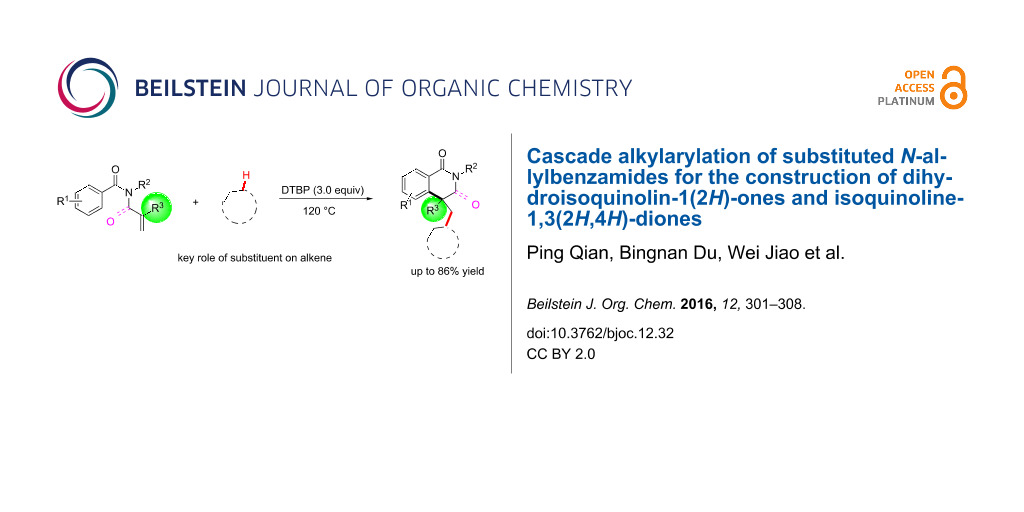
Abstract
An oxidative reaction for the synthesis of 4-alkyl-substituted dihydroisoquinolin-1(2H)-ones with N-allylbenzamide derivatives as starting materials has been developed. The radical alkylarylation reaction proceeds through a sequence of alkylation and intramolecular cyclization. The substituent on the C–C double bond was found to play a key role for the progress of the reaction to give the expected products with good chemical yields. Additionally, N-methacryloylbenzamides were also suitable substrates for the current reaction and provided the alkyl-substituted isoquinoline-1,3(2H,4H)-diones in good yield.
Graphical Abstract
Introduction
The direct and selective functionalization of an unactivated sp3 C–H bond, which belongs to an effective strategic approach in green and sustainable chemistry, has attracted significant research attention [1-4]. This fascinating approach has obvious advantages in functional group transformation and construction of biological heterocycles, due to its high efficiency and waste reduction [5-10]. The pioneering works were focused on the cross-dehydrogenative coupling (CDC) reactions of alkanes, which were reported by Li and other groups [11-15]. Recently, several types of reactions with alkanes as substrates have been developed, such as the Minisci reaction with heteroarenes [16,17], radical addition to unsaturated bonds [18,19], decarboxylative alkenylation of cycloalkanes with aryl vinylic carboxylic acids [20,21], trifluoromethylthiolation [22], thiolation [23,24], alkenylation [25,26], dehydrogenation−olefination and esterification [27,28], radical addition/1,2-aryl migration [29], cascade alkylation-initiated cyclization [30,31] and other radical reactions [32-34]. Due to their low polarity and high bond-dissociation energy, the functionalization of unactivated sp3 C–H bonds in simple alkanes remains as a challenging task.
The direct cascade, 1,2-alkyarylation of alkenes to construct multi-substituted heterocycles has been considered as an efficient organic synthetic strategy, which is often featured by a new ring and dual C–C bond formation in one process [35-42]. Recently, the group of Liu reported a cascade alkylarylation of N-alkyl-N-phenylacryamide with simple alkanes resulting in alkyl-substituted oxindoles (Scheme 1a) [43]. However, cyclization of N-allylbenzamides initiated by the functionalization of sp3 C–H bonds of simple alkanes remains unexplored. Very recently, our group developed a metal-free hydroxyalkylation-initiated radical six-membered heterocycle formation reaction of N-allylbenzamide with alcohols as radical partners. This provided 4-hydroxyalkyl-substituted 3,4-dihydroisoquinolin-1(2H)-one derivatives (Scheme 1b) [44].
Scheme 1: Cascade 1,2-difunctionalization and cyclization to construct heterocycles.
Scheme 1: Cascade 1,2-difunctionalization and cyclization to construct heterocycles.
Based on the knowledge gained from previous reports on the cyclization of N-allylbenzamide [44], we envisioned that the unactivated cycloalkanes (instead of alcohols) could act as radical partners for this system. However, the reaction gave a complex mixture with 15% chemical yield of the expected product (Scheme 1c). Fortunately, when a methyl substituent was introduced onto the C–C double bond of the N-allylbenzamide substrate, the cyclization reaction proceeded smoothly (Scheme 1c). Herein, we report a metal-free cascade 1,2-alkyarylation of substituted N-allylbenzamides with alkanes affording 4-alkyl-substituted dihydroisoquinoline-1(2H)-ones as the product.
Results and Discussion
Initially, we selected N-methyl-N-(2-methylallyl)benzamide (4a) and cyclohexane (2a) as model compounds (Table 1). As shown in Table 1, we found that the reactions did not happen or gave only a trace amount of the desired product with K2S2O8, AIBN, BPO and TBHP as oxidants (Table 1, entries 1, 3–5). PhI(OAc)2 and DCP could be used as oxidants, providing a slightly better yield (Table 1, entries 2 and 6). Dramatically higher chemical yields were found when TBPA and TBPB were used for this reaction (Table 1, entries 7 and 8). DTBP was the best oxidant choice, which afforded the highest chemical yield (53%, Table 1, entry 9). Then, a series of transition metal catalysts, including CuI, FeCl2, FeBr2 and FeCl3, were added into the reaction with DTBP as the oxidant. However, no improvement was observed at all. Finally, an attempt to shorten the reaction time to 24 h or to prolong the reaction time to 72 h resulted in lower yield, thus indicating that 48 h was appropriate for the completion of the reaction (Table 1, entries 14 and 15). Changing the amount of DTBP was also not successful. This is shown by the results presented in Table 1, entries 16 and 17 that clearly suggest that 3.0 equiv is the best choice. Finally, the reaction temperature was examined, and a lower chemical yield was found when the reaction was performed at 100 °C (Table 1, entry 16).
Table 1: Optimization of typical reaction conditions.a
|
|
|||||
| entry | oxidant (equiv) | catalyst (mol %) | temp (°C) | time (h) | yield (%)b |
|---|---|---|---|---|---|
| 1 | K2S2O8 (3.0) | – | 120 | 48 | NR |
| 2 | PhI(OAc)2 (3.0) | – | 120 | 48 | 19 |
| 3c | AIBN (3.0) | – | 120 | 48 | NR |
| 4c | BPO (3.0) | – | 120 | 48 | trace |
| 5c | TBHP (3.0) | – | 120 | 48 | trace |
| 6c | DCP ( 3.0 ) | – | 120 | 48 | 19 |
| 7c | TBPA (3.0) | – | 120 | 48 | 45 |
| 8c | TBPB (3.0) | – | 120 | 48 | 38 |
| 9c | DTBP (3.0) | – | 120 | 48 | 53 |
| 10 | DTBP (3.0) | CuI (10) | 120 | 48 | 49 |
| 11 | DTBP (3.0) | FeCl2 (10) | 120 | 48 | 40 |
| 12 | DTBP (3.0) | FeBr2 (10) | 120 | 48 | 45 |
| 13 | DTBP (3.0) | FeCl3 (10) | 120 | 48 | 33 |
| 14 | DTBP (3.0) | – | 120 | 24 | 25 |
| 15 | DTBP (3.0) | – | 120 | 72 | 50 |
| 16 | DTBP (2.0) | – | 120 | 48 | 30 |
| 17 | DTBP (4.0) | – | 120 | 48 | 46 |
| 18 | DTBP (3.0) | – | 100 | 48 | 43 |
aReaction conditions: 4a (0.2 mmol), cyclohexane (2a, 2.0 mL), oxidant, 120 °C, under N2. bIsolated yield based on 4a. cAIBN = azodiisobutyronitrile; BPO = benzoyl peroxide; TBHP = tert-butyl hydroperoxide, 70% in water; DCP = dicumyl peroxide; TBPA = tert-butyl peracetate; TBPB = tert-butyl peroxybenzoate; DTBP = di-tert-butyl peroxide.
With the optimized conditions developed, we then carried out a substrate generality study using various types of N-(2-methylallyl)benzamides 4 to react with cyclohexane (2a). As shown in Scheme 2, these cascade radical cyclization reactions are of general use for the preparation of 4-alkyldihydroisoquinolin-1(2H)-one derivatives 5. The substrates bearing methyl, methoxy, halo and trifluoromethyl groups on the aromatic ring all worked well in the reaction, providing the target products with 31–65% yield. It should be noted that the reactions of substrates bearing disubstituted aromatic rings were possible but resulted in lower yield (5fa and 5ga). On the other hand, the variation of the substituent on the nitrogen atom has also been examined. In the cases of N-ethyl (4h), N-isopropyl (4i), and N-benzyl (4k), no obvious effect was found and almost the same level of yield was obtained as for 4a. However, a dramatically lower yield was obtained when a substrate with a N-phenyl group (4j) was used.
Scheme 2: Cyclization of cyclohexane (2a) with substituted N-(2-methylallyl)benzamide (reaction conditions: 4 (0.2 mmol), cyclohexane (2a, 2 mL), DTBP (0.6 mmol), 120 °C, 48 h under nitrogen atmosphere. Isolated yield based on 4).
Scheme 2: Cyclization of cyclohexane (2a) with substituted N-(2-methylallyl)benzamide (reaction conditions: 4...
We then carried out another substrate scope examination for the radical reactions using various cycloalkanes 2 and N-methyl-N-(2-methylallyl)benzamide (4a). As indicated in Scheme 3, several cycloalkanes were well-tolerated in this radical reaction resulting in the corresponding product. In the case of cyclopentane (2b), a slightly lower chemical yield was obtained (46%, 5ab), while the reactions of seven- and eight-membered ring cycloalkanes afforded the same level yield of the corresponding product (5ac and 5ad). Finally, methylcyclohexane 2e was used as the substrate for the investigation of the regioselectivity. The reaction almost showed no regio- and stereoselectivity and afforded the corresponding products (5ae1–5ae5) with 42% total yield.
Scheme 3: Cyclization of cycloalkanes with N-methyl-N-(2-methylallyl)benzamide (reaction conditions: 4a (0.2 mmol), cycloalkanes 2 (2 mL), DTBP (0.6 mmol), 120 °C, 48 h under nitrogen atmosphere. Isolated yield based on 4a). aThe total yield of isomers.
Scheme 3: Cyclization of cycloalkanes with N-methyl-N-(2-methylallyl)benzamide (reaction conditions: 4a (0.2 ...
To extend the synthetic utility of this radical cyclization reaction, N-methacryloyl-N-methylbenzamide derivatives 6 were then tried as substrates for this reaction (Scheme 4). It should be mentioned that only a few radical precursors, such as the TMSCF3 reagent [45] and ethers [46], were developed for such a cyclization system. Fortunately, the substrate with the introduction of a carbonyl group worked well in this system with moderate to good chemical yields (33–86%). Due to the existence of the carbonyl group, the reaction time could be shortened to 12 h where all of the starting material 6 is consumed. Firstly, the variation of the substituents on the nitrogen atom was investigated. We found that by changing the methyl group into one of the bulkier groups, the chemical yield significantly decreased (7ba–7ea). It is worth mentioning that the N-phenyl-N-methacryl-substituted substrate 6d works much better than 4j in this system, resulting in higher yield (55%, 5ja). The substituent on the aromatic ring did not greatly affect the reaction efficiency, and methyl (7fa and 7ka), methoxy (7ga, 7la and 7ma), chloro (7ia), bromo (7ja), and phenyl (7na) were well-tolerated in this system. However, in the case of the strong electron-withdrawing group (fluoro, 7ha), the yield clearly decreased, and only 31% yield was obtained. It was noted that the reactions showed almost no evident regioselectivity, and the ratio of 1:4 (7pa-1:7pa-2) was found.
Scheme 4: Cyclization reaction of 6 with cyclohexane 2a (reaction conditions: 6 (0.2 mmol), cyclohexane 2a (2 mL), DTBP (0.6 mmol), 120 °C, 12 h under nitrogen atmosphere. Isolated yield based on 6).
Scheme 4: Cyclization reaction of 6 with cyclohexane 2a (reaction conditions: 6 (0.2 mmol), cyclohexane 2a (2...
The final study of this reaction was the investigation of the mechanism. Firstly, a substrate (8a) bearing a hydrogen atom at the nitrogen was tried for the current system. The cyclohexane radical addition product 9aa’, instead of a cyclization product, was observed with 35% yield (Scheme 5a). This result is consistent with our previous report [19], which discloses that the alkylation of the C–C double bond initiates the radical process. Furthermore, a radical-trapping reagent, 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), was added to the reaction, and the reaction was completely inhibited, affording a cyclohexane radical-trapped compound (Scheme 5b). This implies that the current transformation is a radical process. Finally, an obvious competing kinetic isotope effect (KIE) was found with the ratio of 9.3:1 (kH:kD) when the reaction of 6a was performed with cyclohexane and [D]-cyclohexane (Scheme 5c). This discloses that the cleavage of the C(sp3)−H bond to form the radical may be involved in the turnover-limiting steps of this procedure.
Scheme 5: Control experiments for the mechanism studies. a) Reaction with N-unprotected substrate 8a; b) reaction with the addition of radical-trapping reagent TEMPO; c) KIE study.
Scheme 5: Control experiments for the mechanism studies. a) Reaction with N-unprotected substrate 8a; b) reac...
Based on the previous radical cyclization reactions [44,47,48] and the results obtained above, a plausible mechanism accounting for this cascade radical cyclization reaction was proposed (Scheme 6). Initially, DTBP undergoes homolytic cleavage to form the tert-butoxy radical A, which reacts with cyclohexane (2a) affording intermediate B. Then, intermediate B adds to N-methyl-N-(2-methylallyl)benzamide (4a), giving radical intermediate C. Intermediate C proceeds through intramolecular cyclization to give intermediate D. Finally, H-atom abstraction occurs between D and TBPB directly, which gives the product 5aa and regenerates radical A for the next cycle.
Conclusion
In summary, a metal-free cascade functionalization of unactivated C(sp3)–H bonds and cyclization reactions of N-substituted allylbenzamides were developed. The reaction involved cleavage of the C(sp3)–H bond, alkylation and intramolecular cyclization, affording the 4-alkyl-substituted dihydroisoquinolin-1(2H)-one derivatives with moderate to good chemical yield. The substituent on the C–C double bond was found to play a key role for the formation of the desired products. Also, N-methacryloylbenzamides worked well in the current reaction, which provides an easy way for the preparation of alkyl-subsituted isoquinoline-1,3(2H,4H)-diones.
Experimental
General procedure for the radical cyclization between N-allylbenzamide and N-methacryloylbenzamides with cycloalkanes: Into an oven-dried reaction vial flushed with N2, substrate 4 or 6 (0.2 mmol), cycloalkanes 2 (2 mL), and DTBP (0.6 mmol) were added. Then the reaction mixture was stirred for 12–48 h at 120 °C under nitrogen atmosphere. After cooling, the reaction was quenched by a saturated NaCl solution (1 × 5 mL). Ethyl acetate (30 mL) was added to the system, and the mixture was washed with water (1 × 30 mL) and brine solution (1 × 30 mL). After drying over anhydrous Na2SO4, the solvent was removed. The crude mixture was charged onto silica gel and purified by flash chromatography to furnish the corresponding products 5 and 7.
Supporting Information
| Supporting Information File 1: Experimental details and spectral data. | ||
| Format: PDF | Size: 3.5 MB | Download |
Acknowledgements
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Nos. 21102071 and 21472082) and the Fundamental Research Funds for the Central Universities (020514380018). The Jiangsu 333 program (for Pan) and the Changzhou Jin-Feng-Huang program (for Han) are also acknowledged.
References
-
Girard, S. A.; Knauber, T.; Li, C.-J. Angew. Chem., Int. Ed. 2014, 53, 74–100. doi:10.1002/anie.201304268
Return to citation in text: [1] -
Henrion, M.; Ritleng, V.; Chetcuti, M. J. ACS Catal. 2015, 5, 1283–1302. doi:10.1021/cs5014927
Return to citation in text: [1] -
Kumar, R.; Van der Eycken, E. V. Chem. Soc. Rev. 2013, 42, 1121–1146. doi:10.1039/C2CS35397K
Return to citation in text: [1] -
Cho, S. H.; Kim, J. Y.; Kwak, J.; Chang, S. Chem. Soc. Rev. 2011, 40, 5068–5083. doi:10.1039/c1cs15082k
Return to citation in text: [1] -
Davies, H. M. L.; Morton, D. Chem. Soc. Rev. 2011, 40, 1857–1869. doi:10.1039/c0cs00217h
Return to citation in text: [1] -
Engle, K. M.; Mei, T.-S.; Wasa, M.; Yu, J.-Q. Acc. Chem. Res. 2011, 45, 788–802. doi:10.1021/ar200185g
Return to citation in text: [1] -
Liu, C.; Zhang, H.; Shi, W.; Lei, A. Chem. Rev. 2011, 111, 1780–1824. doi:10.1021/cr100379j
Return to citation in text: [1] -
Newhouse, T.; Baran, P. S. Angew. Chem., Int. Ed. 2011, 50, 3362–3374. doi:10.1002/anie.201006368
Return to citation in text: [1] -
Zhang, S.-Y.; Zhang, F.-M.; Tu, Y.-Q. Chem. Soc. Rev. 2011, 40, 1937–1949. doi:10.1039/c0cs00063a
Return to citation in text: [1] -
Rouquet, G.; Chatani, N. Angew. Chem., Int. Ed. 2013, 52, 11726–11743. doi:10.1002/anie.201301451
Return to citation in text: [1] -
Deng, G.; Zhao, L.; Li, C.-J. Angew. Chem., Int. Ed. 2008, 47, 6278–6282. doi:10.1002/anie.200801544
Return to citation in text: [1] -
Bettinger, H. F.; Filthaus, M.; Bornemann, H.; Oppel, I. M. Angew. Chem., Int. Ed. 2008, 47, 4744–4747. doi:10.1002/anie.200705936
Return to citation in text: [1] -
Ochiai, M.; Miyamato, K.; Kaneaki, T.; Hayashi, S.; Nakanishi, W. Science 2011, 332, 448–451. doi:10.1126/science.1201686
Return to citation in text: [1] -
Tran, B. L.; Li, B.; Driess, M.; Hartwig, J. F. J. Am. Chem. Soc. 2014, 136, 2555–2563. doi:10.1021/ja411912p
Return to citation in text: [1] -
Chuprakov, S.; Malik, J. A.; Zibinsky, M.; Fokin, V. V. J. Am. Chem. Soc. 2011, 133, 10352–10355. doi:10.1021/ja202969z
Return to citation in text: [1] -
Antonchick, A. P.; Burgmann, L. Angew. Chem., Int. Ed. 2013, 52, 3267–3271. doi:10.1002/anie.201209584
Return to citation in text: [1] -
Narayan, R.; Antonchick, A. P. Chem. – Eur. J. 2014, 20, 4568–4572. doi:10.1002/chem.201400186
Return to citation in text: [1] -
Zhao, J.; Fang, H.; Qian, P.; Han, J.; Pan, Y. Org. Lett. 2014, 16, 5342–5345. doi:10.1021/ol502524d
Return to citation in text: [1] -
Zhou, W.; Qian, P.; Zhao, J.; Fang, H.; Han, J.; Pan, Y. Org. Lett. 2015, 17, 1160–1163. doi:10.1021/acs.orglett.5b00088
Return to citation in text: [1] [2] -
Zhao, J.; Fang, H.; Han, J.; Pan, Y. Beilstein J. Org. Chem. 2013, 9, 1718–1723. doi:10.3762/bjoc.9.197
Return to citation in text: [1] -
Cui, Z.; Shang, X.; Shao, X.-F.; Liu, Z.-Q. Chem. Sci. 2012, 3, 2853–2858. doi:10.1039/c2sc20712e
Return to citation in text: [1] -
Wu, H.; Xiao, Z.; Wu, J.; Guo, Y.; Xiao, J.-C.; Liu, C.; Chen, Q.-Y. Angew. Chem., Int. Ed. 2015, 54, 4070–4074. doi:10.1002/anie.201411953
Return to citation in text: [1] -
Du, B.; Jin, B.; Sun, P. Org. Lett. 2014, 16, 3032–3035. doi:10.1021/ol5011449
Return to citation in text: [1] -
Zhao, J.; Fang, H.; Han, J.; Pan, Y.; Li, G. Adv. Synth. Catal. 2014, 356, 2719–2724. doi:10.1002/adsc.201400032
Return to citation in text: [1] -
Zhu, Y.; Wei, Y. Chem. Sci. 2014, 5, 2379–2382. doi:10.1039/c4sc00093e
Return to citation in text: [1] -
Gu, H.; Wang, C. Org. Biomol. Chem. 2015, 13, 5880–5884. doi:10.1039/C5OB00619H
Return to citation in text: [1] -
Zhao, J.; Fang, H.; Han, J.; Pan, Y. Org. Lett. 2014, 16, 2530–2533. doi:10.1021/ol5009119
Return to citation in text: [1] -
Rout, S. K.; Guin, S.; Ali, W.; Gogoi, A.; Patel, B. K. Org. Lett. 2014, 16, 3086–3089. doi:10.1021/ol5011906
Return to citation in text: [1] -
Zhao, J.; Fang, H.; Song, R.; Zhou, J.; Han, J.; Pan, Y. Chem. Commun. 2015, 51, 599–602. doi:10.1039/C4CC07654K
Return to citation in text: [1] -
Li, Z.; Fan, F.; Yang, J.; Liu, Z.-Q. Org. Lett. 2014, 16, 3396–3399. doi:10.1021/ol501461u
Return to citation in text: [1] -
Sha, W.; Yu, J.-T.; Jiang, Y.; Yang, H.; Cheng, J. Chem. Commun. 2014, 50, 9179–9181. doi:10.1039/C4CC03304C
Return to citation in text: [1] -
Wu, C.-J.; Zhong, J.-J.; Meng, Q.-Y.; Lei, T.; Gao, X.-W.; Tung, C.-H.; Wu, L.-Z. Org. Lett. 2015, 17, 884–887. doi:10.1021/ol503744a
Return to citation in text: [1] -
Zhou, L.; Tang, S.; Qi, X.; Lin, C.; Liu, K.; Liu, C.; Lan, Y.; Lei, A. Org. Lett. 2014, 16, 3404–3407. doi:10.1021/ol501485f
Return to citation in text: [1] -
Ni, S.; Zhang, Y.; Xie, C.; Mei, H.; Han, J.; Pan, Y. Org. Lett. 2015, 17, 5524–5527. doi:10.1021/acs.orglett.5b02356
Return to citation in text: [1] -
Wu, T.; Mu, X.; Liu, G. Angew. Chem., Int. Ed. 2011, 50, 12578–12581. doi:10.1002/anie.201104575
Return to citation in text: [1] -
Meng, Y.; Guo, L.-N.; Wang, H.; Duan, X.-H. Chem. Commun. 2013, 49, 7540–7542. doi:10.1039/c3cc44055a
Return to citation in text: [1] -
Wang, J.-Y.; Su, Y.-M.; Yin, F.; Bao, Y.; Zhing, X.; Xu, Y.-M.; Wang, X.-S. Chem. Commun. 2014, 50, 4108–4111. doi:10.1039/c3cc49315f
Return to citation in text: [1] -
Tang, X.-J.; Thomoson, C. S.; Dolbier, W. R., Jr. Org. Lett. 2014, 16, 4594–4597. doi:10.1021/ol502163f
Return to citation in text: [1] -
Li, C.-C.; Yang, S.-D. Org. Lett. 2015, 17, 2142–2145. doi:10.1021/acs.orglett.5b00732
Return to citation in text: [1] -
Lu, M.-Z.; Loh, T.-P. Org. Lett. 2014, 16, 4698–4701. doi:10.1021/ol502411c
Return to citation in text: [1] -
Tang, Q.; Xia, D.; Jin, X.; Zhang, Q.; Sun, X.-Q.; Wang, C. J. Am. Chem. Soc. 2013, 135, 4628–4631. doi:10.1021/ja400020e
Return to citation in text: [1] -
Chen, Y.-H.; Zhang, Y.-H.; Zhang, H.-J.; Liu, D.-Z.; Gu, M.; Li, J.-Y.; Wu, F.; Zhu, X.-Z.; Li, J.; Nan, F.-J. J. Med. Chem. 2006, 49, 1613–1623. doi:10.1021/jm050896o
Return to citation in text: [1] -
Li, Z.; Zhang, Y.; Zhang, L.; Liu, Z.-Q. Org. Lett. 2014, 16, 382–385. doi:10.1021/ol4032478
Return to citation in text: [1] -
Zhou, W.; Ni, S.; Mei, H.; Han, J.; Pan, Y. Org. Lett. 2015, 17, 2724–2727. doi:10.1021/acs.orglett.5b01140
Return to citation in text: [1] [2] [3] -
Li, L.; Deng, M.; Zheng, S.-C.; Xiong, Y.-P.; Tan, B.; Liu, X.-Y. Org. Lett. 2014, 16, 504–507. doi:10.1021/ol403391v
Return to citation in text: [1] -
Zhang, M.; Xie, P.; Zhao, W.; Niu, B.; Wu, W.; Bian, Z.; Pittman, C. U., Jr.; Zhou, A. J. Org. Chem. 2015, 80, 4176–4183. doi:10.1021/acs.joc.5b00158
Return to citation in text: [1] -
Fang, H.; Zhao, J.; Qian, P.; Han, J.; Pan, Y. Asian J. Org. Chem. 2014, 3, 1266–1269. doi:10.1002/ajoc.201402169
Return to citation in text: [1] -
Fang, H.; Zhao, J.; Ni, S.; Mei, H.; Han, J.; Pan, Y. J. Org. Chem. 2015, 80, 3151–3158. doi:10.1021/acs.joc.5b00058
Return to citation in text: [1]
| 46. | Zhang, M.; Xie, P.; Zhao, W.; Niu, B.; Wu, W.; Bian, Z.; Pittman, C. U., Jr.; Zhou, A. J. Org. Chem. 2015, 80, 4176–4183. doi:10.1021/acs.joc.5b00158 |
| 44. | Zhou, W.; Ni, S.; Mei, H.; Han, J.; Pan, Y. Org. Lett. 2015, 17, 2724–2727. doi:10.1021/acs.orglett.5b01140 |
| 45. | Li, L.; Deng, M.; Zheng, S.-C.; Xiong, Y.-P.; Tan, B.; Liu, X.-Y. Org. Lett. 2014, 16, 504–507. doi:10.1021/ol403391v |
| 1. | Girard, S. A.; Knauber, T.; Li, C.-J. Angew. Chem., Int. Ed. 2014, 53, 74–100. doi:10.1002/anie.201304268 |
| 2. | Henrion, M.; Ritleng, V.; Chetcuti, M. J. ACS Catal. 2015, 5, 1283–1302. doi:10.1021/cs5014927 |
| 3. | Kumar, R.; Van der Eycken, E. V. Chem. Soc. Rev. 2013, 42, 1121–1146. doi:10.1039/C2CS35397K |
| 4. | Cho, S. H.; Kim, J. Y.; Kwak, J.; Chang, S. Chem. Soc. Rev. 2011, 40, 5068–5083. doi:10.1039/c1cs15082k |
| 18. | Zhao, J.; Fang, H.; Qian, P.; Han, J.; Pan, Y. Org. Lett. 2014, 16, 5342–5345. doi:10.1021/ol502524d |
| 19. | Zhou, W.; Qian, P.; Zhao, J.; Fang, H.; Han, J.; Pan, Y. Org. Lett. 2015, 17, 1160–1163. doi:10.1021/acs.orglett.5b00088 |
| 43. | Li, Z.; Zhang, Y.; Zhang, L.; Liu, Z.-Q. Org. Lett. 2014, 16, 382–385. doi:10.1021/ol4032478 |
| 16. | Antonchick, A. P.; Burgmann, L. Angew. Chem., Int. Ed. 2013, 52, 3267–3271. doi:10.1002/anie.201209584 |
| 17. | Narayan, R.; Antonchick, A. P. Chem. – Eur. J. 2014, 20, 4568–4572. doi:10.1002/chem.201400186 |
| 44. | Zhou, W.; Ni, S.; Mei, H.; Han, J.; Pan, Y. Org. Lett. 2015, 17, 2724–2727. doi:10.1021/acs.orglett.5b01140 |
| 11. | Deng, G.; Zhao, L.; Li, C.-J. Angew. Chem., Int. Ed. 2008, 47, 6278–6282. doi:10.1002/anie.200801544 |
| 12. | Bettinger, H. F.; Filthaus, M.; Bornemann, H.; Oppel, I. M. Angew. Chem., Int. Ed. 2008, 47, 4744–4747. doi:10.1002/anie.200705936 |
| 13. | Ochiai, M.; Miyamato, K.; Kaneaki, T.; Hayashi, S.; Nakanishi, W. Science 2011, 332, 448–451. doi:10.1126/science.1201686 |
| 14. | Tran, B. L.; Li, B.; Driess, M.; Hartwig, J. F. J. Am. Chem. Soc. 2014, 136, 2555–2563. doi:10.1021/ja411912p |
| 15. | Chuprakov, S.; Malik, J. A.; Zibinsky, M.; Fokin, V. V. J. Am. Chem. Soc. 2011, 133, 10352–10355. doi:10.1021/ja202969z |
| 32. | Wu, C.-J.; Zhong, J.-J.; Meng, Q.-Y.; Lei, T.; Gao, X.-W.; Tung, C.-H.; Wu, L.-Z. Org. Lett. 2015, 17, 884–887. doi:10.1021/ol503744a |
| 33. | Zhou, L.; Tang, S.; Qi, X.; Lin, C.; Liu, K.; Liu, C.; Lan, Y.; Lei, A. Org. Lett. 2014, 16, 3404–3407. doi:10.1021/ol501485f |
| 34. | Ni, S.; Zhang, Y.; Xie, C.; Mei, H.; Han, J.; Pan, Y. Org. Lett. 2015, 17, 5524–5527. doi:10.1021/acs.orglett.5b02356 |
| 5. | Davies, H. M. L.; Morton, D. Chem. Soc. Rev. 2011, 40, 1857–1869. doi:10.1039/c0cs00217h |
| 6. | Engle, K. M.; Mei, T.-S.; Wasa, M.; Yu, J.-Q. Acc. Chem. Res. 2011, 45, 788–802. doi:10.1021/ar200185g |
| 7. | Liu, C.; Zhang, H.; Shi, W.; Lei, A. Chem. Rev. 2011, 111, 1780–1824. doi:10.1021/cr100379j |
| 8. | Newhouse, T.; Baran, P. S. Angew. Chem., Int. Ed. 2011, 50, 3362–3374. doi:10.1002/anie.201006368 |
| 9. | Zhang, S.-Y.; Zhang, F.-M.; Tu, Y.-Q. Chem. Soc. Rev. 2011, 40, 1937–1949. doi:10.1039/c0cs00063a |
| 10. | Rouquet, G.; Chatani, N. Angew. Chem., Int. Ed. 2013, 52, 11726–11743. doi:10.1002/anie.201301451 |
| 35. | Wu, T.; Mu, X.; Liu, G. Angew. Chem., Int. Ed. 2011, 50, 12578–12581. doi:10.1002/anie.201104575 |
| 36. | Meng, Y.; Guo, L.-N.; Wang, H.; Duan, X.-H. Chem. Commun. 2013, 49, 7540–7542. doi:10.1039/c3cc44055a |
| 37. | Wang, J.-Y.; Su, Y.-M.; Yin, F.; Bao, Y.; Zhing, X.; Xu, Y.-M.; Wang, X.-S. Chem. Commun. 2014, 50, 4108–4111. doi:10.1039/c3cc49315f |
| 38. | Tang, X.-J.; Thomoson, C. S.; Dolbier, W. R., Jr. Org. Lett. 2014, 16, 4594–4597. doi:10.1021/ol502163f |
| 39. | Li, C.-C.; Yang, S.-D. Org. Lett. 2015, 17, 2142–2145. doi:10.1021/acs.orglett.5b00732 |
| 40. | Lu, M.-Z.; Loh, T.-P. Org. Lett. 2014, 16, 4698–4701. doi:10.1021/ol502411c |
| 41. | Tang, Q.; Xia, D.; Jin, X.; Zhang, Q.; Sun, X.-Q.; Wang, C. J. Am. Chem. Soc. 2013, 135, 4628–4631. doi:10.1021/ja400020e |
| 42. | Chen, Y.-H.; Zhang, Y.-H.; Zhang, H.-J.; Liu, D.-Z.; Gu, M.; Li, J.-Y.; Wu, F.; Zhu, X.-Z.; Li, J.; Nan, F.-J. J. Med. Chem. 2006, 49, 1613–1623. doi:10.1021/jm050896o |
| 25. | Zhu, Y.; Wei, Y. Chem. Sci. 2014, 5, 2379–2382. doi:10.1039/c4sc00093e |
| 26. | Gu, H.; Wang, C. Org. Biomol. Chem. 2015, 13, 5880–5884. doi:10.1039/C5OB00619H |
| 29. | Zhao, J.; Fang, H.; Song, R.; Zhou, J.; Han, J.; Pan, Y. Chem. Commun. 2015, 51, 599–602. doi:10.1039/C4CC07654K |
| 23. | Du, B.; Jin, B.; Sun, P. Org. Lett. 2014, 16, 3032–3035. doi:10.1021/ol5011449 |
| 24. | Zhao, J.; Fang, H.; Han, J.; Pan, Y.; Li, G. Adv. Synth. Catal. 2014, 356, 2719–2724. doi:10.1002/adsc.201400032 |
| 30. | Li, Z.; Fan, F.; Yang, J.; Liu, Z.-Q. Org. Lett. 2014, 16, 3396–3399. doi:10.1021/ol501461u |
| 31. | Sha, W.; Yu, J.-T.; Jiang, Y.; Yang, H.; Cheng, J. Chem. Commun. 2014, 50, 9179–9181. doi:10.1039/C4CC03304C |
| 22. | Wu, H.; Xiao, Z.; Wu, J.; Guo, Y.; Xiao, J.-C.; Liu, C.; Chen, Q.-Y. Angew. Chem., Int. Ed. 2015, 54, 4070–4074. doi:10.1002/anie.201411953 |
| 19. | Zhou, W.; Qian, P.; Zhao, J.; Fang, H.; Han, J.; Pan, Y. Org. Lett. 2015, 17, 1160–1163. doi:10.1021/acs.orglett.5b00088 |
| 20. | Zhao, J.; Fang, H.; Han, J.; Pan, Y. Beilstein J. Org. Chem. 2013, 9, 1718–1723. doi:10.3762/bjoc.9.197 |
| 21. | Cui, Z.; Shang, X.; Shao, X.-F.; Liu, Z.-Q. Chem. Sci. 2012, 3, 2853–2858. doi:10.1039/c2sc20712e |
| 27. | Zhao, J.; Fang, H.; Han, J.; Pan, Y. Org. Lett. 2014, 16, 2530–2533. doi:10.1021/ol5009119 |
| 28. | Rout, S. K.; Guin, S.; Ali, W.; Gogoi, A.; Patel, B. K. Org. Lett. 2014, 16, 3086–3089. doi:10.1021/ol5011906 |
| 44. | Zhou, W.; Ni, S.; Mei, H.; Han, J.; Pan, Y. Org. Lett. 2015, 17, 2724–2727. doi:10.1021/acs.orglett.5b01140 |
| 47. | Fang, H.; Zhao, J.; Qian, P.; Han, J.; Pan, Y. Asian J. Org. Chem. 2014, 3, 1266–1269. doi:10.1002/ajoc.201402169 |
| 48. | Fang, H.; Zhao, J.; Ni, S.; Mei, H.; Han, J.; Pan, Y. J. Org. Chem. 2015, 80, 3151–3158. doi:10.1021/acs.joc.5b00058 |
© 2016 Qian et al; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)