Abstract
Theoretical and experimental studies of the reaction of isoxazoles with diazo compounds show that the formation of 2H-1,3-oxazines proceeds via the formation of (3Z)-1-oxa-5-azahexa-1,3,5-trienes which undergo a 6π-cyclization. The stationary points corresponding to the probable reaction intermediates, isoxazolium N-ylides, were located by DFT calculations at the B3LYP/6-31G(d) level only for derivatives without a substituent in position 3 of the isoxazole ring. These isoxazolium N-ylides are thermodynamically and kinetically very unstable. According to the calculations and experimental results 2H-1,3-oxazines are usually more thermodynamically stable than the corresponding open-chain isomers, (3Z)-1-oxa-5-azahexa-1,3,5-trienes. The exception are oxaazahexatrienes derived from 5-alkoxyisoxazoles, which are thermodynamically more stable than the corresponding 2H-1,3-oxazines. Therefore, the reaction of diazo esters with 5-alkoxyisoxazoles is a good approach to 1,4-di(alkoxycarbonyl)-2-azabuta-1,3-dienes. The reaction conditions for the preparation of aryl- and halogen-substituted 2H-1,3-oxazines and 1,4-di(alkoxycarbonyl)-2-azabuta-1,3-dienes from isoxazoles were investigated.
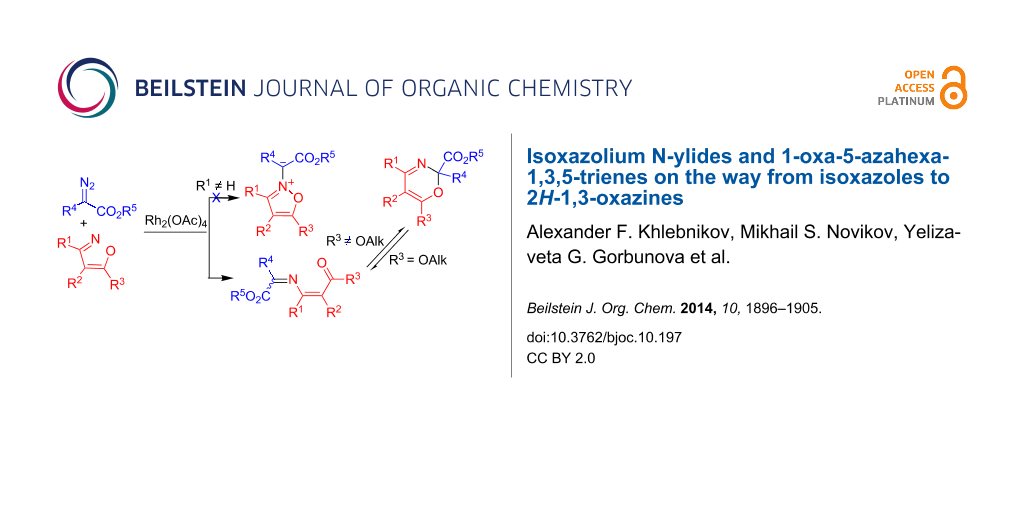
Graphical Abstract
Introduction
Isoxazoles are versatile building blocks, which have found extensive use in organic synthesis [1-3]. However, reactions of isoxazoles with diazo compounds have scarcely been studied [1-5]. In 2008 Davies and Manning [4,5] discovered the Rh-catalyzed reaction of diazo esters with 3,5-dialkylisoxazoles, benzo[d]isoxazole and 3-chlorobenzo[d]isothiazole leading to the corresponding 2H-1,3-oxazines, 2H-benzo[e][1,3]oxazine and 2H-benzo[e][1,3]thiazine.
The authors assumed that the reaction of isoxazoles A with diazo esters B involved an isoxazolium N-ylide intermediate C formed by an attack of the rhodium carbenoid onto the isoxazole nitrogen. Furthermore, ylide C could undergo either a 1,2-shift to directly generate oxazine E or a ring opening to 1-oxa-5-azahexa-1,3,5-triene D, followed by a 6π-electrocyclization to give oxazine E (Scheme 1). At the same time, the third mechanism of the reaction, involving a one-step formation of 1-oxa-5-azahexa-1,3,5-triene D, cannot be excluded.
Scheme 1: Mechanistic scheme of the formation of 2H-1,3-oxazine by the reaction of isoxazoles with a diazo compound.
Scheme 1: Mechanistic scheme of the formation of 2H-1,3-oxazine by the reaction of isoxazoles with a diazo co...
The reaction of a carbenoid with isoxazoles is the only known one-step intermolecular reaction which can in principle produce isoxazolium N-ylides from N-unsubstituted isoxazole derivatives. The formation of such ylides as reactive intermediates in the reactions of bases on isoxazolium salts was earlier supposed [6-9]. However, the detection of isoxazolium N-ylides has never been reported.
Recently, we found an alternative synthetic approach to derivatives of 2H-1,3-oxazines via a Rh2(OAc)4-catalyzed reaction of diazo esters with 2-acyl-2H-azirines F. This reaction involves the intermediate formation of azirinium ylides G, their transformation into 1-oxa-5-azahexa-1,3,5-triene D, and finally the 6π-electrocyclization of the latter to give oxazine E (Scheme 2) [10,11].
Scheme 2: Mechanistic scheme of the formation of 2H-1,3-oxazine by the reaction of azirine with a diazo compound.
Scheme 2: Mechanistic scheme of the formation of 2H-1,3-oxazine by the reaction of azirine with a diazo compo...
Azirinium ylides G, formed by the reaction of azirines F with carbenoids, can transform into (3Z)- and (3E)-1-oxa-5-azahexa-1,3,5-triene D, but only the former can cyclize into 1,3-oxazines E. In contrast, the reaction of isoxazoles with carbenoids results in the exclusive formation of (3Z)-1-oxa-5-azahexa-1,3,5-trienes due to geometrical reasons.
The first aim we set ourselves in the present work was to gain insight into the mechanism of the reaction of isoxazoles with diazo compounds by answering the two following questions: “Are isoxazolium N-ylides really formed in this reaction?” and “What is their reactivity?” To this end, we carried out quantum-chemical calculations of the formation and the ring opening of isoxazolium N-ylides. Probing the type of the mechanistic scheme of the 2H-1,3-oxazine formation was also conducted by searching for the isoxazoles capable of providing stable 1-oxa-5-azahexa-1,3,5-trienes D under reaction with diazo esters and by comparing the experimental results of the reactions of carbenoids with a complementary pair of isoxazole and azirine.
An analysis of recent literature shows that 2H-1,3-oxazine derivatives exhibit various types of bioactivity, e.g., herbicidal [12], inhibition of cell growth and enzyme activity [13-18], inhibition of voltage-gated sodium channels [19] and metabotropic glutamate receptor-5a (hmGluR5a) [20]. Consequently, our second aim was to extend the reaction to the preparation of aryl- and halogen-substituted 1,3-oxazines, taking into account that the latter are potential candidates for metal-catalyzed couplings and thus allow further modifications.
Results and Discussion
The theoretical study of the reaction mechanism was started with an evaluation of the thermodynamic and kinetic stabilities of isoxazolium N-ylides, probable intermediates in a carbenoid- or carbene-mediated one-atom isoxazole ring expansion. Preliminary calculations at the DFT B3LYP/6-31G(d) level with the PCM solvation model for dichloromethane were performed for the model reaction of isoxazoles A with methoxycarbonylcarbene (Figure 1). The stationary points corresponding to isoxazolium N-ylides C formed by an attack from the methoxycarbonylcarbene on the nitrogen of isoxazole A were found only for isoxazoles without substituent R1 in position 3.
![[1860-5397-10-197-1]](/bjoc/content/figures/1860-5397-10-197-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Energy profiles for the transformations of ylides C, (3Z)-1-oxa-5-azahexa-1,3,5-triene D and oxazines E derived from methoxycarbonylcarbene and isoxazole A. Relative free energies [kcal/mol, 298 K, CH2Cl2 (PCM)] computed at DFT B3LYP/6-31G(d) level.
Figure 1: Energy profiles for the transformations of ylides C, (3Z)-1-oxa-5-azahexa-1,3,5-triene D and oxazin...
Further, these ylides undergo a ring opening via very low activation barriers (0.2–1.5 kcal/mol) to give (3Z)-1-oxa-5-azahexa-1,3,5-trienes. This is expected, because the oxazole N–O bond is very weak and the reaction is pseudopericyclic [21,22]. The calculated low thermodynamic and kinetic stabilities of the isoxazolium ylides (Figure 1) give only a small chance of detecting their formation even in cases where they can theoretically be formed. If the starting isoxazole contains substituent R3, an attack of a carbene on the isoxazole nitrogen leads to (3Z)-1-oxa-5-azahexa-1,3,5-triene without an activation barrier. The latter derived from isoxazoles without a methoxy substituent in position 5 can cyclize via a low activation barrier (<12.5 kcal/mol) to the corresponding 2H-1,3-oxazines. All calculated 1-oxa-5-azahexa-1,3,5-trienes, excluding the ones derived from 5-methoxy-substituted isoxazoles, are thermodynamically less stable than 2H-1,3-oxazines. In contrast, 1,4-di(methoxycarbonyl)-2-azabuta-1,3-dienes are much more stable than the corresponding 1,3-oxazines. We also evaluated the possibility of an attack of methoxycarbonylcarbene on the isoxazole oxygen. According to calculations (see Supporting Information File 1) a carbene attack on the isoxazole oxygen is significantly less favorable than an attack on the nitrogen.
The results of the calculations do not fundamentally change if methoxycarbonylcarbene is substituted with (methoxycarbonyl)phenylcarbene or di(methoxycarbonyl)carbene (Figure 2). Again, only oxaazahexatrienes D derived from 5-methoxy-substituted isoxazoles are much more stable than the corresponding oxazines E. Therefore, one can expect the formation of only 1-oxa-5-azahexa-1,3,5-trienes when reacting diazo compounds with 5-methoxyisoxazoles.
![[1860-5397-10-197-2]](/bjoc/content/figures/1860-5397-10-197-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Energy profiles for the transformations of (3Z)-1-oxa-5-azahexa-1,3,5-triene D and oxazines E derived from (methoxycarbonyl)phenylcarbene or di(methoxycarbonyl)carbene and oxazole A. Relative free energies [kcal/mol, 298 K, CH2Cl2 (PCM)] computed at DFT B3LYP/6-31G(d) level.
Figure 2: Energy profiles for the transformations of (3Z)-1-oxa-5-azahexa-1,3,5-triene D and oxazines E deriv...
To start with, we reacted 4-phenyl-substituted isoxazole 1a and phenyldiazoacetate 2a under the reaction conditions used in [4] (catalyst: 1–3 mol % of Rh2(OAc)4, solvent: CH2Cl2 or ClCH2CH2Cl, 40 or 84 °C) (Scheme 3). Unexpectedly, attempts to prepare oxazine 3a under these conditions were unsuccessful (Scheme 3) and isoxazole 1a was completely recovered.
Scheme 3: Reaction of isoxazole 1a and diazo ester 2a.
Scheme 3: Reaction of isoxazole 1a and diazo ester 2a.
Oxazine 3a was obtained in 14% yield when heated under reflux in CH2Cl2 and with the use of dirhodium tetraoctanoate instead of Rh2(OAc)4 as a catalyst. This unsatisfactory result prompted us to test a carbene instead of a Rh(II) carbenoid, since it has been found [23] that carbenes can be successfully generated by thermolysis of diazo compounds without a catalyst in inert solvents with high boiling points, such as trifluoromethylbenzene. These conditions were attempted for the preparation of oxazines from isoxazole 1a and diazo compounds 2a–c (Table 1, entries 1–5). The use of a higher boiling-point solvent may also be a means to overcome the low solubility of arylisoxazoles. The formation of an oxazine occurred only with phenyl diazoacetate 2a under these conditions.
Table 1: Reaction of azirines 1a with diazo compounds 2a–c without a catalyst.
|
|
|||||
| entry | 2 (R1, R2) | ratio 1a:2 | time, h | T, °C | yield of 3, % |
|---|---|---|---|---|---|
| 1 | a (Ph, CO2Me) | 5:1 | 3.5 | 103 | a, 34–42a |
| 2b | a (Ph, CO2Me) | 5:1 | 3.5 | 103 | a, traces |
| 3 | a (Ph, CO2Me) | 10:1 | 3.5 | 103 | a, 88a |
| 4 | b (H, CO2Et) | 5:1 | 12 | 103 | b, traces |
| 5 | c (CO2Me, CO2Me) | 5:1 | 38 | 103 | c, – |
| 6 | b (H, CO2Et) | 3:1 | 0.3 | 120, mw | b, traces |
| 7 | b (H, CO2Et) | 5:1 | 0.3 | 120, mw | b, traces |
| 8b | c (CO2Me, CO2Me) | 2:1 | 0.3 | 120, mw | c, traces |
aBased on consumed 1a, the conversion of 1a was 12–15% (entry 1) and 9% (entry 3); bwithout solvent.
To overcome the inactivity of diazo compounds 2b,c the use of higher temperature and microwave irradiation were investigated, but only traces of oxazines 3b,c were then detected by 1H NMR spectroscopy (Table 1, entries 6–8).
The conditions of choice for the synthesis of aryl-substituted 2H-1,3-oxazines proved to be heating under reflux in PhCF3 and 1.5–3 mol % of Rh2(OAc)2 as a catalyst. Under these conditions oxazines 3a–m were synthesized (Table 2). The yields of oxazines can be improved by using a higher excess of diazo compounds. However, this also leads to an increase of the formation of side products, “carbenoid dimers”, which attribute to a more difficult isolation of the target products in some cases.
Table 2: Synthesis of oxazines 3a–m.
|
|
||||||||
| 1 | 2 | R1 | R2 | R3 | R4 | R5 | ratio 1:2 | 3, yield,a % |
|---|---|---|---|---|---|---|---|---|
| a | a | Ме | Ph | Me | Ph | Me |
1:1.7
1:3.3 |
a, 26
a, 43 |
| a | b | Ме | Ph | Me | H | Et | 1:1.9 | b, 35 |
| a | c | Ме | Ph | Me | CO2Me | Me | 1:1.2 | c, 67 |
| b | a | Ph | H | Ph | Ph | Me | 1:2.3 | d, 66 (70) |
| b | b | Ph | H | Ph | H | Et | 1:3.0 | e, 34 |
| b | c | Ph | H | Ph | CO2Me | Me | 1:3.4 | f, 81 |
| c | b | Ph | Cl | Ph | H | Et | 1:3.7 | g, 27 (73) |
| c | c | Ph | Cl | Ph | CO2Me | Me | 1:1.9 | h , 48 |
| d | b | Ph | Br | Ph | H | Et | 1:3.0 | i, 19 (75) |
| d | c | Ph | Br | Ph | CO2Me | Me | 1:1.5 | k, 21 |
| e | b | Ph | I | Ph | H | Et | 1:3.3 | l, 22 (63) |
| e | c | Ph | I | Ph | CO2Me | Me | 1:1.9 | m, 21 (36) |
aYields based on consumed isoxazole are listed in parentheses.
The structures of compounds 3 were verified by 1H NMR, 13C NMR, IR spectroscopy, HRMS, and elemental analysis. The structures of compounds 3a,k were additionally confirmed by X-ray analysis (Figure 3).
![[1860-5397-10-197-3]](/bjoc/content/figures/1860-5397-10-197-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Molecular structures of compounds 3a,k, displacement parameters are drawn at 50% probability level.
Figure 3: Molecular structures of compounds 3a,k, displacement parameters are drawn at 50% probability level.
The characteristic feature of the structure of compound 3a is the pseudo-axial position of the methoxycarbonyl group. The structure of compound 3a in the crystal corresponds to the most stable conformer according to calculations at DFT B3LYP/6-31G(d) level in vacuo (ΔΔG298 K (equatorial/axial = 1.2 kcal/mol). One of the possible reasons for the higher stability of conformer 3a with a pseudo-axial methoxycarbonyl group in comparison to conformer 3a' with a pseudo-equatorial methoxycarbonyl group, is assumed to be the anomeric effect [24]. The lengthening of the C–CO2Me bond in conformer 3a compared to conformer 3a' (1.563/1.554 Å), corroborates this hypothesis. The pseudo-axia position of the methoxycarbonyl group is preferred for all calculated oxazines.
In the reactions of diazo esters with 5-alkoxy-substituted isoxazoles 1f,h, in contrast to isoxazoles 1a–e, no formation of 1,3-oxazines was detected. Instead, the corresponding 1-oxa-5-azahexa-1,3,5-trienes 4a–f were isolated in moderate to good yields (Table 3).
Table 3: Synthesis of 1-oxa-5-azahexa-1,3,5-trienes 4a–f.
|
|
|||||
| 1 | 2 | R | R1 | R2 | 4, yield,a % |
|---|---|---|---|---|---|
| f | a | Me | Me | Ph | 4a 51 (69) |
| f | c | Me | Me | CO2Me | 4b 54 (61) |
| f | d | Me | Et | CO2Et | 4c 80 (89) |
| f | e | Me | Et | CF3 | 4d 57 |
| g | c | t-Bu | Me | CO2Me | 4e 29 (45) |
| g | d | t-Bu | Et | CO2Et | 4f 25 (38) |
aYields based on consumed isoxazole are listed in parentheses.
The structures of compounds 4a–f were verified by 1H, 13C NMR, IR spectroscopy, and HRMS. Furthermore, the structures of compounds 4a,b were confirmed by X-ray analysis (Figure 4). According to 1H NMR no corresponding 1,3-oxazines were formed.
![[1860-5397-10-197-4]](/bjoc/content/figures/1860-5397-10-197-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Molecular structures of compounds 4a,b, displacement parameters are drawn at 50% probability level.
Figure 4: Molecular structures of compounds 4a,b, displacement parameters are drawn at 50% probability level.
Thus, only reactions of carbenoids with 5-alkoxy-substituted izoxazoles give the corresponding 1-oxa-5-azahexa-1,3,5-trienes instead of oxazines. To reveal the reason for this – either the destabilization of the oxazine or the stabilization of the 2-azabuta-1,3-diene when the phenyl group in 3d,e or 4g,h is exchanged for a methoxy group (compounds 4g,h were not isolated) – the corresponding changes in Gibbs free energy were evaluated from isodesmic reactions 1–4 (Scheme 4). These calculations were based on the Gibbs free energy of the compounds, which were obtained by DFT B3LYP/6-31G(d) calculations (ΔΔG298 K, kcal/mol).
Scheme 4: Isodesmic reactions for 1,3-oxazines 3d,e,n,o and 1-oxa-5-azahexa-1,3,5-trienes 4a,b,g,h.
Scheme 4: Isodesmic reactions for 1,3-oxazines 3d,e,n,o and 1-oxa-5-azahexa-1,3,5-trienes 4a,b,g,h.
Although the substitution of the Ph group to a MeO group in compounds 3d,e results in a stabilization of the oxazines (Scheme 4, reactions 2 and 4), the formation of the corresponding 1-oxa-5-azahexa-1,3,5-trienes from 5-methoxy-substituted isoxazoles is mainly caused by the higher thermodynamic stability of 2-methoxy-substituted 1-oxa-5-azahexa-1,3,5-trienes 4a,b compared to 2-phenyl-substituted 1-oxa-5-azahexa-1,3,5-trienes 4g,h (Scheme 4, reactions 1 and 3). According to the X-ray analysis the R(MeO2C)C=N-group in compound 4 is not in conjugation with the remaining multiple bonds, that is, the methyl cinnamate conjugated system exerts a significantly greater influence on the stabilization than the corresponding chalcone system.
Thus, there is a good correspondence between the theoretical and experimental results, both of which support that Rh(II)-catalyzed reactions of diazo compounds with isoxazoles do not involve the formation of isoxazolium ylides but directly lead to the formation of azadienes, the latter can undergo a 6π-cyclization into the corresponding 1,3-oxazines. The position of the valence isomeric equilibrium depends on the relative thermodynamic stability of cyclic and acyclic isomers.
Additional evidence of a “one-step oxazahexatriene mechanism” of carbenoid-mediated isoxazole ring expansion originates from the results of the interaction of diazo compounds 2a–c with the complimentary isoxazole 1a and azirine 5 (Scheme 5). The reaction of isoxazoles with a carbenoid can only give a (3Z)-1-oxa-5-azahexa-1,3,5-triene due to geometrical restrictions. (3Z)-1-Oxa-5-azahexa-1,3,5-triene can then cyclize into the corresponding oxazine, so that the products of the reaction of isoxazole 1a with diazo compounds 2a–c were only oxazines 3a–c (Table 2). In contrast, azirinium ylides 6a–c formed by the reaction of azirine 5 with diazo compounds 2a–c can transform into (3Z)- and (3E)-1-oxa-5-azahexa-1,3,5-triene 4i–k, but only the former can cyclize into 1,3-oxazines 3a–c (Scheme 5). In accordance with this, the reactions of azirine 5 with diazo compounds 2a–c (3E)-1-oxa-5-azahexa-1,3,5-trienes (E)-4i,k were isolated as well as oxazines 3a–c [10]. The corresponding ethyl 2-((E)-4-oxo-3-phenylpent-2-en-2-ylimino)acetate (E)-4j was not isolated from the reaction of azirine 5 with diazo compound 2b, probably due to its instability.
Scheme 5: Reaction of complementary isoxazole 1a and azirine 5 with diazo esters.
Scheme 5: Reaction of complementary isoxazole 1a and azirine 5 with diazo esters.
Conclusion
According to DFT calculations at the B3LYP/6-31G(d) level and experimental data the formation of 2H-1,3-oxazines from the reaction of isoxazoles with diazo compounds proceeds through an initial formation of (3Z)-1-oxa-5-azahexa-1,3,5-trienes that undergo 6π-cyclization. The stationary points corresponding to isoxazolium N-ylides from isoxazoles and methoxycarbonylcarbene were located only for derivatives without a substituent in position 3 of the isoxazole ring. These isoxazolium N-ylides are thermodynamically and kinetically very unstable and, therefore, there is a low probability to detect them, even though they may theoretically be formed. According to the calculations and experimental results 2H-1,3-oxazines are usually characterized by a greater thermodynamical stability than the corresponding open-chain isomers, (3Z)-1-oxa-5-azahexa-1,3,5-trienes. An exception is oxaazahexatrienes derived from 5-alkoxyisoxazoles which are thermodynamically more stable than the corresponding 2H-1,3-oxazines. Therefore, the reaction of diazo esters with 5-alkoxyisoxazoles is a good approach to yield 1,4-di(alkoxycarbonyl)-2-azabuta-1,3-dienes, which are useful building blocks in heterocyclic synthesis [25,26]. We found reaction conditions which allow for the preparation of aryl- and halogen-substituted 2H-1,3-oxazines as well as 1,4-di(alkoxycarbonyl)-2-azabuta-1,3-dienes starting from isoxazoles and diazo esters.
Experimental
General methods
Melting points were determined on a hot stage microscope and are uncorrected. 1H (300 MHz) and 13C (75 MHz) NMR spectra were determined in CDCl3 with a Bruker DPX 300 and a Bruker AVANCE III 400 spectrometer. Chemical shifts (δ) are reported in parts per million downfield from tetramethylsilane. Mass spectra were recorded on a Bruker maXis HRMS-ESI-QTOF by using electrospray ionization in the positive mode. IR spectra were recorded on a Bruker FTIR spectrometer Tensor 27 by using KBr disks and only characteristic absorption bands are indicated. Single crystal X-ray data were collected by means of an Agilent Technologies Supernova Atlas and an Agilent Technologies Excalibur Eos diffractometer. The crystals were kept at 100 K during data collection. The structures have been solved by the direct methods and refined by means of the SHELXL-97 program [27] incorporated in the OLEX2 program package [28]. Crystallographic data for the structures 3a (CCDC 998319), 3k (CCDC 998318), 4a (CCDC 998317), 4b (CCDC 998316) have been deposited with the Cambridge Crystallographic Data Centre. Isoxazoles 1a [29], 1b [30], 1c–e [31], 1f,g [32] were prepared by the reported procedures.
General procedure of reacting isoxazoles with diazo compounds. A long Schlenk tube containing a mixture of isoxazole (0.3–1.2 mmol) and diazo compound (1 equiv) in PhCF3 (1–2 mL) was put into an oil bath preheated to 110 °C. To the vigorously stirred mixture, Rh2(OAc)4 (1–5 mol %) was added in one portion and stirred until N2 evolution has been stopped (10–15 min). An additional amount of diazo compound was added dropwise, and then the mixture was heated for an additional 15 min. The reaction mixture was cooled, concentrated in vacuo, and the residue was separated by column chromatography on silica with a mixture of hexane/ethyl acetate as eluent.
Methyl 4,6-dimethyl-2,5-diphenyl-2H-1,3-oxazine-2-carboxylate (3a)
Compound 3a (40 mg, 43%) was obtained from isoxazole 1a (50 mg, 0.289 mmol), diazo ester 2a (51 + 117 mg, 0.953 mmol) and Rh2(OAc)4 (3.8 mg, 3 mol %) in PhCF3 (1 mL). Colourless solid; mp 72–74 °C (CF3Ph); 1H NMR (400 MHz, CDCl3) δ 1.89 (s, 3H, Me), 1.96 (s, 3H, Me), 3.76 (s, 3H, MeO), 7.02–7.05 (m, 2H, Ar-H), 7.29–7.44 (m, 6H, Ar-H), 7.78–7.82 (m, 2H, Ar-H); 13C NMR (100 MHz, CDCl3) δ 17.4, 23.6, 53.0, 91.2, 116.2, 126.4, 127.4, 128.2, 128.5, 128.8, 130.3, 135.0, 138.9, 158.7, 165.5, 170.9; ESIMS (m/z): calculated for C20H20NO3+, 322.1438; found, 322.1444; Anal. calcd for C20H19NO3: C, 74.75; H, 5.96; N, 4.36; found: C, 75.04; H, 5.74; N, 4.64; IR (KBr, cm−1) ν: 1743 (C=O); crystal data for 3a: C20H19NO3, M = 321.36, monoclinic, space group P21/n, a = 10.2806(4), b = 10.5164(3), c = 15.3771(4) Å, β = 98.241(3)°, V = 1645.32(9) Å3, Z = 4, F(000) = 680, Dcalc = 1.297 mg m−3, μ = 0.704 mm−1. 8556 reflections were collected yielding 3164 unique (Rint = 0.0191). The final wR2 = 0.1000 (all data) and R1 = 0.0354 for 2848 reflections with I ≥ 2σ, GOF = 1.030.
Ethyl 4,6-dimethyl-5-phenyl-2H-1,3-oxazine-2-carboxylate (3b)
Compound 3b (52 mg, 35%) was obtained from isoxazole 1a (100 mg, 0.577 mmol), diazo ester 2b (66 + 58 mg, 1.09 mmol) and Rh2(OAc)4 (7.6 mg, 3 mol %) in PhCF3 (1 mL). Colorless solid; mp ca. 25 °C; 1H NMR (400 MHz, CDCl3) δ 1.37 (t, J = 7.1 Hz, 3H, Me), 1.83 (d, J = 0.9 Hz, 3H, Me), 1.85 (s, 3H, Me), 4.28–4.42 (m, 2H, CH2O), 5.60 (d, J = 0.9 Hz, 1H, 2-H), 7.11–7.13 (m, 2H, Ar-H), 7.32–7.39 (m, 3H, Ar-H); 13C NMR (100 MHz, CDCl3) δ 14.2, 17.0, 23.3, 62.0, 85.1, 116.0, 127.6, 128.6, 130.4, 135.1, 159.2, 166.2, 168.4; ESIMS (m/z): calcd for C14H18NO3+, 260.1281; found, 260.1281; IR (KBr, cm−1) ν: 1737 (C=O).
Methyl 2,4,6-triphenyl-2H-1,3-oxazine-2-carboxylate (3d)
Compound 3d (245 mg, 66%; 70% based on consumed isoxazole) was obtained from isoxazole 1b (221 mg, 1.00 mmol), diazo ester 2a (176 + 224 mg, 2.27 mmol) and Rh2(OAc)4 (6.6 mg, 1.5 mol %) in PhCF3 (2 mL). Colorless solid; mp 114–115 °C (hexane/ether); 1H NMR (400 MHz, CDCl3) δ 3.72 (s, 3H, MeO), 6.69 (s, 1H, 5-H), 7.46–7.53 (m, 9H, Ar-H), 8.00–8.03 (m, 2H, Ar-H), 8.05–8.07 (m, 2H, Ar-H), 8.07–8.11 (m, 2H, Ar-H); 13C NMR (100 MHz, CDCl3, 323 K) δ 53.0, 93.2, 95.9, 126.7, 126.8, 127.2, 128.3, 128.5, 128.6, 129.0, 130.9, 131.3, 132.2, 136.7, 139.0, 161.5, 162.9, 170.8; ESIMS (m/z): calcd for C24H20NO3+, 370.1438; found, 370.1437; IR (KBr, cm−1) ν: 1735 (C=O).
In addition to the product starting isoxazole 1b (11 mg) and MeO2C(Ph)C=N-N=C(Ph)CO2Me (104 mg, yellowish solid, mp 141–143 °C, ether (lit. [33]: mp 142–143 °C, MeOH)) were isolated.
Methyl 3-((Z)-(2-methoxy-2-oxo-1-phenylethylidene)amino)-3-phenylacrylate (4a)
Compound 4a (139 mg, 51%; 69% based on consumed isoxazole) was obtained from isoxazole 1f (175 mg, 1.00 mmol), diazo ester 2a (176 + 224 mg, 2.27 mmol) and Rh2(OAc)4 (5 mg, 1.5 mol %) in PhCF3 (1 mL). Yellow solid; mp 83–85 °C (hexane/ether); 1H NMR (400 MHz, CDCl3) δ 3.66 (s, 3H, MeO), 3.76 (s, 3H, MeO), 5.66 (s, 1H, 2-H), 7.36–7.41 (m, 3H, Ar-H), 7.44–7.48 (m, 2H, Ar-H), 7.51–7.58 (m, 3H, Ar-H), 7.88–7.90 (m, 2H, Ar-H); 13C NMR (100 MHz, 323 K, CDCl3) δ 51.2, 52.1, 97.0, 126.5, 128.61, 128.62, 130.2, 131.9, 133.1, 135.7, 158.3, 160.8, 163.2, 166.1; ESIMS (m/z): calcd for C19H18NO4+, 324.1230; found, 324.1235; IR (KBr, cm−1) ν: 1743, 1714 (C=O); crystal data for 4a: C19H17NO4, M = 326.36, monoclinic, space group P21/n, a = 9.1055(2), b = 13.7411(3), c = 13.2422(2) Å, β = 101.397(2)°, V = 1624.19(5) Å3, Z = 4, F(000) = 692, Dcalc = 1.335 mg m−3, μ = 0.766 mm−1. 21965 reflections were collected yielding 3407 unique (Rint = 0.0246). The final wR2 = 0.1033 (all data) and R1 = 0.0376 for 3207 reflections with I ≥ 2σ, GOF = 1.061.
Dimethyl (Z)-2-((3-methoxy-3-oxo-1-phenylprop-1-en-1-yl)imino)malonate (4b)
Compound 4b (164 mg, 54%; 61% based on consumed isoxazole) was obtained from isoxazole 1f (175 mg, 1.00 mmol), diazo ester 2c (159 + 63 mg, 1.40 mmol) and Rh2(OAc)4 (5 mg, 1.5 mol %) in PhCF3 (1 mL). Yellow solid; mp 63–65 °C (hexane/ether); 1H NMR (400 MHz, CDCl3) δ 3.26 (s, 3H, MeO), 3.87 (br. s, 6H, MeO), 5.58 (s, 1H, 2’-H), 7.38–7.40 (m, 3H, Ar-H), 7.49–7.51 (m, 2H, Ar-H); 13C NMR (100 MHz, 323 K, CDCl3) δ 51.3, 53.1, 96.9, 126.5, 128.8, 130.6, 134.5, 150.4, 158.8, 160.3, 165.6; ESIMS (m/z): calcd for C15H16NO6+, 306.0972; found, 306.0979; IR (KBr, cm−1) ν: 1751, 1708 (C=O); Crystal data for 4b: C15H15NO6, M = 305.28, triclinic, space group P-1, a = 7.2795(6), b = 9.9865(7), c = 10.7460(5) Å, α = 99.827(5), β = 93.454(6), γ = 105.608(7)°, V = 736.70(9) Å3, Z = 2, F(000) = 320, Dcalc = 1.376 mg m−3, μ = 0.911 mm−1. 6917 reflections were collected yielding 2897 unique (Rint = 0.0627). The final wR2 = 0.2337 (all data) and R1 = 0.0596 for 2689 reflections with I ≥ 2σ, GOF = 0.986.
Calculations. All calculations were carried out at DFT B3LYP/6-31G(d) level [34-36] by using the Gaussian 09 suite of quantum chemical programs [37] at the Resource center ‘Computer center of Saint Petersburg State University’. Geometry optimizations of intermediates, transition states, reactants and products in benzene were performed by means of a PCM model. Intrinsic reaction coordinates were calculated to authenticate all transition states.
Supporting Information
| Supporting Information File 1: Detailed experimental procedures including characterization data for all synthesized compounds, 1H and 13C NMR spectra for all new compounds, and computational details (energies of molecules, transition states, and the Cartesian coordinates of atoms). | ||
| Format: PDF | Size: 5.7 MB | Download |
Acknowledgements
We gratefully acknowledge the financial support from the Russian Foundation for Basic Research (Grant No. 14-03-00187) and Saint Petersburg State University (Grant No. 12.38.78.2012, 12.50.1565.2013, 12.38.239.2014). This research used resources of the resource center ‘Computer Center’, ‘Research resource center for Magnetic Resonance’, ‘Center for Chemical Analysis and Material Research’, and ‘Research resource Centre for X-ray Diffraction Studies’ of the Saint Petersburg State University.
References
-
Baraldi, P. G.; Barco, A.; Benetti, S.; Pollini, G. P.; Simoni, D. Synthesis 1987, 857. doi:10.1055/s-1987-28105
Return to citation in text: [1] [2] -
Pinho e Melo, T. M. V. D. Curr. Org. Chem. 2005, 9, 925. doi:10.2174/1385272054368420
Return to citation in text: [1] [2] -
Hamama, W. S.; Ibrahim, M. E.; Zoorob, H. H. Synth. Commun. 2013, 43, 2393. doi:10.1080/00397911.2012.729281
Return to citation in text: [1] [2] -
Manning, J. R.; Davies, H. M. L. Tetrahedron 2008, 64, 6901. doi:10.1016/j.tet.2008.03.010
Return to citation in text: [1] [2] [3] -
Manning, J. R.; Davies, H. M. L. J. Am. Chem. Soc. 2008, 130, 8602. doi:10.1021/ja803139k
Return to citation in text: [1] [2] -
King, J. F.; Durst, T. Can. J. Chem. 1962, 40, 882. doi:10.1139/v62-134
Return to citation in text: [1] -
Kashima, C.; Tsuda, Y.; Imada, S.; Nishio, T. J. Chem. Soc., Perkin Trans. 1 1980, 1866. doi:10.1039/P19800001866
Return to citation in text: [1] -
DeShong, P.; Cipollina, J. A.; Lowmaster, N. K. J. Org. Chem. 1988, 53, 1356. doi:10.1021/jo00242a003
Return to citation in text: [1] -
González-Nogal, A. M.; Calle, M. Tetrahedron 2009, 65, 5472. doi:10.1016/j.tet.2009.01.114
Return to citation in text: [1] -
Zavyalov, K. V.; Novikov, M. S.; Khlebnikov, A. F.; Yufit, D. S. Tetrahedron 2013, 69, 4546. doi:10.1016/j.tet.2013.04.022
Return to citation in text: [1] [2] -
Zavyalov, K. V.; Novikov, M. S.; Khlebnikov, A. F.; Pakalnis, V. V. Tetrahedron 2014, 70, 3377. doi:10.1016/j.tet.2014.03.101
Return to citation in text: [1] -
Kai, M.; Furuhashi, T.; Masuzawa, Y.; Yano, T.; Saito, F.; Nakaya, Y. Haloalkylsulfonanilide derivative. U.S. Patent 2012/0029187, Feb 2, 2012.
Return to citation in text: [1] -
Heine, N.; Fuchs, K.; Eickmeier, C.; Peters, S.; Dorner-Ciossek, C.; Handschuh, S.; Nar, H.; Klinder, K. Compounds for the treatment of alzheimer's disease. U.S. Patent 2010/0168070, July 1, 2010.
Return to citation in text: [1] -
Claremon, D. A. Cyclic inhibitors of 11beta-hydroxysteroid dehydrogenase 1. W.O. Patent WO2010/091067, Aug 12, 2010.
Return to citation in text: [1] -
Dhar, T. G. M.; Yang, G.; Davies, P.; Malley, M. F.; Gougoutas, J. Z.; Wu, D.-R.; Barrish, J. C.; Carter, P. H. Bioorg. Med. Chem. Lett. 2009, 19, 96. doi:10.1016/j.bmcl.2008.11.002
Return to citation in text: [1] -
Vintonyak, V. V.; Calà, M.; Lay, F.; Kunze, B.; Sasse, F.; Maier, M. E. Chem.–Eur. J. 2008, 14, 3709. doi:10.1002/chem.200701673
Return to citation in text: [1] -
Condon, J. S.; Joseph-McCarthy, D.; Levin, J. I.; Lombart, H.-G.; Lovering, F. E.; Sun, L.; Wang, W.; Xu, W.; Zhang, Y. Bioorg. Med. Chem. Lett. 2007, 17, 34. doi:10.1016/j.bmcl.2006.10.004
Return to citation in text: [1] -
Fries, K. M.; Joswig, C.; Borch, R. F. J. Med. Chem. 1995, 38, 2672. doi:10.1021/jm00014a019
Return to citation in text: [1] -
Marron, B. E.; Fritch, P. C.; Mark-Worth, C. J.; Maynard, A. T.; Swain, N. A. Inhibitors of ion channels. W.O. Patent WO2008/118758, Oct 2, 2008.
Return to citation in text: [1] -
Glatthar, R.; Orain, D.; Spanka, C. Nicotinic acid derivatives as modulators of metabotropic glutamate receptors. W.O. Patemt WO2007/071358, June 28, 2007.
Return to citation in text: [1] -
Ross, J. A.; Seiders, R. P.; Lemal, D. M. J. Am. Chem. Soc. 1976, 98, 4325. doi:10.1021/ja00430a060
Return to citation in text: [1] -
von Ragué Schleyer, P.; Wu, J. I.; Cossio, F. P.; Fernández, I. Chem. Soc. Rev. 2014, 43, 4909. doi:10.1039/c4cs00012a
Return to citation in text: [1] -
Ovalles, S. R.; Hansen, J. H.; Davies, H. M. L. Org. Lett. 2011, 13, 4284. doi:10.1021/ol201628d
Return to citation in text: [1] -
Uehara, F.; Sato, M.; Kaneko, C.; Kurihara, H. J. Org. Chem. 1999, 64, 1436. doi:10.1021/jo970742j
Return to citation in text: [1] -
Monbaliu, J.-C. M.; Masschelein, K. G. R.; Stevens, C. V. Chem. Soc. Rev. 2011, 40, 4708. doi:10.1039/c1cs15070g
Return to citation in text: [1] -
Jayakumar, S.; Ishar, M. P. S.; Mahajan, M. P. Tetrahedron 2002, 58, 379. doi:10.1016/S0040-4020(01)01050-X
Return to citation in text: [1] -
Sheldrick, G. M. Acta Crystallogr., Sect. A 2008, 64, 112. doi:10.1107/S0108767307043930
Return to citation in text: [1] -
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339. doi:10.1107/S0021889808042726
Return to citation in text: [1] -
Bobranski, B.; Wojtowski, R. Rocz. Chem. 1964, 38, 1327.
Return to citation in text: [1] -
Takikawa, H.; Takada, A.; Hikita, K.; Suzuki, K. Angew. Chem., Int. Ed. 2008, 47, 7446. doi:10.1002/anie.200801586
Return to citation in text: [1] -
Day, R. A.; Blake, J. A.; Stephens, C. E. Synthesis 2003, 1586. doi:10.1055/s-2003-40516
Return to citation in text: [1] -
Micetich, R. G.; Chin, C. G. Can. J. Chem. 1970, 48, 1371. doi:10.1139/v70-226
Return to citation in text: [1] -
Singh, B.; Ulman, E. F. J. Am. Chem. Soc. 1967, 89, 6911. doi:10.1021/ja01002a018
Return to citation in text: [1] -
Becke, A. D. J. Chem. Phys. 1993, 98, 5648. doi:10.1063/1.464913
Return to citation in text: [1] -
Becke, A. D. Phys. Rev. A 1998, 38, 3098. doi:10.1103/PhysRevA.38.3098
Return to citation in text: [1] -
Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785. doi:10.1103/PhysRevB.37.785
Return to citation in text: [1] -
Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, 2013.
Return to citation in text: [1]
| 30. | Takikawa, H.; Takada, A.; Hikita, K.; Suzuki, K. Angew. Chem., Int. Ed. 2008, 47, 7446. doi:10.1002/anie.200801586 |
| 28. | Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339. doi:10.1107/S0021889808042726 |
| 1. | Baraldi, P. G.; Barco, A.; Benetti, S.; Pollini, G. P.; Simoni, D. Synthesis 1987, 857. doi:10.1055/s-1987-28105 |
| 2. | Pinho e Melo, T. M. V. D. Curr. Org. Chem. 2005, 9, 925. doi:10.2174/1385272054368420 |
| 3. | Hamama, W. S.; Ibrahim, M. E.; Zoorob, H. H. Synth. Commun. 2013, 43, 2393. doi:10.1080/00397911.2012.729281 |
| 10. | Zavyalov, K. V.; Novikov, M. S.; Khlebnikov, A. F.; Yufit, D. S. Tetrahedron 2013, 69, 4546. doi:10.1016/j.tet.2013.04.022 |
| 11. | Zavyalov, K. V.; Novikov, M. S.; Khlebnikov, A. F.; Pakalnis, V. V. Tetrahedron 2014, 70, 3377. doi:10.1016/j.tet.2014.03.101 |
| 25. | Monbaliu, J.-C. M.; Masschelein, K. G. R.; Stevens, C. V. Chem. Soc. Rev. 2011, 40, 4708. doi:10.1039/c1cs15070g |
| 26. | Jayakumar, S.; Ishar, M. P. S.; Mahajan, M. P. Tetrahedron 2002, 58, 379. doi:10.1016/S0040-4020(01)01050-X |
| 6. | King, J. F.; Durst, T. Can. J. Chem. 1962, 40, 882. doi:10.1139/v62-134 |
| 7. | Kashima, C.; Tsuda, Y.; Imada, S.; Nishio, T. J. Chem. Soc., Perkin Trans. 1 1980, 1866. doi:10.1039/P19800001866 |
| 8. | DeShong, P.; Cipollina, J. A.; Lowmaster, N. K. J. Org. Chem. 1988, 53, 1356. doi:10.1021/jo00242a003 |
| 9. | González-Nogal, A. M.; Calle, M. Tetrahedron 2009, 65, 5472. doi:10.1016/j.tet.2009.01.114 |
| 27. | Sheldrick, G. M. Acta Crystallogr., Sect. A 2008, 64, 112. doi:10.1107/S0108767307043930 |
| 4. | Manning, J. R.; Davies, H. M. L. Tetrahedron 2008, 64, 6901. doi:10.1016/j.tet.2008.03.010 |
| 5. | Manning, J. R.; Davies, H. M. L. J. Am. Chem. Soc. 2008, 130, 8602. doi:10.1021/ja803139k |
| 24. | Uehara, F.; Sato, M.; Kaneko, C.; Kurihara, H. J. Org. Chem. 1999, 64, 1436. doi:10.1021/jo970742j |
| 1. | Baraldi, P. G.; Barco, A.; Benetti, S.; Pollini, G. P.; Simoni, D. Synthesis 1987, 857. doi:10.1055/s-1987-28105 |
| 2. | Pinho e Melo, T. M. V. D. Curr. Org. Chem. 2005, 9, 925. doi:10.2174/1385272054368420 |
| 3. | Hamama, W. S.; Ibrahim, M. E.; Zoorob, H. H. Synth. Commun. 2013, 43, 2393. doi:10.1080/00397911.2012.729281 |
| 4. | Manning, J. R.; Davies, H. M. L. Tetrahedron 2008, 64, 6901. doi:10.1016/j.tet.2008.03.010 |
| 5. | Manning, J. R.; Davies, H. M. L. J. Am. Chem. Soc. 2008, 130, 8602. doi:10.1021/ja803139k |
| 10. | Zavyalov, K. V.; Novikov, M. S.; Khlebnikov, A. F.; Yufit, D. S. Tetrahedron 2013, 69, 4546. doi:10.1016/j.tet.2013.04.022 |
| 20. | Glatthar, R.; Orain, D.; Spanka, C. Nicotinic acid derivatives as modulators of metabotropic glutamate receptors. W.O. Patemt WO2007/071358, June 28, 2007. |
| 4. | Manning, J. R.; Davies, H. M. L. Tetrahedron 2008, 64, 6901. doi:10.1016/j.tet.2008.03.010 |
| 33. | Singh, B.; Ulman, E. F. J. Am. Chem. Soc. 1967, 89, 6911. doi:10.1021/ja01002a018 |
| 19. | Marron, B. E.; Fritch, P. C.; Mark-Worth, C. J.; Maynard, A. T.; Swain, N. A. Inhibitors of ion channels. W.O. Patent WO2008/118758, Oct 2, 2008. |
| 23. | Ovalles, S. R.; Hansen, J. H.; Davies, H. M. L. Org. Lett. 2011, 13, 4284. doi:10.1021/ol201628d |
| 34. | Becke, A. D. J. Chem. Phys. 1993, 98, 5648. doi:10.1063/1.464913 |
| 35. | Becke, A. D. Phys. Rev. A 1998, 38, 3098. doi:10.1103/PhysRevA.38.3098 |
| 36. | Lee, C.; Yang, W.; Parr, R. G. Phys. Rev. B 1988, 37, 785. doi:10.1103/PhysRevB.37.785 |
| 13. | Heine, N.; Fuchs, K.; Eickmeier, C.; Peters, S.; Dorner-Ciossek, C.; Handschuh, S.; Nar, H.; Klinder, K. Compounds for the treatment of alzheimer's disease. U.S. Patent 2010/0168070, July 1, 2010. |
| 14. | Claremon, D. A. Cyclic inhibitors of 11beta-hydroxysteroid dehydrogenase 1. W.O. Patent WO2010/091067, Aug 12, 2010. |
| 15. | Dhar, T. G. M.; Yang, G.; Davies, P.; Malley, M. F.; Gougoutas, J. Z.; Wu, D.-R.; Barrish, J. C.; Carter, P. H. Bioorg. Med. Chem. Lett. 2009, 19, 96. doi:10.1016/j.bmcl.2008.11.002 |
| 16. | Vintonyak, V. V.; Calà, M.; Lay, F.; Kunze, B.; Sasse, F.; Maier, M. E. Chem.–Eur. J. 2008, 14, 3709. doi:10.1002/chem.200701673 |
| 17. | Condon, J. S.; Joseph-McCarthy, D.; Levin, J. I.; Lombart, H.-G.; Lovering, F. E.; Sun, L.; Wang, W.; Xu, W.; Zhang, Y. Bioorg. Med. Chem. Lett. 2007, 17, 34. doi:10.1016/j.bmcl.2006.10.004 |
| 18. | Fries, K. M.; Joswig, C.; Borch, R. F. J. Med. Chem. 1995, 38, 2672. doi:10.1021/jm00014a019 |
| 31. | Day, R. A.; Blake, J. A.; Stephens, C. E. Synthesis 2003, 1586. doi:10.1055/s-2003-40516 |
| 12. | Kai, M.; Furuhashi, T.; Masuzawa, Y.; Yano, T.; Saito, F.; Nakaya, Y. Haloalkylsulfonanilide derivative. U.S. Patent 2012/0029187, Feb 2, 2012. |
| 21. | Ross, J. A.; Seiders, R. P.; Lemal, D. M. J. Am. Chem. Soc. 1976, 98, 4325. doi:10.1021/ja00430a060 |
| 22. | von Ragué Schleyer, P.; Wu, J. I.; Cossio, F. P.; Fernández, I. Chem. Soc. Rev. 2014, 43, 4909. doi:10.1039/c4cs00012a |
| 32. | Micetich, R. G.; Chin, C. G. Can. J. Chem. 1970, 48, 1371. doi:10.1139/v70-226 |
© 2014 Khlebnikov et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)