010.
Return to citation in text: [1] -
Fabre, B. Acc. Chem. Res. 2010, 43, 1509–1518. doi:10.1021/ar100085q
Return to citation in text: [1] -
Moriuchi, T.; Hirao, T. Acc. Chem. Res. 2010, 43, 1040–1051. doi:10.1021/ar100022n
Return to citation in text: [1] -
Halterman, R. L. Chem. Rev. 1992, 92, 965–994. doi:10.1021/cr00013a011
Return to citation in text: [1] -
Togni, A. Angew. Chem., Int. Ed. Engl. 1996, 35, 1475–1477. doi:10.1002/anie.199614751
Return to citation in text: [1] -
Richards, C. J.; Locke, A. J. Tetrahedron: Asymmetry 1998, 9, 2377–2407. doi:10.1016/S0957-4166(98)00251-1
Return to citation in text: [1] -
Fu, G. C. Acc. Chem. Res. 2000, 33, 412–420. doi:10.1021/ar990077w
Return to citation in text: [1] -
Dai, L.-X.; Tu, T.; You, S.-L.; Deng, W.-P.; Hou, X.-L. Acc. Chem. Res. 2003, 36, 659–667. doi:10.1021/ar020153m
Return to citation in text: [1] -
Colacot, T. J. Chem. Rev. 2003, 103, 3101–3118. doi:10.1021/cr000427o
Return to citation in text: [1] -
Fu, G. C. Acc. Chem. Res. 2004, 37, 542–547. doi:10.1021/ar030051b
Return to citation in text: [1] -
Arrayás, R. G.; Adrio, J.; Carretero, J. C. Angew. Chem., Int. Ed. 2006, 45, 7674–7715. doi:10.1002/anie.200602482
Return to citation in text: [1] -
Fu, G. C. Acc. Chem. Res. 2006, 39, 853–860. doi:10.1021/ar068115g
Return to citation in text: [1] -
Roberts, D. J.; Gregg, D. J.; Fitchett, C. M.; Draper, S. M. Organometallics 2010, 29, 6541–6547. doi:10.1021/om100862a
Return to citation in text: [1] -
Arae, S.; Ogasawara, M. J. Synth. Org. Chem., Jpn. 2012, 70, 593–605. doi:10.5059/yukigoseikyokaishi.70.593
Return to citation in text: [1] -
Battelle, L. F.; Bau, R.; Gokel, G. W.; Oyakawa, R. T.; Ugi, I. K. J. Am. Chem. Soc. 1973, 95, 482–486. doi:10.1021/ja00783a030
Return to citation in text: [1] -
Rebière, F.; Riant, O.; Ricard, L.; Kagan, H. B. Angew. Chem., Int. Ed. Engl. 1993, 32, 568–570. doi:10.1002/anie.199305681
Return to citation in text: [1] -
Riant, O.; Samuel, O.; Kagan, H. B. J. Am. Chem. Soc. 1993, 115, 5835–5836. doi:10.1021/ja00066a066
Return to citation in text: [1] -
Richards, C. J.; Damalidis, T.; Hibbs, D. E.; Hursthouse, M. B. Synlett 1995, 74–76. doi:10.1055/s-1995-4864
Return to citation in text: [1] -
Riant, O.; Samuel, O.; Flessner, T.; Taudien, S.; Kagan, H. B. J. Org. Chem. 1997, 62, 6733–6745. doi:10.1021/jo970075u
Return to citation in text: [1] -
Enders, D.; Peters, R.; Lochtman, R.; Raabe, G. Angew. Chem., Int. Ed. 1999, 38, 2421–2423. doi:10.1002/(SICI)1521-3773(19990816)38:16<2421::AID-ANIE2421>3.3.CO;2-I
Return to citation in text: [1] -
Tsukazaki, M.; Tinkl, M.; Roglans, A.; Chapell, B. J.; Taylor, N. J.; Snieckus, V. J. Am. Chem. Soc. 1996, 118, 685–686. doi:10.1021/ja953246q
Return to citation in text: [1] -
Laufer, R. S.; Veith, U.; Taylor, N. J.; Snieckus, V. Org. Lett. 2000, 2, 629–631. doi:10.1021/ol991381s
Return to citation in text: [1] -
Ogasawara, M.; Watanabe, S.; Fan, L.; Nakajima, K.; Takahashi, T. Organometallics 2006, 25, 5201–5203. doi:10.1021/om0608298
Return to citation in text: [1] -
Ogasawara, M.; Watanabe, S.; Nakajima, K.; Takahashi, T. J. Am. Chem. Soc. 2010, 132, 2136–2137. doi:10.1021/ja910348z
Return to citation in text: [1] -
Ogasawara, M.; Arae, S.; Watanabe, S.; Nakajima, K.; Takahashi, T. Chem.–Eur. J. 2013, 19, 4151–4154. doi:10.1002/chem.201300116
Return to citation in text: [1] -
Ogasawara, M.; Watanabe, S.; Nakajima, K.; Takahashi, T. Pure Appl. Chem. 2008, 80, 1109–1113. doi:10.1351/pac200880051109
Return to citation in text: [1] -
Ogasawara, M.; Watanabe, S. Synthesis 2009, 1761–1785. doi:10.1055/s-0029-1216818
Return to citation in text: [1] -
Shi, B.-F.; Maugel, N.; Zhang, Y.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2008, 47, 4882–4886. doi:10.1002/anie.200801030
Return to citation in text: [1] -
Shi, B.-F.; Zhang, Y.-H.; Lam, J. K.; Wang, D.-H.; Yu, J.-Q. J. Am. Chem. Soc. 2010, 132, 460–461. doi:10.1021/ja909571z
Return to citation in text: [1] -
Wasa, M.; Engle, K. M.; Lin, D. W.; Yoo, E. J.; Yu, J.-Q. J. Am. Chem. Soc. 2011, 133, 19598–19601. doi:10.1021/ja207607s
Return to citation in text: [1] -
Musaev, D. G.; Kaledin, A.; Shi, B.-F.; Yu, J.-Q. J. Am. Chem. Soc. 2012, 134, 1690–1698. doi:10.1021/ja208661v
Return to citation in text: [1] -
Cheng, X.-F.; Li, Y.; Su, Y.-M.; Yin, F.; Wang, J.-Y.; Sheng, J.; Vora, H. U.; Wang, X.-S.; Yu, J.-Q. J. Am. Chem. Soc. 2013, 135, 1236–1239. doi:10.1021/ja311259x
Return to citation in text: [1] -
Daugulis, O.; Zaitsev, V. G.; Shabashov, D.; Pham, Q.-N.; Lazareva, A. Synlett 2006, 3382–3388. doi:10.1055/s-2006-956468
Return to citation in text: [1] -
Campeau, L.-C.; Fagnou, K. Chem. Commun. 2006, 1253–1264. doi:10.1039/b515481m
Return to citation in text: [1] -
Li, B.-J.; Yang, S.-D.; Shi, Z.-J. Synlett 2008, 949–957. doi:10.1055/s-2008-1042907
Return to citation in text: [1] -
Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094–5115. doi:10.1002/anie.200806273
Return to citation in text: [1] -
Daugulis, O.; Do, H.-Q.; Shabashov, D. Acc. Chem. Res. 2009, 42, 1074–1086. doi:10.1021/ar9000058
Return to citation in text: [1] -
Giri, R.; Shi, B.-F.; Engle, K. M.; Maugel, N.; Yu, J.-Q. Chem. Soc. Rev. 2009, 38, 3242–3272. doi:10.1039/b816707a
Return to citation in text: [1] -
Ackermann, L.; Vicente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792–9826. doi:10.1002/anie.200902996
Return to citation in text: [1] -
Peng, H. M.; Dai, L.-X.; You, S.-L. Angew. Chem., Int. Ed. 2010, 49, 5826–5828. doi:10.1002/anie.201000799
Return to citation in text: [1] -
Yu, J.-Q.; Shi, Z.-J. Top. Curr. Chem. 2010, 292.
Return to citation in text: [1] -
Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Chem. Commun. 2010, 46, 677–685. doi:10.1039/b908581e
Return to citation in text: [1] -
Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147–1169. doi:10.1021/cr900184e
Return to citation in text: [1] -
Sehnal, P.; Taylor, R. J. K.; Fairlamb, I. J. S. Chem. Rev. 2010, 110, 824–889. doi:10.1021/cr9003242
Return to citation in text: [1] -
Ackermann, L. Chem. Rev. 2011, 111, 1315–1345. doi:10.1021/cr100412j
Return to citation in text: [1] -
Engle, K. M.; Mei, T.-S.; Wasa, M.; Yu, J.-Q. Acc. Chem. Res. 2012, 45, 788–802. doi:10.1021/ar200185g
Return to citation in text: [1] -
Neufeldt, S. R.; Sanford, M. S. Acc. Chem. Res. 2012, 45, 936–946. doi:10.1021/ar300014f
Return to citation in text: [1] -
Yang, L.; Huang, H. Catal. Sci. Technol. 2012, 2, 1099–1112. doi:10.1039/c2cy20111a
Return to citation in text: [1] -
Li, D.; He, C.; Cai, H.; Wang, G. Chin. J. Org. Chem. 2013, 33, 203–223. doi:10.6023/cjoc201209022
Return to citation in text: [1] -
Gao, D.-W.; Shi, Y.-C.; Gu, Q.; Zhao, Z.-L.; You, S.-L. J. Am. Chem. Soc. 2013, 135, 86–89. doi:10.1021/ja311082u
Return to citation in text: [1] [2] [3] -
Pi, C.; Li, Y.; Cui, X.; Zhang, H.; Han, Y.; Wu, Y. Chem. Sci. 2013, 4, 2675–2679. doi:10.1039/c3sc50577d
Return to citation in text: [1] -
Sokolov, V. I.; Troitskaya, L. L.; Reutov, O. A. J. Organomet. Chem. 1979, 182, 537–546. doi:10.1016/S0022-328X(00)83942-X
Return to citation in text: [1] -
Xia, J.-B.; You, S.-L. Organometallics 2007, 26, 4869–4871. doi:10.1021/om700806e
Return to citation in text: [1] -
Takebayashi, S.; Shizuno, T.; Otani, T.; Shibata, T. Beilstein J. Org. Chem. 2012, 8, 1844–1848. doi:10.3762/bjoc.8.212
Return to citation in text: [1] -
Takebayashi, S.; Shibata, T. Organometallics 2012, 31, 4114–4117. doi:10.1021/om300348e
Return to citation in text: [1] -
Singh, K. S.; Dixneuf, P. H. Organometallics 2012, 31, 7320–7323. doi:10.1021/om3008162
Return to citation in text: [1] -
Zhang, H.; Cui, X.; Yao, X.; Wang, H.; Zhang, J.; Wu, Y. Org. Lett. 2012, 14, 3012–3015. doi:10.1021/ol301063k
Return to citation in text: [1] [2] -
Dupont, J.; Pfeffer, M.; Rotteveel, M. A.; De Cian, A.; Fischer, J. Organometallics 1989, 8, 1116–1118. doi:10.1021/om00106a040
Return to citation in text: [1] -
Pfeffer, M.; Rotteveel, M. A.; Sutter, J.-P. J. Organomet. Chem. 1989, 371, C21–C25. doi:10.1016/0022-328X(89)85220-9
Return to citation in text: [1] -
Pfeffer, M.; Sutter, J.-P.; Rotteveel, M. A.; Cian, A. D.; Fischer, J. Tetrahedron 1992, 48, 2427–2440. doi:10.1016/S0040-4020(01)88762-7
Return to citation in text: [1] -
Wu, Y.-T.; Huang, K.-H.; Shin, C.-C.; Wu, T.-C. Chem.–Eur. J. 2008, 14, 6697–6703. doi:10.1002/chem.200800538
Return to citation in text: [1] -
Shi, Z.; Ding, S.; Cui, Y.; Jiao, N. Angew. Chem., Int. Ed. 2009, 48, 7895–7898. doi:10.1002/anie.200903975
Return to citation in text: [1] -
Yamashita, M.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2009, 11, 2337–2340. doi:10.1021/ol900736s
Return to citation in text: [1] -
Yamashita, M.; Horiguchi, H.; Hirano, K.; Satoh, T.; Miura, M. J. Org. Chem. 2009, 74, 7481–7488. doi:10.1021/jo9016698
Return to citation in text: [1] -
Wu, J.; Cui, X.; Mi, X.; Li, Y.; Wu, Y. Chem. Commun. 2010, 46, 6771–6773. doi:10.1039/c0cc01448f
Return to citation in text: [1] -
Günay, M. E.; Richards, C. J. Organometallics 2009, 28, 5833–5836. doi:10.1021/om9005356
Return to citation in text: [1] -
Dendele, N.; Bisaro, F.; Gaumont, A.-C.; Perriob, S.; Richards, C. J. Chem. Commun. 2012, 48, 1991–1993. doi:10.1039/c2cc16864b
Return to citation in text: [1] -
Hayashi, T.; Yamamoto, A.; Hagihara, T.; Ito, Y. Tetrahedron Lett. 1986, 27, 191–194. doi:10.1016/S0040-4039(00)83974-X
Return to citation in text: [1] -
Hayashi, T.; Yamamoto, A.; Ito, Y.; Nishioka, E.; Miura, H.; Yanagi, K. J. Am. Chem. Soc. 1989, 111, 6301–6311. doi:10.1021/ja00198a048
Return to citation in text: [1] -
You, S.-L.; Hou, X.-L.; Dai, L.-X.; Yu, Y.-H.; Xia, W. J. Org. Chem. 2002, 67, 4684–4695. doi:10.1021/jo016330z
Return to citation in text: [1]
| 1. | Hayashi, T.; Togni, A., Eds. Ferrocenes; VCH: Weinheim, Germany, 1995. |
| 2. | Dai, L.-X.; Hou, X.-L., Eds. Chiral Ferrocenes in Asymmetric Catalysis; Wiley-VCH: Weinheim, Germany, 2010. |
| 3. | Fabre, B. Acc. Chem. Res. 2010, 43, 1509–1518. doi:10.1021/ar100085q |
| 4. | Moriuchi, T.; Hirao, T. Acc. Chem. Res. 2010, 43, 1040–1051. doi:10.1021/ar100022n |
| 24. | Ogasawara, M.; Watanabe, S.; Fan, L.; Nakajima, K.; Takahashi, T. Organometallics 2006, 25, 5201–5203. doi:10.1021/om0608298 |
| 25. | Ogasawara, M.; Watanabe, S.; Nakajima, K.; Takahashi, T. J. Am. Chem. Soc. 2010, 132, 2136–2137. doi:10.1021/ja910348z |
| 26. | Ogasawara, M.; Arae, S.; Watanabe, S.; Nakajima, K.; Takahashi, T. Chem.–Eur. J. 2013, 19, 4151–4154. doi:10.1002/chem.201300116 |
| 27. | Ogasawara, M.; Watanabe, S.; Nakajima, K.; Takahashi, T. Pure Appl. Chem. 2008, 80, 1109–1113. doi:10.1351/pac200880051109 |
| 28. | Ogasawara, M.; Watanabe, S. Synthesis 2009, 1761–1785. doi:10.1055/s-0029-1216818 |
| 22. | Tsukazaki, M.; Tinkl, M.; Roglans, A.; Chapell, B. J.; Taylor, N. J.; Snieckus, V. J. Am. Chem. Soc. 1996, 118, 685–686. doi:10.1021/ja953246q |
| 23. | Laufer, R. S.; Veith, U.; Taylor, N. J.; Snieckus, V. Org. Lett. 2000, 2, 629–631. doi:10.1021/ol991381s |
| 16. | Battelle, L. F.; Bau, R.; Gokel, G. W.; Oyakawa, R. T.; Ugi, I. K. J. Am. Chem. Soc. 1973, 95, 482–486. doi:10.1021/ja00783a030 |
| 17. | Rebière, F.; Riant, O.; Ricard, L.; Kagan, H. B. Angew. Chem., Int. Ed. Engl. 1993, 32, 568–570. doi:10.1002/anie.199305681 |
| 18. | Riant, O.; Samuel, O.; Kagan, H. B. J. Am. Chem. Soc. 1993, 115, 5835–5836. doi:10.1021/ja00066a066 |
| 19. | Richards, C. J.; Damalidis, T.; Hibbs, D. E.; Hursthouse, M. B. Synlett 1995, 74–76. doi:10.1055/s-1995-4864 |
| 20. | Riant, O.; Samuel, O.; Flessner, T.; Taudien, S.; Kagan, H. B. J. Org. Chem. 1997, 62, 6733–6745. doi:10.1021/jo970075u |
| 21. | Enders, D.; Peters, R.; Lochtman, R.; Raabe, G. Angew. Chem., Int. Ed. 1999, 38, 2421–2423. doi:10.1002/(SICI)1521-3773(19990816)38:16<2421::AID-ANIE2421>3.3.CO;2-I |
| 5. | Halterman, R. L. Chem. Rev. 1992, 92, 965–994. doi:10.1021/cr00013a011 |
| 6. | Togni, A. Angew. Chem., Int. Ed. Engl. 1996, 35, 1475–1477. doi:10.1002/anie.199614751 |
| 7. | Richards, C. J.; Locke, A. J. Tetrahedron: Asymmetry 1998, 9, 2377–2407. doi:10.1016/S0957-4166(98)00251-1 |
| 8. | Fu, G. C. Acc. Chem. Res. 2000, 33, 412–420. doi:10.1021/ar990077w |
| 9. | Dai, L.-X.; Tu, T.; You, S.-L.; Deng, W.-P.; Hou, X.-L. Acc. Chem. Res. 2003, 36, 659–667. doi:10.1021/ar020153m |
| 10. | Colacot, T. J. Chem. Rev. 2003, 103, 3101–3118. doi:10.1021/cr000427o |
| 11. | Fu, G. C. Acc. Chem. Res. 2004, 37, 542–547. doi:10.1021/ar030051b |
| 12. | Arrayás, R. G.; Adrio, J.; Carretero, J. C. Angew. Chem., Int. Ed. 2006, 45, 7674–7715. doi:10.1002/anie.200602482 |
| 13. | Fu, G. C. Acc. Chem. Res. 2006, 39, 853–860. doi:10.1021/ar068115g |
| 14. | Roberts, D. J.; Gregg, D. J.; Fitchett, C. M.; Draper, S. M. Organometallics 2010, 29, 6541–6547. doi:10.1021/om100862a |
| 15. | Arae, S.; Ogasawara, M. J. Synth. Org. Chem., Jpn. 2012, 70, 593–605. doi:10.5059/yukigoseikyokaishi.70.593 |
| 58. | Zhang, H.; Cui, X.; Yao, X.; Wang, H.; Zhang, J.; Wu, Y. Org. Lett. 2012, 14, 3012–3015. doi:10.1021/ol301063k |
| 69. | Hayashi, T.; Yamamoto, A.; Hagihara, T.; Ito, Y. Tetrahedron Lett. 1986, 27, 191–194. doi:10.1016/S0040-4039(00)83974-X |
| 70. | Hayashi, T.; Yamamoto, A.; Ito, Y.; Nishioka, E.; Miura, H.; Yanagi, K. J. Am. Chem. Soc. 1989, 111, 6301–6311. doi:10.1021/ja00198a048 |
| 71. | You, S.-L.; Hou, X.-L.; Dai, L.-X.; Yu, Y.-H.; Xia, W. J. Org. Chem. 2002, 67, 4684–4695. doi:10.1021/jo016330z |
| 58. | Zhang, H.; Cui, X.; Yao, X.; Wang, H.; Zhang, J.; Wu, Y. Org. Lett. 2012, 14, 3012–3015. doi:10.1021/ol301063k |
| 59. | Dupont, J.; Pfeffer, M.; Rotteveel, M. A.; De Cian, A.; Fischer, J. Organometallics 1989, 8, 1116–1118. doi:10.1021/om00106a040 |
| 60. | Pfeffer, M.; Rotteveel, M. A.; Sutter, J.-P. J. Organomet. Chem. 1989, 371, C21–C25. doi:10.1016/0022-328X(89)85220-9 |
| 61. | Pfeffer, M.; Sutter, J.-P.; Rotteveel, M. A.; Cian, A. D.; Fischer, J. Tetrahedron 1992, 48, 2427–2440. doi:10.1016/S0040-4020(01)88762-7 |
| 62. | Wu, Y.-T.; Huang, K.-H.; Shin, C.-C.; Wu, T.-C. Chem.–Eur. J. 2008, 14, 6697–6703. doi:10.1002/chem.200800538 |
| 63. | Shi, Z.; Ding, S.; Cui, Y.; Jiao, N. Angew. Chem., Int. Ed. 2009, 48, 7895–7898. doi:10.1002/anie.200903975 |
| 64. | Yamashita, M.; Hirano, K.; Satoh, T.; Miura, M. Org. Lett. 2009, 11, 2337–2340. doi:10.1021/ol900736s |
| 65. | Yamashita, M.; Horiguchi, H.; Hirano, K.; Satoh, T.; Miura, M. J. Org. Chem. 2009, 74, 7481–7488. doi:10.1021/jo9016698 |
| 66. | Wu, J.; Cui, X.; Mi, X.; Li, Y.; Wu, Y. Chem. Commun. 2010, 46, 6771–6773. doi:10.1039/c0cc01448f |
| 51. | Gao, D.-W.; Shi, Y.-C.; Gu, Q.; Zhao, Z.-L.; You, S.-L. J. Am. Chem. Soc. 2013, 135, 86–89. doi:10.1021/ja311082u |
| 51. | Gao, D.-W.; Shi, Y.-C.; Gu, Q.; Zhao, Z.-L.; You, S.-L. J. Am. Chem. Soc. 2013, 135, 86–89. doi:10.1021/ja311082u |
| 52. | Pi, C.; Li, Y.; Cui, X.; Zhang, H.; Han, Y.; Wu, Y. Chem. Sci. 2013, 4, 2675–2679. doi:10.1039/c3sc50577d |
| 53. | Sokolov, V. I.; Troitskaya, L. L.; Reutov, O. A. J. Organomet. Chem. 1979, 182, 537–546. doi:10.1016/S0022-328X(00)83942-X |
| 54. | Xia, J.-B.; You, S.-L. Organometallics 2007, 26, 4869–4871. doi:10.1021/om700806e |
| 55. | Takebayashi, S.; Shizuno, T.; Otani, T.; Shibata, T. Beilstein J. Org. Chem. 2012, 8, 1844–1848. doi:10.3762/bjoc.8.212 |
| 56. | Takebayashi, S.; Shibata, T. Organometallics 2012, 31, 4114–4117. doi:10.1021/om300348e |
| 57. | Singh, K. S.; Dixneuf, P. H. Organometallics 2012, 31, 7320–7323. doi:10.1021/om3008162 |
| 29. | Shi, B.-F.; Maugel, N.; Zhang, Y.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2008, 47, 4882–4886. doi:10.1002/anie.200801030 |
| 30. | Shi, B.-F.; Zhang, Y.-H.; Lam, J. K.; Wang, D.-H.; Yu, J.-Q. J. Am. Chem. Soc. 2010, 132, 460–461. doi:10.1021/ja909571z |
| 31. | Wasa, M.; Engle, K. M.; Lin, D. W.; Yoo, E. J.; Yu, J.-Q. J. Am. Chem. Soc. 2011, 133, 19598–19601. doi:10.1021/ja207607s |
| 32. | Musaev, D. G.; Kaledin, A.; Shi, B.-F.; Yu, J.-Q. J. Am. Chem. Soc. 2012, 134, 1690–1698. doi:10.1021/ja208661v |
| 33. | Cheng, X.-F.; Li, Y.; Su, Y.-M.; Yin, F.; Wang, J.-Y.; Sheng, J.; Vora, H. U.; Wang, X.-S.; Yu, J.-Q. J. Am. Chem. Soc. 2013, 135, 1236–1239. doi:10.1021/ja311259x |
| 34. | Daugulis, O.; Zaitsev, V. G.; Shabashov, D.; Pham, Q.-N.; Lazareva, A. Synlett 2006, 3382–3388. doi:10.1055/s-2006-956468 |
| 35. | Campeau, L.-C.; Fagnou, K. Chem. Commun. 2006, 1253–1264. doi:10.1039/b515481m |
| 36. | Li, B.-J.; Yang, S.-D.; Shi, Z.-J. Synlett 2008, 949–957. doi:10.1055/s-2008-1042907 |
| 37. | Chen, X.; Engle, K. M.; Wang, D.-H.; Yu, J.-Q. Angew. Chem., Int. Ed. 2009, 48, 5094–5115. doi:10.1002/anie.200806273 |
| 38. | Daugulis, O.; Do, H.-Q.; Shabashov, D. Acc. Chem. Res. 2009, 42, 1074–1086. doi:10.1021/ar9000058 |
| 39. | Giri, R.; Shi, B.-F.; Engle, K. M.; Maugel, N.; Yu, J.-Q. Chem. Soc. Rev. 2009, 38, 3242–3272. doi:10.1039/b816707a |
| 40. | Ackermann, L.; Vicente, R.; Kapdi, A. R. Angew. Chem., Int. Ed. 2009, 48, 9792–9826. doi:10.1002/anie.200902996 |
| 41. | Peng, H. M.; Dai, L.-X.; You, S.-L. Angew. Chem., Int. Ed. 2010, 49, 5826–5828. doi:10.1002/anie.201000799 |
| 42. | Yu, J.-Q.; Shi, Z.-J. Top. Curr. Chem. 2010, 292. |
| 43. | Sun, C.-L.; Li, B.-J.; Shi, Z.-J. Chem. Commun. 2010, 46, 677–685. doi:10.1039/b908581e |
| 44. | Lyons, T. W.; Sanford, M. S. Chem. Rev. 2010, 110, 1147–1169. doi:10.1021/cr900184e |
| 45. | Sehnal, P.; Taylor, R. J. K.; Fairlamb, I. J. S. Chem. Rev. 2010, 110, 824–889. doi:10.1021/cr9003242 |
| 46. | Ackermann, L. Chem. Rev. 2011, 111, 1315–1345. doi:10.1021/cr100412j |
| 47. | Engle, K. M.; Mei, T.-S.; Wasa, M.; Yu, J.-Q. Acc. Chem. Res. 2012, 45, 788–802. doi:10.1021/ar200185g |
| 48. | Neufeldt, S. R.; Sanford, M. S. Acc. Chem. Res. 2012, 45, 936–946. doi:10.1021/ar300014f |
| 49. | Yang, L.; Huang, H. Catal. Sci. Technol. 2012, 2, 1099–1112. doi:10.1039/c2cy20111a |
| 50. | Li, D.; He, C.; Cai, H.; Wang, G. Chin. J. Org. Chem. 2013, 33, 203–223. doi:10.6023/cjoc201209022 |
| 51. | Gao, D.-W.; Shi, Y.-C.; Gu, Q.; Zhao, Z.-L.; You, S.-L. J. Am. Chem. Soc. 2013, 135, 86–89. doi:10.1021/ja311082u |
| 67. | Günay, M. E.; Richards, C. J. Organometallics 2009, 28, 5833–5836. doi:10.1021/om9005356 |
| 68. | Dendele, N.; Bisaro, F.; Gaumont, A.-C.; Perriob, S.; Richards, C. J. Chem. Commun. 2012, 48, 1991–1993. doi:10.1039/c2cc16864b |
© 2013 Shi et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)