Abstract
The tin(IV)-catalyzed reaction of β-nitrostyrene with (E)-3-methyl-1,3-pentadiene in toluene afforded two major nitronic ester cycloadducts in 27% and 29% yield that arise from the reaction at the less substituted diene double bond. Also present were four cycloadducts from the reaction at the higher substituted diene double bond, two of which were the formal cycloadducts of (Z)-3-methyl-1,3-pentadiene. A Friedel–Crafts alkylation product from the reaction of the diene, β-nitrostyrene, and toluene was also obtained in 10% yield. The tin(IV)-catalyzed reaction of β-nitrostyrene with (Z)-3-methyl-1,3-pentadiene in dichloromethane afforded four nitronic ester cycloadducts all derived from the reaction at the higher substituted double bond. One cycloadduct was isolated in 45% yield and two others are formal adducts of the E-isomer of the diene. The product formation in these reactions is consistent with a stepwise mechanism involving a zwitterionic intermediate. The initially isolated nitronic ester cycloadducts underwent tin(IV)-catalyzed interconversion, presumably via zwitterion intermediates. Cycloadducts derived from the reaction at the less substituted double bond of (E)-3-methyl-1,3-pentadiene underwent a [3,3]-sigmatropic rearrangement on heating to afford 4-nitrocyclohexenes. Cycloadducts derived from the reaction at the higher substituted diene double bond of either diene failed to undergo a thermal rearrangement. Rates and success of the rearrangement are consistent with a concerted mechanism possessing a dipolar transition state. An initial assessment of substituent effects on the rearrangement process is presented.
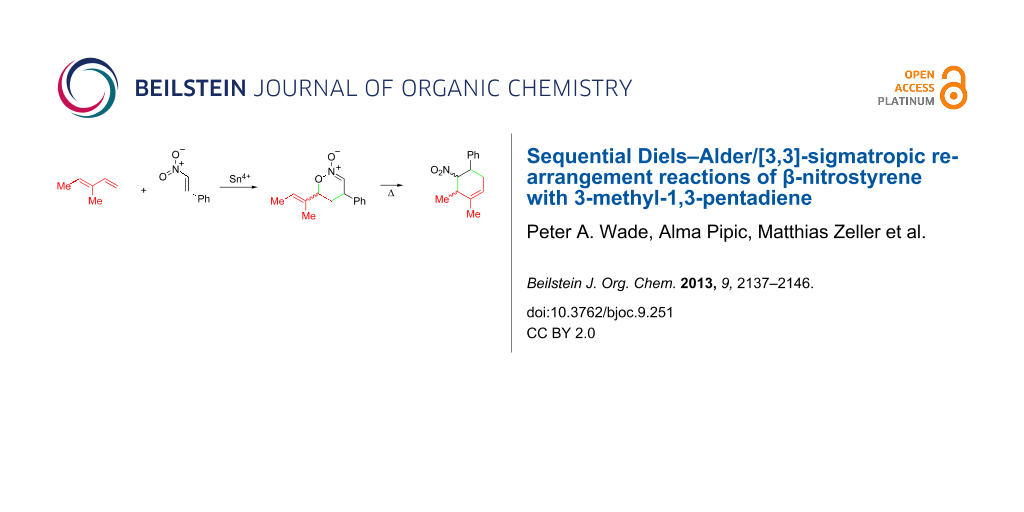
Graphical Abstract
Introduction
We have previously reported examples of a general new [3,3]-sigmatropic rearrangement, the conversion of O-allyl nitronic esters (nitronates) to γ,δ-unsaturated nitro compounds. In the reported examples, 6-membered cyclic O-allyl nitronates were converted to 4-nitrocyclohexenes [1,2]. This rearrangement strongly resembles the classic Claisen rearrangement of allyl vinyl ethers [3-6]. The O-allyl nitronic ester rearrangement has been the subject of several theoretical investigations [7-10]. The necessary nitronic ester precursors are readily preparable by Lewis acid-promoted [4 + 2]-cycloaddition reactions of nitroalkenes with appropriate dienes, a general reaction first reported by Denmark and coworkers (Denmark Diels–Alder reaction) [1,11,12]. Other methods of nitronic ester preparation are known and ongoing studies in our laboratory directed at an application to O-allyl nitronic ester synthesis will be reported elsewhere. In continuing our investigations, we desired additional O-allyl nitronic esters with varied substitution patterns. We were also interested in determining the double bond specificity of the Denmark Diels–Alder reaction for unsymmetrical dienes. Previous work on cyclopentadiene and cyclohexadiene has shown that the reaction can be quite specific to give one of two possible regioisomers [11,12]. In the case of 1-(2-propenyl)cyclohexene only one of four possible regioisomers was observed [1]. However, it was not clear what the outcome would be for a simple open-chain conjugated diene. Consequently, cycloaddition reactions of 3-methyl-1,3-pentadiene were examined. This diene has both trisubstituted and monosubstituted double bonds allowing for cycloaddition to give up to four regioisomers. It is commercially available both as an E,Z-mixture and as the pure E-isomer. The Z-isomer can be obtained from the E,Z-mixture by selective removal of the E-isomer via cycloaddition with maleic anhydride [13].
Results and Discussion
Cycloaddition studies
The tin(IV) chloride-catalyzed reaction of β-nitrostyrene and an E,Z–mixture of 3-methyl-1,3-pentadiene (1a,b, 70:30, respectively) in dichloromethane solution afforded a series of six Diels–Alder nitronic ester cycloadducts, none of which heavily predominated (Table 1). Only cycloadducts resulting from C–C bond formation at the end of the conjugated diene system were observed, in keeping with reported results for other conjugated dienes [1,11,12]. However, cycloaddition was relatively indiscriminant at the two double bonds of the diene in this initial experiment.
Table 1: Cycloaddition products for Sn(IV)-catalyzed reaction of 3-methyl-1,3-pentadiene with β-nitrostyrene.
|
|
|||||||
| diene | solvent | ratioa (yield, %) | |||||
|---|---|---|---|---|---|---|---|
| 2 | 3 | 4 | 5 | 6 | 7 | ||
| 1a/1bb | CH2Cl2 | 24 (14) | 25 (15) | 6 (3) | 12 (7) | 8 (5) | 26 (15) |
| 1a | CH2Cl2 | 29 (16) | 28 (15) | 9 (5) | 11 (6) | <1 | 23 (13) |
| 1bc | CH2Cl2 | 2 (1) | 6 (4) | 19 (12) | 73 (45) | ||
| 1ad | PhMe | 43 (27) | 47 (29) | 3 (2) | 6 (4) | 0.5 (0.3) | 0.4 (0.2) |
aDetermined by 1H NMR. b70:30 ratio. cAdduct 13 (9% yield) also obtained. dTernary adduct 12a,b (10% yield) also obtained.
The reaction with pure E-diene 1a (E/Z, 99.7:0.03) was investigated in the hope of eliminating products derived from the Z-diene. Here, five cycloadducts were detected. Cycloadducts 2 and 3, derived from the reaction at the less substituted diene double bond, predominated and were isolated in 16% and 15% yield, respectively. Cycloadduct 7 which is a formal adduct of Z-diene 1b was the third most prevalent product, being formed in 13% yield. Thus, the E-diene stereochemistry in this experiment was not retained in the cycloaddition products. It was initially thought that the diene might isomerize under the reaction conditions leading to loss of stereointegrity. However, a more likely explanation is that cycloaddition occurs by a stepwise process involving a tin(IV)-coordinated zwitterion intermediate.
The reaction with pure Z-diene 1b gave significantly different results. Only four cycloadducts were obtained, all of which were derived from the attack at the higher substituted diene double bond. Cycloadduct 7 predominated and was isolated in 45% yield. Significantly, the crossover cycloadducts 4 and 5 (formal adducts of the E-diene 1a) were also obtained, albeit in low yield. Here, too, diene stereointegrity was not retained. An open-chain adduct of β-nitrostyrene and the diene was also obtained in 9% yield (vide infra, Scheme 1). The absence of crossover cycloadducts 2 and 3 suggests that diene isomerization is not responsible for the loss of stereointegrity.
Scheme 1: Reaction intermediates, resulting products, and model cations.
Scheme 1: Reaction intermediates, resulting products, and model cations.
Two alternative mechanisms for tin(IV)-catalyzed nitroalkene Diels–Alder reactions have been proposed. Denmark et al. favor a stepwise process proceeding via a zwitterion intermediate [11,14]. Alkene stereointegrity is normally retained, presumably owing to a cyclic conformation dictated by charge interaction between the cation center and tin nitronate, but products derived from carbocation rearrangement of the intermediate have been isolated. Houk et al. favor a concerted mechanism with no intervening energy minimum for cycloaddition based on calculational results [7-9]. The current results are in better agreement with the stepwise mechanism although a competing concerted mechanism is certainly possible. The loss of stereointegrity is not due to simple diene isomerization since cycloadducts 2 and 3 were absent from the product spread derived from Z-diene 1b. The difference in regioselectivity for reactions of E-diene 1a and Z-diene 1b can be rationalized on the basis of the relative stability of allyl cations. The (E,E)- and (E,Z)-1,2,3-trimethylallyl cations 8a and 9a serve as models for projected intermediates 8b and 9b, respectively. A calculational study indicates a lower heat of formation for E,E-cation 8a than for E,Z-cation 9a (ΔHf is 2.3–3 kcal/mol more favorable for 8a) [15]. The analogous intermediate 8b formed from E-diene 1a leads to formation of cycloadducts 2 and 3. The Z-diene 1b should lead to intermediate 9b structurally analogous to E,Z-cation 9a. Presumably the higher heat of formation of 9b is responsible for the absence of products from reaction at the less substituted double bond of diene 1b. However, it is somewhat surprising that E-diene 1a actually affords cycloadducts 4–7. We note that model cation 10 (structurally similar to 11a,b) has a higher heat of formation than 8a (ΔHf is 1.8–5.2 kcal/mol more favorable for 8a) [15]. Nevertheless cycloadducts 4,7 and 5,6 are formed, presumably via intermediates 11a and 11b, respectively.
When the reaction was conducted using pure E-diene in toluene solution, the cycloadducts 2 and 3 derived from the reaction at the less substituted double bond were isolated in 27% and 29% yield, respectively. These cycloadducts are sufficiently major to make the cycloaddition a useful process. Cycloadducts 4 and 5 derived from cycloaddition to the higher substituted double bond of E-diene 1a and crossover cycloadducts 6 and 7 were also obtained, all in low yield.
Also present in reactions run in toluene was the ternary adduct 12a,b in which one molecule each of β-nitrostyrene, diene, and toluene was incorporated. This material was isolated in 10% yield as an inseparable 70:30 mixture in which the p-isomer predominated. Some partial enrichment of the p-isomer was possible through combined chromatographic and recrystallization methods. The structure of nitro compound 12a,b is based on spectral data. However, isomer identification required single-crystal X-ray diffraction analysis, performed on the enriched sample (12a,b 91.4:8.6 ratio, respectively). The ternary adduct 12a,b is a Friedel–Crafts alkylation product of toluene and the zwitterion 11a. Zwitterion 11a would also lead to cycloadducts 4 and 7 if it cyclized. Possibly zwitterion 11a is less prone to cyclization than zwitterion 11b which leads to cycloadducts 5 and 6. The yield of cycloadduct 7 is very low in experiments where ternary adduct 12a,b is formed. Consistent with the presence of these zwitterions, the related open-chain adduct 13 was a minor product obtained from the reaction of Z-diene 1b and β-nitrostyrene in dichloromethane solution.
Nitronic ester structure determination
Structure assignments for nitronic esters 2–7 are based on spectral data. The C=N stretching frequencies of several similar 6-membered cyclic nitronic esters were reported to be 1592–1622 cm−1 [12,14,16]. Table 2 lists the values for nitronic esters obtained here. Reported 1H NMR chemical shifts for HC=N signals (the nitronic H-3 signal) of several cyclic nitronic esters were in the range of δ 6.18–6.74 [12,14,16-18]. Reported 13C NMR chemical shifts for C–O signals of similar 6-membered cyclic nitronic esters were δ 77–87 and for N=C signals were δ 110–126 [14]. Nitronic esters 2–7 exhibit similar signals consistent with the general structural assignment. Stereochemical assignments were made in the following way. The very low amount of nitronic esters 6 and 7 formed from pure E-diene in toluene is attributed to minimal crossover. Thus, retention of cis-dimethyl stereochemistry at C-3, C-4 has been assumed for 4 and 5 and retention of trans-dimethyl stereochemistry from Z-diene precursor has been assumed for nitronic esters 6 and 7. Nitronic esters 5 and 6 exhibit large coupling constants (J4,5 respectively) consistent with trans-diaxial H-4, H-5 stereochemistry. Nitronic ester 2 possesses large coupling constants for one of two H-5 signals (J4,5 = J5,6 = 11.2 Hz) that require an axial–axial–axial H-4, H-5, H-6 atom placement. Thus, the phenyl substituent must be located cis to the alkenyl substituent in 2 and trans in 3. As confirmation of the assignments, nitronic ester 2 is thermally converted to nitro compound 18 and 3 to 19 by [3,3]-sigmatropic rearrangements (vide infra).
Table 2: Characteristic spectral bands for nitronic esters.
| Compd. | C=N (cm−1) | N=CH (ppm) | N=C (ppm) | C-O (ppm) | J3,4 (Hz)a | J4,5 (Hz)a |
|---|---|---|---|---|---|---|
| 2 | 1614 | 6.38 | 113.8 | 86.7 | ||
| 3 | 1615 | 6.48 | 112.7 | 83 | ||
| 4 | 1618 | 6.42 | 113 | 87 | 2.5 | 6.4 |
| 5 | 1619 | 6.36 | 114.1 | 87.3 | 2.9 | 10.7 |
| 6 | 1620 | 6.26 | 114.9 | 87.7 | 2.9 | 11.2 |
| 7 | 1615 | 6.53 | 113 | 86.4 | 2.9 | 6.6 |
aAtom numbering as shown for nitronic ester 2 (Table 1).
Sn(IV)-catalyzed reactions of nitronic esters
The possibility that ternary adduct 12a,b could arise from ring opening of one of the nitronic esters was considered. Neither nitronic ester 4 nor 7 afforded 12a,b when treated with tin(IV) chloride in toluene. That is not to say that the reaction of the nitronic esters with tin(IV) chloride did not occur at all, however. Nitronic ester 7 was converted to nitronic ester 4 (99:1 7/4 final ratio) when it was subjected to tin(IV) chloride for sixteen hours at room temperature (Scheme 2). Similarly, nitronic ester 6 (as a 48:52 mixture with 4) was partially isomerized to nitronic ester 5 (33:17:50 6/5/4 final ratio) when it was subjected to tin(IV) chloride for two hours at room temperature. Nitronic ester 4 did not isomerize under these conditions.
Scheme 2: Sn(IV)-catalyzed isomerization of nitronic esters.
Scheme 2: Sn(IV)-catalyzed isomerization of nitronic esters.
The effect of tin(IV) chloride upon nitronic esters 2 and 3 was also examined. Starting with either nitronic ester 2 or nitronic ester 3 resulted in a final 60:40 ratio of 2,3, respectively, after only 20 minutes. Bond scission to give zwitterions is thought to lead to isomerization in these reactions. These zwitterion intermediates are thought to be characterized by proximate oppositely charged centers (a “cyclo” conformation) as opposed to looser non-proximate oppositely charged centers (an “extended” conformation as shown for 11). In accordance, it is proposed that tin(IV) complexes nitronic ester 7 and bond scission occurs to give the cyclo zwitterion 14. Cyclo zwitterion 14 is structurally identical with zwitterion 11a but remains in a conformation where the opposite charges are in close proximity. Free rotation around the allylic C–C sigma bond of 14 as shown and subsequent reclosure can lead to either regeneration of nitronic ester 7 or formation of nitronic ester 4, presumably depending on relative thermodynamic stabilities of the two nitronic esters. Thus, it is thought that nitronic ester 4 is at least 2 kcal/mol more stable than nitronic ester 7. Interconversion of nitronic esters 5 and 6 is thought to occur via the analogous cyclo zwitterion 15, structurally identical with 11b. Interestingly, these cyclo zwitterions should be capable of closing in multiple ways, either to give nitronic esters or to give isomeric nitro compounds. Nitronic ester 5 was first converted to a mixture of 5 and 6 and eventually in 15% yield to nitro compound 16 after exposure to tin(IV) chloride for two days. It is quite possible that zwitterion 15 is involved but a larger range of motion over the protracted reaction time allows eventual conversion to the more stable nitro compound. It is also possible that a concerted tin(IV)-catalyzed [3,3]-sigmatropic rearrangement of nitronic ester 5 affords nitro compound 16 (vide infra).
The rapid interconversion of nitronic esters 2 and 3 likely reflects the greater ease of rotation for the cyclo zwitterion 17. Here bond breaking and reformation is at a secondary rather than tertiary center. A more rapid change of the face presented to the nitronate-O atom would be expected to occur for cyclo zwitterion 17 because top-to-bottom passage past an H-atom should be more rapid than passage past a methyl group as required for the cyclo zwitterions 14 and 15.
[3,3]-Sigmatropic rearrangement of nitronic esters
A variety of O-allyl nitronic esters have previously been shown to undergo a non-catalyzed thermal rearrangement to γ,δ-unsaturated nitro compounds [1]. Nitronic esters 2 and 3 also undergo this rearrangement (Scheme 3). Thus, nitro compound 18 is obtained in 74% yield when a DMF solution of nitronic ester 2 is heated at 90–100 °C for one hour. The stereochemical assignment is based on the large (J4,5 = 12.2 Hz) and small (J5,6 = 5.8 Hz) 1H NMR coupling constants indicating trans-nitro to phenyl and cis-nitro to methyl substituent placements, respectively. The two-step cycloaddition/sigmatropic rearrangement sequence affords a useful route to this nitro compound. Heating a mixture of β-nitrostyrene and (E)-3-methyl-1,3-pentadiene at 170 °C for five hours gives 18 directly, but only as part of an inseparable mixture containing 16, 20 and at least one other isomer [19]. Thus, tin(IV)-catalyzed nitronic ester cycloaddition and subsequent [3,3]-sigmatropic rearrangement is a superior method to direct thermal Diels–Alder cycloaddition as a means of obtaining pure nitro compound 18.
Scheme 3: Thermal rearrangement of nitronic esters 2 and 3.
Scheme 3: Thermal rearrangement of nitronic esters 2 and 3.
In the rearrangement of nitronic ester 3, more stringent conditions are required and two product isomers are formed. This is thought to be due to the developing unfavorable cis-relationship between nitro and phenyl groups in the transition state. Heating 3 at 130 °C for one hour gave a 70:30 mixture of nitro compounds 19 and 20, respectively, from which 19 could be obtained pure in 48% yield. Longer reaction times at 70–100 °C did afford relatively selective conversion of 3 to nitro compound 19 but the yield was 14% owing to partial decomposition and incomplete rearrangement of the starting material. Nitro compound 19 is the formal Diels–Alder cycloadduct of cis-β-nitrostyrene which is not readily available. When nitro compound 19 is heated in DMF at 130 °C for nine hours, it is completely converted to the trans-isomer 20, obtained in 48% yield after chromatography. Here, too, the stereochemical assignment is based on coupling constants. For nitro compound 19, two small 1H NMR coupling constants (J4,5 = 3.4 Hz; J5,6 = 5.4 Hz) were observed consistent with cis-nitro to phenyl and cis-nitro to methyl substituent placement. Nitro compound 20 exhibited two large diaxial 1H NMR coupling constants (J4,5 = 11.7 Hz; J5,6 = 9.8 Hz) consistent with trans-nitro to phenyl and trans-nitro to methyl substituent placement.
These results parallel previously published observations [1] for rearrangement of nitronic esters 21a and 21b (Scheme 4). The rearrangement of 21b, in which the alkenyl and phenyl groups are located trans was more difficult, requiring higher temperature than the rearrangement of 21a (90 °C and 20 °C, respectively). Apparently, the developing cis-relationship between the phenyl and nitro groups in transition states leading to both 22b and 19 is unfavorable. On prolonged heating, product 22b is converted to the trans-isomer 23. It was postulated that the zwitterion 24 was an intermediate and inversion of the nitro group involved rotation around a C–C single bond. A similar process is likely for conversion of 19 to 20.
Scheme 4: Thermal rearrangement of nitronic esters 21a, 21b and 22b.
Scheme 4: Thermal rearrangement of nitronic esters 21a, 21b and 22b.
Attempts to thermally rearrange nitronic esters 4–7 were unsuccessful. Thus, heating any of these four nitronic esters in the temperature range of 70–150 °C led to decomposition rather than formation of nitro compounds. It is possible that the formation of nitro compound 16 from the mixture of nitronic esters 5 and 6 in the presence of tin(IV) chloride was a catalyzed concerted sigmatropic rearrangement of nitronic ester 5. However, as previously noted this reaction may well involve zwitterion 15 as the intermediate since nitronic esters 5 and 6 equilibrate under these conditions presumably through 15.
The thermal stability of nitronic esters 4–7 appears to be roughly similar to nitronic esters 2 and 3: all have half-life’s greater than one hour at 100 °C with the exception of nitronic ester 2 that largely rearranges under these conditions. However, rearrangement of 4–7 is too slow to occur prior to decomposition. This is particularly surprising for nitronic ester 5 (Scheme 5). Here the rearrangement should occur to give 16 where the nitro and phenyl groups are trans and no other significant steric interactions are apparent for the transition state. Other rearrangements to give products where the nitro and phenyl groups are trans (nitro compounds 18 and previously studied 22a) [1] were successful. Even nitronic ester 26a affords the bicyclo[2.2.2]alkene 28a in 72% yield when heated at 150 °C, presumably through strained tricyclic transition state 27a [1]. In contrast, nitronic ester 26b fails to undergo rearrangement presumably owing to the requirement that the nitro and phenyl groups be cis in the product. The rearrangement of nitronic ester 7 to give nitro compound 25 where nitro and phenyl groups would be trans also fails, but here a methyl group is cis to phenyl in the product.
Scheme 5: Thermal reactions of nitronic esters 5, 7, and 26a–d.
Scheme 5: Thermal reactions of nitronic esters 5, 7, and 26a–d.
Apparently electron-attracting substituents (W-groups) attached to the nitronic C-atom (the C(I) site, Scheme 6) of O-allyl nitronic esters strongly accelerate rearrangement. Nitronic ester 26c with a sulfone group (Hammett σp 0.68) [20] at the C(I) position rearranges at 80 °C to 28b whereas analogous nitronic ester 26d with phenacyl group, a less powerful W-group (Hammett σp 0.43) [20], requires 120 °C for rearrangement to 28c [2]. The [3,3]-sigmatropic rearrangement of 26a, which lacks a W-group at C(I), required especially vigorous conditions (150 °C) [1]. Nitronic ester 26a possesses a phenyl group not present in either 26c or 26d. Thus, the transition state 27a is not strictly comparable to 27b or 27c but should be similar. These results lead to the proposal of dipolar general transition state 29. Negative charge in transition state 29 should be stabilized by a W-group at C(I). Substantial partial charge development would seem to be present from the observed results. In contrast, a W-group attached at C(I) of an allyl vinyl ether slightly decelerates a Claisen rearrangement [3,21].
Scheme 6: General transition state for the [3,3]-sigmatropic rearrangement of O-allyl nitronic esters.
Scheme 6: General transition state for the [3,3]-sigmatropic rearrangement of O-allyl nitronic esters.
Nitronic ester 21a rearranges more readily than nitronic ester 2 (20 °C vs 70 °C, respectively, 1 day) [1]. Likewise, nitronic ester 21b rearranges more easily than nitronic ester 3 (90 °C vs 130 °C, respectively, 1–2 hours). All four of these nitronic esters possess a trisubstituted alkene but only nitronic esters 21a and 21b possess a methyl group at the C(IV) site. Possibly the rate differences are due to an electronic accelerative effect arising from the electron-donating C(IV) methyl group (D-group) as shown in transition state 29. It is also true that 21a and 21b rearrange to tetrasubstituted cycloalkenes 22a and 22b, respectively, whereas 2 and 3 give less stable trisubstituted alkenes 18 and 19 [22] which may explain the rate difference. Significant steric differences between the transition states leading to 22a and 22b versus 18 and19 appear to be absent.
Nitronic esters 4–7 lack methyl substituents at C(V) and C(VI) whereas other nitronic esters that do undergo rearrangement possess methyl or alkyl substituents at these positions. The absence of a methyl substituent in 4–7 at C(VI) is a likely contributing cause of failed rearrangement. In the Claisen rearrangement, a methyl group has a small accelerative effect and alkoxy groups a larger accelerative effect when located at the C(VI) site of the allyl vinyl ether [3,23]. These observations were attributed to an electronic effect whereby the C(VI)-substituent weakened the O–C(IV) bond and hence the rearrangement energy barrier. A similar effect should operate with O-allyl nitronic esters resulting in stabilization of transition state 29 by C(VI) alkyl substituents. It should be noted, however, that the nitronic ester 30 (Scheme 7) also lacks a C(VI) substituent: it undergoes a rearrangement at room temperature and a similar bicyclic transition state must be involved [2]. The high reactivity of nitronic ester 30 is presumably due to the combined presence of a C(I) sulfone group and a C(IV) methyl group. In particular, the presence of an electron-attracting substituent at C(I) appears to have a greater accelerative effect on the rearrangement than the presence of electron-donating substituents at C(IV) or C(VI).
Scheme 7: Thermal rearrangement of nitronic ester 30.
Scheme 7: Thermal rearrangement of nitronic ester 30.
Conclusion
Cycloaddition to (E)-3-methyl-1,3-pentadiene occurred predominantly on the less substituted double bond. However, the diene stereointegrity was compromised. Formation of the ternary adduct 12a,b in 10% yield was a competing reaction in toluene: further studies with additional substrates may lead to a useful new 3-component synthesis of nitro compounds, especially considering the observation that only one diastereomer (mixture of o,p-isomers) was observed. Interestingly, reaction with (Z)-3-methyl-1,3-pentadiene occurred only at the more substituted double bond affording cycloadduct 7 in 45% yield as the major product. These cycloaddition results are consistent with the presence of zwitterionic intermediates.
Tin(IV)-catalyzed nitronic ester isomerization was a generally observed process. Epimerization occurred at C-6 (the C-atom attached to the nitronate O-atom). An intermediate cyclic zwitterion would seem to be present and is consistent with observed relative rates of isomerization.
The rate (and success) of the thermal [3,3]-sigmatropic rearrangement of O-allyl nitronic esters appears to depend on electronic effects. The polar transition state 29 has been proposed and appears to be consistent with the currently available rate data.
Experimental
General methods
3-Methyl-1,3-pentadiene (E/Z mixtures), (E)-3-methyl-1,3-pentadiene (E/Z, 99.7:0.3), tin(IV) chloride, and β-nitrostyrene were purchased commercially and used as received. N,N-Dimethylformamide was distilled (bp 152–153 °C) and a large forerun discarded to ensure solvent dryness. Ethyl acetate was freshly distilled from anhydrous K2CO3. All reactions were run under a nitrogen atmosphere. Organic layers were dried (MgSO4) and concentrated at reduced pressure. Preparative TLC was performed on 250 μm silica gel plates (Analtech). NMR spectra were taken in CDCl3 and recorded on a Varian Inova 500 spectrometer. High-resolution mass spectra (HRMS) were taken on a Waters Autospec Ultima Q spectrometer. Chemical ionization (CI) was performed with CH4 as carrier gas unless otherwise noted. IR samples were recorded as attenuated total reflectance (ATR) spectra on a Perkin-Elmer 1600 spectrometer. Melting points (uncorrected) were taken on a Thomas-Hooover apparatus and elemental analyses (within 0.3% of theory) were performed by Robertson Microlit Laboratories.
Synthesis
Preparation of Z-diene 1b [13]: A stirred mixture of 1a,b (40 mmol, 63:37, 1a/b ratio by 1H NMR) and freshly ground maleic anhydride (3 equiv) warmed to 45–50 °C after 10 min. Stirring was continued at ambient temperature for 24 h. The remaining diene was distilled from the reaction mixture by gradually reducing the pressure to 2 mmHg and was collected in a cold trap (dry ice). A 15 mmol portion of pure (>99.5% by 1H NMR) Z-diene 1b was obtained.
General procedure for carrying out Sn(IV)-catalyzed reactions of β-nitrostyrene and 3-methyl-1,3-pentadiene: A solution of 3-methyl-1,3-pentadiene (either 1a or 1b, 2 mmol) in CH2Cl2 (2 mL) was added dropwise (syringe pump) over 1 h to a cold (−78 °C) mixture of SnCl4 (0.5 equiv), β-nitrostyrene (0.5 equiv), and CH2Cl2 (10 mL). The resulting cold solution was stirred for 40 min. Dichloromethane (10 mL) and then saturated aqueous NaHCO3 (5 mL) were added dropwise over 10 min keeping the temperature below −70 °C. The mixture was allowed to warm and the layers were separated. The aqueous layer was extracted (two 10 mL portions of CH2Cl2) and the combined organic layers were washed with 50:50 brine/water (10 mL), dried, and concentrated to give the crude product. This was chromatographed on a column of silica gel (elution with hexanes/EtOAc from 90:10 to 0:100).
General procedure for reaction of β-nitrostyrene and 3-methyl-1,3-pentadiene in toluene solution: Tin(IV) chloride (2 mmol) was added dropwise over 5 min to a cold (−78 °C) solution of β-nitrostyrene (1 equiv) and 3-methyl-1,3-pentadiene (2 equiv, either pure 1a or a 70:30 1a,b mixture) in toluene (20 mL). The resulting cold (<−70 °C) solution was stirred for 75 min. Ethyl acetate (10 mL) and then saturated aqueous NaHCO3 solution (5 mL) were added dropwise over 5 min keeping the temperature below −50 °C. The mixture was allowed to warm and was extracted with EtOAc (five 10 mL portions). The combined extracts were washed with saturated NaHCO3 solution (five 15 mL portions) and brine (five 10 mL portions), dried, and concentrated to give the crude product which was chromatographed on silica gel (hexanes/EtOAc from 95:5 to 0:100). From the (E)-diene, ternary adduct 12a,b (a/b 66:34 ratio; 0.2 mmol; 10% yield), nitronic ester 2 (0.54 mmol, 27% yield), and nitronic ester 3 (0.58 mmol, 29% yield) were obtained as well as minor amounts of nitronic esters 4–7.
General procedure for Tin(IV) chloride-catalyzed isomerization of nitronic esters: Tin(IV) chloride (0.12 mmol) was added dropwise over 2 min to a solution of nitronic ester (1 equiv) in toluene (5 mL) and the resulting solution was stirred for an appropriate time until further isomerization was not observed (20 min for 2 and 3; 2 h for 6; 16 h for 7). Saturated aqueous NaHCO3 solution (5 mL) and EtOAc (10 mL) were added. The organic layer was separated and the aqueous layer was extracted with EtOAc (five 10 mL portions). The combined organic layers were washed with saturated aqueous NaHCO3 solution (five 10 mL portions) followed by brine (three 10 mL portions), dried, and concentrated to give the crude product which was analyzed spectroscopically.
[3,3]-Sigmatropic rearrangement of nitronic ester 2: A solution of 2 (0.12 mmol) in DMF (10 mL) was heated for 2 h at 90–97 °C. Benzene (20 mL) and EtOAc (20 mL) were added to the cooled solution. The resulting solution was washed with water (twenty 10 mL portions), dried, and concentrated. Preparative TLC (hexanes/EtOAc, 90:10) was performed on the residue to afford 0.09 mmol (74% yield) of pure 18. The analytical sample was recrystallized from benzene/hexanes: mp 100–100.2 °C; TLC Rf = 0.87 (hexanes/EtOAc, 90:10); IR: 1553, 1375 cm−1 (NO2); 1H NMR δ 7.4–7.15 (m, 5H), 5.42 (br m, 1H), 5.14 (dd, J = 5.8, 12.2 Hz, 1H), 3.46 (ddd [24], J = 6.5, 11.4, 11.4 Hz, 1H), 2.83 (m, 1H), 2.48 (m, 1H), 2.21 (m, 1H), 1.80 (s, 3H), 1.11 (d, J = 7.3 Hz, 3H); 13C NMR δ 141.7, 135.2, 128.8, 127.1, 127.1, 120.4, 90.2, 38.3, 37.6, 34.6, 21.8, 14.2; HRMS–CI (m/z): [M + H]+ calcd for C14H18NO2, 232.1338; found, 232.1338; Elemental analysis (%): Anal. calcd C, 72.72; H, 7.36; N, 6.06; found: C, 72.47; H, 7.11; N, 5.89.
Supporting Information
| Supporting Information File 1: Experimental procedures, characterization of compounds 2–7, 12, 13, 16, and 18–20, and crystallographic data for compound 12a,b. | ||
| Format: PDF | Size: 673.6 KB | Download |
| Supporting Information File 2: NMR spectra and signal assignments for compounds 2–7, 12,13, and 18–20. | ||
| Format: PDF | Size: 2.8 MB | Download |
References
-
Wade, P. A.; Pipic, A.; Santhanaraman, M.; Le, H. T. Chem. Commun. 2009, 3531–3532. doi:10.1039/b902870f
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] -
Wade, P. A.; Murray, J. K., Jr.; Shah-Patel, S.; Le, H. T. Chem. Commun. 2002, 1090–1091. doi:10.1039/b200224h
Return to citation in text: [1] [2] [3] -
Castro, A. M. M. Chem. Rev. 2004, 104, 2939–3002. doi:10.1021/cr020703u
Return to citation in text: [1] [2] [3] -
Hiersemann, M.; Nubbemeyer, U., Eds. The Claisen Rearrangement; Wiley-VCH: Weinheim, 2007. doi:10.1002/9783527610549
Return to citation in text: [1] -
Wipf, P. Claisen Rearangemant. Comprehensive Organic Synthesis; Pergamon Press: Oxford, 1991; Vol. 5, pp 827–873. doi:10.1016/B978-0-08-052349-1.00140-2
Return to citation in text: [1] -
Ziegler, F. E. Chem. Rev. 1988, 88, 1423–1452. doi:10.1021/cr00090a001
Return to citation in text: [1] -
Çelebi-Ölçüm, N.; Ess, D. H.; Aviyente, V.; Houk, K. N. J. Org. Chem. 2008, 73, 7472–7480. doi:10.1021/jo801076t
Return to citation in text: [1] [2] -
Ess, D. H.; Wheeler, S. E.; Iafe, R. G.; Xu, L.; Çelebi-Ölçüm, N.; Houk, K. N. Angew. Chem., Int. Ed. 2008, 47, 7592–7601. doi:10.1002/anie.200800918
Return to citation in text: [1] [2] -
Çelebi-Ölçüm, N.; Ess, D. H.; Aviyente, V.; Houk, K. N. J. Am. Chem. Soc. 2007, 129, 4528–4529. doi:10.1021/ja070686w
Return to citation in text: [1] [2] -
Xu, H.; Wade, P. A.; Sohlberg, K. Tetrahedron 2010, 66, 845–851. doi:10.1016/j.tet.2009.11.075
Return to citation in text: [1] -
Denmark, S. E.; Thorarensen, A. Chem. Rev. 1996, 96, 137–166. doi:10.1021/cr940277f
Return to citation in text: [1] [2] [3] [4] -
Denmark, S. E.; Kesler, B. S.; Moon, Y. C. J. Org. Chem. 1992, 57, 4912–4924. doi:10.1021/jo00044a029
Return to citation in text: [1] [2] [3] [4] [5] -
Bartlett, P. D.; Wallbillich, G. E. H.; Montgomery, L. K. J. Org. Chem. 1967, 32, 1290–1297. doi:10.1021/jo01280a601
Return to citation in text: [1] [2] -
Denmark, S. E.; Moon, Y.-C.; Cramer, C. J.; Dappen, M. S.; Senanayake, C. B. W. Tetrahedron 1990, 46, 7373–7392. doi:10.1016/S0040-4020(01)89054-2
Return to citation in text: [1] [2] [3] [4] -
Reindl, B.; Clark, T.; Schleyer, P. v. R. J. Comput. Chem. 1997, 18, 533–551. doi:10.1002/(SICI)1096-987X(199703)18:4<533::AID-JCC7>3.0.CO;2-U
Return to citation in text: [1] [2] -
Denmark, S. E.; Seierstad, M.; Herbert, B. J. Org. Chem. 1999, 64, 884–901. doi:10.1021/jo9818374
Return to citation in text: [1] [2] -
Denmark, S. E.; Baiazitov, R. Y.; Nguyen, S. T. Tetrahedron 2009, 65, 6535–6548. doi:10.1016/j.tet.2009.05.060
Return to citation in text: [1] -
Kornblum, N.; Brown, R. A. J. Am. Chem. Soc. 1964, 86, 2681–2687. doi:10.1021/ja01067a034
1H NMR chemical shifts from δ 5.57–6.25 (E- and Z-isomers) have been reported for a series of HC=N signals in open-chain aliphatic nitronic esters.
Return to citation in text: [1] -
Pipic, A. Mechanistic studies of the sigmatropic rearrangement of O-allyl nitronic esters to γ,δ-unsaturated nitro naphthalene derivatives and nitro substituted cyclohexene derivatives. Ph.D. Thesis, Drexel University, U.S.A., 2010.
Return to citation in text: [1] -
Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004
And references cited therein.
Return to citation in text: [1] [2] -
Burrows, C. J.; Carpenter, B. K. J. Am. Chem. Soc. 1981, 103, 6983–6984. doi:10.1021/ja00413a045
Return to citation in text: [1] -
Allinger, N. L.; Pamphilis, N. A. J. Org. Chem. 1973, 38, 316–319. doi:10.1021/jo00942a024
For example, 1,2-dimethylcyclohexene is reported to be 1 kcal/mol more stable than 1,6-dimethylcyclohexene.
Return to citation in text: [1] -
Curran, D. P.; Suh, Y.-G. J. Am. Chem. Soc. 1984, 106, 5002–5004. doi:10.1021/ja00329a061
Return to citation in text: [1] -
Hoye, T. R.; Hanson, P. R.; Vyvyan, J. R. J. Org. Chem. 1994, 59, 4096–4103. doi:10.1021/jo00094a018
Analyzed following a reported protocol.
Return to citation in text: [1]
| 3. | Castro, A. M. M. Chem. Rev. 2004, 104, 2939–3002. doi:10.1021/cr020703u |
| 23. | Curran, D. P.; Suh, Y.-G. J. Am. Chem. Soc. 1984, 106, 5002–5004. doi:10.1021/ja00329a061 |
| 2. | Wade, P. A.; Murray, J. K., Jr.; Shah-Patel, S.; Le, H. T. Chem. Commun. 2002, 1090–1091. doi:10.1039/b200224h |
| 13. | Bartlett, P. D.; Wallbillich, G. E. H.; Montgomery, L. K. J. Org. Chem. 1967, 32, 1290–1297. doi:10.1021/jo01280a601 |
| 1. | Wade, P. A.; Pipic, A.; Santhanaraman, M.; Le, H. T. Chem. Commun. 2009, 3531–3532. doi:10.1039/b902870f |
| 2. | Wade, P. A.; Murray, J. K., Jr.; Shah-Patel, S.; Le, H. T. Chem. Commun. 2002, 1090–1091. doi:10.1039/b200224h |
| 11. | Denmark, S. E.; Thorarensen, A. Chem. Rev. 1996, 96, 137–166. doi:10.1021/cr940277f |
| 12. | Denmark, S. E.; Kesler, B. S.; Moon, Y. C. J. Org. Chem. 1992, 57, 4912–4924. doi:10.1021/jo00044a029 |
| 14. | Denmark, S. E.; Moon, Y.-C.; Cramer, C. J.; Dappen, M. S.; Senanayake, C. B. W. Tetrahedron 1990, 46, 7373–7392. doi:10.1016/S0040-4020(01)89054-2 |
| 1. | Wade, P. A.; Pipic, A.; Santhanaraman, M.; Le, H. T. Chem. Commun. 2009, 3531–3532. doi:10.1039/b902870f |
| 11. | Denmark, S. E.; Thorarensen, A. Chem. Rev. 1996, 96, 137–166. doi:10.1021/cr940277f |
| 12. | Denmark, S. E.; Kesler, B. S.; Moon, Y. C. J. Org. Chem. 1992, 57, 4912–4924. doi:10.1021/jo00044a029 |
| 1. | Wade, P. A.; Pipic, A.; Santhanaraman, M.; Le, H. T. Chem. Commun. 2009, 3531–3532. doi:10.1039/b902870f |
| 7. | Çelebi-Ölçüm, N.; Ess, D. H.; Aviyente, V.; Houk, K. N. J. Org. Chem. 2008, 73, 7472–7480. doi:10.1021/jo801076t |
| 8. | Ess, D. H.; Wheeler, S. E.; Iafe, R. G.; Xu, L.; Çelebi-Ölçüm, N.; Houk, K. N. Angew. Chem., Int. Ed. 2008, 47, 7592–7601. doi:10.1002/anie.200800918 |
| 9. | Çelebi-Ölçüm, N.; Ess, D. H.; Aviyente, V.; Houk, K. N. J. Am. Chem. Soc. 2007, 129, 4528–4529. doi:10.1021/ja070686w |
| 10. | Xu, H.; Wade, P. A.; Sohlberg, K. Tetrahedron 2010, 66, 845–851. doi:10.1016/j.tet.2009.11.075 |
| 12. | Denmark, S. E.; Kesler, B. S.; Moon, Y. C. J. Org. Chem. 1992, 57, 4912–4924. doi:10.1021/jo00044a029 |
| 14. | Denmark, S. E.; Moon, Y.-C.; Cramer, C. J.; Dappen, M. S.; Senanayake, C. B. W. Tetrahedron 1990, 46, 7373–7392. doi:10.1016/S0040-4020(01)89054-2 |
| 16. | Denmark, S. E.; Seierstad, M.; Herbert, B. J. Org. Chem. 1999, 64, 884–901. doi:10.1021/jo9818374 |
| 3. | Castro, A. M. M. Chem. Rev. 2004, 104, 2939–3002. doi:10.1021/cr020703u |
| 4. | Hiersemann, M.; Nubbemeyer, U., Eds. The Claisen Rearrangement; Wiley-VCH: Weinheim, 2007. doi:10.1002/9783527610549 |
| 5. | Wipf, P. Claisen Rearangemant. Comprehensive Organic Synthesis; Pergamon Press: Oxford, 1991; Vol. 5, pp 827–873. doi:10.1016/B978-0-08-052349-1.00140-2 |
| 6. | Ziegler, F. E. Chem. Rev. 1988, 88, 1423–1452. doi:10.1021/cr00090a001 |
| 12. | Denmark, S. E.; Kesler, B. S.; Moon, Y. C. J. Org. Chem. 1992, 57, 4912–4924. doi:10.1021/jo00044a029 |
| 14. | Denmark, S. E.; Moon, Y.-C.; Cramer, C. J.; Dappen, M. S.; Senanayake, C. B. W. Tetrahedron 1990, 46, 7373–7392. doi:10.1016/S0040-4020(01)89054-2 |
| 16. | Denmark, S. E.; Seierstad, M.; Herbert, B. J. Org. Chem. 1999, 64, 884–901. doi:10.1021/jo9818374 |
| 17. | Denmark, S. E.; Baiazitov, R. Y.; Nguyen, S. T. Tetrahedron 2009, 65, 6535–6548. doi:10.1016/j.tet.2009.05.060 |
| 18. |
Kornblum, N.; Brown, R. A. J. Am. Chem. Soc. 1964, 86, 2681–2687. doi:10.1021/ja01067a034
1H NMR chemical shifts from δ 5.57–6.25 (E- and Z-isomers) have been reported for a series of HC=N signals in open-chain aliphatic nitronic esters. |
| 11. | Denmark, S. E.; Thorarensen, A. Chem. Rev. 1996, 96, 137–166. doi:10.1021/cr940277f |
| 14. | Denmark, S. E.; Moon, Y.-C.; Cramer, C. J.; Dappen, M. S.; Senanayake, C. B. W. Tetrahedron 1990, 46, 7373–7392. doi:10.1016/S0040-4020(01)89054-2 |
| 15. | Reindl, B.; Clark, T.; Schleyer, P. v. R. J. Comput. Chem. 1997, 18, 533–551. doi:10.1002/(SICI)1096-987X(199703)18:4<533::AID-JCC7>3.0.CO;2-U |
| 1. | Wade, P. A.; Pipic, A.; Santhanaraman, M.; Le, H. T. Chem. Commun. 2009, 3531–3532. doi:10.1039/b902870f |
| 11. | Denmark, S. E.; Thorarensen, A. Chem. Rev. 1996, 96, 137–166. doi:10.1021/cr940277f |
| 12. | Denmark, S. E.; Kesler, B. S.; Moon, Y. C. J. Org. Chem. 1992, 57, 4912–4924. doi:10.1021/jo00044a029 |
| 15. | Reindl, B.; Clark, T.; Schleyer, P. v. R. J. Comput. Chem. 1997, 18, 533–551. doi:10.1002/(SICI)1096-987X(199703)18:4<533::AID-JCC7>3.0.CO;2-U |
| 13. | Bartlett, P. D.; Wallbillich, G. E. H.; Montgomery, L. K. J. Org. Chem. 1967, 32, 1290–1297. doi:10.1021/jo01280a601 |
| 24. |
Hoye, T. R.; Hanson, P. R.; Vyvyan, J. R. J. Org. Chem. 1994, 59, 4096–4103. doi:10.1021/jo00094a018
Analyzed following a reported protocol. |
| 1. | Wade, P. A.; Pipic, A.; Santhanaraman, M.; Le, H. T. Chem. Commun. 2009, 3531–3532. doi:10.1039/b902870f |
| 7. | Çelebi-Ölçüm, N.; Ess, D. H.; Aviyente, V.; Houk, K. N. J. Org. Chem. 2008, 73, 7472–7480. doi:10.1021/jo801076t |
| 8. | Ess, D. H.; Wheeler, S. E.; Iafe, R. G.; Xu, L.; Çelebi-Ölçüm, N.; Houk, K. N. Angew. Chem., Int. Ed. 2008, 47, 7592–7601. doi:10.1002/anie.200800918 |
| 9. | Çelebi-Ölçüm, N.; Ess, D. H.; Aviyente, V.; Houk, K. N. J. Am. Chem. Soc. 2007, 129, 4528–4529. doi:10.1021/ja070686w |
| 1. | Wade, P. A.; Pipic, A.; Santhanaraman, M.; Le, H. T. Chem. Commun. 2009, 3531–3532. doi:10.1039/b902870f |
| 19. | Pipic, A. Mechanistic studies of the sigmatropic rearrangement of O-allyl nitronic esters to γ,δ-unsaturated nitro naphthalene derivatives and nitro substituted cyclohexene derivatives. Ph.D. Thesis, Drexel University, U.S.A., 2010. |
| 1. | Wade, P. A.; Pipic, A.; Santhanaraman, M.; Le, H. T. Chem. Commun. 2009, 3531–3532. doi:10.1039/b902870f |
| 1. | Wade, P. A.; Pipic, A.; Santhanaraman, M.; Le, H. T. Chem. Commun. 2009, 3531–3532. doi:10.1039/b902870f |
| 22. |
Allinger, N. L.; Pamphilis, N. A. J. Org. Chem. 1973, 38, 316–319. doi:10.1021/jo00942a024
For example, 1,2-dimethylcyclohexene is reported to be 1 kcal/mol more stable than 1,6-dimethylcyclohexene. |
| 1. | Wade, P. A.; Pipic, A.; Santhanaraman, M.; Le, H. T. Chem. Commun. 2009, 3531–3532. doi:10.1039/b902870f |
| 3. | Castro, A. M. M. Chem. Rev. 2004, 104, 2939–3002. doi:10.1021/cr020703u |
| 21. | Burrows, C. J.; Carpenter, B. K. J. Am. Chem. Soc. 1981, 103, 6983–6984. doi:10.1021/ja00413a045 |
| 20. |
Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004
And references cited therein. |
| 2. | Wade, P. A.; Murray, J. K., Jr.; Shah-Patel, S.; Le, H. T. Chem. Commun. 2002, 1090–1091. doi:10.1039/b200224h |
| 1. | Wade, P. A.; Pipic, A.; Santhanaraman, M.; Le, H. T. Chem. Commun. 2009, 3531–3532. doi:10.1039/b902870f |
| 20. |
Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004
And references cited therein. |
© 2013 Wade et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)