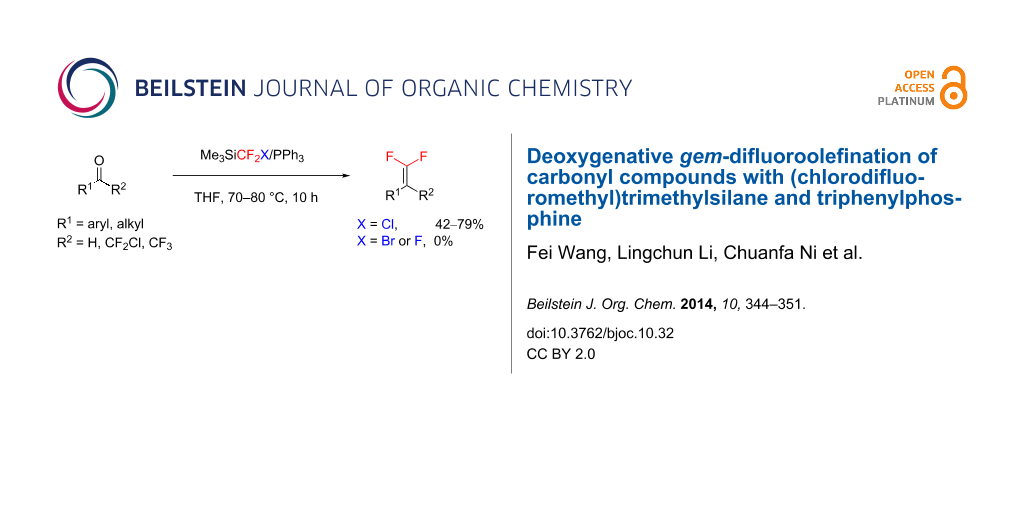
Abstract
Background: 1,1-Difluoroalkenes cannot only be used as valuable precursors for organic synthesis, but also act as bioisosteres for enzyme inhibitors. Among various methods for their preparation, the carbonyl olefination with difluoromethylene phosphonium ylide represents one of the most straightforward methods.
Results: The combination of (chlorodifluoromethyl)trimethylsilane (TMSCF2Cl) and triphenylphosphine (PPh3) can be used for the synthesis of gem-difluoroolefins from carbonyl compounds. Comparative experiments demonstrate that TMSCF2Cl is superior to (bromodifluoromethyl)trimethylsilane (TMSCF2Br) and (trifluoromethyl)trimethylsilane (TMSCF3) in this reaction.
Conclusion: Similar to many other Wittig-type gem-difluoroolefination reactions in the presence of PPh3, the reaction of TMSCF2Cl with aldehydes and activated ketones is effective.
Graphical Abstract
Introduction
The synthesis and application of selectively fluorinated organic molecules have attracted much interest from both organic chemists and biochemists because fluorine can endow these molecules with unique chemical, biological and physical properties [1-3]. 1,1-Difluoroalkenes have been frequently used in the design of potential enzyme inhibitors [4-6], since difluoromethylene functionality (CF2) is known to be isosteric and isopolar to an oxygen atom [7-9], and the gem-difluorovinyl functionality is believed to be a bioisostere for a carbonyl group [10]. More commonly, 1,1-difluoroalkenes, which are highly electrophilic towards many nucleophiles at the terminal difluoromethylene carbon [11], are used as valuable precursors of di- and trifluoromethyl compounds [10,12], monofluoroalkenes [13], monofluorinated heterocycles [14,15], carboxylic acids and esters [16]. Consequently, these relevant applications of 1,1-difluoroalkenes have led to many efforts to develop gem-difluoroolefination methods including β-elimination of functionalized difluoromethyl compounds, transition metal catalysed coupling reactions with gem-difluorovinylation reagents, and deoxygenative gem-difluoroolefination of carbonyl compounds [17,18]. Among these methods, the latter one has been studied with several named reactions, for example Wittig, Horner–Wadsworth–Emmons, and Julia–Kocienski reactions.
In the Wittig gem-difluoroolefination, the reaction is believed to proceed via an undetected difluoromethylene phosphonium ylide, which can be generated in situ either by the transformation of a difluorinated phosphonium salt or by the reaction between difluorocarbene (:CF2) and a phosphine (Scheme 1) [19-26]. In 1964, Fuqua and co-workers first reported the difluoromethylenation of aldehydes by using ClCF2CO2Na/PPh3 [19]. In 1967, Burton and Herkes suggested that the ylide intermediate involved in the olefination process was more likely to be formed by the decarboxylation of a difluorinated phosphonium salt rather than the combination of :CF2 and a phosphine (Scheme 1, reaction 1) [20]. Their suggestion is based on the accelerating effect of PPh3 on the thermal decomposition of ClCF2CO2Na and the unsuccessful capture of :CF2 with an alkene or alcohol during the olefination reaction [20]. Very recently, the successful preparation of (triphenylphosphonio)difluoroacetate (Ph3P+CF2CO2−) and its application in carbonyl gem-difluoroolefination by Xiao and co-workers [21] finally confirmed the mechanism proposed by Burton and others [19,20]. Burton and co-workers also developed another difluorocarbene-free approach using a 1:2 mixture of CF2Br2 and PPh3 or P(NMe2)3 to prepare the ylide intermediate (Scheme 1, reaction 2) [22,23]. Although the difluorocarbene/phosphine procedure for Wittig olefination has been put forward by Fuqua et al. as early as 1964 [19], the formation of difluoromethylene phosphonium ylide in such a way is quite rare [24-26]. Established examples include using bis(trifluoromethyl)mercury (Hg(CF3)2) under the promotion of NaI (Scheme 1, reaction 3) [24] and using methyl 2,2-difluoro-2-(fluorosulfonyl)acetate (MDFA) under the promotion of KI (Scheme 1, reaction 4) [25].
Scheme 1: Various procedures for the generation of difluoromethylene phosphonium ylide [19-25].
Scheme 1: Various procedures for the generation of difluoromethylene phosphonium ylide [19-25].
Our group has focused on the development and application of new difluorocarbene reagents [27-34]. The Prakash group and we have identified that (halodifluoromethyl)trimethylsilanes (TMSCF2X, X = F, Cl, and Br) could serve as difluorocarbene sources under the activation of proper halide initiators or alkaline bases (Scheme 2) [31-34]. Recently, we have developed a relatively environmentally benign method to prepare TMSCF2Br, which can be used as a general carbene source for the difluoromethylenation of alkynes and alkenes and difluoromethylation of heteroatom nucleophiles [34]. In this paper, the novel preparation of TMSCF2Cl from TMSCF2Br and the application of the former in deoxygenative gem-difluoroolefination of carbonyl compounds via Wittig-type reaction are reported.
Scheme 2: Difluoromethylenation of alkenes and alkynes and difluoromethylation of heteroatom nucleophiles with TMSCF2X [31-34].
Scheme 2: Difluoromethylenation of alkenes and alkynes and difluoromethylation of heteroatom nucleophiles wit...
Results and Discussion
(Halodifluoromethyl)trimethylsilanes including TMSCF3 (Ruppert–Prakash reagent), TMSCF2Cl, and TMSCF2Br are initially prepared by reductive silylation of ozone-depleting-substances bromotrifluoromethane (CF3Br) [35], bromochlorodifluoromethane (CF2BrCl) [36,37], and dibromodifluoromethane (CF2Br2) [36,37] with chlorotrimethylsilane (TMSCl). In recent years, Prakash and co-workers have discovered two Freon-free methods for the synthesis of TMSCF3 from fluoroform (CF3H), which paved the way for the synthetic applications of TMSCF3 [38,39]. Moreover, the preparation of TMSCF2Br either by fluoro–bromo exchange reaction of TMSCF3 [34] or by bromination of TMSCF2H [34,40] has also been disclosed. To obtain TMSCF2Cl, we tried the halogen exchange reaction of TMSCF2Br. When a 1:10 mixture of TMSCF2Br and TMSCl was heated in neat in the presence of 5 mol % of tetrabutylammonium chloride (TBAC) for 2 hours, 19F NMR spectroscopy analysis showed that the ratio of TMSCF2Cl to TMSCF2Br was 2.3:1, and prolonging reaction time could not improve the ratio. In view of the difficulty in separating TMSCF2Cl from the reaction mixture because of the approximate boiling points of TMSCF2Cl (~85 °C) [36,37] and TMSCF2Br (~105 °C) [36,37], other chloride sources were tried to achieve a full conversion of TMSCF2Br. Gratifyingly, when the reaction was performed in benzonitrile (bp ~190 °C) at 80 °C using a slight excess of silver chloride under the catalysis of TBAC, a full conversion of TMSCF2Br afforded TMSCF2Cl in 54% yield. Lowering the temperature to room temperature (rt) could improve the yield to 80% (Scheme 3). It is believed that the lower solubility of silver bromide than silver chloride in benzonitrile provides the driving force for this bromo–chloro exchange reaction.
Scheme 3: Bromo–chloro exchange reaction using AgCl.
Scheme 3: Bromo–chloro exchange reaction using AgCl.
At first, the olefination of 1-naphthaldehyde (1a) or benzaldehyde (1b) by using the combination of TMSCF2Cl and PPh3 was tried. Conceiving that the chloride ion might be necessary to promote the decomposition of TMSCF2Cl to release CF2 as reported, a catalytic amount of TBAC was used as the initiator. After heating a reaction mixture of aldehyde 1a, TMSCF2Cl, PPh3, and TBAC in THF at 100 °C for 8 h, 19F NMR spectroscopy analysis showed that difluorinated alkene 2a was formed in 69% yield (Table 1, entry 1). Surprisingly, it was found that in the absence of TBAC, PPh3 could be used both to promote the fragmentation of TMSCF2Cl and combine with the generated :CF2 (Table 1, entry 2). A rough comparison of the reaction temperatures showed that a lower temperature (rt) is detrimental to the olefination process, although the decomposition of TMSCF2Cl could occur to some extent (Table 1, entries 2 and 3).
Table 1: Condition screening of gem-difluoroolefination with TMSCF2X.
|
|
|||||||
| Entrya | Ar | X | Initiator | Temp (°C) | t (h) | Conversion (%)b | Yield (%)b |
|---|---|---|---|---|---|---|---|
| 1 | 1-naphthyl | Cl | TBAC (3 mol %) | 100 | 8 | 100 | 69 |
| 2 | 1-naphthyl | Cl | none | 70 | 10 | 100 | 59c |
| 3 | Ph | Cl | none | rt | 4 | 35 | 0 |
| 4 | Ph | Br | none | 70 | 10 | 100 | 0 |
| 5 | Ph | F | NaI (0.6 equiv) | 70 | 10 | <5 | 0 |
| 6 | Ph | F | NaI (6.0 equiv) | 110 | 10 | <5 | 0 |
aReactions were performed on 0.5 mmol scale in a pressure tube. bConversion of TMSCF2X and yields of 2 were determined by 19F NMR spectroscopy using PhCF3 as an internal standard. cIsolated yield of 2a.
Subsequently, the olefination of aldehyde 1b with TMSCF2Br was examined. Unfortunately, the full consumption of TMSCF2Br did not afford any difluoroolefin 2b (Table 1, entry 4). As determined by 19F NMR, besides the side product (difluoromethyl)triphenylphosponium bromide (δ −127.9, dd, 3JP-F = 80 Hz, 2JF-H = 47 Hz) as reported in the Wittig olefination with FSO2CF2CO2Me [25], a new product which was assigned as difluorinated phosphonium salt 4 (δ −88.8, ddd, 2JF-F = 298 Hz, 3JP-F = 97 Hz, 3JF-H = 3.3 Hz, 1F; δ −106.6, ddd, 2JF-F = 298 Hz, 3JP-F = 101 Hz, 3JF-H = 24 Hz, 1F) was detected as the major product (for details, see Supporting Information File 1). The formation of 4 is supposed to arise from a ready silylation of the addition intermediate betaine 3 by TMSBr. When TMSCF2Cl was used, TMSCl is not reactive enough to trap the betaine 3, thus the oxaphosphetane 5 could be formed to give olefins and triphenylphosphine oxide (Scheme 4).
Scheme 4: Proposed different reaction pathways of the difluorinated ylide in the presence of TMSCl and TMSBr.
Scheme 4: Proposed different reaction pathways of the difluorinated ylide in the presence of TMSCl and TMSBr.
Finally, the olefination of aldehyde 1b with TMSCF3 as the difluoromethylene source was tested. The results showed that no desired reaction took place when PPh3 and either substoichiometric or stoichiometric amounts of NaI were used (Table 1, entries 5 and 6). Although it has been known that TMSCF3 can be used in the difluoromethylenation of alkenes and alkynes initiated by NaI [33], we could not give a reasonable explanation for the failure of the current reaction.
Using the conditions shown in Table 1, entry 2 as standard, the olefination of aldehydes with TMSCF2Cl was investigated. As shown in Figure 1, a variety of structurally diverse aromatic aldehydes were successfully converted into gem-difluoroalkenes 2a–g in moderate to good yields. It should be mentioned that the aromatic aldehydes with substituents such as t-butylthio, methoxy, and bromo groups on the phenyl ring showed similar reactivity. Moreover, this approach is also amenable to enolizable aldehydes, for example, gem-difluoroolefin 2h could be obtained in 47% yield. Although a non-activated ketone such as acetophenone is unreactive under similar conditions, activated ketones could undergo this Wittig olefination reaction. Representative results for the olefination at a slightly elevated temperature (80 °C) are shown in Figure 2. A range of aryl trifluoromethyl (6a–d) and chlorodifluoromethyl aromatic ketones (6e–g) were readily difluoromethylenated to give the corresponding olefins (7a–g) in moderate to good yields. It should be mentioned that in all cases, the formation of gem-difluoroolefins was accompanied by the formation of Ph3PF2 (δ −41.2, d, 1JP-F = 668 Hz) [25], HCF2Cl, fluorotrimethylsilane, and some unidentified byproducts in variable yields (for details, see Supporting Information File 1).
Figure 1: gem-Difluoroolefination of aldehydes. Reactions were performed on 0.5 mmol scale in a pressure tube. aIsolated yield. bYield was determined by 19F NMR spectroscopy using PhCF3 as an internal standard.
Figure 1: gem-Difluoroolefination of aldehydes. Reactions were performed on 0.5 mmol scale in a pressure tube...
Figure 2: gem-Difluoroolefination of activated ketones. Reactions were performed on 0.5 mmol scale in a pressure tube. aYield was determined by 19F NMR spectroscopy using PhCF3 as an internal standard. bIsolated yield.
Figure 2: gem-Difluoroolefination of activated ketones. Reactions were performed on 0.5 mmol scale in a press...
As previously reported, the key mechanistic issue of this Wittig-type reaction is the formation of the presumed difluoromethylene triphenylphosphonium ylide [19-25]. Initially it was speculated that it were trace amounts of nucleophilic impurities (such as chloride ions) that initiated the fragmentation of TMSCF2Cl to release :CF2 [31], which combined with PPh3 to form the ylide. However, the experiment at room temperature showed that PPh3 could significantly accelerate the decomposition of TMSCF2Cl, which indicated that PPh3 should have participated in the activation of TMSCF2Cl. Consequently, two plausible mechanisms are proposed (Scheme 5): one is the initial activation of the C–Si bond by PPh3 (Path A), the other is the initial activation of the C–Cl bond by PPh3 (Path B). In Path A, PPh3 firstly coordinates the silicon atom of TMSCF2Cl to form activated penta-coordinated silicon species 8 [41] and activates both the C–Si and the C–Cl bond. Next, the release of CF2 leads to silylphosphonium salt 9. Finally, the fragmentation of 9 occurs to give TMSCl with regeneration of PPh3; meanwhile, the trapping of :CF2 by PPh3 gives the ylide. In Path B, a phosphonium salt 10, which is formed via a single-electron transfer (SET) mechanism, undergoes a chloride ion-promoted desilylation reaction to afford Ph3P=CF2 [42,43]. However, we could not rule out the possibility of chloride ion-activation in these processes due to the involvement of intermediates 9 and 10 in the proposed mechanisms.
Scheme 5: Plausible mechanisms for the formation of difluoromethylene triphenylphosphonium ylide from TMSCF2Cl and PPh3.
Scheme 5: Plausible mechanisms for the formation of difluoromethylene triphenylphosphonium ylide from TMSCF2C...
Conclusion
In conclusion, a robust difluoromethylenation reagent (chlorodifluoromethyl)trimethylsilane (TMSCF2Cl) has been prepared via a relatively environmentally benign method and has been successfully used in the Wittig difluoroolefination. Similar as many other Wittig-type gem-difluoroolefination reactions in the presence of PPh3, the reaction of TMSCF2Cl with aldehydes and activated ketones is effective. Comparative reactions with TMSCF2Br and TMSCF3 under similar conditions failed to give the gem-difluorinated olefins, which indicate that the halo-substituent of TMSCF2X can influence the reactivity of these fluorinated silanes in difluoromethylene transfer reactions. Further research on the synthetic application of TMSCF2X (X = F, Cl, and Br) is currently underway.
Supporting Information
Full experimental details (difluoromethylation of O, S, and N-nucleophiles and gem-difluoroolefination of carbonyl compounds with TMSCF2Cl) and compound characterization data are given.
| Supporting Information File 1: Experimental procedures and characterization data. | ||
| Format: PDF | Size: 609.7 KB | Download |
References
-
Chambers, R. D. Fluorine in Organic Chemistry; Blackwell: Oxford, 2004.
Return to citation in text: [1] -
Bégué, J.-P.; Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine; John Wiley and Sons: Hoboken, 2008.
Return to citation in text: [1] -
Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, 2nd ed.; Wiley-VCH: Weinheim, 2013.
Return to citation in text: [1] -
McDonald, I. A.; Lacoste, J. M.; Bey, P.; Palfreyman, M. G.; Zreika, M. J. Med. Chem. 1985, 28, 186–193. doi:10.1021/jm00380a007
Return to citation in text: [1] -
Altenburger, J.-M.; Lassalle, G. Y.; Matrougui, M.; Galtier, D.; Jetha, J.-C.; Bocskei, Z.; Berry, C. N.; Lunven, C.; Lorrain, J.; Herault, J.-P.; Schaeffer, P.; O’Connor, S. E.; Herbert, J.-M. Bioorg. Med. Chem. 2004, 12, 1713–1730. doi:10.1016/j.bmc.2004.01.016
Return to citation in text: [1] -
Weintraub, P. M.; Holland, A. K.; Gates, C. A.; Moore, W. R.; Resvick, R. J.; Bey, P.; Peet, N. P. Bioorg. Med. Chem. 2003, 11, 427–431. doi:10.1016/S0968-0896(02)00434-0
Return to citation in text: [1] -
Blackburn, G. M.; England, D. A.; Kolkmann, F. J. Chem. Soc., Chem. Commun. 1981, 930–932. doi:10.1039/C39810000930
Return to citation in text: [1] -
Lapierre, J.; Ahmed, V.; Chen, M.-J.; Ispahany, M.; Guillemette, J. G.; Taylor, S. D. Bioorg. Med. Chem. Lett. 2004, 14, 151–155. doi:10.1016/j.bmcl.2003.09.089
Return to citation in text: [1] -
Navidpour, L.; Lu, W.; Taylor, S. D. Org. Lett. 2006, 8, 5617–5620. doi:10.1021/ol062357z
Return to citation in text: [1] -
Motherwell, W. B.; Tozer, M. J.; Ross, B. C. J. Chem. Soc., Chem. Commun. 1989, 1437–1439. doi:10.1039/C39890001437
Return to citation in text: [1] [2] -
Chambers, R. D.; Vaughan, J. F. S. Top. Curr. Chem. 1997, 192, 1–38. doi:10.1007/BFb0119264
Return to citation in text: [1] -
Nguyen, B. V.; Burton, D. J. J. Org. Chem. 1997, 62, 7758–7764. doi:10.1021/jo971019w
Return to citation in text: [1] -
Hayashi, S.-i.; Nakai, T.; Ichikawa, N.; Burton, D. J.; Naae, D. G.; Kesling, H. S. Chem. Lett. 1979, 8, 983–986. doi:10.1246/cl.1979.983
Return to citation in text: [1] -
Ichikawa, J.; Wada, Y.; Okauchi, T.; Minami, T. Chem. Commun. 1997, 1537–1538. doi:10.1039/A703110F
Return to citation in text: [1] -
Yokota, M.; Fujita, D.; Ichikawa, J. Org. Lett. 2007, 9, 4639–4642. doi:10.1021/ol702279w
Return to citation in text: [1] -
Hayashi, S.-i.; Nakai, T.; Ishikawa, N. Chem. Lett. 1980, 9, 651–654. doi:10.1246/cl.1980.651
Return to citation in text: [1] -
Chelucci, G. Chem. Rev. 2012, 112, 1344–1462. doi:10.1021/cr200165q
Return to citation in text: [1] -
Liu, Y.; Deng, M.; Zhang, Z.; Ding, X.; Dai, Z.; Guan, J. Chin. J. Org. Chem. 2012, 32, 661–666. doi:10.6023/cjoc1104113
Return to citation in text: [1] -
Fuqua, S. A.; Duncan, W. G.; Silverstein, R. M. Tetrahedron Lett. 1964, 5, 1461–1463. doi:10.1016/S0040-4039(01)89512-5
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Herkes, F. E.; Burton, D. J. J. Org. Chem. 1967, 32, 1311–1318. doi:10.1021/jo01280a007
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Zheng, J.; Cai, J.; Lin, J.-H.; Guo, Y.; Xiao, J.-C. Chem. Commun. 2013, 49, 7513–7515. doi:10.1039/C3CC44271C
Return to citation in text: [1] [2] [3] [4] -
Naae, D. G.; Burton, D. J. J. Fluorine Chem. 1971, 1, 123–125. doi:10.1016/S0022-1139(00)82541-5
Return to citation in text: [1] [2] [3] [4] -
Naae, D. G.; Burton, D. J. Synth. Commun. 1973, 3, 197–200. doi:10.1080/00397917308062035
Return to citation in text: [1] [2] [3] [4] -
Nowak, I.; Robins, J. M. Org. Lett. 2005, 7, 721–724. doi:10.1021/ol047416s
Return to citation in text: [1] [2] [3] [4] [5] -
Thomoson, C. S.; Martinez, H.; Dolbier, W. R., Jr. J. Fluorine Chem. 2013, 150, 53–59. doi:10.1016/j.jfluchem.2013.02.026
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] -
Zheng, J.; Lin, J.-H.; Cai, J.; Xiao, J.-C. Chem.–Eur. J. 2013, 19, 15261–15266. doi:10.1002/chem.201303248
This article appeared online after our submission of this article. It is a paper describing the deoxygenative gem-difluoroolefination of carbonyl compounds with the difluorocarbene/phosphine procedures, i.e. HCF2Cl/propylene epoxide/n-Bu4NCl (cat.)/Ph3P.; FSO2CF2CO2TMS/NaF (cat.)/Ph3P.
Return to citation in text: [1] [2] -
Zhang, L.; Zheng, J.; Hu, J. J. Org. Chem. 2006, 71, 9845–9848. doi:10.1021/jo061799l
Return to citation in text: [1] -
Zheng, J.; Li, Y.; Zhang, L.; Hu, J.; Meuzelaar, G. J.; Federsel, H.-J. Chem. Commun. 2007, 5149–5151. doi:10.1039/B713156A
Return to citation in text: [1] -
Zhang, W.; Wang, F.; Hu, J. Org. Lett. 2009, 11, 2109–2112. doi:10.1021/ol900567c
Return to citation in text: [1] -
Wang, F.; Huang, W.; Hu, J. Chin. J. Chem. 2011, 29, 2717–2721. doi:10.1002/cjoc.201100325
Return to citation in text: [1] -
Wang, F.; Zhang, W.; Zhu, J.; Li, H.; Huang, K.-W.; Hu, J. Chem. Commun. 2011, 47, 2411–2413. doi:10.1039/c0cc04548a
Return to citation in text: [1] [2] [3] [4] -
For preliminary results on the difluoromethylation of O, S, and N-nucleophiles with TMSCF2Cl under aqueous basic conditions, see Supporting Information File 1.
Return to citation in text: [1] [2] [3] -
Wang, F.; Luo, T.; Hu, J.; Wang, Y.; Krishnan, H. S.; Jog, P. V.; Ganesh, S. K.; Prakash, G. K. S.; Olah, G. A. Angew. Chem., Int. Ed. 2011, 50, 7153–7157. doi:10.1002/anie.201101691
Return to citation in text: [1] [2] [3] [4] -
Li, L.; Wang, F.; Ni, C.; Hu, J. Angew. Chem., Int. Ed. 2013, 52, 12390–12394. doi:10.1002/anie.201306703
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Ruppert, I.; Schlich, K.; Volbach, W. Tetrahedron Lett. 1984, 25, 2195–2198. doi:10.1016/S0040-4039(01)80208-2
Return to citation in text: [1] -
Broicher, V.; Geffken, D. J. Organomet. Chem. 1990, 381, 315–320. doi:10.1016/0022-328X(90)80061-4
Return to citation in text: [1] [2] [3] [4] -
Yudin, A. K.; Prakash, G. K. S.; Deffieux, D.; Bradley, M.; Bau, R.; Olah, G. A. J. Am. Chem. Soc. 1997, 119, 1572–1581. doi:10.1021/ja962990n
Return to citation in text: [1] [2] [3] [4] -
Prakash, G. K. S.; Hu, J.; Olah, G. A. J. Org. Chem. 2003, 68, 4457–4463. doi:10.1021/jo030110z
Return to citation in text: [1] -
Prakash, G. K. S.; Jog, P. V.; Batamack, P. T. D.; Olah, G. A. Science 2012, 338, 1324–1327. doi:10.1126/science.1227859
Return to citation in text: [1] -
Kosobokov, M. D.; Dilman, A. D.; Levin, V. V.; Struchkova, M. I. J. Org. Chem. 2012, 77, 5850–5855. doi:10.1021/jo301094b
Return to citation in text: [1] -
Matsukawa, S.; Saijo, M. Tetrahedron Lett. 2008, 49, 4655–4657. doi:10.1016/j.tetlet.2008.05.053
Return to citation in text: [1] -
Miller, N. E. J. Am. Chem. Soc. 1965, 87, 390–391. doi:10.1021/ja01080a049
Return to citation in text: [1] -
McNulty, J.; Das, P. Chem.–Eur. J. 2008, 14, 8469–8472. doi:10.1002/chem.200801358
Return to citation in text: [1]
| 36. | Broicher, V.; Geffken, D. J. Organomet. Chem. 1990, 381, 315–320. doi:10.1016/0022-328X(90)80061-4 |
| 37. | Yudin, A. K.; Prakash, G. K. S.; Deffieux, D.; Bradley, M.; Bau, R.; Olah, G. A. J. Am. Chem. Soc. 1997, 119, 1572–1581. doi:10.1021/ja962990n |
| 36. | Broicher, V.; Geffken, D. J. Organomet. Chem. 1990, 381, 315–320. doi:10.1016/0022-328X(90)80061-4 |
| 37. | Yudin, A. K.; Prakash, G. K. S.; Deffieux, D.; Bradley, M.; Bau, R.; Olah, G. A. J. Am. Chem. Soc. 1997, 119, 1572–1581. doi:10.1021/ja962990n |
| 38. | Prakash, G. K. S.; Hu, J.; Olah, G. A. J. Org. Chem. 2003, 68, 4457–4463. doi:10.1021/jo030110z |
| 39. | Prakash, G. K. S.; Jog, P. V.; Batamack, P. T. D.; Olah, G. A. Science 2012, 338, 1324–1327. doi:10.1126/science.1227859 |
| 1. | Chambers, R. D. Fluorine in Organic Chemistry; Blackwell: Oxford, 2004. |
| 2. | Bégué, J.-P.; Bonnet-Delpon, D. Bioorganic and Medicinal Chemistry of Fluorine; John Wiley and Sons: Hoboken, 2008. |
| 3. | Kirsch, P. Modern Fluoroorganic Chemistry: Synthesis, Reactivity, Applications, 2nd ed.; Wiley-VCH: Weinheim, 2013. |
| 11. | Chambers, R. D.; Vaughan, J. F. S. Top. Curr. Chem. 1997, 192, 1–38. doi:10.1007/BFb0119264 |
| 21. | Zheng, J.; Cai, J.; Lin, J.-H.; Guo, Y.; Xiao, J.-C. Chem. Commun. 2013, 49, 7513–7515. doi:10.1039/C3CC44271C |
| 25. | Thomoson, C. S.; Martinez, H.; Dolbier, W. R., Jr. J. Fluorine Chem. 2013, 150, 53–59. doi:10.1016/j.jfluchem.2013.02.026 |
| 10. | Motherwell, W. B.; Tozer, M. J.; Ross, B. C. J. Chem. Soc., Chem. Commun. 1989, 1437–1439. doi:10.1039/C39890001437 |
| 19. | Fuqua, S. A.; Duncan, W. G.; Silverstein, R. M. Tetrahedron Lett. 1964, 5, 1461–1463. doi:10.1016/S0040-4039(01)89512-5 |
| 20. | Herkes, F. E.; Burton, D. J. J. Org. Chem. 1967, 32, 1311–1318. doi:10.1021/jo01280a007 |
| 19. | Fuqua, S. A.; Duncan, W. G.; Silverstein, R. M. Tetrahedron Lett. 1964, 5, 1461–1463. doi:10.1016/S0040-4039(01)89512-5 |
| 20. | Herkes, F. E.; Burton, D. J. J. Org. Chem. 1967, 32, 1311–1318. doi:10.1021/jo01280a007 |
| 21. | Zheng, J.; Cai, J.; Lin, J.-H.; Guo, Y.; Xiao, J.-C. Chem. Commun. 2013, 49, 7513–7515. doi:10.1039/C3CC44271C |
| 22. | Naae, D. G.; Burton, D. J. J. Fluorine Chem. 1971, 1, 123–125. doi:10.1016/S0022-1139(00)82541-5 |
| 23. | Naae, D. G.; Burton, D. J. Synth. Commun. 1973, 3, 197–200. doi:10.1080/00397917308062035 |
| 24. | Nowak, I.; Robins, J. M. Org. Lett. 2005, 7, 721–724. doi:10.1021/ol047416s |
| 25. | Thomoson, C. S.; Martinez, H.; Dolbier, W. R., Jr. J. Fluorine Chem. 2013, 150, 53–59. doi:10.1016/j.jfluchem.2013.02.026 |
| 7. | Blackburn, G. M.; England, D. A.; Kolkmann, F. J. Chem. Soc., Chem. Commun. 1981, 930–932. doi:10.1039/C39810000930 |
| 8. | Lapierre, J.; Ahmed, V.; Chen, M.-J.; Ispahany, M.; Guillemette, J. G.; Taylor, S. D. Bioorg. Med. Chem. Lett. 2004, 14, 151–155. doi:10.1016/j.bmcl.2003.09.089 |
| 9. | Navidpour, L.; Lu, W.; Taylor, S. D. Org. Lett. 2006, 8, 5617–5620. doi:10.1021/ol062357z |
| 20. | Herkes, F. E.; Burton, D. J. J. Org. Chem. 1967, 32, 1311–1318. doi:10.1021/jo01280a007 |
| 25. | Thomoson, C. S.; Martinez, H.; Dolbier, W. R., Jr. J. Fluorine Chem. 2013, 150, 53–59. doi:10.1016/j.jfluchem.2013.02.026 |
| 4. | McDonald, I. A.; Lacoste, J. M.; Bey, P.; Palfreyman, M. G.; Zreika, M. J. Med. Chem. 1985, 28, 186–193. doi:10.1021/jm00380a007 |
| 5. | Altenburger, J.-M.; Lassalle, G. Y.; Matrougui, M.; Galtier, D.; Jetha, J.-C.; Bocskei, Z.; Berry, C. N.; Lunven, C.; Lorrain, J.; Herault, J.-P.; Schaeffer, P.; O’Connor, S. E.; Herbert, J.-M. Bioorg. Med. Chem. 2004, 12, 1713–1730. doi:10.1016/j.bmc.2004.01.016 |
| 6. | Weintraub, P. M.; Holland, A. K.; Gates, C. A.; Moore, W. R.; Resvick, R. J.; Bey, P.; Peet, N. P. Bioorg. Med. Chem. 2003, 11, 427–431. doi:10.1016/S0968-0896(02)00434-0 |
| 20. | Herkes, F. E.; Burton, D. J. J. Org. Chem. 1967, 32, 1311–1318. doi:10.1021/jo01280a007 |
| 33. | Wang, F.; Luo, T.; Hu, J.; Wang, Y.; Krishnan, H. S.; Jog, P. V.; Ganesh, S. K.; Prakash, G. K. S.; Olah, G. A. Angew. Chem., Int. Ed. 2011, 50, 7153–7157. doi:10.1002/anie.201101691 |
| 16. | Hayashi, S.-i.; Nakai, T.; Ishikawa, N. Chem. Lett. 1980, 9, 651–654. doi:10.1246/cl.1980.651 |
| 19. | Fuqua, S. A.; Duncan, W. G.; Silverstein, R. M. Tetrahedron Lett. 1964, 5, 1461–1463. doi:10.1016/S0040-4039(01)89512-5 |
| 20. | Herkes, F. E.; Burton, D. J. J. Org. Chem. 1967, 32, 1311–1318. doi:10.1021/jo01280a007 |
| 21. | Zheng, J.; Cai, J.; Lin, J.-H.; Guo, Y.; Xiao, J.-C. Chem. Commun. 2013, 49, 7513–7515. doi:10.1039/C3CC44271C |
| 22. | Naae, D. G.; Burton, D. J. J. Fluorine Chem. 1971, 1, 123–125. doi:10.1016/S0022-1139(00)82541-5 |
| 23. | Naae, D. G.; Burton, D. J. Synth. Commun. 1973, 3, 197–200. doi:10.1080/00397917308062035 |
| 24. | Nowak, I.; Robins, J. M. Org. Lett. 2005, 7, 721–724. doi:10.1021/ol047416s |
| 25. | Thomoson, C. S.; Martinez, H.; Dolbier, W. R., Jr. J. Fluorine Chem. 2013, 150, 53–59. doi:10.1016/j.jfluchem.2013.02.026 |
| 26. |
Zheng, J.; Lin, J.-H.; Cai, J.; Xiao, J.-C. Chem.–Eur. J. 2013, 19, 15261–15266. doi:10.1002/chem.201303248
This article appeared online after our submission of this article. It is a paper describing the deoxygenative gem-difluoroolefination of carbonyl compounds with the difluorocarbene/phosphine procedures, i.e. HCF2Cl/propylene epoxide/n-Bu4NCl (cat.)/Ph3P.; FSO2CF2CO2TMS/NaF (cat.)/Ph3P. |
| 36. | Broicher, V.; Geffken, D. J. Organomet. Chem. 1990, 381, 315–320. doi:10.1016/0022-328X(90)80061-4 |
| 37. | Yudin, A. K.; Prakash, G. K. S.; Deffieux, D.; Bradley, M.; Bau, R.; Olah, G. A. J. Am. Chem. Soc. 1997, 119, 1572–1581. doi:10.1021/ja962990n |
| 14. | Ichikawa, J.; Wada, Y.; Okauchi, T.; Minami, T. Chem. Commun. 1997, 1537–1538. doi:10.1039/A703110F |
| 15. | Yokota, M.; Fujita, D.; Ichikawa, J. Org. Lett. 2007, 9, 4639–4642. doi:10.1021/ol702279w |
| 19. | Fuqua, S. A.; Duncan, W. G.; Silverstein, R. M. Tetrahedron Lett. 1964, 5, 1461–1463. doi:10.1016/S0040-4039(01)89512-5 |
| 36. | Broicher, V.; Geffken, D. J. Organomet. Chem. 1990, 381, 315–320. doi:10.1016/0022-328X(90)80061-4 |
| 37. | Yudin, A. K.; Prakash, G. K. S.; Deffieux, D.; Bradley, M.; Bau, R.; Olah, G. A. J. Am. Chem. Soc. 1997, 119, 1572–1581. doi:10.1021/ja962990n |
| 13. | Hayashi, S.-i.; Nakai, T.; Ichikawa, N.; Burton, D. J.; Naae, D. G.; Kesling, H. S. Chem. Lett. 1979, 8, 983–986. doi:10.1246/cl.1979.983 |
| 34. | Li, L.; Wang, F.; Ni, C.; Hu, J. Angew. Chem., Int. Ed. 2013, 52, 12390–12394. doi:10.1002/anie.201306703 |
| 10. | Motherwell, W. B.; Tozer, M. J.; Ross, B. C. J. Chem. Soc., Chem. Commun. 1989, 1437–1439. doi:10.1039/C39890001437 |
| 12. | Nguyen, B. V.; Burton, D. J. J. Org. Chem. 1997, 62, 7758–7764. doi:10.1021/jo971019w |
| 17. | Chelucci, G. Chem. Rev. 2012, 112, 1344–1462. doi:10.1021/cr200165q |
| 18. | Liu, Y.; Deng, M.; Zhang, Z.; Ding, X.; Dai, Z.; Guan, J. Chin. J. Org. Chem. 2012, 32, 661–666. doi:10.6023/cjoc1104113 |
| 34. | Li, L.; Wang, F.; Ni, C.; Hu, J. Angew. Chem., Int. Ed. 2013, 52, 12390–12394. doi:10.1002/anie.201306703 |
| 40. | Kosobokov, M. D.; Dilman, A. D.; Levin, V. V.; Struchkova, M. I. J. Org. Chem. 2012, 77, 5850–5855. doi:10.1021/jo301094b |
| 24. | Nowak, I.; Robins, J. M. Org. Lett. 2005, 7, 721–724. doi:10.1021/ol047416s |
| 25. | Thomoson, C. S.; Martinez, H.; Dolbier, W. R., Jr. J. Fluorine Chem. 2013, 150, 53–59. doi:10.1016/j.jfluchem.2013.02.026 |
| 26. |
Zheng, J.; Lin, J.-H.; Cai, J.; Xiao, J.-C. Chem.–Eur. J. 2013, 19, 15261–15266. doi:10.1002/chem.201303248
This article appeared online after our submission of this article. It is a paper describing the deoxygenative gem-difluoroolefination of carbonyl compounds with the difluorocarbene/phosphine procedures, i.e. HCF2Cl/propylene epoxide/n-Bu4NCl (cat.)/Ph3P.; FSO2CF2CO2TMS/NaF (cat.)/Ph3P. |
| 22. | Naae, D. G.; Burton, D. J. J. Fluorine Chem. 1971, 1, 123–125. doi:10.1016/S0022-1139(00)82541-5 |
| 23. | Naae, D. G.; Burton, D. J. Synth. Commun. 1973, 3, 197–200. doi:10.1080/00397917308062035 |
| 31. | Wang, F.; Zhang, W.; Zhu, J.; Li, H.; Huang, K.-W.; Hu, J. Chem. Commun. 2011, 47, 2411–2413. doi:10.1039/c0cc04548a |
| 19. | Fuqua, S. A.; Duncan, W. G.; Silverstein, R. M. Tetrahedron Lett. 1964, 5, 1461–1463. doi:10.1016/S0040-4039(01)89512-5 |
| 41. | Matsukawa, S.; Saijo, M. Tetrahedron Lett. 2008, 49, 4655–4657. doi:10.1016/j.tetlet.2008.05.053 |
| 42. | Miller, N. E. J. Am. Chem. Soc. 1965, 87, 390–391. doi:10.1021/ja01080a049 |
| 43. | McNulty, J.; Das, P. Chem.–Eur. J. 2008, 14, 8469–8472. doi:10.1002/chem.200801358 |
| 31. | Wang, F.; Zhang, W.; Zhu, J.; Li, H.; Huang, K.-W.; Hu, J. Chem. Commun. 2011, 47, 2411–2413. doi:10.1039/c0cc04548a |
| 32. | For preliminary results on the difluoromethylation of O, S, and N-nucleophiles with TMSCF2Cl under aqueous basic conditions, see Supporting Information File 1. |
| 33. | Wang, F.; Luo, T.; Hu, J.; Wang, Y.; Krishnan, H. S.; Jog, P. V.; Ganesh, S. K.; Prakash, G. K. S.; Olah, G. A. Angew. Chem., Int. Ed. 2011, 50, 7153–7157. doi:10.1002/anie.201101691 |
| 34. | Li, L.; Wang, F.; Ni, C.; Hu, J. Angew. Chem., Int. Ed. 2013, 52, 12390–12394. doi:10.1002/anie.201306703 |
| 35. | Ruppert, I.; Schlich, K.; Volbach, W. Tetrahedron Lett. 1984, 25, 2195–2198. doi:10.1016/S0040-4039(01)80208-2 |
| 31. | Wang, F.; Zhang, W.; Zhu, J.; Li, H.; Huang, K.-W.; Hu, J. Chem. Commun. 2011, 47, 2411–2413. doi:10.1039/c0cc04548a |
| 32. | For preliminary results on the difluoromethylation of O, S, and N-nucleophiles with TMSCF2Cl under aqueous basic conditions, see Supporting Information File 1. |
| 33. | Wang, F.; Luo, T.; Hu, J.; Wang, Y.; Krishnan, H. S.; Jog, P. V.; Ganesh, S. K.; Prakash, G. K. S.; Olah, G. A. Angew. Chem., Int. Ed. 2011, 50, 7153–7157. doi:10.1002/anie.201101691 |
| 34. | Li, L.; Wang, F.; Ni, C.; Hu, J. Angew. Chem., Int. Ed. 2013, 52, 12390–12394. doi:10.1002/anie.201306703 |
| 34. | Li, L.; Wang, F.; Ni, C.; Hu, J. Angew. Chem., Int. Ed. 2013, 52, 12390–12394. doi:10.1002/anie.201306703 |
| 19. | Fuqua, S. A.; Duncan, W. G.; Silverstein, R. M. Tetrahedron Lett. 1964, 5, 1461–1463. doi:10.1016/S0040-4039(01)89512-5 |
| 20. | Herkes, F. E.; Burton, D. J. J. Org. Chem. 1967, 32, 1311–1318. doi:10.1021/jo01280a007 |
| 21. | Zheng, J.; Cai, J.; Lin, J.-H.; Guo, Y.; Xiao, J.-C. Chem. Commun. 2013, 49, 7513–7515. doi:10.1039/C3CC44271C |
| 22. | Naae, D. G.; Burton, D. J. J. Fluorine Chem. 1971, 1, 123–125. doi:10.1016/S0022-1139(00)82541-5 |
| 23. | Naae, D. G.; Burton, D. J. Synth. Commun. 1973, 3, 197–200. doi:10.1080/00397917308062035 |
| 24. | Nowak, I.; Robins, J. M. Org. Lett. 2005, 7, 721–724. doi:10.1021/ol047416s |
| 25. | Thomoson, C. S.; Martinez, H.; Dolbier, W. R., Jr. J. Fluorine Chem. 2013, 150, 53–59. doi:10.1016/j.jfluchem.2013.02.026 |
| 27. | Zhang, L.; Zheng, J.; Hu, J. J. Org. Chem. 2006, 71, 9845–9848. doi:10.1021/jo061799l |
| 28. | Zheng, J.; Li, Y.; Zhang, L.; Hu, J.; Meuzelaar, G. J.; Federsel, H.-J. Chem. Commun. 2007, 5149–5151. doi:10.1039/B713156A |
| 29. | Zhang, W.; Wang, F.; Hu, J. Org. Lett. 2009, 11, 2109–2112. doi:10.1021/ol900567c |
| 30. | Wang, F.; Huang, W.; Hu, J. Chin. J. Chem. 2011, 29, 2717–2721. doi:10.1002/cjoc.201100325 |
| 31. | Wang, F.; Zhang, W.; Zhu, J.; Li, H.; Huang, K.-W.; Hu, J. Chem. Commun. 2011, 47, 2411–2413. doi:10.1039/c0cc04548a |
| 32. | For preliminary results on the difluoromethylation of O, S, and N-nucleophiles with TMSCF2Cl under aqueous basic conditions, see Supporting Information File 1. |
| 33. | Wang, F.; Luo, T.; Hu, J.; Wang, Y.; Krishnan, H. S.; Jog, P. V.; Ganesh, S. K.; Prakash, G. K. S.; Olah, G. A. Angew. Chem., Int. Ed. 2011, 50, 7153–7157. doi:10.1002/anie.201101691 |
| 34. | Li, L.; Wang, F.; Ni, C.; Hu, J. Angew. Chem., Int. Ed. 2013, 52, 12390–12394. doi:10.1002/anie.201306703 |
| 25. | Thomoson, C. S.; Martinez, H.; Dolbier, W. R., Jr. J. Fluorine Chem. 2013, 150, 53–59. doi:10.1016/j.jfluchem.2013.02.026 |
© 2014 Wang et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)