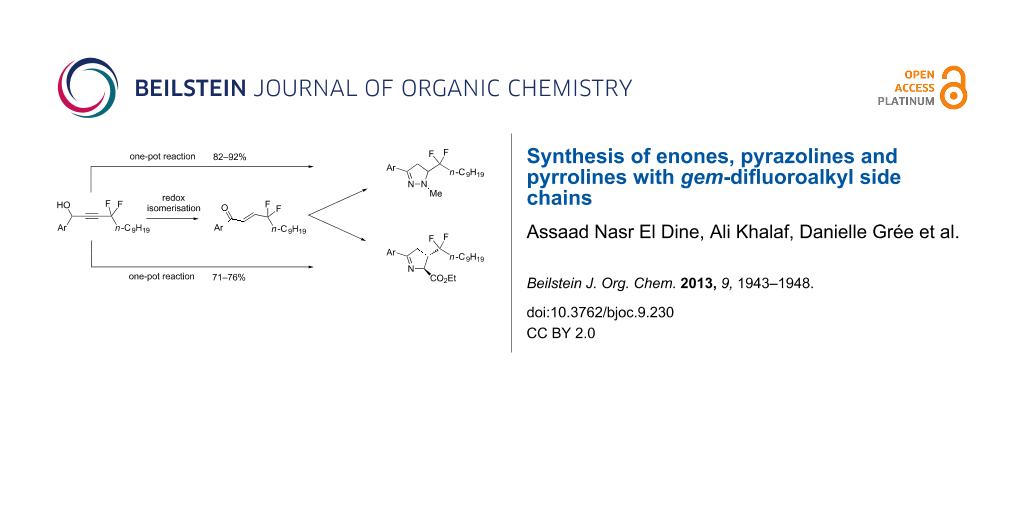
Abstract
Starting from easily accessible gem-difluoropropargylic derivatives, a DBU-mediated isomerisation affords enones in fair yields with a gem-difluoroalkyl chain. These derivatives were used to prepare pyrazolines and pyrrolines with the desired gem-difluoroalkyl side chain by cyclocondensations in good yields and with excellent stereoselectivity. A one-pot process was also successfully developed for these sequential reactions. By carrying out various types of Pd-catalyzed coupling reactions for compounds with a p-bromophenyl substituent a route to focused chemical libraries was demonstrated.
Graphical Abstract
Introduction
A widely used strategy in bioorganic, medicinal chemistry and in chemical biology is the selected introduction of fluorine in organic molecules since it strongly modifies their properties [1-9]. On the other hand, heterocyclic molecules – in particular, the so-called privileged scaffolds – are introduced very classically in the core of pharmaceutical products [10,11]. Therefore, it appears to be of much interest to design novel methodologies for the preparation of new fluorinated heterocyclic molecules. We developed a programme to investigate the preparation and uses of new propargylic fluorides [12-14], which have been employed in the synthesis of fluorinated analogues of lipids [15,16] and in carbocyclic systems [17,18]. They have also been used for the preparation of several 5 and 6-membered heterocycles [19-22]. The goal of the present work is to demonstrate that selected propargylic derivatives [23-25] can be employed for the preparation of enones with a gem-difluoroalkyl chain by using an isomerisation process (Scheme 1).
Scheme 1: Strategy towards the target molecules.
Scheme 1: Strategy towards the target molecules.
These intermediates can be employed for the preparation of representative 5-membered heterocyclic systems with CF2R side chains by using cyclocondensation reactions. Furthermore, selected molecules in these series were functionalized by using appropriate palladium-catalyzed coupling reactions en route to chemical libraries.
Results and Discussion
The first example of a base-mediated isomerisation process for an alkyne activated by an ester group was reported by Nineham and Raphael in 1949 [26]. Later, extension to other electrophilic alkynes was demonstrated by Sonye and Koide [27]. Recently, it has been established by Yamazaki’s group that propargylic alcohols bearing a CF3 group on the triple bond could be isomerised to the corresponding enones. In that case, Et3N proved to be sufficient as a catalyst to perform this transformation [28].
The required starting propargylic alcohols were obtained by a reaction of the lithium salt of easily available gem-difluoro propargylic derivative 1 [18] with aromatic aldehydes, affording compounds 2a–2e in 71–82% yields (Scheme 2 and Table 1). With these gem-difluoro intermediates, Et3N was not an efficient catalyst since only a low conversion was observed and the reaction was not clean. On the contrary, the DBU-mediated isomerisation was successful, affording the desired enones 3a–3e in 60–63% yields. The selectivity was excellent since in all cases the E-isomer was obtained almost exclusively (>98%). Similar reactions were carried out with propargylic derivatives bearing alkyl groups instead of the (Ar) aromatic or heteroaromatic group, but these reactions were not successful. Therefore, this reaction appears limited to derivatives with aryl or heteroaryl substituents as in the case of the CF3-substituted propargylic derivatives [28].
Scheme 2: Synthesis of enones with a gem-difluoroalkyl side chain.
Scheme 2: Synthesis of enones with a gem-difluoroalkyl side chain.
Next, we turned towards the preparation of heterocyclic structures from enones 3. Pyrazolines are well-recognized heterocyclic cores for pharmacologically active molecules [29]. Therefore, they were selected as first examples of 5-membered heterocyclic targets with the fluorinated side chain. Reaction of 3a–3e with methylhydrazine gave the desired pyrazolines 4a–4e in 79–86% yields (Scheme 3).
Scheme 3: Synthesis of pyrazolines with a gem-difluoro side chain.
Scheme 3: Synthesis of pyrazolines with a gem-difluoro side chain.
It appeared that the reaction conditions for the synthesis of these pyrazolines were compatible with the first isomerisation step, therefore the possibility of a "one-pot" reaction was considered. Indeed, by heating a mixture of propargylic alcohols 2a–2e with DBU (1,8-diazabicycloundec-7-ene) in the presence of methylhydrazine (Scheme 4) the pyrazolines 4a–4e were obtained after 3–7 h in excellent yields (82–92%, Table 2). This very short and efficient synthesis of pyrazolines 4 can be related to another excellent one-pot reaction with 3-components where the first step is a Pd-catalyzed coupling–isomerisation process followed by a cyclocondensation [30].
Scheme 4: One-pot synthesis of pyrazolines with a gem-difluoro side chain.
Scheme 4: One-pot synthesis of pyrazolines with a gem-difluoro side chain.
Pyrroline is another noteworthy example of a heterocyclic scaffold useful in bioorganic and medicinal chemistry [31,32]. It is also well-recognized for agrochemicals, especially in combination with CF3 substituents. Recently, the group of Shibata has developed an elegant organocatalyzed asymmetric approach to such pyrrolines [33]. Moreover, an efficient synthesis of β-trifluoromethylated Δ1-pyrrolines has been reported [34]. Therefore, we selected pyrrolines with CF2R side chains as a second example of 5-membered heterocyclic targets. Condensation of the anion of glycine ester diphenylimine 5 with the enones 3a–3e afforded the desired pyrrolines 6a–6e (Scheme 4 and Table 3). In all cases good yields were obtained, and a complete selectivity for the trans-isomer was observed as established by NMR analysis of the crude reaction mixtures. This is different from the results obtained by starting from enones with CF2CF2X side chains, where trans/cis mixtures were reported [34]. The 3JHH (6.3–6.5 Hz, trans) of our pyrrolines were very close to those of similar molecules bearing CF2–CF3 chains (6.4–6.6 Hz), while for latter derivatives the cis coupling constants were larger (≥ 8.3 Hz) [34]. This was confirmed in the case of pyrroline 6a by performing additional 19F/1H hOe 2D experiments which revealed strong correlations between the fluorine atoms of the CF2 group and the cyclic protons Hc and Ha (see Scheme 5 and 2D spectrum in the Supporting Information File 1 for details).
Scheme 5: Synthesis of pyrrolines with a gem-difluoro alkyl side chain.
Scheme 5: Synthesis of pyrrolines with a gem-difluoro alkyl side chain.
The reaction conditions were compatible with both steps and an example of a one-pot reaction was performed starting from 2a–2e. The desired pyrrolines 6a–6e were obtained in excellent yields (71–76%, Scheme 6 and Table 3).
Scheme 6: One-pot synthesis of pyrrolines with a gem-difluoro side chain.
Scheme 6: One-pot synthesis of pyrrolines with a gem-difluoro side chain.
Another important issue was the possibility of using these molecules as scaffolds for the preparation of focused chemical libraries. In order to explore this possibility we developed representative examples of Pd-catalyzed reactions starting from p-bromo derivatives 4e and 6e.
The results are given in Scheme 7 for pyrazoline 4e. Suzuki–Miyaura coupling [35] gave biphenyl derivative 7e in 82% yield, while the Heck [36] and Sonogashira [37] reactions afforded also the desired targets 8e and 9e in 72% and 77% yield respectively. Similar results were obtained in Pd-mediated reactions starting from pyrroline 6e, as indicated in Scheme 8. The desired molecules 10e–12e were obtained in good yields.
Scheme 7: Pd-catalyzed coupling reactions towards chemical libraries of pyrazolines with a gem-difluoro side chain.
Scheme 7: Pd-catalyzed coupling reactions towards chemical libraries of pyrazolines with a gem-difluoro side ...
Scheme 8: Pd-catalyzed coupling reactions towards chemical libraries of pyrrolines with a gem-difluoro side chain.
Scheme 8: Pd-catalyzed coupling reactions towards chemical libraries of pyrrolines with a gem-difluoro side c...
Conclusion
In summary, we developed an efficient access to enones with gem-difluoroalkyl side chains through a base-mediated isomerisation of fluorinated propargylic alcohols. Although this method is, to date, limited to compounds with aryl or heteroaryl substituents, corresponding enones appear as versatile intermediates for the preparation of heterocyclic derivatives. This has been established through the synthesis of pyrazolines and pyrrolines with gem-difluoroalkyl side chains. With appropriate substituents, derivatives of this type can be used for the preparation of chemical libraries.
Supporting Information
| Supporting Information File 1: Experimental details, NMR analysis and characterization data of new compounds. | ||
| Format: PDF | Size: 2.1 MB | Download |
References
-
Ojima, I.; McCarthy, J. R.; Welch, J. T., Eds. Biomedical Frontiers in Fluorine Chemistry, American Chemical Society: Washington, DC, 1996. doi:10.1021/bk-1996-0639
Return to citation in text: [1] -
Welch, J. T.; Eswarakrishnan, S. Fluorine in Bioorganic Chemistry; Wiley-Interscience: New York, NY, USA, 1991.
Return to citation in text: [1] -
Welch, J. T. Tetrahedron 1987, 43, 3123. doi:10.1016/S0040-4020(01)90286-8
Return to citation in text: [1] -
Isanbor, C.; O'Hagan, D. J. Fluorine Chem. 2006, 127, 303. doi:10.1016/j.jfluchem.2006.01.011
Return to citation in text: [1] -
Bégué, J.-P.; Bonnet-Delpon, D. J. Fluorine Chem. 2006, 127, 992. doi:10.1016/j.jfluchem.2006.05.006
Return to citation in text: [1] -
Kirk, K. L. J. Fluorine Chem. 2006, 127, 1013. doi:10.1016/j.jfluchem.2006.06.007
Return to citation in text: [1] -
Hagman, W. K. J. Med. Chem. 2008, 51, 4359. doi:10.1021/jm800219f
Return to citation in text: [1] -
Filler, R.; Saha, R. Future Med. Chem. 2009, 1, 777. doi:10.4155/fmc.09.65
Return to citation in text: [1] -
O’Hagan, D. J. Fluorine Chem. 2010, 131, 1071. doi:10.1016/j.jfluchem.2010.03.003
Return to citation in text: [1] -
Dolle, R. E.; Le Bourdonnec, B.; Worm, K.; Morales, G. A.; Thomas, C. J.; Zhang, W. J. Comb. Chem. 2010, 12, 765. doi:10.1021/cc100128w
And references cited therein.
Return to citation in text: [1] -
Welsh, M. E.; Snyder, S. A.; Stockwell, B. R. Curr. Opin. Chem. Biol. 2010, 14, 347. doi:10.1016/j.cbpa.2010.02.018
And references cited therein.
Return to citation in text: [1] -
Prakesch, M.; Grée, D.; Grée, R. Acc. Chem. Res. 2002, 35, 175. doi:10.1021/ar010055l
Return to citation in text: [1] -
Pacheco, M. C.; Purser, S.; Gouverneur, V. Chem. Rev. 2008, 108, 1943. doi:10.1021/cr068410e
And references cited therein. See for a comprehensive review on the chemistry of propargylic allylic fluorides.
Return to citation in text: [1] -
Prakesch, M.; Kerouredan, E.; Grée, D.; Grée, R.; DeChancie, J.; Houk, K. N. J. Fluorine Chem. 2004, 125, 537. doi:10.1016/j.jfluchem.2003.11.027
Return to citation in text: [1] -
Manthati, V. L.; Grée, D.; Grée, R. Eur. J. Org. Chem. 2005, 3825. doi:10.1002/ejoc.200500200
Return to citation in text: [1] -
Manthati, V. L.; Murthy, A. S. K.; Caijo, F.; Drouin, D.; Lesot, P.; Grée, D.; Grée, R. Tetrahedron: Asymmetry 2006, 17, 2306. doi:10.1016/j.tetasy.2006.08.010
And references cited therein.
Return to citation in text: [1] -
Grée, D.; Grée, R. Tetrahedron Lett. 2007, 48, 5435. doi:10.1016/j.tetlet.2007.06.007
Return to citation in text: [1] -
Pujari, S. A.; Kaliappan, K. P.; Valleix, A.; Grée, D.; Grée, R. Synlett 2008, 2503. doi:10.1055/s-2008-1078179
Return to citation in text: [1] [2] -
Blayo, A.-L.; Le Meur, S.; Grée, D.; Grée, R. Adv. Synth. Catal. 2008, 350, 471. doi:10.1002/adsc.200700488
Return to citation in text: [1] -
Bannwarth, P.; Valleix, A.; Grée, D.; Grée, R. J. Org. Chem. 2009, 74, 4646. doi:10.1021/jo900674u
Return to citation in text: [1] -
Bannwarth, P.; Grée, D.; Grée, R. Tetrahedron Lett. 2010, 51, 2413. doi:10.1016/j.tetlet.2010.02.116
Return to citation in text: [1] -
Bannwarth, P.; Grée, D.; Das, S.; Yadav, J. S.; Grée, R. J. Fluorine Chem. 2012, 134, 180. doi:10.1016/j.jfluchem.2011.03.003
Return to citation in text: [1] -
Arimitsu, S.; Fernández, B.; del Pozo, C.; Fustero, S.; Hammond, G. B. J. Org. Chem. 2008, 73, 2656. doi:10.1021/jo7025965
See for preparation of other series of fluoropropargylic derivatives by a completely different route.
Return to citation in text: [1] -
Xu, B.; Hammond, G. B. Angew. Chem., Int. Ed. 2005, 44, 7404. doi:10.1002/anie.200502807
See for preparation of other series of fluoropropargylic derivatives by a completely different route.
Return to citation in text: [1] -
Wang, Z. G.; Hammond, G. B. J. Org. Chem. 2000, 65, 6547. doi:10.1021/jo000832f
See also references cited therein and for preparation of other series of fluoropropargylic derivatives by a completely different route.
Return to citation in text: [1] -
Nineham, A. W.; Raphael, R. A. J. Chem. Soc. 1949, 118. doi:10.1039/jr9490000118
Return to citation in text: [1] -
Sonye, J. P.; Koide, K. J. Org. Chem. 2007, 72, 1846. doi:10.1021/jo0623944
Return to citation in text: [1] -
Yamazaki, T.; Kawasaki-Takasuka, T.; Furuta, A.; Sakamoto, S. Tetrahedron 2009, 65, 5945. doi:10.1016/j.tet.2009.05.087
Return to citation in text: [1] [2] -
Marella, A.; Ali, Md. R.; Alam, Md. T.; Saha, R.; Tanwar, O.; Akhter, M.; Shaquiquzzaman, Md.; Alam, M. M. Mini-Rev. Med. Chem. 2013, 13, 921. doi:10.2174/1389557511313060012
Return to citation in text: [1] -
Müller, T. J. J.; Ansorge, M.; Aktah, D. Angew. Chem., Int. Ed. 2000, 39, 1253. doi:10.1002/(SICI)1521-3773(20000403)39:7<1253::AID-ANIE1253>3.0.CO;2-X
Return to citation in text: [1] -
Bellina, F.; Rossi, R. Tetrahedron 2006, 62, 7213. doi:10.1016/j.tet.2006.05.024
Return to citation in text: [1] -
Zhang, Y.; Ran, C.; Zhou, G.; Sayre, L. M. Bioorg. Med. Chem. 2007, 15, 1868. doi:10.1016/j.bmc.2006.11.025
Return to citation in text: [1] -
Kawai, H.; Yuan, Z.; Kitayama, T.; Tokunaga, E.; Shibata, N. Angew. Chem., Int. Ed. 2013, 52, 5575. doi:10.1002/anie.201301123
Return to citation in text: [1] -
Marrec, O.; Christophe, C.; Billard, T.; Langlois, B.; Vors, J.-P.; Pazenoc, S. Adv. Synth. Catal. 2010, 352, 2825. doi:10.1002/adsc.201000487
Return to citation in text: [1] [2] [3] -
Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457. doi:10.1021/cr00039a007
Return to citation in text: [1] -
Heck, R. F.; Nolley, J. P., Jr. J. Org. Chem. 1972, 37, 2320. doi:10.1021/jo00979a024
Return to citation in text: [1] -
Sonogashira, K. J. Organomet. Chem. 2002, 653, 46. doi:10.1016/S0022-328X(02)01158-0
Return to citation in text: [1]
| 34. | Marrec, O.; Christophe, C.; Billard, T.; Langlois, B.; Vors, J.-P.; Pazenoc, S. Adv. Synth. Catal. 2010, 352, 2825. doi:10.1002/adsc.201000487 |
| 34. | Marrec, O.; Christophe, C.; Billard, T.; Langlois, B.; Vors, J.-P.; Pazenoc, S. Adv. Synth. Catal. 2010, 352, 2825. doi:10.1002/adsc.201000487 |
| 34. | Marrec, O.; Christophe, C.; Billard, T.; Langlois, B.; Vors, J.-P.; Pazenoc, S. Adv. Synth. Catal. 2010, 352, 2825. doi:10.1002/adsc.201000487 |
| 1. | Ojima, I.; McCarthy, J. R.; Welch, J. T., Eds. Biomedical Frontiers in Fluorine Chemistry, American Chemical Society: Washington, DC, 1996. doi:10.1021/bk-1996-0639 |
| 2. | Welch, J. T.; Eswarakrishnan, S. Fluorine in Bioorganic Chemistry; Wiley-Interscience: New York, NY, USA, 1991. |
| 3. | Welch, J. T. Tetrahedron 1987, 43, 3123. doi:10.1016/S0040-4020(01)90286-8 |
| 4. | Isanbor, C.; O'Hagan, D. J. Fluorine Chem. 2006, 127, 303. doi:10.1016/j.jfluchem.2006.01.011 |
| 5. | Bégué, J.-P.; Bonnet-Delpon, D. J. Fluorine Chem. 2006, 127, 992. doi:10.1016/j.jfluchem.2006.05.006 |
| 6. | Kirk, K. L. J. Fluorine Chem. 2006, 127, 1013. doi:10.1016/j.jfluchem.2006.06.007 |
| 7. | Hagman, W. K. J. Med. Chem. 2008, 51, 4359. doi:10.1021/jm800219f |
| 8. | Filler, R.; Saha, R. Future Med. Chem. 2009, 1, 777. doi:10.4155/fmc.09.65 |
| 9. | O’Hagan, D. J. Fluorine Chem. 2010, 131, 1071. doi:10.1016/j.jfluchem.2010.03.003 |
| 17. | Grée, D.; Grée, R. Tetrahedron Lett. 2007, 48, 5435. doi:10.1016/j.tetlet.2007.06.007 |
| 18. | Pujari, S. A.; Kaliappan, K. P.; Valleix, A.; Grée, D.; Grée, R. Synlett 2008, 2503. doi:10.1055/s-2008-1078179 |
| 31. | Bellina, F.; Rossi, R. Tetrahedron 2006, 62, 7213. doi:10.1016/j.tet.2006.05.024 |
| 32. | Zhang, Y.; Ran, C.; Zhou, G.; Sayre, L. M. Bioorg. Med. Chem. 2007, 15, 1868. doi:10.1016/j.bmc.2006.11.025 |
| 15. | Manthati, V. L.; Grée, D.; Grée, R. Eur. J. Org. Chem. 2005, 3825. doi:10.1002/ejoc.200500200 |
| 16. |
Manthati, V. L.; Murthy, A. S. K.; Caijo, F.; Drouin, D.; Lesot, P.; Grée, D.; Grée, R. Tetrahedron: Asymmetry 2006, 17, 2306. doi:10.1016/j.tetasy.2006.08.010
And references cited therein. |
| 33. | Kawai, H.; Yuan, Z.; Kitayama, T.; Tokunaga, E.; Shibata, N. Angew. Chem., Int. Ed. 2013, 52, 5575. doi:10.1002/anie.201301123 |
| 12. | Prakesch, M.; Grée, D.; Grée, R. Acc. Chem. Res. 2002, 35, 175. doi:10.1021/ar010055l |
| 13. |
Pacheco, M. C.; Purser, S.; Gouverneur, V. Chem. Rev. 2008, 108, 1943. doi:10.1021/cr068410e
And references cited therein. See for a comprehensive review on the chemistry of propargylic allylic fluorides. |
| 14. | Prakesch, M.; Kerouredan, E.; Grée, D.; Grée, R.; DeChancie, J.; Houk, K. N. J. Fluorine Chem. 2004, 125, 537. doi:10.1016/j.jfluchem.2003.11.027 |
| 29. | Marella, A.; Ali, Md. R.; Alam, Md. T.; Saha, R.; Tanwar, O.; Akhter, M.; Shaquiquzzaman, Md.; Alam, M. M. Mini-Rev. Med. Chem. 2013, 13, 921. doi:10.2174/1389557511313060012 |
| 10. |
Dolle, R. E.; Le Bourdonnec, B.; Worm, K.; Morales, G. A.; Thomas, C. J.; Zhang, W. J. Comb. Chem. 2010, 12, 765. doi:10.1021/cc100128w
And references cited therein. |
| 11. |
Welsh, M. E.; Snyder, S. A.; Stockwell, B. R. Curr. Opin. Chem. Biol. 2010, 14, 347. doi:10.1016/j.cbpa.2010.02.018
And references cited therein. |
| 30. | Müller, T. J. J.; Ansorge, M.; Aktah, D. Angew. Chem., Int. Ed. 2000, 39, 1253. doi:10.1002/(SICI)1521-3773(20000403)39:7<1253::AID-ANIE1253>3.0.CO;2-X |
| 18. | Pujari, S. A.; Kaliappan, K. P.; Valleix, A.; Grée, D.; Grée, R. Synlett 2008, 2503. doi:10.1055/s-2008-1078179 |
| 37. | Sonogashira, K. J. Organomet. Chem. 2002, 653, 46. doi:10.1016/S0022-328X(02)01158-0 |
| 26. | Nineham, A. W.; Raphael, R. A. J. Chem. Soc. 1949, 118. doi:10.1039/jr9490000118 |
| 28. | Yamazaki, T.; Kawasaki-Takasuka, T.; Furuta, A.; Sakamoto, S. Tetrahedron 2009, 65, 5945. doi:10.1016/j.tet.2009.05.087 |
| 23. |
Arimitsu, S.; Fernández, B.; del Pozo, C.; Fustero, S.; Hammond, G. B. J. Org. Chem. 2008, 73, 2656. doi:10.1021/jo7025965
See for preparation of other series of fluoropropargylic derivatives by a completely different route. |
| 24. |
Xu, B.; Hammond, G. B. Angew. Chem., Int. Ed. 2005, 44, 7404. doi:10.1002/anie.200502807
See for preparation of other series of fluoropropargylic derivatives by a completely different route. |
| 25. |
Wang, Z. G.; Hammond, G. B. J. Org. Chem. 2000, 65, 6547. doi:10.1021/jo000832f
See also references cited therein and for preparation of other series of fluoropropargylic derivatives by a completely different route. |
| 19. | Blayo, A.-L.; Le Meur, S.; Grée, D.; Grée, R. Adv. Synth. Catal. 2008, 350, 471. doi:10.1002/adsc.200700488 |
| 20. | Bannwarth, P.; Valleix, A.; Grée, D.; Grée, R. J. Org. Chem. 2009, 74, 4646. doi:10.1021/jo900674u |
| 21. | Bannwarth, P.; Grée, D.; Grée, R. Tetrahedron Lett. 2010, 51, 2413. doi:10.1016/j.tetlet.2010.02.116 |
| 22. | Bannwarth, P.; Grée, D.; Das, S.; Yadav, J. S.; Grée, R. J. Fluorine Chem. 2012, 134, 180. doi:10.1016/j.jfluchem.2011.03.003 |
| 28. | Yamazaki, T.; Kawasaki-Takasuka, T.; Furuta, A.; Sakamoto, S. Tetrahedron 2009, 65, 5945. doi:10.1016/j.tet.2009.05.087 |
| 36. | Heck, R. F.; Nolley, J. P., Jr. J. Org. Chem. 1972, 37, 2320. doi:10.1021/jo00979a024 |
© 2013 El Dine et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)