Abstract
Pyrrolo[2,1-a]isoquinoline alkaloids have been prepared via a visible light photoredox catalyzed oxidation/[3 + 2] cycloaddition/oxidative aromatization cascade using Rose Bengal as an organo-photocatalyst. A variety of pyrroloisoquinolines have been obtained in good yields under mild and metal-free reaction conditions.
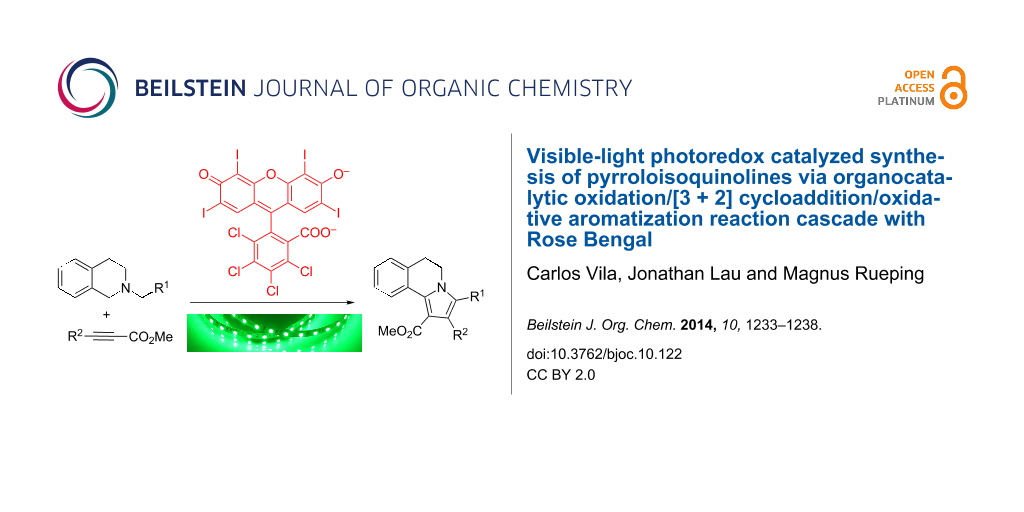
Graphical Abstract
Introduction
Pyrrolo[2,1-a]isoquinolines constitute the core structure of the natural products family lamellarin alkaloids (Figure 1) [1-4]. These alkaloids display numerous biological activities such as inhibitor of human topoisomerase I by lamellarin D [5] or inhibition of HIV integrase by lamellarin α-20-sulfate [6,7]. Moreover lamellarin I and lamellarin K also showed potential antitumor activities [8,9]. Due to their potential biological activities, the synthesis of pyrrolo[2,1-a]isoquinolines has become a very interesting, important and attractive goal in organic synthesis [10-20]. For example, dipolar [3 + 2] cycloaddition using azomethine ylides [21] is a powerful class of reactions that permits the synthesis of structural complex molecules in a straightforward way and has been used for the efficient synthesis of this type of compounds [22-26]. Recently, several metal mediated syntheses using a [3 + 2] cycloaddition reaction have been described in the literature. Porco Jr. et al. [27] described a silver-catalyzed cycloisomerization/dipolar cycloaddition for the synthesis of the pyrrolo[2,1-a]isoquinolines. Wang and co-workers described a copper catalyzed oxidation/[3 + 2] cycloaddition/aromatization cascade [28]. Also, Xiao disclosed a very elegant oxidation/[3 + 2] cycloaddition/aromatization cascade catalyzed by [Ru(bpy)3]3+ under irradiation with visible light [29]. In this context, very recently Zhao reported the same reaction using C60-Bodipy hybrids [30] and porous material immobilized iodo-Bodipy [31] as photocatalysts, obtaining in both cases good yields for different pyrrolo[2,1-a]isoquinolines. Finally, Lu presented in 2013 a dirhodium complex for the synthesis of these compounds [32]. Despite these elegant and important syntheses of pyrrolo[2,1-a]isoquinolines through dipolar [3 + 2] cycloaddition, the development of metal-free syntheses using visible light photoredox catalysis with simple organic dyes remained unexplored. Visible-light photoredox catalysis has emerged as an important field and has attracted increasing attention in recent years [33-42]. Thus, in the last years spectacular advances in visible-light photoredox catalysis have been made and this kind of catalysis has become a powerful tool in organic synthesis. In this context, the use of organic dyes as photoredox catalysts [40-42] has been demonstrated by several groups [43-61] and became a useful alternative to the inorganic photoredox catalysts that are expensive and sometimes toxic. The organic dyes have very important qualities such as being inexpensive, environmentally friendly and easy to handle. As a part of our ongoing research on photoredox catalysis [62-72], we herein present a synthesis of pyrrolo[2,1-a]isoquinolines through an oxidation/[3+2] cycloaddition/aromatization cascade catalyzed by Rose Bengal under irradiation with green LEDs.
Figure 1: Representative examples of lamellarin alkaloids.
Figure 1: Representative examples of lamellarin alkaloids.
Results and Discussion
Initially, we focused on the reaction between methyl dihydroisoquinoline ester 1a and N-methylmaleimide (2a) catalyzed by Rose Bengal. Although the [3 + 2] cycloaddition occurs smoothly in the presence of Rose Bengal (5 mol %) in acetonitrile under irradiation with visible light, the reaction was not selective affording the dihydropyrrolo[2,1-a]isoquinoline 3aa in 35% yield and the hexahydropyrrolo[2,1-a]isoquinoline 4aa in 26% yield, after column chromatography (Scheme 1).
Scheme 1: Photocatalytic metal free construction of pyrrolo[2,1-a]isoquinolines.
Scheme 1: Photocatalytic metal free construction of pyrrolo[2,1-a]isoquinolines.
In order to improve the selectivity of the reaction to the aromatized product 3aa, N-bromosuccinimide was added to the reaction mixture when the starting materials were completely consumed [29-31,73]. In this case the desired product 3aa was obtained in 72% yield (Table 1, entry 1). Other organic dyes such as Rhodamine B or Eosin Y were less efficient compared to Rose Bengal (Table 1, entries 2 and 3, respectively). Several solvents were tested without an improvement in the yield of the product (Table 1, entries 4–9). Finally, after tuning the relative amounts of the reagents, the product 3aa was isolated in 76% yield (Table 1, entry 12).
Table 1: Optimization of the reaction conditions.a
|
|
|||
| Entry | Catalyst | Solvent | Yield (%)b |
|---|---|---|---|
| 1 | Rose Bengal | CH3CN | 72 |
| 2 | Rhodamine B | CH3CN | 11 |
| 3 | Eosin Y | CH3CN | 40 |
| 4 | Rose Bengal | THF | 29 |
| 5 | Rose Bengal | CH2Cl2 | 26 |
| 6 | Rose Bengal | toluene | 14 |
| 7 | Rose Bengal | DMF | 65 |
| 8 | Rose Bengal | MeOH | 52 |
| 9 | Rose Bengal | EtOAc | 16 |
| 10c | Rose Bengal | CH3CN | 64 |
| 11d | Rose Bengal | CH3CN | 60 |
| 12e | Rose Bengal | CH3CN | 76 |
aReaction conditions: 1a (0.2 mmol), 2a (0.2 mmol), organic dye (5 mol %), solvent (1 mL), green LEDs irradiation for 24 hours. NBS (1.1 equiv) was added to the reaction mixture and stirring was continued for 1 hour. bYields of the isolated products after column chromatography. c1.25 equiv of 1a was used. d1.25 equiv of 2a was used. e1.1 equiv of 1a was used.
With the optimal conditions in hand, we examined the substrate scope for the photoreaction catalyzed by Rose Bengal (Scheme 2). Various tetrahydroisoquinolines with different electron-withdrawing groups (R2) such as methyl ester (1a), ethyl ester (1b), tert-butyl ester (1c), cyano (1d) or aromatic ketone (1e) were reacted with N-methylmaleimide (2a) and gave the corresponding products 3 in moderate to good yields. In addition, different N-substituted maleimides were tested under the optimized reaction conditions to give the corresponding products with good yields. Incorporation of methoxy groups at C-6 and C-7 in the dihydroisoquinoline core was well tolerated, affording the corresponding product 3fa in 68% yield.
Scheme 2: Evaluation of the substrate scope.
Scheme 2: Evaluation of the substrate scope.
To demonstrate the synthetic utility of the oxidation/[3 + 2] cycloaddition/aromatization cascade we examined other dipolarophiles such as activated alkynes 5. In this case, the addition of NBS was not necessary, and the corresponding products 6 were isolated in moderate yields (Scheme 3).
Scheme 3: Evaluation of the substrate scope with activated alkynes.
Scheme 3: Evaluation of the substrate scope with activated alkynes.
Conclusion
In conclusion, we have developed a metal-free photoredox oxidation/[3 + 2] dipolar cycloaddition/oxidative aromatization cascade catalyzed by Rose Bengal using visible-light. This protocol offers a “green” and straightforward synthesis of pyrrolo[2,1-a]isoquinolines starting from readily available maleimides and tetrahydroisoquinolines. Further investigations to expand the scope and potential of this methodology are underway in our laboratory.
Supporting Information
| Supporting Information File 1: Experimental details and characterization of the synthesized compounds. | ||
| Format: PDF | Size: 665.1 KB | Download |
References
-
Bailly, C. Curr. Med. Chem.: Anti-Cancer Agents 2004, 4, 363–378. doi:10.2174/1568011043352939
Return to citation in text: [1] -
Handy, S. T.; Zhang, Y. Org. Prep. Proced. Int. 2005, 37, 411–445. doi:10.1080/00304940509354977
Return to citation in text: [1] -
Fan, H.; Peng, J.; Hamann, M. T.; Hu, J.-F. Chem. Rev. 2008, 108, 264–287. doi:10.1021/cr078199m
Return to citation in text: [1] -
Pla, D.; Albericio, F.; Alvarez, M. Anti-Cancer Agents Med. Chem. 2008, 8, 746–760. doi:10.2174/187152008785914789
Return to citation in text: [1] -
Marco, E.; Laine, W.; Tardy, C.; Lansiaux, A.; Iwao, M.; Ishibashi, F.; Bailly, C.; Gago, F. J. Med. Chem. 2005, 48, 3796–3807. doi:10.1021/jm049060w
Return to citation in text: [1] -
Reddy, M. V. R.; Rao, M. R.; Rhodes, D.; Hansen, M. S. T.; Rubins, K.; Bushman, F. D.; Venkateswarlu, Y.; Faulkner, D. J. Med. Chem. 1999, 42, 1901–1907. doi:10.1021/jm9806650
Return to citation in text: [1] -
Aubry, A.; Pan, X.-S.; Fisher, L. M.; Jarlier, V.; Cambau, E. Antimicrob. Agents Chemother. 2004, 48, 1281–1288. doi:10.1128/AAC.48.4.1281-1288.2004
Return to citation in text: [1] -
Reddy, S. M.; Srinivasulu, M.; Satanarayana, N.; Kondapi, A. K.; Venkateswarlu, Y. Tetrahedron 2005, 61, 9242–9247. doi:10.1016/j.tet.2005.07.067
Return to citation in text: [1] -
Quesada, A. R.; Garcia Grávalos, M. D.; Fernández Puentes, J. L. Br. J. Cancer 1996, 74, 677–682. doi:10.1038/bjc.1996.421
Return to citation in text: [1] -
Heim, A.; Terpin, A.; Steglich, W. Angew. Chem., Int. Ed. Engl. 1997, 36, 155–156. doi:10.1002/anie.199701551
Return to citation in text: [1] -
Ploypradith, P.; Mahidol, C.; Sahakitpichan, P.; Wongbundit, S.; Ruchirawat, S. Angew. Chem., Int. Ed. 2004, 43, 866–868. doi:10.1002/anie.200352043
Return to citation in text: [1] -
Boger, D. L.; Boyce, C. W.; Labroli, M. A.; Sehon, C. A.; Jin, Q. J. Am. Chem. Soc. 1999, 121, 54–62. doi:10.1021/ja982078+
Return to citation in text: [1] -
Banwell, M. G.; Flynn, B. L.; Stewart, S. G. J. Org. Chem. 1998, 63, 9139–9144. doi:10.1021/jo9808526
Return to citation in text: [1] -
Handy, S. T.; Zhang, Y.; Bregman, H. J. Org. Chem. 2004, 69, 2362–2366. doi:10.1021/jo0352833
Return to citation in text: [1] -
Ploypradith, P.; Kagan, R. K.; Ruchirawat, S. J. Org. Chem. 2005, 70, 5119–5125. doi:10.1021/jo050388m
Return to citation in text: [1] -
Ohta, T.; Fukuda, T.; Ishibashi, F.; Iwao, M. J. Org. Chem. 2009, 74, 8143–8153. doi:10.1021/jo901589e
Return to citation in text: [1] -
Gupton, J. T.; Clough, S. C.; Miller, R. B.; Lukens, J. R.; Henry, C. A.; Kanters, R. P. F.; Sikorski, J. A. Tetrahedron 2003, 59, 207–215. doi:10.1016/S0040-4020(02)01475-8
Return to citation in text: [1] -
Fujikawa, N.; Ohta, T.; Yamaguchi, T.; Fukuda, T.; Ishibashi, F.; Iwao, M. Tetrahedron 2006, 62, 594–604. doi:10.1016/j.tet.2005.10.014
Return to citation in text: [1] -
Chen, L.; Xu, M.-H. Adv. Synth. Catal. 2009, 351, 2005–2012. doi:10.1002/adsc.200900287
Return to citation in text: [1] -
Yadav, J. S.; Gayathri, K. U.; Reddy, B. V. S.; Prasad, A. R. Synlett 2009, 43–46. doi:10.1055/s-0028-1087387
Return to citation in text: [1] -
Najera, C.; Sansano, J. M. Curr. Org. Chem. 1998, 7, 1105–1150. doi:10.2174/1385272033486594
Return to citation in text: [1] -
Ishibashi, F.; Miyazaki, Y.; Iwao, M. Tetrahedron 1997, 53, 5951–5962. doi:10.1016/S0040-4020(97)00287-1
Return to citation in text: [1] -
Banwell, M. G.; Flynn, B. L.; Hockless, D. C. R. Chem. Commun. 1997, 2259–2260. doi:10.1039/a705874h
Return to citation in text: [1] -
Cironi, P.; Manzanares, I.; Albericio, F.; Álvarez, M. Org. Lett. 2003, 5, 2959–2962. doi:10.1021/ol0351192
Return to citation in text: [1] -
Ploypradith, P.; Petchmanee, T.; Sahakitpichan, P.; Litvinas, N. D.; Ruchirawat, S. J. Org. Chem. 2006, 71, 9440–9448. doi:10.1021/jo061810h
Return to citation in text: [1] -
Grigg, R.; Heaney, F. J. Chem. Soc., Perkin Trans. 1 1989, 198–200. doi:10.1039/p19890000198
Return to citation in text: [1] -
Su, S.; Porco, J. A., Jr. J. Am. Chem. Soc. 2007, 129, 7744–7745. doi:10.1021/ja072737v
Return to citation in text: [1] -
Yu, C.; Zhang, Y.; Zhang, S.; Li, H.; Wang, W. Chem. Commun. 2011, 47, 1036–1038. doi:10.1039/c0cc03186k
Return to citation in text: [1] -
Zou, Y.-Q.; Lu, L.-Q.; Fu, L.; Chang, N.-J.; Rong, J.; Chen, J.-R.; Xiao, W.-J. Angew. Chem., Int. Ed. 2011, 50, 7171–7175. doi:10.1002/anie.201102306
Return to citation in text: [1] [2] -
Huang, L.; Zhao, J. Chem. Commun. 2013, 49, 3751–3753. doi:10.1039/c3cc41494a
Return to citation in text: [1] [2] -
Guo, S.; Zhang, H.; Huang, L.; Guo, Z.; Xiong, G.; Zhao, J. Chem. Commun. 2013, 49, 8689–8691. doi:10.1039/c3cc44486d
Return to citation in text: [1] [2] -
Wang, H.-T.; Lu, C.-D. Tetrahedron Lett. 2013, 54, 3015–3018. doi:10.1016/j.tetlet.2013.04.004
Return to citation in text: [1] -
Zeitler, K. Angew. Chem., Int. Ed. 2009, 48, 9785–9789. doi:10.1002/anie.200904056
Return to citation in text: [1] -
Yoon, T. P.; Ischay, M. A.; Du, J. Nat. Chem. 2010, 2, 527–532. doi:10.1038/nchem.687
Return to citation in text: [1] -
Narayanam, J. M. R.; Stephenson, C. R. J. Chem. Soc. Rev. 2011, 40, 102–113. doi:10.1039/b913880n
Return to citation in text: [1] -
Xuan, J.; Xiao, W.-J. Angew. Chem., Int. Ed. 2012, 51, 6828–6838. doi:10.1002/anie.201200223
Return to citation in text: [1] -
Shi, L.; Xia, W. Chem. Soc. Rev. 2012, 41, 7687–7697. doi:10.1039/c2cs35203f
Return to citation in text: [1] -
Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322–5363. doi:10.1021/cr300503r
Return to citation in text: [1] -
Hu, J.; Wang, J.; Nguyen, T. H.; Zheng, N. Beilstein J. Org. Chem. 2013, 9, 1977–2001. doi:10.3762/bjoc.9.234
Return to citation in text: [1] -
Ravelli, D.; Fagnoni, M. ChemCatChem 2012, 4, 169–171. doi:10.1002/cctc.201100363
Return to citation in text: [1] [2] -
Ravelli, D.; Fagnoni, M.; Albini, A. Chem. Soc. Rev. 2013, 42, 97–113. doi:10.1039/c2cs35250h
Return to citation in text: [1] [2] -
Nicewicz, D. C.; Nguyen, T. M. ACS Catal. 2014, 4, 355–360. doi:10.1021/cs400956a
Return to citation in text: [1] [2] -
Liu, H.; Feng, W.; Kee, C. W.; Zhao, Y.; Leow, D.; Pan, Y.; Tan, C.-H. Green Chem. 2010, 12, 953–956. doi:10.1039/b924609f
Return to citation in text: [1] -
Pan, Y.; Kee, C. W.; Chen, L.; Tan, C.-H. Green Chem. 2011, 13, 2682–2685. doi:10.1039/c1gc15489c
Return to citation in text: [1] -
Pan, Y.; Wang, S.; Kee, C. W.; Dubuisson, E.; Yang, Y.; Loh, K. P.; Tan, C.-H. Green Chem. 2011, 13, 3341–3344. doi:10.1039/c1gc15865a
Return to citation in text: [1] -
Neumann, M.; Füldner, S.; König, B.; Zeitler, K. Angew. Chem., Int. Ed. 2011, 50, 951–954. doi:10.1002/anie.201002992
Return to citation in text: [1] -
Hari, D. P.; König, B. Org. Lett. 2011, 13, 3852–3855. doi:10.1021/ol201376v
Return to citation in text: [1] -
Liu, Q.; Li, Y.-N.; Zhang, H.-H.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Chem.–Eur. J. 2012, 18, 620–627. doi:10.1002/chem.201102299
Return to citation in text: [1] -
Fidaly, K.; Ceballos, C.; Falguières, A.; Veitia, M. S.-I.; Guy, A.; Ferroud, C. Green Chem. 2012, 14, 1293–1297. doi:10.1039/c2gc35118h
Return to citation in text: [1] -
Fu, W.; Guo, W.; Zou, G.; Xu, C. J. Fluorine Chem. 2012, 140, 88–94. doi:10.1016/j.jfluchem.2012.05.009
Return to citation in text: [1] -
Hari, D. P.; Schroll, P.; König, B. J. Am. Chem. Soc. 2012, 134, 2958–2961. doi:10.1021/ja212099r
Return to citation in text: [1] -
Hari, D. P.; Hering, T.; König, B. Org. Lett. 2012, 14, 5334–5337. doi:10.1021/ol302517n
Return to citation in text: [1] -
Neumann, M.; Zeitler, K. Org. Lett. 2012, 14, 2658–2661. doi:10.1021/ol3005529
Return to citation in text: [1] -
Hamilton, D. S.; Nicewicz, D. A. J. Am. Chem. Soc. 2012, 134, 18577–18580. doi:10.1021/ja309635w
Return to citation in text: [1] -
Rueping, M.; Vila, C.; Bootwicha, T. ACS Catal. 2013, 3, 1676–1680. doi:10.1021/cs400350j
Return to citation in text: [1] -
Grandjean, J.; Nicewicz, D. A. Angew. Chem., Int. Ed. 2013, 52, 3967–3971. doi:10.1002/anie.201210111
Return to citation in text: [1] -
Riener, M.; Nicewicz, D. A. Chem. Sci. 2013, 4, 2625–2629. doi:10.1039/c3sc50643f
Return to citation in text: [1] -
Wilger, D. J.; Gesmundo, N. J.; Nicewicz, D. A. Chem. Sci. 2013, 4, 3160–3165. doi:10.1039/c3sc51209f
Return to citation in text: [1] -
Nguyen, T. M.; Nicewicz, D. A. J. Am. Chem. Soc. 2013, 135, 9588–9591. doi:10.1021/ja4031616
Return to citation in text: [1] -
Perkowski, A. J.; Nicewicz, D. A. J. Am. Chem. Soc. 2013, 135, 10334–10337. doi:10.1021/ja4057294
Return to citation in text: [1] -
Pitre, S. P.; McTiernan, C. D.; Ismaili, H.; Scaiano, J. C. J. Am. Chem. Soc. 2013, 135, 13286–13289. doi:10.1021/ja406311g
Return to citation in text: [1] -
Rueping, M.; Vila, C.; Koenings, R. M.; Poscharny, K.; Fabry, D. C. Chem. Commun. 2011, 47, 2360–2362. doi:10.1039/c0cc04539j
Return to citation in text: [1] -
Rueping, M.; Zhu, S.; Koenings, R. M. Chem. Commun. 2011, 47, 8679–8681. doi:10.1039/c1cc12907d
Return to citation in text: [1] -
Rueping, M.; Leonori, D.; Poisson, T. Chem. Commun. 2011, 47, 9615–9617. doi:10.1039/c1cc13660g
Return to citation in text: [1] -
Rueping, M.; Zhu, S.; Koenings, R. M. Chem. Commun. 2011, 47, 12709–12711. doi:10.1039/c1cc15643h
Return to citation in text: [1] -
Rueping, M.; Zoller, J.; Fabry, D. C.; Poscharny, K.; Koenings, R. M.; Weirich, T. E.; Mayer, J. Chem.–Eur. J. 2012, 18, 3478–3481. doi:10.1002/chem.201103242
Return to citation in text: [1] -
Rueping, M.; Koenings, R. M.; Poscharny, K.; Fabry, D. C.; Leonori, D.; Vila, C. Chem.–Eur. J. 2012, 18, 5170–5174. doi:10.1002/chem.201200050
Return to citation in text: [1] -
Rueping, M.; Vila, C.; Szadkowska, A.; Koenigs, R. M.; Fronert, J. ACS Catal. 2012, 2, 2810–2815. doi:10.1021/cs300604k
Return to citation in text: [1] -
Zhu, S.; Rueping, M. Chem. Commun. 2012, 48, 11960–11962. doi:10.1039/c2cc36995h
Return to citation in text: [1] -
Zhu, S.; Das, A.; Bui, L.; Zhou, H.; Curran, D. P.; Rueping, M. J. Am. Chem. Soc. 2013, 135, 1823–1829. doi:10.1021/ja309580a
Return to citation in text: [1] -
Rueping, M.; Vila, C. Org. Lett. 2013, 15, 2092–2095. doi:10.1021/ol400317v
Return to citation in text: [1] -
Vila, C.; Rueping, M. Green Chem. 2013, 15, 2056–2059. doi:10.1039/c3gc40587g
Return to citation in text: [1] -
Tóth, J.; Váradi, L.; Dancsó, A.; Blaskó, G.; Töke, L.; Nyerges, M. Synlett 2007, 1259–1263. doi:10.1055/s-2007-977461
Return to citation in text: [1]
| 62. | Rueping, M.; Vila, C.; Koenings, R. M.; Poscharny, K.; Fabry, D. C. Chem. Commun. 2011, 47, 2360–2362. doi:10.1039/c0cc04539j |
| 63. | Rueping, M.; Zhu, S.; Koenings, R. M. Chem. Commun. 2011, 47, 8679–8681. doi:10.1039/c1cc12907d |
| 64. | Rueping, M.; Leonori, D.; Poisson, T. Chem. Commun. 2011, 47, 9615–9617. doi:10.1039/c1cc13660g |
| 65. | Rueping, M.; Zhu, S.; Koenings, R. M. Chem. Commun. 2011, 47, 12709–12711. doi:10.1039/c1cc15643h |
| 66. | Rueping, M.; Zoller, J.; Fabry, D. C.; Poscharny, K.; Koenings, R. M.; Weirich, T. E.; Mayer, J. Chem.–Eur. J. 2012, 18, 3478–3481. doi:10.1002/chem.201103242 |
| 67. | Rueping, M.; Koenings, R. M.; Poscharny, K.; Fabry, D. C.; Leonori, D.; Vila, C. Chem.–Eur. J. 2012, 18, 5170–5174. doi:10.1002/chem.201200050 |
| 68. | Rueping, M.; Vila, C.; Szadkowska, A.; Koenigs, R. M.; Fronert, J. ACS Catal. 2012, 2, 2810–2815. doi:10.1021/cs300604k |
| 69. | Zhu, S.; Rueping, M. Chem. Commun. 2012, 48, 11960–11962. doi:10.1039/c2cc36995h |
| 70. | Zhu, S.; Das, A.; Bui, L.; Zhou, H.; Curran, D. P.; Rueping, M. J. Am. Chem. Soc. 2013, 135, 1823–1829. doi:10.1021/ja309580a |
| 71. | Rueping, M.; Vila, C. Org. Lett. 2013, 15, 2092–2095. doi:10.1021/ol400317v |
| 72. | Vila, C.; Rueping, M. Green Chem. 2013, 15, 2056–2059. doi:10.1039/c3gc40587g |
| 29. | Zou, Y.-Q.; Lu, L.-Q.; Fu, L.; Chang, N.-J.; Rong, J.; Chen, J.-R.; Xiao, W.-J. Angew. Chem., Int. Ed. 2011, 50, 7171–7175. doi:10.1002/anie.201102306 |
| 30. | Huang, L.; Zhao, J. Chem. Commun. 2013, 49, 3751–3753. doi:10.1039/c3cc41494a |
| 31. | Guo, S.; Zhang, H.; Huang, L.; Guo, Z.; Xiong, G.; Zhao, J. Chem. Commun. 2013, 49, 8689–8691. doi:10.1039/c3cc44486d |
| 73. | Tóth, J.; Váradi, L.; Dancsó, A.; Blaskó, G.; Töke, L.; Nyerges, M. Synlett 2007, 1259–1263. doi:10.1055/s-2007-977461 |
| 1. | Bailly, C. Curr. Med. Chem.: Anti-Cancer Agents 2004, 4, 363–378. doi:10.2174/1568011043352939 |
| 2. | Handy, S. T.; Zhang, Y. Org. Prep. Proced. Int. 2005, 37, 411–445. doi:10.1080/00304940509354977 |
| 3. | Fan, H.; Peng, J.; Hamann, M. T.; Hu, J.-F. Chem. Rev. 2008, 108, 264–287. doi:10.1021/cr078199m |
| 4. | Pla, D.; Albericio, F.; Alvarez, M. Anti-Cancer Agents Med. Chem. 2008, 8, 746–760. doi:10.2174/187152008785914789 |
| 10. | Heim, A.; Terpin, A.; Steglich, W. Angew. Chem., Int. Ed. Engl. 1997, 36, 155–156. doi:10.1002/anie.199701551 |
| 11. | Ploypradith, P.; Mahidol, C.; Sahakitpichan, P.; Wongbundit, S.; Ruchirawat, S. Angew. Chem., Int. Ed. 2004, 43, 866–868. doi:10.1002/anie.200352043 |
| 12. | Boger, D. L.; Boyce, C. W.; Labroli, M. A.; Sehon, C. A.; Jin, Q. J. Am. Chem. Soc. 1999, 121, 54–62. doi:10.1021/ja982078+ |
| 13. | Banwell, M. G.; Flynn, B. L.; Stewart, S. G. J. Org. Chem. 1998, 63, 9139–9144. doi:10.1021/jo9808526 |
| 14. | Handy, S. T.; Zhang, Y.; Bregman, H. J. Org. Chem. 2004, 69, 2362–2366. doi:10.1021/jo0352833 |
| 15. | Ploypradith, P.; Kagan, R. K.; Ruchirawat, S. J. Org. Chem. 2005, 70, 5119–5125. doi:10.1021/jo050388m |
| 16. | Ohta, T.; Fukuda, T.; Ishibashi, F.; Iwao, M. J. Org. Chem. 2009, 74, 8143–8153. doi:10.1021/jo901589e |
| 17. | Gupton, J. T.; Clough, S. C.; Miller, R. B.; Lukens, J. R.; Henry, C. A.; Kanters, R. P. F.; Sikorski, J. A. Tetrahedron 2003, 59, 207–215. doi:10.1016/S0040-4020(02)01475-8 |
| 18. | Fujikawa, N.; Ohta, T.; Yamaguchi, T.; Fukuda, T.; Ishibashi, F.; Iwao, M. Tetrahedron 2006, 62, 594–604. doi:10.1016/j.tet.2005.10.014 |
| 19. | Chen, L.; Xu, M.-H. Adv. Synth. Catal. 2009, 351, 2005–2012. doi:10.1002/adsc.200900287 |
| 20. | Yadav, J. S.; Gayathri, K. U.; Reddy, B. V. S.; Prasad, A. R. Synlett 2009, 43–46. doi:10.1055/s-0028-1087387 |
| 40. | Ravelli, D.; Fagnoni, M. ChemCatChem 2012, 4, 169–171. doi:10.1002/cctc.201100363 |
| 41. | Ravelli, D.; Fagnoni, M.; Albini, A. Chem. Soc. Rev. 2013, 42, 97–113. doi:10.1039/c2cs35250h |
| 42. | Nicewicz, D. C.; Nguyen, T. M. ACS Catal. 2014, 4, 355–360. doi:10.1021/cs400956a |
| 8. | Reddy, S. M.; Srinivasulu, M.; Satanarayana, N.; Kondapi, A. K.; Venkateswarlu, Y. Tetrahedron 2005, 61, 9242–9247. doi:10.1016/j.tet.2005.07.067 |
| 9. | Quesada, A. R.; Garcia Grávalos, M. D.; Fernández Puentes, J. L. Br. J. Cancer 1996, 74, 677–682. doi:10.1038/bjc.1996.421 |
| 43. | Liu, H.; Feng, W.; Kee, C. W.; Zhao, Y.; Leow, D.; Pan, Y.; Tan, C.-H. Green Chem. 2010, 12, 953–956. doi:10.1039/b924609f |
| 44. | Pan, Y.; Kee, C. W.; Chen, L.; Tan, C.-H. Green Chem. 2011, 13, 2682–2685. doi:10.1039/c1gc15489c |
| 45. | Pan, Y.; Wang, S.; Kee, C. W.; Dubuisson, E.; Yang, Y.; Loh, K. P.; Tan, C.-H. Green Chem. 2011, 13, 3341–3344. doi:10.1039/c1gc15865a |
| 46. | Neumann, M.; Füldner, S.; König, B.; Zeitler, K. Angew. Chem., Int. Ed. 2011, 50, 951–954. doi:10.1002/anie.201002992 |
| 47. | Hari, D. P.; König, B. Org. Lett. 2011, 13, 3852–3855. doi:10.1021/ol201376v |
| 48. | Liu, Q.; Li, Y.-N.; Zhang, H.-H.; Chen, B.; Tung, C.-H.; Wu, L.-Z. Chem.–Eur. J. 2012, 18, 620–627. doi:10.1002/chem.201102299 |
| 49. | Fidaly, K.; Ceballos, C.; Falguières, A.; Veitia, M. S.-I.; Guy, A.; Ferroud, C. Green Chem. 2012, 14, 1293–1297. doi:10.1039/c2gc35118h |
| 50. | Fu, W.; Guo, W.; Zou, G.; Xu, C. J. Fluorine Chem. 2012, 140, 88–94. doi:10.1016/j.jfluchem.2012.05.009 |
| 51. | Hari, D. P.; Schroll, P.; König, B. J. Am. Chem. Soc. 2012, 134, 2958–2961. doi:10.1021/ja212099r |
| 52. | Hari, D. P.; Hering, T.; König, B. Org. Lett. 2012, 14, 5334–5337. doi:10.1021/ol302517n |
| 53. | Neumann, M.; Zeitler, K. Org. Lett. 2012, 14, 2658–2661. doi:10.1021/ol3005529 |
| 54. | Hamilton, D. S.; Nicewicz, D. A. J. Am. Chem. Soc. 2012, 134, 18577–18580. doi:10.1021/ja309635w |
| 55. | Rueping, M.; Vila, C.; Bootwicha, T. ACS Catal. 2013, 3, 1676–1680. doi:10.1021/cs400350j |
| 56. | Grandjean, J.; Nicewicz, D. A. Angew. Chem., Int. Ed. 2013, 52, 3967–3971. doi:10.1002/anie.201210111 |
| 57. | Riener, M.; Nicewicz, D. A. Chem. Sci. 2013, 4, 2625–2629. doi:10.1039/c3sc50643f |
| 58. | Wilger, D. J.; Gesmundo, N. J.; Nicewicz, D. A. Chem. Sci. 2013, 4, 3160–3165. doi:10.1039/c3sc51209f |
| 59. | Nguyen, T. M.; Nicewicz, D. A. J. Am. Chem. Soc. 2013, 135, 9588–9591. doi:10.1021/ja4031616 |
| 60. | Perkowski, A. J.; Nicewicz, D. A. J. Am. Chem. Soc. 2013, 135, 10334–10337. doi:10.1021/ja4057294 |
| 61. | Pitre, S. P.; McTiernan, C. D.; Ismaili, H.; Scaiano, J. C. J. Am. Chem. Soc. 2013, 135, 13286–13289. doi:10.1021/ja406311g |
| 6. | Reddy, M. V. R.; Rao, M. R.; Rhodes, D.; Hansen, M. S. T.; Rubins, K.; Bushman, F. D.; Venkateswarlu, Y.; Faulkner, D. J. Med. Chem. 1999, 42, 1901–1907. doi:10.1021/jm9806650 |
| 7. | Aubry, A.; Pan, X.-S.; Fisher, L. M.; Jarlier, V.; Cambau, E. Antimicrob. Agents Chemother. 2004, 48, 1281–1288. doi:10.1128/AAC.48.4.1281-1288.2004 |
| 32. | Wang, H.-T.; Lu, C.-D. Tetrahedron Lett. 2013, 54, 3015–3018. doi:10.1016/j.tetlet.2013.04.004 |
| 5. | Marco, E.; Laine, W.; Tardy, C.; Lansiaux, A.; Iwao, M.; Ishibashi, F.; Bailly, C.; Gago, F. J. Med. Chem. 2005, 48, 3796–3807. doi:10.1021/jm049060w |
| 33. | Zeitler, K. Angew. Chem., Int. Ed. 2009, 48, 9785–9789. doi:10.1002/anie.200904056 |
| 34. | Yoon, T. P.; Ischay, M. A.; Du, J. Nat. Chem. 2010, 2, 527–532. doi:10.1038/nchem.687 |
| 35. | Narayanam, J. M. R.; Stephenson, C. R. J. Chem. Soc. Rev. 2011, 40, 102–113. doi:10.1039/b913880n |
| 36. | Xuan, J.; Xiao, W.-J. Angew. Chem., Int. Ed. 2012, 51, 6828–6838. doi:10.1002/anie.201200223 |
| 37. | Shi, L.; Xia, W. Chem. Soc. Rev. 2012, 41, 7687–7697. doi:10.1039/c2cs35203f |
| 38. | Prier, C. K.; Rankic, D. A.; MacMillan, D. W. C. Chem. Rev. 2013, 113, 5322–5363. doi:10.1021/cr300503r |
| 39. | Hu, J.; Wang, J.; Nguyen, T. H.; Zheng, N. Beilstein J. Org. Chem. 2013, 9, 1977–2001. doi:10.3762/bjoc.9.234 |
| 40. | Ravelli, D.; Fagnoni, M. ChemCatChem 2012, 4, 169–171. doi:10.1002/cctc.201100363 |
| 41. | Ravelli, D.; Fagnoni, M.; Albini, A. Chem. Soc. Rev. 2013, 42, 97–113. doi:10.1039/c2cs35250h |
| 42. | Nicewicz, D. C.; Nguyen, T. M. ACS Catal. 2014, 4, 355–360. doi:10.1021/cs400956a |
| 28. | Yu, C.; Zhang, Y.; Zhang, S.; Li, H.; Wang, W. Chem. Commun. 2011, 47, 1036–1038. doi:10.1039/c0cc03186k |
| 30. | Huang, L.; Zhao, J. Chem. Commun. 2013, 49, 3751–3753. doi:10.1039/c3cc41494a |
| 27. | Su, S.; Porco, J. A., Jr. J. Am. Chem. Soc. 2007, 129, 7744–7745. doi:10.1021/ja072737v |
| 31. | Guo, S.; Zhang, H.; Huang, L.; Guo, Z.; Xiong, G.; Zhao, J. Chem. Commun. 2013, 49, 8689–8691. doi:10.1039/c3cc44486d |
| 22. | Ishibashi, F.; Miyazaki, Y.; Iwao, M. Tetrahedron 1997, 53, 5951–5962. doi:10.1016/S0040-4020(97)00287-1 |
| 23. | Banwell, M. G.; Flynn, B. L.; Hockless, D. C. R. Chem. Commun. 1997, 2259–2260. doi:10.1039/a705874h |
| 24. | Cironi, P.; Manzanares, I.; Albericio, F.; Álvarez, M. Org. Lett. 2003, 5, 2959–2962. doi:10.1021/ol0351192 |
| 25. | Ploypradith, P.; Petchmanee, T.; Sahakitpichan, P.; Litvinas, N. D.; Ruchirawat, S. J. Org. Chem. 2006, 71, 9440–9448. doi:10.1021/jo061810h |
| 26. | Grigg, R.; Heaney, F. J. Chem. Soc., Perkin Trans. 1 1989, 198–200. doi:10.1039/p19890000198 |
| 21. | Najera, C.; Sansano, J. M. Curr. Org. Chem. 1998, 7, 1105–1150. doi:10.2174/1385272033486594 |
| 29. | Zou, Y.-Q.; Lu, L.-Q.; Fu, L.; Chang, N.-J.; Rong, J.; Chen, J.-R.; Xiao, W.-J. Angew. Chem., Int. Ed. 2011, 50, 7171–7175. doi:10.1002/anie.201102306 |
© 2014 Vila et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)