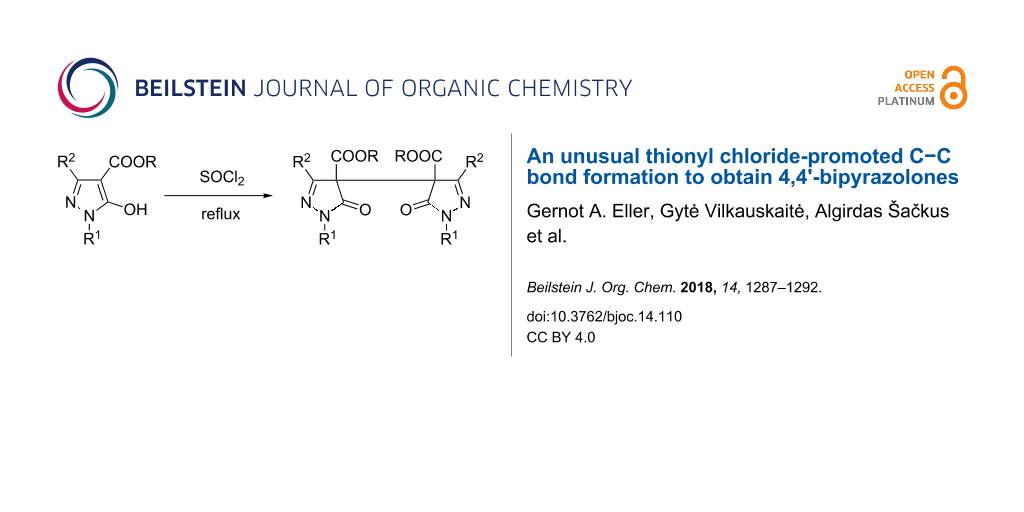
Abstract
Dialkyl 5,5'-dioxo-4,4'-bipyrazole-4,4'-dicarboxylates are readily obtained by the reaction of 5-hydroxypyrazole-4-carboxylates in refluxing thionyl chloride. The obtained diesters can be transformed into the corresponding 4,4'-bipyrazoles via alkaline hydrolysis and subsequent decarboxylation. Detailed NMR spectroscopic investigations (1H, 13C, 15N) were undertaken with all products prepared. Moreover, the structure of a representative 5,5'-dioxo-4,4'-bipyrazole-4,4'-dicarboxylate was confirmed by X-ray crystal structure analysis.
Graphical Abstract
Introduction
Many biologically active substances, therein several drug molecules, agrochemicals, dyestuffs, compounds for optoelectronic purposes, complexing ligands and more contain a pyrazole nucleus [1-8]. Condensed pyrazoles are of special interest, as a commonly used example the phosphodiesterase 5 (PDE5) inhibitor sildenafil (Viagra®) can be mentioned [1]. In a series of former publications we described the synthesis of condensed pyrazole systems using various 4,5-disubstituted pyrazole derivatives as precursors for the annellation reaction [9-16]. Amongst these precursors 5-chloropyrazoles carrying C-substituents at the pyrazole C4 position, like 5-chloropyrazole-4-carbaldehydes or 4-esters, turned out to be particularly useful due to the easy conversion of the chloro substituent into other functional groups or its nature as a good leaving group in ring-closure reactions. In this respect we were interested in a convenient access to 1-substituted or 1,3-disubstituted 5-chloropyrazole-4-carboxylates required as valuable precursors for further functionalizations. Such compounds have been mainly prepared from the corresponding 5-aminopyrazole-4-carboxylates via (non-aqueous) diazotation and subsequent reaction with appropriate chlorine sources [17,18]. Additionally, some years ago we have presented a synthetic approach upon Vilsmeier reaction of 1-phenylpyrazolones with DMF/excessive POCl3 to afford 5-chloropyrazole-4-carbaldehydes, which were oxidized to the corresponding acids (KMnO4) and subsequently converted into the ethyl esters by treatment with EtOH/H2SO4 [15]. However, as the latter approach is tedious and the former one uses toxic substances we envisaged to convert the easily available 5-hydroxypyrazole-4-carboxylates 1 into the corresponding 5-chloro derivatives 2 by the action of an appropriate chlorinating agent such as POCl3 or SOCl2 (Scheme 1). Such conversions of a hydroxy (oxo) into a chloro function is very common with many N-heterocyclic systems, such as, for instance the transformations of 2-pyridones into 2-chloropyridines or 3-pyridazinones into 3-chloropyridazines [19,20]. In the course of the preparation of substituted 6H-pyrazolo[4,3-d][1,2]oxazoles we thus obtained the required 4-benzoyl-5-chloropyrazoles by treatment of the relevant 4-benzoyl-5-hydroxypyrazoles with POCl3 [21].
Scheme 1: Envisaged approach for the synthesis of 5-chloropyrazole-4-carboxylates 2.
Scheme 1: Envisaged approach for the synthesis of 5-chloropyrazole-4-carboxylates 2.
Results and Discussion
Chemistry
However, the attempted reaction of ester 1a (R1 = Ph, R2 = H, R = Et) with POCl3 left the starting material untouched, similarly by treatment of 1a with oxalyl chloride no conversion occurred (Scheme 2). In contrast, treatment of 1a with excessive thionyl chloride at reflux temperature resulted in a defined reaction product which, however, could not be the desired 5-chloro derivative 2a according to – amongst others – a much too large chemical shift of pyrazole C5 (δ 165.6 ppm) compared to the expected one (δ 131.3 ppm) [15]. Moreover, the OCH2 protons revealed to be of diastereotopic character which hints to the presence of a chiral center in the molecule (Figure 1), while the molecular weight obtained by HRMS measurement ([M + Na]+ 485.1432) testified about the possible formation of a dimeric structure.
![[1860-5397-14-110-1]](/bjoc/content/figures/1860-5397-14-110-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: Signal of the OCH2 ester protons of reaction product 3a (500 MHz, CDCl3).
Figure 1: Signal of the OCH2 ester protons of reaction product 3a (500 MHz, CDCl3).
Lastly, by X-ray crystal structure analysis the obtained product could be determined as the dimeric structure 3a (Scheme 2, Figure 2). In addition, HRMS and elemental analysis confirmed the molecular formula. The non-equivalence of the OCH2 protons of the ester functions can be smoothly explained by the presence of an asymmetric carbon atom at pyrazole C4/C4'. As the NMR spectra displayed a single set of signals, regarding the stereochemistry a racemic mixture or the meso-form came into consideration.
![[1860-5397-14-110-2]](/bjoc/content/figures/1860-5397-14-110-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: ORTEP-plot of the crystal structure of compound 3a drawn with 50% displacement ellipsoids [(4S,4'S)-3a enantiomer is shown only]. The length of the C4–C4' bond connecting the two pyrazolone units is 1.544(3) Å. For details see the Supporting Information File 1.
Figure 2: ORTEP-plot of the crystal structure of compound 3a drawn with 50% displacement ellipsoids [(4S,4'S)-...
The single crystal X-ray analysis disclosed that the molecule of the newly obtained compound 3a consists of two pyrazolone residues, which are directly connected to each other by a single covalent carbon–carbon bond between the asymmetric sp3-hybridized C4 and C4' carbon atoms to form a species with relative (4R*,4'R*)-configuration (Figure 2). The bond length of the single C4–C4' bond is 1.544(3) Å, while the dihedral angle C5–C4–C4'–C5' is 47.94°. The packaging of the chiral molecules (4R,4'R)-3a and (4S,4'S)-3a into a racemic crystal occurs in such a way that mirror enantiomers are interconnected to each other by weak intermolecular hydrogen bonds (C–H···O 2.523 Å, 130.64°, Figure 3).
![[1860-5397-14-110-3]](/bjoc/content/figures/1860-5397-14-110-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Arrangement (4R,4'R)- and (4S,4'S)-3a enantiomers in the crystal unit drawn with 50% displacement ellipsoids. The hydrogen bonds are shown by dashed lines. Supplementary crystallographic data for compound 3a can be obtained free of charge from The Cambridge Crystallographic Data Centre via http://www.ccdc.cam.ac.uk/data_request/cif (CCDC 979625).
Figure 3: Arrangement (4R,4'R)- and (4S,4'S)-3a enantiomers in the crystal unit drawn with 50% displacement e...
In the following, related 5-hydroxypyrazol-4-carboxylates 1b–i were subjected to the same reaction conditions (refluxing SOCl2) and in all cases the corresponding dimers of type 3 were obtained in moderate to good yields (Scheme 3).
In order to check if these dimerization reactions also occur with other 5-hydroxypyrazoles carrying a C=O function at pyrazole C4 we subjected ketone 4 and hydrazide 5 to the same reaction conditions. In both cases, a plethora of unidentified products resulted (Scheme 4). In contrast, with aldehyde 6 a reaction product could be isolated in moderate yield, which can be assigned to structure 7 considering NMR data and mass spectra (Scheme 4).
Scheme 4: Reaction of different 5-hydroxypyrazoles with thionyl chloride.
Scheme 4: Reaction of different 5-hydroxypyrazoles with thionyl chloride.
The dehydrogenative homocoupling of 2-pyrazolin-5-ones (or pyrazolidin-5-ones) is well documented in the literature and proceeds under different reaction conditions such as, for instance, by air oxidation [22], under O2 atmosphere using an O2 balloon [23], by organic peroxides [24], phenoxy radicals [25], by treatment with phenylhydrazine at high temperatures [26,27], by nitrosation and subsequent heating [28], by heating with an aqueous NaHSO3 solution [29], and by photochemistry [30,31]. In nearly all cases these reactions have been carried out with 2-pyrazolin-5-ones unsubstituted at pyrazole C4 position or with derivatives carrying an alkyl or aryl substituent at the latter carbon atom. In contrast, only very few examples are described for pyrazolones with a C=O substructure attached to pyrazole C4. Thus, 3-methyl-1-phenyl-4-toluoyl-5-pyrazolone (= (5-hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)(4-methylphenyl)methanone) upon treatment with oxovanadium(V) compounds afforded the corresponding 2,2',4,4'-tetrahydro-3H,3'H-4,4'-bipyrazole-3,3'-dione, whereas it was shown by EPR spectroscopy and by cyclic voltammetry that the reaction obviously proceeds via a radical mechanism [32]. To the best of our knowledge, the only such dimeric species with ester functions at pyrazole C4, namely diethyl 1,1'-dimethyl-5,5'-dioxo-1,1',5,5'-tetrahydro-4H,4'H-4,4'-bipyrazole-4,4'-dicarboxylate (structure 3 with R1 = Me, R2 = H, R = Et) has been obtained – amongst other reaction products – by UV–vis irradiation of 4-ethoxy-2-methyl-5-morpholino-3(2H)-pyridazinone (emorfazone) in acetonitrile [31].
Moreover, we investigated the reaction of 1a with SO2Cl2. Here, two reaction products – 8 and 9 – were isolated, whereas in both cases chlorination not only at the pyrazole C4 but also in the 4-position of the phenyl ring took place (Scheme 5).
Scheme 5: Reaction of 1a with sulfuryl chloride.
Scheme 5: Reaction of 1a with sulfuryl chloride.
The reaction mechanism for the transformation 1 → 3 is unclear. Dimerization by air oxidation can be ruled out as performing the reaction under N2 atmosphere provided the same result. It should be mentioned that for the oxovanadium(V)-mediated dimerization of 4-aroyl-5-hydroxypyrazoles mentioned above a radical mechanism was postulated [32]. However, in Scheme 6 we propose a hypothetical mechanism comprising a redox cyclization of an intermediate di(pyrazolyl) sulfite under elimination of sulfur monoxide.
Scheme 6: Possible reaction mechanism for the transformation 1 → 3.
Scheme 6: Possible reaction mechanism for the transformation 1 → 3.
Finally, it was shown by means of some selected examples, that compounds of type 3 can be converted into the corresponding bipyrazoles 10 upon alkaline hydrolysis and subsequent decarboxylation (Scheme 7). According to the NMR spectra, compounds 10 are obviously present as 5-hydroxypyrazoles due to the absence of a proton attached to pyrazole C4.
NMR spectroscopic investigation
In Supporting Information File 1 the NMR spectroscopic data of all compounds treated within this study are indicated. Full and unambiguous assignment of 1H, 13C and nearly all 15N NMR resonances was achieved by combining standard NMR techniques [33], such as fully 1H-coupled 13C NMR spectra, APT, gs-HSQC, gs-HMBC, gs-HSQC-TOCSY, COSY, TOCSY and NOESY spectroscopy. Figure 4 shows the thus assigned 1H, 13C and 15N NMR chemical shifts for model compound 3a.
Figure 4: 1H NMR (italics), 13C NMR (normal letters) and 15N NMR (in bold) chemical shifts of 3a (in CDCl3).
Figure 4: 1H NMR (italics), 13C NMR (normal letters) and 15N NMR (in bold) chemical shifts of 3a (in CDCl3).
Conclusion
The reaction of 5-hydroxypyrazole-4-carboxylates 1 with thionyl chloride does not lead to the corresponding 5-chloropyrazole congeners but induces dimerization to afford the relevant dialkyl 5,5'-dioxo-4,4'-bipyrazole-4,4'-dicarboxylates of type 3.
Supporting Information
| Supporting Information File 1: Experimental details and compound characterization. | ||
| Format: PDF | Size: 795.1 KB | Download |
Acknowledgements
Ashenafi Damtew Mamuye thanks the University of Sassari (Italy) for awarding a Ph.D. visiting grant for a stay at the University of Vienna (Austria). The authors are grateful to Mr. S. Belyakov (Latvian Institute of Organic Synthesis, Riga) for performing the X-ray analysis and to one of the referees for assistance with the proposed reaction mechanism.
References
-
Kleemann, A.; Engel, J.; Kutscher, B.; Reichert, D. Pharmaceutical Substances, 3rd ed.; Thieme: Stuttgart/New York, Germany/USA, 1999.
Return to citation in text: [1] [2] -
Elguero, J.; Goya, P.; Jagerovic, N.; Silva, A. M. S. Pyrazoles as drugs: facts and fantasies. In Targets In Heterocyclic Systems: Chemistry and Properties; Attanasi, O. A.; Spinelli, D., Eds.; Italian Society of Chemistry: Rome, 2002; Vol. 6, p 52.
Return to citation in text: [1] -
Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. Chem. Rev. 2011, 111, 6984–7034. doi:10.1021/cr2000459
Return to citation in text: [1] -
Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman. New J. Chem. 2017, 41, 16–41. doi:10.1039/C6NJ03181A
Return to citation in text: [1] -
Pèrez-Fernández, R.; Goya, P.; Elguero, J. ARKIVOC 2014, No. ii, 233–293. doi:10.3998/ark.5550190.p008.131
Return to citation in text: [1] -
Schmidt, A.; Dreger, A. Curr. Org. Chem. 2011, 15, 1423–1463. doi:10.2174/138527211795378263
Return to citation in text: [1] -
Lamberth, C. Heterocycles 2007, 71, 1467–1502. doi:10.3987/REV-07-613
Return to citation in text: [1] -
Elguero, J. Pyrazoles and their Benzo Derivatives. In Comprehensive Heterocyclic Chemistry; Katrizky, A. R.; Rees, C. W., Eds.; Pergamon: Oxford, 1984; Vol. 5, pp 167–303.
Return to citation in text: [1] -
Milišiūnaitė, V.; Arbačiauskienė, E.; Bieliauskas, A.; Vilkauskaitė, G.; Šačkus, A.; Holzer, W. Tetrahedron 2015, 71, 3385–3395. doi:10.1016/j.tet.2015.03.092
Return to citation in text: [1] -
Vilkauskaitė, G.; Schaaf, P.; Šačkus, A.; Krystof, V.; Holzer, W. ARKIVOC 2014, No. ii, 135–149. doi:10.3998/ark.5550190.p008.188
Return to citation in text: [1] -
Palka, B.; Di Capua, A.; Anzini, M.; Vilkauskaitė, G.; Šačkus, A.; Holzer, W. Beilstein J. Org. Chem. 2014, 10, 1759–1764. doi:10.3762/bjoc.10.183
Return to citation in text: [1] -
Holzer, W.; Vilkauskaitė, G.; Arbačiauskienė, E.; Šačkus, A. Beilstein J. Org. Chem. 2012, 8, 2223–2229. doi:10.3762/bjoc.8.251
Return to citation in text: [1] -
Arbačiauskienė, E.; Vilkauskaitė, G.; Šačkus, A.; Holzer, W. Eur. J. Org. Chem. 2011, 1880–1890. doi:10.1002/ejoc.201001560
Return to citation in text: [1] -
Eller, G. A.; Vilkauskaitė, G.; Arbačiauskienė, E.; Šačkus, A.; Holzer, W. Synth. Commun. 2011, 41, 541–547. doi:10.1080/00397911003629382
Return to citation in text: [1] -
Datterl, B.; Tröstner, N.; Kucharski, D.; Holzer, W. Molecules 2010, 15, 6106–6126. doi:10.3390/molecules15096106
Return to citation in text: [1] [2] [3] -
Fuchs, F. C.; Eller, G. A.; Holzer, W. Molecules 2009, 14, 3814–3832. doi:10.3390/molecules14093814
Return to citation in text: [1] -
Beck, J. R.; Gajewski, R. P.; Lynch, M. P.; Wright, F. L. J. Heterocycl. Chem. 1987, 24, 267–270. doi:10.1002/jhet.5570240151
Return to citation in text: [1] -
Yamamoto, S.; Morimoto, K.; Sato, T. J. Heterocycl. Chem. 1991, 28, 1545–1547. doi:10.1002/jhet.5570280613
Return to citation in text: [1] -
Comins, D. L.; Joseph, S. P. Pyridines and their Benzo Derivatives: Reactivity at the Ring. In Comprehensive Heterocyclic Chemistry II; Katrizky, A. R.; Rees, C. W.; Scriven, E. F. V., Eds.; Pergamon: Oxford, 1997; Vol. 5, p 63.
Return to citation in text: [1] -
Haider, N.; Holzer, W. Sci. Synth. 2004, 16, 125–249.
Return to citation in text: [1] -
Holzer, W.; Hahn, K. J. Heterocycl. Chem. 2003, 40, 303–308. doi:10.1002/jhet.5570400216
Return to citation in text: [1] -
Veibel, S. Acta Chem. Scand. 1972, 26, 3685–3690. doi:10.3891/acta.chem.scand.26-3685
Return to citation in text: [1] -
Sheng, X.; Zhang, J.; Yang, H.; Jiang, G. Org. Lett. 2017, 19, 2618–2621. doi:10.1021/acs.orglett.7b00951
Return to citation in text: [1] -
Xue, F.; Bao, X.; Zou, L.; Qu, J.; Wang, B. Adv. Synth. Catal. 2016, 358, 3971–3976. doi:10.1002/adsc.201601070
Return to citation in text: [1] -
Schulz, M.; Meske, M. J. Prakt. Chem./Chem.-Ztg. 1993, 335, 607–615. doi:10.1002/prac.19933350706
Return to citation in text: [1] -
Knorr, L.; Haber, F. Ber. Dtsch. Chem. Ges. 1894, 27, 1151–1167. doi:10.1002/cber.189402701233
Return to citation in text: [1] -
Bernstein, J.; Stearns, B.; Dexter, M.; Lott, W. A. J. Am. Chem. Soc. 1947, 69, 1147–1150. doi:10.1021/ja01197a047
Return to citation in text: [1] -
Hüttel, R.; Authaler, A. Chem. Ber. 1963, 96, 2879–2893. doi:10.1002/cber.19630961109
Return to citation in text: [1] -
Li, Z.; Chu, G.; Zeng, Y.; Zhu, L.; Ye, H.; Huang, H.; Li, W.; Chao, Y.; Yu, L. Chin. Pat. CN 10218083 A, Sept 14, 2011.
Chem. Abstr. 2011, 1173728.
Return to citation in text: [1] -
Eğe, S. N.; Tien, C. J.; Dlesk, A.; Potter, B. E.; Eagleson, B. K. J. Chem. Soc., Chem. Commun. 1972, 682–683. doi:10.1039/C39720000682
Return to citation in text: [1] -
Maki, Y.; Shimada, K.; Sako, M.; Kitade, Y.; Hirota, K. Chem. Pharm. Bull. 1988, 36, 1714–1720. doi:10.1248/cpb.36.1714
Return to citation in text: [1] [2] -
Remya, P. N.; Suresh, C. H.; Reddy, M. L. P. Polyhedron 2007, 26, 5016–5022. doi:10.1016/j.poly.2007.07.020
Return to citation in text: [1] [2] -
Braun, S.; Kalinowski, H.-O.; Berger, S. 150 and More Basic NMR Experiments: A Practical Course – Second Expanded Edition; Wiley–VCH: Weinheim, Germany, 1998.
Return to citation in text: [1]
| 32. | Remya, P. N.; Suresh, C. H.; Reddy, M. L. P. Polyhedron 2007, 26, 5016–5022. doi:10.1016/j.poly.2007.07.020 |
| 32. | Remya, P. N.; Suresh, C. H.; Reddy, M. L. P. Polyhedron 2007, 26, 5016–5022. doi:10.1016/j.poly.2007.07.020 |
| 31. | Maki, Y.; Shimada, K.; Sako, M.; Kitade, Y.; Hirota, K. Chem. Pharm. Bull. 1988, 36, 1714–1720. doi:10.1248/cpb.36.1714 |
| 1. | Kleemann, A.; Engel, J.; Kutscher, B.; Reichert, D. Pharmaceutical Substances, 3rd ed.; Thieme: Stuttgart/New York, Germany/USA, 1999. |
| 2. | Elguero, J.; Goya, P.; Jagerovic, N.; Silva, A. M. S. Pyrazoles as drugs: facts and fantasies. In Targets In Heterocyclic Systems: Chemistry and Properties; Attanasi, O. A.; Spinelli, D., Eds.; Italian Society of Chemistry: Rome, 2002; Vol. 6, p 52. |
| 3. | Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. Chem. Rev. 2011, 111, 6984–7034. doi:10.1021/cr2000459 |
| 4. | Ansari, A.; Ali, A.; Asif, M.; Shamsuzzaman. New J. Chem. 2017, 41, 16–41. doi:10.1039/C6NJ03181A |
| 5. | Pèrez-Fernández, R.; Goya, P.; Elguero, J. ARKIVOC 2014, No. ii, 233–293. doi:10.3998/ark.5550190.p008.131 |
| 6. | Schmidt, A.; Dreger, A. Curr. Org. Chem. 2011, 15, 1423–1463. doi:10.2174/138527211795378263 |
| 7. | Lamberth, C. Heterocycles 2007, 71, 1467–1502. doi:10.3987/REV-07-613 |
| 8. | Elguero, J. Pyrazoles and their Benzo Derivatives. In Comprehensive Heterocyclic Chemistry; Katrizky, A. R.; Rees, C. W., Eds.; Pergamon: Oxford, 1984; Vol. 5, pp 167–303. |
| 15. | Datterl, B.; Tröstner, N.; Kucharski, D.; Holzer, W. Molecules 2010, 15, 6106–6126. doi:10.3390/molecules15096106 |
| 29. |
Li, Z.; Chu, G.; Zeng, Y.; Zhu, L.; Ye, H.; Huang, H.; Li, W.; Chao, Y.; Yu, L. Chin. Pat. CN 10218083 A, Sept 14, 2011.
Chem. Abstr. 2011, 1173728. |
| 17. | Beck, J. R.; Gajewski, R. P.; Lynch, M. P.; Wright, F. L. J. Heterocycl. Chem. 1987, 24, 267–270. doi:10.1002/jhet.5570240151 |
| 18. | Yamamoto, S.; Morimoto, K.; Sato, T. J. Heterocycl. Chem. 1991, 28, 1545–1547. doi:10.1002/jhet.5570280613 |
| 30. | Eğe, S. N.; Tien, C. J.; Dlesk, A.; Potter, B. E.; Eagleson, B. K. J. Chem. Soc., Chem. Commun. 1972, 682–683. doi:10.1039/C39720000682 |
| 31. | Maki, Y.; Shimada, K.; Sako, M.; Kitade, Y.; Hirota, K. Chem. Pharm. Bull. 1988, 36, 1714–1720. doi:10.1248/cpb.36.1714 |
| 9. | Milišiūnaitė, V.; Arbačiauskienė, E.; Bieliauskas, A.; Vilkauskaitė, G.; Šačkus, A.; Holzer, W. Tetrahedron 2015, 71, 3385–3395. doi:10.1016/j.tet.2015.03.092 |
| 10. | Vilkauskaitė, G.; Schaaf, P.; Šačkus, A.; Krystof, V.; Holzer, W. ARKIVOC 2014, No. ii, 135–149. doi:10.3998/ark.5550190.p008.188 |
| 11. | Palka, B.; Di Capua, A.; Anzini, M.; Vilkauskaitė, G.; Šačkus, A.; Holzer, W. Beilstein J. Org. Chem. 2014, 10, 1759–1764. doi:10.3762/bjoc.10.183 |
| 12. | Holzer, W.; Vilkauskaitė, G.; Arbačiauskienė, E.; Šačkus, A. Beilstein J. Org. Chem. 2012, 8, 2223–2229. doi:10.3762/bjoc.8.251 |
| 13. | Arbačiauskienė, E.; Vilkauskaitė, G.; Šačkus, A.; Holzer, W. Eur. J. Org. Chem. 2011, 1880–1890. doi:10.1002/ejoc.201001560 |
| 14. | Eller, G. A.; Vilkauskaitė, G.; Arbačiauskienė, E.; Šačkus, A.; Holzer, W. Synth. Commun. 2011, 41, 541–547. doi:10.1080/00397911003629382 |
| 15. | Datterl, B.; Tröstner, N.; Kucharski, D.; Holzer, W. Molecules 2010, 15, 6106–6126. doi:10.3390/molecules15096106 |
| 16. | Fuchs, F. C.; Eller, G. A.; Holzer, W. Molecules 2009, 14, 3814–3832. doi:10.3390/molecules14093814 |
| 26. | Knorr, L.; Haber, F. Ber. Dtsch. Chem. Ges. 1894, 27, 1151–1167. doi:10.1002/cber.189402701233 |
| 27. | Bernstein, J.; Stearns, B.; Dexter, M.; Lott, W. A. J. Am. Chem. Soc. 1947, 69, 1147–1150. doi:10.1021/ja01197a047 |
| 1. | Kleemann, A.; Engel, J.; Kutscher, B.; Reichert, D. Pharmaceutical Substances, 3rd ed.; Thieme: Stuttgart/New York, Germany/USA, 1999. |
| 28. | Hüttel, R.; Authaler, A. Chem. Ber. 1963, 96, 2879–2893. doi:10.1002/cber.19630961109 |
| 22. | Veibel, S. Acta Chem. Scand. 1972, 26, 3685–3690. doi:10.3891/acta.chem.scand.26-3685 |
| 24. | Xue, F.; Bao, X.; Zou, L.; Qu, J.; Wang, B. Adv. Synth. Catal. 2016, 358, 3971–3976. doi:10.1002/adsc.201601070 |
| 15. | Datterl, B.; Tröstner, N.; Kucharski, D.; Holzer, W. Molecules 2010, 15, 6106–6126. doi:10.3390/molecules15096106 |
| 25. | Schulz, M.; Meske, M. J. Prakt. Chem./Chem.-Ztg. 1993, 335, 607–615. doi:10.1002/prac.19933350706 |
| 21. | Holzer, W.; Hahn, K. J. Heterocycl. Chem. 2003, 40, 303–308. doi:10.1002/jhet.5570400216 |
| 33. | Braun, S.; Kalinowski, H.-O.; Berger, S. 150 and More Basic NMR Experiments: A Practical Course – Second Expanded Edition; Wiley–VCH: Weinheim, Germany, 1998. |
| 19. | Comins, D. L.; Joseph, S. P. Pyridines and their Benzo Derivatives: Reactivity at the Ring. In Comprehensive Heterocyclic Chemistry II; Katrizky, A. R.; Rees, C. W.; Scriven, E. F. V., Eds.; Pergamon: Oxford, 1997; Vol. 5, p 63. |
| 20. | Haider, N.; Holzer, W. Sci. Synth. 2004, 16, 125–249. |
| 23. | Sheng, X.; Zhang, J.; Yang, H.; Jiang, G. Org. Lett. 2017, 19, 2618–2621. doi:10.1021/acs.orglett.7b00951 |
© 2018 Eller et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)