Abstract
Hypervalent iodine compounds, in particular aryl-λ3-iodanes, have been used extensively as electrophilic group-transfer reagents. Even though these compounds are superior substrates in terms of reactivity and stability, their utilization is accompanied by stoichiometric amounts of an aryl iodide as waste. This highly nonpolar side product can be tedious to separate from the desired target molecules and significantly reduces the overall atom efficiency of these transformations. In this short review, we want to give a brief summary of recently developed methods, in which this arising former waste is used as an additional reagent in cascade transformations to generate multiple substituted products in one step and with high atom efficiency.
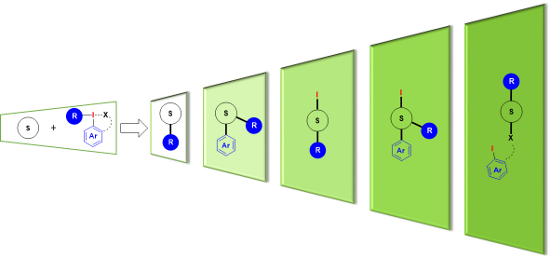
Graphical Abstract
Introduction
Atom economy (AE) is an important parameter which helps to evaluate the overall efficiency of a chemical reaction or a chemical process [1,2]. It is defined as the quotient between the molecular mass of the desired reaction product(s) and the molecular mass of all reactants (Equation 1):
In an ideal reaction with 100% atom economy, every atom of the reactants is becoming part of the desired product. In this short review, we want to discuss recent advances in atom-economical transformations using hypervalent iodine reagents (iodanes) as electrophilic group-transfer reagents. Iodanes, in particular iodonium salts, are well-balanced reagents in terms of stability, reactivity and synthetic and/or commercial availability and therefore it is not surprising to see these compounds as key reagents in a great number of recently developed transformations [3-15]. However, in terms of atom economy, they have an intrinsic problem: their high reactivity is based on the emergence of aryl iodides as supernucleofuges. λ3-Iodanes are the generally preferred hypervalent iodine compounds for electrophilic group transfer reactions, during which the central iodine atom is transformed from a high energy hypervalent state into a normal valent state by a two-electron reduction. The high stability of the newly formed aryl iodide is the thermodynamic driving force for all λ3-iodane-mediated oxidative transformations. Even though this process guarantees the high reactivity of these reagents, it has one major obstacle: after the oxidation process, stoichiometric amounts of the aryl iodide are produced as waste. Aryl iodides, as nonpolar organic waste, are oftentimes hard to separate from the desired reaction products since they cannot be simply washed out with aqueous solutions. Instead, they must be separated by column chromatography. In terms of AE, the high atomic mass of iodine (126.9 u) leads to a dramatic negative impact of iodanes on this “green” reaction parameter. To overcome this obstacle, promising approaches are the use of iodoarenes as precatalysts in combination with external co-oxidants and the utilization of specific hypervalent iodine compounds (polymer-supported as well as non-polymeric species), whose reduced forms are easy to recycle [3,4,16,17]. On the other hand, the additional incorporation of the former iodoarene waste into the reaction product via a cascade reaction does not only improve the overall AE of a chemical reaction but also directly leads to highly functionalized target molecules. A rough estimate about the impact of the incorporation efficiency of the iodane on the AE is depicted in Scheme 1. Here, only the molecular mass of the corresponding iodane substrates is taken into account for AE estimation.
Scheme 1: Overview of different types of iodane-based group-transfer reactions and their atom economy based on the molecular mass of the corresponding λ3-iodane.
Scheme 1: Overview of different types of iodane-based group-transfer reactions and their atom economy based o...
The transfer of only one functional group, for instance in iodane-mediated electrophilic monoarylations, produces not only the aryl iodide, but also stoichiometric amounts of salt side-products, limiting the AE of these transformations to roughly 10–20% (Scheme 1, reaction (a)). In the second case, both organic residues (one carbon or heteroatom ligand plus the arene of the former aryl-λ3-iodane or aryliodonium salt) are transferred to the substrate (Scheme 1, reaction (b)). The loss of iodine and the counterion still limits the overall AE to 30–40%. A dramatic increase in AE arises, if the iodine atom is incorporated in the desired reaction product as well (Scheme 1, reaction (c)). This does not only lead to an overall AE of up to 70% but also produces synthetically versatile intermediates for subsequent transformations, in particular metal-catalyzed cross-coupling reactions. If benziodoxolones or benziodoxoles are used as group-transfer reagents, nearly 100% AE is possible since the counterion (the carboxylate or alcoholate) is covalently attached to the aryl iodide and hence does not get lost during the reaction cascade (Scheme 1, reaction (d)). This is very effective, if this ortho-functionality is a desired part of the final reaction product or if it can be readily transformed into synthetically useful functional groups.
In this short review, we will give a brief summary of very recent efforts toward the atom-economical use of aryl-λ3-iodanes, in particular aryliodonium salts in group-transfer reactions. In our definition, this includes transformations in which at least two of the three ligands attached to the iodane are part of the target molecule or in which the iodane acts as an oxidant and a group-transfer reagent in a consecutive reaction sequence. The chapters are divided by the structure of the transferred functional groups, starting from simple diarylations and oxidative arylations with moderate AE to highly atom efficient transformations using alkynyl and azide-substituted benziodoxolones. The given AE values are simplified and were calculated on the basis of the key substrates, whereas the required equivalents of all starting materials (iodane and usually its reaction partner) are taken into account. Other additives, such as additional bases, acids or catalysts were neglected.
Review
1. Diaryliodonium salts
1.1. Acyclic diaryliodonium salts
Acyclic diaryliodonium salts 1 find widespread application in numerous metal-free and transition metal-mediated electrophilic arylation protocols [18-22]. While in solid state they clearly have a T-shaped pseudotrigonal bipyramid structure, the common structural motif for λ3-iodanes, recent theoretical investigations revealed a potential role of the cationic, normal valent tetrahedral form, in atom transfer processes (Scheme 2a) [23]. Throughout this review, iodonium salts will be shown consequently in the latter structure due to clarity and due to literature habits.
Typically, only one of the two aryl ligands is transferred to the substrate, yielding a monoarylated reaction product and aryl iodide as stoichiometric waste. Examples for their atom-economical utilization, in which at least both aryl ligands are transferred, are still rare. A general approach would involve at first a metal-catalysed or metal-free arylation step of a suitable substrate A with the diaryliodonium salt 1 to give monoarylated intermediate B. Subsequently, a metal-catalysed cross-coupling initiated by an oxidative addition of the metal catalyst into the C–I bond of the emerging iodoarene 2 affords the diarylated product C (Scheme 2b).
Scheme 2: (a) Structure of diaryliodonium salts 1. (b) Diarylation of a suitable substrate A with one equivalent of diaryliodonium salt 1.
Scheme 2: (a) Structure of diaryliodonium salts 1. (b) Diarylation of a suitable substrate A with one equival...
A first report utilizing this strategy was published by Bumagin and co-workers as early as 1995 [24]. Here, symmetrical diaryliodonium salts 1 were used in a palladium-catalysed cross-coupling reaction with sodium tetraphenylborate (Scheme 3). This reaction not only provides excellent yields of the respective biphenyls 3 but also exhibits a high AE (57% for Ar = Ph). However, its general synthetic utility is limited since it requires highly reactive boron compounds as nucleophiles.
Scheme 3: Synthesis of biphenyls 3 and 3’ with symmetrical diaryliodonium salts 1.
Scheme 3: Synthesis of biphenyls 3 and 3’ with symmetrical diaryliodonium salts 1.
Symmetrical biphenyls 3’ can be generated from the corresponding symmetrically substituted diaryliodonium salts 1 and bis(pinacolato)diboron as demonstrated by Muñiz and co-workers [25]. In the first step, a mild carbon–boron bond formation gives one equivalent of arylboronic ester 4 and an iodoarene 2 through a metal-free boron arylation. Subsequent cross coupling under Suzuki conditions affords symmetrical biphenyls 3’ in good yields. Due to the temporary introduction and cleavage of the boron moiety the formal atom economy for this transformation is rather low (26% for Ar = Ph). However, 45% of the molecular weight of the starting iodonium salt 1 is present in the product 3’.
Diaryl thioethers 5 can be synthesized using either cyclic iodonium salts (will be discussed briefly in section 1.2) or their acyclic counterparts 1 (Scheme 4) [26,27]. Jiang and co-workers developed a Cu(II)-catalysed methodology for the conversion of acyclic diaryliodonium salts 1 and potassium thioacetate to the corresponding thioethers 5 (pathway (a)) and later applied the optimized reaction conditions towards cyclic iodonium salts. In an independent work, Li and co-workers successfully utilized a catalytic system based on FeCl3 with comparable results (pathway (b)). However, both procedures have only moderate AE values (28–33% for Ar1 = Ar2 = Ph), since the transferred aryl groups account for only about 35% to the mass of the respective diaryliodonium salts 1.
Scheme 4: Synthesis of diaryl thioethers 5.
Scheme 4: Synthesis of diaryl thioethers 5.
An atom efficient S-arylation of tetraalkylthiuram disulfides 6 was developed by Dong and co-workers under Cu(I)-catalysis (Scheme 5) [28]. This reaction yields two equivalents of S-aryl dithiocarbamates 7 and 7’ in typically high yields applying only one equivalent of a diaryliodonium salt 1. It is worth to mention, that the AE of 51% (for Ar1 = Ar2 = Ph, X = TfO–, R = Me) is only valid if both dithiocarbamates 7 and 7’ are defined as desired reaction products. If symmetrical diaryliodonium salts are used, as also demonstrated, only one arylation product is formed. The proposed reaction mechanism starts with the oxidative addition of the diaryliodonium salt 1 to copper(I) iodide affording the Cu(III) species A, releasing aryl iodide 2. Coordination of the disulfide 6 to the metal centre leads to complex B, followed by the base-induced formation of arylcopper sulfide complex C and potassium salt D upon treatment with KOt-Bu. Reductive elimination from C produces the product 7, under regeneration of the Cu(I) species. The released aryl iodide 2 on the other hand then undergoes oxidative addition forming the Cu(III) species E, which can provide intermediate F upon reaction with the potassium salt D and the release of potassium iodide. Reductive elimination then affords the second S-aryl dithiocarbamate 7’.
Scheme 5: Synthesis of two distinct S-aryl dithiocarbamates 7 and 7’ from one equivalent of diaryliodonium salt 1.
Scheme 5: Synthesis of two distinct S-aryl dithiocarbamates 7 and 7’ from one equivalent of diaryliodonium sa...
If both aryl groups are not transferred to the same reaction centre in metal-mediated diarylations when unsymmetrical diaryliodonium salts are used as aryl-transfer reagents, poor chemoselectivies are often observed. Since electronic differences in many cases exhibit only unsatisfactory levels of selectivity [29,30], sterically demanding groups, such as mesityl moieties, are mostly used to solve this issue. However, the high steric demand can be of disadvantage if the development of an atom-economical transformation is intended through a subsequent functionalization of the emerging, now less reactive, mesityl iodide. A procedure which impressively combines the selectivity of mesityl(aryl)iodonium salts 1a with this difficult second arylation step was developed by Li and co-workers [31]. Under copper-catalysis diarylated isoindolin-1-ones 9 were formed starting from 2-formylbenzonitriles 8 upon treatment with mesityl(aryl)iodonium salts 1a (Scheme 6). A plausible reaction mechanism starts with the selective terminal arylation of the nitrile, forming the arylnitrilium cation A and 2-iodomesitylene (2a). Intramolecular cyclization leads to the iminofuranylium intermediate B which affords the cationic isoindolin-1-one structure C via subsequent intramolecular aza-Michael type rearrangement. This intermediate reacts in the final step with the electron-rich 2-iodomesitylene (2a) in an SEAr reaction to give the diarylated isoindolin-1-one 9. A drawback of these reaction conditions is the use of excess (3 equiv) mesityl(aryl)iodonium salt 1a to achieve satisfying yields. This dramatically lowers the atom-economy to only 29% (for R = H, Ar = Ph), instead of theoretical 75%, if only one equivalent of the iodane 1a would have been sufficient.
Scheme 6: Synthesis of substituted isoindolin-1-ones 9 from 2-formylbenzonitrile 8 and the postulated reaction mechanism.
Scheme 6: Synthesis of substituted isoindolin-1-ones 9 from 2-formylbenzonitrile 8 and the postulated reactio...
In recent years a variety of domino C–H and/or N–H arylations as powerful tools towards selective diarylations of (hetero)arenes was employed by Greaney and co-workers, who were among the first to establish more atom-economical procedures with λ3-iodanes. These methods commonly exhibit good AE for (di)arylation procedures, since only small excesses of diaryliodonium salts are necessary. In an initial study, the selective diarylation of indoles 10 using symmetrical and unsymmetrical diaryliodonium salts 1 was demonstrated (Scheme 7) [32]. A selective C–H arylation at C3 of the indole was realised under copper catalysis before the addition of a ligand and an inorganic base initiated the N-arylation with the in situ formed iodoarene. The desired diarylated indoles 11 are obtained with an AE of 46% (for R1 = R2 = H, Ar1 = Ar2 = Ph). The scope of this transformation is broad, only C2-substituted indoles show poor reactivity. Furthermore, this is a rare example for good chemoselectivities in atom-efficient reaction cascades even if unsymmetrical substituted diaryliodonium salts are applied.
Scheme 7: Domino C-/N-arylation of indoles 10.
Scheme 7: Domino C-/N-arylation of indoles 10.
Based on this initial procedure, an impressive atom-economical biphenylation of N-heterocycles was developed [33]. This method involved a direct N-arylation of pyrazoles or triazoles 12 under basic conditions, followed by a ruthenium-catalysed C–H arylation with the emerging aryl iodide (Scheme 8). Due to the fact that the first step of this reaction sequence proceeds under metal-free conditions, the selective transfer of the more electron-deficient aryl group of the unsymmetrical iodonium salts 1 is achieved. The N-arylated heterocycle NAr1 then serves as the directing group in the following ortho-C–H functionalisation with the iodoarene as coupling reagent to yield biphenyls 13. The optimized reaction conditions are also applicable to alkenyl(aryl)iodonium salts 14 making arylated benzylidenes 15 accessible through the N-alkenylated intermediate NAr2.
Scheme 8: Domino modification of N-heterocycles 12 via in situ-generated directing groups.
Scheme 8: Domino modification of N-heterocycles 12 via in situ-generated directing groups.
The AE for this transformation is 38% (for R1 = H, Y = CH, Ar1 = Ar2 = Ph), a typical value for iodane-mediated double functionalisations with aryl iodonium salts.
Another achievement of Greaney and co-workers is the atom-economical double arylation of anilines 16 with symmetrical diaryliodonium salts 1 (Scheme 9) [34]. The corresponding triarylamines 17 are important structural motifs for organic electronics and can be obtained in good yields and an AE of 48% (Ar1 = p-Tol, Ar2 = Ar3 = Ph) using this Cu(I)-catalysed domino approach. The formation of an intermediately found diarylaniline supposedly follows a Cu(I)/Cu(III) cycle, whereas a radical mechanism is proposed for the second arylation. Unfortunately, a selective aryl transfer using unsymmetrical diaryliodonium salts was not successful under the reaction conditions, giving only mixtures of mono N-arylated anilines in low yields after the first step.
Scheme 9: Synthesis of triarylamines 17 through a double arylation of anilines.
Scheme 9: Synthesis of triarylamines 17 through a double arylation of anilines.
A different approach towards the functionalisation of heterocycles was taken by Shafir, Lledós, and co-workers, who investigated the intrinsic conversion of novel N-heterocyclic aryliodonium salts 1b [35]. Under the influence of base, copper catalyst and N-Me-benzimidazole as a ligand, the heterocyclic aryliodonium salt 1b was converted into the N1-aryl-5-iodoimidazole 18 as the major isomer (Scheme 10). Compound 18 is a valuable intermediate for a variety of coupling reactions to yield 1,5-disubstituted imidazoles – structural motifs which are generally difficult to obtain – in a selective fashion. The proposed reaction mechanism based on DFT calculations starts with the deprotonation of the imidazolyl group to give betain A, which binds to a Cu(I)-OTf fragment leading to complex B. Next, the aryl group is transferred from the iodine to the copper atom leading to the Cu(III) complex D via a five-membered transition state C. Reductive elimination through transition state E provides 18 and restores the active Cu(I) species. Since this reaction follows an intramolecular pathway, it is featured by a remarkable high atom economy of 82% (for Ar1 = Ph, R = H), lowered only by the loss of one formal equivalent of acetic acid.
Scheme 10: Selective conversion of novel aryl(imidazolyl)iodonium salts 1b to 1,5-disubstituted imidazoles 18.
Scheme 10: Selective conversion of novel aryl(imidazolyl)iodonium salts 1b to 1,5-disubstituted imidazoles 18.
1.2. Cyclic diaryliodonium salts
Within the vast scope of hypervalent iodine compounds the class of cyclic diaryliodonium salts 19 is predestined to undergo atom-efficient transformations. Owing to their unique structure in which both carbon ligands attached to the central iodine atom are connected to one another, no iodoarene “waste” can be formed in the reaction process. Depending on the reaction conditions the selective functionalisation of just one aryl iodine moiety or the modification of both carbon ligands in either a symmetrical or unsymmetrical transformation is possible leading to a plethora of useful carbo- and heterocycles (Scheme 11).
Scheme 11: Selected examples for the application of cyclic diaryliodonium salts 19.
Scheme 11: Selected examples for the application of cyclic diaryliodonium salts 19.
The chemistry of cyclic diaryliodonium salts has been reviewed by Grushin in 2000 [36] and more recently by Goswami and co-worker [37]. Therefore, this topic will not be discussed in further detail in this article. However, since iodine and the counterion are inevitable stoichiometric side products, it is worth mentioning that the theoretical AE for these particular transformations is usually limited to 30–40%.
2. (Dicarboxyiodo)benzenes
From a synthetic point of view, (dicarboxyiodo)benzenes 20 are probably one of the most popular hypervalent iodine compounds in organic synthesis [3,9,38-41]. They have been widely applied in C–H oxygenations, nitrene generations, oxidative dearomatisations and dehydrogenative couplings by transferring one of their two carboxyl ligands or external oxygen nucleophiles to a substrate. In all these “classical” reactions aryl iodides are produced as stoichiometric waste products once again. The further utilization of this side product in a subsequent reaction step would dramatically increase the AE for such oxidative transformations.
A general procedure for such a tandem sequence would involve an initial oxidation step of a substrate A followed by a coupling of the formed iodoarene 2 with the oxidised substrate B either under metal-free or metal-catalysed conditions to form the product C (Scheme 12).
Scheme 12: Tandem oxidation–arylation sequence with (dicarboxyiodo)benzenes 20.
Scheme 12: Tandem oxidation–arylation sequence with (dicarboxyiodo)benzenes 20.
The first examples for an oxidation–arylation cascade using (dicarboxyiodo)benzenes were reported by Shafir and co-workers [42,43]. Herein, the authors describe an efficient α-arylation of a variety of 1,3-dicarbonyl derivatives 21 using [bis(trifluoroacetoxy)iodo]benzene (20a, PIFA). In this metal-free approach the target structures 22 are efficiently synthesised even without any initial prefunctionalisation of the arene moiety (Scheme 13). Remarkably, the intact 2-iodoaryl group is transferred via presumed transition state TS1, which leads to a good AE (50% for 22a) and allows the further transformation of the α-arylation product 22 via cross coupling reactions. In addition, the in situ generation of the PIFA reagent proved viable, in order to temporary generate potentially unstable λ3-iodane derivatives and immediately convert them under the established α-arylation conditions.
Scheme 13: Oxidative α-arylation via the transfer of an intact 2-iodoaryl group.
Scheme 13: Oxidative α-arylation via the transfer of an intact 2-iodoaryl group.
A PIDA ((diacetoxyiodo)benzene)-mediated ortho-iodination/O-arylation cascade was developed by Panda and co-workers using diversely functionalised phenols 23 (Scheme 14) [44]. The reaction presumably proceeds via an ortho-addition of the λ3-iodane 20b, followed by an iodine-to-oxygen 1,3-aryl migration via a concerted mechanism through the five-membered intermediate TS2 to afford the corresponding diaryl ether 24. A mechanistically similar ortho-iodination/O-arylation cascade for the modification of 2-arylquinolones 25 was developed by Xie and co-workers, providing the respective products 26 in a one pot sequence [45]. Both methods are characterised by a good AE (49–51% for Ar = Ph) due to the incorporation of both, the aryl group and the iodine atom. However, the usage of 1.5 to 2.0 equivalents of the hypervalent iodine reagent 20b lowers the conclusive atom efficiencies.
Scheme 14: Tandem ortho-iodination/O-arylation cascade with PIDA derivatives 20b.
Scheme 14: Tandem ortho-iodination/O-arylation cascade with PIDA derivatives 20b.
In another closely related procedure, Das and co-workers employed PIDA derivatives 20b for an aromatization–arylation cascade of exocyclic β-enaminones 27 [46]. Under basic reaction conditions meta-N,N-diarylaminophenols 28 were obtained in good to excellent yields using 20b both as the aromatization and arylation agent (Scheme 15). In the first step of the postulated mechanism, a ligand exchange of the starting material 27 and PIDA 20b forms intermediate A, which leads to the formation of the stable N-arylated intermediate B via an iodine-to-nitrogen 1,3-phenyl migration. An equilibrium with its enol form B’ is proposed, which affords the aromatization product 28 via H-shift to intermediate C and subsequent dehydroiodination under basic conditions. Although the iodine atom is not incorporated into the final product, the calculated AE (46% for R1 = R2 = H, R3 = Ar = Ph) is close to the procedures of Panda and Xie due to lower PIDA loadings.
Scheme 15: Synthesis of meta-N,N-diarylaminophenols 28 and the postulated mechanism.
Scheme 15: Synthesis of meta-N,N-diarylaminophenols 28 and the postulated mechanism.
A metal-catalysed procedure for the atom-economical utilization of (dicarboxyiodo)benzenes 20 in a tandem C(sp3)–H amination/sila-Sonogashira–Hagihara reaction was developed by Dauban and co-workers (Scheme 16) [47]. The first step of this sequence includes an iodine(III)-mediated rhodium-catalysed enantioselective amination of an unactivated C(sp3)–H bond with a chiral sulfonimidamide 31. Afterwards, the iodoarene byproduct of the first step is coupled with the alkyne under palladium catalysis. A broad range of TMS-substituted acetylenes 29 was converted efficiently under the optimized reaction conditions, forming the desired arylacetylenes 30 in good yields with high stereoselectivity. The applicable substrate scope is rather broad including thiophene, cyclopentene and adamantyl derivatives 30a–c with good AE values (51% for 30a).
Scheme 16: (Dicarboxyiodo)benzene-mediated metal-catalysed C–H amination and arylation.
Scheme 16: (Dicarboxyiodo)benzene-mediated metal-catalysed C–H amination and arylation.
The proposed mechanism involves two distinct catalytic cycles for the amination and the arylation reaction step (Scheme 17). First, a metallanitrene B is formed via the reaction of the (dicarboxyiodo)benzene 20 with the sulfonimidamide 31 in the presence of the chiral rhodium(II) catalyst A. Hereby, one equivalent of iodoarene 2 is released. The insertion of the nitrene species into the C(sp3)–H bond affords the amination product C, which is the final product of the first reaction step. In the following step, C is subsequently desilylated with TBAF in the presence of CuCl and DABCO to obtain the alkynylcopper species D. In the meantime oxidative addition of the previously released iodoarene 2 to the Pd(0) species occurs and the resulting palladium(II) complex E then undergoes transmetallation with the copper species D to provide complex F. Isomerisation to the cis-palladium complex G and subsequent reductive elimination finally delivers 30.
Scheme 17: Postulated mechanism for the amination–arylation sequence.
Scheme 17: Postulated mechanism for the amination–arylation sequence.
In such sequences, the iodane can also be prone to autooxidation processes, as demonstrated by Dauban and co-workers. Alkyl-substituted PIDA derivatives 20c are the substrates for both the chiral C(sp3)–H amination and for the following cross-coupling reaction (Scheme 18) [48]. In the first step, the C(sp3)–H bonds of PIDA derivatives were efficiently converted via an auto-amination with the external sulfonimidamide 31 to form the corresponding aminated iodoarenes 32. These species smoothly underwent subsequent Suzuki–Miyaura, Sonogashira and Mirozoki–Heck couplings. The respective arylated, alkynylated and alkenylated products 33a–d were obtained in high yields with excellent stereoselectivity. Due to the intramolecular pathway of the first step, high AE values (66% for 33b) are obtained.
Scheme 18: Auto-amination and cross-coupling of PIDA derivatives 20c.
Scheme 18: Auto-amination and cross-coupling of PIDA derivatives 20c.
In another tandem procedure, a metal-free C(sp3)–H olefination of amidines 34 with DMSO and PIDA derivatives 20b was developed by Namitharan and co-workers [49]. A Pd(II)-catalysed Heck reaction of the formed exocyclic double bond with the emerging iodoarene yields substituted acrylamidines 35 (Scheme 19). The proposed reaction mechanism starts with the activation of DMSO (A) via the iodine(III) species 20b. Iodosoarene C is released under basic conditions, forming the sulfonium intermediate D. This intermediate reacts with the amidine 34 to give the sulfide E, which is then oxidized to the sulfoxide G by iodosoarene C via the postulated activated sulfoxide F, releasing one equivalent of aryl iodide 2. A Chugaev-type elimination yields the olefin H, which finally affords acrylamidine 35 via Heck coupling with the iodoarene 2 with 50% AE (for R1 = H, R2 = Bn, Ar1 = Ar2 = Ph).
Scheme 19: Tandem C(sp3)–H olefination/C(sp2)–H arylation.
Scheme 19: Tandem C(sp3)–H olefination/C(sp2)–H arylation.
3. Benziodoxolones
Iodine(III) compounds with a benziodoxolone or a benziodoxole structure are privileged reagents for electrophilic group transfer reactions, in particular in electrophilic alkynylations, azidations, cyanations and trifluoromethylations [3,50-54]. Here, 2-iodobenzoic acid is formed as a quantitative waste product, thus lowering the atom efficiency of these transformations. An atom-economical application of benziodoxolones 36 would involve the utilization of the nucleophilic properties of the released benzoic acid 37, after the initial electrophilic group transfer to form B from substrate A. This would give target structures C that fully include the 2-iodobenzoic acid with a theoretical AE of nearly 100% (Scheme 20). Only molecular hydrogen is produced as formal waste in this process.
Scheme 20: Atom efficient functionalisations with benziodoxolones 36.
Scheme 20: Atom efficient functionalisations with benziodoxolones 36.
The first report for an atom-economical application of benziodoxolones was a reaction of N-arylimines 38 with ethynylbenziodoxolones 36a (EBX), under the influence of a palladium catalyst, developed by Yoshikai and co-workers [55]. Instead of the expected α-alkynylated product, highly substituted furans 39 were observed (Scheme 21). Both, the electrophilic alkyne and the nucleophilic 2-iodobenzoate group took part in this transformation in a complex putative reaction mechanism. However, since two equivalents of EBX (36a) are used, the AE for this transformation is rather low (47% for 39a). The obtained furans 39 are highly useful synthetic intermediates, since the 2-iodoaryl group can be successfully employed in further derivatisations. In example, indenones 40 and phospholes 41 could smoothly be generated.
Scheme 21: Atom-efficient synthesis of furans 39 from benziodoxolones 36a and their further derivatisations.
Scheme 21: Atom-efficient synthesis of furans 39 from benziodoxolones 36a and their further derivatisations.
In contrary to this unexpected reaction pathway, Waser and co-workers particularly aimed for an atom-economical application of EBX reagents 36a utilizing a copper-catalysed reaction with diazo compounds 42 (Scheme 22) [56]. This strategy is not only atom economic regarding the applied hypervalent iodine reagent but also with regard to the chosen substrate. The metal carbene species generated from the diazo compounds displays nucleophilic as well as electrophilic reactivity at the same carbon atom and only gaseous dinitrogen is produced as stoichiometric waste. The reaction provides oxyalkynylated products 43 in high yields and addresses a broad scope of diazo derivatives 42 and EBX compounds 36a. For diazo compounds bearing hydrogen, alkyl or aryl groups (R1), propargyl esters 43a–c are obtained. In contrast, vinyldiazo compounds yielded the corresponding 1,3-enynes 43d–f in good yields. The oxyalkynylation shows an excellent AE regarding the iodine(III) species 36a. Every atom of the benziodoxolone 36a is present in the final product 43 and only one equivalent of elemental nitrogen is lost over the course of the reaction. The overall AE is only diminished by the necessity to use two equivalents of the diazo compound (AE = 74% for 43b).
Scheme 22: Oxyalkynylation of diazo compounds 42.
Scheme 22: Oxyalkynylation of diazo compounds 42.
Furthermore, an enantioselective version of this oxyalkynylation (for R1 = H) was developed [57]. Employing a chiral bisoxazoline ligand, diazo compounds 42’ with various electron-withdrawing groups were efficiently oxyalkynylated affording highly enantioenriched propargyl esters 44 (Scheme 23).
Scheme 23: Enantioselective oxyalkynylation of diazo compounds 42’.
Scheme 23: Enantioselective oxyalkynylation of diazo compounds 42’.
Subsequent reductive cleavage of the ester gave direct access to enantiopure propargyl alcohols. Besides EBX reagents, azidobenziodoxolones 36b (ABX), could be utilized for atom-economical reactions. Gillaizeau and co-workers developed an iron-catalysed oxyazidation of enamides 45 using ABX derivatives 36b (Scheme 24) [58]. The reaction proceeds with complete regio- and stereoselectivity introducing the azide group in C2 and the ester moiety in C3 position, affording the trans-isomer 46 exclusively. The reaction mechanism presumably follows a radical pathway, which begins with a single electron transfer (SET) from Fe(II) to 36b generating a Fe(III) species as well as benziodoxolonyl radical A or benzoyloxy radical A’ and an azide anion. Next, a SET from the enamide 45 to Fe(III) affords again Fe(II) and a further reaction with the radical A or A’ leads to the formation of carbocation B or iminium ion B’, respectively. Recombination of these positively charged intermediates with the azide anion finally affords the oxyazidated product 46. Overall this oxyazidation exhibits the highest AE so far observed for iodane-mediated group transfer reactions with 89% (for Ar = Ph, R1 = Bn, Y = CH2, Z = O).
Scheme 24: Iron-catalysed oxyazidation of enamides 45.
Scheme 24: Iron-catalysed oxyazidation of enamides 45.
Conclusion
In summary, we hope that we could demonstrate in this short focused overview, that aryl-λ3-iodanes are not only stable and highly reactive reagents for electrophilic group-transfer reagents, but that their utilization can be highly atom efficient if the emerging aryl iodides are constructively used in further transformations by cascade processes through a smart reaction design. The small number of discussable transformations, in which at least two of the three ligands attached to the central hypervalent iodine atom are transferred to a given substrate, exemplifies, that this field of research is still in its infancy. Nonetheless, this comparative article should give new impulses to researchers working in the field of iodane-mediated coupling reactions and hopefully will lead to new atom-efficient reaction methods utilizing these highly useful substrates, also in large-scale synthetic applications.
References
-
Trost, B. M. Science 1991, 254, 1471–1477. doi:10.1126/science.1962206
Return to citation in text: [1] -
Trost, B. M. Acc. Chem. Res. 2002, 35, 695–705. doi:10.1021/ar010068z
Return to citation in text: [1] -
Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547
Return to citation in text: [1] [2] [3] [4] -
Yusubov, M. S.; Zhdankin, V. V. Curr. Org. Synth. 2012, 9, 247–272. doi:10.2174/157017912799829021
Return to citation in text: [1] [2] -
Zhdankin, V. V. ARKIVOC 2009, No. i, 1–62. doi:10.3998/ark.5550190.0010.101
Return to citation in text: [1] -
Wirth, T. Angew. Chem., Int. Ed. 2005, 44, 3656–3665. doi:10.1002/anie.200500115
Return to citation in text: [1] -
Moriarty, R. M. J. Org. Chem. 2005, 70, 2893–2903. doi:10.1021/jo050117b
Return to citation in text: [1] -
Wirth, T. Synthesis 1999, 1271–1287. doi:10.1055/s-1999-3540
Return to citation in text: [1] -
Stang, P. J.; Zhdankin, V. V. Chem. Rev. 1996, 96, 1123–1178. doi:10.1021/cr940424+
Return to citation in text: [1] [2] -
Stang, P. J. J. Org. Chem. 2003, 68, 2997–3008. doi:10.1021/jo030022e
Return to citation in text: [1] -
Matveeva, E. D.; Proskurnina, M. V.; Zefirov, N. S. Heteroat. Chem. 2006, 17, 595–617. doi:10.1002/hc.20273
Return to citation in text: [1] -
Sousa e Silva, F. C.; Tierno, A. F.; Wengryniuk, S. E. Molecules 2017, 22, No. 780. doi:10.3390/molecules22050780
Return to citation in text: [1] -
Yoshimura, A.; Yusubov, M. S.; Zhdankin, V. V. Org. Biomol. Chem. 2016, 14, 4771–4781. doi:10.1039/C6OB00773B
Return to citation in text: [1] -
Hypervalent Iodine Chemistry. In Topics in Current Chemistry; Wirth, T., Ed.; Springer International Publishing: Cham, 2016; Vol. 373.
Return to citation in text: [1] -
Silva, L. F., Jr.; Olofsson, B. Nat. Prod. Rep. 2011, 28, 1722–1754. doi:10.1039/C1NP00028D
Return to citation in text: [1] -
Keshavarz, M. Synlett 2011, 2433–2434. doi:10.1055/s-0030-1261234
Return to citation in text: [1] -
Dohi, T.; Kita, Y. Chem. Commun. 2009, 2073–2085. doi:10.1039/b821747e
Return to citation in text: [1] -
Aradi, K.; Tóth, B.; Tolnai, G.; Novák, Z. Synlett 2016, 27, 1456–1485. doi:10.1055/s-0035-1561369
Return to citation in text: [1] -
Olofsson, B. Top. Curr. Chem. 2015, 373, 135–166. doi:10.1007/128_2015_661
Return to citation in text: [1] -
Yusubov, M. S.; Maskaev, A. V.; Zhdankin, V. V. ARKIVOC 2011, No. i, 370–409. doi:10.3998/ark.5550190.0012.107
Return to citation in text: [1] -
Merritt, E. A.; Olofsson, B. Angew. Chem., Int. Ed. 2009, 48, 9052–9070. doi:10.1002/anie.200904689
Return to citation in text: [1] -
Canty, A. J.; Rodemann, T.; Ryan, J. H. Adv. Organomet. Chem. 2007, 55, 279–313. doi:10.1016/S0065-3055(07)55005-7
Return to citation in text: [1] -
Stirling, A. Chem. – Eur. J. 2018, 24, 1709–1713. doi:10.1002/chem.201705285
Return to citation in text: [1] -
Bumagin, N. A.; Luzikova, E. V.; Sukhomlinova, L. I.; Tolstoya, T. P.; Beletskaya, I. P. Russ. Chem. Bull. 1995, 44, 385–386. doi:10.1007/BF00702162
Return to citation in text: [1] -
Miralles, N.; Romero, R. M.; Fernández, E.; Muñiz, K. Chem. Commun. 2015, 51, 14068–14071. doi:10.1039/c5cc04944j
Return to citation in text: [1] -
Wang, M.; Wei, J.; Fan, Q.; Jiang, X. Chem. Commun. 2017, 53, 2918–2921. doi:10.1039/c6cc09201b
Return to citation in text: [1] -
Liu, L.; Qiang, J.; Bai, S.; Li, Y.; Li, J. Appl. Organomet. Chem. 2017, 31, e3810. doi:10.1002/aoc.3810
Return to citation in text: [1] -
Zeng, M.-T.; Xu, W.; Liu, X.; Chang, C.-Z.; Zhu, H.; Dong, Z.-B. Eur. J. Org. Chem. 2017, 6060–6066. doi:10.1002/ejoc.201701056
Return to citation in text: [1] -
Malmgren, J.; Santoro, S.; Jalalian, N.; Himo, F.; Olofsson, B. Chem. – Eur. J. 2013, 19, 10334–10342. doi:10.1002/chem.201300860
Return to citation in text: [1] -
Deprez, N. R.; Sanford, M. S. Inorg. Chem. 2007, 46, 1924–1935. doi:10.1021/ic0620337
Return to citation in text: [1] -
Liu, L.; Qiang, J.; Bai, S.-H.; Sung, H.-L.; Miao, C.-B.; Li, J. Adv. Synth. Catal. 2017, 359, 1283–1289. doi:10.1002/adsc.201601403
Return to citation in text: [1] -
Modha, S. G.; Greaney, M. F. J. Am. Chem. Soc. 2015, 137, 1416–1419. doi:10.1021/ja5124754
Return to citation in text: [1] -
Teskey, C. J.; Sohel, S. M. A.; Bunting, D. L.; Modha, S. G.; Greaney, M. F. Angew. Chem., Int. Ed. 2017, 56, 5263–5266. doi:10.1002/anie.201701523
Return to citation in text: [1] -
Modha, S. G.; Popescu, M. V.; Greaney, M. F. J. Org. Chem. 2017, 82, 11933–11938. doi:10.1021/acs.joc.7b01778
Return to citation in text: [1] -
Wu, Y.; Izquierdo, S.; Vidossich, P.; Lledós, A.; Shafir, A. Angew. Chem., Int. Ed. 2016, 55, 7152–7156. doi:10.1002/anie.201602569
Return to citation in text: [1] -
Grushin, V. V. Chem. Soc. Rev. 2000, 29, 315–324. doi:10.1039/a909041j
Return to citation in text: [1] -
Chatterjee, N.; Goswami, A. Eur. J. Org. Chem. 2017, 3023–3032. doi:10.1002/ejoc.201601651
Return to citation in text: [1] -
Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299–5358. doi:10.1021/cr800332c
Return to citation in text: [1] -
Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2002, 102, 2523–2584. doi:10.1021/cr010003+
Return to citation in text: [1] -
Pouységu, L.; Deffieux, D.; Quideau, S. Tetrahedron 2010, 66, 2235–2261. doi:10.1016/j.tet.2009.12.046
Return to citation in text: [1] -
Moreno, I.; Tellitu, I.; Herrero, M. T.; SanMartin, R.; Dominguez, E. Curr. Org. Synth. 2002, 6, 1433–1452. doi:10.2174/1385272023373383
Return to citation in text: [1] -
Jia, Z.; Gálvez, E.; Sebastián, R. M.; Pleixats, R.; Álvarez-Larena, Á.; Martin, E.; Vallribera, A.; Shafir, A. Angew. Chem., Int. Ed. 2014, 53, 11298–11301. doi:10.1002/anie.201405982
Return to citation in text: [1] -
Wu, Y.; Arenas, I.; Broomfield, L. M.; Martin, E.; Shafir, A. Chem. – Eur. J. 2015, 21, 18779–18784. doi:10.1002/chem.201503987
Return to citation in text: [1] -
Panda, N.; Mattan, I.; Nayak, D. K. J. Org. Chem. 2015, 80, 6590–6597. doi:10.1021/acs.joc.5b00634
Return to citation in text: [1] -
Xie, Y.; Zhong, J.; Li, Y.; Xiao, X.; Zou, L.; Liu, Y.; Zhang, K.; He, J. ChemistrySelect 2018, 3, 1655–1657. doi:10.1002/slct.201800197
Return to citation in text: [1] -
Bhattacherjee, D.; Thakur, V.; Shil, A. K.; Das, P. Adv. Synth. Catal. 2017, 359, 2202–2208. doi:10.1002/adsc.201700004
Return to citation in text: [1] -
Buendia, J.; Darses, B.; Dauban, P. Angew. Chem., Int. Ed. 2015, 54, 5697–5701. doi:10.1002/anie.201412364
Return to citation in text: [1] -
Buendia, J.; Grelier, G.; Darses, B.; Jarvis, A. G.; Taran, F.; Dauban, P. Angew. Chem., Int. Ed. 2016, 55, 7530–7533. doi:10.1002/anie.201602022
Return to citation in text: [1] -
Pothikumar, R.; Sujatha, C.; Namitharan, K. ACS Catal. 2017, 7, 7783–7787. doi:10.1021/acscatal.7b02819
Return to citation in text: [1] -
Brand, J. P.; Fernández González, D.; Nicolai, S.; Waser, J. Chem. Commun. 2011, 47, 102–115. doi:10.1039/c0cc02265a
Return to citation in text: [1] -
Zhdankin, V. V. Curr. Org. Synth. 2005, 2, 121–145. doi:10.2174/1570179052996982
Return to citation in text: [1] -
Le Vaillant, F.; Waser, J. Chimia 2017, 71, 226–230. doi:10.2533/chimia.2017.226
Return to citation in text: [1] -
Li, Y.; Hari, D. P.; Vita, M. V.; Waser, J. Angew. Chem., Int. Ed. 2016, 55, 4436–4454. doi:10.1002/anie.201509073
Return to citation in text: [1] -
Waser, J. Synlett 2016, 27, 2761–2773. doi:10.1055/s-0036-1589409
Return to citation in text: [1] -
Lu, B.; Wu, J.; Yoshikai, N. J. Am. Chem. Soc. 2014, 136, 11598–11601. doi:10.1021/ja5059795
Return to citation in text: [1] -
Hari, D. P.; Waser, J. J. Am. Chem. Soc. 2016, 138, 2190–2193. doi:10.1021/jacs.6b00278
Return to citation in text: [1] -
Hari, D. P.; Waser, J. J. Am. Chem. Soc. 2017, 139, 8420–8423. doi:10.1021/jacs.7b04756
Return to citation in text: [1] -
Bertho, S.; Rey-Rodriguez, R.; Colas, C.; Retailleau, P.; Gillaizeau, I. Chem. – Eur. J. 2017, 23, 17674–17677. doi:10.1002/chem.201704499
Return to citation in text: [1]
| 56. | Hari, D. P.; Waser, J. J. Am. Chem. Soc. 2016, 138, 2190–2193. doi:10.1021/jacs.6b00278 |
| 57. | Hari, D. P.; Waser, J. J. Am. Chem. Soc. 2017, 139, 8420–8423. doi:10.1021/jacs.7b04756 |
| 58. | Bertho, S.; Rey-Rodriguez, R.; Colas, C.; Retailleau, P.; Gillaizeau, I. Chem. – Eur. J. 2017, 23, 17674–17677. doi:10.1002/chem.201704499 |
| 1. | Trost, B. M. Science 1991, 254, 1471–1477. doi:10.1126/science.1962206 |
| 2. | Trost, B. M. Acc. Chem. Res. 2002, 35, 695–705. doi:10.1021/ar010068z |
| 35. | Wu, Y.; Izquierdo, S.; Vidossich, P.; Lledós, A.; Shafir, A. Angew. Chem., Int. Ed. 2016, 55, 7152–7156. doi:10.1002/anie.201602569 |
| 18. | Aradi, K.; Tóth, B.; Tolnai, G.; Novák, Z. Synlett 2016, 27, 1456–1485. doi:10.1055/s-0035-1561369 |
| 19. | Olofsson, B. Top. Curr. Chem. 2015, 373, 135–166. doi:10.1007/128_2015_661 |
| 20. | Yusubov, M. S.; Maskaev, A. V.; Zhdankin, V. V. ARKIVOC 2011, No. i, 370–409. doi:10.3998/ark.5550190.0012.107 |
| 21. | Merritt, E. A.; Olofsson, B. Angew. Chem., Int. Ed. 2009, 48, 9052–9070. doi:10.1002/anie.200904689 |
| 22. | Canty, A. J.; Rodemann, T.; Ryan, J. H. Adv. Organomet. Chem. 2007, 55, 279–313. doi:10.1016/S0065-3055(07)55005-7 |
| 3. | Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547 |
| 4. | Yusubov, M. S.; Zhdankin, V. V. Curr. Org. Synth. 2012, 9, 247–272. doi:10.2174/157017912799829021 |
| 16. | Keshavarz, M. Synlett 2011, 2433–2434. doi:10.1055/s-0030-1261234 |
| 17. | Dohi, T.; Kita, Y. Chem. Commun. 2009, 2073–2085. doi:10.1039/b821747e |
| 33. | Teskey, C. J.; Sohel, S. M. A.; Bunting, D. L.; Modha, S. G.; Greaney, M. F. Angew. Chem., Int. Ed. 2017, 56, 5263–5266. doi:10.1002/anie.201701523 |
| 3. | Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547 |
| 4. | Yusubov, M. S.; Zhdankin, V. V. Curr. Org. Synth. 2012, 9, 247–272. doi:10.2174/157017912799829021 |
| 5. | Zhdankin, V. V. ARKIVOC 2009, No. i, 1–62. doi:10.3998/ark.5550190.0010.101 |
| 6. | Wirth, T. Angew. Chem., Int. Ed. 2005, 44, 3656–3665. doi:10.1002/anie.200500115 |
| 7. | Moriarty, R. M. J. Org. Chem. 2005, 70, 2893–2903. doi:10.1021/jo050117b |
| 8. | Wirth, T. Synthesis 1999, 1271–1287. doi:10.1055/s-1999-3540 |
| 9. | Stang, P. J.; Zhdankin, V. V. Chem. Rev. 1996, 96, 1123–1178. doi:10.1021/cr940424+ |
| 10. | Stang, P. J. J. Org. Chem. 2003, 68, 2997–3008. doi:10.1021/jo030022e |
| 11. | Matveeva, E. D.; Proskurnina, M. V.; Zefirov, N. S. Heteroat. Chem. 2006, 17, 595–617. doi:10.1002/hc.20273 |
| 12. | Sousa e Silva, F. C.; Tierno, A. F.; Wengryniuk, S. E. Molecules 2017, 22, No. 780. doi:10.3390/molecules22050780 |
| 13. | Yoshimura, A.; Yusubov, M. S.; Zhdankin, V. V. Org. Biomol. Chem. 2016, 14, 4771–4781. doi:10.1039/C6OB00773B |
| 14. | Hypervalent Iodine Chemistry. In Topics in Current Chemistry; Wirth, T., Ed.; Springer International Publishing: Cham, 2016; Vol. 373. |
| 15. | Silva, L. F., Jr.; Olofsson, B. Nat. Prod. Rep. 2011, 28, 1722–1754. doi:10.1039/C1NP00028D |
| 34. | Modha, S. G.; Popescu, M. V.; Greaney, M. F. J. Org. Chem. 2017, 82, 11933–11938. doi:10.1021/acs.joc.7b01778 |
| 28. | Zeng, M.-T.; Xu, W.; Liu, X.; Chang, C.-Z.; Zhu, H.; Dong, Z.-B. Eur. J. Org. Chem. 2017, 6060–6066. doi:10.1002/ejoc.201701056 |
| 31. | Liu, L.; Qiang, J.; Bai, S.-H.; Sung, H.-L.; Miao, C.-B.; Li, J. Adv. Synth. Catal. 2017, 359, 1283–1289. doi:10.1002/adsc.201601403 |
| 26. | Wang, M.; Wei, J.; Fan, Q.; Jiang, X. Chem. Commun. 2017, 53, 2918–2921. doi:10.1039/c6cc09201b |
| 27. | Liu, L.; Qiang, J.; Bai, S.; Li, Y.; Li, J. Appl. Organomet. Chem. 2017, 31, e3810. doi:10.1002/aoc.3810 |
| 32. | Modha, S. G.; Greaney, M. F. J. Am. Chem. Soc. 2015, 137, 1416–1419. doi:10.1021/ja5124754 |
| 25. | Miralles, N.; Romero, R. M.; Fernández, E.; Muñiz, K. Chem. Commun. 2015, 51, 14068–14071. doi:10.1039/c5cc04944j |
| 24. | Bumagin, N. A.; Luzikova, E. V.; Sukhomlinova, L. I.; Tolstoya, T. P.; Beletskaya, I. P. Russ. Chem. Bull. 1995, 44, 385–386. doi:10.1007/BF00702162 |
| 29. | Malmgren, J.; Santoro, S.; Jalalian, N.; Himo, F.; Olofsson, B. Chem. – Eur. J. 2013, 19, 10334–10342. doi:10.1002/chem.201300860 |
| 30. | Deprez, N. R.; Sanford, M. S. Inorg. Chem. 2007, 46, 1924–1935. doi:10.1021/ic0620337 |
| 42. | Jia, Z.; Gálvez, E.; Sebastián, R. M.; Pleixats, R.; Álvarez-Larena, Á.; Martin, E.; Vallribera, A.; Shafir, A. Angew. Chem., Int. Ed. 2014, 53, 11298–11301. doi:10.1002/anie.201405982 |
| 43. | Wu, Y.; Arenas, I.; Broomfield, L. M.; Martin, E.; Shafir, A. Chem. – Eur. J. 2015, 21, 18779–18784. doi:10.1002/chem.201503987 |
| 37. | Chatterjee, N.; Goswami, A. Eur. J. Org. Chem. 2017, 3023–3032. doi:10.1002/ejoc.201601651 |
| 3. | Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547 |
| 9. | Stang, P. J.; Zhdankin, V. V. Chem. Rev. 1996, 96, 1123–1178. doi:10.1021/cr940424+ |
| 38. | Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299–5358. doi:10.1021/cr800332c |
| 39. | Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2002, 102, 2523–2584. doi:10.1021/cr010003+ |
| 40. | Pouységu, L.; Deffieux, D.; Quideau, S. Tetrahedron 2010, 66, 2235–2261. doi:10.1016/j.tet.2009.12.046 |
| 41. | Moreno, I.; Tellitu, I.; Herrero, M. T.; SanMartin, R.; Dominguez, E. Curr. Org. Synth. 2002, 6, 1433–1452. doi:10.2174/1385272023373383 |
| 3. | Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547 |
| 50. | Brand, J. P.; Fernández González, D.; Nicolai, S.; Waser, J. Chem. Commun. 2011, 47, 102–115. doi:10.1039/c0cc02265a |
| 51. | Zhdankin, V. V. Curr. Org. Synth. 2005, 2, 121–145. doi:10.2174/1570179052996982 |
| 52. | Le Vaillant, F.; Waser, J. Chimia 2017, 71, 226–230. doi:10.2533/chimia.2017.226 |
| 53. | Li, Y.; Hari, D. P.; Vita, M. V.; Waser, J. Angew. Chem., Int. Ed. 2016, 55, 4436–4454. doi:10.1002/anie.201509073 |
| 54. | Waser, J. Synlett 2016, 27, 2761–2773. doi:10.1055/s-0036-1589409 |
| 55. | Lu, B.; Wu, J.; Yoshikai, N. J. Am. Chem. Soc. 2014, 136, 11598–11601. doi:10.1021/ja5059795 |
| 48. | Buendia, J.; Grelier, G.; Darses, B.; Jarvis, A. G.; Taran, F.; Dauban, P. Angew. Chem., Int. Ed. 2016, 55, 7530–7533. doi:10.1002/anie.201602022 |
| 49. | Pothikumar, R.; Sujatha, C.; Namitharan, K. ACS Catal. 2017, 7, 7783–7787. doi:10.1021/acscatal.7b02819 |
| 46. | Bhattacherjee, D.; Thakur, V.; Shil, A. K.; Das, P. Adv. Synth. Catal. 2017, 359, 2202–2208. doi:10.1002/adsc.201700004 |
| 47. | Buendia, J.; Darses, B.; Dauban, P. Angew. Chem., Int. Ed. 2015, 54, 5697–5701. doi:10.1002/anie.201412364 |
| 44. | Panda, N.; Mattan, I.; Nayak, D. K. J. Org. Chem. 2015, 80, 6590–6597. doi:10.1021/acs.joc.5b00634 |
| 45. | Xie, Y.; Zhong, J.; Li, Y.; Xiao, X.; Zou, L.; Liu, Y.; Zhang, K.; He, J. ChemistrySelect 2018, 3, 1655–1657. doi:10.1002/slct.201800197 |
© 2018 Boelke et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)