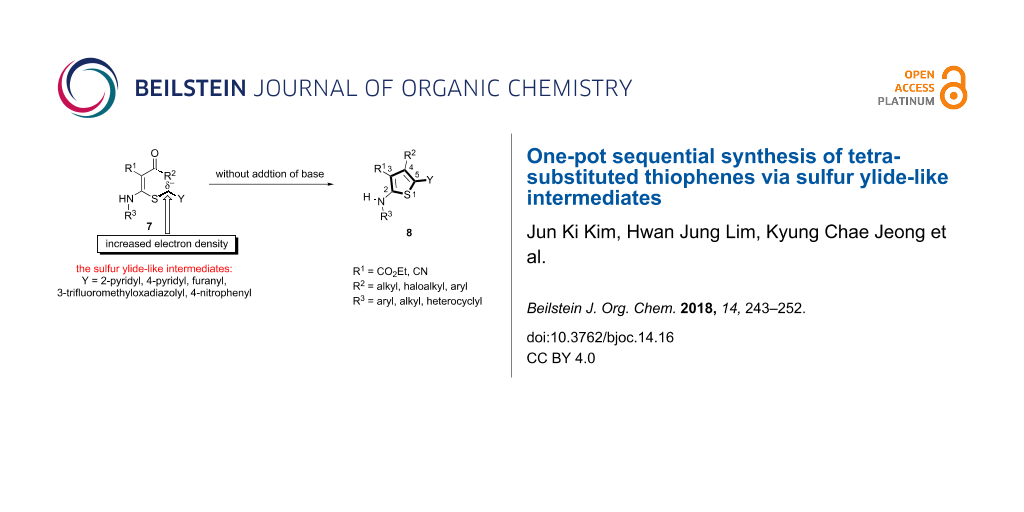
Abstract
Herein, we describe a novel approach for the practical synthesis of tetrasubstituted thiophenes 8. The developed method was particularly used for the facile preparation of thienyl heterocycles 8. The mechanism for this reaction is based on the formation of a sulfur ylide-like intermediate. It was clearly suggested by (i) the intramolecular cyclization of ketene N,S-acetals 7 to the corresponding thiophenes 8, (ii) 1H NMR studies of Meldrum’s acid-substituted aminothioacetals 9, and (iii) substitution studies of the methoxy group on Meldrum’s acid containing N,S-acetals 9b. Notably, in terms of structural effects on the reactivity and stability of sulfur ylide-like intermediates, 2-pyridyl substituted compound 7a exhibited superior properties over those of others.
Graphical Abstract
Introduction
Since the discovery of stable sulfonium ylides 1 in 1930 [1] and the pioneering work of several research groups during the 1960s (2 and 3) [2-9], these carbene precursors have been played an important role in organic chemistry [10-22]. As shown in Figure 1, sulfur(IV) and sulfur(VI) ylides are stable. The stability of sulfonium ylides is determined by the electron delocalization of the carbanionic center and the substituents on the sulfur atom [10]. In general, these reagents are often applied in the preparation of simple small rings [13], such as epoxides [14-18], cyclopropanes [19-22], aziridines [23], indoles [24], pyrroles [24], and indolines [25]. In addition, other reactions involving sulfonium and sulfoxonium ylides have been reported recently [26-32]. For example, Shen and co-workers reported the use of trifluoromethyl-substituted sulfonium ylide 5 in electrophilic trifluoromethylation reactions [33,34]. Moreover, Maulide and co-workers reported an effective ylide transfer reagent, which led to sulfonium ylide 6 [35-38].
Figure 1: The selected examples of sulfur(IV) and sulfur(VI) ylides 1 [1], 2 [5-7], 3 [6,7,9], 4 [11,12], 5 [33,34], 6 [35-38].
Figure 1: The selected examples of sulfur(IV) and sulfur(VI) ylides 1 [1], 2 [5-7], 3 [6,7,9], 4 [11,12], 5 [33,34], 6 [35-38].
As part of our ongoing efforts to discover small molecule modulators of protein–protein interactions (PPIs), we are particularly interested in coplanar compounds that mimic β-strand side-chain distributions [39-43]. Consequently, we are fascinated with thienyl–pyridyl ring systems [43] and have explored facile synthetic procedures to facilitate their production. For the synthesis of heterocyclic–heterocyclic biaryl compounds, numerous studies have been carried out to develop efficient catalytic methods [44-49]. In general, Pd-catalyzed Suzuki–Miyaura cross-coupling reactions are the most popular synthetic strategy for aryl–aryl bond-forming reactions [50-52]. However, it has been reported that the Suzuki cross-coupling of nitrogen- and sulfur-containing heterocycles is more challenging than those of aryl–aryl derivatives. These difficulties resulted from the special properties of thiopheneboronic acids – the sensitivity to polar reaction media and easy degradation by protodeboronation [53].
As a recent example of a metal-free synthesis of the targeted thienylpyridines (Figure 2A and 2B), Al-Showiman and co-workers reported a trisubstituted 5-(pyridin-2-yl)thiophene, obtained from the reaction of 5-(enaminone)thiophene with 2,4-pentanedione in glacial acetic acid in the presence of ammonium acetate [54,55]. Ila and co-workers reported the synthesis of tri- and tetrasubstituted thiophenes via the intramolecular cyclization of S-alkylated heterocyclic–aryl dithioesters [56]. However, these approaches are limited by the multistep synthesis (Figure 2A) [54,55] and the complicated dithioester preparation (Figure 2B) [56]. In general, tetrasubstituted thiophenes have primarily been prepared by base-catalyzed intramolecular Dieckmann-, Thorpe–Ziegler, and aldol-type condensations of the corresponding ketene-N,S-acetals [57-67]. These methods are still need strong bases [60], high temperatures [62,64,65], and are generally low yielding [57,62]. Thus, a new mild synthetic route for the synthesis of 5-(pyridyl)thiophenes is required. We therefore investigated the synthesis of thienylpyridines using a metal-free approach.
Figure 2: Metal-free synthesis of thiophene-based heterocycles (A) [54,55], (B) [56].
Figure 2: Metal-free synthesis of thiophene-based heterocycles (A) [54,55], (B) [56].
Results and Discussion
At first, our efforts focused on the intramolecular cyclization reactions with mild conditions – in the absence of an added base at room temperature. To obtain aminothioacetal 7a, we initially performed the S-alkylation of the intermediate thiolate salt with 2-(bromomethyl)pyridine at room temperature overnight. We interestingly found that the desired 5-(pyridin-2-yl)thiophenes 8a has already been achieved by the intramolecular aldol-type condensation of N,S-acetal 7a (Figure 2C). Subsequently, we investigated the scope of the reaction using our optimized conditions (Scheme 1).
Scheme 1: One-pot sequential synthesis of the trisubstituted 5-(pyridine-2-yl)thiophenes 8a. Substrate: amalonitrile; b5,5-dimethylcyclohexane-1,3-dione.
Scheme 1: One-pot sequential synthesis of the trisubstituted 5-(pyridine-2-yl)thiophenes 8a. Substrate: amalo...
As shown in Scheme 1, various isothiocyanates containing aryl and alkyl groups were applied, and the desired thiophenes (8aa–ai) were obtained in moderate to excellent yields (47–92%). When different 1,3-diketones were applied, the yields were affected by the keto–enol tautomer ratio. Alkyl substituents (isopropyl and cyclopropyl), which promote the enol forms of the ketones, afforded thiophenes 8aj and 8ak in good to excellent yields (68% and 81%). However, a CF3 substituent, which is electron-withdrawing and might promote the keto form, provided the desired compound 8al in a low yield (14%). When the enolate was derived from 3-oxo-3-phenylpropanenitrile, 3-cyano-4-phenylthiophene 8am was obtained in a low yield (32%). Starting from malonitrile, compound 8an was also prepared in a moderate yield (50%) via a Thorpe–Ziegler-type cyclization of N,S-acetal 7an. In this case, the intramolecular cyclization reaction was carried out at 100 °C for 3 h. With 5,5-dimethylcyclohexane-1,3-dione, thiophene 8ao was obtained in a low yield (25%). X-ray crystal structures of thiophenes 8ad and 8an are illustrated in Figure 3 [68].
![[1860-5397-14-16-3]](/bjoc/content/figures/1860-5397-14-16-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: X-ray crystal structures of 8ad and 8an [68].
Figure 3: X-ray crystal structures of 8ad and 8an [68].
Mechanistically, our experimental findings may be attributed to the formation of sulfur ylide-like intermediates. To support this reaction pathway, further studies were performed. By changing the substituent groups on N,S-acetals 7, the effects of the structure on the stability and the reactivity of the intermediates were investigated (Table 1).
Table 1: Examination of N,S-acetals substituted with a heterocycle (7aa–k) or an arene (7l–p).
|
|
|||||
| Entry | Substrates | X | Y | Products | Yield (%)a,b |
| 1 | 7aa | S |
|
8aa | 92 |
| 2 | 7b | S |
|
8b | –c |
| 3d | 7c | S |
|
8c | 80 |
| 4 | 7d | S |
|
8d | 34 |
| 5 | 7e | O |
|
8e | –c |
| 6 | 7f | S |
|
8f | 33 |
| 7 | 7g | S |
|
8g | 20 |
| 8 | 7h | S |
|
8h | –c |
| 9 | 7i | S |
|
8i | 47 |
| 10 | 7j | S |
|
8j | –c |
| 11 | 7k | S |
|
8k | 8 |
| 12 | 7l | S |
|
8l | –c |
| 13 | 7m | S |
|
8m | –c |
| 14 | 7n | S |
|
8n | –c |
| 15 | 7o | S |
|
8o | –c |
| 16 | 7p | S |
|
8p | 42 |
aR3 = 3-methoxyphenyl, one-pot sequential reactions to thiophenes 8: a) ethyl acetoacetate (1 equiv), K2CO3 (1 equiv), DMF, rt, 2 h; b) 3-methoxyphenyl isothiocyanate (1 equiv), DMF, 60 °C, 2 h; c) the corresponding halomethylarenes or halomethyl heterocycles (1 equiv), DMF, 60 °C, 3 h; bAfter column chromatography; cno desired reaction; dbecause N,S-acetal 7c readily transfer to the corresponding thiophene 8c at 0 °C, the substrate 7c could not be isolated.
In terms of inductive and mesomeric effects, we postulated that the electron rich pyridyl N atom could carry a negative charge at the picolinyl position (Table 1, entries 1 to 5). Interestingly, the 2-pyridyl moiety provided stable and reactive N,S-acetal 7aa, which could be isolated and afforded the desired thiophene 8aa in an excellent yield (92%, Table 1, entry 1). The substrate containing a 3-pyridyl group only afforded S-alkylated compound 7b, while 4-pyridyl substituted intermediate 7c could be easily transformed into thiophene 8c at 0 °C (Table 1, entries 2 and 3). Notably, the special properties associated with the 2- and 4-positions of pyridine [69-72] are evident in this study. In the case of 6-methylpyridine-substituted N,S-acetal 7d, the formation of a resonance stabilized enaminate anion had a smaller contribution and this resulted in a reduced yield (34%, Table 1, entry 4) [70]. To identify the effects of sulfur, a reaction with the corresponding isocyanate was performed to introduce an oxygen atom. As a result, only O-alkylation compound 7e was obtained instead of the desired furan (Table 1, entry 5). It is possible to consider that the d orbitals of the sulfur atom in a sulfide group could possibly stabilize the adjacent carbanion [73,74].
To expand the scope of substituted N,S-acetals that could provide the desired sulfur ylide-like intermediates, various heterocycles were subjected to the reaction (Table 1, entries 6–11). The desired thiophenes 8f and 8g were obtained in low yields from the respective furans (33% and 20%, Table 1, entries 6 and 7). With thiophene, however, only N,S-acetal compound 7h was obtained. Thiophene could not generate the desired intermediate because of the lower electronegativity and a weaker inductive effect of sulfur (Table 1, entry 8). Among 1,2,4-oxadiazole moieties, the 3-trifluoromethyloxadiazole group afforded the desired thiophene 8i (Table 1, entry 9), whereas the 5-trifluoromethyloxadiazole substituent was not a viable substrate (Table 1, entry 10). Because of a similar result obtained with the N-methylimidazole substituted compound 7k, the difference between 7i and 7j could be explained by the imidazole-like structure of the 5-trifluoromethyloxadiazole moiety. The reduced inductive effect of the amine might be attributed to the resonance structures of imidazole (Table 1, entry 11) [72].
To determine the influence of substituents on the phenyl group, various arene(methyl)sulfanes 7l–p were tested (Table 1, entries 12–16,). Simple phenyl and electron-donating compounds 7l and 7m did not provide the desired thiophenes 8l and 8m. Although electron-withdrawing groups such as CN and SF5 did not show any effect (Table 1, entries 14 and 15), NO2, the strongest electron-withdrawing group [75-77], provided the desired thiophene 8p in a moderate yield (42%).
While further studies are required, we suggest the sulfur ylide-like intermediates 7aa, 7c, 7p, 7i, and 7f,g after considering the literature [69-72] and McNab’s research on the synthesis of 3-hydroxythiophene and thiphene-3(2H)-ones (Figure 4) [78]. With regards to McNab’s work, the dipolar species [R2C=S+−CH−−R′] were proposed as reaction intermediates [78]. In our studies, it was shown that the order of reactivity was 7c ≥ 7aa > 7i, 7p ≥ 7f, 7g. The different reactivities of the intermediates were related to the presence of heteroatoms, particularly their inductive and mesomeric effects [69-72]. For example, 2-pyridyl-substituted ylide-like intermediate 7aa showed the desired properties in terms of both reactivity and stability, whereas the 4-pyridyl group only displayed high reactivity. For alkylpyridines 7aa and 7c, our observations may be explained by Fraser’s measurements of the pKa values [69,70,79]: among isomeric benzylpyridines, the 4-isomer is more acidic than the 2-isomer, and the 4- and 2-isomers are much more acidic than the 3-isomer. In the case of the oxadiazole-substituted compound 7i, inductive and mesomeric effects facilitated its sulfur ylide-like intermediate formation [71]. For compounds 7f and 7g, the low reactivity resulted from the decreased mesomeric effect of the furan structure: the higher electronegativity of oxygen facilitated the polarized form [71]. Among various arenes, the 4-nitrophenyl substituent 7p only afforded the desired thiophene 8p in a moderated yield (42%) and, the favorable resonance form is illustrated in Figure 4.
Figure 4: The proposed structure of sulfur ylide-like intermediates; resonance contributors (mesomeric structures) [69-72,78].
Figure 4: The proposed structure of sulfur ylide-like intermediates; resonance contributors (mesomeric struct...
According to the recent reports on the multiple isomeric structures of ketene N,S-acetals [80-83], structural assignments of the ketene N,S-aminothioacetals 7 by 1H NMR are not facile. To overcome these difficulties, we prepared N,S-acetals 9a–c since the X-ray crystal structure of Meldrum’s acid-based N,S-acetal was reported by Wentrup [84]. In addition, the intramolecular aldol condensation of Meldrum’s acids did not occur due to the ketone structures. Table 2 displays the 1H NMR result of the sulfur ylide-like intermediate 9b, and demonstrates the effect of increasing electronegativity on the CH2 proton. The 2-pyridyl group caused a downfield shift of 0.13 to 0.14 ppm compared to phenyl and 3-pyridyl groups (Table 2, entry 2).
Table 2: 1H NMR studies of Meldrum’s acid-based N,S-acetals 9a–ca,b [84].
|
|
|||
| Entry | N,S-Acetalsc,d | Structure | -SCH2Y 1H NMR (ppm)e |
| 1 | 9a |
|
4.02 |
| 2 | 9b |
|
4.15 |
| 3 | 9c |
|
4.01 |
aR3 = 3-methoxyphenyl; bS-alkylation of the thiolate with 4-(bromomethyl)pyridine hydrobromide was not successful; cone-pot sequential reactions to N,S-acetals 9: a) Meldrum’s acid (1 equiv), K2CO3 (1 equiv), DMF, rt, 2 h; b) 3-methoxyphenyl isothiocyanate (1 equiv), DMF, 60 °C, 2 h; c) the corresponding benzyl bromide or bromomethylpyridine (1 equiv), DMF, 60 °C, 3 h; dAfter column chromatography; ein CDCl3.
Further 1H NMR studies of pyridin-2-ylmalononitrile 7an, pyridine-2-ylmethyl methanimidothioate 7ao, and time dependent experiments of the intramolecular aldol condensation of N,S-acetal 7aa to 8aa in N,N-dimethylformamide-d7 at room temperature confirmed the formation of the stable sulfur ylide-like intermediates, thus indicating the successful transformation into thiophenes 8an, 8ao, and 8a (see Supporting Information File 1).
In addition to the spectroscopic studies, we attempted to gain additional evidence to support the formation of sulfur ylide-like intermediates via another approach. We selected stable Meldrum’s acid containing N,S-acetals 9a and 9b for further investigation. Based on previous reports regarding carbene generation from sulfonium ylides [6,85,86], compounds 9a and 9b were reacted with excess MeOH (Scheme 2). Interestingly, 2-pyridyl-substituted N,S-acetal 9b only provided N,O-acetal 9ba via a 1,4-Micheal addition, whereas N,S-acetal 9a was completely recovered after the reaction. We believed that these results support the existence of sulfur ylide-like intermediates (Scheme 2) [87].
Scheme 2: The substitution reaction with MeOH.
Scheme 2: The substitution reaction with MeOH.
Conclusion
In conclusion, we have developed a new synthetic pathway for the preparation of 2-amino-5-(heterocyclic)thiophenes 8. We have also shown that sulfur ylide-like intermediates 7, which are easily converted into the desired thiophenes 8, can be generated in situ by S-alkylation of the intermediate thiolate salts. By 1H NMR analysis of N,S-acetals 9 and methoxy group substitution of 9b, the formation of sulfur ylide-like intermediates was successfully demonstrated. The transformation of ylide-like intermediates into the corresponding thiophenes was affected by their electronic properties. Among the various tested residues, the 2-pyridyl motif provided the desired reactivity and stability. This approach could be considered a powerful strategy for the preparation of biologically important thienyl heterocycles. Subsequent studies shall focus on applying this chemistry in other reactions that require sulfur ylides, and the biological activities of thiophenes 8 will also be reported in due course.
Supporting Information
| Supporting Information File 1: Experimental part. | ||
| Format: PDF | Size: 5.9 MB | Download |
References
-
Ingold, C. K.; Jessop, J. A. J. Chem. Soc. 1930, 713. doi:10.1039/JR9300000713
Return to citation in text: [1] [2] -
Johnson, A. W.; LaCount, R. B. J. Am. Chem. Soc. 1961, 83, 417. doi:10.1021/ja01463a040
Return to citation in text: [1] -
Franzen, V.; Schmidt, H.-J.; Mertz, C. Chem. Ber. 1961, 94, 2942. doi:10.1002/cber.19610941117
Return to citation in text: [1] -
Franzen, V.; Driesen, H.-E. Chem. Ber. 1963, 96, 1881. doi:10.1002/cber.19630960722
Return to citation in text: [1] -
Corey, E. J.; Chaykovsky, M. J. Am. Chem. Soc. 1962, 84, 3782. doi:10.1021/ja00878a046
Return to citation in text: [1] [2] -
Corey, E. J.; Chaykovsky, M. J. Am. Chem. Soc. 1964, 86, 1640. doi:10.1021/ja01062a040
Return to citation in text: [1] [2] [3] [4] -
Corey, E. J.; Chaykovsky, M. J. Am. Chem. Soc. 1965, 87, 1353. doi:10.1021/ja01084a034
Return to citation in text: [1] [2] [3] -
Nozaki, H.; Takaku, M.; Kondô, K. Tetrahedron 1966, 22, 2145. doi:10.1016/S0040-4020(01)82134-7
Return to citation in text: [1] -
Ratts, K. W.; Yao, A. N. J. Org. Chem. 1966, 31, 1185. doi:10.1021/jo01342a047
Return to citation in text: [1] [2] -
Burtoloso, A. C. B.; Dias, R. M. P.; Leonarczyk, I. A. Eur. J. Org. Chem. 2013, 5005. doi:10.1002/ejoc.201300581
Return to citation in text: [1] [2] -
Moody, C. J.; Slawin, A. M. Z.; Taylor, R. J.; Williams, D. J. Tetrahedron Lett. 1988, 29, 6009. doi:10.1016/S0040-4039(00)82253-4
Return to citation in text: [1] [2] -
Moody, C. J.; Taylor, R. J. Tetrahedron 1990, 46, 6525. doi:10.1016/S0040-4020(01)96018-1
Return to citation in text: [1] [2] -
Li, A.-H.; Dai, L.-X.; Aggarwal, V. K. Chem. Rev. 1997, 97, 2341. doi:10.1021/cr960411r
Return to citation in text: [1] [2] -
Davoust, M.; Brière, J.-F.; Jaffrès, P.-A.; Metzner, P. J. Org. Chem. 2005, 70, 4166. doi:10.1021/jo0479260
Return to citation in text: [1] [2] -
Piccinini, A.; Kavanagh, S. A.; Connon, P. B.; Connon, S. J. Org. Lett. 2010, 12, 608. doi:10.1021/ol902816w
Return to citation in text: [1] [2] -
Aggarwal, V. K.; Hynd, G.; Picoul, W.; Vasse, J.-L. J. Am. Chem. Soc. 2002, 124, 9964. doi:10.1021/ja0272540
Return to citation in text: [1] [2] -
Chandrasekhar, S.; Narasihmulu, C.; Jagadeshwar, V.; Venkatram Reddy, K. Tetrahedron Lett. 2003, 44, 3629. doi:10.1016/S0040-4039(03)00732-9
Return to citation in text: [1] [2] -
Concellón, J. M.; Bardales, E. J. Org. Chem. 2003, 68, 9492. doi:10.1021/jo0349577
Return to citation in text: [1] [2] -
Paxton, R. J.; Taylor, R. J. K. Synlett 2007, 633. doi:10.1055/s-2007-967966
Return to citation in text: [1] [2] -
Edwards, M. G.; Paxton, R. J.; Pugh, D. S.; Whitwood, A. C.; Taylor, R. J. K. Synthesis 2008, 3279. doi:10.1055/s-0028-1083165
Return to citation in text: [1] [2] -
Robiette, R.; Marchan-Brynaert, J. Synlett 2008, 517. doi:10.1055/s-2008-1032080
Return to citation in text: [1] [2] -
Hartikka, A.; Arvidsson, P. I. J. Org. Chem. 2007, 72, 5874. doi:10.1021/jo070519e
Return to citation in text: [1] [2] -
Recent literature: Marsini, M. A.; Reeves, J. T.; Desrosiers, J.-N.; Herbage, M. A.; Savoie, J.; Li, Z.; Fandrick, K. R.; Sader, C. A.; McKibben, B.; Gao, D. A.; Cui, J.; Gonnella, N. C.; Lee, H.; Wei, X.; Roschangar, F.; Lu, B. Z.; Senanayake, C. H. Org. Lett. 2015, 17, 5614. doi:10.1021/acs.orglett.5b02838
Return to citation in text: [1] -
Vaitla, J.; Bayer, A.; Hopmann, K. H. Angew. Chem., Int. Ed. 2017, 56, 4277. doi:10.1002/anie.201610520
Return to citation in text: [1] [2] -
Boyarskikh, V.; Nyong, A.; Rainier, J. D. Angew. Chem., Int. Ed. 2008, 47, 5374. doi:10.1002/anie.200801336
Return to citation in text: [1] -
Soeta, T.; Ohgai, T.; Sakai, T.; Fujinami, S.; Ukaji, Y. Org. Lett. 2014, 16, 4854. doi:10.1021/ol502347n
Return to citation in text: [1] -
Xu, X.; Li, C.; Tao, Z.; Pan, Y. Green Chem. 2017, 19, 1245. doi:10.1039/C6GC02681H
Return to citation in text: [1] -
Enßle, M.; Buck, S.; Werz, R.; Maas, G. Beilstein J. Org. Chem. 2012, 8, 433. doi:10.3762/bjoc.8.49
Return to citation in text: [1] -
Li, K.; Hu, J.; Liu, H.; Tong, X. Chem. Commun. 2012, 48, 2900. doi:10.1039/c2cc30242j
Return to citation in text: [1] -
Liu, Y.-Y.; Yang, X.-H.; Huang, X.-C.; Wei, W.-T.; Song, R.-J.; Li, J.-H. J. Org. Chem. 2013, 78, 10421. doi:10.1021/jo401851m
Return to citation in text: [1] -
Lu, L.-Q.; Cao, Y.-J.; Liu, X.-P.; An, J.; Yao, C.-J.; Ming, Z.-H.; Xiao, W.-J. J. Am. Chem. Soc. 2008, 130, 6946. doi:10.1021/ja800746q
Return to citation in text: [1] -
Chen, J.-R.; Dong, W.-R.; Candy, M.; Pan, F.-F.; Jörres, M.; Bolm, C. J. Am. Chem. Soc. 2012, 134, 6924. doi:10.1021/ja301196x
Return to citation in text: [1] -
Liu, Y.; Shao, X.; Zhang, P.; Lu, L.; Shen, Q. Org. Lett. 2015, 17, 2752. doi:10.1021/acs.orglett.5b01170
Return to citation in text: [1] [2] -
Zhu, J.; Liu, Y.; Shen, Q. Angew. Chem., Int. Ed. 2016, 55, 9050. doi:10.1002/anie.201603166
Return to citation in text: [1] [2] -
Huang, X.; Goddard, R.; Maulide, N. Angew. Chem., Int. Ed. 2010, 49, 8979. doi:10.1002/anie.201002919
Return to citation in text: [1] [2] -
Huang, X.; Maulide, N. J. Am. Chem. Soc. 2011, 133, 8510. doi:10.1021/ja2031882
Return to citation in text: [1] [2] -
Huang, X.; Klimczyk, S.; Maulide, N. Synthesis 2012, 44, 175. doi:10.1055/s-0031-1289632
Return to citation in text: [1] [2] -
Huang, X.; Klimczyk, S.; Veiros, L. F.; Maulide, N. Chem. Sci. 2013, 4, 1105. doi:10.1039/c2sc21914j
Return to citation in text: [1] [2] -
Jamieson, A. G.; Russell, D.; Hamilton, A. D. Chem. Commun. 2012, 48, 3709. doi:10.1039/c2cc30295k
Return to citation in text: [1] -
German, E. A.; Ross, J. E.; Knipe, P. C.; Don, M. F.; Thompson, S.; Hamilton, A. D. Angew. Chem., Int. Ed. 2015, 54, 2649. doi:10.1002/anie.201410290
Return to citation in text: [1] -
Smith, A. B., III; Keenan, T. P.; Holcomb, R. C.; Sprengeler, P. A.; Guzman, M. C.; Wood, J. L.; Carroll, P. J.; Hirschmann, R. J. Am. Chem. Soc. 1992, 114, 10672. doi:10.1021/ja00052a093
Return to citation in text: [1] -
Wyrembak, P. N.; Hamilton, A. D. J. Am. Chem. Soc. 2009, 131, 4566. doi:10.1021/ja809245t
Return to citation in text: [1] -
Jouanne, M.; Voisin-Chiret, A. S.; Legay, R.; Coufourier, S.; Rault, S.; Santos, J. S. O. Eur. J. Org. Chem. 2016, 5686. doi:10.1002/ejoc.201600882
Return to citation in text: [1] [2] -
Kudo, N.; Perseghini, M.; Fu, G. C. Angew. Chem., Int. Ed. 2006, 45, 1282. doi:10.1002/anie.200503479
Return to citation in text: [1] -
Rao, G. K.; Kumar, A.; Ahmed, J.; Singh, A. K. Chem. Commun. 2010, 46, 5954. doi:10.1039/c0cc01075h
Return to citation in text: [1] -
Billingsley, K. L.; Anderson, K. W.; Buchwald, S. L. Angew. Chem., Int. Ed. 2006, 45, 3484. doi:10.1002/anie.200600493
Return to citation in text: [1] -
Liu, C.; Ni, Q.; Hu, P.; Qiu, J. Org. Biomol. Chem. 2011, 9, 1054. doi:10.1039/C0OB00524J
Return to citation in text: [1] -
Siddle, J. S.; Batsanov, A. S.; Bryce, M. R. Eur. J. Org. Chem. 2008, 2746. doi:10.1002/ejoc.200800018
Return to citation in text: [1] -
Kitamura, Y.; Sako, S.; Tsutsui, A.; Moguchi, Y.; Maegawa, T.; Kitade, Y.; Sajiki, H. Adv. Synth. Catal. 2010, 352, 718. doi:10.1002/adsc.200900638
Return to citation in text: [1] -
Yin, L.; Liebscher, J. Chem. Rev. 2007, 107, 133. doi:10.1021/cr0505674
Return to citation in text: [1] -
Gildner, P. G.; Colacot, T. J. Organometallics 2015, 34, 5497. doi:10.1021/acs.organomet.5b00567
Return to citation in text: [1] -
Ruiz-Castillo, P.; Buchwald, S. L. Chem. Rev. 2016, 116, 12564. doi:10.1021/acs.chemrev.6b00512
Return to citation in text: [1] -
Clavé, G.; Pelissier, F.; Campidelli, S.; Grison, C. Green Chem. 2017, 19, 4093. doi:10.1039/C7GC01672G
Return to citation in text: [1] -
Mabkhot, Y. N.; Kaal, N. A.; Alterary, S.; Al-Showiman, S. S.; Barakat, A.; Ghabbour, H. A.; Frey, W. Molecules 2015, 20, 8712. doi:10.3390/molecules20058712
Return to citation in text: [1] [2] [3] -
Mabkhot, Y. N.; Alatibi, F.; El-Sayed, N. N. E.; Kheder, N. A.; Al-Showiman, S. S. Molecules 2016, 21, 1036. doi:10.3390/molecules21081036
Return to citation in text: [1] [2] [3] -
Acharya, A.; Parameshwarappa, G.; Saraiah, B.; Ila, H. J. Org. Chem. 2015, 80, 414. doi:10.1021/jo502429c
Return to citation in text: [1] [2] [3] -
Sommen, G.; Comel, A.; Kirsch, G. Tetrahedron 2003, 59, 1557. doi:10.1016/S0040-4020(03)00054-1
Return to citation in text: [1] [2] -
Thomae, D.; Perspicace, E.; Henryon, D.; Xu, Z.; Schneider, S.; Hesse, S.; Kirsch, G.; Seck, P. Tetrahedron 2009, 65, 10453. doi:10.1016/j.tet.2009.10.021
Return to citation in text: [1] -
Gaber, H. M.; Bagley, M. C. Eur. J. Chem. 2011, 2, 214. doi:10.5155/eurjchem.2.2.214-222.411
Return to citation in text: [1] -
Apparao, S.; Ila, H.; Junjappa, H. J. Chem. Soc., Perkin Trans. 1 1983, 2837. doi:10.1039/p19830002837
Return to citation in text: [1] [2] -
Okada, E.; Masuda, R.; Hojo, M.; Imazaki, N.; Miya, H. Heterocycles 1992, 34, 103. doi:10.3987/COM-91-5897
Return to citation in text: [1] -
Fadda, A. A.; Abdel-Latif, E.; El-Mekawy, R. E. Eur. J. Med. Chem. 2009, 44, 1250. doi:10.1016/j.ejmech.2008.09.006
Return to citation in text: [1] [2] [3] -
Rudorf, W.-D. Z. Chem. 1979, 19, 100. doi:10.1002/zfch.19790190309
Return to citation in text: [1] -
Fadda, A. A.; Abdel-Latif, E.; El-Mekawy, R. E. Pharmacol. Pharm. 2012, 3, 148. doi:10.4236/pp.2012.32022
Return to citation in text: [1] [2] -
Fadda, A. A.; Abdel-Latif, E.; El-Mekawy, R. E. Phosphorus, Sulfur Silicon Relat. Elem. 2008, 183, 1940. doi:10.1080/10426500701839536
Return to citation in text: [1] [2] -
Rateb, N. M. Phosphorus, Sulfur Silicon Relat. Elem. 2005, 180, 2361. doi:10.1080/104265090921083
Return to citation in text: [1] -
Abu-Melha, S. Heterocycles 2016, 92, 1261. doi:10.3987/COM-16-13461
Return to citation in text: [1] -
CCDC 1813136 (8ad) and CCDC 1813137 (8an) contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Centre.
Return to citation in text: [1] [2] -
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010; p 7.
See for the structure of pyridine.
Return to citation in text: [1] [2] [3] [4] [5] -
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010.
p 120 and p 146. See for the structures of alkylpyridines.
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010.
p 10 and p 42. See for the structures of thiophene, furan, and oxidiazole.
Return to citation in text: [1] [2] [3] [4] [5] [6] -
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester; p 10.
See for the structure of imidazole.
Return to citation in text: [1] [2] [3] [4] [5] -
Doering, W. v. E.; Hoffmann, A. K. J. Am. Chem. Soc. 1955, 77, 521. doi:10.1021/ja01608a003
Return to citation in text: [1] -
Johnson, A. W.; Lacount, R. B. Tetrahedron 1960, 9, 130. doi:10.1016/0040-4020(60)80061-0
Return to citation in text: [1] -
Sheppard, W. A. J. Am. Chem. Soc. 1962, 84, 3072. doi:10.1021/ja00875a007
Return to citation in text: [1] -
Byrne, C. J.; Happer, D. A. R.; Hartshorn, M. P.; Powell, H. K. J. J. Chem. Soc., Perkin Trans. 2 1987, 1649. doi:10.1039/p29870001649
Return to citation in text: [1] -
Shorter, J. Pure Appl. Chem. 1997, 69, 2497. doi:10.1351/pac199769122497
Return to citation in text: [1] -
Hunter, G. A.; McNab, H. J. Chem. Soc., Perkin Trans. 1 1995, 1209. doi:10.1039/p19950001209
Return to citation in text: [1] [2] [3] -
Fraser, R. R.; Mansour, T. S.; Savard, S. J. Org. Chem. 1985, 50, 3232. doi:10.1021/jo00217a050
Return to citation in text: [1] -
Apparao, S.; Ila, H.; Junjappa, H. J. Chem. Soc., Perkin Trans. 1 1983, 2837. doi:10.1039/p19830002837
Return to citation in text: [1] -
Ila, H.; Junjappa, H. Chimia 2013, 67, 17. doi:10.2533/chimia.2013.17
Return to citation in text: [1] -
Pan, L.; Bi, X.; Liu, Q. Chem. Soc. Rev. 2013, 42, 1251. doi:10.1039/C2CS35329F
Return to citation in text: [1] -
Zhang, L.; Dong, J.; Xu, X.; Liu, Q. Chem. Rev. 2016, 116, 287. doi:10.1021/acs.chemrev.5b00360
Return to citation in text: [1] -
Bernhardt, P. V.; Koch, R.; Moloney, D. W. J.; Shtaiwi, M.; Wentrup, C. J. Chem. Soc., Perkin Trans. 2 2002, 515. doi:10.1039/B109624A
Return to citation in text: [1] [2] -
Trost, B. M. J. Am. Chem. Soc. 1966, 88, 1587. doi:10.1021/ja00959a071
Return to citation in text: [1] -
Stoffregen, S. A.; Heying, M.; Jenks, W. S. J. Am. Chem. Soc. 2007, 129, 15746. doi:10.1021/ja076351w
Return to citation in text: [1] -
It cannot be ruled out that the pyridine moiety in 9b could be act as a general base.
Return to citation in text: [1]
| 54. | Mabkhot, Y. N.; Kaal, N. A.; Alterary, S.; Al-Showiman, S. S.; Barakat, A.; Ghabbour, H. A.; Frey, W. Molecules 2015, 20, 8712. doi:10.3390/molecules20058712 |
| 55. | Mabkhot, Y. N.; Alatibi, F.; El-Sayed, N. N. E.; Kheder, N. A.; Al-Showiman, S. S. Molecules 2016, 21, 1036. doi:10.3390/molecules21081036 |
| 56. | Acharya, A.; Parameshwarappa, G.; Saraiah, B.; Ila, H. J. Org. Chem. 2015, 80, 414. doi:10.1021/jo502429c |
| 57. | Sommen, G.; Comel, A.; Kirsch, G. Tetrahedron 2003, 59, 1557. doi:10.1016/S0040-4020(03)00054-1 |
| 58. | Thomae, D.; Perspicace, E.; Henryon, D.; Xu, Z.; Schneider, S.; Hesse, S.; Kirsch, G.; Seck, P. Tetrahedron 2009, 65, 10453. doi:10.1016/j.tet.2009.10.021 |
| 59. | Gaber, H. M.; Bagley, M. C. Eur. J. Chem. 2011, 2, 214. doi:10.5155/eurjchem.2.2.214-222.411 |
| 60. | Apparao, S.; Ila, H.; Junjappa, H. J. Chem. Soc., Perkin Trans. 1 1983, 2837. doi:10.1039/p19830002837 |
| 61. | Okada, E.; Masuda, R.; Hojo, M.; Imazaki, N.; Miya, H. Heterocycles 1992, 34, 103. doi:10.3987/COM-91-5897 |
| 62. | Fadda, A. A.; Abdel-Latif, E.; El-Mekawy, R. E. Eur. J. Med. Chem. 2009, 44, 1250. doi:10.1016/j.ejmech.2008.09.006 |
| 63. | Rudorf, W.-D. Z. Chem. 1979, 19, 100. doi:10.1002/zfch.19790190309 |
| 64. | Fadda, A. A.; Abdel-Latif, E.; El-Mekawy, R. E. Pharmacol. Pharm. 2012, 3, 148. doi:10.4236/pp.2012.32022 |
| 65. | Fadda, A. A.; Abdel-Latif, E.; El-Mekawy, R. E. Phosphorus, Sulfur Silicon Relat. Elem. 2008, 183, 1940. doi:10.1080/10426500701839536 |
| 66. | Rateb, N. M. Phosphorus, Sulfur Silicon Relat. Elem. 2005, 180, 2361. doi:10.1080/104265090921083 |
| 67. | Abu-Melha, S. Heterocycles 2016, 92, 1261. doi:10.3987/COM-16-13461 |
| 68. | CCDC 1813136 (8ad) and CCDC 1813137 (8an) contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Centre. |
| 69. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010; p 7.
See for the structure of pyridine. |
| 70. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010.
p 120 and p 146. See for the structures of alkylpyridines. |
| 71. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010.
p 10 and p 42. See for the structures of thiophene, furan, and oxidiazole. |
| 72. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester; p 10.
See for the structure of imidazole. |
| 56. | Acharya, A.; Parameshwarappa, G.; Saraiah, B.; Ila, H. J. Org. Chem. 2015, 80, 414. doi:10.1021/jo502429c |
| 68. | CCDC 1813136 (8ad) and CCDC 1813137 (8an) contain the supplementary crystallographic data for this paper. These data are provided free of charge by The Cambridge Crystallographic Centre. |
| 57. | Sommen, G.; Comel, A.; Kirsch, G. Tetrahedron 2003, 59, 1557. doi:10.1016/S0040-4020(03)00054-1 |
| 62. | Fadda, A. A.; Abdel-Latif, E.; El-Mekawy, R. E. Eur. J. Med. Chem. 2009, 44, 1250. doi:10.1016/j.ejmech.2008.09.006 |
| 54. | Mabkhot, Y. N.; Kaal, N. A.; Alterary, S.; Al-Showiman, S. S.; Barakat, A.; Ghabbour, H. A.; Frey, W. Molecules 2015, 20, 8712. doi:10.3390/molecules20058712 |
| 55. | Mabkhot, Y. N.; Alatibi, F.; El-Sayed, N. N. E.; Kheder, N. A.; Al-Showiman, S. S. Molecules 2016, 21, 1036. doi:10.3390/molecules21081036 |
| 60. | Apparao, S.; Ila, H.; Junjappa, H. J. Chem. Soc., Perkin Trans. 1 1983, 2837. doi:10.1039/p19830002837 |
| 62. | Fadda, A. A.; Abdel-Latif, E.; El-Mekawy, R. E. Eur. J. Med. Chem. 2009, 44, 1250. doi:10.1016/j.ejmech.2008.09.006 |
| 64. | Fadda, A. A.; Abdel-Latif, E.; El-Mekawy, R. E. Pharmacol. Pharm. 2012, 3, 148. doi:10.4236/pp.2012.32022 |
| 65. | Fadda, A. A.; Abdel-Latif, E.; El-Mekawy, R. E. Phosphorus, Sulfur Silicon Relat. Elem. 2008, 183, 1940. doi:10.1080/10426500701839536 |
| 70. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010.
p 120 and p 146. See for the structures of alkylpyridines. |
| 73. | Doering, W. v. E.; Hoffmann, A. K. J. Am. Chem. Soc. 1955, 77, 521. doi:10.1021/ja01608a003 |
| 74. | Johnson, A. W.; Lacount, R. B. Tetrahedron 1960, 9, 130. doi:10.1016/0040-4020(60)80061-0 |
| 72. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester; p 10.
See for the structure of imidazole. |
| 71. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010.
p 10 and p 42. See for the structures of thiophene, furan, and oxidiazole. |
| 71. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010.
p 10 and p 42. See for the structures of thiophene, furan, and oxidiazole. |
| 69. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010; p 7.
See for the structure of pyridine. |
| 70. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010.
p 120 and p 146. See for the structures of alkylpyridines. |
| 71. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010.
p 10 and p 42. See for the structures of thiophene, furan, and oxidiazole. |
| 72. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester; p 10.
See for the structure of imidazole. |
| 69. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010; p 7.
See for the structure of pyridine. |
| 70. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010.
p 120 and p 146. See for the structures of alkylpyridines. |
| 79. | Fraser, R. R.; Mansour, T. S.; Savard, S. J. Org. Chem. 1985, 50, 3232. doi:10.1021/jo00217a050 |
| 78. | Hunter, G. A.; McNab, H. J. Chem. Soc., Perkin Trans. 1 1995, 1209. doi:10.1039/p19950001209 |
| 78. | Hunter, G. A.; McNab, H. J. Chem. Soc., Perkin Trans. 1 1995, 1209. doi:10.1039/p19950001209 |
| 75. | Sheppard, W. A. J. Am. Chem. Soc. 1962, 84, 3072. doi:10.1021/ja00875a007 |
| 76. | Byrne, C. J.; Happer, D. A. R.; Hartshorn, M. P.; Powell, H. K. J. J. Chem. Soc., Perkin Trans. 2 1987, 1649. doi:10.1039/p29870001649 |
| 77. | Shorter, J. Pure Appl. Chem. 1997, 69, 2497. doi:10.1351/pac199769122497 |
| 69. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010; p 7.
See for the structure of pyridine. |
| 70. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010.
p 120 and p 146. See for the structures of alkylpyridines. |
| 71. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010.
p 10 and p 42. See for the structures of thiophene, furan, and oxidiazole. |
| 72. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester; p 10.
See for the structure of imidazole. |
| 80. | Apparao, S.; Ila, H.; Junjappa, H. J. Chem. Soc., Perkin Trans. 1 1983, 2837. doi:10.1039/p19830002837 |
| 81. | Ila, H.; Junjappa, H. Chimia 2013, 67, 17. doi:10.2533/chimia.2013.17 |
| 82. | Pan, L.; Bi, X.; Liu, Q. Chem. Soc. Rev. 2013, 42, 1251. doi:10.1039/C2CS35329F |
| 83. | Zhang, L.; Dong, J.; Xu, X.; Liu, Q. Chem. Rev. 2016, 116, 287. doi:10.1021/acs.chemrev.5b00360 |
| 84. | Bernhardt, P. V.; Koch, R.; Moloney, D. W. J.; Shtaiwi, M.; Wentrup, C. J. Chem. Soc., Perkin Trans. 2 2002, 515. doi:10.1039/B109624A |
| 69. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010; p 7.
See for the structure of pyridine. |
| 70. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010.
p 120 and p 146. See for the structures of alkylpyridines. |
| 71. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester, 2010.
p 10 and p 42. See for the structures of thiophene, furan, and oxidiazole. |
| 72. |
Joule, J. A.; Mills, K. Heterocyclic Chemistry, 5th ed.; John Wiley & Sons, Ltd.: Chichester; p 10.
See for the structure of imidazole. |
| 78. | Hunter, G. A.; McNab, H. J. Chem. Soc., Perkin Trans. 1 1995, 1209. doi:10.1039/p19950001209 |
| 13. | Li, A.-H.; Dai, L.-X.; Aggarwal, V. K. Chem. Rev. 1997, 97, 2341. doi:10.1021/cr960411r |
| 10. | Burtoloso, A. C. B.; Dias, R. M. P.; Leonarczyk, I. A. Eur. J. Org. Chem. 2013, 5005. doi:10.1002/ejoc.201300581 |
| 5. | Corey, E. J.; Chaykovsky, M. J. Am. Chem. Soc. 1962, 84, 3782. doi:10.1021/ja00878a046 |
| 6. | Corey, E. J.; Chaykovsky, M. J. Am. Chem. Soc. 1964, 86, 1640. doi:10.1021/ja01062a040 |
| 7. | Corey, E. J.; Chaykovsky, M. J. Am. Chem. Soc. 1965, 87, 1353. doi:10.1021/ja01084a034 |
| 10. | Burtoloso, A. C. B.; Dias, R. M. P.; Leonarczyk, I. A. Eur. J. Org. Chem. 2013, 5005. doi:10.1002/ejoc.201300581 |
| 11. | Moody, C. J.; Slawin, A. M. Z.; Taylor, R. J.; Williams, D. J. Tetrahedron Lett. 1988, 29, 6009. doi:10.1016/S0040-4039(00)82253-4 |
| 12. | Moody, C. J.; Taylor, R. J. Tetrahedron 1990, 46, 6525. doi:10.1016/S0040-4020(01)96018-1 |
| 13. | Li, A.-H.; Dai, L.-X.; Aggarwal, V. K. Chem. Rev. 1997, 97, 2341. doi:10.1021/cr960411r |
| 14. | Davoust, M.; Brière, J.-F.; Jaffrès, P.-A.; Metzner, P. J. Org. Chem. 2005, 70, 4166. doi:10.1021/jo0479260 |
| 15. | Piccinini, A.; Kavanagh, S. A.; Connon, P. B.; Connon, S. J. Org. Lett. 2010, 12, 608. doi:10.1021/ol902816w |
| 16. | Aggarwal, V. K.; Hynd, G.; Picoul, W.; Vasse, J.-L. J. Am. Chem. Soc. 2002, 124, 9964. doi:10.1021/ja0272540 |
| 17. | Chandrasekhar, S.; Narasihmulu, C.; Jagadeshwar, V.; Venkatram Reddy, K. Tetrahedron Lett. 2003, 44, 3629. doi:10.1016/S0040-4039(03)00732-9 |
| 18. | Concellón, J. M.; Bardales, E. J. Org. Chem. 2003, 68, 9492. doi:10.1021/jo0349577 |
| 19. | Paxton, R. J.; Taylor, R. J. K. Synlett 2007, 633. doi:10.1055/s-2007-967966 |
| 20. | Edwards, M. G.; Paxton, R. J.; Pugh, D. S.; Whitwood, A. C.; Taylor, R. J. K. Synthesis 2008, 3279. doi:10.1055/s-0028-1083165 |
| 21. | Robiette, R.; Marchan-Brynaert, J. Synlett 2008, 517. doi:10.1055/s-2008-1032080 |
| 22. | Hartikka, A.; Arvidsson, P. I. J. Org. Chem. 2007, 72, 5874. doi:10.1021/jo070519e |
| 33. | Liu, Y.; Shao, X.; Zhang, P.; Lu, L.; Shen, Q. Org. Lett. 2015, 17, 2752. doi:10.1021/acs.orglett.5b01170 |
| 34. | Zhu, J.; Liu, Y.; Shen, Q. Angew. Chem., Int. Ed. 2016, 55, 9050. doi:10.1002/anie.201603166 |
| 2. | Johnson, A. W.; LaCount, R. B. J. Am. Chem. Soc. 1961, 83, 417. doi:10.1021/ja01463a040 |
| 3. | Franzen, V.; Schmidt, H.-J.; Mertz, C. Chem. Ber. 1961, 94, 2942. doi:10.1002/cber.19610941117 |
| 4. | Franzen, V.; Driesen, H.-E. Chem. Ber. 1963, 96, 1881. doi:10.1002/cber.19630960722 |
| 5. | Corey, E. J.; Chaykovsky, M. J. Am. Chem. Soc. 1962, 84, 3782. doi:10.1021/ja00878a046 |
| 6. | Corey, E. J.; Chaykovsky, M. J. Am. Chem. Soc. 1964, 86, 1640. doi:10.1021/ja01062a040 |
| 7. | Corey, E. J.; Chaykovsky, M. J. Am. Chem. Soc. 1965, 87, 1353. doi:10.1021/ja01084a034 |
| 8. | Nozaki, H.; Takaku, M.; Kondô, K. Tetrahedron 1966, 22, 2145. doi:10.1016/S0040-4020(01)82134-7 |
| 9. | Ratts, K. W.; Yao, A. N. J. Org. Chem. 1966, 31, 1185. doi:10.1021/jo01342a047 |
| 35. | Huang, X.; Goddard, R.; Maulide, N. Angew. Chem., Int. Ed. 2010, 49, 8979. doi:10.1002/anie.201002919 |
| 36. | Huang, X.; Maulide, N. J. Am. Chem. Soc. 2011, 133, 8510. doi:10.1021/ja2031882 |
| 37. | Huang, X.; Klimczyk, S.; Maulide, N. Synthesis 2012, 44, 175. doi:10.1055/s-0031-1289632 |
| 38. | Huang, X.; Klimczyk, S.; Veiros, L. F.; Maulide, N. Chem. Sci. 2013, 4, 1105. doi:10.1039/c2sc21914j |
| 24. | Vaitla, J.; Bayer, A.; Hopmann, K. H. Angew. Chem., Int. Ed. 2017, 56, 4277. doi:10.1002/anie.201610520 |
| 25. | Boyarskikh, V.; Nyong, A.; Rainier, J. D. Angew. Chem., Int. Ed. 2008, 47, 5374. doi:10.1002/anie.200801336 |
| 87. | It cannot be ruled out that the pyridine moiety in 9b could be act as a general base. |
| 23. | Recent literature: Marsini, M. A.; Reeves, J. T.; Desrosiers, J.-N.; Herbage, M. A.; Savoie, J.; Li, Z.; Fandrick, K. R.; Sader, C. A.; McKibben, B.; Gao, D. A.; Cui, J.; Gonnella, N. C.; Lee, H.; Wei, X.; Roschangar, F.; Lu, B. Z.; Senanayake, C. H. Org. Lett. 2015, 17, 5614. doi:10.1021/acs.orglett.5b02838 |
| 26. | Soeta, T.; Ohgai, T.; Sakai, T.; Fujinami, S.; Ukaji, Y. Org. Lett. 2014, 16, 4854. doi:10.1021/ol502347n |
| 27. | Xu, X.; Li, C.; Tao, Z.; Pan, Y. Green Chem. 2017, 19, 1245. doi:10.1039/C6GC02681H |
| 28. | Enßle, M.; Buck, S.; Werz, R.; Maas, G. Beilstein J. Org. Chem. 2012, 8, 433. doi:10.3762/bjoc.8.49 |
| 29. | Li, K.; Hu, J.; Liu, H.; Tong, X. Chem. Commun. 2012, 48, 2900. doi:10.1039/c2cc30242j |
| 30. | Liu, Y.-Y.; Yang, X.-H.; Huang, X.-C.; Wei, W.-T.; Song, R.-J.; Li, J.-H. J. Org. Chem. 2013, 78, 10421. doi:10.1021/jo401851m |
| 31. | Lu, L.-Q.; Cao, Y.-J.; Liu, X.-P.; An, J.; Yao, C.-J.; Ming, Z.-H.; Xiao, W.-J. J. Am. Chem. Soc. 2008, 130, 6946. doi:10.1021/ja800746q |
| 32. | Chen, J.-R.; Dong, W.-R.; Candy, M.; Pan, F.-F.; Jörres, M.; Bolm, C. J. Am. Chem. Soc. 2012, 134, 6924. doi:10.1021/ja301196x |
| 19. | Paxton, R. J.; Taylor, R. J. K. Synlett 2007, 633. doi:10.1055/s-2007-967966 |
| 20. | Edwards, M. G.; Paxton, R. J.; Pugh, D. S.; Whitwood, A. C.; Taylor, R. J. K. Synthesis 2008, 3279. doi:10.1055/s-0028-1083165 |
| 21. | Robiette, R.; Marchan-Brynaert, J. Synlett 2008, 517. doi:10.1055/s-2008-1032080 |
| 22. | Hartikka, A.; Arvidsson, P. I. J. Org. Chem. 2007, 72, 5874. doi:10.1021/jo070519e |
| 84. | Bernhardt, P. V.; Koch, R.; Moloney, D. W. J.; Shtaiwi, M.; Wentrup, C. J. Chem. Soc., Perkin Trans. 2 2002, 515. doi:10.1039/B109624A |
| 14. | Davoust, M.; Brière, J.-F.; Jaffrès, P.-A.; Metzner, P. J. Org. Chem. 2005, 70, 4166. doi:10.1021/jo0479260 |
| 15. | Piccinini, A.; Kavanagh, S. A.; Connon, P. B.; Connon, S. J. Org. Lett. 2010, 12, 608. doi:10.1021/ol902816w |
| 16. | Aggarwal, V. K.; Hynd, G.; Picoul, W.; Vasse, J.-L. J. Am. Chem. Soc. 2002, 124, 9964. doi:10.1021/ja0272540 |
| 17. | Chandrasekhar, S.; Narasihmulu, C.; Jagadeshwar, V.; Venkatram Reddy, K. Tetrahedron Lett. 2003, 44, 3629. doi:10.1016/S0040-4039(03)00732-9 |
| 18. | Concellón, J. M.; Bardales, E. J. Org. Chem. 2003, 68, 9492. doi:10.1021/jo0349577 |
| 24. | Vaitla, J.; Bayer, A.; Hopmann, K. H. Angew. Chem., Int. Ed. 2017, 56, 4277. doi:10.1002/anie.201610520 |
| 6. | Corey, E. J.; Chaykovsky, M. J. Am. Chem. Soc. 1964, 86, 1640. doi:10.1021/ja01062a040 |
| 85. | Trost, B. M. J. Am. Chem. Soc. 1966, 88, 1587. doi:10.1021/ja00959a071 |
| 86. | Stoffregen, S. A.; Heying, M.; Jenks, W. S. J. Am. Chem. Soc. 2007, 129, 15746. doi:10.1021/ja076351w |
| 33. | Liu, Y.; Shao, X.; Zhang, P.; Lu, L.; Shen, Q. Org. Lett. 2015, 17, 2752. doi:10.1021/acs.orglett.5b01170 |
| 34. | Zhu, J.; Liu, Y.; Shen, Q. Angew. Chem., Int. Ed. 2016, 55, 9050. doi:10.1002/anie.201603166 |
| 6. | Corey, E. J.; Chaykovsky, M. J. Am. Chem. Soc. 1964, 86, 1640. doi:10.1021/ja01062a040 |
| 7. | Corey, E. J.; Chaykovsky, M. J. Am. Chem. Soc. 1965, 87, 1353. doi:10.1021/ja01084a034 |
| 9. | Ratts, K. W.; Yao, A. N. J. Org. Chem. 1966, 31, 1185. doi:10.1021/jo01342a047 |
| 11. | Moody, C. J.; Slawin, A. M. Z.; Taylor, R. J.; Williams, D. J. Tetrahedron Lett. 1988, 29, 6009. doi:10.1016/S0040-4039(00)82253-4 |
| 12. | Moody, C. J.; Taylor, R. J. Tetrahedron 1990, 46, 6525. doi:10.1016/S0040-4020(01)96018-1 |
| 54. | Mabkhot, Y. N.; Kaal, N. A.; Alterary, S.; Al-Showiman, S. S.; Barakat, A.; Ghabbour, H. A.; Frey, W. Molecules 2015, 20, 8712. doi:10.3390/molecules20058712 |
| 55. | Mabkhot, Y. N.; Alatibi, F.; El-Sayed, N. N. E.; Kheder, N. A.; Al-Showiman, S. S. Molecules 2016, 21, 1036. doi:10.3390/molecules21081036 |
| 56. | Acharya, A.; Parameshwarappa, G.; Saraiah, B.; Ila, H. J. Org. Chem. 2015, 80, 414. doi:10.1021/jo502429c |
| 50. | Yin, L.; Liebscher, J. Chem. Rev. 2007, 107, 133. doi:10.1021/cr0505674 |
| 51. | Gildner, P. G.; Colacot, T. J. Organometallics 2015, 34, 5497. doi:10.1021/acs.organomet.5b00567 |
| 52. | Ruiz-Castillo, P.; Buchwald, S. L. Chem. Rev. 2016, 116, 12564. doi:10.1021/acs.chemrev.6b00512 |
| 53. | Clavé, G.; Pelissier, F.; Campidelli, S.; Grison, C. Green Chem. 2017, 19, 4093. doi:10.1039/C7GC01672G |
| 43. | Jouanne, M.; Voisin-Chiret, A. S.; Legay, R.; Coufourier, S.; Rault, S.; Santos, J. S. O. Eur. J. Org. Chem. 2016, 5686. doi:10.1002/ejoc.201600882 |
| 44. | Kudo, N.; Perseghini, M.; Fu, G. C. Angew. Chem., Int. Ed. 2006, 45, 1282. doi:10.1002/anie.200503479 |
| 45. | Rao, G. K.; Kumar, A.; Ahmed, J.; Singh, A. K. Chem. Commun. 2010, 46, 5954. doi:10.1039/c0cc01075h |
| 46. | Billingsley, K. L.; Anderson, K. W.; Buchwald, S. L. Angew. Chem., Int. Ed. 2006, 45, 3484. doi:10.1002/anie.200600493 |
| 47. | Liu, C.; Ni, Q.; Hu, P.; Qiu, J. Org. Biomol. Chem. 2011, 9, 1054. doi:10.1039/C0OB00524J |
| 48. | Siddle, J. S.; Batsanov, A. S.; Bryce, M. R. Eur. J. Org. Chem. 2008, 2746. doi:10.1002/ejoc.200800018 |
| 49. | Kitamura, Y.; Sako, S.; Tsutsui, A.; Moguchi, Y.; Maegawa, T.; Kitade, Y.; Sajiki, H. Adv. Synth. Catal. 2010, 352, 718. doi:10.1002/adsc.200900638 |
| 35. | Huang, X.; Goddard, R.; Maulide, N. Angew. Chem., Int. Ed. 2010, 49, 8979. doi:10.1002/anie.201002919 |
| 36. | Huang, X.; Maulide, N. J. Am. Chem. Soc. 2011, 133, 8510. doi:10.1021/ja2031882 |
| 37. | Huang, X.; Klimczyk, S.; Maulide, N. Synthesis 2012, 44, 175. doi:10.1055/s-0031-1289632 |
| 38. | Huang, X.; Klimczyk, S.; Veiros, L. F.; Maulide, N. Chem. Sci. 2013, 4, 1105. doi:10.1039/c2sc21914j |
| 39. | Jamieson, A. G.; Russell, D.; Hamilton, A. D. Chem. Commun. 2012, 48, 3709. doi:10.1039/c2cc30295k |
| 40. | German, E. A.; Ross, J. E.; Knipe, P. C.; Don, M. F.; Thompson, S.; Hamilton, A. D. Angew. Chem., Int. Ed. 2015, 54, 2649. doi:10.1002/anie.201410290 |
| 41. | Smith, A. B., III; Keenan, T. P.; Holcomb, R. C.; Sprengeler, P. A.; Guzman, M. C.; Wood, J. L.; Carroll, P. J.; Hirschmann, R. J. Am. Chem. Soc. 1992, 114, 10672. doi:10.1021/ja00052a093 |
| 42. | Wyrembak, P. N.; Hamilton, A. D. J. Am. Chem. Soc. 2009, 131, 4566. doi:10.1021/ja809245t |
| 43. | Jouanne, M.; Voisin-Chiret, A. S.; Legay, R.; Coufourier, S.; Rault, S.; Santos, J. S. O. Eur. J. Org. Chem. 2016, 5686. doi:10.1002/ejoc.201600882 |
© 2018 Kim et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)