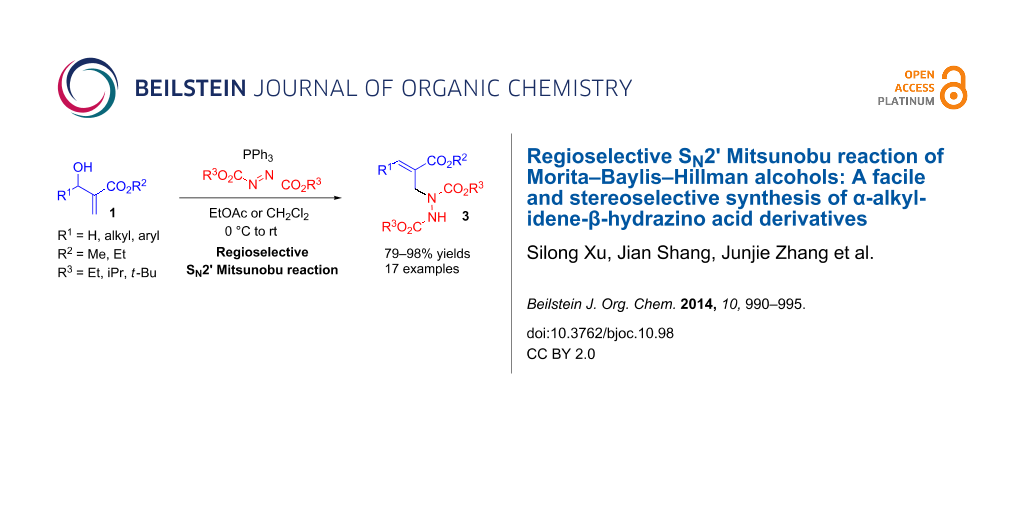
Abstract
A highly regioselective SN2' Mitsunobu reaction between Morita–Baylis–Hillman (MBH) alcohols, azodicarboxylates, and triphenylphosphine is developed, which provides an easy access to α-alkylidene-β-hydrazino acid derivatives in high yields and good stereoselectivity. This reaction represents the first direct transformation of MBH alcohols into hydrazines.
Graphical Abstract
Introduction
Hydrazines and their derivatives are an important class of compounds in organic chemistry. They are widely used in the fields of pesticides, polymers, dyestuff, and pharmaceutical agents [1]. They are also versatile building blocks for accessing many important nitrogen-containing heterocyclic compounds, especially pyrazole derivatives [2-7]. Although various methods detailing the synthesis of hydrazines have been established [8], the development of an efficient synthesis of hydrazines with highly structural diversity from simple starting materials under mild conditions is still desirable.
Morita–Baylis–Hillman (MBH) adducts [9] are a class of unique substrates of great synthetic potential which contain three manipulatable groups, namely, a hydroxy group, a carbon–carbon double bond, and an electron-withdrawing group. Over the past several decades, a myriad of transformations involving MBH adducts have been reported, leading to a wide variety of molecular scaffolds of high diversity and complexity [10-12]. A number of reports have dealt with the conversion of the hydroxy group of MBH adducts into useful functionalities, such as halides [13,14], ethers [15,16], amines [17,18], thioethers [19], phosphonates [20,21], alkyl groups [22,23], and so on. In this context, however, reports on the conversion of MBH alcohols into hydrazine derivatives are scanty.
In 2009, Nair and co-workers [24] reported an interesting reaction of MBH acetates with azodicarboxylates in the presence of PPh3 (Mitsunobu reaction conditions), which gives an efficient access to α-alkylidene-β-hydrazino acid derivatives, an important precursor for many bioactive compounds [25-30] including β-amino acids [25] (Scheme 1, top). However, the reaction exhibited poor chemoselectivity which gave comparable yields of tri- and tetrasubstituted hydrazines. For example, the reaction of MBH acetate 1a' with diisopropyl azodicarboxylate (2a) and PPh3 afforded hydrazines 3a and 4a in 51% and 46% yields, respectively. Inspired by this report, and a pioneering SN2' Mitsunobu reaction of MBH alcohols with carboxylic acids [31,32], we envisioned that direct treatment of simpler MBH alcohols with azodicarboxylates and PPh3 (Mitsunobu conditions) would chemoselectively provide trisubstituted hydrazines of type 3, via a distinct SN2' Mitsunobu reaction approach (Scheme 1, bottom). As part of our interest in exploring new reactivities of MBH adducts [33,34], herein we wish to report the preliminary results from such an investigation.
Scheme 1: Synthesis of α-alkylidene-β-hydrazino acid derivatives from MBH adducts.
Scheme 1: Synthesis of α-alkylidene-β-hydrazino acid derivatives from MBH adducts.
Results and Discussion
In our initial study, MBH alcohol 1a was treated with 2.0 equivalents of diethyl azodicarboxylate (2b) and triphenylphosphine under very mild conditions (Scheme 2). To our delight, the reaction was completed in 30 minutes providing the anticipated trisubstituted hydrazine 3b in 90% isolated yield with excellent E-selectivity (E/Z > 20:1). To the best of our knowledge, this reaction represents the first direct conversion of MBH alcohols into hydrazines. In addition, the normal SN2 Mitsunobu reaction [35,36] product 5, namely, diethyl 1-(2-(ethoxycarbonyl)-1-phenylallyl)hydrazine-1,2-dicarboxylate, could not be detected in the reaction mixture, which suggested a highly regioselective SN2' Mitsunobu process occurred in the reaction (see discussion on mechanism below).
With the encouraging result, the reaction conditions were further optimized using the above reaction as a model (Table 1). Among several common solvents screened, dichloromethane, chloroform, and toluene gave comparable yields to that of THF, while ethyl acetate emerged as the best solvent, offering an excellent 98% yield (Table 1, entries 2–5). However, polar solvents such as DMF, ethanol, and acetonitrile were detrimental to the reaction, giving very low yields (Table 1, entries 6–8). Reducing the amounts of 2b and PPh3 from 2.0 equivalents to 1.5 or 1.0 equivalent resulted in substantial decrease in the yields (Table 1, entries 9 and 10). It was also found that replacing PPh3 with more nucleophilic PBu3 in the reaction could shorten the reaction time but led to an inferior yield (Table 1, entry 11). Therefore PPh3 was chosen as the preferable phosphine due to its high efficiency and cost-effectiveness.
Table 1: Investigation on reaction conditions.a
|
|
|||
| Entry | Solvent | Time (min) | Yieldb (%) |
|---|---|---|---|
| 1 | THF | 30 | 90 |
| 2 | CH2Cl2 | 20 | 80 |
| 3 | CHCl3 | 40 | 86 |
| 4 | toluene | 35 | 88 |
| 5 | EtOAc | 25 | 98 |
| 6 | DMF | 24 | trace |
| 7 | EtOH | 50 | 23 |
| 8 | CH3CN | 30 | 39 |
| 9c | EtOAc | 25 | 29 |
| 10d | EtOAc | 25 | 54 |
| 11e | EtOAc | 10 | 82 |
aDiethyl azodicarboxylate (2b, 0.6 mmol) was slowly added to a solution of MBH alcohol 1a (0.3 mmol) and PPh3 (0.6 mmol) in the specified solvent (2 mL) at 0 °C under N2, and then the mixture was allowed to warm up to room temperature. bIsolated yield. cThe reaction was conducted using 1.0 equiv of both PPh3 and 2b. d1.5 equiv of PPh3 and 2b were adopted. ePBu3 was used instead of PPh3.
Under the optimized conditions, the scope of the SN2' Mitsunobu reaction was examined (Table 2). Variation in the ester alkyl groups of both MBH alcohols and azodicarboxylates had little influence on the reaction; the corresponding hydrazines with different ester groups were generated in excellent yields and stereoselectivity (Table 2, entries 1–3). In addition, a range of aromatic MBH alcohols 1c–g featuring either an electron-donating or an electron-withdrawing group on the benzene ring all worked well, delivering the hydrazines 3e–i in high yields (79–91%) and excellent E/Z selectivity, with an exception of the nitro-substituted 1g giving a moderate E/Z ratio (Table 2, entries 4–8). An ortho substituent on the benzene ring of the MBH alcohol was also well tolerated (Table 2, entry 5 vs 6). Notably, in contrast with Nair’s report [24], it was revealed that aliphatic MBH alcohols were excellent candidates in the reaction. By switching the solvent to dichloromethane, several alkyl MBH alcohols containing ethyl, methyl, or hydrogen substituents (1h–l) readily reacted with representative azodicarboxylates, producing the corresponding α-alkylidene-β-hydrazino esters 3 in high yields (80–90%) and good E/Z selectivity (Table 2, entries 9–18). An exception was observed for the reaction of MBH alcohol 1j with tert-butyl azodicarboxylate (2c), which gave 55% yield and a modest E/Z ratio (5:1) (Table 2, entry 14).
Table 2: Synthesis of α-alkylidene-β-hydrazino esters 3.a
|
|
|||||
| Entry | R1, R2 in 1 | 2 | Time (min) | 3, Yieldb (%) | E/Zc |
|---|---|---|---|---|---|
| 1 | C6H5, Et (1a) | 2b | 25 | 3b, 98 | 20:1 |
| 2 | C6H5, Me (1b) | 2b | 25 | 3c, 95 | 20:1 |
| 3 | C6H5, Me (1b) | 2c | 50 | 3d, 95 | 20:1 |
| 4 | 4-CH3C6H4, Et (1c) | 2b | 35 | 3e, 81 | 20:1 |
| 5 | 2-ClC6H4, Et (1d) | 2b | 35 | 3f, 87 | 20:1 |
| 6 | 4-ClC6H4, Et (1e) | 2b | 30 | 3g, 91 | 20:1 |
| 7 | 4-FC6H4, Me (1f) | 2b | 30 | 3h, 79 | 20:1 |
| 8 | 4-NO2C6H4, Et (1g) | 2c | 40 | 3i, 86 | 3:1 |
| 9 | C2H5, Et (1h) | 2a | 30 | 3j, 80 | 9:1 |
| 10 | C2H5, Et (1h) | 2b | 20 | 3k, 82 | 20:1 |
| 11 | C2H5, Me (1i) | 2b | 25 | 3l, 80 | 20:1 |
| 12 | C2H5, Me (1i) | 2a | 30 | 3m, 81 | 13:1 |
| 13 | CH3, Et (1j) | 2b | 21 | 3n, 83 | 13:1 |
| 14 | CH3, Et (1j) | 2c | 40 | 3o, 55 | 5:1 |
| 15 | H, Et (1k) | 2b | 10 | 3p, 90 | – |
| 16 | H, Et (1k) | 2a | 20 | 3q, 89 | – |
| 17 | H, Et (1k) | 2c | 25 | 3r, 90 | – |
| 18 | H, Me (1l) | 2b | 13 | 3s, 87 | – |
aUnder N2 atmosphere and at 0 °C, to a solution of MBH alcohol 1 (0.3 mmol) and PPh3 (0.6 mmol) in 2 mL ethyl acetate (for entries 9–18, dichloromethane was used as the solvent) was slowly added azodicarboxylates 2 (0.6 mmol), and the mixture was stirred at room temperature and monitored by TLC. bIsolated yield. cDetermined by 1H NMR assay.
To further investigate the scope, a single case using allenic MBH alcohol 6 as the substrate was studied (Scheme 3). However, under similar conditions, the reaction between alcohol 6, tert-butyl azodicarboxylate (2c), and PPh3 afforded an interesting 1H-pyrazole compound 7 in 58% yield. A possible mechanism for the formation of 7 is outlined in Supporting Information File 1.
The above results clearly show that the SN2' Mitsunobu reaction between MBH alcohols, azodicarboxylates, and PPh3 has a broad substrate scope. The reaction is clean and fast, with all the reactions completed in less than one hour. The reaction exhibits good stereoselectivity (E/Z 3:1 to 20:1) and exclusive regioselectivity. In all cases, the normal SN2 Mitsunobu products of type 5 could not be detected by using 1H and 13C DEPT NMR analysis. The structure and stereochemistry of all hydrazines 3 were well identified by 1H, 13C NMR, IR, HRMS, and NOESY analysis for a representative product 3b (for characterization data, see Supporting Information File 1).
A possible mechanism for the SN2' Mitsunobu reaction between MBH alcohols 1, azodicarboxylates 2, and PPh3 is depicted in Scheme 4. Initially, the addition of PPh3 to azodicarboxylates 2 generates the Huisgen zwitterion intermediate 8 [37,38]. Subsequent proton transfer and phosphonium migration between 8 and the MBH alcohol 1 produce an oxophosphonium intermediate 9 and a hydrazine anionic species 10 [36]. Finally, an expedient SN2' attack of species 10 on 9, probably facilitated by the ester group of 9 [31,32], leads to the formation of hydrazines 3 and phosphine oxide. The alternative SN2 displacement of the oxophosphonium moiety of 9 by the species 10 may be retarded by steric hindrance. Recently, SN2' Mitsunobu reactions [31,32,39-45] have attracted considerable interest from the organic chemistry community due to their great synthetic potential being complementary to the Mitsunobu reactions. This report accordingly adds to a new valuable example of SN2' Mitsunobu reactions.
Scheme 4: A plausible mechanism for the formations of 3.
Scheme 4: A plausible mechanism for the formations of 3.
Conclusion
In conclusion, we have developed a highly regioselective SN2' Mitsunobu reaction between Morita–Baylis–Hillman (MBH) alcohols, azodicarboxylates, and triphenylphosphine as an efficient synthetic method for α-alkylidene-β-hydrazino acid derivatives in high yields and good stereoselectivity. This reaction features additional advantages as mild conditions, wide substrate scope, and simple starting materials. The reaction represents the first direct transformation of MBH alcohols into hydrazines, and constitutes a valuable example of regioselective SN2' Mitsunobu reactions. Our future efforts will focus on the application of the current reaction in the synthesis of nitrogen-containing heterocyclic compounds.
Experimental
General information
All reactions were carried out in nitrogen atmosphere under anhydrous conditions. Solvents were purified according to standard procedures. Mortia–Baylis–Hillman (MBH) alcohol 1 were prepared according to literature procedures [46,47]. Benzyl 2-(hydroxymethyl)buta-2,3-dienoate (6) is a known compound and was prepared according to a literature [48]. Reagents from commercial sources were used without further purification. 1H and 13C NMR spectra were recorded on a Bruker AV 400 spectrometer in CDCl3 with tetramethylsilane (TMS) as the internal standard. High resolution ESI mass spectra were acquired with an IonSpec QFT-ESI instrument. IR spectra were recorded on a Nicolet Avatar 330 FTIR spectrometer (in KBr). Column chromatography was performed on silica gel (200–300 mesh) using a mixture of petroleum ether (bp 60–90 °C)/ethyl acetate as the eluant.
General procedure for the synthesis of hydrazines 3
Under N2 atmosphere and at 0 °C, to a stirred solution of MBH alcohols 1 (0.3 mmol) and PPh3 (0.6 mmol) in EtOAc or CH2Cl2 (2 mL) in a Schlenk tube (25 mL) was slowly added azodicarboxylates 2 (0.6 mmol) over 5 minutes by the means of a microsyringe. The resulting reaction mixture was allowed to warm up to room temperature and stirred until the MBH alcohols 1 were completely consumed, as monitored by TLC. The solvent was removed under reduced pressure and the residue was purified by column chromatography on silica gel (gradient eluant: petroleum ether/ethyl acetate 9:1–3:1) to afford the hydrazines 3.
Synthesis of 1H-pyrazole 7
Under N2 atmosphere and at room temperature, PPh3 (157 mg, 0.6 mmol) was slowly added to a stirred solution of allenic MBH alcohol 6 (61 mg, 0.3 mmol) and di-tert-butyl azodicarboxylate 2c (138 mg, 0.6 mmol) in CH2Cl2 (2 mL) in a Schlenk tube (25 mL). The resulting reaction mixture was stirred for 1 hour at that time the alcohol 6 was disappeared as monitored by TLC. The solvent was removed using a rotatory evaporator under reduced pressure. The residue was then directly subject to column chromatography on silica gel (eluant: petroleum ether/ethyl acetate 9:1) to afford the 1H-pyrazole compound 7 in 73 mg, 58% yield, as slightly yellow oil.
Supporting Information
| Supporting Information File 1: Experimental details on the synthesis of all hydrazines 3 and 1H-pyrazole 7, full characterization data and 1H, 13C, DEPT NMR spectra for all compounds 3 and 7, and a mechanistic rationale for the formation of 7. | ||
| Format: PDF | Size: 4.8 MB | Download |
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21305107) and the Fundamental Research Funds for the Central Universities (No. 08143076). We gratefully thank Professor Zhengjie He (Nankai University) for the help with the HRMS measurements and the long-term support.
References
-
Licandro, E.; Perdicchia, D. Eur. J. Org. Chem. 2004, 665–675. doi:10.1002/ejoc.200300416
Return to citation in text: [1] -
LaLonde, R. L.; Wang, Z. J.; Mba, M.; Lackner, A. D.; Toste, F. D. Angew. Chem., Int. Ed. 2010, 49, 598–601. doi:10.1002/anie.200905000
Return to citation in text: [1] -
Suzuki, Y.; Naoe, S.; Oishi, S.; Fujii, N.; Ohno, H. Org. Lett. 2012, 14, 326–329. doi:10.1021/ol203072u
Return to citation in text: [1] -
Fernández, M.; Reyes, E.; Vicario, J. L.; Badía, D.; Carrillo, L. Adv. Synth. Catal. 2012, 354, 371–376. doi:10.1002/adsc.201100722
Return to citation in text: [1] -
Hashimoto, T.; Takiguchi, Y.; Maruoka, K. J. Am. Chem. Soc. 2013, 135, 11473–11476. doi:10.1021/ja405444c
Return to citation in text: [1] -
Dumeunier, R.; Lamberth, C.; Trah, S. Synlett 2013, 24, 1150–1154. doi:10.1055/s-0033-1338433
Return to citation in text: [1] -
Bouvet, S.; Moreau, X.; Coeffard, V.; Greck, C. J. Org. Chem. 2013, 78, 427–437. doi:10.1021/jo302320v
Return to citation in text: [1] -
Ragnarsson, U. Chem. Soc. Rev. 2001, 30, 205–213. doi:10.1039/b010091a
Return to citation in text: [1] -
Morita, K.; Suzuki, Z.; Hirose, H. Bull. Chem. Soc. Jpn. 1968, 41, 2815. doi:10.1246/bcsj.41.2815
Return to citation in text: [1] -
Basavaiah, D.; Reddy, B. S.; Badsara, S. S. Chem. Rev. 2010, 110, 5447–5674. doi:10.1021/cr900291g
Return to citation in text: [1] -
Basavaiah, D.; Veeraraghavaiah, G. Chem. Soc. Rev. 2012, 41, 68–78. doi:10.1039/c1cs15174f
Return to citation in text: [1] -
Wei, Y.; Shi, M. Chem. Rev. 2013, 113, 6659–6690. doi:10.1021/cr300192h
Return to citation in text: [1] -
Basavaiah, D.; Bhavani, A. K. D.; Pandiaraju, S.; Sarma, P. K. S. Synlett 1995, 243–244. doi:10.1055/s-1995-4929
Return to citation in text: [1] -
Das, B.; Banerjee, J.; Ravindranath, N.; Venkataiah, B. Tetrahedron Lett. 2004, 45, 2425–2426. doi:10.1016/j.tetlet.2004.01.101
Return to citation in text: [1] -
Shanmugam, P.; Rajasingh, P. Tetrahedron 2004, 60, 9283–9295. doi:10.1016/j.tet.2004.07.067
Return to citation in text: [1] -
Das, B.; Majhi, A.; Banerjee, J. Tetrahedron Lett. 2006, 47, 7619–7623. doi:10.1016/j.tetlet.2006.08.060
Return to citation in text: [1] -
Rajesh, S.; Banerji, B.; Iqbal, J. J. Org. Chem. 2002, 67, 7852–7857. doi:10.1021/jo010981d
Return to citation in text: [1] -
Ciclosi, M.; Fava, C.; Galeazzi, R.; Orena, M.; Sepulveda-Arques, J. Tetrahedron Lett. 2002, 43, 2199–2202. doi:10.1016/S0040-4039(02)00233-2
Return to citation in text: [1] -
Liu, Y.; Xu, D.; Xu, Z.; Zhang, Y. Heteroat. Chem. 2008, 19, 188–198. doi:10.1002/hc.20394
Return to citation in text: [1] -
Janecki, T.; Bodalski, R. Synthesis 1990, 799–801. doi:10.1055/s-1990-27019
Return to citation in text: [1] -
Basavaiah, D.; Pandiaraju, S. Tetrahedron 1996, 52, 2261–2268. doi:10.1016/0040-4020(95)01055-6
Return to citation in text: [1] -
Basavaiah, D.; Sarma, P. K. S.; Bhavani, A. K. D. J. Chem. Soc., Chem. Commun. 1994, 1091–1092. doi:10.1039/c39940001091
Return to citation in text: [1] -
Basavaiah, D.; Pandiaraju, S. Tetrahedron Lett. 1995, 36, 757–758. doi:10.1016/0040-4039(94)02360-N
Return to citation in text: [1] -
Jose, A.; Paul, R. R.; Mohan, R.; Mathew, S. C.; Biju, A. T.; Suresh, E.; Nair, V. Synthesis 2009, 1829–1833. doi:10.1055/s-0028-1088063
Return to citation in text: [1] [2] -
Liu, M.; Sibi, M. P. Tetrahedron 2002, 58, 7991–8035. doi:10.1016/S0040-4020(02)00991-2
Return to citation in text: [1] [2] -
Abouzid, K. A. M.; El-Abhar, H. S. Arch. Pharmacal Res. 2003, 26, 1–8. doi:10.1007/BF03179922
Return to citation in text: [1] -
Pareek, A. K.; Joseph, P. E.; Seth, D. S. Biomed. Pharmacol. J. 2010, 3, 385–390.
Return to citation in text: [1] -
Hansen, T.; Alst, T.; Havelkova, M.; Strøm, M. B. J. Med. Chem. 2010, 53, 595–606. doi:10.1021/jm901052r
Return to citation in text: [1] -
Sadowsky, J. D.; Murray, J. K.; Tomita, Y.; Gellman, S. H. ChemBioChem 2007, 8, 903–916. doi:10.1002/cbic.200600546
Return to citation in text: [1] -
Sabatino, D.; Proulx, C.; Pohankova, P.; Ong, H.; Lubell, W. D. J. Am. Chem. Soc. 2011, 133, 12493–12506. doi:10.1021/ja203007u
Return to citation in text: [1] -
Charette, A. B.; Côté, B. Tetrahedron Lett. 1993, 34, 6833–6836. doi:10.1016/S0040-4039(00)91807-0
Return to citation in text: [1] [2] [3] -
Charette, A. B.; Cote, B.; Monroc, S.; Prescott, S. J. Org. Chem. 1995, 60, 6888–6894. doi:10.1021/jo00126a046
Return to citation in text: [1] [2] [3] -
Xu, S.; Chen, R.; Qin, Z.; Wu, G.; He, Z. Org. Lett. 2012, 14, 996–999. doi:10.1021/ol2032569
Return to citation in text: [1] -
Chen, R.; Xu, S.; Wang, L.; Tang, Y.; He, Z. Chem. Commun. 2013, 49, 3543–3545. doi:10.1039/c3cc41419a
Return to citation in text: [1] -
Dow, R. L.; Kelly, R. C.; Schletter, I.; Wierenga, W. Synth. Commun. 1981, 11, 43–53. doi:10.1080/00397918108064281
Return to citation in text: [1] -
Swamy, K. C. K.; Kumar, N. N. B.; Balaraman, E.; Kumar, K. V. P. P. Chem. Rev. 2009, 109, 2551–2651. doi:10.1021/cr800278z
Return to citation in text: [1] [2] -
Brunn, E.; Huisgen, R. Angew. Chem., Int. Ed. Engl. 1969, 8, 513–515. doi:10.1002/anie.196905131
Return to citation in text: [1] -
Nair, V.; Biju, A. T.; Mathew, S. C.; Babu, B. P. Chem.–Asian J. 2008, 3, 810–820. doi:10.1002/asia.200700341
Return to citation in text: [1] -
Higashino, M.; Ikeda, N.; Shinada, T.; Sakaguchi, K.; Ohfune, Y. Tetrahedron Lett. 2011, 52, 422–425. doi:10.1016/j.tetlet.2010.11.080
Return to citation in text: [1] -
Berrée, F.; Gernigon, N.; Hercouet, A.; Lin, C. H.; Carboni, B. Eur. J. Org. Chem. 2009, 329–333. doi:10.1002/ejoc.200800965
Return to citation in text: [1] -
Grove, J. J. C.; van Heerden, P. S.; Ferreira, D.; Bezuidenhoudt, B. C. B. Tetrahedron Lett. 2010, 51, 57–59. doi:10.1016/j.tetlet.2009.10.080
Return to citation in text: [1] -
Yoshimura, Y.; Asami, K.; Imamichi, T.; Okuda, T.; Shiraki, K.; Takahata, H. J. Org. Chem. 2010, 75, 4161–4171. doi:10.1021/jo100556u
Return to citation in text: [1] -
Bisegger, P.; Manov, N.; Bienz, S. Tetrahedron 2008, 64, 7531–7536. doi:10.1016/j.tet.2008.05.119
Return to citation in text: [1] -
Zhu, S.; Hudson, T. H.; Kyle, D. E.; Lin, A. J. J. Med. Chem. 2002, 45, 3491–3496. doi:10.1021/jm020104f
Return to citation in text: [1] -
Kwon, Y.-U.; Lee, C.; Chung, S.-K. J. Org. Chem. 2002, 67, 3327–3338. doi:10.1021/jo016237a
Return to citation in text: [1] -
Yu, C.; Liu, B.; Hu, L. J. Org. Chem. 2001, 66, 5413–5418. doi:10.1021/jo015628m
Return to citation in text: [1] -
Deng, J.; Hu, X.-P.; Huang, J.-D.; Yu, S.-B.; Wang, D.-Y.; Duan, Z.-C.; Zheng, Z. J. Org. Chem. 2008, 73, 2015–2017. doi:10.1021/jo702510m
Return to citation in text: [1] -
Hue, B. T. B.; Dijkink, J.; Kuiper, S.; van Schaik, S.; van Maarseveen, J. H.; Hiemstra, H. Eur. J. Org. Chem. 2006, 127–137. doi:10.1002/ejoc.200500609
Return to citation in text: [1]
| 37. | Brunn, E.; Huisgen, R. Angew. Chem., Int. Ed. Engl. 1969, 8, 513–515. doi:10.1002/anie.196905131 |
| 38. | Nair, V.; Biju, A. T.; Mathew, S. C.; Babu, B. P. Chem.–Asian J. 2008, 3, 810–820. doi:10.1002/asia.200700341 |
| 35. | Dow, R. L.; Kelly, R. C.; Schletter, I.; Wierenga, W. Synth. Commun. 1981, 11, 43–53. doi:10.1080/00397918108064281 |
| 36. | Swamy, K. C. K.; Kumar, N. N. B.; Balaraman, E.; Kumar, K. V. P. P. Chem. Rev. 2009, 109, 2551–2651. doi:10.1021/cr800278z |
| 24. | Jose, A.; Paul, R. R.; Mohan, R.; Mathew, S. C.; Biju, A. T.; Suresh, E.; Nair, V. Synthesis 2009, 1829–1833. doi:10.1055/s-0028-1088063 |
| 1. | Licandro, E.; Perdicchia, D. Eur. J. Org. Chem. 2004, 665–675. doi:10.1002/ejoc.200300416 |
| 10. | Basavaiah, D.; Reddy, B. S.; Badsara, S. S. Chem. Rev. 2010, 110, 5447–5674. doi:10.1021/cr900291g |
| 11. | Basavaiah, D.; Veeraraghavaiah, G. Chem. Soc. Rev. 2012, 41, 68–78. doi:10.1039/c1cs15174f |
| 12. | Wei, Y.; Shi, M. Chem. Rev. 2013, 113, 6659–6690. doi:10.1021/cr300192h |
| 31. | Charette, A. B.; Côté, B. Tetrahedron Lett. 1993, 34, 6833–6836. doi:10.1016/S0040-4039(00)91807-0 |
| 32. | Charette, A. B.; Cote, B.; Monroc, S.; Prescott, S. J. Org. Chem. 1995, 60, 6888–6894. doi:10.1021/jo00126a046 |
| 9. | Morita, K.; Suzuki, Z.; Hirose, H. Bull. Chem. Soc. Jpn. 1968, 41, 2815. doi:10.1246/bcsj.41.2815 |
| 33. | Xu, S.; Chen, R.; Qin, Z.; Wu, G.; He, Z. Org. Lett. 2012, 14, 996–999. doi:10.1021/ol2032569 |
| 34. | Chen, R.; Xu, S.; Wang, L.; Tang, Y.; He, Z. Chem. Commun. 2013, 49, 3543–3545. doi:10.1039/c3cc41419a |
| 25. | Liu, M.; Sibi, M. P. Tetrahedron 2002, 58, 7991–8035. doi:10.1016/S0040-4020(02)00991-2 |
| 26. | Abouzid, K. A. M.; El-Abhar, H. S. Arch. Pharmacal Res. 2003, 26, 1–8. doi:10.1007/BF03179922 |
| 27. | Pareek, A. K.; Joseph, P. E.; Seth, D. S. Biomed. Pharmacol. J. 2010, 3, 385–390. |
| 28. | Hansen, T.; Alst, T.; Havelkova, M.; Strøm, M. B. J. Med. Chem. 2010, 53, 595–606. doi:10.1021/jm901052r |
| 29. | Sadowsky, J. D.; Murray, J. K.; Tomita, Y.; Gellman, S. H. ChemBioChem 2007, 8, 903–916. doi:10.1002/cbic.200600546 |
| 30. | Sabatino, D.; Proulx, C.; Pohankova, P.; Ong, H.; Lubell, W. D. J. Am. Chem. Soc. 2011, 133, 12493–12506. doi:10.1021/ja203007u |
| 48. | Hue, B. T. B.; Dijkink, J.; Kuiper, S.; van Schaik, S.; van Maarseveen, J. H.; Hiemstra, H. Eur. J. Org. Chem. 2006, 127–137. doi:10.1002/ejoc.200500609 |
| 2. | LaLonde, R. L.; Wang, Z. J.; Mba, M.; Lackner, A. D.; Toste, F. D. Angew. Chem., Int. Ed. 2010, 49, 598–601. doi:10.1002/anie.200905000 |
| 3. | Suzuki, Y.; Naoe, S.; Oishi, S.; Fujii, N.; Ohno, H. Org. Lett. 2012, 14, 326–329. doi:10.1021/ol203072u |
| 4. | Fernández, M.; Reyes, E.; Vicario, J. L.; Badía, D.; Carrillo, L. Adv. Synth. Catal. 2012, 354, 371–376. doi:10.1002/adsc.201100722 |
| 5. | Hashimoto, T.; Takiguchi, Y.; Maruoka, K. J. Am. Chem. Soc. 2013, 135, 11473–11476. doi:10.1021/ja405444c |
| 6. | Dumeunier, R.; Lamberth, C.; Trah, S. Synlett 2013, 24, 1150–1154. doi:10.1055/s-0033-1338433 |
| 7. | Bouvet, S.; Moreau, X.; Coeffard, V.; Greck, C. J. Org. Chem. 2013, 78, 427–437. doi:10.1021/jo302320v |
| 25. | Liu, M.; Sibi, M. P. Tetrahedron 2002, 58, 7991–8035. doi:10.1016/S0040-4020(02)00991-2 |
| 19. | Liu, Y.; Xu, D.; Xu, Z.; Zhang, Y. Heteroat. Chem. 2008, 19, 188–198. doi:10.1002/hc.20394 |
| 22. | Basavaiah, D.; Sarma, P. K. S.; Bhavani, A. K. D. J. Chem. Soc., Chem. Commun. 1994, 1091–1092. doi:10.1039/c39940001091 |
| 23. | Basavaiah, D.; Pandiaraju, S. Tetrahedron Lett. 1995, 36, 757–758. doi:10.1016/0040-4039(94)02360-N |
| 31. | Charette, A. B.; Côté, B. Tetrahedron Lett. 1993, 34, 6833–6836. doi:10.1016/S0040-4039(00)91807-0 |
| 32. | Charette, A. B.; Cote, B.; Monroc, S.; Prescott, S. J. Org. Chem. 1995, 60, 6888–6894. doi:10.1021/jo00126a046 |
| 39. | Higashino, M.; Ikeda, N.; Shinada, T.; Sakaguchi, K.; Ohfune, Y. Tetrahedron Lett. 2011, 52, 422–425. doi:10.1016/j.tetlet.2010.11.080 |
| 40. | Berrée, F.; Gernigon, N.; Hercouet, A.; Lin, C. H.; Carboni, B. Eur. J. Org. Chem. 2009, 329–333. doi:10.1002/ejoc.200800965 |
| 41. | Grove, J. J. C.; van Heerden, P. S.; Ferreira, D.; Bezuidenhoudt, B. C. B. Tetrahedron Lett. 2010, 51, 57–59. doi:10.1016/j.tetlet.2009.10.080 |
| 42. | Yoshimura, Y.; Asami, K.; Imamichi, T.; Okuda, T.; Shiraki, K.; Takahata, H. J. Org. Chem. 2010, 75, 4161–4171. doi:10.1021/jo100556u |
| 43. | Bisegger, P.; Manov, N.; Bienz, S. Tetrahedron 2008, 64, 7531–7536. doi:10.1016/j.tet.2008.05.119 |
| 44. | Zhu, S.; Hudson, T. H.; Kyle, D. E.; Lin, A. J. J. Med. Chem. 2002, 45, 3491–3496. doi:10.1021/jm020104f |
| 45. | Kwon, Y.-U.; Lee, C.; Chung, S.-K. J. Org. Chem. 2002, 67, 3327–3338. doi:10.1021/jo016237a |
| 17. | Rajesh, S.; Banerji, B.; Iqbal, J. J. Org. Chem. 2002, 67, 7852–7857. doi:10.1021/jo010981d |
| 18. | Ciclosi, M.; Fava, C.; Galeazzi, R.; Orena, M.; Sepulveda-Arques, J. Tetrahedron Lett. 2002, 43, 2199–2202. doi:10.1016/S0040-4039(02)00233-2 |
| 24. | Jose, A.; Paul, R. R.; Mohan, R.; Mathew, S. C.; Biju, A. T.; Suresh, E.; Nair, V. Synthesis 2009, 1829–1833. doi:10.1055/s-0028-1088063 |
| 46. | Yu, C.; Liu, B.; Hu, L. J. Org. Chem. 2001, 66, 5413–5418. doi:10.1021/jo015628m |
| 47. | Deng, J.; Hu, X.-P.; Huang, J.-D.; Yu, S.-B.; Wang, D.-Y.; Duan, Z.-C.; Zheng, Z. J. Org. Chem. 2008, 73, 2015–2017. doi:10.1021/jo702510m |
| 15. | Shanmugam, P.; Rajasingh, P. Tetrahedron 2004, 60, 9283–9295. doi:10.1016/j.tet.2004.07.067 |
| 16. | Das, B.; Majhi, A.; Banerjee, J. Tetrahedron Lett. 2006, 47, 7619–7623. doi:10.1016/j.tetlet.2006.08.060 |
| 36. | Swamy, K. C. K.; Kumar, N. N. B.; Balaraman, E.; Kumar, K. V. P. P. Chem. Rev. 2009, 109, 2551–2651. doi:10.1021/cr800278z |
| 13. | Basavaiah, D.; Bhavani, A. K. D.; Pandiaraju, S.; Sarma, P. K. S. Synlett 1995, 243–244. doi:10.1055/s-1995-4929 |
| 14. | Das, B.; Banerjee, J.; Ravindranath, N.; Venkataiah, B. Tetrahedron Lett. 2004, 45, 2425–2426. doi:10.1016/j.tetlet.2004.01.101 |
| 20. | Janecki, T.; Bodalski, R. Synthesis 1990, 799–801. doi:10.1055/s-1990-27019 |
| 21. | Basavaiah, D.; Pandiaraju, S. Tetrahedron 1996, 52, 2261–2268. doi:10.1016/0040-4020(95)01055-6 |
| 31. | Charette, A. B.; Côté, B. Tetrahedron Lett. 1993, 34, 6833–6836. doi:10.1016/S0040-4039(00)91807-0 |
| 32. | Charette, A. B.; Cote, B.; Monroc, S.; Prescott, S. J. Org. Chem. 1995, 60, 6888–6894. doi:10.1021/jo00126a046 |
© 2014 Xu et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)