Abstract
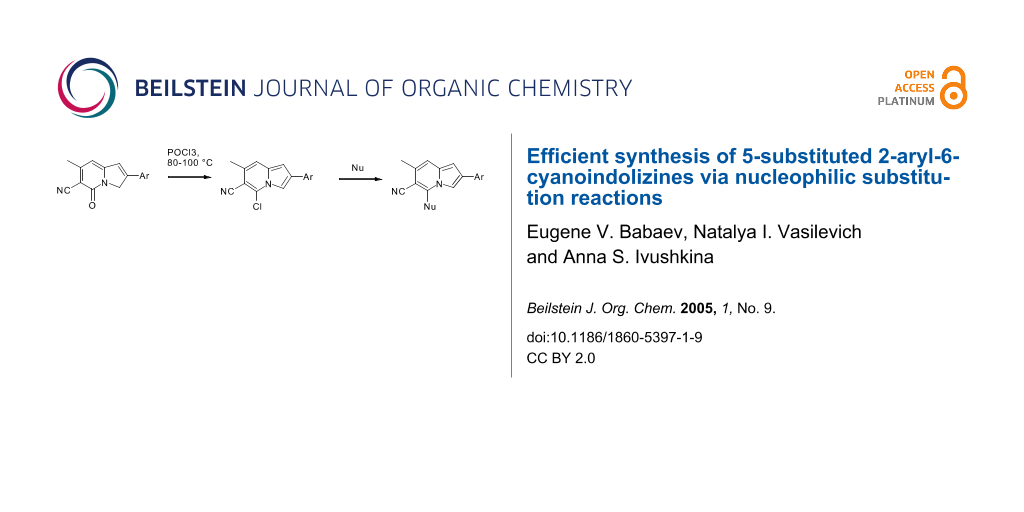
2-Aryl-6-cyano-7-methyl-5-indolizinones were successfully converted into 2-aryl-5-chloro-6-cyano-7-methylindolizines. The obtained 5-chloroindolizines readily underwent nucleophilic substitution at position 5 leading in high yields to novel 5-functionalised indolizines.
Graphical Abstract
Indolizines are an important class of heterocyclic compounds since many natural alkaloids contain in their structure a saturated (swainsonine) or aromatic (camptothecin) indolizine moiety. While the chemistry of indolizines has been widely investigated[1] the chemistry of 5-substituted indolizines remains very poor because there are only a few reliable ways for their synthesis.
8-Nitroindolizines may undergo amination at position 5 (SNH substitution) under the action of secondary amines.[2] 2-Phenylindolizine can be lithiated at position 5, and the resulting indolizyl lithium can react with some electrophiles (CO2, PhCHO, PhCN, Me3SiCl, MeI) leading to variety of new products in good yields.[3] An interesting method for preparing 5-substitutied indolizines by recyclization of oxazolo[3,2-a]pyridinium salts was developed in our laboratory.[4,5] Using this strategy a series of 5-substituted indolizines have been prepared in good yields, but (although the method seems to be quite reliable) it is currently restricted only by secondary amines.
In seeking for a synthetic approach to 5-substituted indolizines we have assumed that indolizines bearing an appropriate leaving group (e.g. halogen) at position 5 may undergo nucleophilic substitution. Herein we discuss the synthesis of previously unknown 5-chloroindolizines and their use as precursors to novel 5-substituted indolizines via nucleophilic displacement reactions.
The synthesis of 2-aryl-5-chloro-6-cyano-7-methylindolizines 2 is shown in Scheme 1. 2-Aryl-6-cyano-7-methyl-5-indolizinones 1 a – d were prepared according to protocol of Gevald.[6] Our modification of the original method included separation of N- and O-isomers of phenacyl pyridines before cyclization (using the difference in their solubility in chloroform). Although 1H-NMR (see Supporting Information File 2) and Nuclear Overhauser Effect confirmed the structure A for indolizinones 1, we assumed the existence of tautomerism between forms A and B involving hydrogen interchange between oxygen and C-3 carbon (Scheme 1). Although the amount of tautomer B is negligibly small, one would expect that treatment of 1 a – d with phosphorous oxychloride may lead to substitution of oxo/oxy-group to chlorine giving the products 2 a – d. (It is well known that analogous 2-hydroxypyridines which exist in the pyridone tautomeric form can be easily converted to 2-chloroderivatives by reaction with POCl3.).[7] Indeed, heating of indolizinones 1 a – d in POCl3 at 80–100°C during 10 hours without any solvent followed by pouring into a mixture of ice/sodium acetate and filtration of the green precipitate afforded crude 5-chloroindolizines. After column chromatography (eluent – carbon tetrachloride) yellow solids were obtained. Performing this reaction in the presence of two-fold molar excess of trimethylbenzylammonium chloride or TEBAC increased the yields of 2 a – d up to 30–75%. In the 1H NMR spectra of these products† the initial signal of 3-CH2 group at ~5 ppm (intensity 2H) disappeared, and a new aromatic signal 3-CH (with intensity 1H) appeared at 7.99 – 8.11 ppm.
Scheme 1: Synthesis of 2-aryl-5-chloro-6-cyano-7-methylindolizines 2. Possible tautomeric structures A and B for 2-aryl-6-cyano-7-methyl-5-indolizinones 1.
Scheme 1: Synthesis of 2-aryl-5-chloro-6-cyano-7-methylindolizines 2. Possible tautomeric structures A and B ...
The halogen atom in 5-chloro-6-cyanoindolizines 2 should be activated to nucleophilic substitution reactions by the suitable ortho-arrangement of the nitrogen atom of the pyridine ring and electron-withdrawing cyano-group. The pattern strongly resembled 2-chloro-3-cyanopyridine, that is why we anticipated successful substitution in reactions of 2 with oxygen, nitrogen, and sulfur nucleophiles. Indeed, 5-chloroindolizines readily underwent nucleophilic substitution to produce previously unknown compounds 3 – 6 in good to excellent yields (Scheme 2). These products are detailed in Table 2. Thus, 5-methoxyindolizines 3 a – c were formed after refluxing 2 b – d in solution of sodium methoxide in methanol overnight in good yields (Scheme 2). Treatment of 2a, d with excess of amines without any solvent gave 5-amino derivatives 4 a – h. In the case of secondary amines (4 a – c, e – g) the reaction proceeded at room temperature, but reaction with less nucleophilic benzylamine (4 d, h) required heating for 30 min. Nucleophilic substitution also occured with sulfur nucleophiles. Thus, 2d reacted with mercaptoethanol under basic conditions leading to 5a. Conversion 2a into 5b was conveniently achieved with ethyl mercaptoacetate in ethanolic sodium hydroxide. Interestingly, 2a reacted also with thiourea in refluxing butanol giving indolizinethione 6. The product 6 seems to be the result of decomposition of unstable isothiouronium salt, and the process resembles the known conversion of 2-chloro-3-cyanopyridines to 3-cyanopyridinethiones under the same conditions via a similar intermediate.[8]1H NMR spectrum of 6, which was very similar to the spectra of 1, indicated disappearance of aromatic proton signal H3 and appearance of a signal at 5.35 ppm with intensity 2H.†
Scheme 2: Nucleophilic substitution in 2-aryl-5-chloro-6-cyano-7-methylindolizines.
Scheme 2: Nucleophilic substitution in 2-aryl-5-chloro-6-cyano-7-methylindolizines.
In conclusion, we are the first to obtain 2-aryl-5-chloro-6-cyano-7-methylindolizines from 2-aryl-6-cyano-7-methyl-5-indolizinones and to prove the possibility to employ them in nucleophilic substitution reactions. Moreover, these reactions are the first examples of preparative nucleophilic substitution in indolizines, and our findings open a new way to functionalize the C-5 position (in most cases considered as inactive). The studies of further cyclizations of 5-substituted indolizines involving neighbouring cyano-group and ring position C3 is underway.
Table 1: Properties of 5-substituted 2-aryl-6-cyano-7-methylindolizines
|
|
||||
| No. | 5-X* | R in 2-Ar | Yield % | m.p., °C |
|---|---|---|---|---|
| 2 a | Cl | p-F | 30 | 173–175 |
| 2 b | Cl | p-Cl | 65 | 198–200 |
| 2 c | Cl | p-Br | 54 | 229–230 |
| 2 d | Cl | p-Me | 74 | 157–158 |
| 3 a | OMe | p-Cl | 50 | 169–172 |
| 3 b | OMe | p-Br | 71 | 197–200 |
| 3 c | OMe | p-Me | 73 | 170–173 |
| 4 a | pyrrolidyl | p-F | 99 | 189–190 |
| 4 b | piperidyl | p-F | 91 | 205–209 |
| 4 c | hexamethy-lenimino | p-F | 88 | 186–188 |
| 4 d | benzylamino | p-F | 58 | 190–194 |
| 4 e | pyrrolidyl | p-Me | 74 | 228–230 |
| 4 f | piperidyl | p-Me | 71 | 212–215 |
| 4 g | hexamethy-lenimino | p-Me | 85 | 213–216 |
| 4 h | benzylamino | p-Me | 83 | 185–187 |
| 5 a | S(CH2)2OH | p-Me | 71 | 132–135 |
| 5 b | SCH2CO2Et | p-F | 70 | 142–145 |
Note
*Characteristics of parent indolizinones 1 a – d were identical to those described in literature.[9]
† Supporting information
References
-
Flitch, W. Pyrroles with fused six-membered heterocyclic rings: a-fused. In Comprehensive Heterocyclic Chemistry; Katritzky, A. R.; Rees, C. W., Eds.; Pergamon Press: London, 1984; Vol. 4, pp 443–478.
Return to citation in text: [1] -
Kost, A. N.; Sagitullin, R. S.; Gromov, S. P. Heterocycles 1977, 7, 997–1001.
Return to citation in text: [1] -
Renard, M.; Gubin, J. Tetrahedron Lett. 1992, 33, 4433–4434. doi:10.1016/S0040-4039(00)60102-8
Return to citation in text: [1] -
Babaev, E. V.; Efimov, A. V.; Maiboroda, D. A.; Jug, K. Eur. J. Org. Chem. 1998, 1, 193–196. doi:10.1002/(SICI)1099-0690(199801)1998:1<193::AID-EJOC193>3.0.CO;2-6
Return to citation in text: [1] -
Babaev, E. V. J. Heterocycl. Chem. 2000, 37, 519–526.
Return to citation in text: [1] -
Gevald, K.; Jansch, H. J. J. Prakt. Chem. 1976, 318, 313–320. doi:10.1002/prac.19763180216
Return to citation in text: [1] -
Kwok, R. J. Heterocycl. Chem. 1978, 15, 877–880.
Return to citation in text: [1] -
Kitaygorodova, E. A.; Konyushkin, L. D.; Mikhaychenko, S. N. Khim. Geterotsikl. Soedin. 1999, 3, 337–341.
(Rus).
Return to citation in text: [1] -
Lin, C. F.; Lin, Y. F.; Lo, Y. C.; Chen, K. T.; Su, T. L. Heterocycles 2000, 1, 15–26.
Return to citation in text: [1]
| 1. | Flitch, W. Pyrroles with fused six-membered heterocyclic rings: a-fused. In Comprehensive Heterocyclic Chemistry; Katritzky, A. R.; Rees, C. W., Eds.; Pergamon Press: London, 1984; Vol. 4, pp 443–478. |
| 6. | Gevald, K.; Jansch, H. J. J. Prakt. Chem. 1976, 318, 313–320. doi:10.1002/prac.19763180216 |
| 4. | Babaev, E. V.; Efimov, A. V.; Maiboroda, D. A.; Jug, K. Eur. J. Org. Chem. 1998, 1, 193–196. doi:10.1002/(SICI)1099-0690(199801)1998:1<193::AID-EJOC193>3.0.CO;2-6 |
| 5. | Babaev, E. V. J. Heterocycl. Chem. 2000, 37, 519–526. |
| 3. | Renard, M.; Gubin, J. Tetrahedron Lett. 1992, 33, 4433–4434. doi:10.1016/S0040-4039(00)60102-8 |
| 9. | Lin, C. F.; Lin, Y. F.; Lo, Y. C.; Chen, K. T.; Su, T. L. Heterocycles 2000, 1, 15–26. |
| 8. |
Kitaygorodova, E. A.; Konyushkin, L. D.; Mikhaychenko, S. N. Khim. Geterotsikl. Soedin. 1999, 3, 337–341.
(Rus). |
© 2005 Babaev et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)