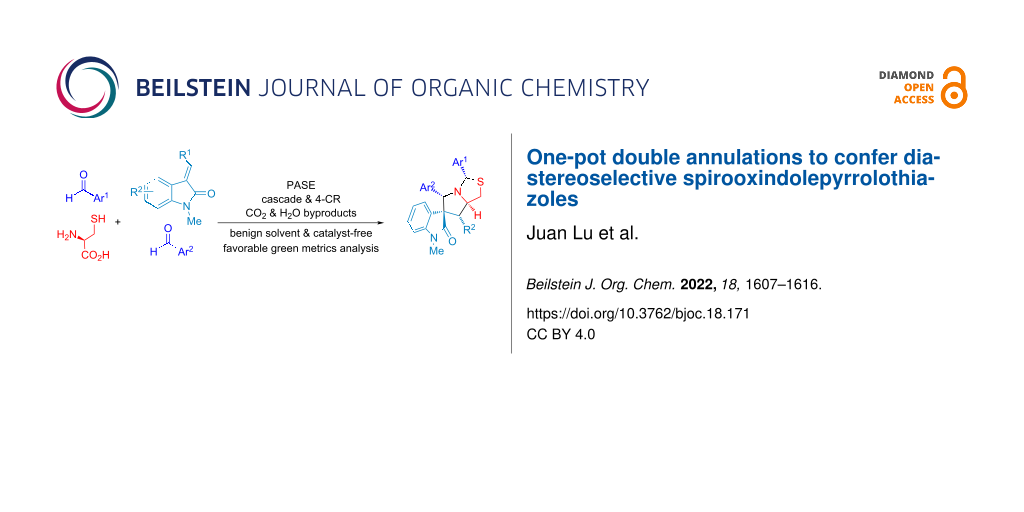
Abstract
A novel four-component reaction in one pot as an atom- and step-economic process was developed to synthesize diastereoselectively spirooxindolepyrrolothiazoles through sequential N,S-acetalation of aldehydes with cysteine and decarboxylative [3 + 2] cycloaddition with olefinic oxindoles. High synthetic efficiency, operational simplification and reaction process economy using EtOH as solvent, and only releasing CO2 and H2O as side products confer this approach favorable in green chemistry metrics analysis.
Graphical Abstract
Introduction
Nitrogen-containing heterocycles play a dominant role as a structural fragment of therapeutic agents in medicinal chemistry and drug discovery [1-9]. The nitrogen-containing heterocyclic moieties are currently discovered in more than 75% of the drugs available in the market approved by the FDA. Thus, the reaction process with synthetic efficiency and operational simplification is a critical factor in the construction of nitrogen-based heterocycles. Normally, some advantageous approaches in green synthesis are in favor of innovating the synthetic methods, optimizing the reaction process and eliminating the step of intermediate purification to save resources and reduce waste [10-12]. The pot, atom, step and economic (PASE) approach [13-17] is one of the most distinguished representatives in the efficient synthesis of nitrogen-based heterocycles, such as multicomponent reactions (MCRs) [18-23], one-pot cascade reactions [24-32] as good examples of PASE synthesis. We have reported a series of multicomponent reactions, like Groebke–Blackburn–Bienayme for making BET inhibitors UMB32 and UMB136 [33,34]. Zhang developed 4-aminoquinolines for the synthesis of fluorinated analogues of acetylcholinesterase (AChE) inhibitors [35] in cascade reactions, such as one-step syntheses of quinolines. Quinolin-4-ols involving histone acetyltransferases (HAT) inhibitors [36,37], as well as one-pot reactions were also developed by Zhang using the 4-aminoquinoline synthesis, for example, in amino acids(esters)-based [3 + 2] cycloadditions [38-48] and in the synthesis of pyrrolidine-containing systems [49-59]. Pyrrolothiazole and spirooxindole moieties occupy exclusive positions as valuable source of natural products and therapeutic agents in organic synthesis and drug discovery [60-68].
We have developed a number of asymmetric reactions to construct spirooxindole-based scaffolds through one-pot reactions with recyclable organocatalysts [69]. Notably, we conferred K10 acid to promote the C–H activation in the synthesis of spirooxindolepyrrolidines, and used Zeolite HY catalyst to synthesize diastereoselective dispiro[oxindolepyrrolidine]s with a butterfly shape (Scheme 1A and 1B) [70,71]. With the promising applications of spirooxindolepyrrolothiazoles in drug discovery (Figure 1) [72-74], the structural integration of spirooxindole and pyrrolothiazole with diverse substituted groups via an efficient synthesis is a challengeable research in green chemistry. The corresponding PASE reactions of making spirooxindolepyrrolothiazoles are even more rare, which only involves three-component reactions with isatins and thioproline (Scheme 2A and 2B) [75,76].
Scheme 1: The diastereoselective synthesis of spirooxindoles through MCRs.
Scheme 1: The diastereoselective synthesis of spirooxindoles through MCRs.
Figure 1: Bioactive Spirooxindole-pyrrolothiazoles.
Figure 1: Bioactive Spirooxindole-pyrrolothiazoles.
Scheme 2: The synthesis of spirooxindolepyrrolothiazoles.
Scheme 2: The synthesis of spirooxindolepyrrolothiazoles.
Four-component double annulations through 2-substituted thioprolines formed in N,S-acetalation of aldehyde and cysteine was introduced in this study. Subsequently one equivalent of aldehyde and olefinic oxindole in situ were followed by decarboxylative 1,3-dipolar cycloaddition for diastereoselective synthesis of spirooxindolepyrrolothiazoles with generating 5 new bonds, 5 stereocenters and two heterocycles (Scheme 1C and Scheme 2C).
Results and Discussion
The optimized reaction conditions of stepwise, one-pot and cascade (two-step with one operational step) processes for N,S-acetalation and decarboxylative 1,3-dipolar cycloaddition were developed by using two equivalents of 4-bromobenzaldehyde (1a), ʟ-cysteine (2) and olefinic oxindole 4a shown in Table 1. In our continuous effort on the reaction optimization of benign solvents, we firstly evaluated the influence of reaction time and protic solvents such as EtOH, iPrOH and MeOH at 25 °C for 6 h, which only results in slightly different LC yield (93–95%) of compound 3a (Table 1, entries 2–4) superior to 86% yield for 3 h (Table 1 entry 1), and followed by decarboxylative [3 + 2] cycloaddition with the second equivalent of compound 1a and olefinic oxindole 4a under reflux heating for 12 h. It indicates that the one-pot reaction process with EtOH and iPrOH afforded the 81% of LC yield for compound 5a slightly better than 78% yield using MeOH as a solvent (Table 1, entries 2–4). After screening the reaction temperature in the 2nd step of the one-pot process (Table 1, entries 4 and 5), it was found that the diastereomeric mixture of thioproline 3a without purification from N,S-acetalation with 1.0:1.15 of 1a/2 at 25 °C for 6 h with EtOH as solvent, in situ followed by addition of 1.1:1.0 of 1a/4a for [3 + 2] cycloaddition at 90 °C for 9 h gave compound 5a with the 81% of LC yield. Next, the stepwise process was also carried out by using the thioproline 3a (1 equiv) with 86% of isolated yield and 1.1:1.0 of 1a/4a through decarboxylative [3 + 2] cycloaddition (Table 1, entry 6), which afforded compound 5a with 73% isolated yield at 90 °C for 9 h. Notably, we conferred cascade reaction process to synthesize compound 5a with 70% isolated yield as one-step four-component reaction (4-CR) with 2.2:1.1:1.0 of 1a/2/4a at 90 °C for 9 h in EtOH after variations of solvents, reaction time and temperature with one operational step (Table 1, entries 7–12). This 4-CR is the first example of double annulations with sequential N,S-acetalation and [3 + 2] cycloaddition for diastereoselective spirooxindolepyrrolothiazoles by the formation of two new rings, 5 bonds, and 5 stereocenters without intermediate purification.
Table 1: Optimization of reaction conditions for double annulations of cysteine.a
|
|
||||||
| entry | solvent | t1 (h) | 3a (%)b | t2 (h) | T1(°C) | 5a (%)b |
| 1 | EtOH | 3 | 86 | – | ||
| 2 | iPrOH | 6 | 95 | 12 | 105 | 81 |
| 3 | MeOH | 6 | 93 | 12 | 70 | 78 |
| 4 | EtOH | 6 | 95 | 12 | 90 | 81 |
| 5 | EtOH | 6 | 95 | 9 | 90 | 81 |
| 6c,e | EtOH | 6 | 95 (86) | 9 | 90 | 83 (73) |
| 7d,e | EtOH | 9 | 90 | 79 (70) | ||
| 8 d | EtOH | 6 | 90 | 67 | ||
| 9 d | EtOH | 18 | 90 | 75 | ||
| 10d | MeOH | 9 | 70 | 76 | ||
| 11d | iPrOH | 9 | 105 | 78 | ||
| 12d | MeCN | 9 | 90 | 67 | ||
aOne-pot reaction of 1.0:1.15 of 1a/2 for N,S-acetalation 3a followed by addition of 1.1:1.0 of 1a/4a for [3 + 2] cycloaddition.bDetected by LC–MS, isolated yield in parenthesis. cIntermediate 3a was isolated in the two-step reaction. dCascade reaction of 2.2:1.1:1.0 of 1a/2/4a. edr > 4:1, Determined by 1H NMR analysis of the crude products after the reaction mixture filtered through a pad of silica gel and removal of solvent.
To explore the reaction scope of 4-CR, different aldehydes 1 (Ar1) were used to react with ʟ-cysteine (2) and olefinic oxindole 4a in the synthesis of substituted spirooxindolepyrrolothiazole analogues 5a–d with 49–70% isolated yield (Scheme 3) under the optimized reaction conditions (Table 1, entry 7). Compounds 5b–d with using heteroaromatic aldehydes resulted in lower yield than 5a.
Scheme 3: Four-component reaction for the synthesis of compound 5.
Scheme 3: Four-component reaction for the synthesis of compound 5.
In addition, according to the one-pot reaction process (Table 1, entry 5) with two operational steps using different aldehydes 1 and 6, products 7a–e were synthesized in 43–72% isolated yields and up to 6:1 dr (Table 2).
Table 2: One-pot reaction for the synthesis of compound 7.
|
|
|||||
| entry | Ar1 | Ar2 | R1 | product | yield (%)b |
| 1 | 2-thiophenyl | 3-OMe-4-FC6H3 | CO2Et | 7a | 66 |
| 2 | 2-thiophenyl | 2-furanyl | CO2Et | 7b | 51 |
| 3 | 2-thiophenyl | 3-pyridinyl | CO2Et | 7c | 43 |
| 4 | 2-FC6H4 | 4-ClC6H4 | CO2Et | 7d | 72 |
| 5 | 4-BrC6H4 | 4-ClC6H4 | CO2Et | 7e | 66 |
| 6 | 4-BrC6H4 | Ph | COMe | 7f | trace |
| 7 | 4-BrC6H4 | Ph | Ph | 7g | – |
| 8 | 4-BrC6H4 | CO2Et | CO2Et | 7h | messy |
| 9 | 4-BrC6H4 | ethyl | CO2Et | 7i | messy |
aIsolated yield. Reaction conditions are same as Table 1, entry 5.
The results indicate that the substituent on Ar2 of the aldehydes could influence the product yield, such as 7c (3-pyridinyl, 43% yield, 4.5:1 dr). In addition, oxindole 4 with different R1 was employed for the synthesis to give 7f with COMe in a trace amount and no product 7g with a Ph group. The following reactions with aliphatic aldehydes gave 7h and 7i as complex mixtures [54-59,71]. The reaction mechanism of the double annulations for sequential N,S-acetalation and decarboxylative [3 + 2] cycloaddition is shown in Scheme 4. With the promotion of the protonic solvent EtOH, compound 3 (N,S-acetal) from the condensation of cysteine and an aldehyde reacts with a second equivalent of aldehyde followed by cyclization to generate thiazolooxazol-1-one I.
Scheme 4: Proposed mechanism for the double [3 + 2] cycloadditions.
Scheme 4: Proposed mechanism for the double [3 + 2] cycloadditions.
Subsequent decarboxylation of thiazolooxazol-1-one I affords non-stabilized azomethine ylide (AY) for 1,3-dipolar cycloaddition with olefinic oxindole 4a to give spirooxindolepyrrolothiazoles 5 and 7. The endo-TS is more favorable than exo-TS for the 1,3-dipolar cycloaddition to afford the major and minor products. The diastereochemistry of non-stabilized azomethine ylides for decarboxylative [3 + 2] cycloaddition could be identified in reported literature [54-59,71]. Through the study of the mechanism, it elucidates that the double annulations using ʟ-cysteine undergoes three stages: compound 3, thiazolooxazol-1-one I and AY in the reducing stereocenter in an ratio of 3 to 1. The mechanistic process indicates that the configuration of ʟ-cysteine didn’t affect the stereoselectivity in the formation of compound 5 and 7. Thus, we further validated the hypothesis through the experimental results using ᴅ- and ʟ-cysteine to synthesize compound 5a (Scheme 5). We conferred green chemistry metrics to evaluate the process efficiency of four-component reaction via comprehensive and quantitative calculation. The metrics analysis is carried out for the two-step synthesis with intermediate separation (process A) and the single-step method (process B) for the synthesis of spirooxindolepyrrolothiazoles 5a according to the reaction conditions shown in Scheme 6. Green chemistry metrics data including atom economy (AE), atom efficiency (AEf), carbon efficiency (CE), reaction mass efficiency (RME), optimum efficiency (OE), mass productivity (MP), mass intensity (MI), process mass intensity (PMI), E factor (E), and solvent intensity (SI) are listed in Table 3 and Table 4 and are shown in Figure 2 and Figure 3 (the green metrics and detailed calculation process is described in Supporting Information File 1).
Scheme 5: The synthesis of compound 5a with ᴅ- and ʟ-cysteine.
Scheme 5: The synthesis of compound 5a with ᴅ- and ʟ-cysteine.
Scheme 6: Two-step (process A) vs cascade (process B) synthesis of 5a. i) 1.0:1.15 of 1a/2, EtOH (0.05 M), 25 °C, 6 h. ii) 1.1:1.0:1.0 of 1a/3a/4a, EtOH (0.5 M), 90 °C, 9 h (Table 1, entry 6). iii) 2.2:1.1:1.0 of 1a/2/4a, EtOH (0.5 M), 90 °C, 9 h (Table 1, entry 7).
Scheme 6: Two-step (process A) vs cascade (process B) synthesis of 5a. i) 1.0:1.15 of 1a/2, EtOH (0.05 M), 25...
![[1860-5397-18-171-2]](/bjoc/content/figures/1860-5397-18-171-2.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 2: Graphical representation of the green metrics (AE, AEf, CE, RME, OE and MP) analysis for processes A and B. The higher the value, the greener the process.
Figure 2: Graphical representation of the green metrics (AE, AEf, CE, RME, OE and MP) analysis for processes ...
![[1860-5397-18-171-3]](/bjoc/content/figures/1860-5397-18-171-3.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 3: Graphical representation of the green metrics (PMI, E-factor, and SI) analysis for processes A and B. The lower value, the better the reaction process.
Figure 3: Graphical representation of the green metrics (PMI, E-factor, and SI) analysis for processes A and ...
Process A is a two-step method involving isolation of intermediates, in which compound 3a was purified before 1,3-dipolar cycloaddition. Process B is a single-step approach without isolation of intermediate 3a. The same substrates for synthesizing product 5a in processes A and B results in 88.9% of AE. The AEf, RME and OE for one-step process B are 62%, 58% and 65%, a little better than those for process A (56%, 57% and 64.1%). In addition, CE and MP are significant references to elucidate reaction process consumption. The CE and MP for process B (115% and 20%) are much better than that for process A (64.4% and 4%). PMI for process A (25) is 5 times larger than that for process B (5). The E-factor for process A (24) is less significant than that for process B (19). Solvent consumption (SI, 3.5) for process B is clearly lower than that for process A (23) with more solvent for intermediate separations.
Conclusion
A readily and efficient four-component synthesis for spirooxindolepyrrolothiazoles is introduced, which involves a sequential N,S-acetalation and decarboxylative [3 + 2] cycloaddition reaction. This one-pot and two-step process with four components generates 5 bonds, 5 stereocenters and two heterocycles in a diastereoselective fashion, and without intermediate purification. The one-pot four-component synthesis in green metrics analysis is compared with the stepwise reaction process to pinpoint the overwhelming advantages of the one-pot approach in the CE, MP, PMI, and SI by eliminating the intermediate purification. It is an efficient way to build up novel spirooxindolepyrrolothiazoles for drug discovery screening.
Supporting Information
| Supporting Information File 1: Experimental and analytical data, copies of NMR spectra, green metrics and the detailed calculation process. | ||
| Format: PDF | Size: 3.0 MB | Download |
References
-
Grover, G.; Nath, R.; Bhatia, R.; Akhtar, M. J. Bioorg. Med. Chem. 2020, 28, 115585. doi:10.1016/j.bmc.2020.115585
Return to citation in text: [1] -
Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K. K.; Jonnalagadda, S. B. Molecules 2020, 25, 1909. doi:10.3390/molecules25081909
Return to citation in text: [1] -
Lang, D. K.; Kaur, R.; Arora, R.; Saini, B.; Arora, S. Anti-Cancer Agents Med. Chem. 2020, 20, 2150–2168. doi:10.2174/1871520620666200705214917
Return to citation in text: [1] -
Heravi, M. M.; Zadsirjan, V. RSC Adv. 2020, 10, 44247–44311. doi:10.1039/d0ra09198g
Return to citation in text: [1] -
Rodriguez del Rey, F. O.; Floreancig, P. E. Org. Lett. 2021, 23, 150–154. doi:10.1021/acs.orglett.0c03868
Return to citation in text: [1] -
Deiters, A.; Martin, S. F. Chem. Rev. 2004, 104, 2199–2238. doi:10.1021/cr0200872
Return to citation in text: [1] -
Shan, Y.; Su, L.; Zhao, Z.; Chen, D. Adv. Synth. Catal. 2021, 363, 906–923. doi:10.1002/adsc.202001283
Return to citation in text: [1] -
Hemmerling, F.; Hahn, F. Beilstein J. Org. Chem. 2016, 12, 1512–1550. doi:10.3762/bjoc.12.148
Return to citation in text: [1] -
Kaur, N. Synth. Commun. 2019, 49, 1633–1658. doi:10.1080/00397911.2018.1542497
Return to citation in text: [1] -
Clarke, P. A.; Santos, S.; Martin, W. H. C. Green Chem. 2007, 9, 438–440. doi:10.1039/b700923b
Return to citation in text: [1] -
Trost, B. M. Acc. Chem. Res. 2002, 35, 695–705. doi:10.1021/ar010068z
Return to citation in text: [1] -
Anastas, P.; Eghbali, N. Chem. Soc. Rev. 2010, 39, 301–312. doi:10.1039/b918763b
Return to citation in text: [1] -
Zhang, X.; Zhang, W. Curr. Opin. Green Sustainable Chem. 2018, 11, 65–69. doi:10.1016/j.cogsc.2018.04.005
Return to citation in text: [1] -
Newhouse, T.; Baran, P. S.; Hoffmann, R. W. Chem. Soc. Rev. 2009, 38, 3010–3021. doi:10.1039/b821200g
Return to citation in text: [1] -
Bhuyan, D.; Sarma, R.; Dommaraju, Y.; Prajapati, D. Green Chem. 2014, 16, 1158–1162. doi:10.1039/c3gc42389a
Return to citation in text: [1] -
Hayashi, Y.; Umemiya, S. Angew. Chem., Int. Ed. 2013, 52, 3450–3452. doi:10.1002/anie.201209380
Return to citation in text: [1] -
Zhang, W.; Yi, W.-B. Introduction to PASE Synthesis. In Pot, Atom, and Step Economy (PASE) Synthesis; Zhang, W.; Yi, W.-B., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp 1–4. doi:10.1007/978-3-030-22596-4_1
Return to citation in text: [1] -
Cioc, R. C.; Ruijter, E.; Orru, R. V. A. Green Chem. 2014, 16, 2958–2975. doi:10.1039/c4gc00013g
Return to citation in text: [1] -
Rotstein, B. H.; Zaretsky, S.; Rai, V.; Yudin, A. K. Chem. Rev. 2014, 114, 8323–8359. doi:10.1021/cr400615v
Return to citation in text: [1] -
Estévez, V.; Villacampa, M.; Menéndez, J. C. Chem. Soc. Rev. 2014, 43, 4633–4657. doi:10.1039/c3cs60015g
Return to citation in text: [1] -
de Graaff, C.; Ruijter, E.; Orru, R. V. A. Chem. Soc. Rev. 2012, 41, 3969–4009. doi:10.1039/c2cs15361k
Return to citation in text: [1] -
Dömling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083–3135. doi:10.1021/cr100233r
Return to citation in text: [1] -
Brauch, S.; van Berkel, S. S.; Westermann, B. Chem. Soc. Rev. 2013, 42, 4948–4962. doi:10.1039/c3cs35505e
Return to citation in text: [1] -
Tietze, L. F.; Brasche, G.; Gericke, K. M. Domino Reactions in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006. doi:10.1002/9783527609925
Return to citation in text: [1] -
Nicolaou, K. C.; Chen, J. S. Chem. Soc. Rev. 2009, 38, 2993–3009. doi:10.1039/b903290h
Return to citation in text: [1] -
Enders, D.; Grondal, C.; Hüttl, M. R. M. Angew. Chem., Int. Ed. 2007, 46, 1570–1581. doi:10.1002/anie.200603129
Return to citation in text: [1] -
Padwa, A.; Bur, S. K. Tetrahedron 2007, 63, 5341–5378. doi:10.1016/j.tet.2007.03.158
Return to citation in text: [1] -
Nicolaou, K. C.; Edmonds, D. J.; Bulger, P. G. Angew. Chem., Int. Ed. 2006, 45, 7134–7186. doi:10.1002/anie.200601872
Return to citation in text: [1] -
Wasilke, J.-C.; Obrey, S. J.; Baker, R. T.; Bazan, G. C. Chem. Rev. 2005, 105, 1001–1020. doi:10.1021/cr020018n
Return to citation in text: [1] -
Hayashi, Y. Chem. Sci. 2016, 7, 866–880. doi:10.1039/c5sc02913a
Return to citation in text: [1] -
Sydnes, M. O. Curr. Green Chem. 2014, 1, 216–226. doi:10.2174/2213346101666140221225404
Return to citation in text: [1] -
Atkinson, M. B. J.; Oyola-Reynoso, S.; Luna, R. E.; Bwambok, D. K.; Thuo, M. M. RSC Adv. 2015, 5, 597–607. doi:10.1039/c4ra13506g
Return to citation in text: [1] -
McKeown, M. R.; Shaw, D. L.; Fu, H.; Liu, S.; Xu, X.; Marineau, J. J.; Huang, Y.; Zhang, X.; Buckley, D. L.; Kadam, A.; Zhang, Z.; Blacklow, S. C.; Qi, J.; Zhang, W.; Bradner, J. E. J. Med. Chem. 2014, 57, 9019–9027. doi:10.1021/jm501120z
Return to citation in text: [1] -
Huang, H.; Liu, S.; Jean, M.; Simpson, S.; Huang, H.; Merkley, M.; Hayashi, T.; Kong, W.; Rodríguez-Sánchez, I.; Zhang, X.; Yosief, H. O.; Miao, H.; Que, J.; Kobie, J. J.; Bradner, J.; Santoso, N. G.; Zhang, W.; Zhu, J. Front. Microbiol. 2017, 8, 1035. doi:10.3389/fmicb.2017.01035
Return to citation in text: [1] -
Zhang, X.; Ma, X.; Qiu, W.; Awad, J.; Evans, J.; Zhang, W. Adv. Synth. Catal. 2020, 362, 5513–5517. doi:10.1002/adsc.202000734
Return to citation in text: [1] -
Zhang, X.; Dhawan, G.; Muthengi, A.; Liu, S.; Wang, W.; Legris, M.; Zhang, W. Green Chem. 2017, 19, 3851–3855. doi:10.1039/c7gc01380a
Return to citation in text: [1] -
Zhang, X.; Ma, X.; Qiu, W.; Evans, J.; Zhang, W. Green Chem. 2019, 21, 349–354. doi:10.1039/c8gc03180k
Return to citation in text: [1] -
Padwa, A.; Pearson, W. H., Eds. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; John Wiley & Sons: New York, NY, USA, 2003; Vol. 59. doi:10.1002/0471221902
Return to citation in text: [1] -
Coldham, I.; Hufton, R. Chem. Rev. 2005, 105, 2765–2810. doi:10.1021/cr040004c
Return to citation in text: [1] -
Pandey, G.; Banerjee, P.; Gadre, S. R. Chem. Rev. 2006, 106, 4484–4517. doi:10.1021/cr050011g
Return to citation in text: [1] -
Hashimoto, T.; Maruoka, K. Chem. Rev. 2015, 115, 5366–5412. doi:10.1021/cr5007182
Return to citation in text: [1] -
Gothelf, K. V.; Jørgensen, K. A. Chem. Rev. 1998, 98, 863–910. doi:10.1021/cr970324e
Return to citation in text: [1] -
Martina, K.; Tagliapietra, S.; Veselov, V. V.; Cravotto, G. Front. Chem. (Lausanne, Switz.) 2019, 7, 95. doi:10.3389/fchem.2019.00095
Return to citation in text: [1] -
Zhang, W. Chem. Lett. 2013, 42, 676–681. doi:10.1246/cl.130504
Return to citation in text: [1] -
Narayan, R.; Potowski, M.; Jia, Z.-J.; Antonchick, A. P.; Waldmann, H. Acc. Chem. Res. 2014, 47, 1296–1310. doi:10.1021/ar400286b
Return to citation in text: [1] -
Selva, V.; Selva, E.; Merino, P.; Nájera, C.; Sansano, J. M. Org. Lett. 2018, 20, 3522–3526. doi:10.1021/acs.orglett.8b01292
Return to citation in text: [1] -
Henke, B. R.; Kouklis, A. J.; Heathcock, C. H. J. Org. Chem. 1992, 57, 7056–7066. doi:10.1021/jo00052a015
Return to citation in text: [1] -
Yildirim, O.; Grigalunas, M.; Brieger, L.; Strohmann, C.; Antonchick, A. P.; Waldmann, H. Angew. Chem., Int. Ed. 2021, 60, 20012–20020. doi:10.1002/anie.202108072
Return to citation in text: [1] -
Lu, Q.; Song, G.; Jasinski, J. P.; Keeley, A. C.; Zhang, W. Green Chem. 2012, 14, 3010–3012. doi:10.1039/c2gc36066g
Return to citation in text: [1] -
Zhang, W.; Lu, Y.; Geib, S. Org. Lett. 2005, 7, 2269–2272. doi:10.1021/ol0507773
Return to citation in text: [1] -
Zhang, X.; Qiu, W.; Ma, X.; Evans, J.; Kaur, M.; Jasinski, J. P.; Zhang, W. J. Org. Chem. 2018, 83, 13536–13542. doi:10.1021/acs.joc.8b02046
Return to citation in text: [1] -
Zhang, X.; Zhi, S.; Wang, W.; Liu, S.; Jasinski, J. P.; Zhang, W. Green Chem. 2016, 18, 2642–2646. doi:10.1039/c6gc00497k
Return to citation in text: [1] -
Zhang, X.; Pham, K.; Liu, S.; Legris, M.; Muthengi, A.; Jasinski, J. P.; Zhang, W. Beilstein J. Org. Chem. 2016, 12, 2204–2210. doi:10.3762/bjoc.12.211
Return to citation in text: [1] -
Zhang, X.; Liu, M.; Zhang, W.; Legris, M.; Zhang, W. J. Fluorine Chem. 2017, 204, 18–22. doi:10.1016/j.jfluchem.2017.10.003
Return to citation in text: [1] [2] [3] -
Zhang, X.; Qiu, W.; Evans, J.; Kaur, M.; Jasinski, J. P.; Zhang, W. Org. Lett. 2019, 21, 2176–2179. doi:10.1021/acs.orglett.9b00487
Return to citation in text: [1] [2] [3] -
Ma, X.; Zhang, X.; Qiu, W.; Zhang, W.; Wan, B.; Evans, J.; Zhang, W. Molecules 2019, 24, 601. doi:10.3390/molecules24030601
Return to citation in text: [1] [2] [3] -
Ma, X.; Zhang, X.; Awad, J. M.; Xie, G.; Qiu, W.; Muriph, R. E.; Zhang, W. Tetrahedron Lett. 2020, 61, 151392. doi:10.1016/j.tetlet.2019.151392
Return to citation in text: [1] [2] [3] -
Ma, X.; Meng, S.; Zhang, X.; Zhang, Q.; Yan, S.; Zhang, Y.; Zhang, W. Beilstein J. Org. Chem. 2020, 16, 1225–1233. doi:10.3762/bjoc.16.106
Return to citation in text: [1] [2] [3] -
Ma, X.; Qiu, W.; Liu, L.; Zhang, X.; Awad, J.; Evans, J.; Zhang, W. Green Synth. Catal. 2021, 2, 74–77. doi:10.1016/j.gresc.2020.11.001
Return to citation in text: [1] [2] [3] -
Spanò, V.; Barreca, M.; Cilibrasi, V.; Genovese, M.; Renda, M.; Montalbano, A.; Galietta, L. J. V.; Barraja, P. Molecules 2021, 26, 1275. doi:10.3390/molecules26051275
Return to citation in text: [1] -
Noda, K.; Terasawa, N.; Murata, M. Food Funct. 2016, 7, 2551–2556. doi:10.1039/c5fo01625h
Return to citation in text: [1] -
Noda, K.; Yamada, S.; Murata, M. Biosci., Biotechnol., Biochem. 2015, 79, 1350–1355. doi:10.1080/09168451.2015.1018127
Return to citation in text: [1] -
Bharkavi, C.; Vivek Kumar, S.; Ashraf Ali, M.; Osman, H.; Muthusubramanian, S.; Perumal, S. Bioorg. Med. Chem. 2016, 24, 5873–5883. doi:10.1016/j.bmc.2016.09.044
Return to citation in text: [1] -
Arulananda Babu, S.; Padmavathi, R.; Ahmad Aslam, N.; Rajkumar, V. Recent Developments on the Synthesis and Applications of Natural Products-Inspired Spirooxindole Frameworks. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, Netherlands, 2015; Vol. 46, pp 227–339. doi:10.1016/b978-0-444-63462-7.00008-7
Return to citation in text: [1] -
Zhou, L.-M.; Qu, R.-Y.; Yang, G.-F. Expert Opin. Drug Discovery 2020, 15, 603–625. doi:10.1080/17460441.2020.1733526
Return to citation in text: [1] -
Panda, S. S.; Jones, R. A.; Bachawala, P.; Mohapatra, P. P. Mini-Rev. Med. Chem. 2017, 17, 1515–1536. doi:10.2174/1389557516666160624125108
Return to citation in text: [1] -
Wang, Y.; Cobo, A. A.; Franz, A. K. Org. Chem. Front. 2021, 8, 4315–4348. doi:10.1039/d1qo00220a
Return to citation in text: [1] -
Ye, N.; Chen, H.; Wold, E. A.; Shi, P.-Y.; Zhou, J. ACS Infect. Dis. 2016, 2, 382–392. doi:10.1021/acsinfecdis.6b00041
Return to citation in text: [1] -
Huang, X.; Zhang, W. Chem. Commun. 2021, 57, 10116–10124. doi:10.1039/d1cc03722f
Return to citation in text: [1] -
Zhang, X.; Liu, M.; Qiu, W.; Evans, J.; Kaur, M.; Jasinski, J. P.; Zhang, W. ACS Sustainable Chem. Eng. 2018, 6, 5574–5579. doi:10.1021/acssuschemeng.8b00555
Return to citation in text: [1] -
Zhang, X.; Qiu, W.; Murray, S. A.; Zhan, D.; Evans, J.; Jasinski, J. P.; Wang, X.; Zhang, W. J. Org. Chem. 2021, 86, 17395–17403. doi:10.1021/acs.joc.1c01797
Return to citation in text: [1] [2] [3] -
Lotfy, G.; Said, M. M.; El Ashry, E. S. H.; El Tamany, E. S. H.; Al-Dhfyan, A.; Abdel Aziz, Y. M.; Barakat, A. Bioorg. Med. Chem. 2017, 25, 1514–1523. doi:10.1016/j.bmc.2017.01.014
Return to citation in text: [1] -
Wu, G.; Ouyang, L.; Liu, J.; Zeng, S.; Huang, W.; Han, B.; Wu, F.; He, G.; Xiang, M. Mol. Diversity 2013, 17, 271–283. doi:10.1007/s11030-013-9432-3
Return to citation in text: [1] -
Ren, W.; Zhao, Q.; Yu, M.; Guo, L.; Chang, H.; Jiang, X.; Luo, Y.; Huang, W.; He, G. Mol. Diversity 2020, 24, 1043–1063. doi:10.1007/s11030-019-10011-2
Return to citation in text: [1] -
Li, J.; Wang, J.; Xu, Z.; Zhu, S. ACS Comb. Sci. 2014, 16, 506–512. doi:10.1021/co500085t
Return to citation in text: [1] -
Feng, T.-T.; Gong, Y.; Wei, Q.-D.; Wang, G.-L.; Liu, H.-H.; Tian, M.-Y.; Liu, X.-L.; Chen, Z.-Y.; Zhou, Y. J. Heterocycl. Chem. 2018, 55, 1136–1146. doi:10.1002/jhet.3145
Return to citation in text: [1]
| 54. | Zhang, X.; Liu, M.; Zhang, W.; Legris, M.; Zhang, W. J. Fluorine Chem. 2017, 204, 18–22. doi:10.1016/j.jfluchem.2017.10.003 |
| 55. | Zhang, X.; Qiu, W.; Evans, J.; Kaur, M.; Jasinski, J. P.; Zhang, W. Org. Lett. 2019, 21, 2176–2179. doi:10.1021/acs.orglett.9b00487 |
| 56. | Ma, X.; Zhang, X.; Qiu, W.; Zhang, W.; Wan, B.; Evans, J.; Zhang, W. Molecules 2019, 24, 601. doi:10.3390/molecules24030601 |
| 57. | Ma, X.; Zhang, X.; Awad, J. M.; Xie, G.; Qiu, W.; Muriph, R. E.; Zhang, W. Tetrahedron Lett. 2020, 61, 151392. doi:10.1016/j.tetlet.2019.151392 |
| 58. | Ma, X.; Meng, S.; Zhang, X.; Zhang, Q.; Yan, S.; Zhang, Y.; Zhang, W. Beilstein J. Org. Chem. 2020, 16, 1225–1233. doi:10.3762/bjoc.16.106 |
| 59. | Ma, X.; Qiu, W.; Liu, L.; Zhang, X.; Awad, J.; Evans, J.; Zhang, W. Green Synth. Catal. 2021, 2, 74–77. doi:10.1016/j.gresc.2020.11.001 |
| 71. | Zhang, X.; Qiu, W.; Murray, S. A.; Zhan, D.; Evans, J.; Jasinski, J. P.; Wang, X.; Zhang, W. J. Org. Chem. 2021, 86, 17395–17403. doi:10.1021/acs.joc.1c01797 |
| 1. | Grover, G.; Nath, R.; Bhatia, R.; Akhtar, M. J. Bioorg. Med. Chem. 2020, 28, 115585. doi:10.1016/j.bmc.2020.115585 |
| 2. | Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K. K.; Jonnalagadda, S. B. Molecules 2020, 25, 1909. doi:10.3390/molecules25081909 |
| 3. | Lang, D. K.; Kaur, R.; Arora, R.; Saini, B.; Arora, S. Anti-Cancer Agents Med. Chem. 2020, 20, 2150–2168. doi:10.2174/1871520620666200705214917 |
| 4. | Heravi, M. M.; Zadsirjan, V. RSC Adv. 2020, 10, 44247–44311. doi:10.1039/d0ra09198g |
| 5. | Rodriguez del Rey, F. O.; Floreancig, P. E. Org. Lett. 2021, 23, 150–154. doi:10.1021/acs.orglett.0c03868 |
| 6. | Deiters, A.; Martin, S. F. Chem. Rev. 2004, 104, 2199–2238. doi:10.1021/cr0200872 |
| 7. | Shan, Y.; Su, L.; Zhao, Z.; Chen, D. Adv. Synth. Catal. 2021, 363, 906–923. doi:10.1002/adsc.202001283 |
| 8. | Hemmerling, F.; Hahn, F. Beilstein J. Org. Chem. 2016, 12, 1512–1550. doi:10.3762/bjoc.12.148 |
| 9. | Kaur, N. Synth. Commun. 2019, 49, 1633–1658. doi:10.1080/00397911.2018.1542497 |
| 24. | Tietze, L. F.; Brasche, G.; Gericke, K. M. Domino Reactions in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2006. doi:10.1002/9783527609925 |
| 25. | Nicolaou, K. C.; Chen, J. S. Chem. Soc. Rev. 2009, 38, 2993–3009. doi:10.1039/b903290h |
| 26. | Enders, D.; Grondal, C.; Hüttl, M. R. M. Angew. Chem., Int. Ed. 2007, 46, 1570–1581. doi:10.1002/anie.200603129 |
| 27. | Padwa, A.; Bur, S. K. Tetrahedron 2007, 63, 5341–5378. doi:10.1016/j.tet.2007.03.158 |
| 28. | Nicolaou, K. C.; Edmonds, D. J.; Bulger, P. G. Angew. Chem., Int. Ed. 2006, 45, 7134–7186. doi:10.1002/anie.200601872 |
| 29. | Wasilke, J.-C.; Obrey, S. J.; Baker, R. T.; Bazan, G. C. Chem. Rev. 2005, 105, 1001–1020. doi:10.1021/cr020018n |
| 30. | Hayashi, Y. Chem. Sci. 2016, 7, 866–880. doi:10.1039/c5sc02913a |
| 31. | Sydnes, M. O. Curr. Green Chem. 2014, 1, 216–226. doi:10.2174/2213346101666140221225404 |
| 32. | Atkinson, M. B. J.; Oyola-Reynoso, S.; Luna, R. E.; Bwambok, D. K.; Thuo, M. M. RSC Adv. 2015, 5, 597–607. doi:10.1039/c4ra13506g |
| 75. | Li, J.; Wang, J.; Xu, Z.; Zhu, S. ACS Comb. Sci. 2014, 16, 506–512. doi:10.1021/co500085t |
| 76. | Feng, T.-T.; Gong, Y.; Wei, Q.-D.; Wang, G.-L.; Liu, H.-H.; Tian, M.-Y.; Liu, X.-L.; Chen, Z.-Y.; Zhou, Y. J. Heterocycl. Chem. 2018, 55, 1136–1146. doi:10.1002/jhet.3145 |
| 18. | Cioc, R. C.; Ruijter, E.; Orru, R. V. A. Green Chem. 2014, 16, 2958–2975. doi:10.1039/c4gc00013g |
| 19. | Rotstein, B. H.; Zaretsky, S.; Rai, V.; Yudin, A. K. Chem. Rev. 2014, 114, 8323–8359. doi:10.1021/cr400615v |
| 20. | Estévez, V.; Villacampa, M.; Menéndez, J. C. Chem. Soc. Rev. 2014, 43, 4633–4657. doi:10.1039/c3cs60015g |
| 21. | de Graaff, C.; Ruijter, E.; Orru, R. V. A. Chem. Soc. Rev. 2012, 41, 3969–4009. doi:10.1039/c2cs15361k |
| 22. | Dömling, A.; Wang, W.; Wang, K. Chem. Rev. 2012, 112, 3083–3135. doi:10.1021/cr100233r |
| 23. | Brauch, S.; van Berkel, S. S.; Westermann, B. Chem. Soc. Rev. 2013, 42, 4948–4962. doi:10.1039/c3cs35505e |
| 54. | Zhang, X.; Liu, M.; Zhang, W.; Legris, M.; Zhang, W. J. Fluorine Chem. 2017, 204, 18–22. doi:10.1016/j.jfluchem.2017.10.003 |
| 55. | Zhang, X.; Qiu, W.; Evans, J.; Kaur, M.; Jasinski, J. P.; Zhang, W. Org. Lett. 2019, 21, 2176–2179. doi:10.1021/acs.orglett.9b00487 |
| 56. | Ma, X.; Zhang, X.; Qiu, W.; Zhang, W.; Wan, B.; Evans, J.; Zhang, W. Molecules 2019, 24, 601. doi:10.3390/molecules24030601 |
| 57. | Ma, X.; Zhang, X.; Awad, J. M.; Xie, G.; Qiu, W.; Muriph, R. E.; Zhang, W. Tetrahedron Lett. 2020, 61, 151392. doi:10.1016/j.tetlet.2019.151392 |
| 58. | Ma, X.; Meng, S.; Zhang, X.; Zhang, Q.; Yan, S.; Zhang, Y.; Zhang, W. Beilstein J. Org. Chem. 2020, 16, 1225–1233. doi:10.3762/bjoc.16.106 |
| 59. | Ma, X.; Qiu, W.; Liu, L.; Zhang, X.; Awad, J.; Evans, J.; Zhang, W. Green Synth. Catal. 2021, 2, 74–77. doi:10.1016/j.gresc.2020.11.001 |
| 71. | Zhang, X.; Qiu, W.; Murray, S. A.; Zhan, D.; Evans, J.; Jasinski, J. P.; Wang, X.; Zhang, W. J. Org. Chem. 2021, 86, 17395–17403. doi:10.1021/acs.joc.1c01797 |
| 13. | Zhang, X.; Zhang, W. Curr. Opin. Green Sustainable Chem. 2018, 11, 65–69. doi:10.1016/j.cogsc.2018.04.005 |
| 14. | Newhouse, T.; Baran, P. S.; Hoffmann, R. W. Chem. Soc. Rev. 2009, 38, 3010–3021. doi:10.1039/b821200g |
| 15. | Bhuyan, D.; Sarma, R.; Dommaraju, Y.; Prajapati, D. Green Chem. 2014, 16, 1158–1162. doi:10.1039/c3gc42389a |
| 16. | Hayashi, Y.; Umemiya, S. Angew. Chem., Int. Ed. 2013, 52, 3450–3452. doi:10.1002/anie.201209380 |
| 17. | Zhang, W.; Yi, W.-B. Introduction to PASE Synthesis. In Pot, Atom, and Step Economy (PASE) Synthesis; Zhang, W.; Yi, W.-B., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp 1–4. doi:10.1007/978-3-030-22596-4_1 |
| 70. | Zhang, X.; Liu, M.; Qiu, W.; Evans, J.; Kaur, M.; Jasinski, J. P.; Zhang, W. ACS Sustainable Chem. Eng. 2018, 6, 5574–5579. doi:10.1021/acssuschemeng.8b00555 |
| 71. | Zhang, X.; Qiu, W.; Murray, S. A.; Zhan, D.; Evans, J.; Jasinski, J. P.; Wang, X.; Zhang, W. J. Org. Chem. 2021, 86, 17395–17403. doi:10.1021/acs.joc.1c01797 |
| 10. | Clarke, P. A.; Santos, S.; Martin, W. H. C. Green Chem. 2007, 9, 438–440. doi:10.1039/b700923b |
| 11. | Trost, B. M. Acc. Chem. Res. 2002, 35, 695–705. doi:10.1021/ar010068z |
| 12. | Anastas, P.; Eghbali, N. Chem. Soc. Rev. 2010, 39, 301–312. doi:10.1039/b918763b |
| 72. | Lotfy, G.; Said, M. M.; El Ashry, E. S. H.; El Tamany, E. S. H.; Al-Dhfyan, A.; Abdel Aziz, Y. M.; Barakat, A. Bioorg. Med. Chem. 2017, 25, 1514–1523. doi:10.1016/j.bmc.2017.01.014 |
| 73. | Wu, G.; Ouyang, L.; Liu, J.; Zeng, S.; Huang, W.; Han, B.; Wu, F.; He, G.; Xiang, M. Mol. Diversity 2013, 17, 271–283. doi:10.1007/s11030-013-9432-3 |
| 74. | Ren, W.; Zhao, Q.; Yu, M.; Guo, L.; Chang, H.; Jiang, X.; Luo, Y.; Huang, W.; He, G. Mol. Diversity 2020, 24, 1043–1063. doi:10.1007/s11030-019-10011-2 |
| 38. | Padwa, A.; Pearson, W. H., Eds. Synthetic Applications of 1,3-Dipolar Cycloaddition Chemistry Toward Heterocycles and Natural Products; John Wiley & Sons: New York, NY, USA, 2003; Vol. 59. doi:10.1002/0471221902 |
| 39. | Coldham, I.; Hufton, R. Chem. Rev. 2005, 105, 2765–2810. doi:10.1021/cr040004c |
| 40. | Pandey, G.; Banerjee, P.; Gadre, S. R. Chem. Rev. 2006, 106, 4484–4517. doi:10.1021/cr050011g |
| 41. | Hashimoto, T.; Maruoka, K. Chem. Rev. 2015, 115, 5366–5412. doi:10.1021/cr5007182 |
| 42. | Gothelf, K. V.; Jørgensen, K. A. Chem. Rev. 1998, 98, 863–910. doi:10.1021/cr970324e |
| 43. | Martina, K.; Tagliapietra, S.; Veselov, V. V.; Cravotto, G. Front. Chem. (Lausanne, Switz.) 2019, 7, 95. doi:10.3389/fchem.2019.00095 |
| 44. | Zhang, W. Chem. Lett. 2013, 42, 676–681. doi:10.1246/cl.130504 |
| 45. | Narayan, R.; Potowski, M.; Jia, Z.-J.; Antonchick, A. P.; Waldmann, H. Acc. Chem. Res. 2014, 47, 1296–1310. doi:10.1021/ar400286b |
| 46. | Selva, V.; Selva, E.; Merino, P.; Nájera, C.; Sansano, J. M. Org. Lett. 2018, 20, 3522–3526. doi:10.1021/acs.orglett.8b01292 |
| 47. | Henke, B. R.; Kouklis, A. J.; Heathcock, C. H. J. Org. Chem. 1992, 57, 7056–7066. doi:10.1021/jo00052a015 |
| 48. | Yildirim, O.; Grigalunas, M.; Brieger, L.; Strohmann, C.; Antonchick, A. P.; Waldmann, H. Angew. Chem., Int. Ed. 2021, 60, 20012–20020. doi:10.1002/anie.202108072 |
| 60. | Spanò, V.; Barreca, M.; Cilibrasi, V.; Genovese, M.; Renda, M.; Montalbano, A.; Galietta, L. J. V.; Barraja, P. Molecules 2021, 26, 1275. doi:10.3390/molecules26051275 |
| 61. | Noda, K.; Terasawa, N.; Murata, M. Food Funct. 2016, 7, 2551–2556. doi:10.1039/c5fo01625h |
| 62. | Noda, K.; Yamada, S.; Murata, M. Biosci., Biotechnol., Biochem. 2015, 79, 1350–1355. doi:10.1080/09168451.2015.1018127 |
| 63. | Bharkavi, C.; Vivek Kumar, S.; Ashraf Ali, M.; Osman, H.; Muthusubramanian, S.; Perumal, S. Bioorg. Med. Chem. 2016, 24, 5873–5883. doi:10.1016/j.bmc.2016.09.044 |
| 64. | Arulananda Babu, S.; Padmavathi, R.; Ahmad Aslam, N.; Rajkumar, V. Recent Developments on the Synthesis and Applications of Natural Products-Inspired Spirooxindole Frameworks. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, Netherlands, 2015; Vol. 46, pp 227–339. doi:10.1016/b978-0-444-63462-7.00008-7 |
| 65. | Zhou, L.-M.; Qu, R.-Y.; Yang, G.-F. Expert Opin. Drug Discovery 2020, 15, 603–625. doi:10.1080/17460441.2020.1733526 |
| 66. | Panda, S. S.; Jones, R. A.; Bachawala, P.; Mohapatra, P. P. Mini-Rev. Med. Chem. 2017, 17, 1515–1536. doi:10.2174/1389557516666160624125108 |
| 67. | Wang, Y.; Cobo, A. A.; Franz, A. K. Org. Chem. Front. 2021, 8, 4315–4348. doi:10.1039/d1qo00220a |
| 68. | Ye, N.; Chen, H.; Wold, E. A.; Shi, P.-Y.; Zhou, J. ACS Infect. Dis. 2016, 2, 382–392. doi:10.1021/acsinfecdis.6b00041 |
| 36. | Zhang, X.; Dhawan, G.; Muthengi, A.; Liu, S.; Wang, W.; Legris, M.; Zhang, W. Green Chem. 2017, 19, 3851–3855. doi:10.1039/c7gc01380a |
| 37. | Zhang, X.; Ma, X.; Qiu, W.; Evans, J.; Zhang, W. Green Chem. 2019, 21, 349–354. doi:10.1039/c8gc03180k |
| 69. | Huang, X.; Zhang, W. Chem. Commun. 2021, 57, 10116–10124. doi:10.1039/d1cc03722f |
| 35. | Zhang, X.; Ma, X.; Qiu, W.; Awad, J.; Evans, J.; Zhang, W. Adv. Synth. Catal. 2020, 362, 5513–5517. doi:10.1002/adsc.202000734 |
| 33. | McKeown, M. R.; Shaw, D. L.; Fu, H.; Liu, S.; Xu, X.; Marineau, J. J.; Huang, Y.; Zhang, X.; Buckley, D. L.; Kadam, A.; Zhang, Z.; Blacklow, S. C.; Qi, J.; Zhang, W.; Bradner, J. E. J. Med. Chem. 2014, 57, 9019–9027. doi:10.1021/jm501120z |
| 34. | Huang, H.; Liu, S.; Jean, M.; Simpson, S.; Huang, H.; Merkley, M.; Hayashi, T.; Kong, W.; Rodríguez-Sánchez, I.; Zhang, X.; Yosief, H. O.; Miao, H.; Que, J.; Kobie, J. J.; Bradner, J.; Santoso, N. G.; Zhang, W.; Zhu, J. Front. Microbiol. 2017, 8, 1035. doi:10.3389/fmicb.2017.01035 |
| 49. | Lu, Q.; Song, G.; Jasinski, J. P.; Keeley, A. C.; Zhang, W. Green Chem. 2012, 14, 3010–3012. doi:10.1039/c2gc36066g |
| 50. | Zhang, W.; Lu, Y.; Geib, S. Org. Lett. 2005, 7, 2269–2272. doi:10.1021/ol0507773 |
| 51. | Zhang, X.; Qiu, W.; Ma, X.; Evans, J.; Kaur, M.; Jasinski, J. P.; Zhang, W. J. Org. Chem. 2018, 83, 13536–13542. doi:10.1021/acs.joc.8b02046 |
| 52. | Zhang, X.; Zhi, S.; Wang, W.; Liu, S.; Jasinski, J. P.; Zhang, W. Green Chem. 2016, 18, 2642–2646. doi:10.1039/c6gc00497k |
| 53. | Zhang, X.; Pham, K.; Liu, S.; Legris, M.; Muthengi, A.; Jasinski, J. P.; Zhang, W. Beilstein J. Org. Chem. 2016, 12, 2204–2210. doi:10.3762/bjoc.12.211 |
| 54. | Zhang, X.; Liu, M.; Zhang, W.; Legris, M.; Zhang, W. J. Fluorine Chem. 2017, 204, 18–22. doi:10.1016/j.jfluchem.2017.10.003 |
| 55. | Zhang, X.; Qiu, W.; Evans, J.; Kaur, M.; Jasinski, J. P.; Zhang, W. Org. Lett. 2019, 21, 2176–2179. doi:10.1021/acs.orglett.9b00487 |
| 56. | Ma, X.; Zhang, X.; Qiu, W.; Zhang, W.; Wan, B.; Evans, J.; Zhang, W. Molecules 2019, 24, 601. doi:10.3390/molecules24030601 |
| 57. | Ma, X.; Zhang, X.; Awad, J. M.; Xie, G.; Qiu, W.; Muriph, R. E.; Zhang, W. Tetrahedron Lett. 2020, 61, 151392. doi:10.1016/j.tetlet.2019.151392 |
| 58. | Ma, X.; Meng, S.; Zhang, X.; Zhang, Q.; Yan, S.; Zhang, Y.; Zhang, W. Beilstein J. Org. Chem. 2020, 16, 1225–1233. doi:10.3762/bjoc.16.106 |
| 59. | Ma, X.; Qiu, W.; Liu, L.; Zhang, X.; Awad, J.; Evans, J.; Zhang, W. Green Synth. Catal. 2021, 2, 74–77. doi:10.1016/j.gresc.2020.11.001 |
© 2022 Lu et al.; licensee Beilstein-Institut.
This is an open access article licensed under the terms of the Beilstein-Institut Open Access License Agreement (https://www.beilstein-journals.org/bjoc/terms), which is identical to the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0). The reuse of material under this license requires that the author(s), source and license are credited. Third-party material in this article could be subject to other licenses (typically indicated in the credit line), and in this case, users are required to obtain permission from the license holder to reuse the material.