Abstract
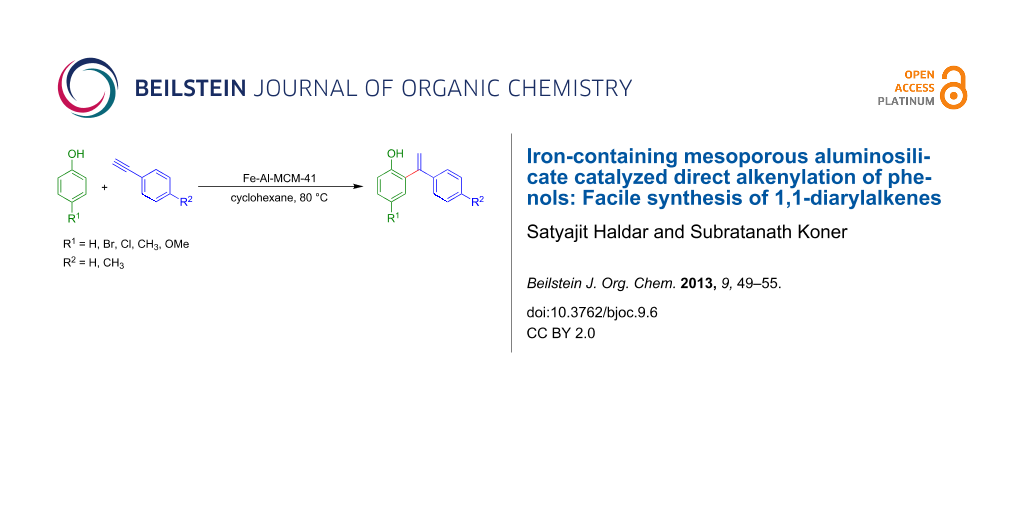
The addition of phenols to aryl-substituted alkynes to form 1,1-diarylalkenes was carried out by using the Fe-Al-MCM-41 catalyst. The catalyst showed remarkable improvement in time and yield in comparison to other solid catalysts. The heterogeneous catalyst can be reused at least three times without a significant loss in activity.
Graphical Abstract
Introduction
The direct vinylation of phenols has received considerable attention by synthetic organic chemists for a long time. The resulting 1,1-disubstituted alkene derivatives (Figure 1) are widely used as key starting materials in the synthesis of fine chemicals [1-7], polymers [8-14], and pharmaceuticals [15-17]. Furthermore, a variety of available reactions to functionalize the double bond, such as reductive (hydrogenation, hydrosilylation, etc.), oxidative (epoxidation, halogenations, dihydroxylation, etc.) or cycloaddition transformations, encourage such vinylation process as an attractive primary tool in organic synthesis. Thus, the development of simple methods concerning such vinylation reactions of phenols always remains an important process.
Figure 1: (RS)-Tolterodine, an important urological drug.
Figure 1: (RS)-Tolterodine, an important urological drug.
Mizoroki–Heck-type reaction could be considered as an efficient procedure for the synthesis of such vinylated phenols [18,19]. There are a number of reports available in the literature that involve Pd-catalyzed cross coupling reactions of various aryl halides with different olefins. However, the major drawback of the Mizoroki–Heck-type reaction in the synthesis of 1,1-disubstituted olefins rests on its poor selectivity toward the formation of α-products [20-23]. In general, the reaction shows β-selectivity, and there are only a few reports available concerned with α-substituted products [24-26]. Sabarre and Love reported a one-pot rhodium-catalyzed alkyne hydrothiolation followed by a nickel-catalyzed Kumada-type cross coupling with Grignard reagents to afford 1,1-disubstituted olefins [27]. Alternatively, Friedel–Crafts-type alkenylation could be a better choice to prepare such regiospecific vinyl aromatic compounds. Yamaguchi et al. reported a direct ortho alkenylation of phenols with 1-alkynes using SnCl4 and Bu3N in acetonitrile under reflux [28]. Further developments include the metal trifluoromethanesulfonate-catalyzed Friedel–Crafts alkenylation of arenes using alkynes by Tsuchimoto et al. [29] and the addition of simple arenes to arylacetylenes to afford exclusive 1,1-diarylethylenes through a C–C coupling reaction catalyzed by a combination of Cu(OTf)2/TMSA (TMSA = trifluoromethanesulfonic acid) [30]. The same reaction was also successfully carried out by Li and co-workers using FeCl3 as catalyst in a homogeneous medium [31]. Further Yadav et al. demonstrated an elegant hydroarylation of different phenols in the presence of gallium(III) chloride [32]. Nevertheless, all of these Lewis acid catalyzed Friedel–Crafts-type reactions have their own limitations. Being homogeneous catalytic systems, these methods suffer from drawbacks, such as difficulty in recovery and reusability of the catalysts and tedious work-up procedures. In addition, in most of the cases the utilization of air-sensitive chemicals also restricts these methods to be carried out under inert conditions. To overcome such limitations, heterogeneous solid catalysts have recieved much attention over the past few decades. The easy separation and possibility of reuse made their employment an attractive choice. Recently, we have successfully employed a Fe-Al-MCM-41 catalyst in a Friedel–Crafts-type hydroarylation reaction of styrenes [33]. The catalyst demonstrated high yields of products with good selectivities within a short reaction time under “open flask conditions”. On further exploration of the use of iron-based mesoporous aluminosilicate catalyst in the direct alkenylation of arenes, we have succeeded in vinylation of various phenols with different phenylacetylenes. In this paper we report a convenient method for the alkenylation of phenols with aryl-substituted alkynes under mild conditions.
Results and Discussion
MCM-41 and Al-MCM-41 were prepared according to the procedure described in our earlier report [33]. The incorporation of iron(III) was achieved in a similar way [33]. The mesoporous patterns of MCM-41, Al-MCM-41 and Fe-Al-MCM-41 were established from the small-angle XRD patterns (see Supporting Information File 1). The BET surface area and the pore width of Fe-Al-MCM-41 were found to be 753 m2/g and 25.83 Å, respectively. The aluminium and iron contents of the Fe-Al-MCM-41 catalyst were estimated by AAS method and found to be 5.5 wt % and 0.80 wt %, respectively.
Initially, the Fe-Al-MCM-41-catalyzed reaction of phenylacetylene and phenol was carried out in different solvent media. The progress of the reaction was monitored by analyzing the products with the help of gas chromatography (Table 1). Amongst various solvents, such as cyclohexane, CH3CN, CH3NO2, CH3OH, CHCl3, 1,2-dichloroethane, and dichloromethane, cyclohexane was found to be the most suitable solvent for the reaction (Table 1, entry 7). Though a moderate yield was obtained in 1,2-dichloroethane, no conversion was observed in DCM (Table 1, entries 5–6). Comparing the reaction result in 1,2-DCE (bp 80 °C) and DCM (bp 40 °C), it seemed that temperature plays a crucial role in the activation of the substrate. Hence, further screening was done by performing the same reaction in cyclohexane at different temperatures (Table 1, entries 8 and 9). The best result was obtained at 80 °C, i.e., in cyclohexane under reflux.
Table 1: Fe-Al-MCM-41-catalyzed reaction of phenylacetylene and phenola.
|
|
|||||
| entry | temp. [°C] | solvent | time | conversion [%]b | yield [%]c |
|---|---|---|---|---|---|
| 1 | 80 | CH3CN | 6 | 0 | 0 |
| 2 | 80 | CH3NO2 | 6 | 0 | 0 |
| 3 | 80 | CH3OH | 6 | 4 | 0 |
| 4 | 80 | CHCl3 | 6 | 0 | 0 |
| 5 | 80 | 1,2-DCE | 2.5 | 88 | 71 |
| 6 | 40 | DCM | 6 | 0 | 0 |
| 7 | 80 | cyclohexane | 2 | 99 | 81 |
| 8 | 70 | cyclohexane | 2.5 | 30 | 25 |
| 9 | 60 | cyclohexane | 9 | 1 | 0 |
aReaction conditions: 1 mmol of phenylacetylene, 1.5 mmol of phenol, 65 mg of Fe-Al-MCM-41, 2 mL of solvent. bGC conversion of phenylacetylene. cGC yield of alkenylated product.
Since a number of pure aluminosilicates are known to catalyze C–C coupling reactions [34-42], a detailed investigation was performed with various available aluminosilicate and siliceous materials to understand the necessity of the iron center in the catalyst Fe-Al-MCM-41. NaY, a microporous aluminosilicate, did not yield any product under the specified reaction conditions (Table 2, entry 1). Sartori et al. reported an electrophilic alkenylation of aromatics with phenylacetylene over zeolite HSZ-360-Y; however, this zeolite is known to have a high number of acidic sites in its porous structure [43]. They showed that among various readily available porous aluminosilicates like HSZ-360-Y, ZSM-5-Y, K10 montmorillonite, etc., HSZ-360-Y was capable of catalyzing the electrophilic substitution process. However, a rigorous pretreatment is necessary to activate the catalyst and a moisture-free medium is essential. Furthermore, a higher temperature (110 °C) and a longer reaction time (14 h) were also required for the catalytic reaction. Sartori et. al. suggested that the external surface of HSZ-360-Y zeolite was responsible for such catalytic activity rather than the pore of the zeolite. As evidence, they reported the acidic-alumina-catalyzed reaction in which only 25% product yield was observed [43]. However, when we used acidic alumina (under our reaction conditions) at a relatively low temperature (80 °C) without any pretreatment, no conversion was observed (Table 2, entry 3). The basic and the neutral alumina also remained inactive in catalyzing the coupling reaction (Table 2, entries 4 and 5). This may be attributed to the low activation energy or the lack of moisture-free conditions. However, when iron-exchanged Al-MCM-41 was employed as catalyst the reaction proceeded smoothly to afford high yields of products within very short reaction times. The reaction was performed under “open-flask” conditions without any moisture-preventing conditions, and no pretreatment of the catalyst was required. Notably, when the alumina support “Al-MCM-41” itself was used as catalyst, the conversion recorded was only 2% (Table 2, entry 7). The aluminium-free pure mesoporous silica, MCM-41, displayed no catalytic activity, as expected (Table 2, entry 8). This clearly indicates that the presence of iron in the catalyst Fe-Al-MCM-41 is directly related to its superior performance in the catalytic reaction. Iron probably has some role in lowering the activation energy of the reaction in synergy with the aluminium moiety present in Al-MCM-41. The short reaction time is probably due to the mesoporous structure of Fe-Al-MCM-41 (pore width = 25.83 Å), which provides a higher surface area to facilitate the reaction. It is well established that the site-isolation of active centers enhances the catalytic efficacy of the materials [44-47]. Owing to the large surface area of catalyst Fe-Al-MCM-41, iron centers are well dispersed in the mesoporous matrix. Thus, the catalyst attained the desired site-isolation of active centers for enhanced activity in catalytic reaction. In fact, in order to investigate the role of mesoporous structure, we further performed the reaction with “degraded-Fe-Al-MCM-41” of which the mesoporous integrity was destroyed by proper treatment [48]. In that case, a long reaction time was observed (Table 2, entry 9), as in the case of HSZ-360-Y.
Table 2: Reactions of phenylacetylene with phenol with different catalystsa.
|
|
||||
| entry | catalyst | time (h) | conversion [%]b | yield [%]c |
|---|---|---|---|---|
| 1 | NaY | 6 | 0 | 0 |
| 2d | HSZ-360-Y | 14 | 55 | 52 |
| 3 | acidic alumina | 6 | 3 | 0 |
| 4 | basic alumina | 6 | 0 | 0 |
| 5 | neutral alumina | 6 | 0 | 0 |
| 6 | Fe-Al-MCM-41 | 2 | 99 | 81 |
| 7 | Al-MCM-41 | 6 | 2 | 0 |
| 8 | MCM-41 | 6 | 0 | 0 |
| 9e | Fe-Al-MCM-41 (degraded) | 12 | 48 | 45 |
| 10f | GaCl3 | 6 | – | 75 |
| 11g | FeCl3·6H2O (10 mol %) | 12 | 0 | 0 |
aReaction conditions: 1 mmol of phenylacetylene, 1.5 mmol of phenol, 65 mg of catalyst, 2 mL of cyclohexane, 80 °C. bGC conversion of phenylacetylene. cGC yield of arylated products. dRef [43]. eSee Experimental for details. fRef [32]. gCatalyst: 2.32 mg of FeCl3·6H2O (0.008 mmol).
On the basis of the optimized reaction conditions, the scope of this Fe-Al-MCM-41 catalyzed C–C coupling reaction was further investigated. Various substituted phenols were used for the alkenylation by phenylacetylene. The reaction preceded smoothly both in the case of electron-donating and -withdrawing groups at the phenol. While p-Br-, p-Me- and p-OMe-phenol took about 1–2 hours for a good conversion, p-Cl-phenol took slightly longer (viz. 2.5 hours) (Table 3, entries 2–5). In all cases a mono-alkenylated product at the ortho position was observed in high yield. Moreover, the catalyst showed regioselectivity in producing the 1,1-diarylalkene product. For alkynes, p-Me-substituted phenylacetylene was further used to explore the general acceptability of this reaction. In its reaction with different substituted phenols a lowering of the reaction time was observed (Table 3, entries 6–9). Thus, Fe-Al-MCM-41 exhibited its efficiency in catalyzing the alkenylation of different phenols by various substituted/nonsubstituted phenylacetylenes to furnish the corresponding 1,1-diarylalkenes in high conversion and selectivity within a short reaction time.
Table 3: Reactions of arenes with phenylalkynesa.
| entry | arene | productb | time [h] | conversion [%]c | yield [%]d |
|---|---|---|---|---|---|
| 1 |
|
1a |
2 | 99 | 81 |
| 2 |
|
1b |
1 | 100 | 83 |
| 3 |
|
1c |
2.5 | 93 | 75 |
| 4 |
|
1d |
1.5 | 99 | 87 |
| 5 |
|
1e |
2 | 99 | 89 |
| 6 |
|
2a |
0.75 | 100 | 86 |
| 7 |
|
2b |
2 | 97 | 81 |
| 8 |
|
2c |
1 | 100 | 87 |
| 9 |
|
2d |
1.5 | 100 | 89 |
aReaction conditions: 1 mmol of arylalkyne, 1.5 mmol of arene, 65 mg of Fe-Al-MCM-41, 2 mL of solvent, 80 °C. bmain product. cGC conversion of phenylalkyne. dGC yield of alkenylated product.
Furthermore, to verify whether the coupling reaction was truly catalyzed by solid Fe-Al-MCM-41 or by the leached iron species from the solid support, a “hot filtration” experiment was undertaken. A reaction between phenol and phenylacetylene was performed until 22% conversion was observed (monitored by GC analysis) and then the catalyst was separated by simple centrifugation under the hot conditions. No conversion in the “catalyst-free centrifugate” was observed upon further heating for 12 hours.
Another most advantageous aspect of this type of solid-support catalyst is their scope for recycling. The reaction between phenol and phenylacetylene under optimized conditions was chosen to evaluate the recyclability of Fe-Al-MCM-41. The catalyst was separated after the completion of the reaction by simple centrifugation. This recovered catalyst was washed with cyclohexane, then methanol followed by dichloromethane, and dried at 80 °C for a few hours prior to its next use. The conversions of successive cycles are given in Table 4.
Conclusion
In summary, Fe-Al-MCM-41 has been proven to be an efficient catalyst for the alkenylation of phenols with aryl-substituted alkynes. Various substituted arylalkynes and phenols afforded the 1,1-diarylethylenes under mild reaction conditions. The reaction did not require any further addition of acids or special reagents such as additives, etc. The catalytic reactions can be carried out under “open-flask conditions”. Furthermore, the catalyst can be easily recovered from the reaction mixture by simple centrifugation and can be reused several times without any special treatment other than washing.
Experimental
General procedure for catalytic reactions: Phenylacetylene (1.0 mmol) was added to a mixture of phenol (1.5 mmol) and Fe-Al-MCM-41 (0.065 g) in 2 mL of cyclohexane. The mixture was stirred at 80 °C in an oil bath. To study the progress of the reaction, the products were collected at different time intervals and identified and quantified by gas chromatography. After completion of the reaction, the solution was cooled down and the catalyst was removed by centrifugation. The resulting crude mixture was gently evaporated under vacuum and purified by flash column chromatography on silica gel 230–400 by using an appropriate solvent.
2-(1-phenylvinyl)phenol (1a) [43]: 1H NMR (300 MHz, CDCl3) δ 7.40–7.32 (m, 5H, CH), 7.30–7.13 (m, 3H, CH), 6.97–6.94 (m, 2H, CH), 5.88 (d, J(H,H) = 0.6 Hz, 1H, CHH), 5.43 (s, 1H, CHH), 5.18 (s, 1H, OH).
Synthesis of the catalyst (Fe-Al-MCM-41): As described in our previous work [33], iron was incorporated into the mesoporous aluminosilicate (Al-MCM-41) by the ion-exchange method. A 0.5 g amount of Al-MCM-41 was added to 100 mL 0.001 M methanolic solution of FeCl3·6H2O, and the mixture was stirred vigorously for 12 h. The resultant solid was then filtered. To remove the excess FeCl3, the isolated solid was washed by Soxhlet extraction using methanol. The resulting solid was dried in an oven at 80 °C and characterized. The catalyst was then used directly in reactions without any further activation.
Disintegration of mesoporous structure: The mesoporous structure is known to disintegrate during boiling with water, due to silicate hydrolysis [48]. The disintegration of the mesoporous structure was achieved by boiling Fe-Al-MCM-41 with millipore water. The liquid-to-sample ratio was fixed at 1 Lg−1. After 12 h of heating, the sample was filtered and dried in an oven for 2 h at 398 K. The XRD patterns of the dried sample revealed that the mesoporous integrity was totally lost. The catalytic reaction was performed with this “degraded-Fe-Al-MCM-41” (Table 2, entry 9).
Supporting Information
| Supporting Information File 1: Experimental procedures with characterization data for all compounds. | ||
| Format: PDF | Size: 1.6 MB | Download |
References
-
Yuki, H.; Okamoto, Y.; Sadamoto, K. Bull. Chem. Soc. Jpn. 1969, 42, 1754–1755. doi:10.1246/bcsj.42.1754
Return to citation in text: [1] -
Brady, W. T.; Giang, Y. F. J. Org. Chem. 1985, 50, 5177–5179. doi:10.1021/jo00225a039
Return to citation in text: [1] -
Hayakawa, K.; Ohara, K.; Akagi, N.; Hirata, Y. Preparation of 1, 1-diphenylethylene derivatives as starting materials for the synthesis of near-infrared-absorbing leuco dyes. Jpn. Kokai Tokkyo Koho JP04338363, A, Nov 25, 1992.
Return to citation in text: [1] -
D'Auria, M.; Esposito, V.; Mauriello, G. Tetrahedron 1996, 52, 14253–14272. doi:10.1016/0040-4020(96)00878-2
Return to citation in text: [1] -
Vesterinen, A.-H.; Rich, J.; Seppälä, J. J. Colloid Interface Sci. 2010, 351, 478–484. doi:10.1016/j.jcis.2010.07.073
Return to citation in text: [1] -
Tavor, D.; Popov, S.; Dlugy, C.; Wolfson, A. Org. Commun. 2010, 3, 70–75.
Return to citation in text: [1] -
Wang, X.; Guram, A.; Caille, S.; Hu, J.; Preston, J. P.; Ronk, M.; Walker, S. Org. Lett. 2011, 13, 1881–1883. doi:10.1021/ol200422p
Return to citation in text: [1] -
Kim, J.; Cho, J. C.; Kim, K. H.; Kim, K. U.; Jo, W. H.; Quirk, R. P. ACS Symp. Ser. 1998, 704, 85–95. doi:10.1021/bk-1998-0704.ch007
Return to citation in text: [1] -
Reutenauer, S.; Hurtrez, G.; Dumas, P. Macromolecules 2001, 34, 755–760. doi:10.1021/ma000676e
Return to citation in text: [1] -
Meyer, N.; Delaite, C.; Hurtrez, G.; Dumas, P. Polymer 2002, 43, 7133–7139. doi:10.1016/S0032-3861(02)00454-8
Return to citation in text: [1] -
Viala, S.; Tauer, K.; Antonietti, M.; Krüger, R.-P.; Bremser, W. Polymer 2002, 43, 7231–7241. doi:10.1016/S0032-3861(02)00676-6
Return to citation in text: [1] -
Kloninger, C.; Rehahn, M. Macromolecules 2004, 37, 1720–1727. doi:10.1021/ma034909o
Return to citation in text: [1] -
Grimsdale, A. C.; Chan, K. L.; Martin, R. E.; Jokisz, P. G.; Holmes, A. B. Chem. Rev. 2009, 109, 897–1091. doi:10.1021/cr000013v
Return to citation in text: [1] -
Tan, J. F.; Blencowe, A.; Goh, T. K.; Qiao, G. G. Macromol. Rapid Commun. 2010, 31, 305–309. doi:10.1002/marc.200900576
Return to citation in text: [1] -
Evans, D.; Cracknell, M. E.; Saunders, J. C.; Smith, C. E.; Williamson, W. R. N.; Dawson, W.; Sweatman, W. J. F. J. Med. Chem. 1987, 30, 1321–1327. doi:10.1021/jm00391a010
Return to citation in text: [1] -
Botteghi, C.; Corrias, T.; Marchetti, M.; Paganelli, S.; Piccolo, O. Org. Process Res. Dev. 2002, 6, 379–383. doi:10.1021/op020014k
Return to citation in text: [1] -
Valcarcel, G.; Cristina, I.; Mantlo, N. B.; Shi, Q.; Wang, M.; Winneroski, L. L., Jr.; Xu, Y.; York, J. S. Preparation of aryloxyalkoxyphenylalkanoic acids and analogs, as PPAR modulators, espeacially PPAR agonists. PCT Int. Appl. WO 2005019151, A1, March 3, 2005.
Return to citation in text: [1] -
Mizoroki, T.; Mori, K.; Ozaki, A. Bull. Chem. Soc. Jpn. 1971, 44, 581. doi:10.1246/bcsj.44.581
Return to citation in text: [1] -
Heck, R. F.; Nolley, J. P. J. Org. Chem. 1972, 37, 2320–2322. doi:10.1021/jo00979a024
Return to citation in text: [1] -
Fristrup, P.; Le Quement, S.; Tanner, D.; Norrby, P.-O. Organometallics 2004, 23, 6160–6165. doi:10.1021/om0494521
Return to citation in text: [1] -
Cabri, W.; Candiani, I. Acc. Chem. Res. 1995, 28, 2–7. doi:10.1021/ar00049a001
Return to citation in text: [1] -
Djakovitch, L.; Koehler, K. J. Am. Chem. Soc. 2001, 123, 5990–5999. doi:10.1021/ja001087r
Return to citation in text: [1] -
Mo, J.; Xu, L.; Xiao, J. J. Am. Chem. Soc. 2005, 127, 751–760. doi:10.1021/ja0450861
and references therein.
Return to citation in text: [1] -
Ruan, J.; Iggo, J. A.; Berry, N. G.; Xiao, J. J. Am. Chem. Soc. 2010, 132, 16689–16699. doi:10.1021/ja1081926
Return to citation in text: [1] -
Matsubara, R.; Gutierrez, A. C.; Jamison, T. F. J. Am. Chem. Soc. 2011, 133, 19020–19023. doi:10.1021/ja209235d
Return to citation in text: [1] -
Ruan, J.; Xiao, J. Acc. Chem. Res. 2011, 44, 614–626. doi:10.1021/ar200053d
Return to citation in text: [1] -
Sabarre, A.; Love, J. Org. Lett. 2008, 10, 3941–3944. doi:10.1021/ol8012843
Return to citation in text: [1] -
Yamaguchi, M.; Hayashi, A.; Hirama, M. J. Am. Chem. Soc. 1995, 117, 1151–1152. doi:10.1021/ja00108a041
Return to citation in text: [1] -
Tsuchimoto, T.; Maeda, T.; Shirakawa, E.; Kawakami, Y. Chem. Commun. 2000, 1573–1574. doi:10.1039/b003702h
Return to citation in text: [1] -
Bhilare, S. V.; Darvatkar, N. B.; Deorukhkar, A. R.; Raut, D. G.; Trivedi, G. K.; Salunkhe, M. M. Tetrahedron Lett. 2009, 50, 893–896. doi:10.1016/j.tetlet.2008.12.031
Return to citation in text: [1] -
Li, R.; Wang, S. R.; Lu, W. Org. Lett. 2007, 9, 2219–2222. doi:10.1021/ol070737u
Return to citation in text: [1] -
Yadav, J. S.; Reddy, B. V. S.; Sengupta, S.; Biswas, S. K. Synthesis 2009, 1301–1304. doi:10.1055/s-0028-1088027
Return to citation in text: [1] [2] -
Haldar, S.; Koner, S. J. Org. Chem. 2010, 75, 6005–6008. doi:10.1021/jo100803y
Return to citation in text: [1] [2] [3] [4] -
Armengol, E.; Cano, M. L.; Corma, A.; García, H.; Navarro, M. T. J. Chem. Soc., Chem. Commun. 1995, 519–520. doi:10.1039/c39950000519
Return to citation in text: [1] -
Corma, A. Chem. Rev. 1997, 97, 2373–2420. doi:10.1021/cr960406n
Return to citation in text: [1] -
Koch, H.; Liepold, A.; Roos, K.; Stöcker, M.; Reschetilowski, W. Chem. Eng. Technol. 1999, 22, 807–811. doi:10.1002/(SICI)1521-4125(199910)22:10<807::AID-CEAT807>3.0.CO;2-4
Return to citation in text: [1] -
Taguchi, A.; Schüth, F. Microporous Mesoporous Mater. 2005, 77, 1–45. doi:10.1016/j.micromeso.2004.06.030
Return to citation in text: [1] -
Agúndez, J.; Díaz, I.; Márquez-Álvarez, C.; Pérez-Pariente, J.; Sastre, E. Chem. Commun. 2003, 150–151. doi:10.1039/B209983G
Return to citation in text: [1] -
Perego, C.; de Angelis, A.; Carati, A.; Flego, C.; Millini, R.; Rizzo, C.; Bellussi, G. Appl. Catal., A 2006, 307, 128–136. doi:10.1016/j.apcata.2006.03.013
Return to citation in text: [1] -
Iwanami, K.; Choi, J.-C.; Lu, B.; Sakakura, T.; Yasuda, H. Chem. Commun. 2008, 1002–1004. doi:10.1039/B718462J
Return to citation in text: [1] -
Pega, S.; Boissière, C.; Grosso, D.; Azaïs, T.; Chaumonnot, A.; Sanchez, C. Angew. Chem., Int. Ed. 2009, 48, 2784–2787. doi:10.1002/anie.200805217
Return to citation in text: [1] -
Robinson, M. W. C.; Davies, A. M.; Mabbett, I.; Davies, T. E.; Apperley, D. C.; Taylor, S. H.; Graham, A. E. J. Mol. Catal. A: Chem. 2010, 329, 57–63. doi:10.1016/j.molcata.2010.06.018
Return to citation in text: [1] -
Sartori, G.; Franca Bigi, F.; Pastorío, A.; Porta, C.; Arienti, A.; Maggi, R.; Moretti, N.; Gnappi, G. Tetrahedron Lett. 1995, 36, 9177–9180. doi:10.1016/0040-4039(95)01934-A
Return to citation in text: [1] [2] [3] [4] -
Sharma, K. K.; Asefa, T. Angew. Chem., Int. Ed. 2007, 46, 2879–2882. doi:10.1002/anie.200604570
Return to citation in text: [1] -
Sharma, K. K.; Anan, A.; Buckley, R. P.; Ouellette, W.; Asefa, T. J. Am. Chem. Soc. 2008, 130, 218–228. doi:10.1021/ja074128t
Return to citation in text: [1] -
Yu, K.; McKittrick, M. W.; Jones, C. W. Organometallics 2004, 23, 4089–4096. doi:10.1021/om049765w
Return to citation in text: [1] -
Müller, C.; Ackerman, L. J.; Reek, J. N. H.; Kamer, P. C. J.; van Leeuwen, P. W. N. M. J. Am. Chem. Soc. 2004, 126, 14960–14963. doi:10.1021/ja046901f
Return to citation in text: [1] -
Kim, J. M.; Ryoo, R. Bull. Korean Chem. Soc. 1996, 17, 66–68.
Return to citation in text: [1] [2]
| 1. | Yuki, H.; Okamoto, Y.; Sadamoto, K. Bull. Chem. Soc. Jpn. 1969, 42, 1754–1755. doi:10.1246/bcsj.42.1754 |
| 2. | Brady, W. T.; Giang, Y. F. J. Org. Chem. 1985, 50, 5177–5179. doi:10.1021/jo00225a039 |
| 3. | Hayakawa, K.; Ohara, K.; Akagi, N.; Hirata, Y. Preparation of 1, 1-diphenylethylene derivatives as starting materials for the synthesis of near-infrared-absorbing leuco dyes. Jpn. Kokai Tokkyo Koho JP04338363, A, Nov 25, 1992. |
| 4. | D'Auria, M.; Esposito, V.; Mauriello, G. Tetrahedron 1996, 52, 14253–14272. doi:10.1016/0040-4020(96)00878-2 |
| 5. | Vesterinen, A.-H.; Rich, J.; Seppälä, J. J. Colloid Interface Sci. 2010, 351, 478–484. doi:10.1016/j.jcis.2010.07.073 |
| 6. | Tavor, D.; Popov, S.; Dlugy, C.; Wolfson, A. Org. Commun. 2010, 3, 70–75. |
| 7. | Wang, X.; Guram, A.; Caille, S.; Hu, J.; Preston, J. P.; Ronk, M.; Walker, S. Org. Lett. 2011, 13, 1881–1883. doi:10.1021/ol200422p |
| 20. | Fristrup, P.; Le Quement, S.; Tanner, D.; Norrby, P.-O. Organometallics 2004, 23, 6160–6165. doi:10.1021/om0494521 |
| 21. | Cabri, W.; Candiani, I. Acc. Chem. Res. 1995, 28, 2–7. doi:10.1021/ar00049a001 |
| 22. | Djakovitch, L.; Koehler, K. J. Am. Chem. Soc. 2001, 123, 5990–5999. doi:10.1021/ja001087r |
| 23. |
Mo, J.; Xu, L.; Xiao, J. J. Am. Chem. Soc. 2005, 127, 751–760. doi:10.1021/ja0450861
and references therein. |
| 33. | Haldar, S.; Koner, S. J. Org. Chem. 2010, 75, 6005–6008. doi:10.1021/jo100803y |
| 18. | Mizoroki, T.; Mori, K.; Ozaki, A. Bull. Chem. Soc. Jpn. 1971, 44, 581. doi:10.1246/bcsj.44.581 |
| 19. | Heck, R. F.; Nolley, J. P. J. Org. Chem. 1972, 37, 2320–2322. doi:10.1021/jo00979a024 |
| 34. | Armengol, E.; Cano, M. L.; Corma, A.; García, H.; Navarro, M. T. J. Chem. Soc., Chem. Commun. 1995, 519–520. doi:10.1039/c39950000519 |
| 35. | Corma, A. Chem. Rev. 1997, 97, 2373–2420. doi:10.1021/cr960406n |
| 36. | Koch, H.; Liepold, A.; Roos, K.; Stöcker, M.; Reschetilowski, W. Chem. Eng. Technol. 1999, 22, 807–811. doi:10.1002/(SICI)1521-4125(199910)22:10<807::AID-CEAT807>3.0.CO;2-4 |
| 37. | Taguchi, A.; Schüth, F. Microporous Mesoporous Mater. 2005, 77, 1–45. doi:10.1016/j.micromeso.2004.06.030 |
| 38. | Agúndez, J.; Díaz, I.; Márquez-Álvarez, C.; Pérez-Pariente, J.; Sastre, E. Chem. Commun. 2003, 150–151. doi:10.1039/B209983G |
| 39. | Perego, C.; de Angelis, A.; Carati, A.; Flego, C.; Millini, R.; Rizzo, C.; Bellussi, G. Appl. Catal., A 2006, 307, 128–136. doi:10.1016/j.apcata.2006.03.013 |
| 40. | Iwanami, K.; Choi, J.-C.; Lu, B.; Sakakura, T.; Yasuda, H. Chem. Commun. 2008, 1002–1004. doi:10.1039/B718462J |
| 41. | Pega, S.; Boissière, C.; Grosso, D.; Azaïs, T.; Chaumonnot, A.; Sanchez, C. Angew. Chem., Int. Ed. 2009, 48, 2784–2787. doi:10.1002/anie.200805217 |
| 42. | Robinson, M. W. C.; Davies, A. M.; Mabbett, I.; Davies, T. E.; Apperley, D. C.; Taylor, S. H.; Graham, A. E. J. Mol. Catal. A: Chem. 2010, 329, 57–63. doi:10.1016/j.molcata.2010.06.018 |
| 15. | Evans, D.; Cracknell, M. E.; Saunders, J. C.; Smith, C. E.; Williamson, W. R. N.; Dawson, W.; Sweatman, W. J. F. J. Med. Chem. 1987, 30, 1321–1327. doi:10.1021/jm00391a010 |
| 16. | Botteghi, C.; Corrias, T.; Marchetti, M.; Paganelli, S.; Piccolo, O. Org. Process Res. Dev. 2002, 6, 379–383. doi:10.1021/op020014k |
| 17. | Valcarcel, G.; Cristina, I.; Mantlo, N. B.; Shi, Q.; Wang, M.; Winneroski, L. L., Jr.; Xu, Y.; York, J. S. Preparation of aryloxyalkoxyphenylalkanoic acids and analogs, as PPAR modulators, espeacially PPAR agonists. PCT Int. Appl. WO 2005019151, A1, March 3, 2005. |
| 33. | Haldar, S.; Koner, S. J. Org. Chem. 2010, 75, 6005–6008. doi:10.1021/jo100803y |
| 8. | Kim, J.; Cho, J. C.; Kim, K. H.; Kim, K. U.; Jo, W. H.; Quirk, R. P. ACS Symp. Ser. 1998, 704, 85–95. doi:10.1021/bk-1998-0704.ch007 |
| 9. | Reutenauer, S.; Hurtrez, G.; Dumas, P. Macromolecules 2001, 34, 755–760. doi:10.1021/ma000676e |
| 10. | Meyer, N.; Delaite, C.; Hurtrez, G.; Dumas, P. Polymer 2002, 43, 7133–7139. doi:10.1016/S0032-3861(02)00454-8 |
| 11. | Viala, S.; Tauer, K.; Antonietti, M.; Krüger, R.-P.; Bremser, W. Polymer 2002, 43, 7231–7241. doi:10.1016/S0032-3861(02)00676-6 |
| 12. | Kloninger, C.; Rehahn, M. Macromolecules 2004, 37, 1720–1727. doi:10.1021/ma034909o |
| 13. | Grimsdale, A. C.; Chan, K. L.; Martin, R. E.; Jokisz, P. G.; Holmes, A. B. Chem. Rev. 2009, 109, 897–1091. doi:10.1021/cr000013v |
| 14. | Tan, J. F.; Blencowe, A.; Goh, T. K.; Qiao, G. G. Macromol. Rapid Commun. 2010, 31, 305–309. doi:10.1002/marc.200900576 |
| 33. | Haldar, S.; Koner, S. J. Org. Chem. 2010, 75, 6005–6008. doi:10.1021/jo100803y |
| 29. | Tsuchimoto, T.; Maeda, T.; Shirakawa, E.; Kawakami, Y. Chem. Commun. 2000, 1573–1574. doi:10.1039/b003702h |
| 31. | Li, R.; Wang, S. R.; Lu, W. Org. Lett. 2007, 9, 2219–2222. doi:10.1021/ol070737u |
| 28. | Yamaguchi, M.; Hayashi, A.; Hirama, M. J. Am. Chem. Soc. 1995, 117, 1151–1152. doi:10.1021/ja00108a041 |
| 32. | Yadav, J. S.; Reddy, B. V. S.; Sengupta, S.; Biswas, S. K. Synthesis 2009, 1301–1304. doi:10.1055/s-0028-1088027 |
| 24. | Ruan, J.; Iggo, J. A.; Berry, N. G.; Xiao, J. J. Am. Chem. Soc. 2010, 132, 16689–16699. doi:10.1021/ja1081926 |
| 25. | Matsubara, R.; Gutierrez, A. C.; Jamison, T. F. J. Am. Chem. Soc. 2011, 133, 19020–19023. doi:10.1021/ja209235d |
| 26. | Ruan, J.; Xiao, J. Acc. Chem. Res. 2011, 44, 614–626. doi:10.1021/ar200053d |
| 30. | Bhilare, S. V.; Darvatkar, N. B.; Deorukhkar, A. R.; Raut, D. G.; Trivedi, G. K.; Salunkhe, M. M. Tetrahedron Lett. 2009, 50, 893–896. doi:10.1016/j.tetlet.2008.12.031 |
| 44. | Sharma, K. K.; Asefa, T. Angew. Chem., Int. Ed. 2007, 46, 2879–2882. doi:10.1002/anie.200604570 |
| 45. | Sharma, K. K.; Anan, A.; Buckley, R. P.; Ouellette, W.; Asefa, T. J. Am. Chem. Soc. 2008, 130, 218–228. doi:10.1021/ja074128t |
| 46. | Yu, K.; McKittrick, M. W.; Jones, C. W. Organometallics 2004, 23, 4089–4096. doi:10.1021/om049765w |
| 47. | Müller, C.; Ackerman, L. J.; Reek, J. N. H.; Kamer, P. C. J.; van Leeuwen, P. W. N. M. J. Am. Chem. Soc. 2004, 126, 14960–14963. doi:10.1021/ja046901f |
| 43. | Sartori, G.; Franca Bigi, F.; Pastorío, A.; Porta, C.; Arienti, A.; Maggi, R.; Moretti, N.; Gnappi, G. Tetrahedron Lett. 1995, 36, 9177–9180. doi:10.1016/0040-4039(95)01934-A |
| 43. | Sartori, G.; Franca Bigi, F.; Pastorío, A.; Porta, C.; Arienti, A.; Maggi, R.; Moretti, N.; Gnappi, G. Tetrahedron Lett. 1995, 36, 9177–9180. doi:10.1016/0040-4039(95)01934-A |
| 33. | Haldar, S.; Koner, S. J. Org. Chem. 2010, 75, 6005–6008. doi:10.1021/jo100803y |
| 32. | Yadav, J. S.; Reddy, B. V. S.; Sengupta, S.; Biswas, S. K. Synthesis 2009, 1301–1304. doi:10.1055/s-0028-1088027 |
| 43. | Sartori, G.; Franca Bigi, F.; Pastorío, A.; Porta, C.; Arienti, A.; Maggi, R.; Moretti, N.; Gnappi, G. Tetrahedron Lett. 1995, 36, 9177–9180. doi:10.1016/0040-4039(95)01934-A |
| 43. | Sartori, G.; Franca Bigi, F.; Pastorío, A.; Porta, C.; Arienti, A.; Maggi, R.; Moretti, N.; Gnappi, G. Tetrahedron Lett. 1995, 36, 9177–9180. doi:10.1016/0040-4039(95)01934-A |
© 2013 Haldar and Koner; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)