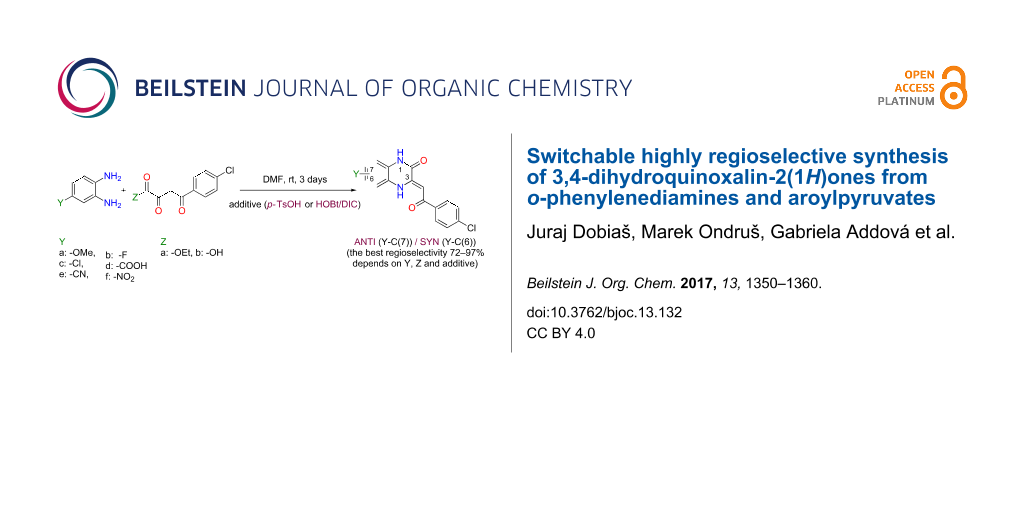
Abstract
3-Acylmethylidene-3,4-dihydroquinoxalin-2(1H)-ones are compounds which possess a wide range of physical and pharmaceutical applications. These compounds can be easily prepared by cyclocondensation of o-phenylenediamines and aroylpyruvates. Unsymmetrically substituted o-phenylenediamines can be obtained form regioisomeric mixtures of 3,4-dihydroquinoxalin-2(1H)-ones. It is often quite difficult to get a pure regioisomer and determine its structure without controlling the reaction selectivity and exploitation of complex NMR techniques (HSQC, NOESY, HMBC). This article examines the regioselectivity of the cyclocondensation between six monosubstituted o-phenylenediamines (-OMe, -F, -Cl, -COOH, -CN, -NO2) and the derivatives of p-chlorobenzoylpyruvate (ester or acid) which we studied. Six regioisomeric 3,4-dihydroquinoxalin-2(1H)-one pairs were selectively prepared and characterised. Based on our experiences, a simplified methodology for determining the structure of the regioisomers was proposed. We developed two general and highly selective methodologies starting from the same o-phenylenediamines and activated 4-chlorobenzoylpyruvates (ester or acid) enabling switching of 3,4-dihydroquinoxalin-2(1H)-one regioselectivity in a predictable manner. For comparison, all regioselective cyclocondensations were performed with the same standardized conditions (DMF, rt, 3 days), differing only by the additives p-TsOH or HOBt/DIC (hydroxybenzotriazole/N,N’-diisopropylcarbodiimide). Both selected methods are simple, general and highly regioselective (72–97%). A mechanism for the regioselectivity was also proposed and discussed. This study can be used as a guide when choosing the most optimal reaction conditions for the synthesis of the desired 3,4-dihydroquinoxalin-2(1H)-one regioisomers with the best selectivity. The demonstrated methodologies in this article may also be applied to differently substituted 3,4-dihydroquinoxalin-2(1H)-ones in general, which could expand the synthetic impact of our results.
Graphical Abstract
Introduction
The quinoxalin-2(1H)-one moiety is frequently found in compounds that exhibit biological activity, particularly antimicrobial, anticancer, anti-HIV, antithrombotic, analgesic and antidiabetic [1-3]. Substitution on C(3) of quinoxalin-2(1H)-ones 1 by substituents that possess a carbonyl group in a β side chain position 2, 3 significantly alters their chemical properties as suggested by 1H NMR, IR spectra and X-ray crystallography of the studied derivatives [4,5]. In this case, their tautomeric equilibrium is shifted to enamines 3 that have been stabilized by an intramolecular hydrogen bond (Scheme 1). A new pseudo-ring is formed via the hydrogen bond in 3, which further spreads the planarity of the 3,4-dihydroquinoxalin-2(1H)-one system.
Scheme 1: The structures of quinoxalin-2(1H)-ones 1, 2 and 3,4-dihydroquinoxalin-2(1H)-ones 3. An acylmethyl group in imines 2 shifts their tautomeric equilibrium to enamines 3.
Scheme 1: The structures of quinoxalin-2(1H)-ones 1, 2 and 3,4-dihydroquinoxalin-2(1H)-ones 3. An acylmethyl ...
The keto–enamine arrangement in 3 is capable of specific binding of Cu(II) ions like in 4, which was proved by red shifts in the UV–vis spectra [6-8]. The pseudo ring can also accommodate BF2 moiety, yielding compounds like 5 with interesting fluorescent properties [9,10]. Compounds 3 have described similar biological activities to the parent quinoxalin-2(1H)-ones 1. Mashevskaya et al. reported about an antimicrobial activity for compound 6 at 1 mg/mL for S. aureus P-209 and E. coli M17 strains [11]. Several recent studies have identified 3,4-dihydroquinoxalin-2(1H)-ones 7–10 as hits for distinct biological targets [12-20], particularly antidiabetic 7 [20], anticancer 8 [18], anti-inflammatory 9 [16], and antibacterial 10 [19] (Figure 1).
Figure 1: The structures including some of their physical and biological properties of 3,4-dihydroqunoxalin-2(1H)-ones 4–10. Abbreviations used: F-1,6-BP = fructose 1,6-bisphosphatase; Eya2 = eyes absent homolog 2; HNE = human neutrophil elastase; MurF = muramyl ligase F; REA = remaining activity.
Figure 1: The structures including some of their physical and biological properties of 3,4-dihydroqunoxalin-2...
The described compounds 4–6 were prepared from o-phenylenediamine. Compounds 7–10 may be prepared from monosubstituted o-phenylenediamines 11. In this case the amino groups usually have different reactivity and thus produce a mixture of regioisomeric products. If both regioisomers are needed, one can separate them [21]. Separation can be difficult to such an extent that some of the Cu(II) chelators were characterised as regioisomeric mixtures [22]. Nevertheless, most of the nonsymmetrical 3,4-dihydroquinoxalin-2(1H)-ones are required in their pure form (Figure 1).
To the best of our knowledge, there is only one paper which deals with the regioselective synthesis of 3-acylmethylidene-3,4-dihydroquinoxalin-2(1H)-ones 3. Andreichikov et al. reported controlled synthesis of both SYN and ANTI regioisomers [23] (Scheme 2). For the sake of simplicity, we introduced SYN and ANTI descriptors for regioisomers according to the relative positions of two substituents on 3,4-dihydroquinoxalin-2(1H)-one skeleton (e.g., -CN and 4-chlorobenzoylmethylidene groups in 16e (SYN) and 17e (ANTI)). The reaction of 3,4-diaminobenzonitrile (11e) with α,γ-diketoester 12a provided the product 16e (SYN) through an enamine intermediate 14, formed by the reaction of the most reactive α-keto group in 12a with the more nucleophilic m-amino group of 11e. The opposite regioisomer 17e (ANTI) was selectively prepared by reaction of the more nucleophilic m-amino group of 11e with the most reactive lactonic carbonyl group in 5-(4-chlorophenyl)furan-2,3-dione (13) through an amide intermediate 15 (Scheme 2).
Scheme 2: Selective synthesis of both 3,4-dihydroquinoxalin-2(1H)-one regioisomers 16e (SYN) and 17e (ANTI).
Scheme 2: Selective synthesis of both 3,4-dihydroquinoxalin-2(1H)-one regioisomers 16e (SYN) and 17e (ANTI).
Abasolo et al. studied the reaction of monosubstituted o-phenylenediamines 11a,f–i with pyruvic ethyl ester (18a) or its acid 18b and postulated that an equilibrium between Z E imines 19, 20 and enamine 21 is quickly reached and the rate-determining reaction step is a subsequent intramolecular cyclization yielding the regioisomeric products 22 and 23 [24] (Scheme 3). The proposed mechanism was based on the isolation of the 4-nitrodiaminobenzene imine intermediate which possessed reduced reactivity for intramolecular cyclisation. This type of reaction was exploited in the literature several times for the synthesis of quinoxalin-2(1H)-one regioisomers [10,25,26].
Scheme 3: The proposed mechanism for the synthesis of 3-methylquinoxalin-2(1H)-one regioisomers 22 and 23. In the case of starting 11g (Y: -H), 22g is the same as 23g and no SYN/ANTI regioisomerism exists.
Scheme 3: The proposed mechanism for the synthesis of 3-methylquinoxalin-2(1H)-one regioisomers 22 and 23. In...
Cyclocondensation between 11f and 24 provided mainly quinoxalin-2(1H)-one 27 (ANTI). Sherman et al. prepared the opposite regioisomer 26 (SYN) by masking the more reactive amino group in 11f through the acetamide intermediate 25 and its subsequent reaction with acid chloride 24, forming a stable N-acetamide-N’-acylamide intermediate (not shown). The acetamide group was then selectively cleaved and the liberated amino group spontaneously cyclised to SYN quinoxalin-2(1H)-one 26 [27] (Scheme 4).
Scheme 4: The regioselective syntheses of both quinoxalin-2(1H)-ones 27 (ANTI) and 26 (SYN).
Scheme 4: The regioselective syntheses of both quinoxalin-2(1H)-ones 27 (ANTI) and 26 (SYN).
Complete regioselectivity can be obtained if one does not begin from the substituted o-phenylenediamine 11. Sakata et al. reported an interesting one-pot procedure yielding 6-substituted SYN quinoxalin-2(1H)-ones from substituted N-(2-nitrophenyl)-3-oxobutanamides [28].
Another example for preparation of the desired regioisomer starts from the nucleophilic substitution of o-fluoronitrobenzenes 28 with the derivatives of α-amino acids [29-31]. The product of spontaneous cyclization of 30 was obtained after reduction of nitro derivative 29. Through mild oxidation, compound 30 provides the required quinoxalin-2(1H)-one 31 (Scheme 5). The obvious disadvantage in this case is the initial aromatic substitution step from 28 to 29 and the additional two reactions to 31, compared to the direct synthesis of both regioisomers depicted in Scheme 2.
Scheme 5: The selective synthesis of substituted quinoxalin-2(1H)-ones 31 from 28 via three reaction steps.
Scheme 5: The selective synthesis of substituted quinoxalin-2(1H)-ones 31 from 28 via three reaction steps.
Results and Discussion
A study to control the regioselectivity in the synthesis of 17d (ANTI) (Table 1) was motivated by our ongoing medicinal chemistry research. According to the literature, the synthesis of 3-acylmethylidene-3,4-dihydroquinoxalin-2(1H)-ones 3 can be performed by simple cyclocondensation of an appropriate o-phenylenediamine 11 with α,γ-diketoester 12a (Scheme 2). In our case, starting from 3,4-diaminobenzoic acid (11d) (natively preferring SYN regioselectivity, see Table 1, entry 4, column 3) complicated the desired reaction. The other possibility was to perform the regioselective synthesis of 17d (ANTI) from an appropriate furan-2,3-dione 13 (Scheme 2). The preparation of 13 failed in our hands. Therefore, we decided to perform the cyclocondensation of 11d with ethyl 4-chlorobenzoylpyruvate (12a) and separate the less abundant 17d (ANTI) from a mixture of regioisomers. The cyclocondensation of 11d with 12a in refluxing dioxane within 1 hour yielded a precipitate that consisted of a balanced (50/50) mixture of both SYN/ANTI regioisomers 16d and 17d (confirmed by 1H NMR). The low solubility of the crude products in DCM, THF, EtOAc or MeOH combined with an unfavourable ratio of regioisomers prevented their separation by FLC or crystallisation. Based on these results we realised that it would be difficult to obtain pure ANTI regioisomer 17d without controlling the reaction selectivity. In order to influence the regioselectivity, cyclocondensation of 11d with 12a was performed in the presence of basic TEA (Et3N) in DMF at rt within 3 days. The reaction yielded a crude mixture with a predominant 17d (ANTI) regioisomer (70/30, not shown elsewhere). For the same reaction, we also used less basic DMAP instead of TEA. This cyclocondensation formed a crude mixture with even better 17d (ANTI) selectivity (88/12) (Table 1, entry 4, column 5). DMAP is also known as an acyl activation reagent (similar to: HOBt, HATU, T3P, etc.). Therefore, we concluded that switching in regioselectivity (from native SYN to ANTI) could be a matter of DMAP activation of the acyl group in 12a.
Table 1: The regioisomeric ratios (17 (ANTI)/16 (SYN)) and conversions depending on used diamine 11a–f, dicarbonyl compound 12a,b and reaction conditions.
|
|
||||||
| Entry | Diamine 11a–f | The below specified conditions dependent ratiosa and conversions.b | ||||
| Ester 12ac | Ester 12ac,d | Acid 12bc,d | Ester 12ac,e | Acid 12bf | ||
| 1 | 11a (-OMe) |
85/15a
(100%)b |
91/09
(100%) |
67/33
(100%) |
14/86
(100%) |
15/85
(100%) |
| 2 | 11b (-F) |
80/20
(95%) |
89/11
(100%) |
85/15
(100%) |
17/83
(80%) |
14/86
(100%) |
| 3 | 11c (-Cl) |
65/35
(100%) |
72/28
(100%) |
70/30
(100%) |
28/72
(75%) |
23/77
(100%) |
| 4 | 11d (-COOH) |
38/62
(100%) |
13/87
(100%) |
12/88
(100%) |
88/12
(50%) |
93/07
(100%) |
| 5 | 11e (-CN) |
17/83
(100%) |
15/85
(100%) |
11/89
(100%) |
100/0
(25%) |
93/07
(100%) |
| 6 | 11f (-NO2) |
18/82
(90%) |
20/80
(100%) |
05/95
(100%) |
68/32
(22%) |
97/03
(100%) |
aThe ratios for both regioisomers 16 and 17 (mol %) were determined by 1H NMR spectra from evaporated reaction mixtures (based on characteristic singlet signals of each regioisomer =CHCO– (6.8–6.9 ppm)) and are depicted here in the order 17 (ANTI)/16 (SYN). The ratios marked in bold represent the best selectivities with cut-off ≥85% for the main ANTI or SYN regioisomer. bThe % number in brackets describes conversion of the appropriate starting o-phenylenediamine 11a–f obtained from the crude 1H NMR spectra. cGeneral procedure A was used. dTsOH (1 mol equiv) was added. eDMAP (1 mol equiv) was added. Reaction proceeds through in situ formed intermediate 12c (Scheme 6). fGeneral procedure B (HOBt/DIC) was used. Reaction proceeds through in situ formed intermediate 12d (Scheme 6).
Scheme 6: Regioselectivity switching based on carbonyl activation of 4-chlorobenzoylpyruvates 12a,b by p-TsOH or acyl activation in 12c,d (prepared in situ from ester 12a by DMAP – 12c or from acid 12b and HOBt/DIC – 12d).
Scheme 6: Regioselectivity switching based on carbonyl activation of 4-chlorobenzoylpyruvates 12a,b by p-TsOH...
The above controlled ANTI cyclocondensation (88/12) performed in the presence of DMAP enabled us to obtain pure 17d (ANTI) regioisomer in a 36% yield after crystallisation from DMSO. To prove the exact structure of 17d (ANTI) was not easy and we had to combine the more complex 2D NMR techniques (HSQC, NOESY and HMBC). At first, we assigned hydrogens and carbons in the main regioisomer. Next, we proved for 17d its ANTI isomerism by HMBC analysis, which showed an important four-bonded interaction between the hydrogen of the methylene C(3)=CH- group and the quaternary carbon C(4a) (Figure 2). The carbon C(4a) was interconnected in the HMBC with 2 other three-bonded interactions with hydrogen H-C(8) and H-C(6), whereas for the 16d (SYN) isomer the H-C(6) interaction should be missing (see also the HMBC diagrams and spectra in Supporting Information File 1).
Figure 2: The interactions and assignments, obtained after analyses of NMR spectra, allowed us to distinguish between the 16d and 17d regioisomers. The important 1H and 13C NMR chemical shifts are depicted close to each appropriate atom in the structures accompanied with an coupling constant in brackets, if there was any. The double-headed red arrows represent important space interactions observed in NOESY spectra. The green-coloured bonds in the structures represent some of the most important HMBC interactions that helped us when assigning the right regioisomers. The HMBC signals between the skeletal proton and carbon are limited to 2, 3 and sometimes also to 4 bonds.
Figure 2: The interactions and assignments, obtained after analyses of NMR spectra, allowed us to distinguish...
After we had proved the structure of the 17d (ANTI) regioisomer, we decided to elucidate the structure for the 16d (SYN) isomer as well, in order to complementary validate the above assignments. In regards to the absence of regioselectivity by heating of 3,4-diaminobenzoic acid (11d, -COOH) with 12a in dioxane for 1 hour (50/50), a reaction under milder conditions (DMF, rt, 3 days) was performed resulting in ANTI/SYN selectivity (38/62, Table 1, entry 4, columns 3 and 4). In order to positively influence the regioselectivity, cyclocondensation was performed in the presence of p-TsOH (DMF, rt, 3 days). This reaction resulted in high SYN regioselectivity (13/87). The enhanced selectivity was explained by the elevation of electrophilicity of the α-ketone in 12a by p-TsOH. After the crystallisation from DMSO, pure 16d (SYN) was isolated. We elucidated its structure in similar manner as for the 17d (ANTI) isomer (Figure 2) and confirmed that the 1H NMR (DMSO-d6) higher shifted signal for the H-N(4) group is the one that forms a intramolecular hydrogen bond with the carbonyl group from a side chain like in 3 (Scheme 1). This knowledge, in combination with a simple NOE experiment (interaction H-N(4) with a narrow doublet of H-C(5) (ca 1.5 Hz) in SYN or H-N(4) with a broad doublet of H-C(5) (8.4 Hz) in ANTI, allows for the determination of the regioisomerism more effectively (no need for HMBC), in the case that the NOE experiment was performing (which was not always the case). The same approach to distinguish regioisomers can be applied on space interactions between H-N(1) and H-C(8) signals (NOESY in Figure 2 or for NOE see the Supporting Information File 1).
Finally, all six regioisomeric 3,4-dihydroquinoxalin-2(1H)-one pairs were selectively prepared and characterised (Table 1 and Supporting Information File 1). Their condensations were investigated under five different reaction conditions:
a) Cyclocondensations of diamines 11a–f and ethyl ester of 12a were performed by our standardized conditions: DMF, rt, 3 days (General procedure A). In all cases, the most nucleophilic amine from diamine 11 predominantly reacts with the most reactive α-carbonyl group from ester 12a to form an imine/enamine bond followed by amidic intramolecular cyclization to yield the main regioisomer of 3,4-dihydroquinoxalin-2(1H)-one. The regioselectivity was dependent on the character of substituent Y in diamines 11a–f (Table 1). According to obtained selectivities (Table 1), the substituents on diamines 11 were divided into two clusters causing either the same or the contrary regioselectivity: (a) with electron-donating groups and halogens (EDG or HG) or (b) with electron-withdrawing (EWG) groups (Table 1, Scheme 6). Using the general procedure A without any additives, diamine 11 was always almost quantitatively consumed (90–100%), and for both clusters of substituents on 11, the main regioisomer of 3,4-dihydroquinoxalin-2(1H)-one was formed with average to good selectivity (62–85%) (Table 1, column 3).
b) Utilizing the general procedure A with p-TsOH as an additive to diamine from 11a–f and ester 12a caused greater reactivity of the α-carbonyl group in 12a (Scheme 6), quantitative consumption of diamine 11, and production of the main regioisomer of 3,4-dihydroquinoxalin-2(1H)-one with good to excellent selectivity (72–91%) for both clusters of substituents (Table 1, column 4).
c) Ester 12a to acid 12b modification with other parameters as in (b) resulted in the preservation of complete conversions of 11, a decrease of ANTI selectivity (67–85%) for EDG/HG substituents in 11a–c (Table 1, entries 1–3, column 5), however, a SYN selectivity boost (88–95%) for EWG substituents in 11d–f (Table 1, entries 4–6, column 5).
d) In order to investigate the contrary (switched) regioselectivity, DMAP was added to diamine from 11a–f and ester 12a, and standard general procedure A was used. In these cases, DMAP activated the acyl moiety in ester 12a via 12c (Scheme 6) and caused the formation of the predominant regioisomer of 3,4-dihydroquinoxalin-2(1H)-one with reversed selectivity as determined before (a)–(c). The consumption of 11a–c (Table 1, entries 1–3, column 6) was good (75–100%), but insufficient for 11d–f (22–50%, Table 1, entries 4–6, column 6). Our explanation for the low conversions is a diminished amine nucleophilicity in the EWG-substituted dianilines 11d–f and reduced electrophilicity of the 12a due to a partial formation of a stabilized enolate anion by DMAP deprotonation. Therefore, using DMAP activation is not recommended for EWG-substituted diamines.
e) The reaction of activated ester 12d (obtained in situ after treatment of carboxylic acid 12b with HOBt/DIC, Scheme 6) and a diamine 11a–f (general procedure B) was found to be the most convenient method to produce contrary product regioselelectivity. In this case, the consumption of diamine 11 was always quantitative. The regioselectivity was opposite in comparison to the reactions from (a)–(c) and similar to the reaction exploiting DMAP activation of ester 12a in (d). HOBt/DIC activation of acid 12b resulted in the synthesis of the main regioisomers of 3,4-dihydroquinoxalin-2(1H)-ones with good to excellent SYN selectivity (77–86%) for EDG/HG cluster of substituents in 11a–c (Table 1, entries 1–3, column 7) and excellent ANTI selectivity (93–97%) for EWG substituents in 11d–f (Table 1, entries 4–6, column 7). The HOBt/DIC methodology seems to be better than the one described with DMAP due to its clean reaction course and high regioselectivity in general (Table 1, column 7). The HOBt methodology is complementary in regioselectivity to cycloadditions performed by p-TsOH (Table 1, column 4).
From the above experimental results, we have proved that the regioselectivity of the cyclocondensation depends on both (a) the different nucleophilicity of the two amines from o-phenylenediamines 11a–f and (b) the electrophilicity of the α-carbonyl in 12a or the contrary regioselectivity of activated species 12c,d (prepared in situ from appropriate 12 by DMAP – 12c or HOBt/DIC additives – 12d) (Scheme 6).
Conclusion
Simple reaction conditions were discovered for predictable and switchable highly regioselective synthesis of 3,4-dihydroquinoxaline-2(1H)-ones 16 or 17 starting from monosubstituted o-phenylenediamines 11 and 4-chlorobenzoylpyruvates 12 in DMF at rt. These conditions were tested by cyclocondensations on six diamines 11a–f with two pyruvates 12a,b and allowed us to prepare, purify and characterise twelve (six pairs) of regioisomeric 3,4-dihydroquinoxaline-2(1H)-ones. Their ANTI (17) or SYN (16) structures were assigned by complex 2D NMR techniques (HSQC, NOESY and HMBC) or by a proposed simplified method (NOE interaction between higher 1H NMR (DMSO-d6) shifted H-N(4), bonded by intramolecular hydrogen bond, and H-C(5) or interaction of lower shifted H-N(1) and H-C(8), whereby coupling of HC(5 and 8) depends on type of the regioisomer). It was proved that observed regioselectivity of performed cyclocondensations depends on both a/ the different nucleophilicity of amine groups in diamine 11 (with two clusters of substituents: EDGs + halogens (HG) and complementary operating EWGs) and (b) the different activation of 4-benzoylpyruvates 12a,b (p-TsOH; contrarily by DMAP or HOBt/DIC). Obtained selectivities were discussed and their mechanism proposed (Scheme 6). Our study can act as a guide for choosing the optimal reaction conditions for the synthesis of the desired regioisomer of 3,4-dihydroquinoxaline-2(1H)-one 16 or 17 with the best selectivity (activation of 12a,b by acid or opposite selectivity obtained from activated species 12c,d) (Scheme 6, Table 2).
Table 2: The guide for reaction conditions for obtaining the desired regioisomer of 3,4-dihydroquinoxaline-2(1H)one 16 (SYN) or 17 (ANTI) with the best selectivity.
| Starting pyruvate 12 with additive/ substituted diamine 11 |
Ester 12a Acid 12b
p-TsOH |
Acid 12b
HOBt/DIC |
|---|---|---|
| 11a–c (EDG or halogen) | 17 (ANTI) (72–91%) | 16 (SYN) (77–86%) |
| 11d–f (EWG) | 16 (SYN) (88–95%) | 17 (ANTI) (93–97%) |
A limitation for regioselectivity is the fact that it is dependent on the character of the substituent in aryldiamine 11. If the nucleophilicity of the two amino groups in 11 are not differentiated enough, the obtained selectivity will be less synthetically useful. In that case, a different synthesis will be required, e.g., masking amino with the nitro group. The HOBt/DIC procedure performs with contrary regioselectivity to p-TsOH. We believe that the other acyl activated agents like T3P (propylphosphonic anhydride) or HATU should produce similar results as well. The demonstrated findings could be applied also to differently substituted 3,4-dihydroquinoxalin-2(1H)-ones in general, which can expand the synthetic impact of our study.
Experimental
Syntheses and characterisation of compounds 16d (SYN) and 17d (ANTI) are described below. The synthesis of all prepared compounds 12a,b, 16a–f (SYN) and 17a–f (ANTI) together with their characterisation, spectral diagrams and spectra can be found in Supporting Information File 1.
General procedure A
A solution of ethyl 4-chlorobenzoylpyruvate (100 mg, 0.39 mmol, 1.00 equiv) (12a) or 4-chlorobenzoylpyruvic acid (12b) (88.4 mg, 0.39 mmol, 1.00 equiv), o-phenylenediamine 11a–f (1.00 equiv) with or without an additive (1.00 equiv) (p-TsOH or DMAP) was stirred in 3.0 mL of DMF (abs) at rt under Ar for 72 hours. A low soluble mixture of ANTI/SYN regioisomers slowly precipitated within the reaction. The precipitate was collected by filtration or centrifugation, triturated by 3 mL of Et2O and crystallized from DMSO (if not otherwise stated) to yield the main solid regioisomer 16 or 17.
General procedure B
Diisopropylcarbodiimide 82 μL (66.9 mg, 0.53 mmol, 1.20 equiv, DIC) was added to a solution of 4-chlorobenzoylpyruvic acid (100 mg, 0.44 mmol, 1.00 equiv, 12b) and 73.8 mg (0.53 mmol, 1.20 mol equiv) of HOBt [CAS: 123333-53-9, 97% wetted with ≥14 wt % H2O] in 3.0 mL of DMF (abs) under Ar. The reaction mixture was stirred for 5 min. Then o-phenylenediamine (1.00 equiv, 11a–f) was added and the mixture was stirred at rt under Ar for 72 h. The precipitated product mixture obtained after filtration (or centrifugation) was triturated by 3 mL of Et2O and crystallized from DMSO (if not otherwise stated) to yield the main solid regioisomer 16 or 17.
Compound 16d (SYN)
The compound 16d (SYN) (Figure 3) was prepared according the general procedure A from diamine 11d, ester 12a and p-TsOH as an additive. The crude mixture of ANTI/SYN regioisomers was purified by crystallization from DMSO to yield 64.6 mg (0.19 mmol, 48%) of 16d (SYN). Mp: 363.0–365.0 °C (DMSO), yellow solid compound. 1H NMR (600 MHz, DMSO-d6) δ 13.48 (s, 1H, H-NA(4)), 12.92 (br s, 1H, -COOH), 12.26 (s, 1H, H-NA(1)), 8.04 (d, J(A5,A7) = 1.5 Hz, 1H, H-CA(5)), 7.99 (d, J(B2,B3) = 8.4 Hz, 2H, 2 × H-CB(2)), 7.69 (dd, J(A7,A8) = 8.3 Hz, J(A5,A7) = 1.5 Hz, 1H, H-CA(7)), 7.57 (d, J(B2,B3) = 8.4 Hz, 2H, 2 × H-CB(3)), 7.18 (d, J(A7,A8) = 8.3 Hz, 1H, H-CA(8)), 6.79 (s, 1H, -COCH=); 13C NMR (150 MHz, DMSO-d6) δ 187.7 (CB(1)C=O), 167.0 (-COOH), 156.4 (CA(2)), 145.8 (CA(3)), 137.8 (CB(1)), 137.2 (CB(4)), 130.8 (CA(8a)), 129.4 and 129.3 (2 × CB(2 and 3)), 126.4 (CA(6)), 125.6 (CA(7)), 124.4 (CA(4a)), 118.3 (CA(5)), 115.8 (CA(8)), 90.0 (-COCH=). FTIR cm−1 (solid): 3184 (s, -COOH), 2925 (m), 1732 (w), 1688 (s, C=O), 1615 (s), 1586 (s), 1550 (w), 1486 (w), 1366 (m), 1247 (m), 1218 (m), 1095 (m), 1065 (w), 1011 (w), 787 (w), 750 (m) cm−1; MS (ESI m/z): 341.2 [M − H]−; anal. calcd for C17H11ClN2O4 (342.73): C, 59.57; H, 3.23; Cl, 10.34; N, 8.17; found: C, 59.75; H, 3.38; Cl, 10.23; N, 8.01.
Figure 3: NMR assignments for compound 16d.
Figure 3: NMR assignments for compound 16d.
Compound 17d (ANTI)
Compound 17d (ANTI) (Figure 4) was prepared according general procedure A from diamine 11d, ester 12a and (1.00 equiv) of DMAP as an additive. The crude product was crystallized from DMSO and obtained as salt with DMAP. To liberate the free acid 17d (ANTI), the salt was suspended in 1 M HCl, stirred for 24 hours, the solid material was filtered off, washed with water and dried to yield 48.4 mg (0.14 mmol, 36%) of 17d (ANTI).
Alternatively 17d (ANTI) was also prepared according to general procedure B from diamine 11d, acid 12b and HOBt/DIC. The crude product was crystallized from DMSO to yield 105.9 mg (0.31 mmol, 70%) of 17d (ANTI). Mp: 391.0–392.0 °C [DMSO], yellow solid compound. 1H NMR (300 MHz, DMSO-d6) δ 13.44 (s, 1H, H-NA(4)), 12.95 (br s, 1H, -COOH), 12.14 (s, 1H, H-NA(1)), 8.02 (d, J(B2,B3) = 8.6 Hz, 2H, 2 × H-CB(2)), 7.73 (d, J(A6,A8) = 1.6 Hz, 1H, H-CA(8)), 7.67 (dd, J(A5,A6) = 8.4 Hz, J(A6,A8) = 1.6 Hz, 1H, H-CA(6)), 7.61 (d, J(A5,A6) = 8.4 Hz, 1H, H-CA(5)), 7.60 (d, J(B2,B3) = 8.6 Hz, 2H, 2 × H-CB(3)), 6.87 (s, 1H, -COCH=); 13C NMR (150 MHz, DMSO-d6) δ 188.3 (CB(1)C=O), 167.0 (-COOH), 156.1 (CA(2)=O), 145.6 (CA(3)), 2 × 137.6 (CB(1 and 4)), 129.5 and 129.3 (2 × CB(2 and 3)), 128.1 (CA(4a)), 2 × 126.9 (CA(7 and 8a)), 125.2 (CA(6)), 2 × 116.9 (CA(5 and 8)), 90.8 (-COCH=); FTIR cm−1 (solid): 3486 (m), 3206 (s, -COOH), 2634 (w), 1706 (s, C=O), 1661 (w), 1628 (m), 1586 (s, C=O), 1522 (w), 1398 (m), 1374 (w), 1291 (m), 1248 (m), 1184 (m), 1093 (w), 1056 (m), 1009 (w), 899 (w), 781 (w), 764 (w), 721 (w) cm−1; MS (ESI m/z): 341.0 [M − H]−; anal. calcd for C17H11ClN2O4 (342.73): C, 59.57; H, 3.23; N, 8.17; found: C, 59.82; H, 3.10; N, 8.32.
Figure 4: NMR assignments for compound 17d.
Figure 4: NMR assignments for compound 17d.
Supporting Information
| Supporting Information File 1: Additional experimental and characterisation data. | ||
| Format: PDF | Size: 2.8 MB | Download |
References
-
Qin, X.; Hao, X.; Han, H.; Zhu, S.; Yang, Y.; Wu, B.; Hussain, S.; Parveen, S.; Jing, C.; Ma, B.; Zhu, C. J. Med. Chem. 2015, 58, 1254–1267. doi:10.1021/jm501484b
Return to citation in text: [1] -
Carta, A.; Piras, S.; Loriga, G.; Paglietti, G. Mini-Rev. Med. Chem. 2006, 6, 1179–1200. doi:10.2174/138955706778742713
Return to citation in text: [1] -
Abu-Hashem, A. A. Am. J. Org. Chem. 2015, 5, 14–56.
Return to citation in text: [1] -
Kurasawa, Y.; Miyashita, R.; Takada, A.; Kim, H. S.; Okamoto, Y. J. Heterocycl. Chem. 1995, 32, 671–674. doi:10.1002/jhet.5570320249
Return to citation in text: [1] -
Seki, T.; Iwanami, Y.; Kuwatani, Y.; Iyoda, M. J. Heterocycl. Chem. 1997, 34, 773–780. doi:10.1002/jhet.5570340312
Return to citation in text: [1] -
Xu, Y.-S.; Zeng, C.-C.; Li, X.-M.; Zhong, R.-G.; Zeng, Y. Chin. J. Chem. 2006, 24, 1086–1094. doi:10.1002/cjoc.200690203
Return to citation in text: [1] -
Badawy, M. A.; Mohamed, G. G.; Omar, M. M.; Nassar, M. M.; Kamel, A. B. Eur. J. Chem. 2010, 1, 282–288. doi:10.5155/eurjchem.1.4.282-288.113
Return to citation in text: [1] -
Korin, E.; Cohen, B.; Liu, Y.-D.; Zeng, C.-C.; Shames, A. I.; Becker, J. Y. J. Coord. Chem. 2013, 66, 2351–2366. doi:10.1080/00958972.2013.803535
Return to citation in text: [1] -
Xia, M.; Wu, B.; Xiang, G. J. Fluorine Chem. 2008, 129, 402–408. doi:10.1016/j.jfluchem.2008.01.019
Return to citation in text: [1] -
Yao, Q.-C.; Wu, D.-E.; Ma, R.-Z.; Xia, M. J. Organomet. Chem. 2013, 743, 1–9. doi:10.1016/j.jorganchem.2013.06.012
Return to citation in text: [1] [2] -
Mashevskaya, I. V.; Tolmacheva, I. A.; Voronova, E. V.; Odegova, T. F.; Aleksandrova, G. A.; Goleneva, A. F.; Kol’tsova, S. V.; Maslivets, A. N. Khim.-Farm. Zh. 2002, 36, 86–88.
Return to citation in text: [1] -
Ambaye, N. D.; Gunzburg, M. J.; Lim, R. C. C.; Price, J. T.; Wilce, M. C. J.; Wilce, J. A. Bioorg. Med. Chem. 2011, 19, 693–701. doi:10.1016/j.bmc.2010.10.030
Return to citation in text: [1] -
Ciustea, M.; Silverman, J. E. Y.; Druck Shudofsky, A. M.; Ricciardi, R. P. J. Med. Chem. 2008, 51, 6563–6570. doi:10.1021/jm800366g
Return to citation in text: [1] -
Drwal, M. N.; Marinello, J.; Manzo, S. G.; Wakelin, L. P. G.; Capranico, G.; Griffith, R. PLoS One 2014, 9, e114904. doi:10.1371/journal.pone.0114904
Return to citation in text: [1] -
Fan, C.; Clay, M. D.; Deyholos, M. K.; Vederas, J. C. Bioorg. Med. Chem. 2010, 18, 2141–2151. doi:10.1016/j.bmc.2010.02.001
Return to citation in text: [1] -
Habash, M.; Abdelazeem, A. H.; Taha, M. O. Med. Chem. Res. 2014, 23, 3876–3896. doi:10.1007/s00044-014-0966-4
Return to citation in text: [1] [2] -
Krueger, B. A.; Weil, T.; Schneider, G. J. Comput.-Aided Mol. Des. 2009, 23, 869–881. doi:10.1007/s10822-009-9304-1
Return to citation in text: [1] -
Park, H.; Jung, S.-K.; Yu, K. R.; Kim, J. H.; Kim, Y.-S.; Ko, J. H.; Park, B. C.; Kim, S. J. Chem. Biol. Drug Des. 2011, 78, 642–650. doi:10.1111/j.1747-0285.2011.01192.x
Return to citation in text: [1] [2] -
Taha, M. O.; Atallah, N.; Al-Bakri, A. G.; Paradis-Bleau, C.; Zalloum, H.; Younis, K. S.; Levesque, R. C. Bioorg. Med. Chem. 2008, 16, 1218–1235. doi:10.1016/j.bmc.2007.10.076
Return to citation in text: [1] [2] -
Tayyem, R. F.; Zalloum, H. M.; Elmaghrabi, M. R.; Yousef, A.-M.; Mubarak, M. S. Eur. J. Med. Chem. 2012, 56, 70–95. doi:10.1016/j.ejmech.2012.08.004
Return to citation in text: [1] [2] -
Sanna, P.; Carta, A.; Loriga, M.; Zanetti, S.; Sechi, L. Farmaco 1999, 54, 169–177. doi:10.1016/S0014-827X(99)00011-7
Return to citation in text: [1] -
Korin, E.; Cohen, B.; Bai, Y.-X.; Zeng, C.-C.; Becker, J. Y. Tetrahedron 2012, 68, 7450–7455. doi:10.1016/j.tet.2012.06.071
Return to citation in text: [1] -
Andreichikov, Y. S.; Nekrasov, D. D.; Pitirimova, S. G.; Zaks, A. S.; Korsheninnikova, M. I.; Plaksina, P. N.; Semenova, Z. N.; Kopeikin, V. A. Khim.-Farm. Zh. 1989, 23, 946–949.
Return to citation in text: [1] -
Abasolo, M. I.; Gaozza, C. H.; Fernández, B. M. J. Heterocycl. Chem. 1987, 24, 1771–1775. doi:10.1002/jhet.5570240651
Return to citation in text: [1] -
Mondieig, D.; Negrier, P.; Massip, S.; Leger, J. M.; Jarmoumi, C.; Lakhrissi, B. J. Phys. Org. Chem. 2011, 24, 1193–1200. doi:10.1002/poc.1846
Return to citation in text: [1] -
Xia, Q.-H.; Hu, W.; Li, C.; Wu, J.-F.; Yang, L.; Han, X.-M.; Shen, Y.-M.; Li, Z.-Y.; Li, X. Eur. J. Med. Chem. 2016, 124, 311–325. doi:10.1016/j.ejmech.2016.08.010
Return to citation in text: [1] -
Sherman, D.; Kawakami, J.; He, H.-Y.; Dhun, F.; Rios, R.; Liu, H.; Pan, W.; Xu, Y.-J.; Hong, S.-p.; Arbour, M.; Labelle, M.; Duncton, M. A. J. Tetrahedron Lett. 2007, 48, 8943–8946. doi:10.1016/j.tetlet.2007.10.117
Return to citation in text: [1] -
Sakata, G.; Makino, K.; Morimoto, K. Heterocycles 1985, 23, 143–151. doi:10.3987/R-1985-01-0143
Return to citation in text: [1] -
Cushing, T. D.; Hao, X.; Shin, Y.; Andrews, K.; Brown, M.; Cardozo, M.; Chen, Y.; Duquette, J.; Fisher, B.; Gonzalez-Lopez de Turiso, F.; He, X.; Henne, K. R.; Hu, Y.-L.; Hungate, R.; Johnson, M. G.; Kelly, R. C.; Lucas, B.; McCarter, J. D.; McGee, L. R.; Medina, J. C.; San Miguel, T.; Mohn, D.; Pattaropong, V.; Pettus, L. H.; Reichelt, A.; Rzasa, R. M.; Seganish, J.; Tasker, A. S.; Wahl, R. C.; Wannberg, S.; Whittington, D. A.; Whoriskey, J.; Yu, G.; Zalameda, L.; Zhang, D.; Metz, D. P. J. Med. Chem. 2015, 58, 480–511. doi:10.1021/jm501624r
Return to citation in text: [1] -
Liu, R.; Huang, Z.; Murray, M. G.; Guo, X.; Liu, G. J. Med. Chem. 2011, 54, 5747–5768. doi:10.1021/jm200394x
Return to citation in text: [1] -
Kher, S. M.; Cai, S. X.; Weber, E.; Keana, J. F. W. J. Org. Chem. 1995, 60, 5838–5842. doi:10.1021/jo00123a020
Return to citation in text: [1]
| 28. | Sakata, G.; Makino, K.; Morimoto, K. Heterocycles 1985, 23, 143–151. doi:10.3987/R-1985-01-0143 |
| 29. | Cushing, T. D.; Hao, X.; Shin, Y.; Andrews, K.; Brown, M.; Cardozo, M.; Chen, Y.; Duquette, J.; Fisher, B.; Gonzalez-Lopez de Turiso, F.; He, X.; Henne, K. R.; Hu, Y.-L.; Hungate, R.; Johnson, M. G.; Kelly, R. C.; Lucas, B.; McCarter, J. D.; McGee, L. R.; Medina, J. C.; San Miguel, T.; Mohn, D.; Pattaropong, V.; Pettus, L. H.; Reichelt, A.; Rzasa, R. M.; Seganish, J.; Tasker, A. S.; Wahl, R. C.; Wannberg, S.; Whittington, D. A.; Whoriskey, J.; Yu, G.; Zalameda, L.; Zhang, D.; Metz, D. P. J. Med. Chem. 2015, 58, 480–511. doi:10.1021/jm501624r |
| 30. | Liu, R.; Huang, Z.; Murray, M. G.; Guo, X.; Liu, G. J. Med. Chem. 2011, 54, 5747–5768. doi:10.1021/jm200394x |
| 31. | Kher, S. M.; Cai, S. X.; Weber, E.; Keana, J. F. W. J. Org. Chem. 1995, 60, 5838–5842. doi:10.1021/jo00123a020 |
| 1. | Qin, X.; Hao, X.; Han, H.; Zhu, S.; Yang, Y.; Wu, B.; Hussain, S.; Parveen, S.; Jing, C.; Ma, B.; Zhu, C. J. Med. Chem. 2015, 58, 1254–1267. doi:10.1021/jm501484b |
| 2. | Carta, A.; Piras, S.; Loriga, G.; Paglietti, G. Mini-Rev. Med. Chem. 2006, 6, 1179–1200. doi:10.2174/138955706778742713 |
| 3. | Abu-Hashem, A. A. Am. J. Org. Chem. 2015, 5, 14–56. |
| 11. | Mashevskaya, I. V.; Tolmacheva, I. A.; Voronova, E. V.; Odegova, T. F.; Aleksandrova, G. A.; Goleneva, A. F.; Kol’tsova, S. V.; Maslivets, A. N. Khim.-Farm. Zh. 2002, 36, 86–88. |
| 10. | Yao, Q.-C.; Wu, D.-E.; Ma, R.-Z.; Xia, M. J. Organomet. Chem. 2013, 743, 1–9. doi:10.1016/j.jorganchem.2013.06.012 |
| 25. | Mondieig, D.; Negrier, P.; Massip, S.; Leger, J. M.; Jarmoumi, C.; Lakhrissi, B. J. Phys. Org. Chem. 2011, 24, 1193–1200. doi:10.1002/poc.1846 |
| 26. | Xia, Q.-H.; Hu, W.; Li, C.; Wu, J.-F.; Yang, L.; Han, X.-M.; Shen, Y.-M.; Li, Z.-Y.; Li, X. Eur. J. Med. Chem. 2016, 124, 311–325. doi:10.1016/j.ejmech.2016.08.010 |
| 9. | Xia, M.; Wu, B.; Xiang, G. J. Fluorine Chem. 2008, 129, 402–408. doi:10.1016/j.jfluchem.2008.01.019 |
| 10. | Yao, Q.-C.; Wu, D.-E.; Ma, R.-Z.; Xia, M. J. Organomet. Chem. 2013, 743, 1–9. doi:10.1016/j.jorganchem.2013.06.012 |
| 27. | Sherman, D.; Kawakami, J.; He, H.-Y.; Dhun, F.; Rios, R.; Liu, H.; Pan, W.; Xu, Y.-J.; Hong, S.-p.; Arbour, M.; Labelle, M.; Duncton, M. A. J. Tetrahedron Lett. 2007, 48, 8943–8946. doi:10.1016/j.tetlet.2007.10.117 |
| 6. | Xu, Y.-S.; Zeng, C.-C.; Li, X.-M.; Zhong, R.-G.; Zeng, Y. Chin. J. Chem. 2006, 24, 1086–1094. doi:10.1002/cjoc.200690203 |
| 7. | Badawy, M. A.; Mohamed, G. G.; Omar, M. M.; Nassar, M. M.; Kamel, A. B. Eur. J. Chem. 2010, 1, 282–288. doi:10.5155/eurjchem.1.4.282-288.113 |
| 8. | Korin, E.; Cohen, B.; Liu, Y.-D.; Zeng, C.-C.; Shames, A. I.; Becker, J. Y. J. Coord. Chem. 2013, 66, 2351–2366. doi:10.1080/00958972.2013.803535 |
| 23. | Andreichikov, Y. S.; Nekrasov, D. D.; Pitirimova, S. G.; Zaks, A. S.; Korsheninnikova, M. I.; Plaksina, P. N.; Semenova, Z. N.; Kopeikin, V. A. Khim.-Farm. Zh. 1989, 23, 946–949. |
| 4. | Kurasawa, Y.; Miyashita, R.; Takada, A.; Kim, H. S.; Okamoto, Y. J. Heterocycl. Chem. 1995, 32, 671–674. doi:10.1002/jhet.5570320249 |
| 5. | Seki, T.; Iwanami, Y.; Kuwatani, Y.; Iyoda, M. J. Heterocycl. Chem. 1997, 34, 773–780. doi:10.1002/jhet.5570340312 |
| 24. | Abasolo, M. I.; Gaozza, C. H.; Fernández, B. M. J. Heterocycl. Chem. 1987, 24, 1771–1775. doi:10.1002/jhet.5570240651 |
| 16. | Habash, M.; Abdelazeem, A. H.; Taha, M. O. Med. Chem. Res. 2014, 23, 3876–3896. doi:10.1007/s00044-014-0966-4 |
| 21. | Sanna, P.; Carta, A.; Loriga, M.; Zanetti, S.; Sechi, L. Farmaco 1999, 54, 169–177. doi:10.1016/S0014-827X(99)00011-7 |
| 18. | Park, H.; Jung, S.-K.; Yu, K. R.; Kim, J. H.; Kim, Y.-S.; Ko, J. H.; Park, B. C.; Kim, S. J. Chem. Biol. Drug Des. 2011, 78, 642–650. doi:10.1111/j.1747-0285.2011.01192.x |
| 22. | Korin, E.; Cohen, B.; Bai, Y.-X.; Zeng, C.-C.; Becker, J. Y. Tetrahedron 2012, 68, 7450–7455. doi:10.1016/j.tet.2012.06.071 |
| 20. | Tayyem, R. F.; Zalloum, H. M.; Elmaghrabi, M. R.; Yousef, A.-M.; Mubarak, M. S. Eur. J. Med. Chem. 2012, 56, 70–95. doi:10.1016/j.ejmech.2012.08.004 |
| 12. | Ambaye, N. D.; Gunzburg, M. J.; Lim, R. C. C.; Price, J. T.; Wilce, M. C. J.; Wilce, J. A. Bioorg. Med. Chem. 2011, 19, 693–701. doi:10.1016/j.bmc.2010.10.030 |
| 13. | Ciustea, M.; Silverman, J. E. Y.; Druck Shudofsky, A. M.; Ricciardi, R. P. J. Med. Chem. 2008, 51, 6563–6570. doi:10.1021/jm800366g |
| 14. | Drwal, M. N.; Marinello, J.; Manzo, S. G.; Wakelin, L. P. G.; Capranico, G.; Griffith, R. PLoS One 2014, 9, e114904. doi:10.1371/journal.pone.0114904 |
| 15. | Fan, C.; Clay, M. D.; Deyholos, M. K.; Vederas, J. C. Bioorg. Med. Chem. 2010, 18, 2141–2151. doi:10.1016/j.bmc.2010.02.001 |
| 16. | Habash, M.; Abdelazeem, A. H.; Taha, M. O. Med. Chem. Res. 2014, 23, 3876–3896. doi:10.1007/s00044-014-0966-4 |
| 17. | Krueger, B. A.; Weil, T.; Schneider, G. J. Comput.-Aided Mol. Des. 2009, 23, 869–881. doi:10.1007/s10822-009-9304-1 |
| 18. | Park, H.; Jung, S.-K.; Yu, K. R.; Kim, J. H.; Kim, Y.-S.; Ko, J. H.; Park, B. C.; Kim, S. J. Chem. Biol. Drug Des. 2011, 78, 642–650. doi:10.1111/j.1747-0285.2011.01192.x |
| 19. | Taha, M. O.; Atallah, N.; Al-Bakri, A. G.; Paradis-Bleau, C.; Zalloum, H.; Younis, K. S.; Levesque, R. C. Bioorg. Med. Chem. 2008, 16, 1218–1235. doi:10.1016/j.bmc.2007.10.076 |
| 20. | Tayyem, R. F.; Zalloum, H. M.; Elmaghrabi, M. R.; Yousef, A.-M.; Mubarak, M. S. Eur. J. Med. Chem. 2012, 56, 70–95. doi:10.1016/j.ejmech.2012.08.004 |
| 19. | Taha, M. O.; Atallah, N.; Al-Bakri, A. G.; Paradis-Bleau, C.; Zalloum, H.; Younis, K. S.; Levesque, R. C. Bioorg. Med. Chem. 2008, 16, 1218–1235. doi:10.1016/j.bmc.2007.10.076 |
© 2017 Dobiaš et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)