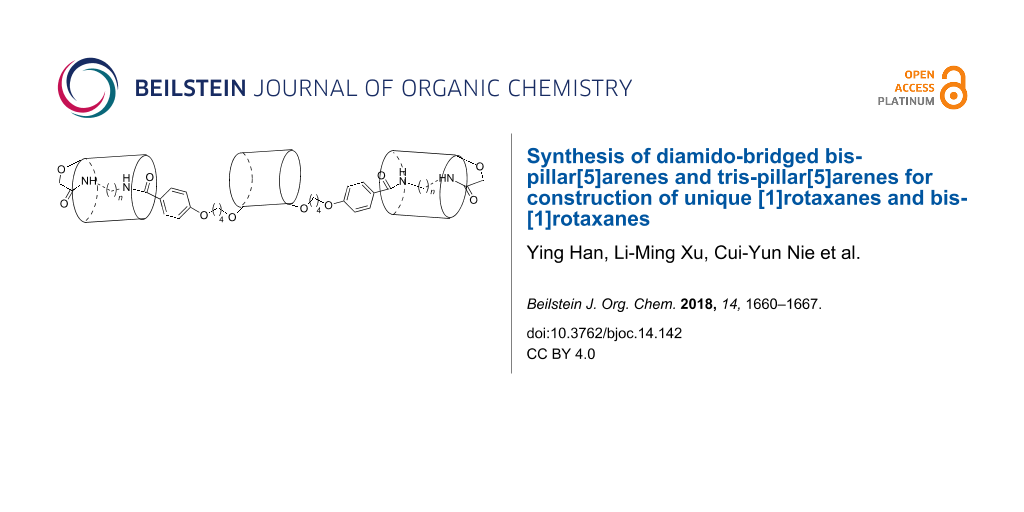
Abstract
The pillar[5]arene mono- and di(oxyalkoxy)benzoic acids were successfully prepared in high yields by sequential alkylation of ω-bromoalkoxy-substituted pillar[5]arenes with methyl or ethyl p-hydroxybenzoate followed by a hydrolytic reaction under basic conditions. Under catalysis of HOBt/EDCl, the amidation reaction of pillar[5]arene mono(oxybutoxy)benzoic acid with monoamido-functionalized pillar[5]arenes afforded diamido-bridged bis-pillar[5]arenes. 1H NMR and 2D NOESY spectra clearly indicated that [1]rotaxanes were formed by insertion of longer diaminoalkylene unit into the cavity of one pillar[5]arene with another pillar[5]arene acting as a stopper. The similar catalysed amidation reaction of pillar[5]arene di(oxybutoxy)benzoic acid with monoamido-functionalized pillar[5]arenes resulted in the diamido-bridged tris-pillar[5]arenes, which successfully form the unique bis-[1]rotaxanes bearing longer than diaminopropylene diamido bridges.
Graphical Abstract
Introduction
The construction and dynamic motion of the mechanically interlocked molecules (MIMs) have attracted significant research interests due to their intrinsic self-assembled nature and potential applications in various aspects [1-4]. Pseudo[1]rotaxane and [1]rotaxane are one of particular supramolecular assembly system and are considered as an important building block in the construction of diverse MIMs [5-10]. [1]Rotaxane has a macrocyclic wheel component connected with a self-locked chain axle, and a bulky stopper at the terminal axle to prevent dissociation of the subcomponents. In recent years, many effects have been devoted to the construction and functionalization of pseudo[1]rotaxanes and [1]rotaxanes [11-20]. For this purpose, the well-known macrocycles such as crown ether [21-23], cyclodextrin [24-26], calixarene [27-29] and pillararene have been successfully employed as the wheel subcomponent. Pillararenes are new star macrocyclic compounds with aromatic rings para-bridged by methylene units and have unique tubular shape rather than cone [30-32]. In recent years, an explosive development on the construction of various supramolecular devices and diverse responsive materials has been reported by using diverse functionalized pillararenes [33-35]. Due to easily preparation and suitable cavity, functionalized pillar[5]arenes were widely used as wheel component for constructing of the various interlocked molecules [36-42]. In the past few years, many elegant works on the construction of pseudo[1]rotaxanes and [1]rotaxanes have been developed on the basis of various mono-functionalized pillar[5]arenes [43-57]. Recently, we have successfully constructed a couple of pseudo[1]rotaxane and [1]rotaxane both in solution and in solid state developed by using mono-functionalized pillar[5]arene Schiff base, urea and pyridylimine derivatives [58-63]. In continuation of our effort on the development on the construction of [1]rotaxanes via various mono-functionalized pillar[5]arene derivatives, herein we wish to report the convenient synthesis of diamido-bridged bis-pillar[5]arenes and tris-pillar[5]arenes as well as formation of unique [1]rotaxanes and bis-[1]rotaxanes.
Results and Discussion
The synthetic route for the pillar[5]arene mono(oxyalkoxy)benzoic acids was illustrated in Scheme 1. Firstly, the alkylation of mono(bromoalkoxy)pillar[5]arene 1a–c (n = 4, 5, 6) [64] with methyl or ethyl p-hydroxybenzoate was carried out in the refluxed medium of KI/K2CO3/CH3CN for one day. The pillar[5]arene mono(oxyalkoxy)benzoates 2a–f were successfully prepared in high yields. Then, basic hydrolysis of pillar[5]arene mono(oxyalkoxy)benzoates 2a–f in ethanol in the presence of potassium hydroxide afforded the desired pillar[5]arene mono(oxyalkoxy)benzoic acids 3a–c. The structures of the prepared pillar[5]arenes 2a–f and 3a–c were fully characterized by the spectroscopic methods. The single crystal structures of the pillar[5]arenes 2a (Figure 1), 2c, 2d, 2e (Supporting Information File 1, Figure S1–S3) and 2f (Figure 2) were successfully determined by X-ray diffraction. The same structural feature was obtained in the five single crystals. That is, the longer chain of methyl (ethyl) oxyalkoxybenzoate not only does not inserted in the cavity of the pillar[5]arene to form the pseudo[1]rotaxane, but also does not thread to the cavity of the neighbouring pillar[5]arene to form the supramolecular polymer. This result is consistent to the Cao’s previously reported results in the series of pillar[5]arenes bearing aliphatic esters [49], in that they found the chain of methyl oxybutyrate did not threaded into the cavity of pillar[5]arene.
Scheme 1: Synthesis of pillar[5]arene mono(oxyalkoxy)benzoic acids 3a–c.
Scheme 1: Synthesis of pillar[5]arene mono(oxyalkoxy)benzoic acids 3a–c.
![[1860-5397-14-142-1]](/bjoc/content/figures/1860-5397-14-142-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: single crystal structure of pillar[5]arene 2a.
Figure 1: single crystal structure of pillar[5]arene 2a.
![[1860-5397-14-142-2]](/bjoc/content/figures/1860-5397-14-142-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Single crystal structure of pillar[5]arene 2f.
Figure 2: Single crystal structure of pillar[5]arene 2f.
The above synthetic pillar[5]arene mono(oxyalkoxy)benzoic acids have a longer chain functionalized group and a large macrocycle, which enabled them to be a good candidate as an efficient terminal stopper for the construction of rotaxanes. Therefore, the amidation reaction of pillar[5]arene mono(oxybutoxy)benzoic acid 3a with our previously reported amido-functionalized pillar[5]arenes 4a–d (n = 2, 3, 4, 6) [58] was carried out in chloroform under the combined catalysis of 1-hydroxybenzotrizole (HOBt) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCl). The reaction proceeded smoothly to give diamido-bridged bis-pillar[5]arenes 5a–d (n = 2, 3, 4, 6) in moderate yields (Scheme 2). It has been reported that the chain of N-(ω-aminoalkyl)oxyacetamide inserted in the cavity of pillar[5]arene in the amido-functionalized pillar[5]arene 4a–d (n = 2, 3, 4, 6) to form pseudo[1]rotaxanes both in solution and in solid state [58]. The diamido-bridged bis-pillar[5]arenes 5a–d might form the expected [1]rotaxanes. 1H NMR spectrum of the bis-pillar[5]arenes 5a clearly showed that there is no any signals at very high magnetic field (δ < 0), which indicated that the diaminoethylene chain does not inserted in the cavity of pillar[5]arene to form the expected [1]rotaxane. Therefore, the two moieties of pillar[5]arenes are just connected by the diaminoethylene chain from the outside in diamido-bridged bis-pillar[5]arenes 5a. However, a couple of characteristic signals at very high magnetic field can be seen in the 1H NMR spectra of the bis-pillar[5]arenes 5b–d. There is a broad singlet at −1.82 ppm in 5b, a mixed peak at −1.88 to −2.14 ppm in 5c and several peaks in the range of 0.07 to −2.07 ppm in 5d. This result clearly displayed that the unique [1]rotaxane structures were actually formed by threading the longer diaminoalkylene bridge in the cavity of one molecular pillar[5]arene, while another pillar[5]arene as the bigger stopper. Additionally, 2D NOESY spectra of the compound 5d provided more strong evidence for the formation of [1]rotaxane (Figure 3). The NOE correlations were clearly observed between Ha, Hb, Hc, Hd, and He protons of the bridging hexylene chain with the proton Hf in the core of pillar[5]arene. The proton Hb of the bridging hexylene chain also correlated with protons of the aromatic protons Hg and Hf.
Scheme 2: Synthesis of diamido-bridged bis-pillar[5]arenes 5a–d.
Scheme 2: Synthesis of diamido-bridged bis-pillar[5]arenes 5a–d.
![[1860-5397-14-142-3]](/bjoc/content/figures/1860-5397-14-142-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: The 2D NOSEY spectrum of bis-pillar[5]arene 5d.
Figure 3: The 2D NOSEY spectrum of bis-pillar[5]arene 5d.
According to similar reaction procedure for the synthesis of pillar[5]arene mono(oxyalkoxy)benzoic acids 3a–c, pillar[5]arene di(oxybutoxy)benzoic acid 8 was prepared in moderate yield from sequential alkylation and basic hydrolysis reaction (Scheme 3). The single crystal structure of the pillar[5]arene di(oxybutoxy)benzoate 7 showed that the two chains of methyl oxybutoxybenzoate did not insert in the cavity of pillar[5]arene (Figure 4) as that of the above mentioned pillar[5]arene mono(oxybutoxy)benzoates 2a–f. The two chains straight stretched to the opposite direction of central pillar[5]arene. It might be attribute to the electron-rich effect of the methyl oxybutoxybenzoate unit, which kept it away from the electron-rich cavity of pillar[5]arene.
Scheme 3: Synthesis of pillar[5]arene di(oxyalkoxy)benzoic acid 8.
Scheme 3: Synthesis of pillar[5]arene di(oxyalkoxy)benzoic acid 8.
![[1860-5397-14-142-4]](/bjoc/content/figures/1860-5397-14-142-4.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 4: Single crystal structure of pillar[5]arene 7.
Figure 4: Single crystal structure of pillar[5]arene 7.
Under the combined catalysis of HOBT and EDCl, the amide reaction of pillar[5]arene di(oxybutoxy)benzoic acid 8a with two molecular amido-functionalized pillar[5]arenes 4a–d in chloroform afforded tris-pillar[5]arenes 9a–d in moderate yields (Scheme 4). The structures of the synthetic tris-pillar[5]arenes 9a–d were fully characterized by IR, HRMS, 1H and 13C NMR spectra. The 1H NMR spectra provided stronger evidence for the formation of fascinating bis-[1]rotaxanes. Because there are no peaks with negative chemical shift in the 1H NMR spectra of the tris-pillar[5]arene 9a, it can be concluded that the three pillar[5]arenes are connected from the outsides by two diamidoethylene-bridges. There is one broad peak at −1.80 ppm in tris-pillar[5]arene 9b, a mixed peak at −2.00 ppm in tris-pillar[5]arene 9c, and five broad peaks at −0.29 ppm, −0.74 ppm, −0.97 ppm, −1.62 ppm and −2.08 ppm in tris-pillar[5]arene 9d. Therefore, 1H NMR spectra of 9b–d indicated that the diaminoalkylene chain ambiguously inserted in the cavity of the pillar[5]arene. In other words, the fascinating bis-[1]rotaxane structures were formed in the tris-pillar[5]arenes 9b–d. Here, the lengths of bridging chains played the critical role in the selflocked behaviour of pillar[5]arene-based [1]rotaxanes.
Scheme 4: Synthesis of diamido-bridged tris-pillar[5]arenes 9a–d.
Scheme 4: Synthesis of diamido-bridged tris-pillar[5]arenes 9a–d.
In order to confirm the formation of the bis-[1]rotaxanes, 2D NOESY spectra of the compounds 9a–d were recorded. The 2D NOESY spectrum of compound 9d was showed in Figure 5. There it can be seen that the NOE correlations were clearly observed between Ha, Hb, Hc, Hd, Hf, Hg, Hh protons of the bridging diaminohexylene chain and the protons Hi, Hj in the core of pillar[5]arene. Additionally, some correlations exists between protons Ha, He, Hd and Hh and active amino (N–H) group. These NOE correlations clearly indicated the two bridged diaminohexylene chain threading into the cavity of the two pillar[5]arenes to form the bis-[1]rotaxane. The similar correlations were also observed in the NOESY spectra of the tris-pillar[5]arene 9b and 9c (see Supporting Information File 1, Figures S5 and S6). However, there is no such correlation in the 2D NOESY spectrum of the compound 9a (see Supporting Information File 1, Figure S4), which confirmed that the diamidoethylene bridge did not insert to the cavity of the pillar[5]arene to form [1]rotaxanes. Thus, the 2D NOESY spectra provided stronger evidence for the formation of novel bis-[1]rotaxanes for the tris-pillar[5]arenes 9c–d bearing longer than diaminopropylene diamido-bridges.
![[1860-5397-14-142-5]](/bjoc/content/figures/1860-5397-14-142-5.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 5: The 2D NOSEY spectra of tris-pillar[5]arene 9d.
Figure 5: The 2D NOSEY spectra of tris-pillar[5]arene 9d.
Conclusion
In summary, we have conveniently prepared several pillar[5]arene mono- and di(oxyalkoxy)benzoic acids and found that the chain of alkyl oxyalkoxybenzoate did not inserted to the cavity of pillar[5]arene. More importantly, a series of diamido-bridged bis-pillar[5]arenes and tris-pillar[5]arenes were efficiently synthesized by catalyzed amidation reaction of pillar[5]arene mono- and di(oxybutoxy)benzoic acids with monoamide-functionalized pillar[5]arenes. On the basis of 1H NMR and 2D NOESY spectra, we successfully concluded that the chains longer than diaminopropylene threaded into the one or two cavities of the pillar[5]arenes to form the unique [1]rotaxane and bis-[1]rotaxanes. This work not only provided a fundamental self-assembly of the mechanically interlocked molecules, but also developed the potential applications of pillar[5]arene in supramolecular chemistry. The design and construction of diverse mechanically interlocked molecules are underway in our laboratory.
Supporting Information
Experimental procedures, analytical data, and copies of the 1H and 13C NMR spectra, HRMS spectra for all new products. Single crystal data for 2a (CCDC: 1837205), 2c (CCDC: 1837206), 2d (CCDC: 1837207), 2e (CCDC: 1837208), 2f (CCDC: 1837209) and 7 (CCDC: 1846692) have been deposited at the Cambridge Crystallographic Data Centre.
| Supporting Information File 1: Experimental and analytical data. | ||
| Format: PDF | Size: 2.0 MB | Download |
References
-
Wenz, G.; Han, B. H.; Müller, A. Chem. Rev. 2006, 106, 782–817. doi:10.1021/cr970027+
Return to citation in text: [1] -
Erbas-Cakmak, S.; Leigh, D. A.; McTernan, C. T.; Nussbaumer, A. L. Chem. Rev. 2015, 115, 10081–10206. doi:10.1021/acs.chemrev.5b00146
Return to citation in text: [1] -
Fahrenbach, A. C.; Bruns, C. J.; Li, H.; Trabolsi, A.; Coskun, A.; Stoddart, J. F. Acc. Chem. Res. 2014, 47, 482–493. doi:10.1021/ar400161z
Return to citation in text: [1] -
Bruns, C. J.; Stoddart, J. F. Acc. Chem. Res. 2014, 47, 2186–2199. doi:10.1021/ar500138u
Return to citation in text: [1] -
Langton, M. J.; Beer, P. D. Acc. Chem. Res. 2014, 47, 1935–1949. doi:10.1021/ar500012a
Return to citation in text: [1] -
Zhang, M.; Yan, X.; Huang, F.; Niu, Z.; Gibson, H. W. Acc. Chem. Res. 2014, 47, 1995–2005. doi:10.1021/ar500046r
Return to citation in text: [1] -
Qu, D.-H.; Wang, Q.-C.; Zhang, Q.-W.; Ma, X.; Tian, H. Chem. Rev. 2015, 115, 7543–7588. doi:10.1021/cr5006342
Return to citation in text: [1] -
Xue, M.; Yang, Y.; Chi, X.; Yan, X.; Huang, F. Chem. Rev. 2015, 115, 7398–7501. doi:10.1021/cr5005869
Return to citation in text: [1] -
Ogoshi, T.; Yamagishi, T.-a.; Nakamoto, Y. Chem. Rev. 2016, 116, 7937–8002. doi:10.1021/acs.chemrev.5b00765
Return to citation in text: [1] -
Wang, Y.; Ping, G.; Li, C. Chem. Commun. 2016, 52, 9858–9872. doi:10.1039/C6CC03999E
Return to citation in text: [1] -
Ma, X.; Tian, H. Chem. Soc. Rev. 2010, 39, 70–80. doi:10.1039/B901710K
Return to citation in text: [1] -
Lewis, J. E. M.; Galli, M.; Goldup, S. M. Chem. Commun. 2017, 53, 298–312. doi:10.1039/C6CC07377H
Return to citation in text: [1] -
Roberts, D. A.; Pilgrim, B. S.; Nitschke, J. R. Chem. Soc. Rev. 2018, 47, 626–644. doi:10.1039/C6CS00907G
Return to citation in text: [1] -
Han, X.; Liu, G.; Liu, S. H.; Yin, J. Org. Biomol. Chem. 2016, 14, 10331–10351. doi:10.1039/C6OB01581F
Return to citation in text: [1] -
Lewis, J. E. M.; Beer, P. D.; Loeb, S. J.; Goldup, S. M. Chem. Soc. Rev. 2017, 46, 2577–2591. doi:10.1039/C7CS00199A
Return to citation in text: [1] -
Li, S.-H.; Zhang, H.-Y.; Xu, X.; Liu, Y. Nat. Commun. 2015, 6, No. 7590. doi:10.1038/ncomms8590
Return to citation in text: [1] -
Chi, X.; Yu, G.; Shao, L.; Chen, J.; Huang, F. J. Am. Chem. Soc. 2016, 138, 3168–3174. doi:10.1021/jacs.5b13173
Return to citation in text: [1] -
Eichstaedt, K.; Jaramillo-Garcia, J.; Leigh, D. A.; Marcos, V.; Pisano, S.; Singleton, T. A. J. Am. Chem. Soc. 2017, 139, 9376–9381. doi:10.1021/jacs.7b04955
Return to citation in text: [1] -
De Bo, G.; Dolphijn, G.; McTernan, C. T.; Leigh, D. A. J. Am. Chem. Soc. 2017, 139, 8455–8457. doi:10.1021/jacs.7b05640
Return to citation in text: [1] -
Wang, Y.; Sun, J.; Liu, Z.; Nassar, M. S.; Botros, Y. Y.; Stoddart, J. F. Chem. Sci. 2017, 8, 2562–2568. doi:10.1039/C6SC05035B
Return to citation in text: [1] -
Hiratani, K.; Kaneyama, M.; Nagawa, Y.; Koyama, E.; Kanesato, M. J. Am. Chem. Soc. 2004, 126, 13568–13569. doi:10.1021/ja046929r
Return to citation in text: [1] -
Ogawa, T.; Nakazono, K.; Aoki, D.; Uchida, S.; Takata, T. ACS Macro Lett. 2015, 4, 343–347. doi:10.1021/acsmacrolett.5b00067
Return to citation in text: [1] -
Ogawa, T.; Usuki, N.; Nakazono, K.; Koyama, Y.; Takata, T. Chem. Commun. 2015, 51, 5606–5609. doi:10.1039/C4CC08982K
Return to citation in text: [1] -
Xue, Z.; Mayer, M. F. J. Am. Chem. Soc. 2010, 132, 3274–3276. doi:10.1021/ja9077655
Return to citation in text: [1] -
Waelés, P.; Clavel, C.; Fournel-Marotte, K.; Coutrot, F. Chem. Sci. 2015, 6, 4828–4836. doi:10.1039/C5SC01722J
Return to citation in text: [1] -
Schröder, H. V.; Wollschläger, J. M.; Schalley, C. A. Chem. Commun. 2017, 53, 9218–9221. doi:10.1039/C7CC05259F
Return to citation in text: [1] -
Li, H.; Zhang, H.; Zhang, Q.; Zhang, Q.-W.; Qu, D.-H. Org. Lett. 2012, 14, 5900–5903. doi:10.1021/ol302826g
Return to citation in text: [1] -
Li, H.; Zhang, J.-N.; Zhou, W.; Zhang, H.; Zhang, Q.; Qu, D.-H.; Tian, H. Org. Lett. 2013, 15, 3070–3073. doi:10.1021/ol401251u
Return to citation in text: [1] -
Li, H.; Li, X.; Agren, H.; Qu, D.-H. Org. Lett. 2014, 16, 4940–4943. doi:10.1021/ol502466x
Return to citation in text: [1] -
Ma, X.; Qu, D.; Ji, F.; Wang, Q.; Zhu, L.; Xu, Y.; Tian, H. Chem. Commun. 2007, 1409–1411. doi:10.1039/b615900a
Return to citation in text: [1] -
Ma, X.; Wang, Q.; Tian, H. Tetrahedron Lett. 2007, 48, 7112–7116. doi:10.1016/j.tetlet.2007.07.209
Return to citation in text: [1] -
Yamauchi, K.; Miyawaki, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. Org. Lett. 2010, 12, 1284–1286. doi:10.1021/ol1001736
Return to citation in text: [1] -
Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.-a.; Nakamoto, Y. J. Am. Chem. Soc. 2008, 130, 5022–5023. doi:10.1021/ja711260m
Return to citation in text: [1] -
Cao, D.; Kou, Y.; Liang, J.; Chen, Z.; Wang, L.; Meier, H. Angew. Chem., Int. Ed. 2009, 48, 9721–9723. doi:10.1002/anie.200904765
Return to citation in text: [1] -
Liu, Z.; Nalluri, S. K. M.; Stoddart, J. F. Chem. Soc. Rev. 2017, 46, 2459–2478. doi:10.1039/C7CS00185A
Return to citation in text: [1] -
Zhang, H.; Strutt, N. L.; Stoll, R. S.; Li, H.; Zhu, Z.; Stoddart, J. F. Chem. Commun. 2011, 47, 11420–11422. doi:10.1039/c1cc14934b
Return to citation in text: [1] -
Zhang, H.; Liu, Z.; Xin, F.; Hao, A. Chin. J. Org. Chem. 2012, 32, 219–229. doi:10.6023/cjoc1107141
Return to citation in text: [1] -
Zhang, H.; Zhao, Y. Chem. – Eur. J. 2013, 19, 16862–16879. doi:10.1002/chem.201301635
Return to citation in text: [1] -
Zhang, H.; Ma, X.; Nguyen, K. T.; Zhao, Y. ACS Nano 2013, 7, 7853–7863. doi:10.1021/nn402777x
Return to citation in text: [1] -
Zhang, H.; Ma, X.; Guo, J.; Nguyen, K. T.; Zhang, Q.; Wang, X.-J.; Yan, H.; Zhu, L.; Zhao, Y. RSC Adv. 2013, 3, 368–371. doi:10.1039/C2RA22123C
Return to citation in text: [1] -
Zhang, H.; Nguyen, K. T.; Ma, X.; Yan, H.; Guo, J.; Zhu, L.; Zhao, Y. Org. Biomol. Chem. 2013, 11, 2070–2074. doi:10.1039/c2ob27340c
Return to citation in text: [1] -
Zhang, H.; Ma, X.; Nguyen, K. T.; Zeng, Y.; Tai, S.; Zhao, Y. ChemPlusChem 2014, 79, 462–469. doi:10.1002/cplu.201300408
Return to citation in text: [1] -
Chen, H.; Fan, J.; Hu, X.; Ma, J.; Wang, S.; Li, J.; Yu, Y.; Jia, X.; Li, C. Chem. Sci. 2015, 6, 197–202. doi:10.1039/C4SC02422B
Return to citation in text: [1] -
Ma, J.; Meng, Q.; Hu, X.; Li, B.; Ma, S.; Hu, B.; Li, J.; Jia, X.; Li, C. Org. Lett. 2016, 18, 5740–5743. doi:10.1021/acs.orglett.6b03005
Return to citation in text: [1] -
Sun, Y.; Fu, W.; Chen, C.; Wang, J.; Yao, Y. Chem. Commun. 2017, 53, 3725–3728. doi:10.1039/C7CC00291B
Return to citation in text: [1] -
Li, B.; Meng, Z.; Li, Q.; Huang, X.; Kang, Z.; Dong, H.; Chen, J.; Sun, J.; Dong, Y.; Li, J.; Jia, X.; Sessler, J. L.; Meng, Q.; Li, C. Chem. Sci. 2017, 8, 4458–4464. doi:10.1039/C7SC01438D
Return to citation in text: [1] -
Ping, G.; Wang, Y.; Shen, L.; Wang, Y.; Hu, X.; Chen, J.; Hu, B.; Cui, L.; Meng, Q.; Li, C. Chem. Commun. 2017, 53, 7381–7384. doi:10.1039/C7CC02799K
Return to citation in text: [1] -
Ogoshi, T.; Demachi, K.; Kitajima, K.; Yamagishi, T.-a. Chem. Commun. 2011, 47, 7164–7166. doi:10.1039/c1cc12333e
Return to citation in text: [1] -
Chen, Y.; Cao, D.; Wang, L.; He, M.; Zhou, L.; Schollmeyer, D.; Meier, H. Chem. – Eur. J. 2013, 19, 7064–7070. doi:10.1002/chem.201204628
Return to citation in text: [1] [2] -
Xia, B.; Xue, M. Chem. Commun. 2014, 50, 1021–1023. doi:10.1039/C3CC48014C
Return to citation in text: [1] -
Ni, M.; Hu, X.-Y.; Jiang, J.; Wang, L. Chem. Commun. 2014, 50, 1317–1319. doi:10.1039/C3CC47823H
Return to citation in text: [1] -
Guan, Y.; Liu, P.; Deng, C.; Ni, M.; Xiong, S.; Lin, C.; Hu, X.-Y.; Ma, J.; Wang, L. Org. Biomol. Chem. 2014, 12, 1079–1089. doi:10.1039/c3ob42044b
Return to citation in text: [1] -
Wu, X.; Ni, M.; Xia, W.; Hu, X.-Y.; Wang, L. Org. Chem. Front. 2015, 2, 1013–1017. doi:10.1039/C5QO00159E
Return to citation in text: [1] -
Wu, X.; Gao, L.; Sun, J.; Hu, X.-Y.; Wang, L. Chin. Chem. Lett. 2016, 27, 1655–1660. doi:10.1016/j.cclet.2016.05.004
Return to citation in text: [1] -
Sun, C.-L.; Xu, J.-F.; Chen, Y.-Z.; Niu, L.-Y.; Wu, L.-Z.; Tung, C.-H.; Yang, Q.-Z. Chin. Chem. Lett. 2015, 26, 843–846. doi:10.1016/j.cclet.2015.05.030
Return to citation in text: [1] -
Du, X.-S.; Wang, C.-Y.; Jia, Q.; Deng, R.; Tian, H.-S.; Zhang, H.-Y.; Meguellati, K.; Yang, Y.-W. Chem. Commun. 2017, 53, 5326–5329. doi:10.1039/C7CC02364B
Return to citation in text: [1] -
Cheng, M.; Wang, Q.; Cao, Y.; Pan, Y.; Yang, Z.; Jiang, J.; Wang, L. Tetrahedron Lett. 2016, 57, 4133–4137. doi:10.1016/j.tetlet.2016.07.038
Return to citation in text: [1] -
Han, Y.; Huo, G.-F.; Sun, J.; Xie, J.; Yan, C.-G.; Zhao, Y.; Wu, X.; Lin, C.; Wang, L. Sci. Rep. 2016, 6, No. 28748. doi:10.1038/srep28748
Return to citation in text: [1] [2] [3] -
Huo, G.-F.; Han, Y.; Sun, J.; Yan, C.-G. J. Inclusion Phenom. Macrocyclic Chem. 2016, 86, 231–240. doi:10.1007/s10847-016-0652-x
Return to citation in text: [1] -
Han, Y.; Huo, G.-F.; Sun, J.; Yan, C.-G.; Lu, Y.; Lin, C.; Wang, L. Supramol. Chem. 2017, 29, 547–552. doi:10.1080/10610278.2017.1287367
Return to citation in text: [1] -
Jiang, S.; Han, Y.; Sun, J.; Yan, C.-G. Tetrahedron 2017, 73, 5107–5114. doi:10.1016/j.tet.2017.07.001
Return to citation in text: [1] -
Jiang, S.; Han, Y.; Zhao, L.-L.; Sun, J.; Yan, C.-G. Supramol. Chem. 2018, 30, 642–647. doi:10.1080/10610278.2018.1427238
Return to citation in text: [1] -
Jiang, S.; Han, Y.; Cheng, M.; Sun, J.; Yan, C.-G.; Jiang, J.; Wang, L. New J. Chem. 2018, 42, 7603–7606. doi:10.1039/c7nj05192a
Return to citation in text: [1] -
Yin, C.-B.; Han, Y.; Huo, G.-F.; Sun, J.; Yan, C.-G. Chin. Chem. Lett. 2017, 28, 431–436. doi:10.1016/j.cclet.2016.09.008
Return to citation in text: [1]
| 1. | Wenz, G.; Han, B. H.; Müller, A. Chem. Rev. 2006, 106, 782–817. doi:10.1021/cr970027+ |
| 2. | Erbas-Cakmak, S.; Leigh, D. A.; McTernan, C. T.; Nussbaumer, A. L. Chem. Rev. 2015, 115, 10081–10206. doi:10.1021/acs.chemrev.5b00146 |
| 3. | Fahrenbach, A. C.; Bruns, C. J.; Li, H.; Trabolsi, A.; Coskun, A.; Stoddart, J. F. Acc. Chem. Res. 2014, 47, 482–493. doi:10.1021/ar400161z |
| 4. | Bruns, C. J.; Stoddart, J. F. Acc. Chem. Res. 2014, 47, 2186–2199. doi:10.1021/ar500138u |
| 24. | Xue, Z.; Mayer, M. F. J. Am. Chem. Soc. 2010, 132, 3274–3276. doi:10.1021/ja9077655 |
| 25. | Waelés, P.; Clavel, C.; Fournel-Marotte, K.; Coutrot, F. Chem. Sci. 2015, 6, 4828–4836. doi:10.1039/C5SC01722J |
| 26. | Schröder, H. V.; Wollschläger, J. M.; Schalley, C. A. Chem. Commun. 2017, 53, 9218–9221. doi:10.1039/C7CC05259F |
| 58. | Han, Y.; Huo, G.-F.; Sun, J.; Xie, J.; Yan, C.-G.; Zhao, Y.; Wu, X.; Lin, C.; Wang, L. Sci. Rep. 2016, 6, No. 28748. doi:10.1038/srep28748 |
| 21. | Hiratani, K.; Kaneyama, M.; Nagawa, Y.; Koyama, E.; Kanesato, M. J. Am. Chem. Soc. 2004, 126, 13568–13569. doi:10.1021/ja046929r |
| 22. | Ogawa, T.; Nakazono, K.; Aoki, D.; Uchida, S.; Takata, T. ACS Macro Lett. 2015, 4, 343–347. doi:10.1021/acsmacrolett.5b00067 |
| 23. | Ogawa, T.; Usuki, N.; Nakazono, K.; Koyama, Y.; Takata, T. Chem. Commun. 2015, 51, 5606–5609. doi:10.1039/C4CC08982K |
| 11. | Ma, X.; Tian, H. Chem. Soc. Rev. 2010, 39, 70–80. doi:10.1039/B901710K |
| 12. | Lewis, J. E. M.; Galli, M.; Goldup, S. M. Chem. Commun. 2017, 53, 298–312. doi:10.1039/C6CC07377H |
| 13. | Roberts, D. A.; Pilgrim, B. S.; Nitschke, J. R. Chem. Soc. Rev. 2018, 47, 626–644. doi:10.1039/C6CS00907G |
| 14. | Han, X.; Liu, G.; Liu, S. H.; Yin, J. Org. Biomol. Chem. 2016, 14, 10331–10351. doi:10.1039/C6OB01581F |
| 15. | Lewis, J. E. M.; Beer, P. D.; Loeb, S. J.; Goldup, S. M. Chem. Soc. Rev. 2017, 46, 2577–2591. doi:10.1039/C7CS00199A |
| 16. | Li, S.-H.; Zhang, H.-Y.; Xu, X.; Liu, Y. Nat. Commun. 2015, 6, No. 7590. doi:10.1038/ncomms8590 |
| 17. | Chi, X.; Yu, G.; Shao, L.; Chen, J.; Huang, F. J. Am. Chem. Soc. 2016, 138, 3168–3174. doi:10.1021/jacs.5b13173 |
| 18. | Eichstaedt, K.; Jaramillo-Garcia, J.; Leigh, D. A.; Marcos, V.; Pisano, S.; Singleton, T. A. J. Am. Chem. Soc. 2017, 139, 9376–9381. doi:10.1021/jacs.7b04955 |
| 19. | De Bo, G.; Dolphijn, G.; McTernan, C. T.; Leigh, D. A. J. Am. Chem. Soc. 2017, 139, 8455–8457. doi:10.1021/jacs.7b05640 |
| 20. | Wang, Y.; Sun, J.; Liu, Z.; Nassar, M. S.; Botros, Y. Y.; Stoddart, J. F. Chem. Sci. 2017, 8, 2562–2568. doi:10.1039/C6SC05035B |
| 49. | Chen, Y.; Cao, D.; Wang, L.; He, M.; Zhou, L.; Schollmeyer, D.; Meier, H. Chem. – Eur. J. 2013, 19, 7064–7070. doi:10.1002/chem.201204628 |
| 5. | Langton, M. J.; Beer, P. D. Acc. Chem. Res. 2014, 47, 1935–1949. doi:10.1021/ar500012a |
| 6. | Zhang, M.; Yan, X.; Huang, F.; Niu, Z.; Gibson, H. W. Acc. Chem. Res. 2014, 47, 1995–2005. doi:10.1021/ar500046r |
| 7. | Qu, D.-H.; Wang, Q.-C.; Zhang, Q.-W.; Ma, X.; Tian, H. Chem. Rev. 2015, 115, 7543–7588. doi:10.1021/cr5006342 |
| 8. | Xue, M.; Yang, Y.; Chi, X.; Yan, X.; Huang, F. Chem. Rev. 2015, 115, 7398–7501. doi:10.1021/cr5005869 |
| 9. | Ogoshi, T.; Yamagishi, T.-a.; Nakamoto, Y. Chem. Rev. 2016, 116, 7937–8002. doi:10.1021/acs.chemrev.5b00765 |
| 10. | Wang, Y.; Ping, G.; Li, C. Chem. Commun. 2016, 52, 9858–9872. doi:10.1039/C6CC03999E |
| 58. | Han, Y.; Huo, G.-F.; Sun, J.; Xie, J.; Yan, C.-G.; Zhao, Y.; Wu, X.; Lin, C.; Wang, L. Sci. Rep. 2016, 6, No. 28748. doi:10.1038/srep28748 |
| 36. | Zhang, H.; Strutt, N. L.; Stoll, R. S.; Li, H.; Zhu, Z.; Stoddart, J. F. Chem. Commun. 2011, 47, 11420–11422. doi:10.1039/c1cc14934b |
| 37. | Zhang, H.; Liu, Z.; Xin, F.; Hao, A. Chin. J. Org. Chem. 2012, 32, 219–229. doi:10.6023/cjoc1107141 |
| 38. | Zhang, H.; Zhao, Y. Chem. – Eur. J. 2013, 19, 16862–16879. doi:10.1002/chem.201301635 |
| 39. | Zhang, H.; Ma, X.; Nguyen, K. T.; Zhao, Y. ACS Nano 2013, 7, 7853–7863. doi:10.1021/nn402777x |
| 40. | Zhang, H.; Ma, X.; Guo, J.; Nguyen, K. T.; Zhang, Q.; Wang, X.-J.; Yan, H.; Zhu, L.; Zhao, Y. RSC Adv. 2013, 3, 368–371. doi:10.1039/C2RA22123C |
| 41. | Zhang, H.; Nguyen, K. T.; Ma, X.; Yan, H.; Guo, J.; Zhu, L.; Zhao, Y. Org. Biomol. Chem. 2013, 11, 2070–2074. doi:10.1039/c2ob27340c |
| 42. | Zhang, H.; Ma, X.; Nguyen, K. T.; Zeng, Y.; Tai, S.; Zhao, Y. ChemPlusChem 2014, 79, 462–469. doi:10.1002/cplu.201300408 |
| 58. | Han, Y.; Huo, G.-F.; Sun, J.; Xie, J.; Yan, C.-G.; Zhao, Y.; Wu, X.; Lin, C.; Wang, L. Sci. Rep. 2016, 6, No. 28748. doi:10.1038/srep28748 |
| 59. | Huo, G.-F.; Han, Y.; Sun, J.; Yan, C.-G. J. Inclusion Phenom. Macrocyclic Chem. 2016, 86, 231–240. doi:10.1007/s10847-016-0652-x |
| 60. | Han, Y.; Huo, G.-F.; Sun, J.; Yan, C.-G.; Lu, Y.; Lin, C.; Wang, L. Supramol. Chem. 2017, 29, 547–552. doi:10.1080/10610278.2017.1287367 |
| 61. | Jiang, S.; Han, Y.; Sun, J.; Yan, C.-G. Tetrahedron 2017, 73, 5107–5114. doi:10.1016/j.tet.2017.07.001 |
| 62. | Jiang, S.; Han, Y.; Zhao, L.-L.; Sun, J.; Yan, C.-G. Supramol. Chem. 2018, 30, 642–647. doi:10.1080/10610278.2018.1427238 |
| 63. | Jiang, S.; Han, Y.; Cheng, M.; Sun, J.; Yan, C.-G.; Jiang, J.; Wang, L. New J. Chem. 2018, 42, 7603–7606. doi:10.1039/c7nj05192a |
| 33. | Ogoshi, T.; Kanai, S.; Fujinami, S.; Yamagishi, T.-a.; Nakamoto, Y. J. Am. Chem. Soc. 2008, 130, 5022–5023. doi:10.1021/ja711260m |
| 34. | Cao, D.; Kou, Y.; Liang, J.; Chen, Z.; Wang, L.; Meier, H. Angew. Chem., Int. Ed. 2009, 48, 9721–9723. doi:10.1002/anie.200904765 |
| 35. | Liu, Z.; Nalluri, S. K. M.; Stoddart, J. F. Chem. Soc. Rev. 2017, 46, 2459–2478. doi:10.1039/C7CS00185A |
| 64. | Yin, C.-B.; Han, Y.; Huo, G.-F.; Sun, J.; Yan, C.-G. Chin. Chem. Lett. 2017, 28, 431–436. doi:10.1016/j.cclet.2016.09.008 |
| 30. | Ma, X.; Qu, D.; Ji, F.; Wang, Q.; Zhu, L.; Xu, Y.; Tian, H. Chem. Commun. 2007, 1409–1411. doi:10.1039/b615900a |
| 31. | Ma, X.; Wang, Q.; Tian, H. Tetrahedron Lett. 2007, 48, 7112–7116. doi:10.1016/j.tetlet.2007.07.209 |
| 32. | Yamauchi, K.; Miyawaki, A.; Takashima, Y.; Yamaguchi, H.; Harada, A. Org. Lett. 2010, 12, 1284–1286. doi:10.1021/ol1001736 |
| 27. | Li, H.; Zhang, H.; Zhang, Q.; Zhang, Q.-W.; Qu, D.-H. Org. Lett. 2012, 14, 5900–5903. doi:10.1021/ol302826g |
| 28. | Li, H.; Zhang, J.-N.; Zhou, W.; Zhang, H.; Zhang, Q.; Qu, D.-H.; Tian, H. Org. Lett. 2013, 15, 3070–3073. doi:10.1021/ol401251u |
| 29. | Li, H.; Li, X.; Agren, H.; Qu, D.-H. Org. Lett. 2014, 16, 4940–4943. doi:10.1021/ol502466x |
| 43. | Chen, H.; Fan, J.; Hu, X.; Ma, J.; Wang, S.; Li, J.; Yu, Y.; Jia, X.; Li, C. Chem. Sci. 2015, 6, 197–202. doi:10.1039/C4SC02422B |
| 44. | Ma, J.; Meng, Q.; Hu, X.; Li, B.; Ma, S.; Hu, B.; Li, J.; Jia, X.; Li, C. Org. Lett. 2016, 18, 5740–5743. doi:10.1021/acs.orglett.6b03005 |
| 45. | Sun, Y.; Fu, W.; Chen, C.; Wang, J.; Yao, Y. Chem. Commun. 2017, 53, 3725–3728. doi:10.1039/C7CC00291B |
| 46. | Li, B.; Meng, Z.; Li, Q.; Huang, X.; Kang, Z.; Dong, H.; Chen, J.; Sun, J.; Dong, Y.; Li, J.; Jia, X.; Sessler, J. L.; Meng, Q.; Li, C. Chem. Sci. 2017, 8, 4458–4464. doi:10.1039/C7SC01438D |
| 47. | Ping, G.; Wang, Y.; Shen, L.; Wang, Y.; Hu, X.; Chen, J.; Hu, B.; Cui, L.; Meng, Q.; Li, C. Chem. Commun. 2017, 53, 7381–7384. doi:10.1039/C7CC02799K |
| 48. | Ogoshi, T.; Demachi, K.; Kitajima, K.; Yamagishi, T.-a. Chem. Commun. 2011, 47, 7164–7166. doi:10.1039/c1cc12333e |
| 49. | Chen, Y.; Cao, D.; Wang, L.; He, M.; Zhou, L.; Schollmeyer, D.; Meier, H. Chem. – Eur. J. 2013, 19, 7064–7070. doi:10.1002/chem.201204628 |
| 50. | Xia, B.; Xue, M. Chem. Commun. 2014, 50, 1021–1023. doi:10.1039/C3CC48014C |
| 51. | Ni, M.; Hu, X.-Y.; Jiang, J.; Wang, L. Chem. Commun. 2014, 50, 1317–1319. doi:10.1039/C3CC47823H |
| 52. | Guan, Y.; Liu, P.; Deng, C.; Ni, M.; Xiong, S.; Lin, C.; Hu, X.-Y.; Ma, J.; Wang, L. Org. Biomol. Chem. 2014, 12, 1079–1089. doi:10.1039/c3ob42044b |
| 53. | Wu, X.; Ni, M.; Xia, W.; Hu, X.-Y.; Wang, L. Org. Chem. Front. 2015, 2, 1013–1017. doi:10.1039/C5QO00159E |
| 54. | Wu, X.; Gao, L.; Sun, J.; Hu, X.-Y.; Wang, L. Chin. Chem. Lett. 2016, 27, 1655–1660. doi:10.1016/j.cclet.2016.05.004 |
| 55. | Sun, C.-L.; Xu, J.-F.; Chen, Y.-Z.; Niu, L.-Y.; Wu, L.-Z.; Tung, C.-H.; Yang, Q.-Z. Chin. Chem. Lett. 2015, 26, 843–846. doi:10.1016/j.cclet.2015.05.030 |
| 56. | Du, X.-S.; Wang, C.-Y.; Jia, Q.; Deng, R.; Tian, H.-S.; Zhang, H.-Y.; Meguellati, K.; Yang, Y.-W. Chem. Commun. 2017, 53, 5326–5329. doi:10.1039/C7CC02364B |
| 57. | Cheng, M.; Wang, Q.; Cao, Y.; Pan, Y.; Yang, Z.; Jiang, J.; Wang, L. Tetrahedron Lett. 2016, 57, 4133–4137. doi:10.1016/j.tetlet.2016.07.038 |
© 2018 Han et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)