Abstract
Small differences in the reactivity of weakly nucleophilic potassium aryltrifluoroborates are revealed in the silver-assisted Pd-catalyzed cross-coupling of K[4-RC6F4BF3] (R = H, Bu, MeO, EtO, PrO, iPrO, BuO, t-BuO, CH2=CHCH2O, PhCH2O, PhCH2CH2O, PhO, F, pyrazol-1-yl, pyrrol-1-yl, and indol-1-yl) with ArX (4-BrC6H4CH3, 4-IC6H4F and 3-IC6H4F). An assumed role of silver(I) compounds AgmY (Y = O, NO3, SO4, BF4, F) consists in polarization of the Pd–X bond in neutral complex ArPdLnX with the generation of the related transition state or formation of [ArPdLn][XAgmY] with a highly electrophilic cation and subsequent transmetallation with the weakly nucleophilic borate. Efficiency of AgmY as a polarizing agent decreases in order Ag2O > AgNO3 ≈ Ag2SO4 > Ag[BF4] > AgF. No clear correlation between the reactivity of K[4-RC6F4BF3] and substituent electron parameters, σI and σR°, of the aryl group 4-RC6F4 was found.
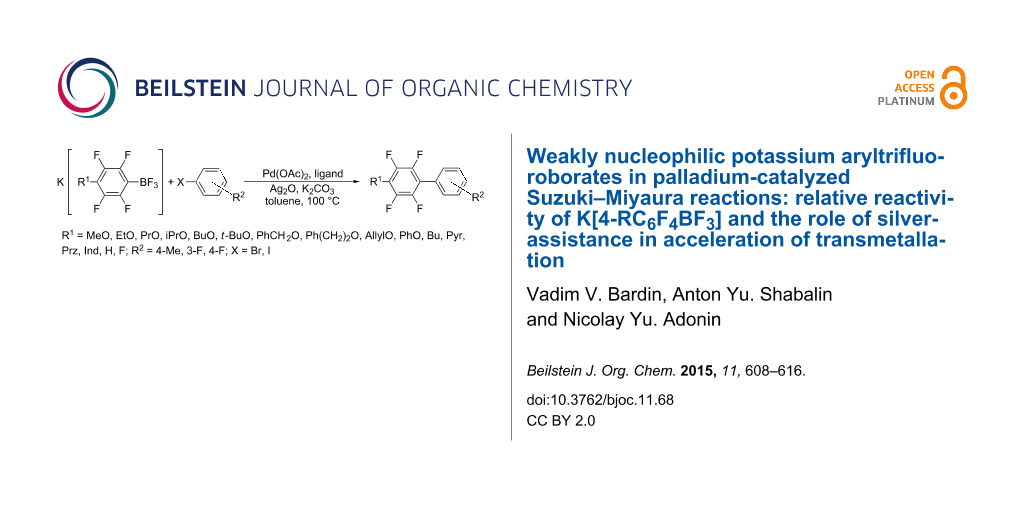
Graphical Abstract
Introduction
The palladium-catalyzed reaction of organoboron compounds with C-electrophiles (Suzuki–Miyaura reaction) is one of the most intensively studied processes of the carbon–carbon bond formation. Organoboronic acids, their esters and organotrifluoroborates are partners in these reactions and the choice of the desired reagent depends on the specific requirements in each particular case [1-3]. Organoboron reagents containing an electron-poor organic moiety exhibit a low reactivity under the usual cross-coupling conditions [3-9] and the target products are formed in low yield and/or are contaminated with byproducts.
Reactions of weakly nucleophilic organoboron reagents (alkyl- and cyclopropylboronic acids and esters [10-14], alken-1-ylboronic acids and esters [15,16], some arylboronic acids [17-19], K[CF2=CFBF3] [19]) often are accelerated by the addition of stoichiometric amounts of Ag2O. Initially this phenomenon was reported by Kishi et al. [16] for the cross-coupling of alkenylboronic acids with alkenyl iodides in the presence of Ag2O and elucidated by formation of AgOH which acts like aqueous KOH or TlOH. Korenaga et al. [20] have studied Pd-catalyzed cross-coupling of C6F5B(OH)2 with aryl halides in the presence of Ag2O and assumed the generation of a hydroxy–palladium complex with higher ability to transmetallation under the action of the corresponding organoboron reagent than ArPdL2X. For example, complex trans-C6F5Pd(PEt3)2OH is formed in the reaction of trans-C6F5Pd(PEt3)2I with Ag2O in toluene–water and undergoes transmetallation with 4-MeOC6H4B(OH)2 [21]. The subsequent reductive elimination leads to the corresponding polyfluorobiphenyl. In contrary, the reaction of trans-C6F5Pd(PEt3)2I with 2,4,6-C6F3H2B(OH)2 and Ag2O leads to an unsymmetrical diarylpalladium complex trans-(C6F5)Pd(PEt3)2(2,4,6-C6F3H2) in 92% yield. The latter is thermally stable and does not produce the cross-coupling product even upon heating in toluene at 100 °C for 24 h [21]. The authors suggested that acceleration of these reactions by silver(I) oxide results in the generation of a hydroxy–palladium complex with a higher ability to transmetallation, to coordinate to the organoboronic acid with the three-coordinated boron atom to ArPdL2OH and subsequent transmetallation (Scheme 1).
Scheme 1: The assumed silver(I) oxide assisted transmetallation with organoboronic acids.
Scheme 1: The assumed silver(I) oxide assisted transmetallation with organoboronic acids.
However, some experimental facts indicate the multilateral role of silver(I) compounds AgmY in the acceleration of the Suzuki–Miyaura cross-coupling. Beside generation of ArPdLnOH, AgmY may act as a Lewis acid producing complex [RPdLn][XAgmY] with a highly electrophilic cation. The reactions often are performed in polar aprotic solvents (MeCN, THF, acetone) and the complexes exist in solutions as solvates [RPdLn(solv.)][XAgmY]. Some complexes are much more stable and can be isolated [22,23], although for preparative aims they usually are generated in situ. This approach has been successfully used for arylation of alkenes, alkynes, insertion of CO species [23-31], polyfluoroarenes and thiophenes [32].
The effect of the counterion Y in silver(I) compounds AgmY on the rate of the cross-coupling was slightly investigated. For example, the rates of the CO insertion into cationic methylpalladium complex [MePd(PMe3)2][XAgmY] generated from MePd(PMe3)2Cl, Ag[BF4] or Ag[PF6] in acetone are equal to 23 ∙ 10−5 and 24 ∙ 10−5 s−1. The use of AgOTf (11 ∙ 10−5 s−1) or AgNO3 (5.9 ∙ 10−5 s–1) causes a decrease in the reactivity towards the CO insertion [28,33]. The similar effect of anions Y = BF4, OTf, PF6 was observed in the formation of complexes [RPd(PMe3)2(solv.)][XAgmY] and [RPd(PMe3)(solv.)2][XAgmY] [34].
In continuation of systematic research of the Suzuki–Miyaura cross-coupling reaction of weakly nucleophilic organotrifluoroborates we report here the study of the relative reactivity of K[4-RC6F4BF3] in the Pd-catalyzed reactions with some aryl bromides and iodides in the presence of Ag2O. The borates were chosen as model organoboron reagents because of tuning electronic properties of 4-RC6F4 groups with varied R = H, Bu, MeO, EtO, PrO, iPrO, BuO, t-BuO, CH2=CHCH2O, PhCH2O, Ph(CH2)2O, PhO, pyrazol-1-yl (Prz), pyrrol-1-yl (Pyr), indol-1-yl (Ind), imidazol-1-yl (Im), and benzamidazol-1-yl (Bim) and the equal steric requirements near reaction site C1–BF3. The relative reactivity was estimated (a) from the yield of biphenyl 4-RC6F4–Ar vs C6F5–Ar and (b) from the rate of consumption of K[4-RC6F4BF3] and compared with the substituent electron parameters (SEP) of the 4-RC6F4 group. The efficiency of some other silver(I) compounds (AgNO3, Ag2SO4, Ag[BF4], and AgF) in the cross-coupling was examined too.
Results
All reactions were carried out under the previously elaborated optimal conditions [35]. The cross-coupling of K[4-RC6F4BF3] (1a–r) with 1-fluoro-3-iodobenzene (2) produces pentafluorobiphenyls 4-(3'-FC6H4)C6F4R in 80–99% yield (Scheme 2 and Table 1). Pentafluorobiphenyls 4-(4'-FC6H4)C6F4R were obtained from K[4-RC6F4BF3] and 1-fluoro-4-iodobenzene (3). Notably borates K[4-RC6F4BF3] with R = H (1b), AllylO (1c) and azolides (1n–r) (see Table 1, entries 2, 3, 14–18) give much lower yields of the corresponding biphenyls than K[C6F5BF3] (1a) (Table 1, entry 1) [19].
Scheme 2: Cross-coupling of K[4-RC6F4BF3] (1a–r) with 3-IC6H4F (2) and 4-IC6H4F (3).
Scheme 2: Cross-coupling of K[4-RC6F4BF3] (1a–r) with 3-IC6H4F (2) and 4-IC6H4F (3).
Table 1: Preparation of biphenyls 4a–r and 5a–r.
| Entry | K[4-RC6F4BF3] | Isolated yield (%) | |
|---|---|---|---|
| 4 | 5 | ||
| 1 | 1a (R = F) | 99 | 99 |
| 2 | 1b (R = H) | 26 | 24 |
| 3 | 1c (R = AllylO) | 34 | 18 |
| 4 | 1d (R = MeO) | 90 | 94 |
| 5 | 1e (R = EtO) | 98 | 97 |
| 6 | 1f (R = PrO) | 82 | 96 |
| 7 | 1g (R = Bu) | 89 | 50 |
| 8 | 1h (R = PhCH2O) | 86 | 73 |
| 9 | 1i (R = BuO) | 87 | 99 |
| 10 | 1j (R = iPrO) | 82 | 97 |
| 11 | 1k (R = t-BuO) | 98 | 99 |
| 12 | 1l (R = Ph(CH2)2O) | 79 | 71 |
| 13 | 1m (R = PhO) | 98 | 96 |
| 14 | 1n (R = Ind) | 91 | 42 |
| 15 | 1o (R = Pyr) | 84 | 20 |
| 16 | 1p (R = Prz) | 47 | 35a |
| 17 | 1q (R = Im) | 10b, c | 12b, d |
| 18 | 1r (R = Bim) | 20b, e | 20b, f |
aByproduct 2,3,5,6-C6F4HPrz (3%); bphosphine XPhos was used instead of PPh3; cbyproduct 2,3,5,6-C6F4HIm (7%); dbyproduct 2,3,5,6-C6F4HIm (7%); ebyproduct 2,3,5,6-C6F4HBim (5%); fbyproduct 2,3,5,6-C6F4HBim (14%).
Potassium 2,3,5,6-tetrafluoropyridyltrifluoroborate (6) exhibits extremely low reactivity toward both 2 and 3 gives the corresponding 4-(3'-fluorophenyl)- (7) and 4-(4'-fluorophenyl)- (8) -2,3,5,6-tetrafluoropyridines with yields no more than 5% (19F NMR) (Scheme 3). It should be noted that 2,3,5,6-tetrafluoropyridine was not observed in the reaction mixtures in both cases.
Scheme 3: Attempted synthesis of 7 (3’-F) and 8 (4’-F) by cross-coupling reaction.
Scheme 3: Attempted synthesis of 7 (3’-F) and 8 (4’-F) by cross-coupling reaction.
The use of the electron-rich C-electrophile, 4-IC6H4CH3 (9), instead of 3 leads to biphenyls 4-(4'-CH3C6H4)C6F4R (10c–f,h–p) in 60–80% preparative yields (see Table 2, entries 2–14) while 4-CH3C6H4C6F5 (10a) was isolated in 93% yield (see Table 2, entry 1) [19]. Although aryl bromides are less reactive than iodides, the substitution of 4-IC6H4CH3 (9) for 4-BrC6H4CH3 (11) does not affect the yields of the corresponding biphenyls (Scheme 4).
Scheme 4: Synthesis of biphenyls 10a,c–f,h–p.
Scheme 4: Synthesis of biphenyls 10a,c–f,h–p.
Table 2: Preparation of biphenyls 10a,c–f,h–p.
| Entry | K[4-RC6F4BF3] | Isolated yield of 10 (%) | Entry | K[4-RC6F4BF3] | Isolated yield of 10 (%) |
|---|---|---|---|---|---|
| 1 | 1a (R = F) | 93 | 8 | 1j (R = iPrO) | 71 |
| 2 | 1c (R = AllylO) | 25a | 9 | 1k (R = t-BuO) | 62 |
| 3 | 1d (R = MeO) | 80 | 10 | 1l (R = Ph(CH2)2O) | 93 |
| 4 | 1e (R = EtO) | 70 | 11 | 1m (R = PhO) | 65 |
| 5 | 1f (R = PrO) | 83 | 12 | 1n (R = Ind) | 91 |
| 6 | 1h (R = PhCH2O) | 43b | 13 | 1o (R = Pyr) | 73 |
| 7 | 1i (R = BuO) | 97 | 14 | 1p (R = Prz) | 99 |
aByproduct 2,3,5,6-C6F4HOCH2CH=CH2 (18%); bbyproduct 2,3,5,6-C6F4HOCH2Ph (28%).
The obtained results show a similar or slightly reduced reactivity of the majority of K[4-RC6F4BF3] (R ≠ F) with respect to salt 1a. Exceptions are borates with R = H (1b), CH2=CHCH2O (1c) and azolides (1n–r), which produce cross-coupling products in low to moderate yields because of the side reactions. Highly tolerant borate 6 also gives the product in a low yield, but its conversion is low too. Hence, the data based on an isolated yield of biphenyls 4a–r, 5a–r, or 10a,c–f,h–p are not a convenient measure for the quantitative estimation of the relative reactivity. We hoped to get more accurate data by the concurrent cross-coupling of equimolar mixtures of K[C6F5BF3] (1a) and K[4-RC6F4BF3] (1b–p) with 11. The reactions were carried out over a short period (5–15 min) and the product ratios were determined by analyzing the crude reaction mixtures with 19F NMR spectroscopy. The relative reactivity was determined as Crel = CR / CF where CR and CF are the yield (in mmol) of 4-(4'-CH3C6H4)C6F4R from salts 1b–p and 4'-CH3C6H4C6F5 from 1a, respectively (Scheme 5, Table 3).
Scheme 5: Pd-catalyzed cross-coupling of 1a and salts 1b–p (1:1) with 11 (the results are presented in Table 3 and outlined in the Discussion section).
Scheme 5: Pd-catalyzed cross-coupling of 1a and salts 1b–p (1:1) with 11 (the results are presented in Table 3 and o...
Table 3: Relative reactivity Crel of K[4-RC6F4BF3] in the cross coupling with 11.a,b
| Entry | K[4-RC6F4BF3] | CR | CF | Crel |
|---|---|---|---|---|
| 1 | 1c (R = AllylO) | 13 | 22 | 0.59 |
| 2 | 1g (R = Bu) | 27 | 40 | 0.68 |
| 3 | 1f (R = PrO) | 22 | 32 | 0.68 |
| 4 | 1d (R = MeO) | 22 | 29 | 0.76 |
| 5 | 1h (R = PhCH2O) | 25 | 32 | 0.78 |
| 6 | 1b (R = H) | 22 | 28 | 0.79 |
| 7 | 1i (R = BuO) | 23 | 28 | 0.82 |
| 8 | 1k (R = t-BuO) | 26 | 29 | 0.90 |
| 9 | 1j (R = iPrO) | 23 | 25 | 0.92 |
| 10 | 1e (R = EtO) | 31 | 33 | 0.94 |
| 11 | 1m (R = PhO) | 20 | 21 | 0.95 |
| 12 | 1n (R = Ind) | 26 | 27 | 0.96 |
| 13 | 1l (R = Ph(CH2)2O) | 22 | 22 | 1.00 |
| 14 | 1o (R = Pyr) | 26 | 25 | 1.04 |
| 15 | 1p (R = Prz) | 26 | 25 | 1.04 |
aConditions: Pd(OAc)2, P(t-Bu)3, Ag2O, K2CO3 in toluene, 100 °C, 5–15 min; bCrel = CR/CF, where CR = 4-(4'-CH3C6H4)C6F4R (mmol)/K[4-RC6F4BF3] (0.20 mmol) and CF = 4'-CH3C6H4C6F5 (mmol)/K[C6F5BF3] (0.20 mmol).
The above experiments were performed using silver(I) oxide. For better understanding the role of Ag+ we estimated the relative efficiency of some other silver(I) compounds under identical conditions (Scheme 6) (Table 4).
Scheme 6: The cross-coupling of 1a with 11 in the presence of different silver(I) compounds.
Scheme 6: The cross-coupling of 1a with 11 in the presence of different silver(I) compounds.
Table 4: Cross-coupling of 1a with 11 in the presence of different silver(I) compounds.
| Entry | Conversion of 1a ( %) | AgmY | Yield (%)a,b | ||
|---|---|---|---|---|---|
| 10a | 12 | 13 | |||
| 1 | 69 | Ag2O | 99 | 1 | 0 |
| 2 | 79 | AgNO3 | 20 | 27 | 27 |
| 3 | 75 | Ag2SO4 | 20 | 28 | 26 |
| 4 | 40 | Ag[BF4] | 40 | 60 | 0 |
| 5 | 19 | AgF | 51 | 43 | 3 |
aYields were determined by 19F NMR; bcalculated on reacted 1a.
The presented data demonstrate clearly that AgNO3, Ag2SO4, Ag[BF4], and AgF salts are less appropriate promotors for the palladium catalyzed cross-coupling than Ag2O. The significant contribution of the side reactions (hydrodeboration and homo-coupling) results in decreasing yields of 10a and hinders isolation of the desired product (see Table 4).
Discussion
The general concept of the Pd-catalyzed Suzuki–Miyaura (SM) reaction applied to the cross-coupling of K[4-RC6F4BF3] with ArX is presented in Scheme 7.
Scheme 7: General concept of Pd-catalyzed Suzuki–Miyaura reaction.
Scheme 7: General concept of Pd-catalyzed Suzuki–Miyaura reaction.
The first step is the generation of neutral complex ArPdX by the oxidative addition of ArX to Pd(0) species. This step precedes the subsequent transformation of ArPdX and does not influence the reactivity of organoboron partner K[4-RC6F4BF3] as well as its behavior in transmetallation and/or reductive elimination steps. For the further consideration we need to clarify the nature of the active organoboron partner. Despite of many publications [2,6,7,36,37] in this field it is not yet fully understood. For illustration, we refer to two examples. Molander argues for direct transmetallation of ArPdBr by aryltrifluoroborates in aprotic anhydrous THF in the presence of trialkylamine as a base or in alcohols [4]. Alternatively, the Pd-catalyzed cross-coupling of K[ArBF3] in aqueous THF is proved to proceed through stepwise hydrolysis to produce more reactive [ArBFn(OH)3-n]− or ArB(OH)2 [35,38]. However, special experiments showed that K[C6F5BF3] retards toward K2CO3 [39] as well as K2CO3 in a mixture with catalytic amounts of Pd(OAc)2 in refluxing MeOH [19]. Resistance of K[C6F5BF3] (1a) towards K2CO3 and Ag2O in toluene (without palladium catalyst and phosphine ligand) is confirmed by stirring the corresponding suspensions at 100 °C for 10 min. These facts make it possible to reject any transformation of the [BF3]− moiety before the transmetallation of the palladium-containing intermediate generated in the previous step of the catalytic cycle by K[4-RC6F4BF3]. Potassium carbonate scavenges toluene-soluble acidic impurities formed in the reaction and does not participate in the other steps [19,35].
The boron atom in K[4-RC6F4BF3] is coordinately saturated and transmetallation via transition state A (Scheme 1) is impossible. An alternative pathway is the polarization of the palladium–X bond by Ag+ and the generation of complex [ArPdLn][XAgmY] with a highly electrophilic cation which is attacked by carbon atom C-1 of K[C6F5BF3] (1a). In toluene salt 1a is consumed within 3 h to give 2,3,4,4’,5,6-hexafluorobiphenyl (5a) in 70–92% yield [19], while in the polar coordinating solvents (DME, DMF) the Ag2O-assisted cross-coupling of 1a with 3 leads to formation of 5a at unsatisfactory conversions (22–38%) and low yields (10–22%). This indicates that the strong solvation of the Pd atom with DME or DMF reduces electrophilicity of [ArPdLn][X] as compared with the electrophilicity in the case of weakly coordinating toluene.
K[C6F5BF3] (1a) and AgmY are insoluble in toluene and, very likely, the transmetallation proceeds on the surface of the silver(I) compounds. Both the aryltrifluoroborate anion and ArPdLnX can be adsorbed on the surface of solid AgmY due to the interaction with Ag atoms (acidic Lewis centers). This phenomenon causes an increase in the electrophilicity of the Pd atom and bond rearrangement through transition state B to give the diarylpalladium species (Scheme 8). Perhaps, transformations presented in Scheme 8 are parallel, i.e., the transmetallation proceeds simultaneously with polarization of ArPdLnX.
Scheme 8: Assumed silver(I)-assisted transmetallation of weakly nucleophilic aryitrifluoroborates.
Scheme 8: Assumed silver(I)-assisted transmetallation of weakly nucleophilic aryitrifluoroborates.
There is a popular notion that the transmetallation gives both cis- and trans-ArPdLnAr'. First of them is low stable and undergoes easy reductive elimination to biphenyl. trans-ArPdLnAr' can react only after transformation to the corresponding cis-isomer [40,41]. Complexes with polyfluorinated aryl groups are highly stable and do not isomerize to the cis-isomer as well as do not form the cross-coupling products. Thus, independently prepared complexes trans-C6F5PdL2(2,4,6-C6F3H2) (L = PEt3, PMe2Ph, PMePh2) do not undergo any changes even after heating in toluene at 100 °C for 24 h [21]. These facts lead to assume either instability of trans-(4-RC6F4)PdL2Ar towards isomerisation to cis-isomer or the direct formation of reactive complex cis-(4-RC6F4)PdL2Ar during the transmetallation with closely related borates K[4-RC6F4BF3] (Scheme 8).
At the next step, cis-(4-RC6F4)PdL2Ar undergoes reductive elimination which includes the carbon–palladium bond cleavage in both 4-RC6F4-Pd and Pd-Ar moieties. If the substituent Ar is the same for all cis-diarylpalladium, the rate of the intramolecular transformation from cis-(4-RC6F4)PdL2Ar to 4-RC6F4Ar should depend on the specific property of the 4-RC6F4 group. It may depend on the substituent electron parameters (SEP), σI and σR°, of substituent R or 4-RC6F4 groups. We compared the relative rates of consumption Crel (Table 3) of K[4-RC6F4BF3] with σI (R) and σR° (R) (Table 5) and did not find any correlation between the obtained values.
SEP of the 4-RC6F4 groups are not reported as yet except inductive constants σI (C6F5) [43,45,46] and σI (2,3,5,6-C6F4H) [43], and resonance constants σR° (C6F5) [45]. We bridge this gap using a series of biphenyls 4a–r and 5a–r and determine SEP of 4-RC6F4 groups using the Taft's method [42]. The 19F NMR spectra were measured in CHCl3 (non-polar weakly coordinating solvent) and in toluene (solvent for the present research). The calculated SEP values in both solvents are closely related to one another to indicate no specific intermolecular interaction biphenyl–solvent (Table 6) and obtained results are in agreement with the data by Sheppard for CCl3F or benzene [45]. When R = H, Bu, or alkoxy group, inductive constants σI (4-RC6F4) consist of 0.16–0.18. The 4-RC6F4 groups with R = F, pyrazol-1-yl, pyrrol-1-yl, indol-1-yl, imidazol-1-yl and benzimidazol-1-yl possess a higher electron-withdrawing effect (σI = 0.21 − 0.27) which achieves maximum for the 2,3,5,6-tetrafluoropyridyl group (σI = 0.35). Resonance constants σR° of all tetrafluoroaryl groups are insignificant and reflect the substantial non-coplanarity of aryl moieties [45].
Table 6: The substituent electron parameters (SEP) of 4-RC6F4 groups.
| R | σI | σR° | ||
|---|---|---|---|---|
| In toluene | In CHCl3 | In toluene | In CHCl3 | |
| 4-PhCH2O | 0.160 | 0.172 | 0.010 | 0.014 |
| 4-iPrO | 0.161 | 0.009 | ||
| 4-PrO | 0.163 | 0.008 | ||
| 4-t-BuO | 0.163 | 0.165 | 0.011 | 0.015 |
| 4-BuO | 0.164 | 0.165 | 0.008 | 0.011 |
| 4-EtO | 0.165 | 0.167 | 0.008 | 0.011 |
| 4-Bu | 0.165 | 0.158 | 0.013 | 0.015 |
| 4-AllylO | 0.166 | 0.010 | ||
| 4-MeO | 0.168 | 0.009 | ||
| 4-PhCH2CH2O | 0.170 | 0.008 | ||
| 4-Ha | 0.179 | 0.193 | 0.029 | |
| 4-PhO | 0.201 | 0.211 | 0.021 | 0.023 |
| 4-Prz | 0.214 | 0.253 | 0.033 | 0,037 |
| 4-Pyr | 0.218 | 0.240 | 0.027 | 0.030 |
| 4-Fb | 0.220 | 0.235 | 0.022 | 0.025 |
| 4-Ind | 0.235 | 0.250 | 0.032 | 0.034 |
| 4-Im | 0.260 | 0.036 | ||
| 4-Bim | 0.270 | 0.293 | 0.040 | 0.044 |
| 4-CF3C6F4 | 0.300 | 0.056 | ||
| 4-C5NF4 | 0.346 | 0.064 | ||
| 2-C5NF4 | 0.248 | 0.070 | ||
aσI = 0.33 (water, 25 °C) [43]; bσI = 0.31 (water, 25 °C) [43,46], 0.25 (CCl3F) [45]; σR° = 0.02 (CCl3F) [45].
Unfortunately, the search for possible dependence between the relative rates of consumption of K[4-RC6F4BF3] and SEP of the respective 4-RC6F4 group does not show certain correlation.
Conclusion
1. The relative reactivities of K[4-RC6F4BF3] in the Ag(I)-assisted Pd-catalyzed cross-coupling reactions with 3-IC6H4F, 4-IC6H4F, 4-IC6H4CH3 or 4-BrC6H4CH3 in the presence of P(t-Bu)3 or PPh3 (toluene, 100 °C, 8 h) differ negligibly from each other. The same results show the competitive cross-coupling of mixtures of K[4-RC6F4BF3] and K[C6F5BF3] (1:1, mol) with 4-BrC6H4CH3 at a low conversion of borates (<40%). K[2,3,5,6-C5NF4BF3] is extremely weakly nucleophilic and conversion in the cross-coupling product is no more than 5% in 8 h.
2. It is very likely that transmetallation of ArPdLnX with K[4-RC6F4BF3] proceeds on the surface of the silver(I) compounds (K[4-RC6F4BF3] and AgmY are insoluble in toluene). Neutral complex ArPdLnX is adsorbed on the acidic center (Ag+) of the solid surface to form highly reactive complex [ArPdLn···X···AgmY] which facilitates exchange of group BF3 in K[4-RC6F4BF3] by ArPdLn and thus accelerates the formation of (4-RC6F4)PdLnAr.
3. Verification of the assumed correlation between reactivity (Crel) of K[4-RC6F4BF3] and the substituent electron parameters (SEP) (σI and σR°) as the measure of the electron-withdrawing properties of 4-RC6F4 gives an unclear result. Intervals of change of both Crel (0.6–1.0) and σI (0.16–0.27) are too narrow and small experimental errors of measurements may corrupt or mask electronic effect of 4-RC6F4 with respect to C6F5 group.
Supporting Information
| Supporting Information File 1: Full experimental details and compound characterization data. | ||
| Format: PDF | Size: 239.4 KB | Download |
References
-
Lennox, A. J. J.; Lloyd-Jones, G. C. Chem. Soc. Rev. 2014, 43, 412–443. doi:10.1039/C3CS60197H
Return to citation in text: [1] -
Molander, G. A.; Ellis, N. Acc. Chem. Res. 2007, 40, 275–286. doi:10.1021/ar050199q
Return to citation in text: [1] [2] -
Molander, G. A.; Canturk, B. Angew. Chem., Int. Ed. 2009, 48, 9240–9261. doi:10.1002/anie.200904306
Return to citation in text: [1] [2] -
Molander, G. A.; Biolatto, B. J. Org. Chem. 2003, 68, 4302–4314. doi:10.1021/jo0342368
Return to citation in text: [1] [2] -
Miyaura, N. Organoboron Compounds. In Cross-Coupling Reactions; Miyaura, N., Ed.; Topics in Current Chemistry, Vol. 219; Springer: Berlin, Germany, 2002; pp 11–59. doi:10.1007/3-540-45313-X_2
Return to citation in text: [1] -
Darses, S.; Genet, J.-P. Chem. Rev. 2008, 108, 288–325. doi:10.1021/cr0509758
Return to citation in text: [1] [2] -
Stefani, H. A.; Cella, R.; Vieira, A. S. Tetrahedron 2007, 63, 3623–3658. doi:10.1016/j.tet.2007.01.061
Return to citation in text: [1] [2] -
Adonin, N. Y.; Bardin, V. V. Chim. Oggi 2009, 27, 31–33.
Return to citation in text: [1] -
Adonin, N. Yu.; Bardin, V. V. Russ. Chem. Rev. 2010, 79, 757–785. doi:10.1070/RC2010v079n09ABEH004136
Return to citation in text: [1] -
Chen, H.; Deng, M.-Z. Org. Lett. 2000, 2, 1649–1651. doi:10.1021/ol000013h
Return to citation in text: [1] -
Chen, H.; Deng, M.-Z. J. Chem. Soc., Perkin Trans. 1 2000, 1609–1613. doi:10.1039/B000121J
Return to citation in text: [1] -
Yao, M.-L.; Deng, M.-Z. J. Org. Chem. 2000, 65, 5034–5036. doi:10.1021/jo000195t
Return to citation in text: [1] -
Zhou, S.-m.; Deng, M.-z. Tetrahedron Lett. 2000, 41, 3951–3954. doi:10.1016/S0040-4039(00)00490-1
Return to citation in text: [1] -
Zou, G.; Reddy, Y. K.; Falck, J. R. Tetrahedron Lett. 2001, 42, 7213–7215. doi:10.1016/S0040-4039(01)01536-2
Return to citation in text: [1] -
Occhiato, E. G.; Trabocchi, A.; Guarna, A. J. Org. Chem. 2001, 66, 2459–2465. doi:10.1021/jo001807c
Return to citation in text: [1] -
Uenishi, J.; Beau, J. M.; Armstrong, R. W.; Kishi, Y. J. Am. Chem. Soc. 1987, 109, 4756–4758. doi:10.1021/ja00249a069
Return to citation in text: [1] [2] -
Chen, J.; Cammers-Goodwin, A. Tetrahedron Lett. 2003, 44, 1503–1506. doi:10.1016/S0040-4039(02)02793-4
Return to citation in text: [1] -
Gillmann, T.; Weeber, T. Synlett 1994, 649–650. doi:10.1055/s-1994-22961
Return to citation in text: [1] -
Frohn, H.-J.; Adonin, N. Yu.; Bardin, V. V.; Starichenko, V. F. Tetrahedron Lett. 2002, 43, 8111–8114. doi:10.1016/S0040-4039(02)01922-6
Return to citation in text: [1] [2] [3] [4] [5] [6] [7] -
Korenaga, T.; Kosaki, T.; Fukumura, R.; Ema, T.; Sakai, T. Org. Lett. 2005, 7, 4915–4917. doi:10.1021/ol051866i
Return to citation in text: [1] -
Nishihara, Y.; Onodera, H.; Osakada, K. Chem. Commun. 2004, 192–193. doi:10.1039/B308741G
Return to citation in text: [1] [2] [3] -
Yagyu, T.; Hamada, M.; Osakada, K.; Yamamoto, T. Organometallics 2001, 20, 1087–1101. doi:10.1021/om0007466
Return to citation in text: [1] -
Mecking, S. Coord. Chem. Rev. 2000, 203, 325–351. doi:10.1016/S0010-8545(99)00229-5
Return to citation in text: [1] [2] -
van Asselt, R.; Gielens, E. E. C. G.; Rulke, R. E.; Vrieze, K.; Elsevier, C. J. J. Am. Chem. Soc. 1994, 116, 977–985. doi:10.1021/ja00082a020
Return to citation in text: [1] -
Kawataka, F.; Kayaki, Y.; Shimizu, I.; Yamamoto, A. Organometallics 1994, 13, 3517–3524. doi:10.1021/om00021a027
Return to citation in text: [1] -
Dekker, G. P. C. M.; Elsevier, C. J.; Vrieze, K.; Van Leeuwen, P. W. N. M. Organometallics 1992, 11, 1598–1603. doi:10.1021/om00040a034
Return to citation in text: [1] -
Ozawa, F.; Sugimoto, T.; Yuasa, Y.; Santra, M.; Yamamoto, T.; Yamamoto, A. Organometallics 1984, 3, 683–692. doi:10.1021/om00083a007
Return to citation in text: [1] -
Kayaki, Y.; Shimizu, I.; Yamamoto, A. Chem. Lett. 1995, 24, 1089–1090. doi:10.1246/cl.1995.1089
Return to citation in text: [1] [2] -
Kawataka, F.; Shimizu, I.; Yamamoto, A. Bull. Chem. Soc. Jpn. 1995, 68, 654–660. doi:10.1246/bcsj.68.654
Return to citation in text: [1] -
Kayaki, Y.; Kawataka, F.; Shimizu, I.; Yamamoto, A. Chem. Lett. 1994, 23, 2171–2174. doi:10.1246/cl.1994.2171
Return to citation in text: [1] -
Denmark, S. E.; Schnute, M. E. J. Org. Chem. 1995, 60, 1013–1019. doi:10.1021/jo00109a037
Return to citation in text: [1] -
René, O.; Fagnou, K. Org. Lett. 2010, 12, 2116–2119. doi:10.1021/ol1006136
Return to citation in text: [1] -
Kayaki, Y.; Shimizu, I.; Yamamoto, A. Bull. Chem. Soc. Jpn. 1997, 70, 917–927. doi:10.1246/bcsj.70.917
Return to citation in text: [1] -
Kayaki, Y.; Shimizu, I.; Yamamoto, A. Bull. Chem. Soc. Jpn. 1997, 70, 1135–1140. doi:10.1246/bcsj.70.1135
Return to citation in text: [1] -
Shabalin, A. Yu.; Adonin, N. Yu.; Bardin, V. V.; Parmon, V. N. Tetrahedron 2014, 70, 3720–3725. doi:10.1016/j.tet.2014.04.019
Return to citation in text: [1] [2] [3] -
Lennox, A. J. J.; Lloyd-Jones, G. C. J. Am. Chem. Soc. 2012, 134, 7431–7441. doi:10.1021/ja300236k
Return to citation in text: [1] -
Ting, R.; Harwig, C. W.; Lo, J.; Li, Y.; Adam, M. J.; Ruth, T. J.; Perrin, D. M. J. Org. Chem. 2008, 73, 4662–4670. doi:10.1021/jo800681d
Return to citation in text: [1] -
Butters, M.; Harvey, J. N.; Jover, J.; Lennox, A. J. J.; Lloyd-Jones, G. C.; Murray, P. M. Angew. Chem., Int. Ed. 2010, 49, 5156–5160. doi:10.1002/anie.201001522
Return to citation in text: [1] -
Adonin, N. Yu.; Shabalin, A. Yu.; Bardin, V. V. J. Fluorine Chem. 2014, 168, 111–120. doi:10.1016/j.jfluchem.2014.09.016
Return to citation in text: [1] -
Ozawa, F.; Hidaka, T.; Yamamoto, T.; Yamamoto, A. J. Organomet. Chem. 1987, 330, 253–263. doi:10.1016/0022-328X(87)80292-9
Return to citation in text: [1] -
Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457–2483. doi:10.1021/cr00039a007
Return to citation in text: [1] -
Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004
Return to citation in text: [1] [2] -
Charton, M. Electrical Effect Substituent Constants for Correlation Analysis. In Progress in Physical Organic Chemistry; Taft, R. W., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1981; Vol. 13, pp 119–251. doi:10.1002/9780470171929.ch3
Return to citation in text: [1] [2] [3] [4] [5] -
Elguĕro, J.; Estopá, C.; Ilavský, D. J. Chem. Res., Miniprint 1981, 4237–4245.
Return to citation in text: [1] [2] [3] -
Sheppard, W. A. J. Am. Chem. Soc. 1970, 92, 5419–5422. doi:10.1021/ja00721a021
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Petrov, V. P.; Koptyug, V. A. Org. React. (N. Y., Engl. Transl.) 1966, 3, 135–142.
Return to citation in text: [1] [2]
| 42. | Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004 |
| 43. | Charton, M. Electrical Effect Substituent Constants for Correlation Analysis. In Progress in Physical Organic Chemistry; Taft, R. W., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1981; Vol. 13, pp 119–251. doi:10.1002/9780470171929.ch3 |
| 44. | Elguĕro, J.; Estopá, C.; Ilavský, D. J. Chem. Res., Miniprint 1981, 4237–4245. |
| 1. | Lennox, A. J. J.; Lloyd-Jones, G. C. Chem. Soc. Rev. 2014, 43, 412–443. doi:10.1039/C3CS60197H |
| 2. | Molander, G. A.; Ellis, N. Acc. Chem. Res. 2007, 40, 275–286. doi:10.1021/ar050199q |
| 3. | Molander, G. A.; Canturk, B. Angew. Chem., Int. Ed. 2009, 48, 9240–9261. doi:10.1002/anie.200904306 |
| 17. | Chen, J.; Cammers-Goodwin, A. Tetrahedron Lett. 2003, 44, 1503–1506. doi:10.1016/S0040-4039(02)02793-4 |
| 18. | Gillmann, T.; Weeber, T. Synlett 1994, 649–650. doi:10.1055/s-1994-22961 |
| 19. | Frohn, H.-J.; Adonin, N. Yu.; Bardin, V. V.; Starichenko, V. F. Tetrahedron Lett. 2002, 43, 8111–8114. doi:10.1016/S0040-4039(02)01922-6 |
| 34. | Kayaki, Y.; Shimizu, I.; Yamamoto, A. Bull. Chem. Soc. Jpn. 1997, 70, 1135–1140. doi:10.1246/bcsj.70.1135 |
| 45. | Sheppard, W. A. J. Am. Chem. Soc. 1970, 92, 5419–5422. doi:10.1021/ja00721a021 |
| 15. | Occhiato, E. G.; Trabocchi, A.; Guarna, A. J. Org. Chem. 2001, 66, 2459–2465. doi:10.1021/jo001807c |
| 16. | Uenishi, J.; Beau, J. M.; Armstrong, R. W.; Kishi, Y. J. Am. Chem. Soc. 1987, 109, 4756–4758. doi:10.1021/ja00249a069 |
| 35. | Shabalin, A. Yu.; Adonin, N. Yu.; Bardin, V. V.; Parmon, V. N. Tetrahedron 2014, 70, 3720–3725. doi:10.1016/j.tet.2014.04.019 |
| 45. | Sheppard, W. A. J. Am. Chem. Soc. 1970, 92, 5419–5422. doi:10.1021/ja00721a021 |
| 10. | Chen, H.; Deng, M.-Z. Org. Lett. 2000, 2, 1649–1651. doi:10.1021/ol000013h |
| 11. | Chen, H.; Deng, M.-Z. J. Chem. Soc., Perkin Trans. 1 2000, 1609–1613. doi:10.1039/B000121J |
| 12. | Yao, M.-L.; Deng, M.-Z. J. Org. Chem. 2000, 65, 5034–5036. doi:10.1021/jo000195t |
| 13. | Zhou, S.-m.; Deng, M.-z. Tetrahedron Lett. 2000, 41, 3951–3954. doi:10.1016/S0040-4039(00)00490-1 |
| 14. | Zou, G.; Reddy, Y. K.; Falck, J. R. Tetrahedron Lett. 2001, 42, 7213–7215. doi:10.1016/S0040-4039(01)01536-2 |
| 45. | Sheppard, W. A. J. Am. Chem. Soc. 1970, 92, 5419–5422. doi:10.1021/ja00721a021 |
| 3. | Molander, G. A.; Canturk, B. Angew. Chem., Int. Ed. 2009, 48, 9240–9261. doi:10.1002/anie.200904306 |
| 4. | Molander, G. A.; Biolatto, B. J. Org. Chem. 2003, 68, 4302–4314. doi:10.1021/jo0342368 |
| 5. | Miyaura, N. Organoboron Compounds. In Cross-Coupling Reactions; Miyaura, N., Ed.; Topics in Current Chemistry, Vol. 219; Springer: Berlin, Germany, 2002; pp 11–59. doi:10.1007/3-540-45313-X_2 |
| 6. | Darses, S.; Genet, J.-P. Chem. Rev. 2008, 108, 288–325. doi:10.1021/cr0509758 |
| 7. | Stefani, H. A.; Cella, R.; Vieira, A. S. Tetrahedron 2007, 63, 3623–3658. doi:10.1016/j.tet.2007.01.061 |
| 8. | Adonin, N. Y.; Bardin, V. V. Chim. Oggi 2009, 27, 31–33. |
| 9. | Adonin, N. Yu.; Bardin, V. V. Russ. Chem. Rev. 2010, 79, 757–785. doi:10.1070/RC2010v079n09ABEH004136 |
| 28. | Kayaki, Y.; Shimizu, I.; Yamamoto, A. Chem. Lett. 1995, 24, 1089–1090. doi:10.1246/cl.1995.1089 |
| 33. | Kayaki, Y.; Shimizu, I.; Yamamoto, A. Bull. Chem. Soc. Jpn. 1997, 70, 917–927. doi:10.1246/bcsj.70.917 |
| 42. | Hansch, C.; Leo, A.; Taft, R. W. Chem. Rev. 1991, 91, 165–195. doi:10.1021/cr00002a004 |
| 21. | Nishihara, Y.; Onodera, H.; Osakada, K. Chem. Commun. 2004, 192–193. doi:10.1039/B308741G |
| 22. | Yagyu, T.; Hamada, M.; Osakada, K.; Yamamoto, T. Organometallics 2001, 20, 1087–1101. doi:10.1021/om0007466 |
| 23. | Mecking, S. Coord. Chem. Rev. 2000, 203, 325–351. doi:10.1016/S0010-8545(99)00229-5 |
| 43. | Charton, M. Electrical Effect Substituent Constants for Correlation Analysis. In Progress in Physical Organic Chemistry; Taft, R. W., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1981; Vol. 13, pp 119–251. doi:10.1002/9780470171929.ch3 |
| 45. | Sheppard, W. A. J. Am. Chem. Soc. 1970, 92, 5419–5422. doi:10.1021/ja00721a021 |
| 46. | Petrov, V. P.; Koptyug, V. A. Org. React. (N. Y., Engl. Transl.) 1966, 3, 135–142. |
| 20. | Korenaga, T.; Kosaki, T.; Fukumura, R.; Ema, T.; Sakai, T. Org. Lett. 2005, 7, 4915–4917. doi:10.1021/ol051866i |
| 23. | Mecking, S. Coord. Chem. Rev. 2000, 203, 325–351. doi:10.1016/S0010-8545(99)00229-5 |
| 24. | van Asselt, R.; Gielens, E. E. C. G.; Rulke, R. E.; Vrieze, K.; Elsevier, C. J. J. Am. Chem. Soc. 1994, 116, 977–985. doi:10.1021/ja00082a020 |
| 25. | Kawataka, F.; Kayaki, Y.; Shimizu, I.; Yamamoto, A. Organometallics 1994, 13, 3517–3524. doi:10.1021/om00021a027 |
| 26. | Dekker, G. P. C. M.; Elsevier, C. J.; Vrieze, K.; Van Leeuwen, P. W. N. M. Organometallics 1992, 11, 1598–1603. doi:10.1021/om00040a034 |
| 27. | Ozawa, F.; Sugimoto, T.; Yuasa, Y.; Santra, M.; Yamamoto, T.; Yamamoto, A. Organometallics 1984, 3, 683–692. doi:10.1021/om00083a007 |
| 28. | Kayaki, Y.; Shimizu, I.; Yamamoto, A. Chem. Lett. 1995, 24, 1089–1090. doi:10.1246/cl.1995.1089 |
| 29. | Kawataka, F.; Shimizu, I.; Yamamoto, A. Bull. Chem. Soc. Jpn. 1995, 68, 654–660. doi:10.1246/bcsj.68.654 |
| 30. | Kayaki, Y.; Kawataka, F.; Shimizu, I.; Yamamoto, A. Chem. Lett. 1994, 23, 2171–2174. doi:10.1246/cl.1994.2171 |
| 31. | Denmark, S. E.; Schnute, M. E. J. Org. Chem. 1995, 60, 1013–1019. doi:10.1021/jo00109a037 |
| 43. | Charton, M. Electrical Effect Substituent Constants for Correlation Analysis. In Progress in Physical Organic Chemistry; Taft, R. W., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1981; Vol. 13, pp 119–251. doi:10.1002/9780470171929.ch3 |
| 16. | Uenishi, J.; Beau, J. M.; Armstrong, R. W.; Kishi, Y. J. Am. Chem. Soc. 1987, 109, 4756–4758. doi:10.1021/ja00249a069 |
| 44. | Elguĕro, J.; Estopá, C.; Ilavský, D. J. Chem. Res., Miniprint 1981, 4237–4245. |
| 19. | Frohn, H.-J.; Adonin, N. Yu.; Bardin, V. V.; Starichenko, V. F. Tetrahedron Lett. 2002, 43, 8111–8114. doi:10.1016/S0040-4039(02)01922-6 |
| 21. | Nishihara, Y.; Onodera, H.; Osakada, K. Chem. Commun. 2004, 192–193. doi:10.1039/B308741G |
| 44. | Elguĕro, J.; Estopá, C.; Ilavský, D. J. Chem. Res., Miniprint 1981, 4237–4245. |
| 2. | Molander, G. A.; Ellis, N. Acc. Chem. Res. 2007, 40, 275–286. doi:10.1021/ar050199q |
| 6. | Darses, S.; Genet, J.-P. Chem. Rev. 2008, 108, 288–325. doi:10.1021/cr0509758 |
| 7. | Stefani, H. A.; Cella, R.; Vieira, A. S. Tetrahedron 2007, 63, 3623–3658. doi:10.1016/j.tet.2007.01.061 |
| 36. | Lennox, A. J. J.; Lloyd-Jones, G. C. J. Am. Chem. Soc. 2012, 134, 7431–7441. doi:10.1021/ja300236k |
| 37. | Ting, R.; Harwig, C. W.; Lo, J.; Li, Y.; Adam, M. J.; Ruth, T. J.; Perrin, D. M. J. Org. Chem. 2008, 73, 4662–4670. doi:10.1021/jo800681d |
| 19. | Frohn, H.-J.; Adonin, N. Yu.; Bardin, V. V.; Starichenko, V. F. Tetrahedron Lett. 2002, 43, 8111–8114. doi:10.1016/S0040-4039(02)01922-6 |
| 43. | Charton, M. Electrical Effect Substituent Constants for Correlation Analysis. In Progress in Physical Organic Chemistry; Taft, R. W., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1981; Vol. 13, pp 119–251. doi:10.1002/9780470171929.ch3 |
| 19. | Frohn, H.-J.; Adonin, N. Yu.; Bardin, V. V.; Starichenko, V. F. Tetrahedron Lett. 2002, 43, 8111–8114. doi:10.1016/S0040-4039(02)01922-6 |
| 43. | Charton, M. Electrical Effect Substituent Constants for Correlation Analysis. In Progress in Physical Organic Chemistry; Taft, R. W., Ed.; John Wiley & Sons: Hoboken, NJ, USA, 1981; Vol. 13, pp 119–251. doi:10.1002/9780470171929.ch3 |
| 46. | Petrov, V. P.; Koptyug, V. A. Org. React. (N. Y., Engl. Transl.) 1966, 3, 135–142. |
| 45. | Sheppard, W. A. J. Am. Chem. Soc. 1970, 92, 5419–5422. doi:10.1021/ja00721a021 |
| 40. | Ozawa, F.; Hidaka, T.; Yamamoto, T.; Yamamoto, A. J. Organomet. Chem. 1987, 330, 253–263. doi:10.1016/0022-328X(87)80292-9 |
| 41. | Miyaura, N.; Suzuki, A. Chem. Rev. 1995, 95, 2457–2483. doi:10.1021/cr00039a007 |
| 21. | Nishihara, Y.; Onodera, H.; Osakada, K. Chem. Commun. 2004, 192–193. doi:10.1039/B308741G |
| 19. | Frohn, H.-J.; Adonin, N. Yu.; Bardin, V. V.; Starichenko, V. F. Tetrahedron Lett. 2002, 43, 8111–8114. doi:10.1016/S0040-4039(02)01922-6 |
| 35. | Shabalin, A. Yu.; Adonin, N. Yu.; Bardin, V. V.; Parmon, V. N. Tetrahedron 2014, 70, 3720–3725. doi:10.1016/j.tet.2014.04.019 |
| 19. | Frohn, H.-J.; Adonin, N. Yu.; Bardin, V. V.; Starichenko, V. F. Tetrahedron Lett. 2002, 43, 8111–8114. doi:10.1016/S0040-4039(02)01922-6 |
| 39. | Adonin, N. Yu.; Shabalin, A. Yu.; Bardin, V. V. J. Fluorine Chem. 2014, 168, 111–120. doi:10.1016/j.jfluchem.2014.09.016 |
| 19. | Frohn, H.-J.; Adonin, N. Yu.; Bardin, V. V.; Starichenko, V. F. Tetrahedron Lett. 2002, 43, 8111–8114. doi:10.1016/S0040-4039(02)01922-6 |
| 4. | Molander, G. A.; Biolatto, B. J. Org. Chem. 2003, 68, 4302–4314. doi:10.1021/jo0342368 |
| 45. | Sheppard, W. A. J. Am. Chem. Soc. 1970, 92, 5419–5422. doi:10.1021/ja00721a021 |
| 35. | Shabalin, A. Yu.; Adonin, N. Yu.; Bardin, V. V.; Parmon, V. N. Tetrahedron 2014, 70, 3720–3725. doi:10.1016/j.tet.2014.04.019 |
| 38. | Butters, M.; Harvey, J. N.; Jover, J.; Lennox, A. J. J.; Lloyd-Jones, G. C.; Murray, P. M. Angew. Chem., Int. Ed. 2010, 49, 5156–5160. doi:10.1002/anie.201001522 |
© 2015 Bardin et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)