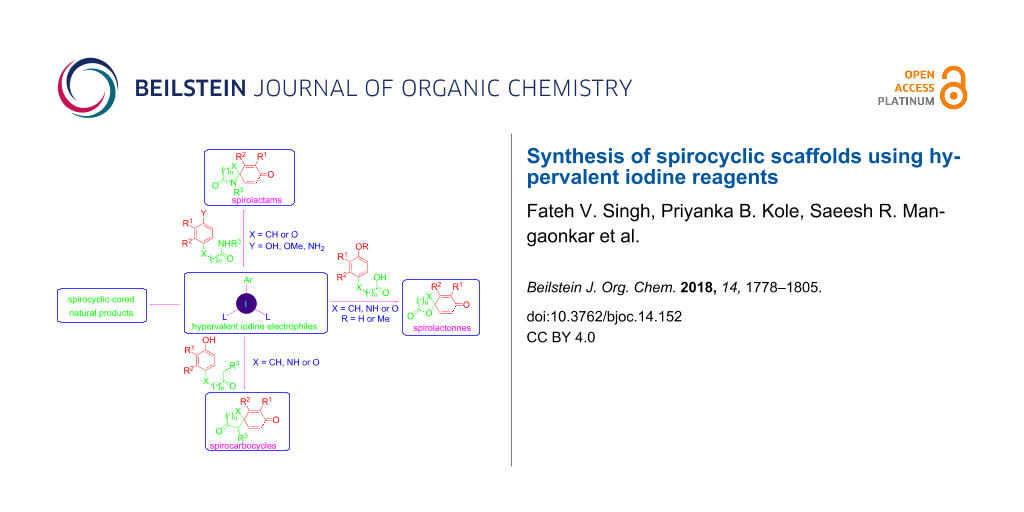
Abstract
Hypervalent iodine reagents have been developed as highly valuable reagents in synthetic organic chemistry during the past few decades. These reagents have been identified as key replacements of various toxic heavy metals in organic synthesis. Various synthetically and biologically important scaffolds have been developed using hypervalent iodine reagents either in stoichiometric or catalytic amounts. In addition, hypervalent iodine reagents have been employed for the synthesis of spirocyclic scaffolds via dearomatization processes. In this review, various approaches for the synthesis of spirocyclic scaffolds using hypervalent iodine reagents are covered including their stereoselective synthesis. Additionally, the applications of these reagents in natural product synthesis are also covered.
Graphical Abstract
Review
1. Introduction
The chemistry of spirocyclic compounds is a well established research area of organic and medicinal chemistry [1-5]. These scaffolds are common structural motifs found in various classes of naturally occurring systems [6-8]. More importantly, various natural and synthetic products containing a spirocyclic ring are currently used as commercial drugs for the treatment of several health problems [9,10]. Annosqualine (1) is an isoquinoline-cored alkaloid and it was isolated in 2004 from the stem of Annona squamosa [11] (Figure 1).
Figure 1: The structures of biologically active natural and synthetic products having spirocyclic moiety.
Figure 1: The structures of biologically active natural and synthetic products having spirocyclic moiety.
Griseofulvin (2) is a spirobenzofuranone-based naturally occurring compound which was isolated from Penicillium griseofulvum in 1939 [12]. In 1959, it was launched in the market as antifungal agent for the treatment of ringworm in human beings and animals [4,13]. Stepharine (3) is a member of the proaporphine alkaloid family and isolated from an angiosperm Stephania glabra [14]. Tofogliflozin (4) is a synthetic spirocyclic glycoside that was launched as antidiabetic agent in 2012 in Japan [15]. Rolapitant (5) is a marketed drug that was approved in 2015 for the treatment of nausea and vomiting [16]. Compound 6 is a spiropyrimidinetrione analogue which is currently in clinical trials for the treatment of gonorrhea [17]. There are several ways available in literature for the synthesis of spirocyclic compounds but most of them are associated either with transition metals or hypervalent iodine reagents [1-3].
Hypervalent iodine reagents provide various functional group transformation opportunities in organic chemistry. Their environment-friendly nature and mild reaction conditions makes them more attractive candidates for the replacements of various toxic metals in organic synthesis [18-31]. These reagents are more popular for their oxidizing properties [32-38] and electrophilic nature of different iodine(III) reagents has been explored to developed various synthetic transformation including rearrangements [39-62]. Hypervalent iodine chemistry has now become a well-established research area and various book chapters [19,20,27] and review articles [21-24,31-35,60,63,64] appeared to explain the chemistry of these reagents. In the past two decades, a number of organic chemists used these reagents for the construction of a variety of spirocyclic scaffolds. In 2008, Quideau and co-workers published a nice review article where they have described various spirocyclization reactions using hypervalent iodine reagents via dearomatizations of aromatic phenolic species [32]. This review article is quite useful for readers who want to know the chemistry involved during the dearomatization of phenols and to find the relevant literature available until 2008. In this review article, various approaches for the synthesis of spirocyclic scaffolds using hypervalent iodine reagents are covered including stereoselective reactions.
Hypervalent iodine reagents are mainly popular for their oxidative properties but various iodine(III) reagents have been used as electrophiles. Numerous iodine(III) reagents have been successfully used to achieve diverse spirocyclic scaffolds. Phenols 7 or 11 having an internal nucleophile at ortho- or para-position can be used as starting material for the synthesis of ortho- and para-spirocyclic compounds in the presence of iodine(III)-based electrophiles (Scheme 1). Phenolic oxygen of compound 7 attacks to the iodine of 8 to form intermediate 9. Furthermore, on nucleophilic attack of the internal nucleophile to the ortho-position intermediate 9 converts to ortho-spirocyclic compound 10 with the elimination of the hypervalent iodine moiety. Similarly, para-spirocyclic compounds 13 can be achieved starting from compounds 11 and iodine(III) reagent 8 (Scheme 1). The synthesis of spirocyclic compounds can be achieved using stoichiometric or catalytic amounts of iodine(III) reagents. According to literature reports, both heterocyclic and carbocyclic spirocyclic compounds can be achieved using these reagents [27,32].
![[1860-5397-14-152-i1]](/bjoc/content/inline/1860-5397-14-152-i1.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 1: Iodine(III)-mediated spirocyclization of substituted phenols 7 and 11 to 10 and 13, respectively.
Scheme 1: Iodine(III)-mediated spirocyclization of substituted phenols 7 and 11 to 10 and 13, respectively.
2. Synthesis of spirolactones
2.1. Using stoichiometric amounts of iodine(III) reagents
The history of the utility of hypervalent iodine reagents in the synthesis of spirocyclic compounds is going to become quite old now. Initially, iodine(III) reagents were applied for synthesis of spirocyclic in 1990s [65,66]. In 1991, Kita and co-workers [67] established the synthesis of spirohexadiones from N-acyltyramines using iodine(III) reagent. After these reports, numerous hypervalent iodine-mediated spirocyclizations were investigated and phenolic oxidations of substrates have been explored for the construction of spirodienone motifs [21,64].
In 1993, Wipf and Kim [68] employed PIDA (15) for spirocyclization of N-protected tyrosine 14 to spirolactone 16. The spirocyclization reaction was carried out in methanol using stoichiometric amounts of PIDA (15) and spirolactone 16 was isolated in 35% yield (Scheme 2). Probably, the cyclization reaction proceeded via dearomatizaion of phenolic substrate 14 followed by nucleophilic attack of the carbonyl moiety of carboxylic group.
Scheme 2: PIDA-mediated spirolactonization of N-protected tyrosine 14 to spirolactone 16.
Scheme 2: PIDA-mediated spirolactonization of N-protected tyrosine 14 to spirolactone 16.
Furthermore, Giannis and co-workers [69] reported the synthesis of novel aminomethylpolystyrene-supported (diacetoxyiodo)benzene (PSDIB) reagents 17a and 17b starting from aminomethylated polystyrene with 4-iodobenzoic acid and 4-iodophenylacetic acid in two steps (Figure 2).
Figure 2: The structures of polymer-supported iodine(III) reagents 17a and 17b.
Figure 2: The structures of polymer-supported iodine(III) reagents 17a and 17b.
Both polymer-supported reagents 17a and 17b were used in similar spirocyclizations of tyrosine 14. Both tyrosine 14a and N-protected tyrosine derivatives 14b,c were used as starting material and results of their spirolactonization are summarized in Table 1 (Scheme 3).
Scheme 3: Spirolactonization of substrates 14 to spirolactones 16 using polymer-supported reagents 17a and 17b.
Scheme 3: Spirolactonization of substrates 14 to spirolactones 16 using polymer-supported reagents 17a and 17b...
The spirolactonization products 16 were isolated in excellent yields when reactions were performed with substrates 14 (R = NH2) having free amino group (Table 1, entries 1 and 2). Notably, the poor yields were observed during the spirolactonization of N-protected tyrosine derivatives 14b and 14c (Table 1, entries 3–6). The advantage of this reaction is that the polymer-supported reagent can be regenerated and reused without loss of any significant activity [69].
Table 1: Spirolactonization of substrates 14 to spirolactones 16 using polymer-supported reagents 17a and 17b.
| entry | substrate 14 | PS-iodine(III) reagent | 16 yields (%) |
|---|---|---|---|
| 1 | 14a: R = NH2 | 17a | 82 |
| 2 | 14a: R = NH2 | 17b | 80 |
| 3 | 14b: R = Cbz-NH | 17a | 25 |
| 4 | 14b: R = Cbz-NH | 17b | 26 |
| 5 | 14c: R = Boc-NH | 17a | 24 |
| 6 | 14c: R = Boc-NH | 17b | 25 |
In 2010, Kita and co-workers [70] developed another approach for PIDA-mediated spirolactonization of 1-(p-hydroxyaryl)cyclobutanols 18 to spirolactones 19 in good yields (Scheme 4). The reaction was initiated with formation of an intermediate 20 by the oxidation of the phenolic hydroxy group of 18, which rearranged to compound 21. Furthermore, water attacks the ketone moiety of 21 to form para-substituted phenol 22. The phenolic intermediate 22 is further oxidized with another molecule of PIDA (15) to form intermediate 23, which yielded the final product 19 on intramolecular cyclization [70].
![[1860-5397-14-152-i4]](/bjoc/content/inline/1860-5397-14-152-i4.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 4: PIDA-mediated spirolactonization of 1-(p-hydroxyaryl)cyclobutanols 18 to spirolactones 19.
Scheme 4: PIDA-mediated spirolactonization of 1-(p-hydroxyaryl)cyclobutanols 18 to spirolactones 19.
Furthermore, Kita and his research group [71] reported an iodine(III)-mediated cyclization of arylalkynes 24 to spirocyclic products 26 by in situ-generated active hypervalent iodine species. In this report, para-substituted esters 24 were cyclized to corresponding spirolactones 26 using stoichiometric amount of bis(iodoarene) 25 with terminal oxidant mCPBA in the presence of TsOH·H2O in TFE (Scheme 5). In this reaction, active hypervalent iodine species was generated in situ by the oxidation of bis(iodoarene) 25 using mCPBA as terminal oxidant.
Scheme 5: Iodine(III)-mediated spirocyclization of aryl alkynes 24 to spirolactones 26 by the reaction with bis(iodoarene) 25 in the presence of mCPBA.
Scheme 5: Iodine(III)-mediated spirocyclization of aryl alkynes 24 to spirolactones 26 by the reaction with b...
In 2011, Kita and co-workers [72] investigated a more reactive µ-oxo bridged hypervalent iodine(III) reagent used in the spirocyclization of phenolic substrates 27 to spirolactones 29. The reaction products were obtained in excellent yields using 0.55 equivalents of bridged iodine(III) reagent 28 in acetonitrile at room temperature (Scheme 6). Furthermore, a comparative study was done between bridged iodine(III) reagent 28 with PIFA. It was found that the reaction products 29 were obtained in higher yield using the bridged iodine(III) reagent compared to that using PIFA. Probably, the iodine-OCOCF3 bond of the bridged compound 28 has a significant ionic character as the iodine–oxygen bond distance is larger than in PIFA which intends to make it more reactive than PIFA.
Scheme 6: Bridged iodine(III)-mediated spirocyclization of phenols 27 to spirodienones 29.
Scheme 6: Bridged iodine(III)-mediated spirocyclization of phenols 27 to spirodienones 29.
PIFA (31) is a more electrophilic iodine(III) reagent than PIDA (15) due to the presence of two trifluoroacetoxy groups. There are some approaches for the synthesis of spirocyclic compounds where PIFA (31) is used as electrophile.
Recently, Lewis and co-workers [73] reported the conversion of arnottin I (30) to its spirocyclic analogue arnottin II (32) by reaction with LiOH followed by PIFA (31). The spirocyclic product arnottin II (32) was isolated in 56% yield (Scheme 7). This approach is based on a tandem oxidative dearomatization process and will be quite useful for the conversion of functionalized benzocoumarins to spirocyclic lactones.
Scheme 7: Iodine(III)-mediated spirocyclization of arnottin I (30) to its spirocyclic analogue arnottin II (32) using stoichiometric amount of PIFA (31).
Scheme 7: Iodine(III)-mediated spirocyclization of arnottin I (30) to its spirocyclic analogue arnottin II (32...
In 2015, Du and co-workers [74] reported a spirocyclization of diarylacetylenes to fused spiro polycyclic compounds through a hypervalent iodine-mediated cascade annulation reaction. In this reaction, the Lewis acid BF3·Et2O acts as catalyst which activates the substrate. A further treatment with PIDA (15) forms the spirocyclic products through intramolecular cyclization.
2.2. Using hypervalent iodine reagents as catalyst
The hypervalent iodine-catalyzed synthesis of spirocylic compounds can be achieved either by using catalytic amounts of a hypervalent iodine species or by generation of a similar active catalytic species in situ by the oxidation of iodoarene using a terminal oxidant. More commonly, m-chloroperbenzoic acid (mCPBA) and oxone are used as oxidant to generate the hypervalent iodine species in situ via oxidation of iodoarenes. In 2014, Singh and Wirth have compiled a review article where they have covered various aspects of hypervalent iodine catalyzed reactions [75].
In 2005, Kita and his research group investigated a hypervalent iodine-catalyzed spirocyclization reaction by generating the catalytic hypervalent iodine species via in situ oxidation of iodoarene using mCPBA as terminal oxidant [76]. In this report, p-substituted phenols 27 were cyclized to the corresponding spirolactones 29 using iodotoluene 33 as precatalyst, mCPBA as oxidant and TFA as an additive. The spirolactones 29 were isolated as reaction products in excellent yields (Scheme 8). Probably, the iodine(III) species was generated in situ as the active catalytic species that was playing the key role for the dearomatization of phenol. In addition, a similar reaction was also achieved by using various PIFA analogues as catalyst directly in the presence of 1.5 equivalents of mCPBA. Since this report, several iodine(III)-catalyzed oxidative spirocyclization reactions have been successfully developed.
Scheme 8: Iodine(III)-catalyzed spirolactonization of p-substituted phenols 27 to spirolactones 29 using iodotoluene 33 as a precatalyst and mCPBA as an oxidant.
Scheme 8: Iodine(III)-catalyzed spirolactonization of p-substituted phenols 27 to spirolactones 29 using iodo...
In 2009, Ishihara and co-workers [77] developed an oxylactonization of ketocarboxylic acid 34 to spirolactone 36 using 10 mol % of iodobenzene (35) as precatalyst, 20 mol % of TsOH·H2O as additive and 1.8 equivalents of mCPBA as oxidant. The catalytic reaction was carried out in nitromethane at 50 °C for 23 h and spirolactone 36 was isolated in 74% yield (Scheme 9). It was noted that 20 mol % of additive was essential to initiate the reaction efficiently. The reaction was quite slow when 10 mol % of additive was used. Once again, iodine(III) species was generated in situ which was probably working as active catalytic species.
Scheme 9: Iodine(III)-catalyzed oxylactonization of ketocarboxylic acid 34 to spirolactone 36 using iodobenzene 35 as precatalyst in the presence of mCPBA.
Scheme 9: Iodine(III)-catalyzed oxylactonization of ketocarboxylic acid 34 to spirolactone 36 using iodobenze...
2.3. Stereoselective synthesis of spirolactones
Recently, Kita and co-workers [78] reported a new type of binaphthyl-based chiral iodine(III) species 38 and its efficient utilization in the spirocyclization of naphthols containing carboxylic acids. 1-Naphthol-2-propionic acids 37 were cyclized to corresponding spirolactone derivatives 39 using chiral-8,8’-diiodonaphthyl reagent 38 as precatalyst, mCPBA as an oxidant in chloroform at low temperature. The reaction products 39 were isolated in good yields with more than 78% enantiomeric excess (Scheme 10). The active catalytic hypervalent iodine species was generated in situ by oxidation of optically active iodoarene 38 using mCPBA as an oxidant.
Scheme 10: Iodine(III)-mediated asymmetric oxidative spirocyclization of naphthyl acids 37 to naphthyl spirolactones 39 using chiral iodoarene 38 as precatalyst.
Scheme 10: Iodine(III)-mediated asymmetric oxidative spirocyclization of naphthyl acids 37 to naphthyl spirola...
2.4. Application of spirolactones in natural products synthesis
In 2005, Wipf and Spencer [79] reported the first total synthesis of the Stemona alkaloid (−)-tuberostemonine (40). In this report, PIDA (15) was used as an electrophile for the synthesis of spirolactone 16 in 35% yield by the cyclization of L-tyrosine 14 in nitromethane at room temperature for 2.5 h (Scheme 11). Additionally, the synthesized spirocyclic precursor 16 was transfered to (−)-tuberostemonine (40) in three chemical steps.
Scheme 11: Oxidative cyclization of L-tyrosine 14 to spirocyclic lactone 16 using PIDA (15).
Scheme 11: Oxidative cyclization of L-tyrosine 14 to spirocyclic lactone 16 using PIDA (15).
3. Synthesis of spirolactams
3.1. Using stoichiometric amounts of iodine(III) reagents
In 1998, Ciufolini and co-workers [80] reported the oxidative cyclization of tyrosine derivatives to spirolactams using iodine(III) reagents. In this reaction, oxazoline derivatives 41 were cyclized to spirocyclic products 42 using PIDA (15) as an electrophile in trifluoroethanol at room temperature for 30 minutes. The desired products 42 were isolated in moderate yields (Scheme 12).
Scheme 12: Oxidative cyclization of oxazoline derivatives 41 to spirolactams 42 using PIDA (15).
Scheme 12: Oxidative cyclization of oxazoline derivatives 41 to spirolactams 42 using PIDA (15).
Additionally, the same research group [81] reported the oxidative cyclization of a phenolic substrate to a spirolactam using PIDA as electrophile. In this methodology, oxazoline 43 was cyclized to spirolactam 44 in 50% yield using PIDA (15) in trifluoroethanol at room temperature (Scheme 13). Furthermore, spirolactam was used as intermediate in the synthesis of tricyclic compound 43 possessing a similar structure like that of the naturally occurring heterocyclic compound FR901483 [82].
Scheme 13: Oxidative cyclization of oxazoline 43 to spirolactam 44 using PIDA 15 as oxidant.
Scheme 13: Oxidative cyclization of oxazoline 43 to spirolactam 44 using PIDA 15 as oxidant.
Wardrop and co-workers [83] developed a new method for the preparation of 1-azaspiranes 47 by treatment of α- and β-substituted 3-(methoxyphenyl)-N-methoxypropionamides 46 with [bis(trifluoroacetoxy)iodo]benzene (PIFA, 31) in dichloromethane (Scheme 14). The reactions were carried out at low temperature and spirolactams 47 were achieved in high yields with up to 96% enantiomeric excess. Furthermore, these compounds have been employed as important synthetic intermediates for the construction of biologically active molecules such as histrionicotoxins and the cytotoxic marine alkaloid fasicularin [84].
Scheme 14: PIFA-mediated spirocyclization of amides 46 to N-spirolactams 47 using PIFA (31) as an electrophile.
Scheme 14: PIFA-mediated spirocyclization of amides 46 to N-spirolactams 47 using PIFA (31) as an electrophile....
In 2010, Honda [85] reported the synthesis of isoquinoline alkaloids possessing spirocyclic framework using PIDA (15) as an electrophile in hexafluroisopropanol solvent. The p-substituted phenolic compound 48 was used as starting material for the construction of spirolactam 49 in 69% yield (Scheme 15). This is an important intermediate in the synthesis of various naturally occurring alkaloids such as TAN1251A, TAN1251C and TAN1251D [86].
Scheme 15: Synthesis of spirolactam 49 from phenolic enamide 48 using PIDA (15).
Scheme 15: Synthesis of spirolactam 49 from phenolic enamide 48 using PIDA (15).
Wardrop and Burge [87] reported a iodine(III)-mediated oxidative spirocyclization of hydroxamates 50. The azaspirans 51 containing quaternary carbon centers were synthesized in good to excellent yields on treating substrates 50 with PIFA (31) in dichloromethane/methanol (1:1, Scheme 16). The reaction products (spirolactams 51) were obtained as inseparable mixture of anti- and syn-diastereomers.
Scheme 16: Iodine(III)-mediated spirocyclization of alkyl hydroxamates 50 to spirolactams 51 using stoichiometric amount of PIFA (31).
Scheme 16: Iodine(III)-mediated spirocyclization of alkyl hydroxamates 50 to spirolactams 51 using stoichiomet...
Haroutounian and co-workers [88] investigated a PIFA-mediated synthesis of spirocyclic lactam 54 as side product by treating substrate 52 with 1.5 equivalents of PIFA (31) in presence of 3.0 equivalents of TFA as an additive in dichloromethane (Scheme 17). The fused tricyclic compound 53 was obtained as major product in 55% yield along with the spiro compound 54 as a minor product in 8% yield.
Scheme 17: PIFA-mediated cyclization of substrate 52 to spirocyclic product 54.
Scheme 17: PIFA-mediated cyclization of substrate 52 to spirocyclic product 54.
In 2009, Zhang and co-workers [89] reported an efficient method for the synthesis of spiro β-lactams via oxidative dearomatization reactions. In this report, the synthesis of spiro β-lactams 56 were achieved successfully by the oxidative cyclization of p-substituted phenols 55 using PIDA (15) as an electrophile and copper(II) sulfate pentahydrate as an additive in the presence of DMAP base. The spirocyclization reactions were performed in MeOH for 2 h at 0 °C and spirocyclic products 56 were isolated in good yields (Scheme 18). Additionally, fused bicyclic compounds 57 were also observed in few reactions in traces. The structure of the spiro β-lactam was confirmed by single crystal X-ray crystallography.
Scheme 18: Synthesis of spiro β-lactams 56 by oxidative coupling reaction of p-substituted phenols 55 using PIDA (15) and CuSO4·5H2O in the presence of base in methanol.
Scheme 18: Synthesis of spiro β-lactams 56 by oxidative coupling reaction of p-substituted phenols 55 using PI...
Dong and co-workers [90] developed a novel way for the synthesis of five membered spiro pyrazolin-5-ones using amide and amine-containing precursors. Herein, five-membered azaheterocyclic derivatives were synthesized efficiently in presence of PIFA and with TFA as an additive.
Furthermore, Kita and his research group [71] displayed a method for the cyclization of alkyne derivative 58 to spirolactam 59 by an in situ-generated active hypervalent iodine species. In this method, para-substituted amide 58 was cyclized to the corresponding spirolactam 59 in 92% yield using a stoichiometric amount of bis(iodoarene) 25 with the terminal oxidant mCPBA in the presence of TsOH·H2O in TFE (Scheme 19).
Scheme 19: Iodine(III)-mediated spirocyclization of para-substituted amide 58 to spirolactam 59 by the reaction with bis(iodoarene) 25 in the presence of mCPBA.
Scheme 19: Iodine(III)-mediated spirocyclization of para-substituted amide 58 to spirolactam 59 by the reactio...
In 2012, Zhao and co-workers [91] developed a new approach for the construction of spirooxindoles 61 through tandem cascade oxidation of substituted anilides 60. In this methodology, anilide derivatives 60 were reacted with [bis(trifluoroacetoxy)iodo]benzene (31, PIFA) in TFE at room temperature to afford functionalized lactams 61 in good yields (Scheme 20). Various electron-donating and withdrawing groups at the phenyl ring in anilides were successfully tolerated.
Scheme 20: Iodine(III)-mediated synthesis of spirolactams 61 from anilide derivatives 60.
Scheme 20: Iodine(III)-mediated synthesis of spirolactams 61 from anilide derivatives 60.
Furthermore, Sunoj and Sreenithya [92] developed a metal-free approach for the synthesis of 1,1'-dimethyl-3,3'-spirobi[indoline]-2,2'-dione (61) from N1,N3-dimethyl-N1,N3-diphenylmalonamide (60) using PIFA (31) in trifluoroethanol at room temperature. The spirolactam 61 was isolated in 75% yield (Scheme 21). According to the proposed mechanistic pathway, the reaction was initiated with formation of an intermediate 63 by the attack of the carbonyl oxygen to electrophilic iodine(III) reagent 31 which could be rearranged to compound 64. Finally, the acetate anion attacks the β-hydrogen of 64 to form spirolactam product 61.
Scheme 21: PIFA-mediated oxidative cyclization of anilide 60 to bis-spirobisoxindole 61.
Scheme 21: PIFA-mediated oxidative cyclization of anilide 60 to bis-spirobisoxindole 61.
In 2014, Xu and Abdellaoui [93] reported a nucleophilic intramolecular cyclization of phenylacetamides 65 to spirocyclic lactams 66 via iodine(III)-mediated spirocarbocyclizations. In literature, there are limited methods available for the synthesis of spiro-β-lactam-3-carbonitrile which is widely used as an antibiotic [94]. In this methodology, N-(p-hydroxyphenyl)cyanoacetamides 65 were cyclized to corresponding 4-spiro-β-lactam-3-carbonitriles 66 in useful yields using PIDA (15) as an electrophile in the presence of KOH as base in anhydrous ethanol at room temperature (Scheme 22).
Scheme 22: PIDA-mediated spirocyclization of phenylacetamides 65 to spirocyclic lactams 66.
Scheme 22: PIDA-mediated spirocyclization of phenylacetamides 65 to spirocyclic lactams 66.
In 2014, Fan and co-workers [95] investigated an efficient approach for the synthesis of a spirocyclic-skeleton-containing dieniminium moiety. Herein, arylamines 67 were cyclized to spirocyclic dieniminium salts 68 using PIFA (31) as an electrophilic species in nitromethane (Scheme 23). All the reactions were completed within a minute and desired lactams were isolated in good yields. The presence of electron-withdrawing groups at the aromatic ring shows a negative effect on the yield while the presence of electron-enriched groups afforded the products 68 in high yields.
Scheme 23: Oxidative dearomatization of arylamines 67 with PIFA (31) to give dieniminium salts 68.
Scheme 23: Oxidative dearomatization of arylamines 67 with PIFA (31) to give dieniminium salts 68.
In addition, Zhu and co-workers [96] developed another hypervalent iodine-mediated intermolecular spirocarbocyclization approach for synthesis of spirolactam. In this approach, N-methoxybenzamide 69 and diphenylacetylene (70) were treated in presence of PIFA (31) in dichloromethane to corresponding spirodienone compound 71 in 48% yield (Scheme 24). Additionally, trifluoroacetic acid (TFA) was used as an additive in the reaction.
Scheme 24: PIFA-mediated oxidative spirocarbocyclization of 4-methoxybenzamide 69 with diphenylacetylene (70) to spirolactam 71.
Scheme 24: PIFA-mediated oxidative spirocarbocyclization of 4-methoxybenzamide 69 with diphenylacetylene (70) ...
In 2015, Wang’s group [97] reported an iodine(III)-mediated approach for the intermolecular spirocyclization of amides 72 with sulfonylhydrazides 73 to spirolactams 75. In this method, functionalized amides 72 containing an alkyne moiety and sulfonylhydrazides 73 undergo intermolecular spirocyclization in presence of I2O5/TBHP oxidative system to give the sulfonated spirolactams 75 in high yields (Scheme 25). This oxidative system found to be more efficient and could sustain the presence of diverse functional groups. The structure of 75 was confirmed by single crystal X-ray crystallography.
Scheme 25: Synthesis of spiroxyindole 75 using I2O5/TBHP oxidative system.
Scheme 25: Synthesis of spiroxyindole 75 using I2O5/TBHP oxidative system.
3.2. Using hypervalent iodine reagents as catalysts
In 2007, Kita and co-workers [98] investigated the first iodoarene-catalyzed spirocyclization of functionalized amides 76 to spirocyclic systems 77 by carbon–nitrogen bond formation using 10 mol % of iodotoluene 33 as precatalyst, 1.0 equivalent of CF3COOH as an additive and mCPBA as terminal oxidant in trifluoroethanol (Scheme 26). The spirocyclic compounds 77 were isolated in high yields. The cyclization reaction was probably initiated by in situ generated active iodine(III) species by the oxidation of iodotoluene 33 in the presence of mCPBA.
Scheme 26: Iodine(III)-catalyzed spirolactonization of functionalized amides 76 to spirolactones 77 using iodotoluene 33 as a precatalyst and mCPBA as an oxidant.
Scheme 26: Iodine(III)-catalyzed spirolactonization of functionalized amides 76 to spirolactones 77 using iodo...
In 2010, Zhu’s research group [99] achieved a Pd-catalyzed synthesis of spirolactams 80 by the cyclization of functionalized amides 78 using 10 mol % PdCl2 (79) in presence of PhI(OAc)2 (15) in acetonitrile solvent at 80 °C. The spirocyclic products 80 were obtained in moderate yields (Scheme 27). It was observed that the introduction of electron-donating group at para-position in substrates 78 gave the desired products in good yields whereas introduction of strong electron withdrawing groups resulted in a decrease in the yield of spirocyclic products.
Scheme 27: Intramolecular cyclization of alkenes 78 to spirolactams 80 using Pd(II) 79 and PIDA (15) as the oxidative system in acetonitrile.
Scheme 27: Intramolecular cyclization of alkenes 78 to spirolactams 80 using Pd(II) 79 and PIDA (15) as the ox...
Kita and co-workers [100] developed another catalytic approach for the cyclization of amides 76 to spirolactams 77. In this approach, 2 mol % of bis(iodoarene) 81 was used as precatalyst and peracetic acid (PAA) as an oxidant instead of mCPBA, which plays an important role in generation of active iodine(III) species. The bis(iodoarene) 81 was oxidized to a unique µ-oxo-bridged hypervalent iodine(III) species in situ, wherein PAA is used as extremely green oxidant which releases non-toxic co-products (Scheme 28).
Scheme 28: Iodine(III)-catalyzed spiroaminocyclization of amides 76 to spirolactam 77 using bis(iodoarene) 81 as a precatalyst in the presence of PAA.
Scheme 28: Iodine(III)-catalyzed spiroaminocyclization of amides 76 to spirolactam 77 using bis(iodoarene) 81 ...
In 2011, Yu and co-workers [101] developed an intramolecular lactonization of p-substituted phenols 82 to spirooxindoles 83 using 10 mol % of iodobenzene (35) as precatalyst, mCPBA as an external oxidant and TFA as additive. All the catalytic reactions were performed in dichloromethane and spirolactams 83 were isolated in good to excellent yields (Scheme 29). It was noted that mCPBA/TFA combination did not work well for some transformations and it was replaced with oxidant urea·H2O2 and TFAA as an additive.
Scheme 29: Iodine(III)-catalyzed spirolactonization of N-phenyl benzamides 82 to spirolactams 83 using iodobenzene 35 as a precatalyst.
Scheme 29: Iodine(III)-catalyzed spirolactonization of N-phenyl benzamides 82 to spirolactams 83 using iodoben...
3.3. Stereoselective synthesis of spirolactams
Gong and co-workers [102] efficiently cyclized 1-hydroxy-N-aryl-2-naphthamides 84 to corresponding spirolactam derivatives 86 using chiral iodoarene 85 as precatalyst, mCPBA as an oxidant and TFE as an additive. The presence of 10.0 equivalents of H2O was required to get the reaction products in high yields with up to 92% ee (Scheme 30). The chiral hypervalent-λ3-iodanes were generated in situ by the oxidation of the chiral C2-symmetric iodoarene 85 that was playing the key role for the oxidative spirocyclization of phenols.
Scheme 30: Iodine(III)-mediated asymmetric oxidative spirocyclization of phenols 84 to spirolactams 86 using chiral iodoarene 85 as precatalyst.
Scheme 30: Iodine(III)-mediated asymmetric oxidative spirocyclization of phenols 84 to spirolactams 86 using c...
In addition, N-methyl-N-(2-naphthyl)-2-naphthamides 87 were also cyclized to corresponding spiro compounds 88 in high yields and with upto 84% enantiomeric excess (Scheme 31). Furthermore, the absolute configuration of 88 was assigned by its single crystal X-ray analysis.
Scheme 31: Iodine(III)-catalyzed asymmetric oxidative spirocyclization of N-aryl naphthamides 87 to spirocyclic compounds 88 using chiral iodoarene 85 as precatalyst.
Scheme 31: Iodine(III)-catalyzed asymmetric oxidative spirocyclization of N-aryl naphthamides 87 to spirocycli...
3.3. Application of spirolactams in natural product synthesis
In 2001, Ciufolini and co-workers [103] employed PIDA (15) as an electrophile during the synthesis of naturally occurring tricyclic azaspirane derivative TAN1251C. In this report, phenolic 3-arylpropionamide 89 was cyclized to spirolactam 90 in 41% yield using PIDA (15) as an electrophile in the presence of NaHCO3 in trifluoroethanol (TFE) at room temperature followed by addition of acetic anhydride and pyridine in the presence of 10 mol % DMAP (Scheme 32). In addition, spirocyclic product 90 was used as key precursor in the synthesis of naturally occurring tricyclic azaspirane derivative TAN1251C 91 in a sequence of steps.
Scheme 32: Cyclization of p-substituted phenolic compound 89 to spirolactam 90 using PIDA (15) in TFE.
Scheme 32: Cyclization of p-substituted phenolic compound 89 to spirolactam 90 using PIDA (15) in TFE.
Furthermore, PIDA (15) was used as an electrophile during the synthesis of biologically active molecule FR901483 by the same research group [104]. In this report, spirocyclic oxazoline 93 was prepared by starting from para-substituted phenolic compound 92 under the reaction conditions mentioned in Scheme 32 (Scheme 33).
Scheme 33: Iodine(III)-mediated synthesis of spirocyclic compound 93 from substrates 92 using PIDA (15) as an electrophile.
Scheme 33: Iodine(III)-mediated synthesis of spirocyclic compound 93 from substrates 92 using PIDA (15) as an ...
In 2002, Honda and co-workers [105] reported the synthesis of naturally occurring (−)-TAN1251A (95) employing an oxidation of phenols via an dearomatization process. In this report, para-substituted phenolic compound 48 was cyclized to spirocyclic lactam 49 using PIDA (15) as an electrophile. The spirocyclic compound 49 was achieved in 69% yield (Scheme 34). Additionally, synthesized spirocyclic compound 49 was converted to natural product 95 in few chemical steps.
Scheme 34: Iodine(III)-mediated spirocyclization of p-substituted phenol 48 to spirocyclic compound 49 using PIDA (15) as an electrophile.
Scheme 34: Iodine(III)-mediated spirocyclization of p-substituted phenol 48 to spirocyclic compound 49 using P...
4. Synthesis of spirocarbocycles
4.1. Using stoichiometric amounts of iodine(III) reagents
Furthermore, O-silylated phenolic compound 96 was spirocyclized to spirocarbocyclic compound 97 in 95% yield using bridged iodine(III) reagent 28 as an electrophile and trifluoroethanol (TFE) as the solvent at room temperature (Scheme 35). Compound 97 was further used as substrate for the synthesis of discorhabdin alkaloids [106,107].
Scheme 35: Bridged iodine(III)-mediated spirocyclization of O-silylated phenolic compound 96 in the synthesis of spirodienone 97.
Scheme 35: Bridged iodine(III)-mediated spirocyclization of O-silylated phenolic compound 96 in the synthesis ...
In 1996, Kita and co-workers [108] developed an intramolecular cyclization of ortho-substituted phenols 98 to aza-spirocarbocyclic compounds 101 via hypervalent iodine-mediated spirocarbocyclization reactions using 31 as an electrophile. In this methodology, ortho-substituted phenolic derivatives 98 were treated with stoichiometric amounts of PIFA (31) in trifluoroethanol at room temperature for 0.5 h to afford spirocyclic compounds 101 in good yields (Scheme 36).
![[1860-5397-14-152-i36]](/bjoc/content/inline/1860-5397-14-152-i36.png?scale=2.0&max-width=1024&background=FFFFFF)
Scheme 36: PIFA-mediated approach for the spirocyclization of ortho-substituted phenols 98 to aza-spirocarbocyclic products 101.
Scheme 36: PIFA-mediated approach for the spirocyclization of ortho-substituted phenols 98 to aza-spirocarbocy...
Like PIDA and PIFA, Koser reagents are other iodine(III) reagents known to behave as electrophiles. In 2000, Spyroudis and co-workers [109] reported the spirocyclization of para-substituted phenols 102 to corresponding spirocarbocyclic derivatives 104 via dearomatization process using Koser reagent. In this reaction, substrates 102 were reacted with a stoichiometric amount of [(hydroxy)(tosyloxy)iodo]benzene (103) in dichloromethane at 0 °C. The spirocyclic products 104 were obtained in poor yields (Scheme 37).
Scheme 37: Oxidative cyclization of para-substituted phenols 102 to spirocarbocyclic compounds 104 using Koser reagent 103.
Scheme 37: Oxidative cyclization of para-substituted phenols 102 to spirocarbocyclic compounds 104 using Koser...
Furthermore, Kita and his research group [71] reported the synthesis of spirocarbocyclic compounds 106 from arylalkynes 105 using a hypervalent iodine reagent generated in situ by the oxidation of bis(iodoarene) 25 in the presence of mCPBA as an terminal oxidant (Scheme 38).
Scheme 38: Iodine(III)-mediated spirocyclization of aryl alkynes 105 to spirocarbocyclic compound 106 by the reaction with bis(iodoarene) 25 in the presence of mCPBA.
Scheme 38: Iodine(III)-mediated spirocyclization of aryl alkynes 105 to spirocarbocyclic compound 106 by the r...
Wang and co-workers [110] developed a hypervalent iodine-mediated synthesis of ortho-spirocarbocyclic compounds via dearomatization of ortho-substituted phenols. In this reaction, ortho-substituted phenols 107 were cyclized to form spirocarbocyclic compounds 109 in useful yields. All the reactions were performed in a CF3CH2OH/CH2Cl2 (1:1) solvent combination using PIDA (15) as an electrophile at −40 °C for 10–15 minutes (Scheme 39). This is an example of an ortho-oxidative phenol dearomatization reaction wherein there is the formation of the steriogenic center at the spiro-ring junction. This approach provides an easy and direct method for the construction of ortho-spirocarbocyclic compounds which is broadly found to originate in most of bioactive natural products [111,112].
Scheme 39: Iodine(III)-mediated spirocarbocyclization of ortho-substituted phenols 107 to spirocarbocyclic compounds 109 using PIDA 15.
Scheme 39: Iodine(III)-mediated spirocarbocyclization of ortho-substituted phenols 107 to spirocarbocyclic com...
4.3. Application of spirocarbocyclic compounds in natural product synthesis
In 2003, Kita and co-workers [113,114] employed a iodine(III) reagent during the total synthesis of sulfur-containing alkaloid 112. Initially, the substrates 110 were cyclized to spirodienone derivatives 111 in useful yields using PIFA (31) as source of electrophile in trifluoroethanol at room temperature (Scheme 40). Furthermore, synthesized compounds 111 were converted into the natural product discorhabdin A (112).
Scheme 40: PIFA-mediated oxidative cyclization of substrates 110 to spirocarbocyclic compounds 111.
Scheme 40: PIFA-mediated oxidative cyclization of substrates 110 to spirocarbocyclic compounds 111.
In 2006, Honda and co-workers [115] reported the total synthesis of spiro-isoquinoline alkaloid (±)-annosqualine (1). In this report, the substrate 113 was cyclized to form spirocyclic compound 114 via desilylation with TBAF in THF followed by reaction with n-BuLi in hexafluoroisopropanol using PIDA (15) at 4 °C (Scheme 41). This oxidative cyclization of enamide substrate 113 afforded synthetically useful spiroenamide 114, which was used as key intermediate for total synthesis of annosqualine (1). The synthesis of natural product 1 was achieved in two steps starting from synthesized compound 114.
Scheme 41: Iodine(III)-mediated cyclization of substrate 113 to spirocyclic compound 114.
Scheme 41: Iodine(III)-mediated cyclization of substrate 113 to spirocyclic compound 114.
Honda and Shigehisa [116] reported the total synthesis of naturally occuring compound stepharine (3) starting from aromatic aldehyde 115. Initially, substituted phenolic compound 116 was prepared in seven steps from aldehyde 115. Furthermore, the synthesized compound 116 was converted into the natural product stepharine (3) by reaction with PIDA (15) in trifluoroethanol (TFE) followed by the reduction with NaBH4. The synthesis of the natural product stepharine (3) was obtained in 90% yield by starting from phenolic substrate 116 (Scheme 42).
Scheme 42: Iodine(III)-mediated spirocyclization of phenolic substrate 116 to the spirocarbocyclic natural product stepharine 3 using PIDA (15).
Scheme 42: Iodine(III)-mediated spirocyclization of phenolic substrate 116 to the spirocarbocyclic natural pro...
In 2008, Kita and co-workers [117] developed an iodine(III)-catalyzed approach for the spirocyclization of p-substituted phenols 117 to spirocarbocyclic products 119 in good yields using a catalytic amount of iodoarene 118 and urea·H2O2 as an oxidant. Probably, the active hypervalent iodine(III) species was generated in situ by the oxidation of iodoaerene 118 in the presence of urea·H2O2 oxidant (Scheme 43). Furthermore, the synthesized spirocyclic compounds were used as synthetic intermediate for the synthesis of biologically active natural product amaryllidaceae alkaloids such as (±)-maritidine (120) [118-120].
Scheme 43: Iodine(III)-catalyzed spirocyclization of phenols 117 to spirocarbocyclic products 119 using iodoarene 118 in the presence of the oxidant urea·H2O2.
Scheme 43: Iodine(III)-catalyzed spirocyclization of phenols 117 to spirocarbocyclic products 119 using iodoar...
In 2009, Kita and co-workers [121] reported the synthesis of various oxygen analogues of naturally occurring compound discorhabdin A starting from substrate 110 in few chemical steps. Discorhabdin A is an alkaloid that shows various biological activities including strong cytotoxic activity [122]. During the first step, starting substrates 110 were cyclized to spirocyclic compounds 111 in useful yields using PIFA (31) in presence of montmorillonite K10 in trifluoroethanol (Scheme 44). Furthermore, synthesized spirocyclic compounds 111 were used as key precursors for the synthesis of oxygen analogues of discorhabdin A (121).
Scheme 44: PIFA-mediated spirocyclization of 110 to spirocyclic compound 111 using PIFA (31) as electrophile.
Scheme 44: PIFA-mediated spirocyclization of 110 to spirocyclic compound 111 using PIFA (31) as electrophile.
5. Synthesis of miscellaneous spirocyclic compounds
5.1. Using stoichiometric amounts of iodine(III) reagents
In 2002, Ciufolini and co-workers [123] reported the spirocyclization of various phenolic sulfonamides 122 to spiropyrrolidines 123 using PIDA (15). In this reaction, sulfonamides 122 undergo N-acylation, wherein various homotyramine sulfonamides were treated with electrophile PIDA (15) in hexafluoroisopropanol to give the spirocyclic products 123 in high yields (Scheme 45). However, the similar spirocyclization could not successfully applied for the construction of six-membered spiropiperidine systems.
Scheme 45: PIDA-mediated spirocyclization of phenolic sulfonamide 122 to spiroketones 123.
Scheme 45: PIDA-mediated spirocyclization of phenolic sulfonamide 122 to spiroketones 123.
In 2015, Jain and Ciufolini [124] developed PIDA-mediated spirocyclization of 2-naphtholic sulfonamides 124 to spiropyrrolidine derivatives 125. The spirocyclization reactions were carried out by treating N-sulfonamide substrates 124 with (diacetoxyiodo)benzene (15) in trifluoroacetic acid (TFA) and spiropyrrolidines 125 were isolated in good to excellent yields (Scheme 46). However, the presence of an electron-donating functionality at para-position to the phenolic group induced no spirocyclization product.
Scheme 46: Iodine(III)-mediated oxidative spirocyclization of 2-naphthol derivatives 124 to spiropyrrolidines 125.
Scheme 46: Iodine(III)-mediated oxidative spirocyclization of 2-naphthol derivatives 124 to spiropyrrolidines ...
In 2016, Bray and Shirley [125] reported the oxidative spirocyclization of meta-substituted phenol 126 to tricyclic spiroketals 127a,b in 56% yield using PIDA (15) as electrophilic species in acetonitrile at room temperature (Scheme 47). The mixture of both isomers was separated by flash column chromatography and the stereochemistry of major isomer 127a was assigned on the basis of NOE. This spirocyclic functionality is the basic nucleus found in the phorbaketal family of natural products.
Scheme 47: PIDA-mediated oxidative spirocyclization of m-substituted phenols 126 to tricyclic spiroketals 127.
Scheme 47: PIDA-mediated oxidative spirocyclization of m-substituted phenols 126 to tricyclic spiroketals 127.
5.2. Stereoselective synthesis of chiral spirocyclic ketals
Recently, Ishihara and co-workers [126] synthesized chiral C2-symmetric iodoarene 129a and 129b (Figure 3) in few steps and used as precatalyst in iodine(III)-catalyzed enantioselective synthesis of spiroketals with high selectivities.
Figure 3: The structures of chiral organoiodine(III) catalysts 129a and 129b.
Figure 3: The structures of chiral organoiodine(III) catalysts 129a and 129b.
In this report, substrates 128 were reacted with 10 mol % of chiral iodoarene 129a and 129b in the presence of mCPBA oxidant in chloroform at 0 °C. The desired ortho-spirocyclic ketals 130 were obtained in high yields with more than 93% enantiomeric excess (Scheme 48). Interestingly, the higher selectivities were observed with chiral hypervalent iodine(III) reagent 129b compared to 129a.
Scheme 48: Iodine(III)-catalyzed oxidative spirocyclization of substituted phenols 128 to spirocyclic ketals 130.
Scheme 48: Iodine(III)-catalyzed oxidative spirocyclization of substituted phenols 128 to spirocyclic ketals 1...
5.3. Application of miscellaneous spirocyclic compounds in natural product synthesis
Various hypervalent iodine reagents have been proved as vital reagents during the synthesis of several natural products containing spirocyclic skeleton. In 1999, Ley and co-workers [127] used polymeric PIDA reagent 132 to achieve the synthesis of spirocyclic core of natural product (+)-epidihydromaritidine (134). In this report, para-substituted phenol 131 was cyclized to spirodienone 133 using polymer supported (diacetoxy)iodobenzene reagent 132 (Scheme 49). The desired product 133 was obtained in 70% yield without conventional work-up procedure and purification by chromatographic technique. Furthermore, synthesized spirocyclic compound 133 was converted into the alkaloid (+)-epidihydromaritidine (134) in three chemical steps.
Scheme 49: Oxidative spirocyclization of para-substituted phenol 131 to spirodienone 133 using polymer supported iodine(III) reagent 132.
Scheme 49: Oxidative spirocyclization of para-substituted phenol 131 to spirodienone 133 using polymer support...
Furthermore, Wipf and co-workers [128] reported a new synthetic route for the synthesis of deoxypreussomerin A (137) and palmarumycin CP1 (138). During the first step, the synthesis of spirocyclic compound 136 was achieved in 87% yield by the reaction of PIDA (15) with the naphthol derivative 135 in trifluoroethanol at room temperature. Additionally, synthesized compound 136 was used as key intermediate in the total synthesis of natural products 137 and 138 (Scheme 50). Additionally, more analogues of palmarumycin CP1 were synthesized later which were showing good thioredoxin–thioredoxin reductase (Trx-1/TrxR) inhibitory activity [129]. It was observed that the introduction of enone functionality in naphthoquinone spiroketal enhances the biological activity of palmarumycin 138.
Scheme 50: Oxidative cyclization of bis-hydroxynaphthyl ether 135 to spiroketal 136 using PIDA (15) as an electrophile.
Scheme 50: Oxidative cyclization of bis-hydroxynaphthyl ether 135 to spiroketal 136 using PIDA (15) as an elec...
Furthermore, Ley and co-workers [130] employed polymer-supported iodine(III) reagent during the total synthesis of Amaryllidaceae alkaloid (+)-plicamine (141). In this report, spirodienone 140 was synthesized in 82% yield by the oxidative spirocyclization of p-substituted phenolic substrate 139 using polymer-supported iodonium diacetate 132 in 2,2,2-trifluoroethanol/DCM at −10 °C (Scheme 51). Additionally, the synthesized functionalized spirodienone 140 was used as precursor for the synthesis of (+)-plicamine (141).
Scheme 51: Oxidative spirocyclization of phenolic compound 139 to spirodienone 140 using polymer-supported PIDA 132.
Scheme 51: Oxidative spirocyclization of phenolic compound 139 to spirodienone 140 using polymer-supported PID...
In 2002, Quideau and co-workers [131] developed the synthesis of marine sesquiterpenoid (+)-puupehenone starting from catechol derivative 142. Marine sesquiterpenoids are mainly known for their biological importance such as antitumor, antiviral and antibiotic properties [132]. In this report, the catechol-derived starting substrate 142 was cyclized to spirocyclic product 143 in 67% yield using PIFA (31) as suitable electrophile in dichloromethane at −25 °C (Scheme 52). Furthermore, the spirocyclic product 143 assists as the key synthetic intermediate in the synthesis of the marine natural product (+)-puupehenone (144).
Scheme 52: PIFA-mediated oxidative cyclization of catechol derived substrate 142 to spirocyclic product 143.
Scheme 52: PIFA-mediated oxidative cyclization of catechol derived substrate 142 to spirocyclic product 143.
In 2005, Marco and co-workers [133] reported the synthesis of naturally occurring spiroacetals aculeatin A (146a) and aculeatin B (146b) and iodine(III) reagent was used as an electrophile in one step during their synthesis. In this report, p-substituted phenolic substrate 145 was directly cyclized to naturally occuring spirocyclic optical isomers 146a and 146b using PIFA (31) in solvent combination of CH3COCH3/H2O (9:1) at room temperature for 24 h. The spirocyclic compound 146 was obtained as two optical isomers in 5.5:1 ratio with overall 65% yield (Scheme 53).
Scheme 53: Oxidative spirocyclization of p-substituted phenolic substrate 145 to aculeatin A (146a) and aculeatin B (146b) using PIFA (31).
Scheme 53: Oxidative spirocyclization of p-substituted phenolic substrate 145 to aculeatin A (146a) and aculea...
Furthermore, Peuchmaur and Wong [134] developed a new synthetic route for the total synthesis of the natural product (±)-aculeatin starting from substrate 147. (±)-Aculeatin and its derivatives possessing spirocyclic skeleton are known for their antibacterial and antiprotozoal properties [135]. In this report, substrate 147 was cyclized to spiroketals, i.e., (−)-aculeatin (146a) and (+)-146b in 3:2 ratio. Herein, 1.0 equivalent of PIFA (31) was used as an electrophile, 0.4 equivalents of TFA as non-nucleophilic counter anion in solvent combination of Me2CO/H2O (10:1) at room temperature for 15 minutes (Scheme 54). The reaction proceeds through phenolic oxidative cyclization of phenolic substrate 147 which is the key step in the overall synthesis. The absolute configuration of the synthesised compound was determined by comparing the optical rotary values with that of natural compound (−)-aculeatin (146a) and (+)-aculeatin (146b).
Scheme 54: Oxidative spirocyclization of p-substituted phenolic substrate 147 to aculeatin A (146a) and aculeatin B (146b) using PIFA (31).
Scheme 54: Oxidative spirocyclization of p-substituted phenolic substrate 147 to aculeatin A (146a) and aculea...
In the continuation to previous work, the same research group [136] reported the synthesis of aculeatin D. In this report, the p-substituted phenolic compound 148 was directly cyclized to natural product aculeatin D (149) in 77% yield using PIFA (31) (Scheme 55).
Scheme 55: Oxidative spirocyclization of p-substituted phenolic substrate 148 to aculeatin D (149) using electrophilic species PIFA (31).
Scheme 55: Oxidative spirocyclization of p-substituted phenolic substrate 148 to aculeatin D (149) using elect...
In 2006, Ley and co-workers [137] reported the total synthesis of natural product (±)-oxomaritidine (151) starting from phenolic substrates and polymer-supported hypervalent iodine reagent was used in one step. In this report, p-substituted phenolic compound 131 was cyclized to spirocyclic compound 133 in 50% yield containing a seven membered ring system. The cyclization reaction was carried out using polymer-supported PIFA reagent 150 as an electrophile and trifluoroacetic anhydride (TFAA) as an additive at 80 °C in a microreactor without using any solvent (Scheme 56). Additionally, synthesised compound 133 was used as precursor for the synthesis of (±)-oxomaritidine (151).
Scheme 56: Cyclization of phenolic substrate 131 to spirocyclic product 133 using polymer-supported PIFA 150.
Scheme 56: Cyclization of phenolic substrate 131 to spirocyclic product 133 using polymer-supported PIFA 150.
In 2007, Lalic and Corey [138] reported the synthetic pathway for the synthesis of the naturally occurring antibiotic platensimycin (154) which is isolated from Streptomyces platensis. In this report, 6-methoxy-1,4-naphthoquinone-4-ethylene ketal (153) was synthesized by intermolecular oxidative cyclization of 7-methoxy-α-naphthol (152) with ethylene glycol in the presence of PIFA (31) in acetonitrile. The reaction product 153 was isolated in 80% yield (Scheme 57). Additionally, the synthesised compound 153 was converted into the antibiotic platensimycin (154) after nine chemical steps.
Scheme 57: Iodine(III)-mediated oxidative intermolecular spirocyclization of 7-methoxy-α-naphthol (152) to spirocyclic compound 153.
Scheme 57: Iodine(III)-mediated oxidative intermolecular spirocyclization of 7-methoxy-α-naphthol (152) to spi...
Furthermore, the same electrophilic species 15 was used to cyclize ortho-substituted phenolic compounds 155 to spiroketals 156 by Quideau and co-workers [139]. The cyclization reactions were performed in trifluoroethanol and spirocyclic ketals 156 were isolated in useful yields (Scheme 58). Additionally, the synthesized spiroketal 156 (R = iPr; R1 = iPr) was used as substrate for the synthesis of natural product (+)-biscarvacrol (157).
Scheme 58: Oxidative cyclization of phenols 155 to spiro-ketals 156 using electrophilic species PIDA (15).
Scheme 58: Oxidative cyclization of phenols 155 to spiro-ketals 156 using electrophilic species PIDA (15).
Koag and Lee [140] reported the synthesis of a spiroketal by radical cyclization of a steroidal alkylamine in presence of PIDA (15) as oxidant and molecular iodine in dichloromethane at low temperature. It is an example of hypoiodite-mediated radical cyclization wherein the oxazaspiroketal moiety is formed which is further used as key intermediate for the synthesis of the natural product cephalostatin.
Additionally, spiroketals 159 were also synthesised by enatioselective spirocyclization of ortho-substituted phenols 158 using similar chiral auxiliaries 129a or 129b under similar reaction conditions mentioned in Scheme 48. Furthermore, the synthesized spiroketal 159 (R2 = iPr; R4 = SiMe3) was used as synthetic intermediate for enantioselective synthesis of natural product (−)-biscarvacrol [8] (Scheme 59). Additionally, Parra and Reboredo compiled a review article where authors have covered various aspects of stereoselective spirocyclizations using chiral hypervalent iodine reagents [44]. This review article would be more interesting for readers and provides some significant information about the utility of chiral iodine(III) reagents in enantioselective spirocyclizations with suitable detail.
Scheme 59: Iodine(III)-catalyzed oxidative spirocyclization of ortho-substituted phenols 158 to spirocyclic ketals 159.
Scheme 59: Iodine(III)-catalyzed oxidative spirocyclization of ortho-substituted phenols 158 to spirocyclic ke...
Conclusion
In this review article, we have summarized different approaches for the synthesis of spirocyclic scaffolds using hypervalent iodine reagents in stoichiometric or catalytic amounts. Various iodine(III) reagents such as (diacetoxyiodo)benzene, [bis(trifluoroacetoxy)iodo]benzene and Koser’s reagent have been used to achieve a variety of spirocyclization reactions under mild reaction conditions. Various hypervalent iodine-catalyzed spirocyclization of functionalized phenols and aromatic amines have been successfully developed using iodoarenes as precatalyst in the presence of terminal oxidants. In addition, this review highlights various stereoselective spirocyclizations using chiral hypervalent iodine reagents. Finally, the recent applications of hypervalent iodine reagents in natural product synthesis are also covered.
References
-
Nemoto, T.; Hamada, Y. Yuki Gosei Kagaku Kyokaishi 2015, 73, 977–986. doi:10.5059/yukigoseikyokaishi.73.977
Return to citation in text: [1] [2] -
Li, Y.; Cheng, L.; Liu, X.; Li, B.; Sun, N. Beilstein J. Org. Chem. 2014, 10, 2886–2891. doi:10.3762/bjoc.10.305
Return to citation in text: [1] [2] -
Zuo, Z.; Yang, X.; Liu, J.; Nan, J.; Bai, L.; Wang, Y.; Luan, X. J. Org. Chem. 2015, 80, 3349–3356. doi:10.1021/acs.joc.5b00316
Return to citation in text: [1] [2] -
Sun, W.; Li, G.; Hong, L.; Wang, R. Org. Biomol. Chem. 2016, 14, 2164–2176. doi:10.1039/C5OB02526E
Return to citation in text: [1] [2] -
Petersen, A. B.; Rønnest, M. H.; Larsen, T. O.; Clausen, M. H. Chem. Rev. 2014, 114, 12088–12107. doi:10.1021/cr400368e
Return to citation in text: [1] -
Bhakuni, D. S.; Gupta, S. J. Nat. Prod. 1982, 45, 407–411. doi:10.1021/np50022a007
Return to citation in text: [1] -
Pouységu, L.; Deffieux, D.; Quideau, S. Tetrahedron 2010, 66, 2235–2261. doi:10.1016/j.tet.2009.12.046
Return to citation in text: [1] -
Pouységu, L.; Chassaing, S.; Dejugnac, D.; Lamidey, A.-M.; Miqueu, K.; Sotiropoulos, J.-M.; Quideau, S. Angew. Chem., Int. Ed. 2008, 47, 3552–3555. doi:10.1002/anie.200705816
Return to citation in text: [1] [2] -
Zheng, Y.; Tice, C. M.; Singh, S. B. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. doi:10.1016/j.bmcl.2014.06.081
Return to citation in text: [1] -
Zheng, Y.-J.; Tice, C. M. Expert Opin. Drug Discovery 2016, 11, 831–834. doi:10.1080/17460441.2016.1195367
Return to citation in text: [1] -
Yang, Y.-L.; Chang, F.-R.; Wu, Y.-C. Helv. Chim. Acta 2004, 87, 1392–1399. doi:10.1002/hlca.200490127
Return to citation in text: [1] -
Oxford, A. E.; Raistrick, H.; Simonart, P. Biochem. J. 1939, 33, 240–248. doi:10.1042/bj0330240
Return to citation in text: [1] -
Davenport-Hines, R. P. T.; Slinn, J. Glaxo: A History to 1962; Cambridge University Press: Cambridge, 1992; p 219.
Return to citation in text: [1] -
Cava, M. P.; Nomura, K.; Schlessinger, R. H.; Buck, K. T.; Douglas, B.; Raffauf, R. F.; Weisbach, J. A. Chem. Ind. 1964, 282–283.
Return to citation in text: [1] -
Ohtake, Y.; Sato, T.; Kobayashi, T.; Nishimoto, M.; Taka, N.; Takano, K.; Yamamoto, K.; Ohmori, M.; Yamaguchi, M.; Takami, K.; Yeu, S.-Y.; Ahn, K.-H.; Matsuoka, H.; Morikawa, K.; Suzuki, M.; Hagita, H.; Ozawa, K.; Yamaguchi, K.; Kato, M.; Ikeda, S. J. Med. Chem. 2012, 55, 7828–7840. doi:10.1021/jm300884k
Return to citation in text: [1] -
Olver, I. Lancet Oncol. 2015, 16, 1006–1007. doi:10.1016/S1470-2045(15)00096-0
Return to citation in text: [1] -
Basarab, G. S.; Doig, P.; Galullo, V.; Kern, G.; Kimzey, A.; Kutschke, A.; Newman, J. P.; Morningstar, M.; Mueller, J.; Otterson, L.; Vishwanathan, K.; Zhou, F.; Gowravaram, M. J. Med. Chem. 2015, 58, 6264–6282. doi:10.1021/acs.jmedchem.5b00863
Return to citation in text: [1] -
Wirth, T. Angew. Chem. 2001, 113, 2893–2895. doi:10.1002/1521-3757(20010803)113:15<2893::aid-ange2893>3.0.co;2-f
Angew. Chem. Int. Ed. 2001, 40, 2812–2814. doi:10.1002/1521-3773(20010803)40:15<2812::AID-ANIE2812>3.0.CO;2-X
Return to citation in text: [1] -
Wirth, T. In Organic Synthesis Highlights; Schmalz, H.-G.; Wirth, T., Eds.; Wiley-VCH: Weinheim, 2003; p 144.
Return to citation in text: [1] [2] -
Wirth, T. Hypervalent Iodine Chemistry. In Topics in Current Chemistry; Wirth, T., Ed.; Springer-Verlag: Berlin Heidelberg, 2003; Vol. 224. doi:10.1007/3-540-46114-0
Return to citation in text: [1] [2] -
Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2002, 102, 2523–2584. doi:10.1021/cr010003+
Return to citation in text: [1] [2] [3] -
Wirth, T. Angew. Chem. 2005, 117, 3722–3731. doi:10.1002/ange.200500115
Angew. Chem. Int. Ed. 2005, 44, 3656–3665. doi:10.1002/anie.200500115
Return to citation in text: [1] [2] -
Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299–5358. doi:10.1021/cr800332c
Return to citation in text: [1] [2] -
Zhdankin, V. V. ARKIVOC 2009, No. 1, 1–62. doi:10.3998/ark.5550190.0010.101
Return to citation in text: [1] [2] -
Farooq, U.; Shah, A.-u.-H. A.; Wirth, T. Angew. Chem. 2009, 121, 1036–1038. doi:10.1002/ange.200805027
Angew. Chem. Int. Ed. 2009, 48, 1018-1020. doi:10.1002/anie.200805027
Return to citation in text: [1] -
Zhdankin, V. V. J. Org. Chem. 2011, 76, 1185–1197. doi:10.1021/jo1024738
Return to citation in text: [1] -
Singh, F. V.; Wirth, T. Oxidative Functionalization with Hypervalent Halides. In Comprehensive Organic Synthesis II, 2nd ed.; Molander, G. A.; Knochel, P., Eds.; Elsevier: Oxford, 2014; Vol. 7, pp 880–933. doi:10.1016/B978-0-08-097742-3.00735-7
Return to citation in text: [1] [2] [3] -
Moriarty, R. M. J. Org. Chem. 2005, 70, 2893–2903. doi:10.1021/jo050117b
Return to citation in text: [1] -
Ladziata, U.; Zhdankin, V. V. Synlett 2007, 527–537. doi:10.1055/s-2007-967983
Return to citation in text: [1] -
Merritt, E. A.; Olofsson, B. Angew. Chem. 2009, 121, 9214–9234. doi:10.1002/ange.200904689
Angew. Chem. Int. Ed. 2009, 48, 9052-9070. doi:10.1002/anie.200904689
Return to citation in text: [1] -
Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547
Return to citation in text: [1] [2] -
Quideau, S.; Pouységu, L.; Deffieux, D. Synlett 2008, 467–495. doi:10.1055/s-2008-1032094
Return to citation in text: [1] [2] [3] [4] -
Uyanik, M.; Ishihara, K. Chem. Commun. 2009, 2086–2099. doi:10.1039/B823399C
Return to citation in text: [1] [2] -
Liang, H.; Ciufolini, M. A. Tetrahedron 2010, 66, 5884–5892. doi:10.1016/j.tet.2010.05.020
Return to citation in text: [1] [2] -
Satam, V.; Harad, A.; Rajule, R.; Pati, H. Tetrahedron 2010, 66, 7659–7706. doi:10.1016/j.tet.2010.07.014
Return to citation in text: [1] [2] -
Guérard, K. C.; Sabot, C.; Beaulieu, M.-A.; Giroux, M.-A.; Canesi, S. Tetrahedron 2010, 66, 5893–5901. doi:10.1016/j.tet.2010.03.096
Return to citation in text: [1] -
Singh, F. V.; Wirth, T. Org. Lett. 2011, 13, 6504–6507. doi:10.1021/ol202800k
Return to citation in text: [1] -
Mangaonkar, S. R.; Kole, P. B.; Singh, F. V. Synlett 2018, 199–202. doi:10.1055/s-0036-1588575
Return to citation in text: [1] -
Merritt, E. A.; Olofsson, B. Synthesis 2011, 517–538. doi:10.1055/s-0030-1258328
Return to citation in text: [1] -
Tinnis, F.; Stridfeldt, E.; Lundberg, H.; Adolfsson, H.; Olofsson, B. Org. Lett. 2015, 17, 2688–2691. doi:10.1021/acs.orglett.5b01079
Return to citation in text: [1] -
Ghosh, R.; Stridfeldt, E.; Olofsson, B. Chem. – Eur. J. 2014, 20, 8888–8892. doi:10.1002/chem.201403523
Return to citation in text: [1] -
Ghosh, R.; Lindstedt, E.; Jalalian, N.; Olofsson, B. ChemistryOpen 2014, 3, 54–57. doi:10.1002/open.201402006
Return to citation in text: [1] -
Brown, M.; Delorme, M.; Malmedy, F.; Malmgren, J.; Olofsson, B.; Wirth, T. Synlett 2015, 26, 1573–1577. doi:10.1055/s-0034-1380687
Return to citation in text: [1] -
Parra, A.; Reboredo, S. Chem. – Eur. J. 2013, 19, 17244–17260. doi:10.1002/chem.201302220
Return to citation in text: [1] [2] -
Wang, H.; Fan, R. J. Org. Chem. 2010, 75, 6994–6997. doi:10.1021/jo1014245
Return to citation in text: [1] -
Moriarty, R. M.; Tyagi, S.; Kinch, M. Tetrahedron 2010, 66, 5801–5810. doi:10.1016/j.tet.2010.05.005
Return to citation in text: [1] -
Bose, D. S.; Idrees, M. Synthesis 2010, 393–402. doi:10.1055/s-0029-1217136
Return to citation in text: [1] -
Pardo, L. M.; Tellitu, I.; Domínguez, E. Synthesis 2010, 971–978. doi:10.1055/s-0029-1219218
Return to citation in text: [1] -
Du, X.; Chen, H.; Chen, Y.; Chen, J.; Liu, Y. Synlett 2011, 1010–1014. doi:10.1055/s-0030-1259717
Return to citation in text: [1] -
Wardrop, D. J.; Yermolina, M. V.; Bowen, E. G. Synthesis 2012, 44, 1199–1207. doi:10.1055/s-0031-1290750
Return to citation in text: [1] -
Singh, F. V.; Wirth, T. Synthesis 2012, 44, 1171–1177. doi:10.1055/s-0031-1290588
Return to citation in text: [1] -
Paz, N. R.; Santana, A. G.; Francisco, C. G.; Suárez, E.; González, C. C. Org. Lett. 2012, 14, 3388–3391. doi:10.1021/ol3013638
Return to citation in text: [1] -
Kajiyama, D.; Saitoh, T.; Yamaguchi, S.; Nishiyama, S. Synthesis 2012, 44, 1667–1671. doi:10.1055/s-0031-1291006
Return to citation in text: [1] -
Hempel, C.; Weckenmann, N. M.; Maichle-Moessmer, C.; Nachtsheim, B. J. Org. Biomol. Chem. 2012, 10, 9325–9329. doi:10.1039/C2OB26815A
Return to citation in text: [1] -
Mizar, P.; Wirth, T. Angew. Chem. 2014, 126, 6103–6107. doi:10.1002/ange.201400405
Angew. Chem. Int. Ed. 2014, 53, 5993-5997. doi:10.1002/anie.201400405
Return to citation in text: [1] -
Qian, G.; Liu, B.; Tan, Q.; Zhang, S.; Xu, B. Eur. J. Org. Chem. 2014, 4837–4843. doi:10.1002/ejoc.201402456
Return to citation in text: [1] -
Mizar, P.; Burrelli, A.; Günther, E.; Söftje, M.; Farooq, U.; Wirth, T. Chem. – Eur. J. 2014, 20, 13113–13116. doi:10.1002/chem.201404762
Return to citation in text: [1] -
Yoshimura, A.; Koski, S. R.; Fuchs, J. M.; Saito, A.; Nemykin, V. N.; Zhdankin, V. V. Chem. – Eur. J. 2015, 21, 5328–5331. doi:10.1002/chem.201500335
Return to citation in text: [1] -
Mizar, P.; Niebuhr, R.; Hutchings, M.; Farooq, U.; Wirth, T. Chem. – Eur. J. 2016, 22, 1614–1617. doi:10.1002/chem.201504636
Return to citation in text: [1] -
Singh, F. V.; Wirth, T. Synthesis 2013, 45, 2499–2511. doi:10.1055/s-0033-1339679
Return to citation in text: [1] [2] -
Brown, M.; Kumar, R.; Rehbein, J.; Wirth, T. Chem. – Eur. J. 2016, 22, 4030–4035. doi:10.1002/chem.201504844
Return to citation in text: [1] -
Malmedy, F.; Wirth, T. Chem. – Eur. J. 2016, 22, 16072–16077. doi:10.1002/chem.201603022
Return to citation in text: [1] -
Maertens, G.; L'Homme, C.; Canesi, S. Front. Chem. (Lausanne, Switz.) 2015, 2, No. 115. doi:10.3389/fchem.2014.00115
Return to citation in text: [1] -
Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215–1292. doi:10.1021/cr100280d
Return to citation in text: [1] [2] -
Tamura, Y.; Yakura, T.; Haruta, J.; Kita, Y. J. Org. Chem. 1987, 52, 3927–3930. doi:10.1021/jo00226a041
Return to citation in text: [1] -
Rama Krishna, K. V.; Sujatha, K.; Kapil, R. S. Tetrahedron Lett. 1990, 31, 1351–1352. doi:10.1016/S0040-4039(00)88804-8
Return to citation in text: [1] -
Kita, Y.; Tohma, H.; Kikuchi, K.; Inagaki, M.; Yakura, T. J. Org. Chem. 1991, 56, 435–438. doi:10.1021/jo00001a082
Return to citation in text: [1] -
Wipf, P.; Kim, Y. J. Org. Chem. 1993, 58, 1649–1650. doi:10.1021/jo00059a004
Return to citation in text: [1] -
Ficht, S.; Mülbaier, M.; Giannis, A. Tetrahedron 2001, 57, 4863–4866. doi:10.1016/S0040-4020(01)00424-0
Return to citation in text: [1] [2] -
Fujioka, H.; Komatsu, H.; Nakamura, T.; Miyoshi, A.; Hata, K.; Ganesh, J.; Murai, K.; Kita, Y. Chem. Commun. 2010, 46, 4133–4135. doi:10.1039/B925687C
Return to citation in text: [1] [2] -
Dohi, T.; Nakae, T.; Ishikado, Y.; Kato, D.; Kita, Y. Org. Biomol. Chem. 2011, 9, 6899–6902. doi:10.1039/c1ob06199b
Return to citation in text: [1] [2] [3] -
Dohi, T.; Uchiyama, T.; Yamashita, D.; Washimi, N.; Kita, Y. Tetrahedron Lett. 2011, 52, 2212–2215. doi:10.1016/j.tetlet.2010.12.037
Return to citation in text: [1] -
Moschitto, M. J.; Anthony, D. R.; Lewis, C. A. J. Org. Chem. 2015, 80, 3339–3342. doi:10.1021/acs.joc.5b00107
Return to citation in text: [1] -
Zhang, X.; Hou, W.; Zhang-Negrerie, D.; Zhao, K.; Du, Y. Org. Lett. 2015, 17, 5252–5255. doi:10.1021/acs.orglett.5b02611
Return to citation in text: [1] -
Singh, F. V.; Wirth, T. Chem. – Asian J. 2014, 9, 950–971. doi:10.1002/asia.201301582
Return to citation in text: [1] -
Dohi, T.; Maruyama, A.; Yoshimura, M.; Morimoto, K.; Tohma, H.; Kita, Y. Angew. Chem., Int. Ed. 2005, 44, 6193–6196. doi:10.1002/anie.200501688
Return to citation in text: [1] -
Uyanik, M.; Yasui, T.; Ishihara, K. Bioorg. Med. Chem. Lett. 2009, 19, 3848–3851. doi:10.1016/j.bmcl.2009.03.148
Return to citation in text: [1] -
Dohi, T.; Sasa, H.; Miyazaki, K.; Fujitake, M.; Takenaga, N.; Kita, Y. J. Org. Chem. 2017, 82, 11954–11960. doi:10.1021/acs.joc.7b02037
Return to citation in text: [1] -
Wipf, P.; Spencer, S. R. J. Am. Chem. Soc. 2005, 127, 225–235. doi:10.1021/ja044280k
Return to citation in text: [1] -
Braun, N. A.; Ciufolini, M. A.; Peters, K.; Peters, E.-M. Tetrahedron Lett. 1998, 39, 4667–4670. doi:10.1016/S0040-4039(98)00874-0
Return to citation in text: [1] -
Braun, N. A.; Ousmer, M.; Bray, J. D.; Bouchu, D.; Peters, K.; Peters, E.-M.; Ciufolini, M. A. J. Org. Chem. 2000, 65, 4397–4408. doi:10.1021/jo000341v
Return to citation in text: [1] -
Diaba, F.; Ricou, E.; Solé, D.; Teixidó, E.; Valls, N.; Bonjoch, J. ARKIVOC 2007, No. iv, 320–330. doi:10.3998/ark.5550190.0008.429
Return to citation in text: [1] -
Wardrop, D. J.; Burge, M. S.; Zhang, W.; Ortíz, J. A. Tetrahedron Lett. 2003, 44, 2587–2591. doi:10.1016/S0040-4039(03)00227-2
Return to citation in text: [1] -
Patil, A. D.; Freyer, A. J.; Reichwein, R.; Carte, B.; Killmer, L. B.; Faucette, L.; Johnson, R. K.; Faulkner, D. J. Tetrahedron Lett. 1997, 38, 363–364. doi:10.1016/S0040-4039(96)02304-0
Return to citation in text: [1] -
Honda, T. Pure Appl. Chem. 2010, 82, 1773–1783. doi:10.1351/PAC-CON-09-08-06
Return to citation in text: [1] -
Mizutani, H.; Takayama, J.; Honda, T. Heterocycles 2004, 62, 343–355. doi:10.3987/COM-03-S(P)14
Return to citation in text: [1] -
Wardrop, D. J.; Burge, M. S. J. Org. Chem. 2005, 70, 10271–10284. doi:10.1021/jo051252r
Return to citation in text: [1] -
Christodoulou, M. S.; Kasiotis, K. M.; Fokialakis, N.; Tellitu, I.; Haroutounian, S. A. Tetrahedron Lett. 2008, 49, 7100–7102. doi:10.1016/j.tetlet.2008.09.098
Return to citation in text: [1] -
Liang, J.; Chen, J.; Du, F.; Zeng, X.; Li, L.; Zhang, H. Org. Lett. 2009, 11, 2820–2823. doi:10.1021/ol901005x
Return to citation in text: [1] -
Wang, K.; Fu, X.; Liu, J.; Liang, Y.; Dong, D. Org. Lett. 2009, 11, 1015–1018. doi:10.1021/ol802952e
Return to citation in text: [1] -
Wang, J.; Yuan, Y.; Xiong, R.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Org. Lett. 2012, 14, 2210–2213. doi:10.1021/ol300418h
Return to citation in text: [1] -
Sreenithya, A.; Sunoj, R. B. Org. Lett. 2014, 16, 6224–6227. doi:10.1021/ol503161g
Return to citation in text: [1] -
Abdellaoui, H.; Xu, J. Tetrahedron 2014, 70, 4323–4330. doi:10.1016/j.tet.2014.05.008
Return to citation in text: [1] -
Southgate, R.; Branch, C.; Coulton, S.; Hunt, E. In Recent Progress in the Chemical Synthesis of Antibiotics and Related Microbial Products; Luckacs, G., Ed.; Spirnger: Berlin, 1993; Vol. 2, pp 621–630.
Return to citation in text: [1] -
Jin, C.-Y.; Du, J.-Y.; Zeng, C.; Zhao, X.-H.; Cao, Y.-X.; Zhang, X.-Z.; Lu, X.-Y.; Fan, C.-A. Adv. Synth. Catal. 2014, 356, 2437–2444. doi:10.1002/adsc.201400191
Return to citation in text: [1] -
Chen, Z.-W.; Zhu, Y.-Z.; Ou, J.-W.; Wang, Y.-P.; Zheng, J.-Y. J. Org. Chem. 2014, 79, 10988–10998. doi:10.1021/jo5020307
Return to citation in text: [1] -
Wen, J.; Wei, W.; Xue, S.; Yang, D.; Lou, Y.; Gao, C.; Wang, H. J. Org. Chem. 2015, 80, 4966–4972. doi:10.1021/acs.joc.5b00361
Return to citation in text: [1] -
Dohi, T.; Maruyama, A.; Minamitsuji, Y.; Takenaga, N.; Kita, Y. Chem. Commun. 2007, 1224–1226. doi:10.1039/B616510A
Return to citation in text: [1] -
Jaegli, S.; Dufour, J.; Wei, H.-l.; Piou, T.; Duan, X.-H.; Vors, J.-P.; Neuville, L.; Zhu, J. Org. Lett. 2010, 12, 4498–4501. doi:10.1021/ol101778c
Return to citation in text: [1] -
Dohi, T.; Takenaga, N.; Fukushima, K.-i.; Uchiyama, T.; Kato, D.; Motoo, S.; Fujioka, H.; Kita, Y. Chem. Commun. 2010, 46, 7697–7699. doi:10.1039/C0CC03213A
Return to citation in text: [1] -
Yu, Z.; Ju, X.; Wang, J.; Yu, W. Synthesis 2011, 6, 860–866. doi:10.1055/s-0030-1259444
Return to citation in text: [1] -
Zhang, D.-Y.; Xu, L.; Wu, H.; Gong, L.-Z. Chem. – Eur. J. 2015, 21, 10314–10317. doi:10.1002/chem.201501583
Return to citation in text: [1] -
Ousmer, M.; Braun, N. A.; Bavoux, C.; Perrin, M.; Ciufolini, M. A. J. Am. Chem. Soc. 2001, 123, 7534–7538. doi:10.1021/ja016030z
Return to citation in text: [1] -
Ousmer, M.; Braun, N. A.; Ciufolini, M. A. Org. Lett. 2001, 3, 765–767. doi:10.1021/ol015526i
Return to citation in text: [1] -
Mizutani, H.; Takayama, J.; Soeda, Y.; Honda, T. Tetrahedron Lett. 2002, 43, 2411–2414. doi:10.1016/S0040-4039(02)00296-4
Return to citation in text: [1] -
Kita, Y.; Tohma, H.; Inagaki, M.; Hatanaka, K.; Kikuchi, K.; Yakura, T. Tetrahedron Lett. 1991, 32, 2035–2038. doi:10.1016/S0040-4039(00)78901-5
Return to citation in text: [1] -
Kita, Y.; Tohma, H.; Inagaki, M.; Hatanaka, K.; Yakura, T. J. Am. Chem. Soc. 1992, 114, 2175–2180. doi:10.1021/ja00032a036
Return to citation in text: [1] -
Kita, Y.; Takada, T.; Ibaraki, M.; Gyoten, M.; Mihara, S.; Fujita, S.; Tohma, H. J. Org. Chem. 1996, 61, 223–227. doi:10.1021/jo951439q
Return to citation in text: [1] -
Asmanidou, A.; Papoutsis, I.; Spyroudis, S.; Varvoglis, A. Molecules 2000, 5, 874–879. doi:10.3390/50600874
Return to citation in text: [1] -
Zheng, C.; Wang, L.; Li, J.; Wang, L.; Wang, D. Z. Org. Lett. 2013, 15, 4046–4049. doi:10.1021/ol401863k
Return to citation in text: [1] -
Huang, J.; Wang, H.; Wu, C.; Wulff, W. D. Org. Lett. 2007, 9, 2799–2802. doi:10.1021/ol070904q
Return to citation in text: [1] -
Phipps, R. J.; Toste, F. D. J. Am. Chem. Soc. 2013, 135, 1268–1271. doi:10.1021/ja311798q
Return to citation in text: [1] -
Tohma, H.; Harayama, Y.; Hashizume, M.; Iwata, M.; Kiyono, Y.; Egi, M.; Kita, Y. J. Am. Chem. Soc. 2003, 125, 11235–11240. doi:10.1021/ja0365330
Return to citation in text: [1] -
Kita, Y.; Yakura, T.; Tohma, H.; Kikuchi, K.; Tamura, Y. Tetrahedron Lett. 1989, 30, 1119–1120. doi:10.1016/S0040-4039(01)80375-0
Return to citation in text: [1] -
Shigehisa, H.; Takayama, J.; Honda, T. Tetrahedron Lett. 2006, 47, 7301–7306. doi:10.1016/j.tetlet.2006.08.028
Return to citation in text: [1] -
Honda, T.; Shigehisa, H. Org. Lett. 2006, 8, 657–659. doi:10.1021/ol052841m
Return to citation in text: [1] -
Dohi, T.; Minamitsuji, Y.; Maruyama, A.; Hirose, S.; Kita, Y. Org. Lett. 2008, 10, 3559–3562. doi:10.1021/ol801321f
Return to citation in text: [1] -
Kita, Y.; Takada, T.; Gyoten, M.; Tohma, H.; Zenk, M. H.; Eichhorn, J. J. Org. Chem. 1996, 61, 5857–5864. doi:10.1021/jo9606766
Return to citation in text: [1] -
Kita, Y.; Arisawa, M.; Gyoten, M.; Nakajika, M.; Hamada, R.; Tohma, H.; Takada, T. J. Org. Chem. 1998, 63, 6625–6635. doi:10.1021/jo9807868
Return to citation in text: [1] -
Roe, C.; Stephenson, G. R. Org. Lett. 2008, 10, 189–192. doi:10.1021/ol702550z
Return to citation in text: [1] -
Wada, Y.; Otani, K.; Endo, N.; Harayama, Y.; Kamimura, D.; Yoshida, M.; Fujioka, H.; Kita, Y. Org. Lett. 2009, 11, 4048–4050. doi:10.1021/ol901471r
Return to citation in text: [1] -
Harayama, Y.; Kita, Y. Curr. Org. Chem. 2005, 9, 1567–1588. doi:10.2174/138527205774370568
Return to citation in text: [1] -
Canesi, S.; Belmont, P.; Bouchu, D.; Rousset, L.; Ciufolini, M. A. Tetrahedron Lett. 2002, 43, 5193–5195. doi:10.1016/S0040-4039(02)00949-8
Return to citation in text: [1] -
Jain, N.; Ciufolini, M. A. Synlett 2015, 26, 631–634. doi:10.1055/s-0034-1379960
Return to citation in text: [1] -
Shirley, H. J.; Bray, C. D. Eur. J. Org. Chem. 2016, 1504–1507. doi:10.1002/ejoc.201501370
Return to citation in text: [1] -
Uyanik, M.; Sasakura, N.; Mizuno, M.; Ishihara, K. ACS Catal. 2017, 7, 872–876. doi:10.1021/acscatal.6b03380
Return to citation in text: [1] -
Ley, S. V.; Schucht, O.; Thomas, A. W.; Murray, P. J. J. Chem. Soc., Perkin Trans. 1 1999, 1251–1252. doi:10.1039/A901798D
Return to citation in text: [1] -
Wipf, P.; Jung, J.-K.; Rodríguez, S.; Lazo, J. S. Tetrahedron 2001, 57, 283–296. doi:10.1016/S0040-4020(00)00936-4
Return to citation in text: [1] -
Wipf, P.; Lynch, S. M.; Birmingham, A.; Tamayo, G.; Jiménez, A.; Campos, N.; Powis, G. Org. Biomol. Chem. 2004, 2, 1651–1658. doi:10.1039/B402431A
Return to citation in text: [1] -
Baxendale, I. R.; Ley, S. V.; Nessi, M.; Piutti, C. Tetrahedron Lett. 2002, 58, 6285–6304. doi:10.1016/S0040-4020(02)00628-2
Return to citation in text: [1] -
Quideau, S.; Lebon, M.; Lamidey, A.-M. Org. Lett. 2002, 4, 3975–3978. doi:10.1021/ol026855t
Return to citation in text: [1] -
El Sayed, K. A.; Bartyzel, P.; Shen, X.; Perry, T. L.; Zjawiony, J. K.; Hamman, M. T. Tetrahedron 2000, 56, 949–953. doi:10.1016/S0040-4020(99)01093-5
Return to citation in text: [1] -
Falomir, E.; Álvarez-Bercedo, P.; Carda, M.; Marco, J. A. Tetrahedron Lett. 2005, 46, 8407–8410. doi:10.1016/j.tetlet.2005.09.146
Return to citation in text: [1] -
Peuchmaur, M.; Wong, Y.-S. J. Org. Chem. 2007, 72, 5374–5379. doi:10.1021/jo0707986
Return to citation in text: [1] -
Wong, Y.-S. Chem. Commun. 2002, 686–687. doi:10.1039/B200740A
Return to citation in text: [1] -
Álvarez-Bercedo, P.; Falomir, E.; Carda, M.; Marco, J. A. Tetrahedron 2006, 62, 9641–9649. doi:10.1016/j.tet.2006.07.076
Return to citation in text: [1] -
Baxendale, I. R.; Deeley, J.; Griffiths-Jones, C. M.; Ley, S. V.; Saaby, S.; Tranmer, G. K. Chem. Commun. 2006, 2566–2568. doi:10.1039/B600382F
Return to citation in text: [1] -
Lalic, G.; Corey, E. J. Org. Lett. 2007, 9, 4921–4923. doi:10.1021/ol702323s
Return to citation in text: [1] -
Pouységu, L.; Sylla, T.; Garnier, T.; Rojas, L.; Charris, J.; Deffieux, D.; Quideau, S. Tetrahedron 2010, 66, 5908–5917. doi:10.1016/j.tet.2010.05.078
Return to citation in text: [1] -
Koag, M.; Lee, S. Org. Lett. 2011, 13, 4766–4769. doi:10.1021/ol2017033
Return to citation in text: [1]
| 72. | Dohi, T.; Uchiyama, T.; Yamashita, D.; Washimi, N.; Kita, Y. Tetrahedron Lett. 2011, 52, 2212–2215. doi:10.1016/j.tetlet.2010.12.037 |
| 73. | Moschitto, M. J.; Anthony, D. R.; Lewis, C. A. J. Org. Chem. 2015, 80, 3339–3342. doi:10.1021/acs.joc.5b00107 |
| 118. | Kita, Y.; Takada, T.; Gyoten, M.; Tohma, H.; Zenk, M. H.; Eichhorn, J. J. Org. Chem. 1996, 61, 5857–5864. doi:10.1021/jo9606766 |
| 119. | Kita, Y.; Arisawa, M.; Gyoten, M.; Nakajika, M.; Hamada, R.; Tohma, H.; Takada, T. J. Org. Chem. 1998, 63, 6625–6635. doi:10.1021/jo9807868 |
| 120. | Roe, C.; Stephenson, G. R. Org. Lett. 2008, 10, 189–192. doi:10.1021/ol702550z |
| 74. | Zhang, X.; Hou, W.; Zhang-Negrerie, D.; Zhao, K.; Du, Y. Org. Lett. 2015, 17, 5252–5255. doi:10.1021/acs.orglett.5b02611 |
| 121. | Wada, Y.; Otani, K.; Endo, N.; Harayama, Y.; Kamimura, D.; Yoshida, M.; Fujioka, H.; Kita, Y. Org. Lett. 2009, 11, 4048–4050. doi:10.1021/ol901471r |
| 116. | Honda, T.; Shigehisa, H. Org. Lett. 2006, 8, 657–659. doi:10.1021/ol052841m |
| 117. | Dohi, T.; Minamitsuji, Y.; Maruyama, A.; Hirose, S.; Kita, Y. Org. Lett. 2008, 10, 3559–3562. doi:10.1021/ol801321f |
| 115. | Shigehisa, H.; Takayama, J.; Honda, T. Tetrahedron Lett. 2006, 47, 7301–7306. doi:10.1016/j.tetlet.2006.08.028 |
| 81. | Braun, N. A.; Ousmer, M.; Bray, J. D.; Bouchu, D.; Peters, K.; Peters, E.-M.; Ciufolini, M. A. J. Org. Chem. 2000, 65, 4397–4408. doi:10.1021/jo000341v |
| 82. | Diaba, F.; Ricou, E.; Solé, D.; Teixidó, E.; Valls, N.; Bonjoch, J. ARKIVOC 2007, No. iv, 320–330. doi:10.3998/ark.5550190.0008.429 |
| 79. | Wipf, P.; Spencer, S. R. J. Am. Chem. Soc. 2005, 127, 225–235. doi:10.1021/ja044280k |
| 126. | Uyanik, M.; Sasakura, N.; Mizuno, M.; Ishihara, K. ACS Catal. 2017, 7, 872–876. doi:10.1021/acscatal.6b03380 |
| 80. | Braun, N. A.; Ciufolini, M. A.; Peters, K.; Peters, E.-M. Tetrahedron Lett. 1998, 39, 4667–4670. doi:10.1016/S0040-4039(98)00874-0 |
| 77. | Uyanik, M.; Yasui, T.; Ishihara, K. Bioorg. Med. Chem. Lett. 2009, 19, 3848–3851. doi:10.1016/j.bmcl.2009.03.148 |
| 124. | Jain, N.; Ciufolini, M. A. Synlett 2015, 26, 631–634. doi:10.1055/s-0034-1379960 |
| 78. | Dohi, T.; Sasa, H.; Miyazaki, K.; Fujitake, M.; Takenaga, N.; Kita, Y. J. Org. Chem. 2017, 82, 11954–11960. doi:10.1021/acs.joc.7b02037 |
| 125. | Shirley, H. J.; Bray, C. D. Eur. J. Org. Chem. 2016, 1504–1507. doi:10.1002/ejoc.201501370 |
| 75. | Singh, F. V.; Wirth, T. Chem. – Asian J. 2014, 9, 950–971. doi:10.1002/asia.201301582 |
| 122. | Harayama, Y.; Kita, Y. Curr. Org. Chem. 2005, 9, 1567–1588. doi:10.2174/138527205774370568 |
| 76. | Dohi, T.; Maruyama, A.; Yoshimura, M.; Morimoto, K.; Tohma, H.; Kita, Y. Angew. Chem., Int. Ed. 2005, 44, 6193–6196. doi:10.1002/anie.200501688 |
| 123. | Canesi, S.; Belmont, P.; Bouchu, D.; Rousset, L.; Ciufolini, M. A. Tetrahedron Lett. 2002, 43, 5193–5195. doi:10.1016/S0040-4039(02)00949-8 |
| 83. | Wardrop, D. J.; Burge, M. S.; Zhang, W.; Ortíz, J. A. Tetrahedron Lett. 2003, 44, 2587–2591. doi:10.1016/S0040-4039(03)00227-2 |
| 84. | Patil, A. D.; Freyer, A. J.; Reichwein, R.; Carte, B.; Killmer, L. B.; Faucette, L.; Johnson, R. K.; Faulkner, D. J. Tetrahedron Lett. 1997, 38, 363–364. doi:10.1016/S0040-4039(96)02304-0 |
| 131. | Quideau, S.; Lebon, M.; Lamidey, A.-M. Org. Lett. 2002, 4, 3975–3978. doi:10.1021/ol026855t |
| 132. | El Sayed, K. A.; Bartyzel, P.; Shen, X.; Perry, T. L.; Zjawiony, J. K.; Hamman, M. T. Tetrahedron 2000, 56, 949–953. doi:10.1016/S0040-4020(99)01093-5 |
| 129. | Wipf, P.; Lynch, S. M.; Birmingham, A.; Tamayo, G.; Jiménez, A.; Campos, N.; Powis, G. Org. Biomol. Chem. 2004, 2, 1651–1658. doi:10.1039/B402431A |
| 130. | Baxendale, I. R.; Ley, S. V.; Nessi, M.; Piutti, C. Tetrahedron Lett. 2002, 58, 6285–6304. doi:10.1016/S0040-4020(02)00628-2 |
| 127. | Ley, S. V.; Schucht, O.; Thomas, A. W.; Murray, P. J. J. Chem. Soc., Perkin Trans. 1 1999, 1251–1252. doi:10.1039/A901798D |
| 128. | Wipf, P.; Jung, J.-K.; Rodríguez, S.; Lazo, J. S. Tetrahedron 2001, 57, 283–296. doi:10.1016/S0040-4020(00)00936-4 |
| 91. | Wang, J.; Yuan, Y.; Xiong, R.; Zhang-Negrerie, D.; Du, Y.; Zhao, K. Org. Lett. 2012, 14, 2210–2213. doi:10.1021/ol300418h |
| 92. | Sreenithya, A.; Sunoj, R. B. Org. Lett. 2014, 16, 6224–6227. doi:10.1021/ol503161g |
| 90. | Wang, K.; Fu, X.; Liu, J.; Liang, Y.; Dong, D. Org. Lett. 2009, 11, 1015–1018. doi:10.1021/ol802952e |
| 71. | Dohi, T.; Nakae, T.; Ishikado, Y.; Kato, D.; Kita, Y. Org. Biomol. Chem. 2011, 9, 6899–6902. doi:10.1039/c1ob06199b |
| 88. | Christodoulou, M. S.; Kasiotis, K. M.; Fokialakis, N.; Tellitu, I.; Haroutounian, S. A. Tetrahedron Lett. 2008, 49, 7100–7102. doi:10.1016/j.tetlet.2008.09.098 |
| 89. | Liang, J.; Chen, J.; Du, F.; Zeng, X.; Li, L.; Zhang, H. Org. Lett. 2009, 11, 2820–2823. doi:10.1021/ol901005x |
| 136. | Álvarez-Bercedo, P.; Falomir, E.; Carda, M.; Marco, J. A. Tetrahedron 2006, 62, 9641–9649. doi:10.1016/j.tet.2006.07.076 |
| 86. | Mizutani, H.; Takayama, J.; Honda, T. Heterocycles 2004, 62, 343–355. doi:10.3987/COM-03-S(P)14 |
| 133. | Falomir, E.; Álvarez-Bercedo, P.; Carda, M.; Marco, J. A. Tetrahedron Lett. 2005, 46, 8407–8410. doi:10.1016/j.tetlet.2005.09.146 |
| 87. | Wardrop, D. J.; Burge, M. S. J. Org. Chem. 2005, 70, 10271–10284. doi:10.1021/jo051252r |
| 134. | Peuchmaur, M.; Wong, Y.-S. J. Org. Chem. 2007, 72, 5374–5379. doi:10.1021/jo0707986 |
| 94. | Southgate, R.; Branch, C.; Coulton, S.; Hunt, E. In Recent Progress in the Chemical Synthesis of Antibiotics and Related Microbial Products; Luckacs, G., Ed.; Spirnger: Berlin, 1993; Vol. 2, pp 621–630. |
| 44. | Parra, A.; Reboredo, S. Chem. – Eur. J. 2013, 19, 17244–17260. doi:10.1002/chem.201302220 |
| 95. | Jin, C.-Y.; Du, J.-Y.; Zeng, C.; Zhao, X.-H.; Cao, Y.-X.; Zhang, X.-Z.; Lu, X.-Y.; Fan, C.-A. Adv. Synth. Catal. 2014, 356, 2437–2444. doi:10.1002/adsc.201400191 |
| 93. | Abdellaoui, H.; Xu, J. Tetrahedron 2014, 70, 4323–4330. doi:10.1016/j.tet.2014.05.008 |
| 8. | Pouységu, L.; Chassaing, S.; Dejugnac, D.; Lamidey, A.-M.; Miqueu, K.; Sotiropoulos, J.-M.; Quideau, S. Angew. Chem., Int. Ed. 2008, 47, 3552–3555. doi:10.1002/anie.200705816 |
| 1. | Nemoto, T.; Hamada, Y. Yuki Gosei Kagaku Kyokaishi 2015, 73, 977–986. doi:10.5059/yukigoseikyokaishi.73.977 |
| 2. | Li, Y.; Cheng, L.; Liu, X.; Li, B.; Sun, N. Beilstein J. Org. Chem. 2014, 10, 2886–2891. doi:10.3762/bjoc.10.305 |
| 3. | Zuo, Z.; Yang, X.; Liu, J.; Nan, J.; Bai, L.; Wang, Y.; Luan, X. J. Org. Chem. 2015, 80, 3349–3356. doi:10.1021/acs.joc.5b00316 |
| 4. | Sun, W.; Li, G.; Hong, L.; Wang, R. Org. Biomol. Chem. 2016, 14, 2164–2176. doi:10.1039/C5OB02526E |
| 5. | Petersen, A. B.; Rønnest, M. H.; Larsen, T. O.; Clausen, M. H. Chem. Rev. 2014, 114, 12088–12107. doi:10.1021/cr400368e |
| 138. | Lalic, G.; Corey, E. J. Org. Lett. 2007, 9, 4921–4923. doi:10.1021/ol702323s |
| 139. | Pouységu, L.; Sylla, T.; Garnier, T.; Rojas, L.; Charris, J.; Deffieux, D.; Quideau, S. Tetrahedron 2010, 66, 5908–5917. doi:10.1016/j.tet.2010.05.078 |
| 137. | Baxendale, I. R.; Deeley, J.; Griffiths-Jones, C. M.; Ley, S. V.; Saaby, S.; Tranmer, G. K. Chem. Commun. 2006, 2566–2568. doi:10.1039/B600382F |
| 12. | Oxford, A. E.; Raistrick, H.; Simonart, P. Biochem. J. 1939, 33, 240–248. doi:10.1042/bj0330240 |
| 19. | Wirth, T. In Organic Synthesis Highlights; Schmalz, H.-G.; Wirth, T., Eds.; Wiley-VCH: Weinheim, 2003; p 144. |
| 20. | Wirth, T. Hypervalent Iodine Chemistry. In Topics in Current Chemistry; Wirth, T., Ed.; Springer-Verlag: Berlin Heidelberg, 2003; Vol. 224. doi:10.1007/3-540-46114-0 |
| 27. | Singh, F. V.; Wirth, T. Oxidative Functionalization with Hypervalent Halides. In Comprehensive Organic Synthesis II, 2nd ed.; Molander, G. A.; Knochel, P., Eds.; Elsevier: Oxford, 2014; Vol. 7, pp 880–933. doi:10.1016/B978-0-08-097742-3.00735-7 |
| 102. | Zhang, D.-Y.; Xu, L.; Wu, H.; Gong, L.-Z. Chem. – Eur. J. 2015, 21, 10314–10317. doi:10.1002/chem.201501583 |
| 11. | Yang, Y.-L.; Chang, F.-R.; Wu, Y.-C. Helv. Chim. Acta 2004, 87, 1392–1399. doi:10.1002/hlca.200490127 |
| 21. | Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2002, 102, 2523–2584. doi:10.1021/cr010003+ |
| 22. |
Wirth, T. Angew. Chem. 2005, 117, 3722–3731. doi:10.1002/ange.200500115
Angew. Chem. Int. Ed. 2005, 44, 3656–3665. doi:10.1002/anie.200500115 |
| 23. | Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299–5358. doi:10.1021/cr800332c |
| 24. | Zhdankin, V. V. ARKIVOC 2009, No. 1, 1–62. doi:10.3998/ark.5550190.0010.101 |
| 31. | Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547 |
| 32. | Quideau, S.; Pouységu, L.; Deffieux, D. Synlett 2008, 467–495. doi:10.1055/s-2008-1032094 |
| 33. | Uyanik, M.; Ishihara, K. Chem. Commun. 2009, 2086–2099. doi:10.1039/B823399C |
| 34. | Liang, H.; Ciufolini, M. A. Tetrahedron 2010, 66, 5884–5892. doi:10.1016/j.tet.2010.05.020 |
| 35. | Satam, V.; Harad, A.; Rajule, R.; Pati, H. Tetrahedron 2010, 66, 7659–7706. doi:10.1016/j.tet.2010.07.014 |
| 60. | Singh, F. V.; Wirth, T. Synthesis 2013, 45, 2499–2511. doi:10.1055/s-0033-1339679 |
| 63. | Maertens, G.; L'Homme, C.; Canesi, S. Front. Chem. (Lausanne, Switz.) 2015, 2, No. 115. doi:10.3389/fchem.2014.00115 |
| 64. | Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215–1292. doi:10.1021/cr100280d |
| 9. | Zheng, Y.; Tice, C. M.; Singh, S. B. Bioorg. Med. Chem. Lett. 2014, 24, 3673–3682. doi:10.1016/j.bmcl.2014.06.081 |
| 10. | Zheng, Y.-J.; Tice, C. M. Expert Opin. Drug Discovery 2016, 11, 831–834. doi:10.1080/17460441.2016.1195367 |
| 32. | Quideau, S.; Pouységu, L.; Deffieux, D. Synlett 2008, 467–495. doi:10.1055/s-2008-1032094 |
| 33. | Uyanik, M.; Ishihara, K. Chem. Commun. 2009, 2086–2099. doi:10.1039/B823399C |
| 34. | Liang, H.; Ciufolini, M. A. Tetrahedron 2010, 66, 5884–5892. doi:10.1016/j.tet.2010.05.020 |
| 35. | Satam, V.; Harad, A.; Rajule, R.; Pati, H. Tetrahedron 2010, 66, 7659–7706. doi:10.1016/j.tet.2010.07.014 |
| 36. | Guérard, K. C.; Sabot, C.; Beaulieu, M.-A.; Giroux, M.-A.; Canesi, S. Tetrahedron 2010, 66, 5893–5901. doi:10.1016/j.tet.2010.03.096 |
| 37. | Singh, F. V.; Wirth, T. Org. Lett. 2011, 13, 6504–6507. doi:10.1021/ol202800k |
| 38. | Mangaonkar, S. R.; Kole, P. B.; Singh, F. V. Synlett 2018, 199–202. doi:10.1055/s-0036-1588575 |
| 100. | Dohi, T.; Takenaga, N.; Fukushima, K.-i.; Uchiyama, T.; Kato, D.; Motoo, S.; Fujioka, H.; Kita, Y. Chem. Commun. 2010, 46, 7697–7699. doi:10.1039/C0CC03213A |
| 6. | Bhakuni, D. S.; Gupta, S. J. Nat. Prod. 1982, 45, 407–411. doi:10.1021/np50022a007 |
| 7. | Pouységu, L.; Deffieux, D.; Quideau, S. Tetrahedron 2010, 66, 2235–2261. doi:10.1016/j.tet.2009.12.046 |
| 8. | Pouységu, L.; Chassaing, S.; Dejugnac, D.; Lamidey, A.-M.; Miqueu, K.; Sotiropoulos, J.-M.; Quideau, S. Angew. Chem., Int. Ed. 2008, 47, 3552–3555. doi:10.1002/anie.200705816 |
| 39. | Merritt, E. A.; Olofsson, B. Synthesis 2011, 517–538. doi:10.1055/s-0030-1258328 |
| 40. | Tinnis, F.; Stridfeldt, E.; Lundberg, H.; Adolfsson, H.; Olofsson, B. Org. Lett. 2015, 17, 2688–2691. doi:10.1021/acs.orglett.5b01079 |
| 41. | Ghosh, R.; Stridfeldt, E.; Olofsson, B. Chem. – Eur. J. 2014, 20, 8888–8892. doi:10.1002/chem.201403523 |
| 42. | Ghosh, R.; Lindstedt, E.; Jalalian, N.; Olofsson, B. ChemistryOpen 2014, 3, 54–57. doi:10.1002/open.201402006 |
| 43. | Brown, M.; Delorme, M.; Malmedy, F.; Malmgren, J.; Olofsson, B.; Wirth, T. Synlett 2015, 26, 1573–1577. doi:10.1055/s-0034-1380687 |
| 44. | Parra, A.; Reboredo, S. Chem. – Eur. J. 2013, 19, 17244–17260. doi:10.1002/chem.201302220 |
| 45. | Wang, H.; Fan, R. J. Org. Chem. 2010, 75, 6994–6997. doi:10.1021/jo1014245 |
| 46. | Moriarty, R. M.; Tyagi, S.; Kinch, M. Tetrahedron 2010, 66, 5801–5810. doi:10.1016/j.tet.2010.05.005 |
| 47. | Bose, D. S.; Idrees, M. Synthesis 2010, 393–402. doi:10.1055/s-0029-1217136 |
| 48. | Pardo, L. M.; Tellitu, I.; Domínguez, E. Synthesis 2010, 971–978. doi:10.1055/s-0029-1219218 |
| 49. | Du, X.; Chen, H.; Chen, Y.; Chen, J.; Liu, Y. Synlett 2011, 1010–1014. doi:10.1055/s-0030-1259717 |
| 50. | Wardrop, D. J.; Yermolina, M. V.; Bowen, E. G. Synthesis 2012, 44, 1199–1207. doi:10.1055/s-0031-1290750 |
| 51. | Singh, F. V.; Wirth, T. Synthesis 2012, 44, 1171–1177. doi:10.1055/s-0031-1290588 |
| 52. | Paz, N. R.; Santana, A. G.; Francisco, C. G.; Suárez, E.; González, C. C. Org. Lett. 2012, 14, 3388–3391. doi:10.1021/ol3013638 |
| 53. | Kajiyama, D.; Saitoh, T.; Yamaguchi, S.; Nishiyama, S. Synthesis 2012, 44, 1667–1671. doi:10.1055/s-0031-1291006 |
| 54. | Hempel, C.; Weckenmann, N. M.; Maichle-Moessmer, C.; Nachtsheim, B. J. Org. Biomol. Chem. 2012, 10, 9325–9329. doi:10.1039/C2OB26815A |
| 55. |
Mizar, P.; Wirth, T. Angew. Chem. 2014, 126, 6103–6107. doi:10.1002/ange.201400405
Angew. Chem. Int. Ed. 2014, 53, 5993-5997. doi:10.1002/anie.201400405 |
| 56. | Qian, G.; Liu, B.; Tan, Q.; Zhang, S.; Xu, B. Eur. J. Org. Chem. 2014, 4837–4843. doi:10.1002/ejoc.201402456 |
| 57. | Mizar, P.; Burrelli, A.; Günther, E.; Söftje, M.; Farooq, U.; Wirth, T. Chem. – Eur. J. 2014, 20, 13113–13116. doi:10.1002/chem.201404762 |
| 58. | Yoshimura, A.; Koski, S. R.; Fuchs, J. M.; Saito, A.; Nemykin, V. N.; Zhdankin, V. V. Chem. – Eur. J. 2015, 21, 5328–5331. doi:10.1002/chem.201500335 |
| 59. | Mizar, P.; Niebuhr, R.; Hutchings, M.; Farooq, U.; Wirth, T. Chem. – Eur. J. 2016, 22, 1614–1617. doi:10.1002/chem.201504636 |
| 60. | Singh, F. V.; Wirth, T. Synthesis 2013, 45, 2499–2511. doi:10.1055/s-0033-1339679 |
| 61. | Brown, M.; Kumar, R.; Rehbein, J.; Wirth, T. Chem. – Eur. J. 2016, 22, 4030–4035. doi:10.1002/chem.201504844 |
| 62. | Malmedy, F.; Wirth, T. Chem. – Eur. J. 2016, 22, 16072–16077. doi:10.1002/chem.201603022 |
| 101. | Yu, Z.; Ju, X.; Wang, J.; Yu, W. Synthesis 2011, 6, 860–866. doi:10.1055/s-0030-1259444 |
| 16. | Olver, I. Lancet Oncol. 2015, 16, 1006–1007. doi:10.1016/S1470-2045(15)00096-0 |
| 1. | Nemoto, T.; Hamada, Y. Yuki Gosei Kagaku Kyokaishi 2015, 73, 977–986. doi:10.5059/yukigoseikyokaishi.73.977 |
| 2. | Li, Y.; Cheng, L.; Liu, X.; Li, B.; Sun, N. Beilstein J. Org. Chem. 2014, 10, 2886–2891. doi:10.3762/bjoc.10.305 |
| 3. | Zuo, Z.; Yang, X.; Liu, J.; Nan, J.; Bai, L.; Wang, Y.; Luan, X. J. Org. Chem. 2015, 80, 3349–3356. doi:10.1021/acs.joc.5b00316 |
| 98. | Dohi, T.; Maruyama, A.; Minamitsuji, Y.; Takenaga, N.; Kita, Y. Chem. Commun. 2007, 1224–1226. doi:10.1039/B616510A |
| 15. | Ohtake, Y.; Sato, T.; Kobayashi, T.; Nishimoto, M.; Taka, N.; Takano, K.; Yamamoto, K.; Ohmori, M.; Yamaguchi, M.; Takami, K.; Yeu, S.-Y.; Ahn, K.-H.; Matsuoka, H.; Morikawa, K.; Suzuki, M.; Hagita, H.; Ozawa, K.; Yamaguchi, K.; Kato, M.; Ikeda, S. J. Med. Chem. 2012, 55, 7828–7840. doi:10.1021/jm300884k |
| 18. |
Wirth, T. Angew. Chem. 2001, 113, 2893–2895. doi:10.1002/1521-3757(20010803)113:15<2893::aid-ange2893>3.0.co;2-f
Angew. Chem. Int. Ed. 2001, 40, 2812–2814. doi:10.1002/1521-3773(20010803)40:15<2812::AID-ANIE2812>3.0.CO;2-X |
| 19. | Wirth, T. In Organic Synthesis Highlights; Schmalz, H.-G.; Wirth, T., Eds.; Wiley-VCH: Weinheim, 2003; p 144. |
| 20. | Wirth, T. Hypervalent Iodine Chemistry. In Topics in Current Chemistry; Wirth, T., Ed.; Springer-Verlag: Berlin Heidelberg, 2003; Vol. 224. doi:10.1007/3-540-46114-0 |
| 21. | Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2002, 102, 2523–2584. doi:10.1021/cr010003+ |
| 22. |
Wirth, T. Angew. Chem. 2005, 117, 3722–3731. doi:10.1002/ange.200500115
Angew. Chem. Int. Ed. 2005, 44, 3656–3665. doi:10.1002/anie.200500115 |
| 23. | Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2008, 108, 5299–5358. doi:10.1021/cr800332c |
| 24. | Zhdankin, V. V. ARKIVOC 2009, No. 1, 1–62. doi:10.3998/ark.5550190.0010.101 |
| 25. |
Farooq, U.; Shah, A.-u.-H. A.; Wirth, T. Angew. Chem. 2009, 121, 1036–1038. doi:10.1002/ange.200805027
Angew. Chem. Int. Ed. 2009, 48, 1018-1020. doi:10.1002/anie.200805027 |
| 26. | Zhdankin, V. V. J. Org. Chem. 2011, 76, 1185–1197. doi:10.1021/jo1024738 |
| 27. | Singh, F. V.; Wirth, T. Oxidative Functionalization with Hypervalent Halides. In Comprehensive Organic Synthesis II, 2nd ed.; Molander, G. A.; Knochel, P., Eds.; Elsevier: Oxford, 2014; Vol. 7, pp 880–933. doi:10.1016/B978-0-08-097742-3.00735-7 |
| 28. | Moriarty, R. M. J. Org. Chem. 2005, 70, 2893–2903. doi:10.1021/jo050117b |
| 29. | Ladziata, U.; Zhdankin, V. V. Synlett 2007, 527–537. doi:10.1055/s-2007-967983 |
| 30. |
Merritt, E. A.; Olofsson, B. Angew. Chem. 2009, 121, 9214–9234. doi:10.1002/ange.200904689
Angew. Chem. Int. Ed. 2009, 48, 9052-9070. doi:10.1002/anie.200904689 |
| 31. | Yoshimura, A.; Zhdankin, V. V. Chem. Rev. 2016, 116, 3328–3435. doi:10.1021/acs.chemrev.5b00547 |
| 99. | Jaegli, S.; Dufour, J.; Wei, H.-l.; Piou, T.; Duan, X.-H.; Vors, J.-P.; Neuville, L.; Zhu, J. Org. Lett. 2010, 12, 4498–4501. doi:10.1021/ol101778c |
| 14. | Cava, M. P.; Nomura, K.; Schlessinger, R. H.; Buck, K. T.; Douglas, B.; Raffauf, R. F.; Weisbach, J. A. Chem. Ind. 1964, 282–283. |
| 96. | Chen, Z.-W.; Zhu, Y.-Z.; Ou, J.-W.; Wang, Y.-P.; Zheng, J.-Y. J. Org. Chem. 2014, 79, 10988–10998. doi:10.1021/jo5020307 |
| 4. | Sun, W.; Li, G.; Hong, L.; Wang, R. Org. Biomol. Chem. 2016, 14, 2164–2176. doi:10.1039/C5OB02526E |
| 13. | Davenport-Hines, R. P. T.; Slinn, J. Glaxo: A History to 1962; Cambridge University Press: Cambridge, 1992; p 219. |
| 17. | Basarab, G. S.; Doig, P.; Galullo, V.; Kern, G.; Kimzey, A.; Kutschke, A.; Newman, J. P.; Morningstar, M.; Mueller, J.; Otterson, L.; Vishwanathan, K.; Zhou, F.; Gowravaram, M. J. Med. Chem. 2015, 58, 6264–6282. doi:10.1021/acs.jmedchem.5b00863 |
| 97. | Wen, J.; Wei, W.; Xue, S.; Yang, D.; Lou, Y.; Gao, C.; Wang, H. J. Org. Chem. 2015, 80, 4966–4972. doi:10.1021/acs.joc.5b00361 |
| 65. | Tamura, Y.; Yakura, T.; Haruta, J.; Kita, Y. J. Org. Chem. 1987, 52, 3927–3930. doi:10.1021/jo00226a041 |
| 66. | Rama Krishna, K. V.; Sujatha, K.; Kapil, R. S. Tetrahedron Lett. 1990, 31, 1351–1352. doi:10.1016/S0040-4039(00)88804-8 |
| 32. | Quideau, S.; Pouységu, L.; Deffieux, D. Synlett 2008, 467–495. doi:10.1055/s-2008-1032094 |
| 27. | Singh, F. V.; Wirth, T. Oxidative Functionalization with Hypervalent Halides. In Comprehensive Organic Synthesis II, 2nd ed.; Molander, G. A.; Knochel, P., Eds.; Elsevier: Oxford, 2014; Vol. 7, pp 880–933. doi:10.1016/B978-0-08-097742-3.00735-7 |
| 32. | Quideau, S.; Pouységu, L.; Deffieux, D. Synlett 2008, 467–495. doi:10.1055/s-2008-1032094 |
| 105. | Mizutani, H.; Takayama, J.; Soeda, Y.; Honda, T. Tetrahedron Lett. 2002, 43, 2411–2414. doi:10.1016/S0040-4039(02)00296-4 |
| 106. | Kita, Y.; Tohma, H.; Inagaki, M.; Hatanaka, K.; Kikuchi, K.; Yakura, T. Tetrahedron Lett. 1991, 32, 2035–2038. doi:10.1016/S0040-4039(00)78901-5 |
| 107. | Kita, Y.; Tohma, H.; Inagaki, M.; Hatanaka, K.; Yakura, T. J. Am. Chem. Soc. 1992, 114, 2175–2180. doi:10.1021/ja00032a036 |
| 103. | Ousmer, M.; Braun, N. A.; Bavoux, C.; Perrin, M.; Ciufolini, M. A. J. Am. Chem. Soc. 2001, 123, 7534–7538. doi:10.1021/ja016030z |
| 104. | Ousmer, M.; Braun, N. A.; Ciufolini, M. A. Org. Lett. 2001, 3, 765–767. doi:10.1021/ol015526i |
| 70. | Fujioka, H.; Komatsu, H.; Nakamura, T.; Miyoshi, A.; Hata, K.; Ganesh, J.; Murai, K.; Kita, Y. Chem. Commun. 2010, 46, 4133–4135. doi:10.1039/B925687C |
| 71. | Dohi, T.; Nakae, T.; Ishikado, Y.; Kato, D.; Kita, Y. Org. Biomol. Chem. 2011, 9, 6899–6902. doi:10.1039/c1ob06199b |
| 69. | Ficht, S.; Mülbaier, M.; Giannis, A. Tetrahedron 2001, 57, 4863–4866. doi:10.1016/S0040-4020(01)00424-0 |
| 111. | Huang, J.; Wang, H.; Wu, C.; Wulff, W. D. Org. Lett. 2007, 9, 2799–2802. doi:10.1021/ol070904q |
| 112. | Phipps, R. J.; Toste, F. D. J. Am. Chem. Soc. 2013, 135, 1268–1271. doi:10.1021/ja311798q |
| 70. | Fujioka, H.; Komatsu, H.; Nakamura, T.; Miyoshi, A.; Hata, K.; Ganesh, J.; Murai, K.; Kita, Y. Chem. Commun. 2010, 46, 4133–4135. doi:10.1039/B925687C |
| 113. | Tohma, H.; Harayama, Y.; Hashizume, M.; Iwata, M.; Kiyono, Y.; Egi, M.; Kita, Y. J. Am. Chem. Soc. 2003, 125, 11235–11240. doi:10.1021/ja0365330 |
| 114. | Kita, Y.; Yakura, T.; Tohma, H.; Kikuchi, K.; Tamura, Y. Tetrahedron Lett. 1989, 30, 1119–1120. doi:10.1016/S0040-4039(01)80375-0 |
| 71. | Dohi, T.; Nakae, T.; Ishikado, Y.; Kato, D.; Kita, Y. Org. Biomol. Chem. 2011, 9, 6899–6902. doi:10.1039/c1ob06199b |
| 69. | Ficht, S.; Mülbaier, M.; Giannis, A. Tetrahedron 2001, 57, 4863–4866. doi:10.1016/S0040-4020(01)00424-0 |
| 110. | Zheng, C.; Wang, L.; Li, J.; Wang, L.; Wang, D. Z. Org. Lett. 2013, 15, 4046–4049. doi:10.1021/ol401863k |
| 67. | Kita, Y.; Tohma, H.; Kikuchi, K.; Inagaki, M.; Yakura, T. J. Org. Chem. 1991, 56, 435–438. doi:10.1021/jo00001a082 |
| 108. | Kita, Y.; Takada, T.; Ibaraki, M.; Gyoten, M.; Mihara, S.; Fujita, S.; Tohma, H. J. Org. Chem. 1996, 61, 223–227. doi:10.1021/jo951439q |
| 21. | Zhdankin, V. V.; Stang, P. J. Chem. Rev. 2002, 102, 2523–2584. doi:10.1021/cr010003+ |
| 64. | Yeung, C. S.; Dong, V. M. Chem. Rev. 2011, 111, 1215–1292. doi:10.1021/cr100280d |
| 109. | Asmanidou, A.; Papoutsis, I.; Spyroudis, S.; Varvoglis, A. Molecules 2000, 5, 874–879. doi:10.3390/50600874 |
© 2018 Singh et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)