Abstract
The use of sonochemistry is described in the organocatalytic enamine–azide [3 + 2] cycloaddition between 1,3-diketones and aryl azidophenyl selenides. These sonochemically promoted reactions were found to be amenable to a range of 1,3-diketones or aryl azidophenyl selenides, providing an efficient access to new ((arylselanyl)phenyl-1H-1,2,3-triazol-4-yl)ketones in good to excellent yields and short reaction times. In addition, this protocol was extended to β-keto esters, β-keto amides and α-cyano ketones. Selanyltriazoyl carboxylates, carboxamides and carbonitriles were synthesized in high yields at short times of reaction under very mild reaction conditions.
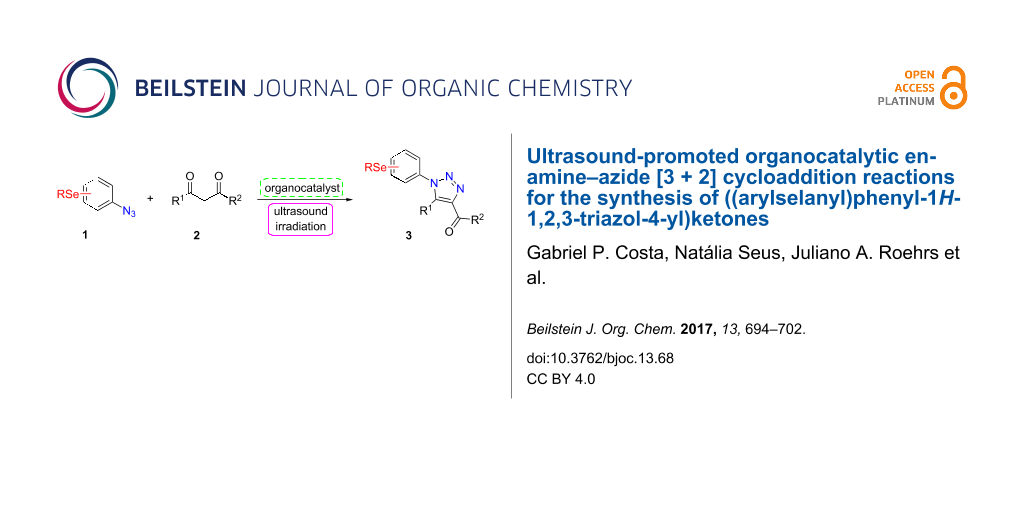
Graphical Abstract
Introduction
Substituted 1,2,3-triazoles are an interesting class of heterocyclic compounds distinguished by their biological activities [1-3] as well as in various fields of chemistry [4-15]. The most attractive way for their preparation is the thermal 1,3-dipolar cycloaddition of alkynes and azides, introduced by Huisgen which usually gives rise to a mixture of 1,4 and 1,5-isomers [16-19]. More recently, transition metal catalysts based on copper, ruthenium, silver and iridium salts have been used for this cycloaddition reaction [20-29].
Organocatalytic approaches based on β-enamine–azide or enolate–azide cycloadditions have been employed to synthesize 1,2,3-triazole scaffolds [30-32]. Depending on the organocatalyst employed, different carbonyl compounds could successfully generate an enamine or an enolate, and these species react as dipolarophiles with organic azides in organocatalyzed 1,3-dipolar cycloadditions. Our research group has demonstrated β-enamine–azide cycloaddition reactions for the synthesis of selenium-functionalized 1,2,3-triazoles [33-37]. Selanyltriazoyl carboxylates, carboxamides, carbonitriles or sulfones were synthesized in good to excellent yields using catalytic amounts of an organocatalyst.
Organoselenium compounds are attractive synthetic targets because of their selective reactions [38-43], photophysical properties [44-49] and interesting biological activities [50-52]. An interesting class of molecules are the selanyl-1,2,3-triazoles [53-61] which can present some biological applications. As example, 4-phenyl-1-(phenylselanylmethyl)-1,2,3-triazole A (Se-TZ) demonstrated an antidepressant-like effect (Figure 1) [60]. In another example, 5-phenyl-1-(2-(phenylselanyl)phenyl)-1H-1,2,3-triazole-4-carbonitrile B (Se-TZCN) was reported to exhibit antioxidant activities in different in vitro assays (Figure 1) [36]. Selenanyl-quinone-based 1,2,3-triazoles C and D were synthesized and evaluated against six types of cancer cell lines. The synthesized compounds emerge as promising molecules for the therapeutic use of cancers overexpressing NQO1 (Figure 1) [61].
Figure 1: Biologically relevant selanyl-1,2,3-triazoles.
Figure 1: Biologically relevant selanyl-1,2,3-triazoles.
Thus, the search for efficient methods using appropriate and environmentally sound substrates for the preparation of selenium-functionalized 1,2,3-triazoles still remains a challenge in organic synthesis.
Ultrasonic irradiation has emerged in the past decades as a versatile tool in industrial and academic applications [62-67]. The use of sonication in organic synthesis (sonochemistry) is well documented and is generally considered as an environmentally sound energy source, comparatively less energy intensive to conventional heating and microwave irradiation, also able to reduce the number and quantities of side reaction products [62-67].
There are only a few contributions describing the use of sonochemistry for the preparation of functionalized 1,2,3-triazoles [68-74]. As a recent example, our research group described the use of sonochemistry in the organocatalytic enamine–azide [3 + 2] cycloadditions of β-oxo-amides with a range of substituted aryl azides providing and efficient access to new N-aryl-1,2,3-triazoyl carboxamides in good to excellent yields and short reaction times of [75].
However, to the best of our knowledge, the use of sonochemistry to synthesize complex selenium-functionalized 1,2,3-triazoles via organocatalytic enamine–azide cycloaddition has not been explored to date. As a continuation of our ongoing studies towards the development of new 1,2,3-triazoles bearing organoselenium moieties, this contribution was aimed to disclose a sonochemical approach for the organocatalyzed synthesis of ((arylselanyl)phenyl-1H-1,2,3-triazol-4-yl)ketones by reacting a range of 1,3-diketones with substituted aryl azidophenyl selenides (Scheme 1).
Scheme 1: General scheme of the reaction.
Scheme 1: General scheme of the reaction.
Results and Discussion
Due to the fact that organocatalyzed β-enamine–azide cycloaddition reactions between azidophenyl aryl selenides and 1,3-diketones were not described, preliminary studies were attempted to react 2-azidophenyl phenyl selenide (1a) and 2,4-pentanedione (2a) as model reaction substrates. Based on our previous report on such reaction [33], a mixture of substrates 1a (0.3 mmol) and 2a (0.3 mmol) in DMSO (0.6 mL) was stirred at room temperature in the presence of 1 mol % of Et2NH as organocatalyst, providing an excellent yield (98%) of the desired product 3a after 2 h (conditions A, Scheme 2).
Scheme 2: Comparative study of the conventional conditions and ultrasound irradiation. Conditions A: Reaction at 25 °C for 2 h (3a, 98%); Conditions B: Reaction under ultrasound irradiation (20% of the amplitude) at 25 °C for 20 min (3a, 92%).
Scheme 2: Comparative study of the conventional conditions and ultrasound irradiation. Conditions A: Reaction...
With the aim to compare the effect of different energy sources in this β-enamine–azide cycloaddition, the reaction between substrates 1a and 2a in DMSO using Et2NH (1 mol %) was also performed under ultrasound irradiation.
The reaction performed under ultrasound irradiation with 20% of the amplitude for 20 minutes (followed by TLC until the total consumption of the starting materials) yielded product 3a in 92% (conditions B, Scheme 2). Inspired by results described under conditions B, we performed additional experiments using ultrasound irradiation with Et2NH as organocatalyst (Table 1).
Table 1: Optimization of reaction conditions.a
|
|
||||
| Entry | Amplitude | Et2NH (mol %) | Time (min) | Yield 3a (%)b |
|---|---|---|---|---|
| 1 | 20 | 1 | 20 | 92 |
| 2 | 25 | 1 | 20 | 92 |
| 3 | 30 | 1 | 20 | 93 |
| 4 | 40 | 1 | 20 | 96 |
| 5 | 20 | 1 | 10 | 70 |
| 6 | 40 | 1 | 10 | 95 |
| 7 | 40 | 1 | 5 | 93 |
| 8 | 40 | 0.5 | 25 | 85 |
| 9 | 40 | 0.1 | 60 | n.d. |
| 10 | 40 | – | 60 | n.d. |
| 11c | 40 | 1 | 5 | 27 |
| 12d | 40 | 1 | 5 | 85 |
aReactions were performed with 2-azidophenyl phenyl selenide (1a, 0.3 mmol) and 2,4-pentanedione (2a, 0.3 mmol) in DMSO (0.6 mL) as solvent under ultrasound irradiation at 25 °C. bYields are given for isolated products. cReaction was performed with L-proline as a catalyst. dReaction was performed with pyrrolidine (1 mol %). n.d.: not detected.
Initially, substrates 1a and 2a were reacted in DMSO under ultrasound irradiation for 20 min using different amplitudes (Table 1, entries 1–4). We observed that the desired product 3a was obtained in excellent yields in all reactions. However, product yield of 3a decreased to 70% (Table 1, entry 5) in 10 minutes under 20% sonochemistry amplitude. To our delight, reactions performed using 40% of amplitude during 10 or 5 min gave excellent yields of selanyltriazole 3a (Table 1, entries 6 and 7). We observed that the amplitude effect could be correlated to the product formation time, since that in reaction carried out in 40% of amplitude the yield of compound 3a was excellent (93%) after 5 min reaction time (Table 1, entry 5 vs 7). A slight decrease in reaction yields could be observed after decreasing the loading of organocatalyst to 0.5 mol % (Table 1, entry 8). Finally, in blank runs (in the absence of organocatalyst) or performed using 0.1 mol % of catalyst the reaction did not occur, even under sonication for 60 min using 40% of amplitude (Table 1, entries 9 and 10). Reactions performed with other catalysts (L-proline and pyrrolidine) gave lower yields of 3a than those using 1 mol % of Et2NH (Table 1, entry 7 vs entries 11 and 12).
From Table 1, optimum reaction conditions to obtain 1-(5-methyl-1-(2-(phenylselanyl)phenyl)-1H-1,2,3-triazol-4-yl)ethan-1-one (3a) were clearly present in entry 7, in which a mixture of azidophenyl phenyl selenide (1a, 0.3 mmol), 2,4-pentanedione (2a, 0.3 mmol) and Et2NH (1 mol %) in DMSO (0.6 mL) was sonicated using 40% of amplitude at room temperature for 5 minutes. In order to extend the scope of the reaction, optimum reaction conditions were extended to other 1,3-diketones 2a–e with different substitution patterns (Table 2). High yields of desired 1,2,3-triazoles were obtained using β-diketones 2a, 2b and 2c bearing methyl, ethyl and phenyl substituents (Table 2, entries 1–3). However, we observed that the steric hindrance effect in 2,2,6,6-tetramethyl-3,5-heptanedione 2d displays an important role in the overall reaction and only traces of product 3d was observed (Table 2, entries 1–3 vs 4). Unfortunately, no reaction occurred when cyclic β-diketone 2e was employed as substrate (Table 2, entry 5). We next evaluated the reactivity of 2,4-pentanedione (2a) with different functionalized aryl azidophenyl selenides 1b–f under identical reaction conditions. Aryl azidophenyl selenides containing either an EDG or an EWG on the aromatic ring delivered the expected selanyltriazoles 3f–i in good isolated yields (Table 2, entries 6–9). However, a decrease in yield was observed when the reaction was performed with aryl azidophenyl selenide containing a –CF3 group (Table 2, entry 9). In addition, 4-azidophenyl phenyl selenide (1f) was treated with 2,4-pentanedione (2a) to afford the desired product 3j in 92% yield as a mixture of regioisomers (6:1) (Table 2, entry 10).
Table 2: Scope of substrates: Variation of the aryl azidophenyl selenides 1 and 1,3-diketones 2.a
|
|
||||
| Entry | Aryl azidophenyl selenides 1 | 1,3-Diketone 2 | Product 3 | Isolated Yield (%)b |
|---|---|---|---|---|
| 1 |
1a |
2a |
3a |
93 |
| 2 |
1a |
2b |
3b |
91 |
| 3 |
1a |
2c |
3c |
85 |
| 4 |
1a |
2d |
3d |
traces |
| 5 |
1a |
2e |
3e |
n.d. |
| 6 |
1b |
2a |
3f |
74 |
| 7 |
1c |
2a |
3g |
84 |
| 8 |
1d |
2a |
3h |
87 |
| 9 |
1e |
2a |
3i |
56 |
| 10 |
1f |
2a |
3j |
92 |
aReactions were performed with aryl azidophenyl selenides 1a–f (0.3 mmol) and 1,3-diketones 2a–e (0.3 mmol), using Et2NH (1 mol %) as catalyst in DMSO (0.6 mL) as solvent under ultrasound irradiation (40% of amplitude) at room temperature for 5 min. bYields are given for isolated products. n.d.: not detected.
In addition, the possibility to perform the reaction of 2-azidophenyl phenyl selenide (1a) with β-keto-esters, β-keto-amides and α-cyano-ketones 2f–k was also investigated. The reaction conditions optimized for 1,3-diketone 2a were employed, but independently using as substrates ethyl acetoacetate (2f), ethyl benzoylacetate (2g), 3-oxo-N-phenylbutanamide (2h), 3-oxo-N-(p-tolyl)butanamide (2i), benzoylacetonitrile (2j) and 4-toluoylacetonitrile (2k). The corresponding esters 3k,l [33], amides 3m,n [34] and nitriles 3o,p [36] were obtained in good yields (Scheme 3) after 5 minutes reaction under ultrasound irradiation (40% of amplitude) at room temperature. Comparing these results with already published ones under conventional conditions, our methodology using ultrasound irradiation affords the products in 5 minutes and in comparable yields while the other methods mostly provide the products in times above 60 minutes [33,34,36].
Scheme 3: Reaction of 2-azidophenyl phenyl selenide 1a with activated ketones 2f–k.
Scheme 3: Reaction of 2-azidophenyl phenyl selenide 1a with activated ketones 2f–k.
Conclusion
In summary, we have described the use of sonochemistry in the organocatalytic enamine–azide [3 + 2] cycloaddition between 1,3-diketones and aryl azidophenyl selenides. These sonochemical promoted reactions were found to be amenable to a range of 1,3-diketones or aryl azidophenyl selenides, providing an efficient access to novel selenium-containing 1,2,3-triazole compounds in good to excellent yields, in a few minutes of reaction at room temperature. The protocol was extended to activated ketones and selanyltriazoyl carboxylates, with carboxamides and carbonitriles synthesized in high yields and short times of reaction.
Experimental
General information
The reactions were monitored by TLC carried out on Merck silica gel (60 F254) by using UV light as visualizing agent and 5% vanillin in 10% H2SO4 and heat as developing agents. Baker silica gel (particle size 0.040–0.063 mm) was used for flash chromatography. A Cole Parmer-ultrasonic processor Model CPX 130, with a maximum power of 130 W, operating at an amplitude of 40% and a frequency of 20 kHz was used. The temperature of the reaction was monitored using an Incoterm digital infrared thermometer Model Infraterm (Brazil) (in most reactions the temperature was in the range between 60 and 65 °C). Proton nuclear magnetic resonance spectra (1H NMR) were obtained at 400 MHz on Bruker DPX 400 spectrometer. Spectra were recorded in CDCl3 solutions. Chemical shifts are reported in ppm, referenced to tetramethylsilane (TMS) as the external reference. Coupling constants (J) are reported in Hertz. Abbreviations to denote the multiplicity of a particular signal are s (singlet), d (doublet), t (triplet), q (quartet) and m (multiplet). Carbon-13 nuclear magnetic resonance spectra (13C NMR) were obtained at 100 MHz on Bruker DPX 400 spectrometer. Chemical shifts are reported in ppm, referenced to the solvent peak of CDCl3. Low-resolution mass spectra were obtained with a Shimadzu GC-MS-QP2010 mass spectrometer. High resolution mass spectra (HRMS) were recorded on a Bruker Micro TOF-QII spectrometer 10416.
General procedure for the synthesis of selanyltriazoles 3a–r under ultrasound irradiation
Aryl azidophenyl selenides 1a–f (0.3 mmol), activated ketones 2a–k (0.3 mmol), Et2NH (1 mol %) and DMSO (0.6 mL) were added to a glass tube. The ultrasound probe was placed in a glass vial containing the reaction mixture. The amplitude of the ultrasound waves was fixed in 40%. Then, the reaction mixture was sonicated for 5 min. The crude product obtained was subsequently purified by column chromatography on silica gel using a mixture of hexane/ethyl acetate (5:1) as eluent to afford the desired products 3a–p.
Supporting Information
| Supporting Information File 1: Experimental and analytical data. | ||
| Format: PDF | Size: 571.1 KB | Download |
Acknowledgements
We thank the CNPq (Grants 306430/2013-4, 400150/2014-0 and 447595/2014-8), CAPES and FAPERGS (PRONEM 6/2551-0000240-1) for the financial support. Rafael Luque gratefully acknowledges support from Ciência sem Fronteiras Program (Grant 303415/2014-2) as Visiting Scientist to Universidade Federal de Pelotas, RS.
References
-
Dehaen, W.; Bakulev, V. A. Chemistry of 1,2,3-triazoles, 1st ed.; Springer International Publishing: New York, 2014.
Return to citation in text: [1] -
For a set of reviews in this area see the themed issue: Click chemistry: function follows form, in Chem. Soc. Rev. 2010, 39, 1221. doi:10.1039/C003926H
Return to citation in text: [1] -
For a set of reviews in this area see the themed issue: Bioorthogonal Chemistry in Biology, in Acc. Chem. Res. 2011, 44, 651. doi:10.1021/ar200193f
Return to citation in text: [1] -
Nandivada, H.; Jiang, X.; Lahann, J. Adv. Mater. 2007, 19, 2197. doi:10.1002/adma.200602739
Return to citation in text: [1] -
Lee, B. S.; Lee, J. K.; Kim, W.-J.; Jung, Y. H.; Sim, S. J.; Lee, J.; Choi, I. S. Biomacromolecules 2007, 8, 744. doi:10.1021/bm060782+
Return to citation in text: [1] -
Deobald, A. M.; Camargo, L. R. S.; Alves, D.; Zukerman-Schpector, J.; Corrêa, A. G.; Paixão, M. W. Synthesis 2011, 4003. doi:10.1055/s-0031-1289606
Return to citation in text: [1] -
Pérez-Labrada, K.; Brovard, I.; Morera, C.; Estévez, F.; Bermejo, J.; Rivera, D. G. Tetrahedron 2011, 67, 7713. doi:10.1016/j.tet.2011.08.003
Return to citation in text: [1] -
Días, D. D.; Rajagopal, K.; Strable, E.; Schneider, J.; Finn, M. G. J. Am. Chem. Soc. 2006, 128, 6056. doi:10.1021/ja061251w
Return to citation in text: [1] -
Astruc, D.; Liang, L.; Rapakousiou, A.; Ruiz, J. Acc. Chem. Res. 2012, 45, 630. doi:10.1021/ar200235m
Return to citation in text: [1] -
Tasdelen, M. A.; Yilmaz, G.; Iskin, B.; Yagci, Y. Macromolecules 2012, 45, 56. doi:10.1021/ma202438w
Return to citation in text: [1] -
Font, D.; Jimeno, C.; Pericàs, M. A. Org. Lett. 2006, 8, 4653. doi:10.1021/ol061964j
Return to citation in text: [1] -
Font, D.; Bastero, A.; Sayalero, S.; Jimeno, C.; Pericàs, M. A. Org. Lett. 2007, 9, 1943. doi:10.1021/ol070526p
Return to citation in text: [1] -
Font, D.; Sayalero, S.; Bastero, A.; Jimeno, C.; Pericàs, M. A. Org. Lett. 2008, 10, 337. doi:10.1021/ol702901z
Return to citation in text: [1] -
Lallana, E.; Riguera, R.; Fernandez-Megia, E. Angew. Chem., Int. Ed. 2011, 50, 8794. doi:10.1002/anie.201101019
Return to citation in text: [1] -
Hong, V.; Presolski, S. I.; Ma, C.; Finn, M. G. Angew. Chem., Int. Ed. 2009, 48, 9879. doi:10.1002/anie.200905087
Return to citation in text: [1] -
Huisgen, R. Angew. Chem. 1963, 75, 604. doi:10.1002/ange.19630751304
Return to citation in text: [1] -
Huisgen, R. Proc. Chem. Soc., London 1961, 357. doi:10.1039/PS9610000357
Return to citation in text: [1] -
Huisgen, R. In 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley: New York, 1984; Vol. 1, 1.
Return to citation in text: [1] -
Huisgen, R. Pure Appl. Chem. 1989, 61, 613. doi:10.1351/pac198961040613
Return to citation in text: [1] -
Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4
Return to citation in text: [1] -
Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057. doi:10.1021/jo011148j
Return to citation in text: [1] -
Krasiński, A.; Radić, Z.; Manetsch, R.; Raushel, J.; Taylor, P.; Sharpless, K. B.; Kolb, H. C. J. Am. Chem. Soc. 2005, 127, 6686. doi:10.1021/ja043031t
Return to citation in text: [1] -
Lee, L. V.; Mitchell, M. L.; Huang, S.-J.; Fokin, V. V.; Sharpless, K. B.; Wong, C.-H. J. Am. Chem. Soc. 2003, 125, 9588. doi:10.1021/ja0302836
Return to citation in text: [1] -
Hein, J. E.; Tripp, J. C.; Krasnova, L. B.; Sharpless, K. B.; Fokin, V. V. Angew. Chem., Int. Ed. 2009, 48, 8018–8021. doi:10.1002/anie.200903558
Return to citation in text: [1] -
Zhang, L.; Chen, X.; Xue, P.; Sun, H. H. Y.; Williams, I. D.; Sharpless, K. B.; Fokin, V. V.; Jia, G. J. Am. Chem. Soc. 2005, 127, 15998. doi:10.1021/ja054114s
Return to citation in text: [1] -
Boren, B. C.; Narayan, S.; Rasmussen, L. K.; Zhang, L.; Zhao, H.; Lin, Z.; Jia, G.; Fokin, V. V. J. Am. Chem. Soc. 2008, 130, 8923. doi:10.1021/ja0749993
Return to citation in text: [1] -
McNulty, J.; Keskar, K.; Vemula, R. Chem. – Eur. J. 2011, 17, 14727. doi:10.1002/chem.201103244
Return to citation in text: [1] -
McNulty, J.; Keskar, K. Eur. J. Org. Chem. 2012, 5462. doi:10.1002/ejoc.201200930
Return to citation in text: [1] -
Ding, S.; Jia, G.; Sun, J. Angew. Chem., Int. Ed. 2014, 53, 1877. doi:10.1002/anie.201309855
Return to citation in text: [1] -
Lima, C. G. S.; Ali, A.; van Berkel, S. S.; Westermann, B.; Paixão, M. W. Chem. Commun. 2015, 51, 10784. doi:10.1039/C5CC04114G
Return to citation in text: [1] -
Ramasastry, S. S. V. Angew. Chem., Int. Ed. 2014, 53, 14310–14312. doi:10.1002/anie.201409410
Return to citation in text: [1] -
John, J.; Thomas, J.; Dehaen, W. Chem. Commun. 2015, 51, 10797. doi:10.1039/C5CC02319J
Return to citation in text: [1] -
Seus, N.; Gonçalves, L. C.; Deobald, A. M.; Savegnago, L.; Alves, D.; Paixão, M. W. Tetrahedron 2012, 68, 10456. doi:10.1016/j.tet.2012.10.007
Return to citation in text: [1] [2] [3] [4] -
Seus, N.; Goldani, B.; Lenardão, E. J.; Savegnago, L.; Paixão, M. W.; Alves, D. Eur. J. Org. Chem. 2014, 1059. doi:10.1002/ejoc.201301547
Return to citation in text: [1] [2] [3] -
Saraiva, M. T.; Costa, G. P.; Seus, N.; Schumacher, R. F.; Perin, G.; Paixão, M. W.; Luque, R.; Alves, D. Org. Lett. 2015, 17, 6206. doi:10.1021/acs.orglett.5b03196
Return to citation in text: [1] -
Savegnago, L.; do Sacramento, M.; Brod, L. M. P.; Fronza, M. G.; Seus, N.; Lenardão, E. J.; Paixão, M. W.; Alves, D. RSC Adv. 2016, 6, 8021. doi:10.1039/C5RA22445D
Return to citation in text: [1] [2] [3] [4] -
Saraiva, M. T.; Krüger, R.; Baldinotti, R. S. M.; Lenardão, E. J.; Luchese, C.; Savegnago, L.; Wilhelm, E. A.; Alves, D. J. Braz. Chem. Soc. 2016, 27, 41. doi:10.5935/0103-5053.20150239
Return to citation in text: [1] -
Alberto, E. E.; Braga, A. L. In Selenium and Tellurium Chemistry - From Small Molecules to Biomolecules and Materials; Derek, W. J.; Risto, L., Eds.; Springer-Verlag: Berlin Heidelberg, 2011.
Return to citation in text: [1] -
Wirth, T. Organoselenium Chemistry: Synthesis and Reactions; Wiley-VCH: Weinheim, 2011. doi:10.1002/9783527641949
Return to citation in text: [1] -
Menezes, P. H.; Zeni, G. Vinyl Selenides. Patai’s Chemistry of Functional Groups; John Wiley & Sons: Oxford, 2011. doi:10.1002/9780470682531.pat0568
Return to citation in text: [1] -
Perin, G.; Lenardão, E. J.; Jacob, R. G.; Panatieri, R. B. Chem. Rev. 2009, 109, 1277. doi:10.1021/cr8004394
Return to citation in text: [1] -
Perin, G.; Alves, D.; Jacob, R. G.; Barcellos, A. M.; Soares, L. K.; Lenardão, E. J. ChemistrySelect 2016, 1, 205. doi:10.1002/slct.201500031
Return to citation in text: [1] -
Freudendahl, D. M.; Santoro, S.; Shahzad, S. A.; Santi, C.; Wirth, T. Angew. Chem., Int. Ed. 2009, 48, 8409. doi:10.1002/anie.200903893
Return to citation in text: [1] -
Rampon, D. S.; Santos, F. S.; Descalzo, R. R.; Toldo, J. M.; Gonçalves, P. F. B.; Schneider, P. H.; Rodembusch, F. S. J. Phys. Org. Chem. 2014, 27, 336. doi:10.1002/poc.3229
Return to citation in text: [1] -
Rampon, D. S.; Rodembusch, F. S.; Schneider, J. M. F. M.; Bechtold, I. H.; Gonçalves, P. F. B.; Merlo, A.; Schneider, P. H. J. Mater. Chem. 2010, 20, 715. doi:10.1039/B917366H
Return to citation in text: [1] -
Samb, I.; Bell, J.; Toullec, P. Y.; Michelet, V.; Leray, I. Org. Lett. 2011, 13, 1182. doi:10.1021/ol200066p
Return to citation in text: [1] -
Goswami, S.; Hazra, A.; Chakrabarty, R.; Fun, H.-K. Org. Lett. 2009, 11, 4350. doi:10.1021/ol901737s
Return to citation in text: [1] -
Tang, B.; Xing, Y.; Li, P.; Zhang, N.; Yu, F.; Yang, G. J. Am. Chem. Soc. 2007, 129, 11666. doi:10.1021/ja072572q
Return to citation in text: [1] -
Balaguez, R. A.; Ricordi, V. G.; Duarte, R. C.; Toldo, J. M.; Santos, C. M.; Schneider, P. H.; Gonçalves, P. F. B.; Rodembusch, F. S.; Alves, D. RSC Adv. 2016, 6, 49613. doi:10.1039/C6RA04157D
Return to citation in text: [1] -
Nogueira, C. W.; Rocha, J. B. T. Organoselenium and organotellurium compounds: Toxicology and pharmacology. In Patai's Chemistry of Functional Groups; Rappoport, Z., (Org)., Ed.; Wiley: Chichester, 2011. doi:10.1002/9780470682531.pat0567
Return to citation in text: [1] -
Santoro, S.; Azeredo, J. B.; Nascimento, V.; Sancineto, L.; Braga, A. L.; Santi, C. RSC Adv. 2014, 4, 31521. doi:10.1039/C4RA04493B
Return to citation in text: [1] -
Nogueira, C. W.; Zeni, G.; Rocha, J. B. T. Chem. Rev. 2004, 104, 6255. doi:10.1021/cr0406559
Return to citation in text: [1] -
Tiecco, M.; Testaferri, L.; Santi, C.; Tomassini, C.; Marini, F.; Bagnoli, L.; Temperini, A. Angew. Chem., Int. Ed. 2003, 42, 3131. doi:10.1002/anie.200351229
Return to citation in text: [1] -
Back, T. G.; Bethell, R. J.; Parvez, M.; Taylor, J. A.; Wehrli, D. J. Org. Chem. 1999, 64, 7426. doi:10.1021/jo990730t
Return to citation in text: [1] -
Stefani, H. A.; Silva, N. C. S.; Manarin, F.; Lüdtke, D. S.; Zukerman-Schpector, J.; Madureira, L. S.; Tiekink, E. R. T. Tetrahedron Lett. 2012, 53, 1742. doi:10.1016/j.tetlet.2012.01.102
Return to citation in text: [1] -
Stefani, H. A.; Leal, D. M.; Manarin, F. Tetrahedron Lett. 2012, 53, 6495. doi:10.1016/j.tetlet.2012.09.062
Return to citation in text: [1] -
Deobald, A. M.; Camargo, L. R. S.; Hörner, M.; Rodrigues, O. E. D.; Alves, D.; Braga, A. L. Synthesis 2011, 2397. doi:10.1055/s-0030-1260083
Return to citation in text: [1] -
Seus, N.; Saraiva, M. T.; Alberto, E. E.; Savegnago, L.; Alves, D. Tetrahedron 2012, 68, 10419. doi:10.1016/j.tet.2012.07.019
Return to citation in text: [1] -
Saraiva, M. T.; Seus, N.; de Souza, D.; Rodrigues, O. E. D.; Paixão, M. W.; Jacob, R. G.; Lenardão, E. J.; Perin, G.; Alves, D. Synthesis 2012, 44, 1997. doi:10.1055/s-0031-1291135
Return to citation in text: [1] -
Donato, F.; de Gomes, M. G.; Goes, A. T. R.; Seus, N.; Alves, D.; Jesse, C. R.; Savegnago, L. Life Sci. 2013, 93, 393. doi:10.1016/j.lfs.2013.07.024
Return to citation in text: [1] [2] -
da Cruz, E. H. G.; Silvers, M. A.; Jardim, G. A. M.; Resende, J. M.; Cavalcanti, B. C.; Bomfim, I. S.; Pessoa, C.; de Simone, C. A.; Botteselle, G. V.; Braga, A. L.; Nair, D. K.; Namboothiri, I. N. N.; Boothman, D. A.; da Silva Júnior, E. N. Eur. J. Med. Chem. 2016, 122, 1. doi:10.1016/j.ejmech.2016.06.019
Return to citation in text: [1] [2] -
Mojtahedi, M. M.; Abaee, M. S. Ultrasound applications in synthetic organic chemistry. In Handbook on Applications of Ultrasound Sonochemistry for Sustainability; Chen, D.; Sharma, S. K.; Mudhoo, A., Eds.; CRC Press: New York, 2012; pp 281 ff.
Return to citation in text: [1] [2] -
Li, Z.; Hong, J.; Zhou, X. Tetrahedron 2011, 67, 3690. doi:10.1016/j.tet.2011.03.067
Return to citation in text: [1] [2] -
Cravotto, G.; Cintas, P. Chem. Soc. Rev. 2006, 35, 180. doi:10.1039/B503848K
Return to citation in text: [1] [2] -
Mason, T. J. Ultrason. Sonochem. 2007, 14, 476. doi:10.1016/j.ultsonch.2006.10.008
Return to citation in text: [1] [2] -
Nüchter, M.; Ondruschka, B.; Jungnickel, A.; Müller, U. J. Phys. Org. Chem. 2000, 13, 579. doi:10.1002/1099-1395(200010)13:10<579::AID-POC272>3.0.CO;2-M
Return to citation in text: [1] [2] -
Mason, T. J. Chem. Soc. Rev. 1997, 26, 443. doi:10.1039/cs9972600443
Return to citation in text: [1] [2] -
Cravotto, G.; Fokin, V. V.; Garella, D.; Binello, A.; Boffa, L.; Barge, A. J. Comb. Chem. 2010, 12, 13. doi:10.1021/cc900150d
Return to citation in text: [1] -
Mady, M. F.; Awad, G. E. A.; Jørgensen, K. B. Eur. J. Med. Chem. 2014, 84, 433. doi:10.1016/j.ejmech.2014.07.042
Return to citation in text: [1] -
Marzag, H.; Alaoui, S.; Amdouni, H.; Martin, A. R.; Bougrin, K.; Benhida, R. New J. Chem. 2015, 39, 5437. doi:10.1039/C5NJ00624D
Return to citation in text: [1] -
Nallapati, S. B.; Sreenivas, B. Y.; Bankala, R.; Parsa, K. V. L.; Sripelly, S.; Mukkanti, K.; Pal, M. RSC Adv. 2015, 5, 94623. doi:10.1039/C5RA20380E
Return to citation in text: [1] -
Naeimi, H.; Dadashzadeh, S.; Moradian, M. Res. Chem. Intermed. 2015, 41, 2687. doi:10.1007/s11164-013-1379-6
Return to citation in text: [1] -
Stefani, H. A.; Canduzini, H. A.; Manarin, F. Tetrahedron Lett. 2011, 52, 6086. doi:10.1016/j.tetlet.2011.09.004
Return to citation in text: [1] -
Jiang, Y.; Chen, X.; Qu, L.; Wang, J.; Yuan, J.; Chen, S.; Li, X.; Qu, C. Ultrason. Sonochem. 2011, 18, 527. doi:10.1016/j.ultsonch.2010.09.009
Return to citation in text: [1] -
Xavier, D. M.; Goldani, B. S.; Seus, N.; Jacob, R. G.; Barcellos, T.; Paixão, M. W.; Luque, R.; Alves, D. Ultrason. Sonochem. 2017, 34, 107. doi:10.1016/j.ultsonch.2016.05.007
Return to citation in text: [1]
| 33. | Seus, N.; Gonçalves, L. C.; Deobald, A. M.; Savegnago, L.; Alves, D.; Paixão, M. W. Tetrahedron 2012, 68, 10456. doi:10.1016/j.tet.2012.10.007 |
| 75. | Xavier, D. M.; Goldani, B. S.; Seus, N.; Jacob, R. G.; Barcellos, T.; Paixão, M. W.; Luque, R.; Alves, D. Ultrason. Sonochem. 2017, 34, 107. doi:10.1016/j.ultsonch.2016.05.007 |
| 33. | Seus, N.; Gonçalves, L. C.; Deobald, A. M.; Savegnago, L.; Alves, D.; Paixão, M. W. Tetrahedron 2012, 68, 10456. doi:10.1016/j.tet.2012.10.007 |
| 1. | Dehaen, W.; Bakulev, V. A. Chemistry of 1,2,3-triazoles, 1st ed.; Springer International Publishing: New York, 2014. |
| 2. | For a set of reviews in this area see the themed issue: Click chemistry: function follows form, in Chem. Soc. Rev. 2010, 39, 1221. doi:10.1039/C003926H |
| 3. | For a set of reviews in this area see the themed issue: Bioorthogonal Chemistry in Biology, in Acc. Chem. Res. 2011, 44, 651. doi:10.1021/ar200193f |
| 30. | Lima, C. G. S.; Ali, A.; van Berkel, S. S.; Westermann, B.; Paixão, M. W. Chem. Commun. 2015, 51, 10784. doi:10.1039/C5CC04114G |
| 31. | Ramasastry, S. S. V. Angew. Chem., Int. Ed. 2014, 53, 14310–14312. doi:10.1002/anie.201409410 |
| 32. | John, J.; Thomas, J.; Dehaen, W. Chem. Commun. 2015, 51, 10797. doi:10.1039/C5CC02319J |
| 62. | Mojtahedi, M. M.; Abaee, M. S. Ultrasound applications in synthetic organic chemistry. In Handbook on Applications of Ultrasound Sonochemistry for Sustainability; Chen, D.; Sharma, S. K.; Mudhoo, A., Eds.; CRC Press: New York, 2012; pp 281 ff. |
| 63. | Li, Z.; Hong, J.; Zhou, X. Tetrahedron 2011, 67, 3690. doi:10.1016/j.tet.2011.03.067 |
| 64. | Cravotto, G.; Cintas, P. Chem. Soc. Rev. 2006, 35, 180. doi:10.1039/B503848K |
| 65. | Mason, T. J. Ultrason. Sonochem. 2007, 14, 476. doi:10.1016/j.ultsonch.2006.10.008 |
| 66. | Nüchter, M.; Ondruschka, B.; Jungnickel, A.; Müller, U. J. Phys. Org. Chem. 2000, 13, 579. doi:10.1002/1099-1395(200010)13:10<579::AID-POC272>3.0.CO;2-M |
| 67. | Mason, T. J. Chem. Soc. Rev. 1997, 26, 443. doi:10.1039/cs9972600443 |
| 20. | Rostovtsev, V. V.; Green, L. G.; Fokin, V. V.; Sharpless, K. B. Angew. Chem., Int. Ed. 2002, 41, 2596. doi:10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4 |
| 21. | Tornøe, C. W.; Christensen, C.; Meldal, M. J. Org. Chem. 2002, 67, 3057. doi:10.1021/jo011148j |
| 22. | Krasiński, A.; Radić, Z.; Manetsch, R.; Raushel, J.; Taylor, P.; Sharpless, K. B.; Kolb, H. C. J. Am. Chem. Soc. 2005, 127, 6686. doi:10.1021/ja043031t |
| 23. | Lee, L. V.; Mitchell, M. L.; Huang, S.-J.; Fokin, V. V.; Sharpless, K. B.; Wong, C.-H. J. Am. Chem. Soc. 2003, 125, 9588. doi:10.1021/ja0302836 |
| 24. | Hein, J. E.; Tripp, J. C.; Krasnova, L. B.; Sharpless, K. B.; Fokin, V. V. Angew. Chem., Int. Ed. 2009, 48, 8018–8021. doi:10.1002/anie.200903558 |
| 25. | Zhang, L.; Chen, X.; Xue, P.; Sun, H. H. Y.; Williams, I. D.; Sharpless, K. B.; Fokin, V. V.; Jia, G. J. Am. Chem. Soc. 2005, 127, 15998. doi:10.1021/ja054114s |
| 26. | Boren, B. C.; Narayan, S.; Rasmussen, L. K.; Zhang, L.; Zhao, H.; Lin, Z.; Jia, G.; Fokin, V. V. J. Am. Chem. Soc. 2008, 130, 8923. doi:10.1021/ja0749993 |
| 27. | McNulty, J.; Keskar, K.; Vemula, R. Chem. – Eur. J. 2011, 17, 14727. doi:10.1002/chem.201103244 |
| 28. | McNulty, J.; Keskar, K. Eur. J. Org. Chem. 2012, 5462. doi:10.1002/ejoc.201200930 |
| 29. | Ding, S.; Jia, G.; Sun, J. Angew. Chem., Int. Ed. 2014, 53, 1877. doi:10.1002/anie.201309855 |
| 68. | Cravotto, G.; Fokin, V. V.; Garella, D.; Binello, A.; Boffa, L.; Barge, A. J. Comb. Chem. 2010, 12, 13. doi:10.1021/cc900150d |
| 69. | Mady, M. F.; Awad, G. E. A.; Jørgensen, K. B. Eur. J. Med. Chem. 2014, 84, 433. doi:10.1016/j.ejmech.2014.07.042 |
| 70. | Marzag, H.; Alaoui, S.; Amdouni, H.; Martin, A. R.; Bougrin, K.; Benhida, R. New J. Chem. 2015, 39, 5437. doi:10.1039/C5NJ00624D |
| 71. | Nallapati, S. B.; Sreenivas, B. Y.; Bankala, R.; Parsa, K. V. L.; Sripelly, S.; Mukkanti, K.; Pal, M. RSC Adv. 2015, 5, 94623. doi:10.1039/C5RA20380E |
| 72. | Naeimi, H.; Dadashzadeh, S.; Moradian, M. Res. Chem. Intermed. 2015, 41, 2687. doi:10.1007/s11164-013-1379-6 |
| 73. | Stefani, H. A.; Canduzini, H. A.; Manarin, F. Tetrahedron Lett. 2011, 52, 6086. doi:10.1016/j.tetlet.2011.09.004 |
| 74. | Jiang, Y.; Chen, X.; Qu, L.; Wang, J.; Yuan, J.; Chen, S.; Li, X.; Qu, C. Ultrason. Sonochem. 2011, 18, 527. doi:10.1016/j.ultsonch.2010.09.009 |
| 16. | Huisgen, R. Angew. Chem. 1963, 75, 604. doi:10.1002/ange.19630751304 |
| 17. | Huisgen, R. Proc. Chem. Soc., London 1961, 357. doi:10.1039/PS9610000357 |
| 18. | Huisgen, R. In 1,3-Dipolar Cycloaddition Chemistry; Padwa, A., Ed.; Wiley: New York, 1984; Vol. 1, 1. |
| 19. | Huisgen, R. Pure Appl. Chem. 1989, 61, 613. doi:10.1351/pac198961040613 |
| 61. | da Cruz, E. H. G.; Silvers, M. A.; Jardim, G. A. M.; Resende, J. M.; Cavalcanti, B. C.; Bomfim, I. S.; Pessoa, C.; de Simone, C. A.; Botteselle, G. V.; Braga, A. L.; Nair, D. K.; Namboothiri, I. N. N.; Boothman, D. A.; da Silva Júnior, E. N. Eur. J. Med. Chem. 2016, 122, 1. doi:10.1016/j.ejmech.2016.06.019 |
| 4. | Nandivada, H.; Jiang, X.; Lahann, J. Adv. Mater. 2007, 19, 2197. doi:10.1002/adma.200602739 |
| 5. | Lee, B. S.; Lee, J. K.; Kim, W.-J.; Jung, Y. H.; Sim, S. J.; Lee, J.; Choi, I. S. Biomacromolecules 2007, 8, 744. doi:10.1021/bm060782+ |
| 6. | Deobald, A. M.; Camargo, L. R. S.; Alves, D.; Zukerman-Schpector, J.; Corrêa, A. G.; Paixão, M. W. Synthesis 2011, 4003. doi:10.1055/s-0031-1289606 |
| 7. | Pérez-Labrada, K.; Brovard, I.; Morera, C.; Estévez, F.; Bermejo, J.; Rivera, D. G. Tetrahedron 2011, 67, 7713. doi:10.1016/j.tet.2011.08.003 |
| 8. | Días, D. D.; Rajagopal, K.; Strable, E.; Schneider, J.; Finn, M. G. J. Am. Chem. Soc. 2006, 128, 6056. doi:10.1021/ja061251w |
| 9. | Astruc, D.; Liang, L.; Rapakousiou, A.; Ruiz, J. Acc. Chem. Res. 2012, 45, 630. doi:10.1021/ar200235m |
| 10. | Tasdelen, M. A.; Yilmaz, G.; Iskin, B.; Yagci, Y. Macromolecules 2012, 45, 56. doi:10.1021/ma202438w |
| 11. | Font, D.; Jimeno, C.; Pericàs, M. A. Org. Lett. 2006, 8, 4653. doi:10.1021/ol061964j |
| 12. | Font, D.; Bastero, A.; Sayalero, S.; Jimeno, C.; Pericàs, M. A. Org. Lett. 2007, 9, 1943. doi:10.1021/ol070526p |
| 13. | Font, D.; Sayalero, S.; Bastero, A.; Jimeno, C.; Pericàs, M. A. Org. Lett. 2008, 10, 337. doi:10.1021/ol702901z |
| 14. | Lallana, E.; Riguera, R.; Fernandez-Megia, E. Angew. Chem., Int. Ed. 2011, 50, 8794. doi:10.1002/anie.201101019 |
| 15. | Hong, V.; Presolski, S. I.; Ma, C.; Finn, M. G. Angew. Chem., Int. Ed. 2009, 48, 9879. doi:10.1002/anie.200905087 |
| 62. | Mojtahedi, M. M.; Abaee, M. S. Ultrasound applications in synthetic organic chemistry. In Handbook on Applications of Ultrasound Sonochemistry for Sustainability; Chen, D.; Sharma, S. K.; Mudhoo, A., Eds.; CRC Press: New York, 2012; pp 281 ff. |
| 63. | Li, Z.; Hong, J.; Zhou, X. Tetrahedron 2011, 67, 3690. doi:10.1016/j.tet.2011.03.067 |
| 64. | Cravotto, G.; Cintas, P. Chem. Soc. Rev. 2006, 35, 180. doi:10.1039/B503848K |
| 65. | Mason, T. J. Ultrason. Sonochem. 2007, 14, 476. doi:10.1016/j.ultsonch.2006.10.008 |
| 66. | Nüchter, M.; Ondruschka, B.; Jungnickel, A.; Müller, U. J. Phys. Org. Chem. 2000, 13, 579. doi:10.1002/1099-1395(200010)13:10<579::AID-POC272>3.0.CO;2-M |
| 67. | Mason, T. J. Chem. Soc. Rev. 1997, 26, 443. doi:10.1039/cs9972600443 |
| 50. | Nogueira, C. W.; Rocha, J. B. T. Organoselenium and organotellurium compounds: Toxicology and pharmacology. In Patai's Chemistry of Functional Groups; Rappoport, Z., (Org)., Ed.; Wiley: Chichester, 2011. doi:10.1002/9780470682531.pat0567 |
| 51. | Santoro, S.; Azeredo, J. B.; Nascimento, V.; Sancineto, L.; Braga, A. L.; Santi, C. RSC Adv. 2014, 4, 31521. doi:10.1039/C4RA04493B |
| 52. | Nogueira, C. W.; Zeni, G.; Rocha, J. B. T. Chem. Rev. 2004, 104, 6255. doi:10.1021/cr0406559 |
| 60. | Donato, F.; de Gomes, M. G.; Goes, A. T. R.; Seus, N.; Alves, D.; Jesse, C. R.; Savegnago, L. Life Sci. 2013, 93, 393. doi:10.1016/j.lfs.2013.07.024 |
| 33. | Seus, N.; Gonçalves, L. C.; Deobald, A. M.; Savegnago, L.; Alves, D.; Paixão, M. W. Tetrahedron 2012, 68, 10456. doi:10.1016/j.tet.2012.10.007 |
| 34. | Seus, N.; Goldani, B.; Lenardão, E. J.; Savegnago, L.; Paixão, M. W.; Alves, D. Eur. J. Org. Chem. 2014, 1059. doi:10.1002/ejoc.201301547 |
| 36. | Savegnago, L.; do Sacramento, M.; Brod, L. M. P.; Fronza, M. G.; Seus, N.; Lenardão, E. J.; Paixão, M. W.; Alves, D. RSC Adv. 2016, 6, 8021. doi:10.1039/C5RA22445D |
| 44. | Rampon, D. S.; Santos, F. S.; Descalzo, R. R.; Toldo, J. M.; Gonçalves, P. F. B.; Schneider, P. H.; Rodembusch, F. S. J. Phys. Org. Chem. 2014, 27, 336. doi:10.1002/poc.3229 |
| 45. | Rampon, D. S.; Rodembusch, F. S.; Schneider, J. M. F. M.; Bechtold, I. H.; Gonçalves, P. F. B.; Merlo, A.; Schneider, P. H. J. Mater. Chem. 2010, 20, 715. doi:10.1039/B917366H |
| 46. | Samb, I.; Bell, J.; Toullec, P. Y.; Michelet, V.; Leray, I. Org. Lett. 2011, 13, 1182. doi:10.1021/ol200066p |
| 47. | Goswami, S.; Hazra, A.; Chakrabarty, R.; Fun, H.-K. Org. Lett. 2009, 11, 4350. doi:10.1021/ol901737s |
| 48. | Tang, B.; Xing, Y.; Li, P.; Zhang, N.; Yu, F.; Yang, G. J. Am. Chem. Soc. 2007, 129, 11666. doi:10.1021/ja072572q |
| 49. | Balaguez, R. A.; Ricordi, V. G.; Duarte, R. C.; Toldo, J. M.; Santos, C. M.; Schneider, P. H.; Gonçalves, P. F. B.; Rodembusch, F. S.; Alves, D. RSC Adv. 2016, 6, 49613. doi:10.1039/C6RA04157D |
| 36. | Savegnago, L.; do Sacramento, M.; Brod, L. M. P.; Fronza, M. G.; Seus, N.; Lenardão, E. J.; Paixão, M. W.; Alves, D. RSC Adv. 2016, 6, 8021. doi:10.1039/C5RA22445D |
| 38. | Alberto, E. E.; Braga, A. L. In Selenium and Tellurium Chemistry - From Small Molecules to Biomolecules and Materials; Derek, W. J.; Risto, L., Eds.; Springer-Verlag: Berlin Heidelberg, 2011. |
| 39. | Wirth, T. Organoselenium Chemistry: Synthesis and Reactions; Wiley-VCH: Weinheim, 2011. doi:10.1002/9783527641949 |
| 40. | Menezes, P. H.; Zeni, G. Vinyl Selenides. Patai’s Chemistry of Functional Groups; John Wiley & Sons: Oxford, 2011. doi:10.1002/9780470682531.pat0568 |
| 41. | Perin, G.; Lenardão, E. J.; Jacob, R. G.; Panatieri, R. B. Chem. Rev. 2009, 109, 1277. doi:10.1021/cr8004394 |
| 42. | Perin, G.; Alves, D.; Jacob, R. G.; Barcellos, A. M.; Soares, L. K.; Lenardão, E. J. ChemistrySelect 2016, 1, 205. doi:10.1002/slct.201500031 |
| 43. | Freudendahl, D. M.; Santoro, S.; Shahzad, S. A.; Santi, C.; Wirth, T. Angew. Chem., Int. Ed. 2009, 48, 8409. doi:10.1002/anie.200903893 |
| 34. | Seus, N.; Goldani, B.; Lenardão, E. J.; Savegnago, L.; Paixão, M. W.; Alves, D. Eur. J. Org. Chem. 2014, 1059. doi:10.1002/ejoc.201301547 |
| 33. | Seus, N.; Gonçalves, L. C.; Deobald, A. M.; Savegnago, L.; Alves, D.; Paixão, M. W. Tetrahedron 2012, 68, 10456. doi:10.1016/j.tet.2012.10.007 |
| 34. | Seus, N.; Goldani, B.; Lenardão, E. J.; Savegnago, L.; Paixão, M. W.; Alves, D. Eur. J. Org. Chem. 2014, 1059. doi:10.1002/ejoc.201301547 |
| 35. | Saraiva, M. T.; Costa, G. P.; Seus, N.; Schumacher, R. F.; Perin, G.; Paixão, M. W.; Luque, R.; Alves, D. Org. Lett. 2015, 17, 6206. doi:10.1021/acs.orglett.5b03196 |
| 36. | Savegnago, L.; do Sacramento, M.; Brod, L. M. P.; Fronza, M. G.; Seus, N.; Lenardão, E. J.; Paixão, M. W.; Alves, D. RSC Adv. 2016, 6, 8021. doi:10.1039/C5RA22445D |
| 37. | Saraiva, M. T.; Krüger, R.; Baldinotti, R. S. M.; Lenardão, E. J.; Luchese, C.; Savegnago, L.; Wilhelm, E. A.; Alves, D. J. Braz. Chem. Soc. 2016, 27, 41. doi:10.5935/0103-5053.20150239 |
| 53. | Tiecco, M.; Testaferri, L.; Santi, C.; Tomassini, C.; Marini, F.; Bagnoli, L.; Temperini, A. Angew. Chem., Int. Ed. 2003, 42, 3131. doi:10.1002/anie.200351229 |
| 54. | Back, T. G.; Bethell, R. J.; Parvez, M.; Taylor, J. A.; Wehrli, D. J. Org. Chem. 1999, 64, 7426. doi:10.1021/jo990730t |
| 55. | Stefani, H. A.; Silva, N. C. S.; Manarin, F.; Lüdtke, D. S.; Zukerman-Schpector, J.; Madureira, L. S.; Tiekink, E. R. T. Tetrahedron Lett. 2012, 53, 1742. doi:10.1016/j.tetlet.2012.01.102 |
| 56. | Stefani, H. A.; Leal, D. M.; Manarin, F. Tetrahedron Lett. 2012, 53, 6495. doi:10.1016/j.tetlet.2012.09.062 |
| 57. | Deobald, A. M.; Camargo, L. R. S.; Hörner, M.; Rodrigues, O. E. D.; Alves, D.; Braga, A. L. Synthesis 2011, 2397. doi:10.1055/s-0030-1260083 |
| 58. | Seus, N.; Saraiva, M. T.; Alberto, E. E.; Savegnago, L.; Alves, D. Tetrahedron 2012, 68, 10419. doi:10.1016/j.tet.2012.07.019 |
| 59. | Saraiva, M. T.; Seus, N.; de Souza, D.; Rodrigues, O. E. D.; Paixão, M. W.; Jacob, R. G.; Lenardão, E. J.; Perin, G.; Alves, D. Synthesis 2012, 44, 1997. doi:10.1055/s-0031-1291135 |
| 60. | Donato, F.; de Gomes, M. G.; Goes, A. T. R.; Seus, N.; Alves, D.; Jesse, C. R.; Savegnago, L. Life Sci. 2013, 93, 393. doi:10.1016/j.lfs.2013.07.024 |
| 61. | da Cruz, E. H. G.; Silvers, M. A.; Jardim, G. A. M.; Resende, J. M.; Cavalcanti, B. C.; Bomfim, I. S.; Pessoa, C.; de Simone, C. A.; Botteselle, G. V.; Braga, A. L.; Nair, D. K.; Namboothiri, I. N. N.; Boothman, D. A.; da Silva Júnior, E. N. Eur. J. Med. Chem. 2016, 122, 1. doi:10.1016/j.ejmech.2016.06.019 |
| 36. | Savegnago, L.; do Sacramento, M.; Brod, L. M. P.; Fronza, M. G.; Seus, N.; Lenardão, E. J.; Paixão, M. W.; Alves, D. RSC Adv. 2016, 6, 8021. doi:10.1039/C5RA22445D |
© 2017 Costa et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (http://www.beilstein-journals.org/bjoc)