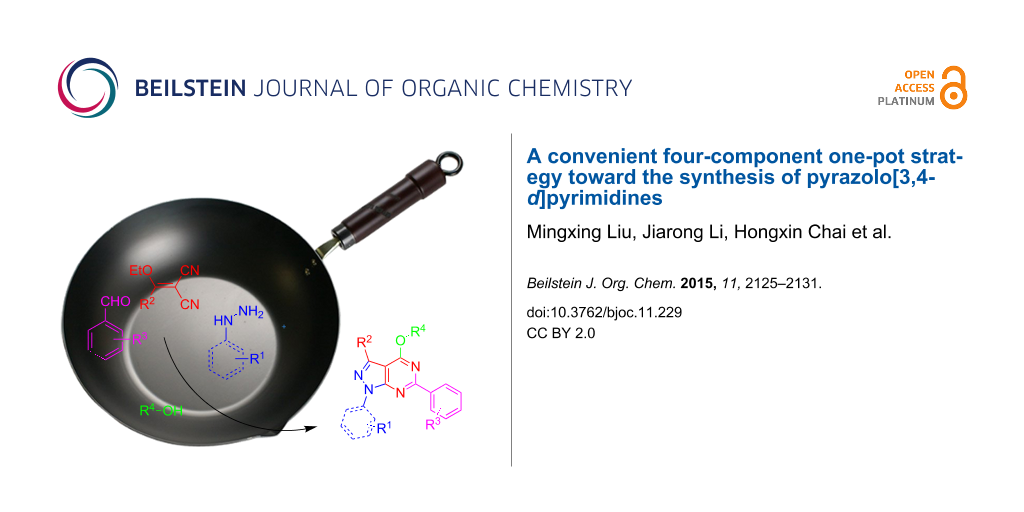
Abstract
An efficient one-pot synthesis of pyrazolo[3,4-d]pyrimidine derivatives by the four-component condensation of hydrazines, methylenemalononitriles, aldehydes and alcohols has been developed via two different reaction pathways. The structures of target products were characterized by IR spectroscopy, NMR (1H and 13C) spectroscopy and HRMS (ESI) spectrometry. The crystal structure of 4-ethoxy-6-(2-nitrophenyl)-1-phenyl-1H-pyrazolo[3,4-d]pyrimidine was determined by single crystal X-ray diffraction.
Graphical Abstract
Introduction
Heterocycles containing a pyrimidine ring are extensively present in natural products and are very important because of their biological activity [1-6]. They have shown a wide range of pharmacological potential such as kinase inhibitors [1], antitumor [7,8], anti-inflammatory [9,10], antimicrobial [11-13], pesticides [14], radio protectant [15] and cardiovascular activity [16,17]. For example, ibrutinib, sildenafil, allopurinol and zaleplon are famous pyrazolopyrimidine drugs.
Because of the importance of pyrazolo[3,4-d]pyrimidines, many methods for the synthesis of pyrazolo[3,4-d]pyrimidines have been explored. Some examples include the condensation of 5-aminopyrazole-4-carbonitrile with amides [18-21], carboxylic acids [22-24], amidines [25,26], nitriles [27,28], ketones [29,30] and halohydrocarbon [31], the cyclization of 5-aminopyrazole-4-carboxamides with amides [32], ureas [33-36], esters [37-39] and acyl chloride [40], and the reaction of aminopyrazoles and amides [41,42].
In our previous studies, dihydropyrimidinone was synthesized through the condensation of 5-aminopyrazole-4-carbonitrile and ketones [29,30]. 5-Aminopyrazole-4-carbonitrile was prepared from the reaction of ethoxymethylenemalononitrile with phenylhydrazine in a step-wise fashion [43-47]. During the course of previous studies, we envisioned that we could combine these reactions and embarked on designing a strategy toward a one-pot synthesis by combining the three reactants. When benzaldehyde was used as the reactant, the target product was obtained (Scheme 1A). But when benzaldehyde was switched to anisaldehyde, the expected product was not obtained and pyrazolo[3,4-d]pyrimidines was isolated (Scheme 1B). Inspired by this phenomenon, we conducted detailed studies and found a new convenient synthesis of pyrazolo[3,4-d]pyrimidines. To the best of our knowledge, this is a novel methodology for the synthesis of pyrazolo[3,4-d]pyrimidines by the reaction of hydrazines, methylenemalononitriles, aldehydes and alcohols. During the preparation of this manuscript, Liu et al. reported the synthesis of pyrazolo[3,4-d]pyrimidines from 5-aminopyrazole-4-carbonitrile [48]. The differences between their and our strategy are that we developed a four-component combined reaction to synthesize pyrazolo[3,4-d]pyrimidines, the catalyst that we use is different, the universality of the substrates are very broad and the substrates are more readily available.
Scheme 1: The synthesis of pyrazolo[3,4-d]pyrimidines.
Scheme 1: The synthesis of pyrazolo[3,4-d]pyrimidines.
Results and Discussion
Phenylhydrazine, 2-(ethoxymethylene)malononitrile, ethanol and benzaldehyde were selected as the model reactants. The influence of the reaction conditions was studied and the results are summarized in Table 1. No target product was afforded in the presence of an inorganic weak base or without a catalyst (Table 1, entries 1 and 2). Sodium hydroxide could catalyze this reaction, but pyrazolo[3,4-d]pyrimidinone 5aa was obtained instead of pyrazolo[3,4-d]pyrimidine 5a (Table 1, entry 3). This shows that the catalytic properties of sodium hydroxide have some limitations. Fortunately, some strong bases could promote the reaction to produce 5a, though DBU needed a higher reaction temperature (Table 1, entries 4–6). Taking into account the yield of the reaction, sodium alkoxide was the best choice. The reaction performed in alcohol resulted in the highest yield (Table 1, entries 6–9). The reaction temperature was screened and the appropriate temperature was found to be 60 °C (Table 1, entries 6, 10 and 11). The amount of catalyst had an effect on the reaction and 1.2 equivalents of sodium alkoxide was the most appropriate choice (Table 1, entries 12 and 13). This means that sodium alkoxide is not only a catalyst, but also participates in the reaction.
Table 1: Optimization of the reaction conditionsa.
|
|
||||
| Entry | Solvent | Cat. (eqiv.) | Temp. (°C) | Yield (%)b |
|---|---|---|---|---|
| 1 | EtOH | – | 60 | 0 |
| 2 | EtOH | Na2CO3 (1.2) | 60 | 0 |
| 3 | EtOH | NaOH (1.2) | 60 | 82 (5aa) |
| 4 | EtOH | DBU (1.2) | reflux | 42 (5a) |
| 5 | EtOH | NaH (1.2) | 60 | 62 (5a) |
| 6 | EtOH | NaOEt (1.2) | 60 | 85 (5a) |
| 7 | DMSO | NaOEt (1.2) | 60 | 57 (5a) |
| 8 | toluene | NaOEt (1.2) | 60 | 70 (5a) |
| 9 | 1,4-dioxane | NaOEt (1.2) | 60 | 35 (5a) |
| 10 | EtOH | NaOEt (1.2) | 25 | 63 (5a) |
| 11 | EtOH | NaOEt (1.2) | reflux | 85 (5a) |
| 12 | EtOH | NaOEt (0.5) | 60 | 47 (5a) |
| 13 | EtOH | NaOEt (2.0) | 60 | 85 (5a) |
aReaction conditions: 1a (1.2 mmol), 2a (1.0 mmol), 3a (1.2 mmol) and catalyst in solvent (15 mL). bIsolated yields.
A series of hydrazines, methylenemalononitriles, aldehydes and alcohols were investigated under the optimal reaction conditions. As shown in Figure 1, the influence of different aldehydes on the reaction was studied first. The results show that aldehydes with substituents such as p-MeO, p-Me, 3,4,5-(MeO)3, 2-MeO-5-Br, m-NO2 and o-NO2 are all compatible under optimal conditions. The corresponding products were obtained in good yield (Figure 1, 5a–g). Then a set of hydrazines were selected and the target products were obtained. However, the yield of aromatic hydrazines bearing electron-withdrawing groups or electron-donating groups was higher than that of methylhydrazine (Figure 1, 5h–j). This is possibly due to the electronic effect of the substituents. Though the steric hindrance could affect the reaction, 3-substituted pyrazolo[3,4-d]pyrimidine was also obtained in good yield (Figure 1, 5k). In order to further broaden the scope of this one-pot methodology, a series of alcohols such as methanol, n-butanol, n-propanol and isopropanol were investigated. The expected products were also obtained in good yield (Figure 1, 5i–p). This fact revealed the universality and advantages of this method for the synthesis of pyrazolo[3,4-d]pyrimidines.
Figure 1: Four-component one-pot synthesis of 5. Reactions conditions: 1 (1.2 mmol), 2 (1.0 mmol), 3 (1.2 mmol) and 4 (1.2 mmol) in alcohol (15 mL).
Figure 1: Four-component one-pot synthesis of 5. Reactions conditions: 1 (1.2 mmol), 2 (1.0 mmol), 3 (1.2 mmo...
To rationalize the possible reaction mechanism, we successfully separated three intermediates (6a, 7a and 5aa). 4-Ethoxy-1,6-diphenyl-1H-pyrazolo[3,4-d]pyrimidine (5a) was obtained from the condensation of 5-amino-1-phenyl-1H-pyrazole-4-carbonitrile (6a) with benzaldehyde and ethanol, the cyclization of (E)-5-(benzylideneamino)-1-phenyl-1H-pyrazole-4-carbonitrile (7a) with ethanol or the reaction of 1,6-diphenyl-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one (5aa) with ethanol, respectively (Scheme 2).
Scheme 2: Synthesis of 5a from different intermediates.
Scheme 2: Synthesis of 5a from different intermediates.
With those results in hand, two possible reaction mechanisms were proposed and shown in Scheme 3. 5-Aminopyrazole-4-carbonitrile 6 was obtained from the reaction of hydrazine 1 and methylenemalononitrile 2 through nucleophilic addition, cyclization and aromatization. The nucleophilic attack of the amino group of 6 on the carbonyl group of the aldehyde affords 7. Then 7 provides the target product via two different reaction pathways. The first route is that 7 loses a water molecule to afford the Schiff base 8. Then 8 undergoes a Pinner reaction and imine 9 is formed, and then 9 turns into 10 through intramolecular cyclization. Finally, 10 is oxidized to give pyrazolo[3,4-d]pyrimidine 5. Another route is that 7 undergoes an intramolecular Pinner reaction to form 11. Then 11 rearranges to dihydropyrazolo[3,4-d]pyrimidin-4-ones 13 via Dimroth rearrangement and 13 is oxidized to provide 14 [49]. Finally, 14 undergoes a nucleophilic addition and loses a water molecule to afford the final product 5.
Scheme 3: Possible reaction mechanisms for the formation of pyrazolo[3,4-d]pyrimidiine.
Scheme 3: Possible reaction mechanisms for the formation of pyrazolo[3,4-d]pyrimidiine.
All products were characterized by IR, 1H NMR, 13C NMR and HRMS. A final confirmation of the structure of the reaction product 5g was determined by X-ray diffraction (Figure 2) [50].
![[1860-5397-11-229-2]](/bjoc/content/figures/1860-5397-11-229-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Molecular structure (from X-ray diffraction data) of 5g.
Figure 2: Molecular structure (from X-ray diffraction data) of 5g.
Conclusion
In summary, we have disclosed an efficient one-pot four-component synthesis of pyrazolo[3,4-d]pyrimidines. The simplicity of execution, readily available substrates and the potentially important use of the products make this synthetic protocol attractive for academic research and practical applications. Further studies towards the detailed mechanism and synthetic application of this protocol are in progress.
Supporting Information
| Supporting Information File 1: Experimental section and copies of 1H and 13C NMR spectra of compounds. | ||
| Format: PDF | Size: 2.6 MB | Download |
References
-
Schenone, S.; Radi, M.; Musumeci, F.; Brullo, C.; Botta, M. Chem. Rev. 2014, 114, 7189–7238. doi:10.1021/cr400270z
Return to citation in text: [1] [2] -
Parker, W. B. Chem. Rev. 2009, 109, 2880–2893. doi:10.1021/cr900028p
Return to citation in text: [1] -
Martin, M. W.; Newcomb, J.; Nunes, J. J.; Boucher, C.; Chai, L.; Epstein, L. F.; Faust, T.; Flores, S.; Gallant, P.; Gore, A.; Gu, Y.; Hsieh, F.; Huang, X.; Kim, J. L.; Middleton, S.; Oliveira-dos-Santos, A.; Patel, V. F.; Powers, D.; Rose, P.; Tudor, Y.; Turci, S. M.; Welcher, A. A.; Zack, D.; Zhao, H.; Zhu, L.; Zhu, X.; Ghiron, C.; Ermann, M.; Johnston, D.; Saluste, C.-G. P. J. Med. Chem. 2008, 51, 1637–1648. doi:10.1021/jm701095m
Return to citation in text: [1] -
Chauhan, M.; Kumar, R. Bioorg. Med. Chem. 2013, 21, 5657–5668. doi:10.1016/j.bmc.2013.07.027
Return to citation in text: [1] -
Palanisamy, P.; Jenniefer, S. J.; Muthiah, P. T.; Kumaresan, S. RSC Adv. 2013, 3, 19300–19310. doi:10.1039/c3ra42283f
Return to citation in text: [1] -
Wang, L.; Zhou, X.; Fredimoses, M.; Liao, S.; Liu, Y. RSC Adv. 2014, 4, 57350–57376. doi:10.1039/C4RA09833A
Return to citation in text: [1] -
Hsu, T. C.; Robins, R. K.; Cheng, C. C. Science 1956, 123, 848–849. doi:10.1126/science.123.3202.848-a
Return to citation in text: [1] -
Hafez, T. S.; Osman, S. A.; Yosef, H. A. A.; Abd El-All, A. S.; Hassan, A. S.; El-Sawy, A. A.; Abdallah, M. M.; Youns, M. Sci. Pharm. 2013, 81, 339–357. doi:10.3797/scipharm.1211-07
Return to citation in text: [1] -
Bahashwan, S. A.; Fayed, A. A.; Amr, A. E. E.; Flefel, E. M.; Kalmouch, A. Molecules 2013, 18, 15051–15063. doi:10.3390/molecules181215051
Return to citation in text: [1] -
Nugent, R. A.; Dunn, C. J.; Staite, N. D.; Murphy, M. J.; Schlachter, S. T.; Aspar, D. G.; Shields, S. K.; Galinet, L. A. Phosphorus, Sulfur Silicon Relat. Elem. 1996, 109, 229–232. doi:10.1080/10426509608545132
Return to citation in text: [1] -
Shamroukh, A. H.; Rashd, A. E.; Ali, H. S.; Abdel-Megeid, F. M. E. J. Heterocycl. Chem. 2013, 50, 758–765. doi:10.1002/jhet.1550
Return to citation in text: [1] -
Holla, B. S.; Mahalinga, M.; Karthikeyan, M. S.; Akberali, P. M.; Shetty, N. S. Bioorg. Med. Chem. 2006, 14, 2040–2047. doi:10.1016/j.bmc.2005.10.053
Return to citation in text: [1] -
Ali, A.; Taylor, G. E.; Ellsworth, K.; Harris, G.; Painter, R.; Silver, L. L.; Young, K. J. Med. Chem. 2003, 46, 1824–1830. doi:10.1021/jm020483c
Return to citation in text: [1] -
Ram, V. J.; Pandey, H. N.; Mishra, L. Arch. Pharm. 1979, 312, 586–590. doi:10.1002/ardp.19793120705
Return to citation in text: [1] -
Ghorab, M. M.; Ragab, F. A.; Alqasoumi, S. I.; Alafeefy, A. M.; Aboulmagd, S. A. Eur. J. Med. Chem. 2010, 45, 171–178. doi:10.1016/j.ejmech.2009.09.039
Return to citation in text: [1] -
Xia, Y.; Chackalamannil, S.; Czarniecki, M.; Tsai, H.; Vaccaro, H.; Cleven, R.; Cook, J.; Fawzi, A.; Watkins, R.; Zhang, H. J. Med. Chem. 1997, 40, 4372–4377. doi:10.1021/jm970495b
Return to citation in text: [1] -
Guccione, S.; Modica, M.; Longmore, J.; Shaw, D.; Barretta, G. U.; Santagati, A.; Santagati, M.; Russo, F. Bioorg. Med. Chem. Lett. 1996, 6, 59–64. doi:10.1016/0960-894X(95)00558-B
Return to citation in text: [1] -
Todorovic, N.; Awuah, E.; Shakya, T.; Wright, G. D.; Capretta, A. Tetrahedron Lett. 2011, 52, 5761–5763. doi:10.1016/j.tetlet.2011.08.103
Return to citation in text: [1] -
Da Settimo, F.; Primofiore, G.; La Motta, C.; Taliani, S.; Simorini, F.; Marini, A. M.; Mugnaini, L.; Lavecchia, A.; Novellino, E.; Tuscano, D.; Martini, C. J. Med. Chem. 2005, 48, 5162–5174. doi:10.1021/jm050136d
Return to citation in text: [1] -
Kulkarni, A.; Quang, P.; Curry, V.; Keyes, R.; Zhou, W.; Cho, H.; Baffoe, J.; Török, B.; Stieglitz, K. Chem. Biol. Drug Des. 2014, 84, 270–281. doi:10.1111/cbdd.12319
Return to citation in text: [1] -
El Hedi Jellali, M.; Van Bac, N.; Dat-Xuong, N. Tetrahedron 1975, 31, 587–591. doi:10.1016/0040-4020(75)85034-4
Return to citation in text: [1] -
La Motta, C.; Sartini, S.; Mugnaini, L.; Salerno, S.; Simorini, F.; Taliani, S.; Marini, A. M.; Da Settimo, F.; Lavecchia, A.; Novellino, E.; Antonioli, L.; Fornai, M.; Blandizzi, C.; Del Tacca, M. J. Med. Chem. 2009, 52, 1681–1692. doi:10.1021/jm801427r
Return to citation in text: [1] -
Shamroukh, A. H.; Rashad, A. E.; Abdel-Megeid, R. E.; Ali, H. S.; Ali, M. M. Arch. Pharm. 2014, 347, 559–565. doi:10.1002/ardp.201400064
Return to citation in text: [1] -
Semple, G.; Ren, A.; Fioravanti, B.; Pereira, G.; Calderon, I.; Choi, K.; Xiong, Y.; Shin, Y.-J.; Gharbaoui, T.; Sage, C. R.; Morgan, M.; Xing, C.; Chu, Z.-L.; Leonard, J. N.; Grottick, A. J.; Al-Shamma, H.; Liang, Y.; Demarest, K. T.; Jones, R. M. Bioorg. Med. Chem. Lett. 2011, 21, 3134–3141. doi:10.1016/j.bmcl.2011.03.007
Return to citation in text: [1] -
Kreutzberger, A.; Burgwitz, K. Eur. J. Med. Chem. 1979, 14, 539–544.
Return to citation in text: [1] -
Bacon, E. R.; Bailey, T.; Becknell, N. C.; Gingrich, D. E.; Hostetler, G.; Hudkins, R. L.; Learn, K. S.; Wagner, J. C. Substituted pyrazolopyrimidines. U.S. Patent US20070281949, Dec 6, 2007.
Return to citation in text: [1] -
Salaheldin, A. M.; Oliveira-Campos, A. M. F.; Rodrigues, L. M. Synth. Commun. 2009, 39, 1186–1195. doi:10.1080/00397910802517814
Return to citation in text: [1] -
Biggadike, K.; House, D.; Inglis, G. G. A.; Macdonald, S. J. F.; Mclay, I. M.; Skone, P. A. Pyrazolo-pyrimidine derivatives as anti-inflammatory agents. PCT Patent WO2007054294, May 18, 2007.
Return to citation in text: [1] -
Zhang, L.-J.; Shi, D.-X.; Li, J.-R. Synth. Commun. 2009, 39, 4010–4018. doi:10.1080/00397910902883629
Return to citation in text: [1] [2] -
Liu, M.; Li, J.; Chen, S.; Huang, D.; Chai, H.; Zhang, Q.; Shi, D. RSC Adv. 2014, 4, 35629–35634. doi:10.1039/C4RA05346J
Return to citation in text: [1] [2] -
Baraldi, P. G.; Pavani, M. G.; del Carmen Nuñez, M.; Brigidi, P.; Vitali, B.; Gambari, R.; Romagnoli, R. Bioorg. Med. Chem. 2002, 10, 449–456. doi:10.1016/S0968-0896(01)00294-2
Return to citation in text: [1] -
Anderson, J. D.; Cottam, H. B.; Larson, S. B.; Nord, L. D.; Revankar, G. R.; Robins, R. K. J. Heterocycl. Chem. 1990, 27, 439–453. doi:10.1002/jhet.5570270262
Return to citation in text: [1] -
Wamhoff, H.; Ertas, M.; Atta, S. M. S. Liebigs Ann. Chem. 1985, 1910–1916. doi:10.1002/jlac.198519850918
Return to citation in text: [1] -
Schmidt, P.; Eichenberger, K.; Druey, J. Helv. Chim. Acta 1958, 41, 1052–1060. doi:10.1002/hlca.19580410418
Return to citation in text: [1] -
Ferroni, R.; Simoni, D.; Orlandini, P.; Bardi, A.; Franze, G. P.; Guarneri, M. Arzneim. Forsch. 1990, 40, 1328–1331.
Return to citation in text: [1] -
Cheng, C. C.; Robins, R. K. J. Org. Chem. 1958, 23, 852–861. doi:10.1021/jo01100a025
Return to citation in text: [1] -
Miyashita, A.; Iijima, C.; Higashino, T.; Matsuda, H. Heterocycles 1990, 31, 1309–1314. doi:10.3987/COM-90-5407
Return to citation in text: [1] -
Tavakoli-Hoseini, N.; Moloudi, R.; Davoodnia, A.; Shaker, M. Chin. J. Chem. 2011, 29, 2421–2426. doi:10.1002/cjoc.201180411
Return to citation in text: [1] -
Zhang, X. Q.; Abad, M. G.; Gibbs, A. C.; Kuo, G.-H.; Kuo, L. C.; Song, F.; Sui, Z. Indazole compounds useful as ketohexokinase inhibitors. U.S. Patent US20110263559, Oct 27, 2011.
Return to citation in text: [1] -
Xu, L. Pyrimidine derivatives and analogs, preparation method and use thereof. U.S. Patent US20120178915, July 12, 2012.
Return to citation in text: [1] -
Chang, C.-H.; Tsai, H. J.; Huang, Y.-Y.; Lin, H.-Y.; Wang, L.-Y.; Wu, T.-S.; Wong, F. F. Tetrahedron 2013, 69, 1378–1386. doi:10.1016/j.tet.2012.11.002
Return to citation in text: [1] -
Simay, A.; Takacs, K.; Horvath, K.; Dvortsak, P. Acta Chim. Acad. Sci. Hung. 1980, 105, 127–139.
Return to citation in text: [1] -
Srivastava, M.; Rai, P.; Singh, J.; Singh, J. RSC Adv. 2013, 3, 16994–16998. doi:10.1039/c3ra42493f
Return to citation in text: [1] -
Bobko, M. A.; Kaura, A. C.; Evans, K. A.; Su, D.-S. Org. Lett. 2012, 14, 3906–3908. doi:10.1021/ol301655f
Return to citation in text: [1] -
Apsel, B.; Blair, J. A.; Gonzalez, B.; Nazif, T. M.; Feldman, M. E.; Aizenstein, B.; Hoffman, R.; Williams, R. L.; Shokat, K. M.; Knight, Z. A. Nat. Chem. Biol. 2008, 4, 691–699. doi:10.1038/nchembio.117
Return to citation in text: [1] -
Meng, F.; Hou, J.; Shao, Y.-X.; Wu, P.-Y.; Huang, M.; Zhu, X.; Cai, Y.; Li, Z.; Xu, J.; Liu, P.; Luo, H.-B.; Wan, Y.; Ke, H. J. Med. Chem. 2012, 55, 8549–8558. doi:10.1021/jm301189c
Return to citation in text: [1] -
Harden, F. A.; Quinn, R. J.; Scammells, P. J. J. Med. Chem. 1991, 34, 2892–2898. doi:10.1021/jm00113a031
Return to citation in text: [1] -
Liu, J.; Zhang, X.-w.; Wang, Y.; Chen, Y.; Zhang, M.-r.; Cai, Z.-q.; Zhou, Y.-p.; Xu, L.-f. Synth. Commun. 2015, 45, 1009–1017. doi:10.1080/00397911.2014.996296
Return to citation in text: [1] -
Liu, C.; Yu, Q.; Tang, J.; Li, J. Chin. J. Org. Chem. 2012, 32, 532–537. doi:10.6023/cjoc1108102
Return to citation in text: [1] -
Full details have been deposited the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-1401076. Copies of the datacan be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44(0)-1223336033 or e-mail: deposit@ccdc.cam.ac.uk).
Return to citation in text: [1]
| 41. | Chang, C.-H.; Tsai, H. J.; Huang, Y.-Y.; Lin, H.-Y.; Wang, L.-Y.; Wu, T.-S.; Wong, F. F. Tetrahedron 2013, 69, 1378–1386. doi:10.1016/j.tet.2012.11.002 |
| 42. | Simay, A.; Takacs, K.; Horvath, K.; Dvortsak, P. Acta Chim. Acad. Sci. Hung. 1980, 105, 127–139. |
| 37. | Miyashita, A.; Iijima, C.; Higashino, T.; Matsuda, H. Heterocycles 1990, 31, 1309–1314. doi:10.3987/COM-90-5407 |
| 38. | Tavakoli-Hoseini, N.; Moloudi, R.; Davoodnia, A.; Shaker, M. Chin. J. Chem. 2011, 29, 2421–2426. doi:10.1002/cjoc.201180411 |
| 39. | Zhang, X. Q.; Abad, M. G.; Gibbs, A. C.; Kuo, G.-H.; Kuo, L. C.; Song, F.; Sui, Z. Indazole compounds useful as ketohexokinase inhibitors. U.S. Patent US20110263559, Oct 27, 2011. |
| 40. | Xu, L. Pyrimidine derivatives and analogs, preparation method and use thereof. U.S. Patent US20120178915, July 12, 2012. |
| 1. | Schenone, S.; Radi, M.; Musumeci, F.; Brullo, C.; Botta, M. Chem. Rev. 2014, 114, 7189–7238. doi:10.1021/cr400270z |
| 2. | Parker, W. B. Chem. Rev. 2009, 109, 2880–2893. doi:10.1021/cr900028p |
| 3. | Martin, M. W.; Newcomb, J.; Nunes, J. J.; Boucher, C.; Chai, L.; Epstein, L. F.; Faust, T.; Flores, S.; Gallant, P.; Gore, A.; Gu, Y.; Hsieh, F.; Huang, X.; Kim, J. L.; Middleton, S.; Oliveira-dos-Santos, A.; Patel, V. F.; Powers, D.; Rose, P.; Tudor, Y.; Turci, S. M.; Welcher, A. A.; Zack, D.; Zhao, H.; Zhu, L.; Zhu, X.; Ghiron, C.; Ermann, M.; Johnston, D.; Saluste, C.-G. P. J. Med. Chem. 2008, 51, 1637–1648. doi:10.1021/jm701095m |
| 4. | Chauhan, M.; Kumar, R. Bioorg. Med. Chem. 2013, 21, 5657–5668. doi:10.1016/j.bmc.2013.07.027 |
| 5. | Palanisamy, P.; Jenniefer, S. J.; Muthiah, P. T.; Kumaresan, S. RSC Adv. 2013, 3, 19300–19310. doi:10.1039/c3ra42283f |
| 6. | Wang, L.; Zhou, X.; Fredimoses, M.; Liao, S.; Liu, Y. RSC Adv. 2014, 4, 57350–57376. doi:10.1039/C4RA09833A |
| 11. | Shamroukh, A. H.; Rashd, A. E.; Ali, H. S.; Abdel-Megeid, F. M. E. J. Heterocycl. Chem. 2013, 50, 758–765. doi:10.1002/jhet.1550 |
| 12. | Holla, B. S.; Mahalinga, M.; Karthikeyan, M. S.; Akberali, P. M.; Shetty, N. S. Bioorg. Med. Chem. 2006, 14, 2040–2047. doi:10.1016/j.bmc.2005.10.053 |
| 13. | Ali, A.; Taylor, G. E.; Ellsworth, K.; Harris, G.; Painter, R.; Silver, L. L.; Young, K. J. Med. Chem. 2003, 46, 1824–1830. doi:10.1021/jm020483c |
| 32. | Anderson, J. D.; Cottam, H. B.; Larson, S. B.; Nord, L. D.; Revankar, G. R.; Robins, R. K. J. Heterocycl. Chem. 1990, 27, 439–453. doi:10.1002/jhet.5570270262 |
| 9. | Bahashwan, S. A.; Fayed, A. A.; Amr, A. E. E.; Flefel, E. M.; Kalmouch, A. Molecules 2013, 18, 15051–15063. doi:10.3390/molecules181215051 |
| 10. | Nugent, R. A.; Dunn, C. J.; Staite, N. D.; Murphy, M. J.; Schlachter, S. T.; Aspar, D. G.; Shields, S. K.; Galinet, L. A. Phosphorus, Sulfur Silicon Relat. Elem. 1996, 109, 229–232. doi:10.1080/10426509608545132 |
| 33. | Wamhoff, H.; Ertas, M.; Atta, S. M. S. Liebigs Ann. Chem. 1985, 1910–1916. doi:10.1002/jlac.198519850918 |
| 34. | Schmidt, P.; Eichenberger, K.; Druey, J. Helv. Chim. Acta 1958, 41, 1052–1060. doi:10.1002/hlca.19580410418 |
| 35. | Ferroni, R.; Simoni, D.; Orlandini, P.; Bardi, A.; Franze, G. P.; Guarneri, M. Arzneim. Forsch. 1990, 40, 1328–1331. |
| 36. | Cheng, C. C.; Robins, R. K. J. Org. Chem. 1958, 23, 852–861. doi:10.1021/jo01100a025 |
| 7. | Hsu, T. C.; Robins, R. K.; Cheng, C. C. Science 1956, 123, 848–849. doi:10.1126/science.123.3202.848-a |
| 8. | Hafez, T. S.; Osman, S. A.; Yosef, H. A. A.; Abd El-All, A. S.; Hassan, A. S.; El-Sawy, A. A.; Abdallah, M. M.; Youns, M. Sci. Pharm. 2013, 81, 339–357. doi:10.3797/scipharm.1211-07 |
| 29. | Zhang, L.-J.; Shi, D.-X.; Li, J.-R. Synth. Commun. 2009, 39, 4010–4018. doi:10.1080/00397910902883629 |
| 30. | Liu, M.; Li, J.; Chen, S.; Huang, D.; Chai, H.; Zhang, Q.; Shi, D. RSC Adv. 2014, 4, 35629–35634. doi:10.1039/C4RA05346J |
| 50. | Full details have been deposited the Cambridge Crystallographic Data Centre as supplementary publication no. CCDC-1401076. Copies of the datacan be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44(0)-1223336033 or e-mail: deposit@ccdc.cam.ac.uk). |
| 1. | Schenone, S.; Radi, M.; Musumeci, F.; Brullo, C.; Botta, M. Chem. Rev. 2014, 114, 7189–7238. doi:10.1021/cr400270z |
| 31. | Baraldi, P. G.; Pavani, M. G.; del Carmen Nuñez, M.; Brigidi, P.; Vitali, B.; Gambari, R.; Romagnoli, R. Bioorg. Med. Chem. 2002, 10, 449–456. doi:10.1016/S0968-0896(01)00294-2 |
| 18. | Todorovic, N.; Awuah, E.; Shakya, T.; Wright, G. D.; Capretta, A. Tetrahedron Lett. 2011, 52, 5761–5763. doi:10.1016/j.tetlet.2011.08.103 |
| 19. | Da Settimo, F.; Primofiore, G.; La Motta, C.; Taliani, S.; Simorini, F.; Marini, A. M.; Mugnaini, L.; Lavecchia, A.; Novellino, E.; Tuscano, D.; Martini, C. J. Med. Chem. 2005, 48, 5162–5174. doi:10.1021/jm050136d |
| 20. | Kulkarni, A.; Quang, P.; Curry, V.; Keyes, R.; Zhou, W.; Cho, H.; Baffoe, J.; Török, B.; Stieglitz, K. Chem. Biol. Drug Des. 2014, 84, 270–281. doi:10.1111/cbdd.12319 |
| 21. | El Hedi Jellali, M.; Van Bac, N.; Dat-Xuong, N. Tetrahedron 1975, 31, 587–591. doi:10.1016/0040-4020(75)85034-4 |
| 25. | Kreutzberger, A.; Burgwitz, K. Eur. J. Med. Chem. 1979, 14, 539–544. |
| 26. | Bacon, E. R.; Bailey, T.; Becknell, N. C.; Gingrich, D. E.; Hostetler, G.; Hudkins, R. L.; Learn, K. S.; Wagner, J. C. Substituted pyrazolopyrimidines. U.S. Patent US20070281949, Dec 6, 2007. |
| 48. | Liu, J.; Zhang, X.-w.; Wang, Y.; Chen, Y.; Zhang, M.-r.; Cai, Z.-q.; Zhou, Y.-p.; Xu, L.-f. Synth. Commun. 2015, 45, 1009–1017. doi:10.1080/00397911.2014.996296 |
| 16. | Xia, Y.; Chackalamannil, S.; Czarniecki, M.; Tsai, H.; Vaccaro, H.; Cleven, R.; Cook, J.; Fawzi, A.; Watkins, R.; Zhang, H. J. Med. Chem. 1997, 40, 4372–4377. doi:10.1021/jm970495b |
| 17. | Guccione, S.; Modica, M.; Longmore, J.; Shaw, D.; Barretta, G. U.; Santagati, A.; Santagati, M.; Russo, F. Bioorg. Med. Chem. Lett. 1996, 6, 59–64. doi:10.1016/0960-894X(95)00558-B |
| 27. | Salaheldin, A. M.; Oliveira-Campos, A. M. F.; Rodrigues, L. M. Synth. Commun. 2009, 39, 1186–1195. doi:10.1080/00397910802517814 |
| 28. | Biggadike, K.; House, D.; Inglis, G. G. A.; Macdonald, S. J. F.; Mclay, I. M.; Skone, P. A. Pyrazolo-pyrimidine derivatives as anti-inflammatory agents. PCT Patent WO2007054294, May 18, 2007. |
| 49. | Liu, C.; Yu, Q.; Tang, J.; Li, J. Chin. J. Org. Chem. 2012, 32, 532–537. doi:10.6023/cjoc1108102 |
| 15. | Ghorab, M. M.; Ragab, F. A.; Alqasoumi, S. I.; Alafeefy, A. M.; Aboulmagd, S. A. Eur. J. Med. Chem. 2010, 45, 171–178. doi:10.1016/j.ejmech.2009.09.039 |
| 29. | Zhang, L.-J.; Shi, D.-X.; Li, J.-R. Synth. Commun. 2009, 39, 4010–4018. doi:10.1080/00397910902883629 |
| 30. | Liu, M.; Li, J.; Chen, S.; Huang, D.; Chai, H.; Zhang, Q.; Shi, D. RSC Adv. 2014, 4, 35629–35634. doi:10.1039/C4RA05346J |
| 14. | Ram, V. J.; Pandey, H. N.; Mishra, L. Arch. Pharm. 1979, 312, 586–590. doi:10.1002/ardp.19793120705 |
| 22. | La Motta, C.; Sartini, S.; Mugnaini, L.; Salerno, S.; Simorini, F.; Taliani, S.; Marini, A. M.; Da Settimo, F.; Lavecchia, A.; Novellino, E.; Antonioli, L.; Fornai, M.; Blandizzi, C.; Del Tacca, M. J. Med. Chem. 2009, 52, 1681–1692. doi:10.1021/jm801427r |
| 23. | Shamroukh, A. H.; Rashad, A. E.; Abdel-Megeid, R. E.; Ali, H. S.; Ali, M. M. Arch. Pharm. 2014, 347, 559–565. doi:10.1002/ardp.201400064 |
| 24. | Semple, G.; Ren, A.; Fioravanti, B.; Pereira, G.; Calderon, I.; Choi, K.; Xiong, Y.; Shin, Y.-J.; Gharbaoui, T.; Sage, C. R.; Morgan, M.; Xing, C.; Chu, Z.-L.; Leonard, J. N.; Grottick, A. J.; Al-Shamma, H.; Liang, Y.; Demarest, K. T.; Jones, R. M. Bioorg. Med. Chem. Lett. 2011, 21, 3134–3141. doi:10.1016/j.bmcl.2011.03.007 |
| 43. | Srivastava, M.; Rai, P.; Singh, J.; Singh, J. RSC Adv. 2013, 3, 16994–16998. doi:10.1039/c3ra42493f |
| 44. | Bobko, M. A.; Kaura, A. C.; Evans, K. A.; Su, D.-S. Org. Lett. 2012, 14, 3906–3908. doi:10.1021/ol301655f |
| 45. | Apsel, B.; Blair, J. A.; Gonzalez, B.; Nazif, T. M.; Feldman, M. E.; Aizenstein, B.; Hoffman, R.; Williams, R. L.; Shokat, K. M.; Knight, Z. A. Nat. Chem. Biol. 2008, 4, 691–699. doi:10.1038/nchembio.117 |
| 46. | Meng, F.; Hou, J.; Shao, Y.-X.; Wu, P.-Y.; Huang, M.; Zhu, X.; Cai, Y.; Li, Z.; Xu, J.; Liu, P.; Luo, H.-B.; Wan, Y.; Ke, H. J. Med. Chem. 2012, 55, 8549–8558. doi:10.1021/jm301189c |
| 47. | Harden, F. A.; Quinn, R. J.; Scammells, P. J. J. Med. Chem. 1991, 34, 2892–2898. doi:10.1021/jm00113a031 |
© 2015 Liu et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)