Abstract
Substituted 3,4-dihydro-1,8-naphthyridin-2(1H)-ones have been synthesized with the inverse electron-demand Diels–Alder reaction from 1,2,4-triazines bearing an acylamino group with a terminal alkyne side chain. Alkynes were first subjected to the Sonogashira cross-coupling reaction with aryl halides, the product of which then underwent an intramolecular inverse electron-demand Diels–Alder reaction to yield 5-aryl-3,4-dihydro-1,8-naphthyridin-2(1H)-ones by an efficient synthetic route.
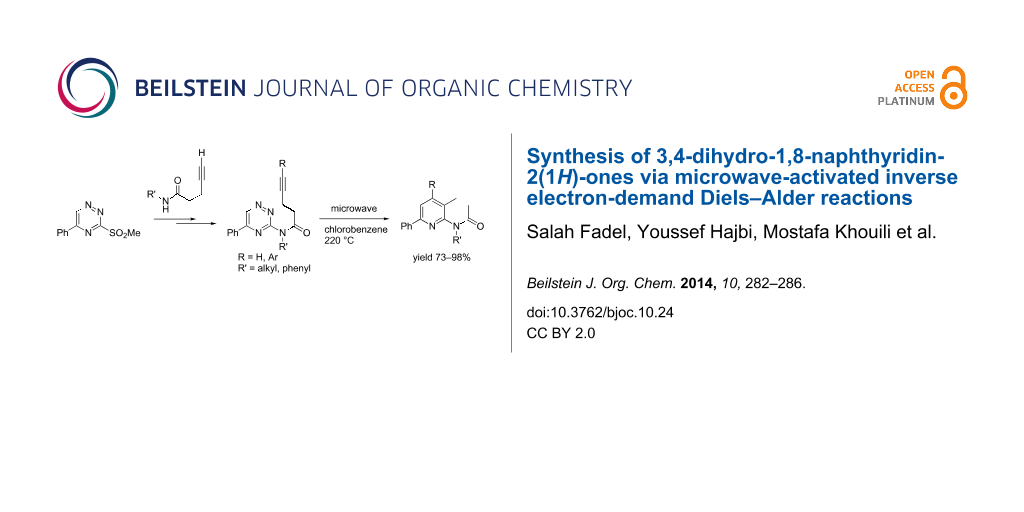
Graphical Abstract
Introduction
1,8-Naphthyridine derivatives are an important class of heterocyclic compounds and include many substances of both biological and chemical interest [1-4]. Prevention and treatment of angiogenic disorders and cancers were realized with this class of heterocyclic derivatives [5]. They show anti-allergic [6], anti-inflammatory [7], antibacterial [8] and gastric antisecretory activities [9]. Many other remarkable applications are reported in the literature [10-14], such as the selective inhibition of p38 mitogen-activated protein kinase [15] and the potent inhibition of protein kinase C isozymes [16]. Much attention has been devoted to the synthesis of 1,8-naphthyridin-2(1H)-ones because of their acyl-CoA:cholesterol acyltransferase (ACAT) inhibitory activity [17] and their role as phosphodiesterase inhibitors [18,19]. To date, 1,8-naphthyridin-2(1H)-ones have been prepared mainly by the Knorr or the Friedländer reaction [20,21]. However, these methods cannot give access to various polysubstituted 1,8-naphthyridin-2-ones. Recently, we reported an efficient method for the synthesis of polysubstituted 2,3-dihydrofuro[2,3-b]pyridines and 3,4-dihydro-2H-pyrano[2,3-b]pyridines from 1,2,4-triazines via an inverse electron-demand Diels–Alder reaction under microwave irradiation [22-24]. The use of 1,2,4-triazines in inverse electron-demand Diels–Alder reactions proved to be an efficient strategy for the construction of various heterocyclic compounds [25-27], such as azacarbazoles [28-33], polycyclic condensed pyrazines [34,35], dihydropyrrolopyridines [36,37], thienopyridines and thiopyranopyridines [38,39], as well as furo- and pyranopyridines [22-24,40-42]. Reactions with microwave irradiation are well-known for their ability to reduce reaction times, increase product yields, and reduce unwanted side reactions compared to conventional heating methods [43-49]. In the continuation of our studies on the synthesis of fused heterocyclic systems we decided to extend this methodology to the synthesis of substituted 3,4-dihydro-1,8-naphthyridin-2(1H)-ones.
Results and Discussion
Synthesis of 1,7-disubstituted 3,4-dihydro-1,8-naphthyridin-2(1H)-ones
3-Methylsulfonyl-5-phenyl-1,2,4-triazine
Our strategy was first based on the 3-methylsulfonyl-1,2,4-triazine 1 (Scheme 1). This key triazine 1 was prepared according to the procedure described by Taylor and Paudler [34,50], i.e., the phenylglyoxal was condensed with the S-methylthiosemicarbazide followed by an oxidation reaction with MCPBA.
Scheme 1: (a) MeI, EtOH, reflux, 3 h (87%); (b) phenylglyoxal, Na2CO3, H2O, 5 °C, 6 h (96%); (c) MCPBA, CH2Cl2, rt, 4 h (82%).
Scheme 1: (a) MeI, EtOH, reflux, 3 h (87%); (b) phenylglyoxal, Na2CO3, H2O, 5 °C, 6 h (96%); (c) MCPBA, CH2Cl2...
Synthesis of N-substituted pent-4-ynamides
N-Alkyl or N-aryl-pent-4-ynamides were prepared by amide coupling reactions between pent-4-ynoic acid and various amines in THF in the presence of EDCI and DMAP. The corresponding amides 2–5 were obtained in excellent yields (Scheme 2). The results are shown in Table 1.
Scheme 2: Coupling of pent-4-ynoic acid with different amines. Conditions: (a) EDCI, DMAP, THF, rt, 36 h.
Scheme 2: Coupling of pent-4-ynoic acid with different amines. Conditions: (a) EDCI, DMAP, THF, rt, 36 h.
Table 1: Amide coupling reactions of pent-4-ynoic acid with different amines.
aYield of pure isolated product.
Preparation of N-substituted N-triazinylpent-4-ynamides
The nucleophilic substitution of the methylsulfonyl leaving group from 1 by the lithium salt of ynamides 2–5 [22-24,53] afforded triazinylpent-4-ynamides 6–9 in moderate to good yields (Scheme 3, Table 2).
Scheme 3: Reaction of triazine 1 with different pent-4-ynamides. Conditions: n-BuLi, THF, −30 °C, 2 h.
Scheme 3: Reaction of triazine 1 with different pent-4-ynamides. Conditions: n-BuLi, THF, −30 °C, 2 h.
Table 2: Substitution of 1,2,4-triazine 1 by different amides 2–5.
| Entry | R | Product | Yield (%)a |
|---|---|---|---|
| 1 | butyl | 6 | 74 |
| 2 | propenyl | 7 | 56 |
| 3 | isopropyl | 8 | 24 |
| 4 | phenyl | 9 | 79 |
aYield of pure isolated product.
Intramolecular inverse electron-demand Diels–Alder reactions
With the tethered triazines 6–9 in hand, we were able to study the cycloaddition reaction under microwave heating following the optimal experimental conditions already reported with triazines [22-24]. In chlorobenzene at 220 °C (optimal reaction temperature for six-membered-ring formation), the corresponding cycloadducts 10–13 were obtained in high yields (Scheme 4, Table 3).
Scheme 4: Reaction of triazines 6–9 under microwave irradiation. Conditions: Chorobenzene, 220 °C, 1 h.
Scheme 4: Reaction of triazines 6–9 under microwave irradiation. Conditions: Chorobenzene, 220 °C, 1 h.
Table 3: Intramolecular inverse electron-demand Diels–Alder reactions under microwave irradiation.
| Entry | R | Product | Yield (%)a |
|---|---|---|---|
| 1 | butyl | 10 | 97 |
| 2 | propenyl | 11 | 96 |
| 3 | isopropyl | 12 | 93 |
| 4 | phenyl | 13 | 98 |
aYield of pure isolated product.
We therefore developed an efficient method for the synthesis of 1-substituted 3,4-dihydro-1,8-naphthyridin-2(1H)-ones by using 1,2,4-triazine and alkyne tethered together by an amide linker.
Synthesis of 1,5,7-trisubstituted-3,4-dihydro-1,8-naphthyridin-2(1H)-ones
In order to functionalize the 4-position of the pyridine ring and to extend diversity, we envisaged to evaluate the reactivity of internal alkynes towards the inverse electron-demand Diels–Alder reaction. To reach this goal, we decided to functionalize the alkynes 6–9 employing the Sonogashira cross-coupling reaction.
Preparation of aryl-N-triazinylpentynamides
The terminal alkynes 6–9 were then subjected to a Sonogashira cross-coupling reaction. Thus, treating compounds 6–9 in DME with Pd(PPh3)2Cl2 (5 mol %), CuI, Et3N and aryl iodide, gave the cross-coupling products 14–21 in very good yields (Scheme 5). The results are summarized in Table 4.
Scheme 5: Preparation of aryl-N-triazinylpentynamides. Conditions: CuI (10 mol %), Pd(PPh3)2Cl2 (5 mol %), DME, Et3N, rt, 3 h.
Scheme 5: Preparation of aryl-N-triazinylpentynamides. Conditions: CuI (10 mol %), Pd(PPh3)2Cl2 (5 mol %), DM...
Table 4: Sonogashira cross-coupling reactions from alkynes 6–9.
| Entry | R | Aryl | Product | Yield (%)a |
|---|---|---|---|---|
| 1 | butyl | 2-thienyl | 14 | 95 |
| 2 | 4-methoxyphenyl | 15 | 95 | |
| 3 | propenyl | 2-thienyl | 16 | 95 |
| 4 | 4-methoxyphenyl | 17 | 89 | |
| 5 | isopropyl | 2-thienyl | 18 | 91 |
| 6 | 4-methoxyphenyl | 19 | 85 | |
| 7 | phenyl | 2-thienyl | 20 | 86 |
| 8 | 4-methoxyphenyl | 21 | 82 | |
aYield of pure isolated product.
Intramolecular inverse electron-demand Diels–Alder reactions
Finally, the inverse electron-demand Diels–Alder reaction with tethered triazine 14–21 was carried out under microwave irradiation in a sealed tube at 220 °C (Scheme 6) as previously mentioned [22-24]. The corresponding substituted naphthyridin-2(1H)-ones 22–29 were obtained in excellent yields. The results are given in Table 5.
Scheme 6: Preparation of 3,4-dihydro-1,8-naphthridin-2(1H)-ones. Conditions: Chlorobenzene, 220 °C, 1 h.
Scheme 6: Preparation of 3,4-dihydro-1,8-naphthridin-2(1H)-ones. Conditions: Chlorobenzene, 220 °C, 1 h.
Conclusion
In this article, we report the successful application of a new synthesis strategy leading to 1-substituted 3,4-dihydro-1,8-naphthyridin-2(1H)-ones by inverse electron-demand Diels–Alder reactions under microwave activation. We also synthesized 5-substituted 3,4-dihydro-1,8-naphthyridin-2(1H)-ones via the Sonogashira cross-coupling reaction followed by intramolecular inverse electron-demand Diels–Alder reactions. The developed approaches allow a high diversity of substituents on the bicyclic scaffold.
Supporting Information
| Supporting Information File 1: Experimental section. | ||
| Format: PDF | Size: 346.3 KB | Download |
References
-
Settimo, A.; Biagi, G.; Primofiore, G.; Ferrarini, P. L.; Livi, O. Farmaco 1978, 33, 770.
Return to citation in text: [1] -
Roma, G.; Di Braccio, M.; Grossi, G.; Piras, D.; Ballabeni, V.; Tognolini, M.; Bertoni, S.; Barocelli, E. Eur. J. Med. Chem. 2010, 45, 352. doi:10.1016/j.ejmech.2009.10.020
Return to citation in text: [1] -
Li, C.; Mu, X.-Y.; Li, Y.-L.; Liu, Y.; Wang, X.-S. ACS Comb. Sci. 2013, 15, 267. doi:10.1021/co400020w
Return to citation in text: [1] -
Bunce, R.; Squires, S. T.; Nammalwar, B. J. Org. Chem. 2013, 78, 2144. doi:10.1021/jo3018632
Return to citation in text: [1] -
Tsuzuki, Y.; Tomita, K.; Sato, Y.; Kashimoto, S.; Chiba, K. Bioorg. Med. Chem. Lett. 2004, 14, 3189. doi:10.1016/j.bmcl.2004.04.011
Return to citation in text: [1] -
Sherlock, M. H.; Kaminski, J. J.; Tom, W. C.; Lee, J. F.; Wong, S. C.; Kreutner, W.; Bryant, R. W.; McPhail, A. T. J. Med. Chem. 1988, 31, 2108. doi:10.1021/jm00119a010
Return to citation in text: [1] -
Kuroda, T.; Suzuki, F.; Tamura, T.; Ohmori, K.; Hosoe, H. J. Med. Chem. 1992, 35, 1130. doi:10.1021/jm00084a019
Return to citation in text: [1] -
Santilli, A. A.; Scotese, A. C.; Yurchenco, J. A. J. Med. Chem. 1975, 18, 1038. doi:10.1021/jm00244a021
Return to citation in text: [1] -
Santilli, A. A.; Scotese, A. C.; Bauer, R. F.; Bell, S. C. J. Med. Chem. 1987, 30, 2270. doi:10.1021/jm00395a015
Return to citation in text: [1] -
Carboni, S.; Da Settimo, A.; Ferrarini, P. L.; Primofiore, G.; Livi, O.; Menichetti, V.; Del Tacca, M.; Martinotti, E.; Bernardini, C.; Bertelli, A. Eur. J. Med. Chem. 1982, 17, 159.
Return to citation in text: [1] -
Ferrarini, P. L.; Mori, C.; Primofiore, G.; Da Settimo, A.; Breschi, M. C.; Martinotti, E.; Nieri, P.; Ciucci, M. A. Eur. J. Med. Chem. 1990, 25, 489. doi:10.1016/0223-5234(90)90143-Q
Return to citation in text: [1] -
Saccomanni, G.; Badawneh, M.; Adinolfi, B.; Calderone, V.; Cavallini, T.; Ferrarini, P. L.; Greco, R.; Manera, C.; Testai, L. Bioorg. Med. Chem. 2003, 11, 4921. doi:10.1016/j.bmc.2003.09.017
Return to citation in text: [1] -
Debenham, J. S.; Madsen-Duggan, C. B.; Walsh, T. F.; Wang, J.; Tong, X.; Doss, G. A.; Lao, J.; Fong, T. M.; Schaeffer, M.-T.; Xiao, J. C.; Huang, C. R.-R. C.; Shen, C.-P.; Feng, Y.; Marsh, D. J.; Stribling, D. S.; Shearman, L. P.; Strack, A. M.; MacIntyre, D. E.; Van der Ploeg, L. H. T.; Goulet, M. T. Bioorg. Med. Chem. Lett. 2006, 16, 681. doi:10.1016/j.bmcl.2005.10.028
Return to citation in text: [1] -
Manera, C.; Saccomanni, G.; Adinolfi, B.; Benetti, V.; Ligresti, A.; Cascio, M. G.; Tuccinardi, T.; Lucchesi, V.; Martinelli, A.; Nieri, P.; Masini, E.; Di Marzo, V.; Ferrarini, P. L. J. Med. Chem. 2009, 52, 3644. doi:10.1021/jm801563d
Return to citation in text: [1] -
Lumeras, W.; Vidal, L.; Vidal, B.; Balagué, C.; Orellana, A.; Maldonado, M.; Dominguez, M.; Segarra, V.; Caturla, F. J. Med. Chem. 2011, 54, 7899. doi:10.1021/jm200975u
Return to citation in text: [1] -
van Eis, M. J.; Evenou, J.-P.; Floersheim, P.; Gaul, C.; Cowan-Jacob, S. W.; Monovich, L.; Rummel, G.; Schuler, W.; Stark, W.; Strauss, A.; von Matt, A.; Vangrevelinghe, E.; Wagner, J.; Soldermann, N. Bioorg. Med. Chem. Lett. 2011, 21, 7367. doi:10.1016/j.bmcl.2011.10.025
Return to citation in text: [1] -
Ban, H.; Muraoka, M.; Ioriya, K.; Ohashi, N. Bioorg. Med. Chem. Lett. 2006, 16, 44. doi:10.1016/j.bmcl.2005.09.056
Return to citation in text: [1] -
Aoki, M.; Isomura, Y.; Iwata, M.; Niwa, A.; Okamoto, Y.; Takayama, K. Novel naphthyridine derivative and medicinal composition thereof. WO Patent WO1996006843A1, March 7, 1996.
Chem. Abstr. 1996, 125, 86620.
Return to citation in text: [1] -
Takayama, K.; Iwata, M.; Hisamichi, H.; Okamoto, Y.; Aoki, M.; Niwa, A. Chem. Pharm. Bull. 2002, 50, 1050. doi:10.1248/cpb.50.1050
Return to citation in text: [1] -
Cheng, C. C.; Yan, S. J. Org. React. 1982, 28, 37.
Return to citation in text: [1] -
Zhou, J.; Li, B.; Hu, F.; Shi, B.-F. Org. Lett. 2013, 15, 3460. doi:10.1021/ol401540k
Return to citation in text: [1] -
Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Tetrahedron 2007, 63, 8286. doi:10.1016/j.tet.2007.05.112
Return to citation in text: [1] [2] [3] [4] [5] -
Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Synlett 2009, 92. doi:10.1055/s-0028-1087485
Return to citation in text: [1] [2] [3] [4] [5] -
Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Synthesis 2010, 1349. doi:10.1055/s-0029-1218665
Return to citation in text: [1] [2] [3] [4] [5] -
Foster, R. A. A.; Willis, M. C. Chem. Soc. Rev. 2013, 42, 63. doi:10.1039/c2cs35316d
Return to citation in text: [1] -
Prokhorov, A. M.; Kozhevnikov, D. N. Prog. Heterocycl. Chem. 2011, 23, 403.
Return to citation in text: [1] -
Raw, S. A.; Taylor, R. J. K. Adv. Heterocycl. Chem. 2010, 100, 75. doi:10.1016/S0065-2725(10)10003-8
Return to citation in text: [1] -
Boger, D. L.; Duff, S. R.; Panek, J. S.; Yasuda, M. J. Org. Chem. 1985, 50, 5782. doi:10.1021/jo00350a069
Return to citation in text: [1] -
Gribble, G. W.; Barden, T. C.; Johnson, D. A. Tetrahedron 1988, 44, 3195. doi:10.1016/S0040-4020(01)85951-2
Return to citation in text: [1] -
Benson, S. C.; Gross, J. L.; Snyder, J. K. J. Org. Chem. 1990, 55, 3257. doi:10.1021/jo00297a050
Return to citation in text: [1] -
Li, J. H.; Snyder, J. K. J. Org. Chem. 1993, 58, 516. doi:10.1021/jo00054a044
Return to citation in text: [1] -
Fan, W.-H.; Parikh, M.; Snyder, J. K. Tetrahedron Lett. 1995, 36, 6591. doi:10.1016/00404-0399(50)1347-K
Return to citation in text: [1] -
Lahue, B. R.; Lo, S.-M.; Wan, Z.-K.; Woo, G. H. C.; Snyder, J. K. J. Org. Chem. 2004, 69, 7171. doi:10.1021/jo040193z
Return to citation in text: [1] -
Taylor, E. C.; Pont, J. L.; Warner, J. C. Tetrahedron 1987, 43, 5159. doi:10.1016/S0040-4020(01)87691-2
Return to citation in text: [1] [2] -
Taylor, E. C.; French, L. G. J. Org. Chem. 1989, 54, 1245. doi:10.1021/jo00267a006
Return to citation in text: [1] -
Seitz, G.; Görge, L.; Dietrich, S. Tetrahedron Lett. 1985, 26, 4355. doi:10.1016/S0040-4039(00)98733-1
Return to citation in text: [1] -
Taylor, E. C.; Pont, J. L. Tetrahedron Lett. 1987, 28, 379. doi:10.1016/S0040-4039(00)95733-2
Return to citation in text: [1] -
Taylor, E. C.; Macor, J. E. J. Org. Chem. 1987, 52, 4280. doi:10.1021/jo00228a023
Return to citation in text: [1] -
Taylor, E. C.; Macor, J. E. J. Org. Chem. 1989, 54, 4984. doi:10.1021/jo00282a006
Return to citation in text: [1] -
Taylor, E. C.; Macor, J. E. Tetrahedron Lett. 1986, 27, 431. doi:10.1016/S0040-4039(00)85497-0
Return to citation in text: [1] -
Taylor, E. C.; Macor, J. E.; Pont, J. L. Tetrahedron 1987, 43, 5145. doi:10.1016/S0040-4020(01)87690-0
Return to citation in text: [1] -
Haenel, F.; John, R.; Seitz, G. Arch. Pharm. 1992, 325, 349. doi:10.1002/ardp.19923250608
Return to citation in text: [1] -
de la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Chem. Soc. Rev. 2005, 34, 164. doi:10.1039/b411438h
Return to citation in text: [1] -
Loupy, A., Ed. Microwaves in Organic Synthesis; Wiley-VCH: Weinhein, 2006.
Return to citation in text: [1] -
Kostakis, I. K.; Elomri, A.; Seguin, E.; Iannelli, M.; Besson, T. Tetrahedron Lett. 2007, 48, 6609. doi:10.1016/j.tetlet.2007.07.114
Return to citation in text: [1] -
Herrero, M. A.; Kremsner, J. M.; Kappe, C. O. J. Org. Chem. 2008, 73, 36. doi:10.1021/jo7022697
Return to citation in text: [1] -
Caddick, S.; Fitzmaurice, R. Tetrahedron 2009, 65, 3325. doi:10.1016/j.tet.2009.01.105
Return to citation in text: [1] -
Kappe, C. O.; Dallinger, D. Mol. Diversity 2009, 13, 71. doi:10.1007/s11030-009-9138-8
Return to citation in text: [1] -
Mikhailichenko, S. S.; Bouillon, J.-P.; Besson, T.; Shermolovich, Y. G. Tetrahedron Lett. 2010, 51, 990. doi:10.1016/j.tetlet.2009.12.064
Return to citation in text: [1] -
Paudler, W. W.; Chen, T.-K. J. Heterocycl. Chem. 1970, 7, 767. doi:10.1002/jhet.5570070403
Return to citation in text: [1] -
Pardo, L. M.; Tellitu, I.; Domínguez, E. Tetrahedron 2012, 68, 3692. doi:10.1016/j.tet.2012.03.033
Return to citation in text: [1] -
Zhou, Y.; Feng, E.; Liu, G.; Ye, D.; Li, J.; Jiang, H.; Liu, H. J. Org. Chem. 2009, 74, 7344. doi:10.1021/jo901418m
Return to citation in text: [1] -
Benson, S. C.; Lee, L.; Yang, L.; Snyder, J. K. Tetrahedron 2000, 56, 1165. doi:10.1016/S0040-4020(00)00003-X
Return to citation in text: [1]
| 1. | Settimo, A.; Biagi, G.; Primofiore, G.; Ferrarini, P. L.; Livi, O. Farmaco 1978, 33, 770. |
| 2. | Roma, G.; Di Braccio, M.; Grossi, G.; Piras, D.; Ballabeni, V.; Tognolini, M.; Bertoni, S.; Barocelli, E. Eur. J. Med. Chem. 2010, 45, 352. doi:10.1016/j.ejmech.2009.10.020 |
| 3. | Li, C.; Mu, X.-Y.; Li, Y.-L.; Liu, Y.; Wang, X.-S. ACS Comb. Sci. 2013, 15, 267. doi:10.1021/co400020w |
| 4. | Bunce, R.; Squires, S. T.; Nammalwar, B. J. Org. Chem. 2013, 78, 2144. doi:10.1021/jo3018632 |
| 8. | Santilli, A. A.; Scotese, A. C.; Yurchenco, J. A. J. Med. Chem. 1975, 18, 1038. doi:10.1021/jm00244a021 |
| 28. | Boger, D. L.; Duff, S. R.; Panek, J. S.; Yasuda, M. J. Org. Chem. 1985, 50, 5782. doi:10.1021/jo00350a069 |
| 29. | Gribble, G. W.; Barden, T. C.; Johnson, D. A. Tetrahedron 1988, 44, 3195. doi:10.1016/S0040-4020(01)85951-2 |
| 30. | Benson, S. C.; Gross, J. L.; Snyder, J. K. J. Org. Chem. 1990, 55, 3257. doi:10.1021/jo00297a050 |
| 31. | Li, J. H.; Snyder, J. K. J. Org. Chem. 1993, 58, 516. doi:10.1021/jo00054a044 |
| 32. | Fan, W.-H.; Parikh, M.; Snyder, J. K. Tetrahedron Lett. 1995, 36, 6591. doi:10.1016/00404-0399(50)1347-K |
| 33. | Lahue, B. R.; Lo, S.-M.; Wan, Z.-K.; Woo, G. H. C.; Snyder, J. K. J. Org. Chem. 2004, 69, 7171. doi:10.1021/jo040193z |
| 7. | Kuroda, T.; Suzuki, F.; Tamura, T.; Ohmori, K.; Hosoe, H. J. Med. Chem. 1992, 35, 1130. doi:10.1021/jm00084a019 |
| 34. | Taylor, E. C.; Pont, J. L.; Warner, J. C. Tetrahedron 1987, 43, 5159. doi:10.1016/S0040-4020(01)87691-2 |
| 35. | Taylor, E. C.; French, L. G. J. Org. Chem. 1989, 54, 1245. doi:10.1021/jo00267a006 |
| 6. | Sherlock, M. H.; Kaminski, J. J.; Tom, W. C.; Lee, J. F.; Wong, S. C.; Kreutner, W.; Bryant, R. W.; McPhail, A. T. J. Med. Chem. 1988, 31, 2108. doi:10.1021/jm00119a010 |
| 22. | Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Tetrahedron 2007, 63, 8286. doi:10.1016/j.tet.2007.05.112 |
| 23. | Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Synlett 2009, 92. doi:10.1055/s-0028-1087485 |
| 24. | Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Synthesis 2010, 1349. doi:10.1055/s-0029-1218665 |
| 5. | Tsuzuki, Y.; Tomita, K.; Sato, Y.; Kashimoto, S.; Chiba, K. Bioorg. Med. Chem. Lett. 2004, 14, 3189. doi:10.1016/j.bmcl.2004.04.011 |
| 25. | Foster, R. A. A.; Willis, M. C. Chem. Soc. Rev. 2013, 42, 63. doi:10.1039/c2cs35316d |
| 26. | Prokhorov, A. M.; Kozhevnikov, D. N. Prog. Heterocycl. Chem. 2011, 23, 403. |
| 27. | Raw, S. A.; Taylor, R. J. K. Adv. Heterocycl. Chem. 2010, 100, 75. doi:10.1016/S0065-2725(10)10003-8 |
| 16. | van Eis, M. J.; Evenou, J.-P.; Floersheim, P.; Gaul, C.; Cowan-Jacob, S. W.; Monovich, L.; Rummel, G.; Schuler, W.; Stark, W.; Strauss, A.; von Matt, A.; Vangrevelinghe, E.; Wagner, J.; Soldermann, N. Bioorg. Med. Chem. Lett. 2011, 21, 7367. doi:10.1016/j.bmcl.2011.10.025 |
| 18. |
Aoki, M.; Isomura, Y.; Iwata, M.; Niwa, A.; Okamoto, Y.; Takayama, K. Novel naphthyridine derivative and medicinal composition thereof. WO Patent WO1996006843A1, March 7, 1996.
Chem. Abstr. 1996, 125, 86620. |
| 19. | Takayama, K.; Iwata, M.; Hisamichi, H.; Okamoto, Y.; Aoki, M.; Niwa, A. Chem. Pharm. Bull. 2002, 50, 1050. doi:10.1248/cpb.50.1050 |
| 15. | Lumeras, W.; Vidal, L.; Vidal, B.; Balagué, C.; Orellana, A.; Maldonado, M.; Dominguez, M.; Segarra, V.; Caturla, F. J. Med. Chem. 2011, 54, 7899. doi:10.1021/jm200975u |
| 20. | Cheng, C. C.; Yan, S. J. Org. React. 1982, 28, 37. |
| 21. | Zhou, J.; Li, B.; Hu, F.; Shi, B.-F. Org. Lett. 2013, 15, 3460. doi:10.1021/ol401540k |
| 10. | Carboni, S.; Da Settimo, A.; Ferrarini, P. L.; Primofiore, G.; Livi, O.; Menichetti, V.; Del Tacca, M.; Martinotti, E.; Bernardini, C.; Bertelli, A. Eur. J. Med. Chem. 1982, 17, 159. |
| 11. | Ferrarini, P. L.; Mori, C.; Primofiore, G.; Da Settimo, A.; Breschi, M. C.; Martinotti, E.; Nieri, P.; Ciucci, M. A. Eur. J. Med. Chem. 1990, 25, 489. doi:10.1016/0223-5234(90)90143-Q |
| 12. | Saccomanni, G.; Badawneh, M.; Adinolfi, B.; Calderone, V.; Cavallini, T.; Ferrarini, P. L.; Greco, R.; Manera, C.; Testai, L. Bioorg. Med. Chem. 2003, 11, 4921. doi:10.1016/j.bmc.2003.09.017 |
| 13. | Debenham, J. S.; Madsen-Duggan, C. B.; Walsh, T. F.; Wang, J.; Tong, X.; Doss, G. A.; Lao, J.; Fong, T. M.; Schaeffer, M.-T.; Xiao, J. C.; Huang, C. R.-R. C.; Shen, C.-P.; Feng, Y.; Marsh, D. J.; Stribling, D. S.; Shearman, L. P.; Strack, A. M.; MacIntyre, D. E.; Van der Ploeg, L. H. T.; Goulet, M. T. Bioorg. Med. Chem. Lett. 2006, 16, 681. doi:10.1016/j.bmcl.2005.10.028 |
| 14. | Manera, C.; Saccomanni, G.; Adinolfi, B.; Benetti, V.; Ligresti, A.; Cascio, M. G.; Tuccinardi, T.; Lucchesi, V.; Martinelli, A.; Nieri, P.; Masini, E.; Di Marzo, V.; Ferrarini, P. L. J. Med. Chem. 2009, 52, 3644. doi:10.1021/jm801563d |
| 9. | Santilli, A. A.; Scotese, A. C.; Bauer, R. F.; Bell, S. C. J. Med. Chem. 1987, 30, 2270. doi:10.1021/jm00395a015 |
| 17. | Ban, H.; Muraoka, M.; Ioriya, K.; Ohashi, N. Bioorg. Med. Chem. Lett. 2006, 16, 44. doi:10.1016/j.bmcl.2005.09.056 |
| 22. | Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Tetrahedron 2007, 63, 8286. doi:10.1016/j.tet.2007.05.112 |
| 23. | Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Synlett 2009, 92. doi:10.1055/s-0028-1087485 |
| 24. | Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Synthesis 2010, 1349. doi:10.1055/s-0029-1218665 |
| 40. | Taylor, E. C.; Macor, J. E. Tetrahedron Lett. 1986, 27, 431. doi:10.1016/S0040-4039(00)85497-0 |
| 41. | Taylor, E. C.; Macor, J. E.; Pont, J. L. Tetrahedron 1987, 43, 5145. doi:10.1016/S0040-4020(01)87690-0 |
| 42. | Haenel, F.; John, R.; Seitz, G. Arch. Pharm. 1992, 325, 349. doi:10.1002/ardp.19923250608 |
| 36. | Seitz, G.; Görge, L.; Dietrich, S. Tetrahedron Lett. 1985, 26, 4355. doi:10.1016/S0040-4039(00)98733-1 |
| 37. | Taylor, E. C.; Pont, J. L. Tetrahedron Lett. 1987, 28, 379. doi:10.1016/S0040-4039(00)95733-2 |
| 38. | Taylor, E. C.; Macor, J. E. J. Org. Chem. 1987, 52, 4280. doi:10.1021/jo00228a023 |
| 39. | Taylor, E. C.; Macor, J. E. J. Org. Chem. 1989, 54, 4984. doi:10.1021/jo00282a006 |
| 22. | Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Tetrahedron 2007, 63, 8286. doi:10.1016/j.tet.2007.05.112 |
| 23. | Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Synlett 2009, 92. doi:10.1055/s-0028-1087485 |
| 24. | Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Synthesis 2010, 1349. doi:10.1055/s-0029-1218665 |
| 22. | Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Tetrahedron 2007, 63, 8286. doi:10.1016/j.tet.2007.05.112 |
| 23. | Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Synlett 2009, 92. doi:10.1055/s-0028-1087485 |
| 24. | Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Synthesis 2010, 1349. doi:10.1055/s-0029-1218665 |
| 53. | Benson, S. C.; Lee, L.; Yang, L.; Snyder, J. K. Tetrahedron 2000, 56, 1165. doi:10.1016/S0040-4020(00)00003-X |
| 22. | Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Tetrahedron 2007, 63, 8286. doi:10.1016/j.tet.2007.05.112 |
| 23. | Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Synlett 2009, 92. doi:10.1055/s-0028-1087485 |
| 24. | Hajbi, Y.; Suzenet, F.; Khouili, M.; Lazar, S.; Guillaumet, G. Synthesis 2010, 1349. doi:10.1055/s-0029-1218665 |
| 51. | Pardo, L. M.; Tellitu, I.; Domínguez, E. Tetrahedron 2012, 68, 3692. doi:10.1016/j.tet.2012.03.033 |
| 52. | Zhou, Y.; Feng, E.; Liu, G.; Ye, D.; Li, J.; Jiang, H.; Liu, H. J. Org. Chem. 2009, 74, 7344. doi:10.1021/jo901418m |
| 43. | de la Hoz, A.; Diaz-Ortiz, A.; Moreno, A. Chem. Soc. Rev. 2005, 34, 164. doi:10.1039/b411438h |
| 44. | Loupy, A., Ed. Microwaves in Organic Synthesis; Wiley-VCH: Weinhein, 2006. |
| 45. | Kostakis, I. K.; Elomri, A.; Seguin, E.; Iannelli, M.; Besson, T. Tetrahedron Lett. 2007, 48, 6609. doi:10.1016/j.tetlet.2007.07.114 |
| 46. | Herrero, M. A.; Kremsner, J. M.; Kappe, C. O. J. Org. Chem. 2008, 73, 36. doi:10.1021/jo7022697 |
| 47. | Caddick, S.; Fitzmaurice, R. Tetrahedron 2009, 65, 3325. doi:10.1016/j.tet.2009.01.105 |
| 48. | Kappe, C. O.; Dallinger, D. Mol. Diversity 2009, 13, 71. doi:10.1007/s11030-009-9138-8 |
| 49. | Mikhailichenko, S. S.; Bouillon, J.-P.; Besson, T.; Shermolovich, Y. G. Tetrahedron Lett. 2010, 51, 990. doi:10.1016/j.tetlet.2009.12.064 |
| 34. | Taylor, E. C.; Pont, J. L.; Warner, J. C. Tetrahedron 1987, 43, 5159. doi:10.1016/S0040-4020(01)87691-2 |
| 50. | Paudler, W. W.; Chen, T.-K. J. Heterocycl. Chem. 1970, 7, 767. doi:10.1002/jhet.5570070403 |
© 2014 Fadel et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)