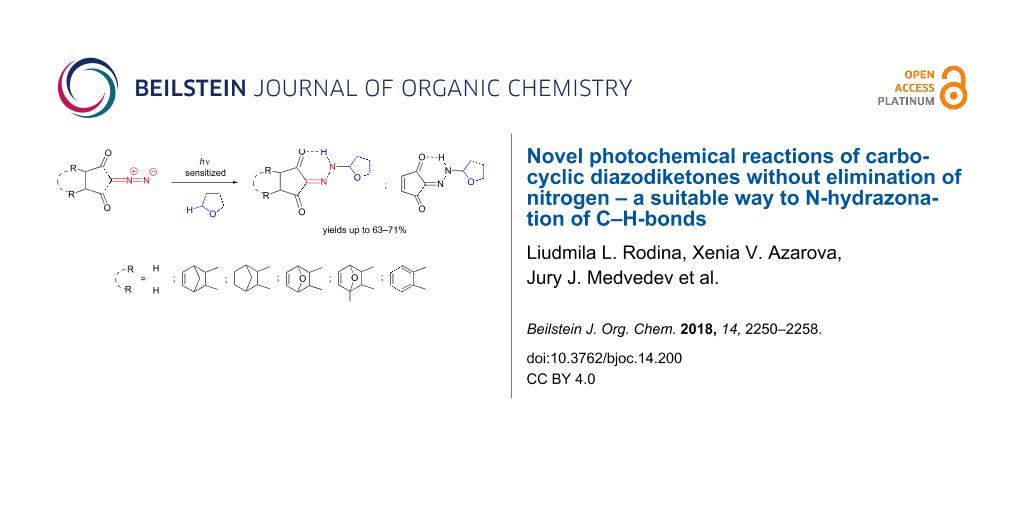
Abstract
The sensitized photoexcitation of 2-diazocyclopentane-1,3-diones in the presence of THF leads to the insertion of the terminal N-atom of the diazo group into the α-С–Н bond of THF, producing the associated N-alkylhydrazones in yields of up to 63–71%. Further irradiation of hydrazones derived from furan-fused tricyclic diazocyclopentanediones culminates in the cycloelimination of furans to yield 2-N-(alkyl)hydrazone of cyclopentene-1,2,3-trione. By contrast, the direct photolysis of carbocyclic diazodiketones gives only Wolff rearrangement products with up to 90–97% yield.
Graphical Abstract
Introduction
Photochemical reactions of diazocarbonyl compounds are well-known transformations in the synthesis of the diversified acyclic, carbo- and heterocyclic structures [1-5]. A direct irradiation of diazo compounds by UV light usually gives rise to nitrogen elimination and generation of carbenes [6-10] or ketenes [11-18] and their ensuing transformations. On the other hand, photochemical reactions of diazocarbonyl compounds without elimination of dinitrogen are essentially limited to their isomerization into α-ketodiazirines [19-26] which is usually observed upon irradiation of diazo compounds with longer wavelength UV light.
Recently a new light-induced reaction of diazo compounds with retention of the diazo group nitrogen atoms in the structure of the reaction products was discovered by our group. The sensitized photoexcitation of heterocyclic diazoketones – diazotetrahydrofuranones resulted in the formation of N-alkyl-substituted hydrazones and other nitrogen-containing compounds [27-30]. This photochemical process is assumed to proceed through the triplet excited states of diazoketones via ‘insertion’ of the terminal nitrogen atom of the diazo group into the С–Н bond of the organic substrates [27,30].
Hence it was principally demonstrated that by carefully varying the irradiation conditions one can direct the photochemical reaction of diazo compounds at the alternative way, which provides the retention of ‘dinitrogen’ in the structure of molecule formed. It is reasonable that a question arises on the scope and limitations of this new light-induced reaction of diazo compounds.
The main objective of our current research was to elucidate the possibility of using carbocyclic diazodiketones in this photochemical process. For this purpose, diazocyclopentanediones 1a–g were tested in the study including unsubstituted diazocyclopentanedione 1a, tricyclic diazodiketones 1b–e with CH2- and O-bridges in their structure, diazoindandione 1f, diazocyclopentenedione 1g, and as a С–Н donor tetrahydrofuran was employed in the study (Figure 1).
Figure 1: The structures of carbocyclic diazodiketones 1a–g and C–H-donating tetrahydrofuran used in the project.
Figure 1: The structures of carbocyclic diazodiketones 1a–g and C–H-donating tetrahydrofuran used in the proj...
To determine the most efficient conditions for this reaction with diazodiketones 1, three sensitizers, acetophenone, benzophenone, and Michler’s ketone, with different levels of triplet state energy were tested. Furthermore, to clearly demonstrate the difference of light-induced reactions of diazo compounds 1 in the triplet and singlet excited states, the direct photolysis of diazodiketones 1a-c without sensitizers was also examined.
Results and Discussion
The starting diazocyclopentanediones (DDK, 1a–f) were synthesized from the corresponding 1,3-dicarbonyl compounds [31-33] employing a diazotransfer reaction [34-39]. Diazocyclopentenedione 1g was prepared by thermal cycloelimination from diazodiketones 1d,e [38,39]. The structures of diazodiketones 1a–g were established using a standard set of spectroscopic methods (1Н and 13С NMR, IR and UV; for details, see Supporting Information File 1, Table S1) and in the case of the tricyclic diazodiketone 1с the structure was also confirmed by means of X-ray analysis (Supporting Information File 1, Figure S1). As it is evident from these data, the tricyclic diazodiketones 1b–e all have endo-configuration of the molecular structure.
Analysis of the UV spectra of diazodiketones 1 shows that they have four absorption bands: two intense ones at 216–222 and 248–250 nm, and two bands with very week intensities at 311–316 nm (215 < ε < 290) and 363–367 nm (30 < ε < 49) (see Supporting Information File 1, Table S1). By analogy with the literature data [40], the absorption bands in the short wavelength region (216–250 nm) are attributed to π–π* electronic transitions, whereas the long wavelength bands are most likely caused by n–π* transitions. Based on the position of the long wavelength bands (363–367 nm) the energy of the singlet excited state 1S1 of diazodiketones 1 can be estimated at about 78–79 kcal/mol [41].
The characteristics of the absorption bands of the sensitizers used in this study (acetophenone, benzophenone and Michler’s ketone) demonstrate that they show appropriate absorption of the actinic light at the irradiation conditions of diazodiketones 1 (Supporting Information File 1, Table S2).
The sensitized photoreactions of diazodiketones 1a–g were carried out in THF solutions with added sensitizers (Table 1). In the case of diazodiketone 1а the only isolated product was hydrazone 2а with 33–49% yields (Table 1, entries 1–3). Increasing the amount of sensitizer (up to 10:1) enhanced the yield of hydrazone 2а by 1/2 while reducing the irradiation time by 50% (Table 1, entries 1 and 3).
Table 1: Sensitized light-induced reactions of diazodiketones 1а–f.a
|
|
|||||
| Entry | DDKb | Sensitizer | Ratio sensitizer/DDK | Time, h | Yields of 2a–d,f and 3 [%] |
|---|---|---|---|---|---|
| 1 | 2:1 | 1.5 | 2a; 33 | ||
| 2 | 1a | Ph2CO | 4:1 | 0.75 | 2a; 42 |
| 3 | 10:1 | 0.75 | 2a; 49 | ||
| 4 | Ph2CO | 1:1 | 1.3 | 2b; 32 | |
| 5c | Ph2CO | 1:1 | 6.5 | 2b; 34 | |
| 6 | 1b | Ph2CO | 4:1 | 2 | 2b; 52 |
| 7 | Ph2CO | 10:1 | 1.5 | 2b; 71 | |
| 8 | MePhCO | 4:1 | 1.5 | 2b; 40 | |
| 9 | M Kd | 2:1 | 3.5 | n.d.e | |
| 10 | 1c | Ph2CO | 10:1 | 0.8 | 2c; 63 |
| 11 | 1d | Ph2CO | 4:1 | 0.5 | 2d; 35 + 3; 47 (total yield 82%) |
| 12 | 1e | Ph2CO | 4:1 | 1.0 | 3; 34 |
| 13 | 1f | Ph2CO | 4:1 | 0.8 | 2f; 52 |
aIrradiation at λ > 210 nm. bDiazodiketone. cIrradiation at λ > 310 nm. dMichler’s ketone. eInseparable complex reaction mixture was obtained with Michler’s ketone.
The irradiation of tricyclic diazodiketone 1b in the presence of 1 equiv of benzophenone in THF solution led to the formation of hydrazone 2b in a yield of 32% (Table 1, entry 4), whose structure was unambiguously established by X-ray analysis (Figure 2).
![[1860-5397-14-200-2]](/bjoc/content/figures/1860-5397-14-200-2.png?scale=3.28&max-width=1024&background=FFFFFF)
Figure 2: Molecular structure of hydrazone 2b as determined by X-ray analysis data (Olex2 plot with 50% probability level of ellipsoids).
Figure 2: Molecular structure of hydrazone 2b as determined by X-ray analysis data (Olex2 plot with 50% proba...
To increase the yields of hydrazones 2, an effort was undertaken to optimize the photochemical reaction conditions by varying the wavelength of irradiation, sensitizer, as well as the ratio of sensitizer/diazodiketone 1b (Table 1, entries 4–9).
As can be seen from the data in Table 1 the most effective sensitizer for the light-induced reaction studied in the series of acetophenone, benzophenone, and Michler’s ketone was found to be benzophenone. The application of acetophenone reduced the yield of hydrazone 2b by about 1/4 as compared to benzophenone (from 52 to 40%; Table 1, entries 6 and 8). With Michler’s ketone, a complex product mixture was obtained, from which it was impossible to isolate pure compounds.
When varying the ratio of sensitizer/diazodiketone 1b, the best result was obtained with a 10-fold excess of benzophenone (Table 1, entry 7; 71% yield). Application of a 4-fold excess of this sensitizer diminished the yield of hydrazone 2b to 52% (Table 1, entry 6). In going from short wavelength (λ > 210 nm) to longer wavelength (λ > 310 nm) irradiation, the yield of hydrazone 2b remained practically unchanged, however the reaction time considerably increased from 1.3 to 6.5 h; Table 1, entries 4 and 5).
The reactions of tricyclic diazodiketone 1c and diazoindandione 1f in the presence of benzophenone as sensitizer proceeded in a similar way to diazo compound 1b under the optimal conditions affording hydrazones 2c,f in yields of 63 and 52%, respectively (Table 1, entries 10 and 13).
An unexpected side reaction was observed during irradiation of furan-fused tricyclic diazodiketones 1d,e (Scheme 1). In the case of diazodiketone 1d, hydrazone 3 (47%) was isolated from reaction mixture in addition to the expected hydrazone 2d (35%; total yield 82%, Table 1, entry 11), whereas the same reaction with diazodiketone 1e afforded hydrazone 3 exclusively (Table 1, entry 12).
Scheme 1: Photochemical cycloelimination of furans from hydrazones 2d,e.
Scheme 1: Photochemical cycloelimination of furans from hydrazones 2d,e.
Additional experiments showed that hydrazone 3 was not formed on sensitized photoexcitation of diazocyclopentenedione 1g in the presence of the C–H-donor THF (Scheme 1). Thus one can conclude that product 3 is generated by a light-induced cycloelimination of furan from the initially generated tricyclic hydrazones 2d,e.
Hence it was established that the sensitized photoexcitation of diazodiketones 1a–d,f in THF solution proceeds through the insertion of the terminal N-atom of the diazo group in the α-С–Н-bond of THF with the formation of the appropriate hydrazones 2a–d,f and the most effective sensitizer for this reaction was found to be benzophenone.
The direct photolysis of diazodiketones 1a–c was carried out by UV light (λ > 210 nm) in THF solution containing a small amount of H2O or MeOH to trap the proposed intermediate α-oxoketene. These light-induced processes with diazodiketones 1a–c resulted in formation of 2-oxocyclobutanecarboxylic acid 4a or methyl esters 5a–c in high isolated yields of up to 90% (Table 2). At the same time no insertion products of the possible intermediate dioxocarbenes into O–H-bonds of the nucleophilic reagents (H2O, MeOH) or C–H-bonds at С5 or С6 atoms in 1 [26,42-45] were detected in the reaction mixtures. Thus, it was experimentally shown that the direct photolysis of diazodiketones 1а–c employing UV light with λ > 210 nm produces only Wolff rearrangement products in high yields.
Table 2: Direct photolysis of diazodiketones 1a–с by UV light with λ > 210 nm.
|
|
||||
| Entry | DDK 1a–c | Solvent/NuH (ratio) | Time, min | Yields of 4,5 [%]a |
|---|---|---|---|---|
| 1 | 1a | THF/Н2О (45:1) | 50 | 4a; 90 |
| 2 | 1a | THF/МеОН (20:1) | 55 | 5a; 86 |
| 3 | 1b | THF/МеОН (200:1) | 55 | 5b; 79 (97) |
| 4 | 1c | THF/МеОН (50:1) | 45 | 5с; 82 |
aYield determined by 1H NMR spectroscopy is given in parenthesis.
The structure of 2-oxocyclobutanecarboxylic acid 4a and the methyl esters 5a–c was established by spectroscopic data (1Н, 13С NMR, and HRMS), and in case of 4a and 5a, also confirmed by comparison with the known literature data [46-48].
It was found that 2-oxocyclobutanecarboxylic acid 4a and ester 5a were easily hydrolyzed in the presence of H2O or during chromatography on silica gel to produce glutaric acid and the corresponding methyl ester (Table 2). By contrast, esters 5b,c proved to be much more stable to hydrolysis on SiO2 and in the presence of nucleophilic reagents. For example, when heating in boiling MeOH for 2 h no hydrolysis of ketoester 5с occurs according to 1H NMR spectra.
The assumed ways for light-induced reactions of diazodiketones 1 can be represented as follows. The direct irradiation (λ > 210 nm) of diazodiketones 1 gives rise to accumulation of one of their upper singlet excited states 1(1)* [13,15,18,20,49-51] which turn into usual Wolff rearrangement products, i.e., highly reactive oxoketenes 6 (Scheme 2, reaction I). The intermediate ketenes 6 react with H2O or MeOH present in the reaction mixture to give 2-oxocarboxylic acid 4a or esters 5a–c.
Scheme 2: Different pathways of diazodiketones 1 light-induced reactions in the singlet (reaction I) and triplet (reaction II) excited states.
Scheme 2: Different pathways of diazodiketones 1 light-induced reactions in the singlet (reaction I) and trip...
On the other hand, the sensitized photoexcitation of diazodiketones 1 gives rise to generation of triplet excited states of diazo compounds 3(1)1 which interact with the H-donor (tetrahydrofuran) producing N-alkyl-substituted hydrazones 2 (Scheme 2, reaction II). Based on the comparison study of the sensitizers’ efficiency in this reaction (Table 2) one can conclude [41,52], that the energy of the triplet excited states 3T1 of diazodiketones 1 are about 68 kcal/mol. This provides a possible explanation why the use of Michler’s ketone was found to be ineffective as a photosensitizer in the process. The energy of the triplet excited state 3T1 of this sensitizer (64 kcal/mol) is somewhat lower than the corresponding energy of the diazodiketones 1 making it inadequate to initiate the C–H insertion reaction. On the other hand, the triplet excited state energy of benzophenone (69 kcal/mol) renders it a suitable sensitizer for this reaction.
In the case of tricyclic diazodiketones 1d,f (with annulated furan and 2-methylfuran-motifs in their structures), a tandem photochemical process of C–H-insertion followed by cycloelimination of furan from the initially generated hydrazones 2d,f apparently occurs, giving rise to the formation of hydrazone 3 as the final reaction product (Scheme 1).
On direct irradiation of tricyclic diazodiketones 1 possessing endo-configuration (Figure 2), one would expect in addition to the Wolff rearrangement also the occurrence of an intramolecular cyclopropanation (with 1b,d,e) [53-55] or an insertion of the assumed intermediate dioxocarbenes in C–H-bonds at С5 or С6-atoms (with 1c) [26,42-45]. However, the performed experiments did not confirm the formation of corresponding reaction products in the photochemical reactions studied. This can be considered as an argument in favor of the concerted Wolff rearrangement [11-18] in the case of these diazodiketones.
Conclusion
The sensitized photoexcitation of carbocyclic diazodiketones proceeds without elimination of dinitrogen. Instead, the reaction leads to the insertion of the terminal N-atom of the diazo group into the C–H bond of THF giving rise to the appropriate N-alkyl-substituted hydrazones in yields of up to 63–71%. The most powerful sensitizer for this light-induced process in the series of acetophenone, benzophenone and Michler’s ketone was found to be benzophenone. Hence, the diazodicarbonyl compounds can be successfully used for a photochemical N-functionalization of C–H-bonds of organic compounds. In contrast to this, the direct photolysis of carbocyclic diazoketones produces exclusively Wolff rearrangement products in yields of 90–97%.
Experimental
General methods
All reactions were carried out under argon atmosphere in solvents that were purified and dried before use by common methods. Monitoring of the reaction course was accomplished by thin layer chromatography (TLC) on silica gel SIL G/UV254 plates (Marcherey, Nagel & Co.). Chromatography was performed using Merck silica gel 60, 230–400 mesh. 1H and 13C NMR spectra were recorded using a Bruker-400 Avance NMR spectrometer. All HRMS spectra were recorded on a «MaXis» (Bruker Daltonik GmbH), the HPLC system (UHPLC) with the combined high-resolution quadrupole-time-of-flight mass spectrometer with electrospray ionization (ESI-QTOF). Chemical shifts are reported in ppm, and coupling constants are given in hertz (Hz). All signals in NMR spectra were normalized relative to signals of CНCl3 (δ = 7.26 ppm in 1H NMR) and CDCl3 (δ = 77.0 in 13C NMR spectra).
For single crystal X-ray diffraction experiments of 1c (CCDC 1584937) and 2b (CCDC 1584938) an Agilent Technologies «Xcalibur» diffractometer with monochromated MoKα radiation was used. All samples were measured at 100 K. The unit cell parameters were refined by least square techniques in CrysAlisPro (Agilent Technologies, 2012) program complex. Empirical absorption correction was applied using spherical harmonics, implemented in SCALE3 ABSPACK scaling algorithm. The structures were solved by the Superflip [56-58] and ShelXS [59] structure solution programs using Charge Flipping and Direct Methods, respectively, then refined by means of the ShelXL [60] program, incorporated in the Olex2 program package [61].
Sensitized reactions of diazodiketones 1a–g
General procedure. The solution of diazodiketone 1a–g (1 equiv) and benzophenone (up to 10 equiv) in THF (10–30 ml) was irradiated in a quartz reactor (λ > 210 nm) under an argon atmosphere for up to 6.5 h (control of the reacted diazodiketone 1 by TLC). Thereupon the reaction mixture was dried over magnesium sulfate, the solvent completely distilled off in vacuum and the residue separated by column chromatography (SiO2, eluent: petroleum ether → petroleum ether/acetone 8:1) to give the hydrazones 2 and 3.
A. Sensitized photoexcitation of 2-diazocyclopentane-1,3-dione (1a). The reaction was performed according to the general procedure with 496 mg (4 mmol, 1 equiv) of diazodiketone 1a and 2.912 g (16 mmol, 4 equiv) of benzophenone in 30 ml of THF with an irradiation time of 45 min. After the workup procedure hydrazone 2a and unreacted diazoketone 1a were isolated.
2-(2-(Tetrahydrofuran-2-yl)hydrazono)cyclopentane-1,3-dione (2a). Yield 168 mg (42%, calculated on the reacted diazodiketone 1a), bright-yellow oil; 1H NMR (400 MHz, CDCl3, δ) 12.93 (s, 1H), 5.61–5.53 (m, 1H), 4.08–3.99 (m, 1H), 3.95–3.88 (m, 1H), 2.69–2.60 (m, 4H), 2.33–2.19 (m, 2H), 2.08–1.96 (m, 2H) ppm; 13C NMR (101 MHz, CDCl3, δ) 200.3, 198.7, 130.9, 92.5, 69.2, 33.3, 31.8, 31.2, 24.4 ppm; HRMS–ESI (m/z): [M + Na]+ calcd for C9H12N2O3, 219.0746; found, 219.0746.
B. Sensitized photoexcitation of 2-diazo-3a,4,7,7a-tetrahydro-1H-4,7-methanoindene-1,3(2H)-dione (1b). The reaction was performed according to the general procedure with 380 mg (2 mmol, 1 equiv) of diazodiketone 1b and 3.64 g (20 mmol, 10 equiv) of benzophenone in 20 ml of THF with an irradiation time of 90 min. After the standard workup procedure hydrazone 2b was isolated.
2-(2-(Tetrahydrofuran-2-yl)hydrazono)-3a,4,7,7a-tetrahydro-1H-4,7-methanoindene-1,3(2H)-dione (2b). Yield 374 mg (71%); bright-yellow solid; mp 127–129 °С; 1H NMR (400 MHz, CDCl3, δ) 12.78 (s, 1H), 6.11–5.98 (m, 1H), 6.00–5.87 (m, 1H), 5.54–5.41 (m, 1H), 4.05–3.94 (m, 1H), 3.92–3.82 (m, 1H), 3.38 (d, J = 20.3 Hz, 2H), 3.20–3.07 (m, 2H), 2.32–2.10 (m, 2H), 2.03–1.92 (m, 2H), 1.63 (dt, J = 8.6, 1.5 Hz, 1H), 1.48 (d, J = 8.6 Hz, 1H) ppm; 13C NMR (101 MHz, CDCl3, δ) 201.3, 199.51, 199.49, 134.9, 134.8, 133.7, 133.63, 133.55, 133.53, 92.44, 92.39, 69.0, 68.9, 52.39, 52.37, 49.8, 48.2, 48.14, 46.14, 46.11, 46.04, 46.00, 31.2, 31.1, 24.4, 24.3 ppm; 1H NMR (400 MHz, acetone-d6, δ) 6.36–5.71 (m, 2H), 5.48 (dd, J = 6.6, 3.6 Hz, 1H), 4.14–3.75 (m, 2H), 3.46–3.01 (m, 5H), 2.46–2.12 (m, 2H), 2.10–1.73 (m, 2H), 1.62–1.52 (m, 2H) ppm; 13C NMR (101 MHz, acetone-d6, δ) 199.8, 134.1, 133.7, 92.1, 68.5, 52.0, 48.8, 45.9, 30.3, 24.4 ppm; HRMS–ESI (m/z): [M + Na]+ calcd for C14H16N2O3, 283.1059; found, 283.1065.
C. Sensitized photoexcitation of 2-diazohexahydro-1H-4,7-methanoindene-1,3(2H)-dione (1c). The reaction was performed according to the general procedure with 376 mg (2 mmol, 1 equiv) of diazodiketone 1c and 3.64 g (20 mmol, 10 equiv) of benzophenone in 20 ml of THF with an irradiation time of 1 h. After the standard workup procedure hydrazone 2c was isolated.
2-(2-(Tetrahydrofuran-2-yl)hydrazono)hexahydro-1H-4,7-methanoindene-1,3(2H)-dione (2c). Yield 328 mg (63%); bright-yellow oil; 1H NMR (400 MHz, CDCl3, δ) 13.10 (broad, 1H, NH), 5.66–5.51 (m, 1H), 4.11–4.01 (m, 1H), 3.98–3.89 (m, 1H), 3.02–2.90 (m, 2H), 2.75–2.86 (m, 2H), 2.37–2.19 (m, 2H), 2.11–1.99 (m, 2H), 1.65–1.54 (m, 2H), 1.53–1.44 (m, 2H), 1.39–1.31 (m, 1H), 1.27–1.15 (m, 1H) ppm; 13C NMR (101 MHz, CDCl3, δ) 202.96, 202.95, 202.0, 201.0, 133.2, 133.0, 92.7, 92.6, 69.2, 69.1, 52.68, 52.65, 50.99, 50.98, 42.49, 42.47, 40.62, 40.59, 40.55, 40.52, 31.34, 31.26, 24.7, 24.59, 24.56, 24.5, 24.4 ppm; HRMS–ESI (m/z): [M + Na]+ calcd for C14H18N2O3, 285.1215; found, 285.1210.
D. Sensitized photoexcitation of 2-diazo-3a,4,7,7a-tetrahydro-1H-4,7-epoxyindene-1,3(2H)-dione (1d). The reaction was performed according to the general procedure with 380 mg (2 mmol, 1 equiv) of diazodiketone 1d and 1.46 g (8 mmol, 4 equiv) of benzophenone in 20 ml of THF with an irradiation time of 0.5 h. After the standard workup procedure hydrazones 2d, 3 and unreacted diazoketone 1d were isolated.
2-(2-(Tetrahydrofuran-2-yl)hydrazono)-3a,4,7,7a-tetrahydro-1H-4,7-epoxyindene-1,3(2H)-dione (2d). Yield 134 mg (35% based on reacted diazodiketone 1d); yellow oil; 1H NMR (400 MHz, CDCl3, δ) 12.83 (broad, 1H), 6.67–6.31 (m, 2H), 5.60–5.49 (m, 1H), 5.29 (s, 1H), 5.23 (s, 1H), 4.10–3.95 (m, 1H), 3.98–3.85 (m, 1H), 2.76–2.60 (m, 2H), 2.34–2.20 (m, 2H), 2.11–1.89 (m, 2H) ppm; 13C NMR (101 MHz, CDCl3, δ) 198.73, 198.70, 197.37, 197.36, 137.4, 136.8 133.50, 133.46, 92.7, 92.4, 82.5, 81.8, 69.25, 69.22, 51.01, 50.99, 49.51, 49.48, 31.3, 31.2, 24.3 ppm; HRMS–ESI (m/z): [M + H]+ calcd for C13H14N2O4, 263.1032; found, 263.1029.
2-(2-(Tetrahydrofuran-2-yl)hydrazono)cyclopent-4-ene-1,3-dione (3). Yield 135 mg (47%, on the reacted diazodiketone 1d); yellow oil; 1H NMR (400 MHz, CDCl3, δ) 11.07 (s, 1H), 7.09 (d, J = 6.7 Hz, 1H), 7.05 (d, J = 6.7 Hz, 1H), 5.62–5.45 (m, 1H), 4.13–3.79 (m, 1H), 2.40–1.75 (m, 1H) ppm; 13C NMR (101 MHz, CDCl3, δ) 191.9, 189.9, 145.8, 143.5, 137.0, 91.5, 68.5, 30.7, 24.7 ppm; HRMS–ESI (m/z): [M + H]+ calcd for C9H10N2O3, 195.0770; found, 195.0774.
E. Sensitized photoexcitation of 2-diazo-4-methyl-3a,4,7,7a-tetrahydro-1H-4,7-epoxyindene-1,3(2H)-dione (2e). The reaction was performed according to the general procedure with 204 mg (2 mmol, 1 equiv) of diazoketone 1e and 728 mg (8 mmol, 4 equiv) of benzophenone in 10 ml of THF with an irradiation time of 1 h. After the workup procedure hydrazone 3 [66 mg (34%)] was isolated exclusively.
F. Sensitized photoexcitation of 2-diazo-1H-indene-1,3(2H)-dione (2f). The reaction was performed according to the general procedure with 344 mg (2 mmol, 1 equiv) of diazodiketone 1f and 1.46 g (8 mmol, 4 equiv) of benzophenone in 20 ml of THF with and irradiation time of 0.8 h. After standard workup procedure hydrazone 2f and unreacted diazodiketone 1f were isolated.
2-(2-(Tetrahydrofuran-2-yl)hydrazono)-1H-indene-1,3(2H)-dione (2f). Yield 70 mg (52%, calculated on the reacted diazodiketone 1f); yellow oil; 1H NMR (400 MHz, CDCl3, δ) 11.79 (broad, 1H), 7.96–7.85 (m, 1H), 7.84–7.77 (m, 1H), 7.77–7.65 (m, 2H), 5.71–5.45 (m, 1H), 4.14–3.95 (m, 1H), 3.96–3.67 (m, 1H), 2.44–2.12 (m, 2H), 2.15–1.70 (m, 2H) ppm; 13C NMR (101 MHz, CDCl3, δ) 188.3, 186.4, 140.2, 138.6, 135.1, 134.8, 130.1, 123.1, 122.7, 91.9, 68.6, 31.0, 24.7 ppm; HRMS–ESI (m/z): [M + H]+ calcd for C13H12N2O3, 245.0926; found, 245.0926.
Direct photolysis of diazodiketones 1a–c
Photolysis of diazodiketone 1a (with H2O). A solution of sublimated diazodiketone 1а (1.24 g, 0.01 mol) in 90 ml of pure anhydrous THF and distilled H2O (1.8 ml, 0.1 mol) was irradiated in a quartz reactor (λ > 210 nm) for 50 min until complete disappearance of diazodiketone 1a (control by TLC). Then the reaction mixture was dried with magnesium sulfate and the solvent distilled off in vacuum. The obtained residue was dissolved in 25 ml of anhydrous Et2O, cooled to −75 to −78 °C. The precipitated resinous matter was separated by decantation and the solvent was completely removed in vacuum. The resulting oily residue was taken up in 2–3 ml of anhydrous Et2O and separated by column chromatography (5 g of anhydrous neutral SiO2; eluent: anhydrous diethyl ether) to give acid 4a as a crystalline solid after usual workup and careful removal of solvent in vacuum from the proper fractions (0.5–0.1 mm Hg).
Cyclobutane-2-one carboxylic acid (4а) [46,47]. Yield 1.03 g (90%); mp 43–44.5 °C (distilled); bp 93.5–94 °C (0.2–0.1 mm Hg). It is noteworthy that acid 4а is a very hygroscopic compound which is easily hydrolyzed to produce glutaric acid.
Photolysis of diazodiketone 1a (with MeOH). A solution of sublimated diazodiketone 1а (2.48 g, 0.02 mol) in 80 ml of anhydrous THF and pure CH3OH (4 ml, 0.10 mol) was irradiated in a quartz reactor (λ > 210 nm) for 55 min and worked up similarly as described in the previous experiment. The resulting reaction mixture after removal of THF and methanol in vacuum was distilled to give methyl ester 5а.
Methyl cyclobutane-2-one carboxylate (5а) [48]. Yield 2.20 g (86%); bp 43.5–45 °С (1 mm Hg); nD20 1.5111. During column chromatography with SiO2 of I or II activity, methyl ester 5а considerably hydrolyzes to give Me ester of glutaric acid.
Photolysis of diazodiketone 1b (with MeOH). A solution of diazodiketone 1b (141 mg, 0.75 mmol) in 10 ml of THF/CH3OH 200:1 was irradiated for 55 min in a quartz reactor (λ > 210 nm). Then the reaction mixture was dried with magnesium sulfate and the solvent and excess of CH3OH were completely removed in vacuum. The residue containing ester 5b (140 mg; 97% by 1H NMR) was separated by preparative TLC to furnish Me ester 5b.
Methyl 4-oxotricyclo[4.2.1.02,5]non-7-ene-3-carboxylate (5b). Yield 114 mg (79%; 97% by 1H NMR); colorless oil; 1Н NMR (400 MHz, CDCl3, δ) 6.23–6.19 (m, 2H), 4.02–3.80 (m, 1H), 3.71 (s, 3H), 3.32–2.99 (m, 4H), 1.82–1.75 (m, 1H), 1.53–1.47 (m, 1H) ppm; 13C NMR (101 MHz, CDCl3, δ) 201.4, 167.8, 136.2, 133.4, 67.1, 63.9, 54.3, 52.4, 46.6, 43.9, 30.8 ppm; HRMS–ESI (m/z): [M + Na]+ calcd for C11H12O3, 215.0684; found, 215.0681.
Photolysis of diazodiketone 1c (with MeOH). A solution of diazodiketone 1c (190 mg, 1 mmol) in 10 ml of THF/MeOH 50:1 was irradiated in a quartz reactor (λ > 210 nm) for 45 min. Then the reaction mixture was dried with magnesium sulfate and the solvent and excess of CH3OH were completely distilled off in vacuum. The residue, containing ester 5c (189 mg; 98% by 1H NMR) was separated by preparative TLC to give pure methyl ester 5c.
Methyl 4-oxotricyclo[4.2.1.02,5]nonane-3-carboxylate (5c). Yield 158 mg (82%); colorless oil; 1Н NMR (400 MHz, CDCl3, δ) 3.92–3.87 (m, 1H), 3.75–3.66 (m, 4H), 3.06–2.99 (m, 1H), 2.63–2.57 (m, 1H), 2.59–2.50 (m, 1H), 1.65–1.53 (m, 5H), 1.41–1.33 (m, 1H) ppm; 13C NMR (101 MHz, CDCl3, δ) 203.7, 168.2, 69.6, 61.6, 52.5, 43.7, 40.8, 38.9, 34.9, 25.7, 24.6 ppm; HRMS–ESI (m/z): [M + Na]+ calcd for C11H14O3, 217.0841; found, 217.0835.
Supporting Information
| Supporting Information File 1: NMR spectra of all new compounds and data of X-ray analysis for compounds 1c (CCDC 1584937) and 2b (CCDC 1584938). | ||
| Format: PDF | Size: 2.7 MB | Download |
Acknowledgements
The authors express their gratitude to the SPbSU resource centers: "Center for Magnetic Resonance", "Chemical Analysis and Materials Research Centre" and "Resource Education Center" and "Research Centre for X-Ray Diffraction Methods of Investigation". J.J.M. gratefully acknowledges generous financial support from "Russian Foundation for Basic Research" (RFBR, # 16-33-00059 mol_a).
References
-
Ford, A.; Miel, H.; Ring, A.; Slattery, C. N.; Maguire, A. R.; McKervey, M. A. Chem. Rev. 2015, 115, 9981–10080. doi:10.1021/acs.chemrev.5b00121
Return to citation in text: [1] -
Galkina, O. S.; Rodina, L. L. Russ. Chem. Rev. 2016, 85, 537–555. doi:10.1070/RCR4519
Return to citation in text: [1] -
Candeias, N. R.; Afonso, C. A. M. Curr. Org. Chem. 2009, 13, 763–787. doi:10.2174/138527209788167231
Return to citation in text: [1] -
Zhang, Z.; Wang, J. Tetrahedron 2008, 64, 6577–6605. doi:10.1016/j.tet.2008.04.074
Return to citation in text: [1] -
Kirmse, W. Eur. J. Org. Chem. 2002, 2193–2256. doi:10.1002/1099-0690(200207)2002:14<2193::aid-ejoc2193>3.0.co;2-d
Return to citation in text: [1] -
Bertrand, G., Ed. Carbene Chemistry; Marcel Dekker: New York, NY, U.S.A., 2002; p 320. doi:10.1201/9780203910924
Return to citation in text: [1] -
Platz, M. S., Ed. Kinetics and Spectroscopy of Carbenes and Biradicals; Plenum Press: New York, NY, U.S.A., 1990; p 372. doi:10.1007/978-1-4899-3707-0
Return to citation in text: [1] -
Ross, M. J.; Moss, R. A. Carbene Chemistry; John Wiley & Sons Inc.: New York, NY, U.S.A., 1975.
Return to citation in text: [1] -
Moss, R. A.; Jones, M., Jr. Carbenes; John Wiley & Sons: New York, NY, U.S.A., 1973.
Return to citation in text: [1] -
Kirmse, W. Carbenes, 2nd ed.; Academic Press: New York, NY, U.S.A., 1971.
Return to citation in text: [1] -
Tidwell, T. T. Angew. Chem., Int. Ed. 2005, 44, 5778–5785. doi:10.1002/anie.200500098
Return to citation in text: [1] [2] -
Garbarino, S.; Banfi, L.; Riva, R.; Basso, A. J. Org. Chem. 2014, 79, 3615–3622. doi:10.1021/jo500535f
Return to citation in text: [1] [2] -
Burdzinski, G.; Kubicki, J.; Sliwa, M.; Réhault, J.; Zhang, Y.; Vyas, S.; Luk, H. L.; Hadad, C. M.; Platz, M. S. J. Org. Chem. 2013, 78, 2026–2032. doi:10.1021/jo302023a
Return to citation in text: [1] [2] [3] -
Willumstad, T. P.; Haze, O.; Mak, X. Y.; Lam, T. Y.; Wang, Y.-P.; Danheiser, R. L. J. Org. Chem. 2013, 78, 11450–11469. doi:10.1021/jo402010b
Return to citation in text: [1] [2] -
Burdzinski, G.; Zhang, Y.; Wang, J.; Platz, M. S. J. Phys. Chem. A 2010, 114, 13065–13068. doi:10.1021/jp108690n
Return to citation in text: [1] [2] [3] -
Wolpert, D.; Schade, M.; Brixner, T. J. Chem. Phys. 2008, 129, 094504. doi:10.1063/1.2971037
Return to citation in text: [1] [2] -
Nikolaev, V. A.; Shevchenko, V. V.; Platz, M. S.; Khimich, N. N. Russ. J. Org. Chem. 2006, 42, 815–827. doi:10.1134/S1070428006060029
Return to citation in text: [1] [2] -
Popik, V. V. Can. J. Chem. 2005, 83, 1382–1390. doi:10.1139/v05-152
Return to citation in text: [1] [2] [3] -
Korneev, S. M. Eur. J. Org. Chem. 2011, 6153–6175. doi:10.1002/ejoc.201100224
Return to citation in text: [1] -
Shevchenko, V. V.; Khimich, N. N.; Platz, M. S.; Nikolaev, V. A. Tetrahedron Lett. 2005, 46, 435–438. doi:10.1016/j.tetlet.2004.11.102
Return to citation in text: [1] [2] -
Bogdanova, A.; Popik, V. V. J. Am. Chem. Soc. 2004, 126, 11293–11302. doi:10.1021/ja047824r
Return to citation in text: [1] -
Meier, H. Diazirine-Diazoalkane Interconversions. In Chemistry of diazirines; Liu, M. T. H., Ed.; CRC Press: Boca Raton, FL, U.S.A., 1987; pp 1–19.
Return to citation in text: [1] -
Nikolaev, V. A.; Khimich, N. N.; Korobitsyna, I. K. Chem. Heterocycl. Compd. 1985, 21, 264–268. doi:10.1007/bf00506661
Khim. Geterotsikl. Soedin. 1985, 321–325.
Return to citation in text: [1] -
Livinghouse, T.; Stevens, R. V. J. Am. Chem. Soc. 1978, 100, 6479–6482. doi:10.1021/ja00488a036
Return to citation in text: [1] -
Voigt, E.; Meier, H. Chem. Ber. 1975, 108, 3326–3335. doi:10.1002/cber.19751081025
Return to citation in text: [1] -
Lowe, G.; Parker, J. J. Chem. Soc. D 1971, 577–578. doi:10.1039/c29710000577
Return to citation in text: [1] [2] [3] -
Rodina, L. L.; Baranovskii, V. I.; Galkina, O. S.; Nikolaev, V. A.; Tonogina, N. L.; Povolotskiy, A. V. J. Org. Chem. 2017, 82, 11399–11405. doi:10.1021/acs.joc.7b01848
Return to citation in text: [1] [2] -
Rodina, L. L.; Galkina, O. S.; Maas, G.; Platz, M. S.; Nikolaev, V. A. Asian J. Org. Chem. 2016, 5, 691–698. doi:10.1002/ajoc.201600050
Return to citation in text: [1] -
Nikolaev, V. A.; Galkina, O. S.; Sieler, J.; Rodina, L. L. Tetrahedron Lett. 2010, 51, 2713–2716. doi:10.1016/j.tetlet.2010.03.050
Return to citation in text: [1] -
Galkina, O. S. Ph.D. Thesis, Saint-Petersburg State University, 2015.
Return to citation in text: [1] [2] -
Presset, M.; Mailhol, D.; Coquerel, Y.; Rodriguez, J. Synthesis 2011, 2549–2552. doi:10.1055/s-0030-1260107
Return to citation in text: [1] -
Wu, C.; Mooring, A. M.; Yardley, J. T. UV photoresist containing polycyclic cyclopentanediazodione derivative. Eur. Pat. Appl. EP405957A1, Jan 2, 1991.
Return to citation in text: [1] -
Korobitsyna, I. K.; Rodina, L. L.; Nikolaev, V. A. The method of producing bicyclic diazocyclopentanediones. Patent of the USSR 1004359, March 15, 1983.
Return to citation in text: [1] -
Regitz, M.; Maas, G. Diazo Compounds. Properties and Synthesis; Academic Press: New York, NY, U.S.A., 1986. doi:10.1016/b978-0-12-585840-3.x5001-6
Return to citation in text: [1] -
Doyle, M. P.; McKervey, M. A.; Ye, T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds; John Wiley & Sons Inc.: New York, NY, U.S.A., 1998.
Return to citation in text: [1] -
Baum, J. S.; Shook, D. A.; Davies, H. M. L.; Smith, H. D. Synth. Commun. 1987, 17, 1709–1716. doi:10.1080/00397918708063988
Return to citation in text: [1] -
Popic, V. V.; Korneev, S. M.; Nikolaev, V. A.; Korobitsyna, I. K. Synthesis 1991, 195–198. doi:10.1055/s-1991-26416
Return to citation in text: [1] -
Nikolaev, V. A.; Korobitsyna, I. K. Procedure for preparation of 2-diazo-1,3-dioxoceclopentene. Patent of the USSR 1172224, May 13, 1985.
Return to citation in text: [1] [2] -
Oda, M.; Kasai, M.; Kitahara, Y. Chem. Lett. 1977, 6, 307–310. doi:10.1246/cl.1977.307
Return to citation in text: [1] [2] -
Csizmadia, I. G.; Houlden, S. A.; Meresz, O.; Yates, P. Tetrahedron 1969, 25, 2121–2138. doi:10.1016/s0040-4020(01)82763-0
Return to citation in text: [1] -
Turro, N. J.; Ramamurthy, V.; Scaiano, J. C. Principles of molecular photochemistry: an introduction; University Science Books: Sausalito, CA, U.S.A., 2009.
Return to citation in text: [1] [2] -
Candeias, N. R.; Gois, P. M. P.; Veiros, L. F.; Afonso, C. A. M. J. Org. Chem. 2008, 73, 5926–5932. doi:10.1021/jo800980c
Return to citation in text: [1] [2] -
Buchner, M. R.; Wahl, B.; Ruhland, K. J. Photochem. Photobiol., A 2013, 252, 183–193. doi:10.1016/j.jphotochem.2012.12.005
Return to citation in text: [1] [2] -
Rau, H.; Bokel, M. J. Photochem. Photobiol., A 1990, 53, 311–322. doi:10.1016/1010-6030(90)87135-x
Return to citation in text: [1] [2] -
Corey, E. J.; Felix, A. M. J. Am. Chem. Soc. 1965, 87, 2518–2519. doi:10.1021/ja01089a055
Return to citation in text: [1] [2] -
Chang, J. A.; Chiang, Y.; Keeffe, J. R.; Kresge, A. J.; Nikolaev, V. A.; Popik, V. V. J. Org. Chem. 2006, 71, 4460–4467. doi:10.1021/jo060253w
Return to citation in text: [1] [2] -
Amice, P.; Conia, J. M. Tetrahedron Lett. 1974, 15, 479–482. doi:10.1016/s0040-4039(01)82249-8
Return to citation in text: [1] [2] -
Kniep, C. S.; Padias, A. B.; Hall, H. K., Jr. Tetrahedron 2000, 56, 4279–4288. doi:10.1016/s0040-4020(00)00354-9
Return to citation in text: [1] [2] -
Bogdanova, A.; Popik, V. V. J. Am. Chem. Soc. 2003, 125, 1456–1457. doi:10.1021/ja029528p
Return to citation in text: [1] -
Bogdanova, A.; Popik, V. V. J. Am. Chem. Soc. 2003, 125, 14153–14162. doi:10.1021/ja037637d
Return to citation in text: [1] -
Nikolaev, V. A.; Frenkh, Y.; Korobitsyna, I. K. J. Org. Chem. USSR 1978, 14, 1338–1346.
Return to citation in text: [1] -
Klan, P.; Wirz, J. Photochemistry of organic compounds: from concepts to practice; Wiley: West Sussex, United Kingdom, 2009. doi:10.1002/9781444300017
Return to citation in text: [1] -
Maas, G.; Krebs, F.; Werle, T.; Gettwert, V.; Striegler, R. Eur. J. Org. Chem. 1999, 1939–1946. doi:10.1002/(sici)1099-0690(199908)1999:8<1939::aid-ejoc1939>3.0.co;2-a
Return to citation in text: [1] -
Hudlicky, T.; Ranu, B. C.; Naqvi, S. M.; Srnak, A. J. Org. Chem. 1985, 50, 123–127. doi:10.1021/jo00201a026
Return to citation in text: [1] -
Hudlicky, T.; Sheth, J. P.; Gee, V.; Barnvos, D. Tetrahedron Lett. 1979, 20, 4889–4892. doi:10.1016/s0040-4039(01)86741-1
Return to citation in text: [1] -
Palatinus, L.; Chapuis, G. J. Appl. Crystallogr. 2007, 40, 786–790. doi:10.1107/S0021889807029238
Return to citation in text: [1] -
Palatinus, L.; van der Lee, A. J. Appl. Crystallogr. 2008, 41, 975–984. doi:10.1107/s0021889808028185
Return to citation in text: [1] -
Palatinus, L.; Prathapa, S. J.; van Smaalen, S. J. Appl. Crystallogr. 2012, 45, 575–580. doi:10.1107/S0021889812016068
Return to citation in text: [1] -
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, 64, 112–122. doi:10.1107/s0108767307043930
Return to citation in text: [1] -
Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. doi:10.1107/s2053273314026370
Return to citation in text: [1] -
Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339–341. doi:10.1107/S0021889808042726
Return to citation in text: [1]
| 56. | Palatinus, L.; Chapuis, G. J. Appl. Crystallogr. 2007, 40, 786–790. doi:10.1107/S0021889807029238 |
| 57. | Palatinus, L.; van der Lee, A. J. Appl. Crystallogr. 2008, 41, 975–984. doi:10.1107/s0021889808028185 |
| 58. | Palatinus, L.; Prathapa, S. J.; van Smaalen, S. J. Appl. Crystallogr. 2012, 45, 575–580. doi:10.1107/S0021889812016068 |
| 26. | Lowe, G.; Parker, J. J. Chem. Soc. D 1971, 577–578. doi:10.1039/c29710000577 |
| 42. | Candeias, N. R.; Gois, P. M. P.; Veiros, L. F.; Afonso, C. A. M. J. Org. Chem. 2008, 73, 5926–5932. doi:10.1021/jo800980c |
| 43. | Buchner, M. R.; Wahl, B.; Ruhland, K. J. Photochem. Photobiol., A 2013, 252, 183–193. doi:10.1016/j.jphotochem.2012.12.005 |
| 44. | Rau, H.; Bokel, M. J. Photochem. Photobiol., A 1990, 53, 311–322. doi:10.1016/1010-6030(90)87135-x |
| 45. | Corey, E. J.; Felix, A. M. J. Am. Chem. Soc. 1965, 87, 2518–2519. doi:10.1021/ja01089a055 |
| 11. | Tidwell, T. T. Angew. Chem., Int. Ed. 2005, 44, 5778–5785. doi:10.1002/anie.200500098 |
| 12. | Garbarino, S.; Banfi, L.; Riva, R.; Basso, A. J. Org. Chem. 2014, 79, 3615–3622. doi:10.1021/jo500535f |
| 13. | Burdzinski, G.; Kubicki, J.; Sliwa, M.; Réhault, J.; Zhang, Y.; Vyas, S.; Luk, H. L.; Hadad, C. M.; Platz, M. S. J. Org. Chem. 2013, 78, 2026–2032. doi:10.1021/jo302023a |
| 14. | Willumstad, T. P.; Haze, O.; Mak, X. Y.; Lam, T. Y.; Wang, Y.-P.; Danheiser, R. L. J. Org. Chem. 2013, 78, 11450–11469. doi:10.1021/jo402010b |
| 15. | Burdzinski, G.; Zhang, Y.; Wang, J.; Platz, M. S. J. Phys. Chem. A 2010, 114, 13065–13068. doi:10.1021/jp108690n |
| 16. | Wolpert, D.; Schade, M.; Brixner, T. J. Chem. Phys. 2008, 129, 094504. doi:10.1063/1.2971037 |
| 17. | Nikolaev, V. A.; Shevchenko, V. V.; Platz, M. S.; Khimich, N. N. Russ. J. Org. Chem. 2006, 42, 815–827. doi:10.1134/S1070428006060029 |
| 18. | Popik, V. V. Can. J. Chem. 2005, 83, 1382–1390. doi:10.1139/v05-152 |
| 1. | Ford, A.; Miel, H.; Ring, A.; Slattery, C. N.; Maguire, A. R.; McKervey, M. A. Chem. Rev. 2015, 115, 9981–10080. doi:10.1021/acs.chemrev.5b00121 |
| 2. | Galkina, O. S.; Rodina, L. L. Russ. Chem. Rev. 2016, 85, 537–555. doi:10.1070/RCR4519 |
| 3. | Candeias, N. R.; Afonso, C. A. M. Curr. Org. Chem. 2009, 13, 763–787. doi:10.2174/138527209788167231 |
| 4. | Zhang, Z.; Wang, J. Tetrahedron 2008, 64, 6577–6605. doi:10.1016/j.tet.2008.04.074 |
| 5. | Kirmse, W. Eur. J. Org. Chem. 2002, 2193–2256. doi:10.1002/1099-0690(200207)2002:14<2193::aid-ejoc2193>3.0.co;2-d |
| 27. | Rodina, L. L.; Baranovskii, V. I.; Galkina, O. S.; Nikolaev, V. A.; Tonogina, N. L.; Povolotskiy, A. V. J. Org. Chem. 2017, 82, 11399–11405. doi:10.1021/acs.joc.7b01848 |
| 28. | Rodina, L. L.; Galkina, O. S.; Maas, G.; Platz, M. S.; Nikolaev, V. A. Asian J. Org. Chem. 2016, 5, 691–698. doi:10.1002/ajoc.201600050 |
| 29. | Nikolaev, V. A.; Galkina, O. S.; Sieler, J.; Rodina, L. L. Tetrahedron Lett. 2010, 51, 2713–2716. doi:10.1016/j.tetlet.2010.03.050 |
| 30. | Galkina, O. S. Ph.D. Thesis, Saint-Petersburg State University, 2015. |
| 41. | Turro, N. J.; Ramamurthy, V.; Scaiano, J. C. Principles of molecular photochemistry: an introduction; University Science Books: Sausalito, CA, U.S.A., 2009. |
| 52. | Klan, P.; Wirz, J. Photochemistry of organic compounds: from concepts to practice; Wiley: West Sussex, United Kingdom, 2009. doi:10.1002/9781444300017 |
| 19. | Korneev, S. M. Eur. J. Org. Chem. 2011, 6153–6175. doi:10.1002/ejoc.201100224 |
| 20. | Shevchenko, V. V.; Khimich, N. N.; Platz, M. S.; Nikolaev, V. A. Tetrahedron Lett. 2005, 46, 435–438. doi:10.1016/j.tetlet.2004.11.102 |
| 21. | Bogdanova, A.; Popik, V. V. J. Am. Chem. Soc. 2004, 126, 11293–11302. doi:10.1021/ja047824r |
| 22. | Meier, H. Diazirine-Diazoalkane Interconversions. In Chemistry of diazirines; Liu, M. T. H., Ed.; CRC Press: Boca Raton, FL, U.S.A., 1987; pp 1–19. |
| 23. |
Nikolaev, V. A.; Khimich, N. N.; Korobitsyna, I. K. Chem. Heterocycl. Compd. 1985, 21, 264–268. doi:10.1007/bf00506661
Khim. Geterotsikl. Soedin. 1985, 321–325. |
| 24. | Livinghouse, T.; Stevens, R. V. J. Am. Chem. Soc. 1978, 100, 6479–6482. doi:10.1021/ja00488a036 |
| 25. | Voigt, E.; Meier, H. Chem. Ber. 1975, 108, 3326–3335. doi:10.1002/cber.19751081025 |
| 26. | Lowe, G.; Parker, J. J. Chem. Soc. D 1971, 577–578. doi:10.1039/c29710000577 |
| 53. | Maas, G.; Krebs, F.; Werle, T.; Gettwert, V.; Striegler, R. Eur. J. Org. Chem. 1999, 1939–1946. doi:10.1002/(sici)1099-0690(199908)1999:8<1939::aid-ejoc1939>3.0.co;2-a |
| 54. | Hudlicky, T.; Ranu, B. C.; Naqvi, S. M.; Srnak, A. J. Org. Chem. 1985, 50, 123–127. doi:10.1021/jo00201a026 |
| 55. | Hudlicky, T.; Sheth, J. P.; Gee, V.; Barnvos, D. Tetrahedron Lett. 1979, 20, 4889–4892. doi:10.1016/s0040-4039(01)86741-1 |
| 11. | Tidwell, T. T. Angew. Chem., Int. Ed. 2005, 44, 5778–5785. doi:10.1002/anie.200500098 |
| 12. | Garbarino, S.; Banfi, L.; Riva, R.; Basso, A. J. Org. Chem. 2014, 79, 3615–3622. doi:10.1021/jo500535f |
| 13. | Burdzinski, G.; Kubicki, J.; Sliwa, M.; Réhault, J.; Zhang, Y.; Vyas, S.; Luk, H. L.; Hadad, C. M.; Platz, M. S. J. Org. Chem. 2013, 78, 2026–2032. doi:10.1021/jo302023a |
| 14. | Willumstad, T. P.; Haze, O.; Mak, X. Y.; Lam, T. Y.; Wang, Y.-P.; Danheiser, R. L. J. Org. Chem. 2013, 78, 11450–11469. doi:10.1021/jo402010b |
| 15. | Burdzinski, G.; Zhang, Y.; Wang, J.; Platz, M. S. J. Phys. Chem. A 2010, 114, 13065–13068. doi:10.1021/jp108690n |
| 16. | Wolpert, D.; Schade, M.; Brixner, T. J. Chem. Phys. 2008, 129, 094504. doi:10.1063/1.2971037 |
| 17. | Nikolaev, V. A.; Shevchenko, V. V.; Platz, M. S.; Khimich, N. N. Russ. J. Org. Chem. 2006, 42, 815–827. doi:10.1134/S1070428006060029 |
| 18. | Popik, V. V. Can. J. Chem. 2005, 83, 1382–1390. doi:10.1139/v05-152 |
| 46. | Chang, J. A.; Chiang, Y.; Keeffe, J. R.; Kresge, A. J.; Nikolaev, V. A.; Popik, V. V. J. Org. Chem. 2006, 71, 4460–4467. doi:10.1021/jo060253w |
| 47. | Amice, P.; Conia, J. M. Tetrahedron Lett. 1974, 15, 479–482. doi:10.1016/s0040-4039(01)82249-8 |
| 48. | Kniep, C. S.; Padias, A. B.; Hall, H. K., Jr. Tetrahedron 2000, 56, 4279–4288. doi:10.1016/s0040-4020(00)00354-9 |
| 48. | Kniep, C. S.; Padias, A. B.; Hall, H. K., Jr. Tetrahedron 2000, 56, 4279–4288. doi:10.1016/s0040-4020(00)00354-9 |
| 6. | Bertrand, G., Ed. Carbene Chemistry; Marcel Dekker: New York, NY, U.S.A., 2002; p 320. doi:10.1201/9780203910924 |
| 7. | Platz, M. S., Ed. Kinetics and Spectroscopy of Carbenes and Biradicals; Plenum Press: New York, NY, U.S.A., 1990; p 372. doi:10.1007/978-1-4899-3707-0 |
| 8. | Ross, M. J.; Moss, R. A. Carbene Chemistry; John Wiley & Sons Inc.: New York, NY, U.S.A., 1975. |
| 9. | Moss, R. A.; Jones, M., Jr. Carbenes; John Wiley & Sons: New York, NY, U.S.A., 1973. |
| 10. | Kirmse, W. Carbenes, 2nd ed.; Academic Press: New York, NY, U.S.A., 1971. |
| 13. | Burdzinski, G.; Kubicki, J.; Sliwa, M.; Réhault, J.; Zhang, Y.; Vyas, S.; Luk, H. L.; Hadad, C. M.; Platz, M. S. J. Org. Chem. 2013, 78, 2026–2032. doi:10.1021/jo302023a |
| 15. | Burdzinski, G.; Zhang, Y.; Wang, J.; Platz, M. S. J. Phys. Chem. A 2010, 114, 13065–13068. doi:10.1021/jp108690n |
| 18. | Popik, V. V. Can. J. Chem. 2005, 83, 1382–1390. doi:10.1139/v05-152 |
| 20. | Shevchenko, V. V.; Khimich, N. N.; Platz, M. S.; Nikolaev, V. A. Tetrahedron Lett. 2005, 46, 435–438. doi:10.1016/j.tetlet.2004.11.102 |
| 49. | Bogdanova, A.; Popik, V. V. J. Am. Chem. Soc. 2003, 125, 1456–1457. doi:10.1021/ja029528p |
| 50. | Bogdanova, A.; Popik, V. V. J. Am. Chem. Soc. 2003, 125, 14153–14162. doi:10.1021/ja037637d |
| 51. | Nikolaev, V. A.; Frenkh, Y.; Korobitsyna, I. K. J. Org. Chem. USSR 1978, 14, 1338–1346. |
| 38. | Nikolaev, V. A.; Korobitsyna, I. K. Procedure for preparation of 2-diazo-1,3-dioxoceclopentene. Patent of the USSR 1172224, May 13, 1985. |
| 39. | Oda, M.; Kasai, M.; Kitahara, Y. Chem. Lett. 1977, 6, 307–310. doi:10.1246/cl.1977.307 |
| 41. | Turro, N. J.; Ramamurthy, V.; Scaiano, J. C. Principles of molecular photochemistry: an introduction; University Science Books: Sausalito, CA, U.S.A., 2009. |
| 61. | Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. J. Appl. Crystallogr. 2009, 42, 339–341. doi:10.1107/S0021889808042726 |
| 34. | Regitz, M.; Maas, G. Diazo Compounds. Properties and Synthesis; Academic Press: New York, NY, U.S.A., 1986. doi:10.1016/b978-0-12-585840-3.x5001-6 |
| 35. | Doyle, M. P.; McKervey, M. A.; Ye, T. Modern Catalytic Methods for Organic Synthesis with Diazo Compounds; John Wiley & Sons Inc.: New York, NY, U.S.A., 1998. |
| 36. | Baum, J. S.; Shook, D. A.; Davies, H. M. L.; Smith, H. D. Synth. Commun. 1987, 17, 1709–1716. doi:10.1080/00397918708063988 |
| 37. | Popic, V. V.; Korneev, S. M.; Nikolaev, V. A.; Korobitsyna, I. K. Synthesis 1991, 195–198. doi:10.1055/s-1991-26416 |
| 38. | Nikolaev, V. A.; Korobitsyna, I. K. Procedure for preparation of 2-diazo-1,3-dioxoceclopentene. Patent of the USSR 1172224, May 13, 1985. |
| 39. | Oda, M.; Kasai, M.; Kitahara, Y. Chem. Lett. 1977, 6, 307–310. doi:10.1246/cl.1977.307 |
| 26. | Lowe, G.; Parker, J. J. Chem. Soc. D 1971, 577–578. doi:10.1039/c29710000577 |
| 42. | Candeias, N. R.; Gois, P. M. P.; Veiros, L. F.; Afonso, C. A. M. J. Org. Chem. 2008, 73, 5926–5932. doi:10.1021/jo800980c |
| 43. | Buchner, M. R.; Wahl, B.; Ruhland, K. J. Photochem. Photobiol., A 2013, 252, 183–193. doi:10.1016/j.jphotochem.2012.12.005 |
| 44. | Rau, H.; Bokel, M. J. Photochem. Photobiol., A 1990, 53, 311–322. doi:10.1016/1010-6030(90)87135-x |
| 45. | Corey, E. J.; Felix, A. M. J. Am. Chem. Soc. 1965, 87, 2518–2519. doi:10.1021/ja01089a055 |
| 46. | Chang, J. A.; Chiang, Y.; Keeffe, J. R.; Kresge, A. J.; Nikolaev, V. A.; Popik, V. V. J. Org. Chem. 2006, 71, 4460–4467. doi:10.1021/jo060253w |
| 47. | Amice, P.; Conia, J. M. Tetrahedron Lett. 1974, 15, 479–482. doi:10.1016/s0040-4039(01)82249-8 |
| 31. | Presset, M.; Mailhol, D.; Coquerel, Y.; Rodriguez, J. Synthesis 2011, 2549–2552. doi:10.1055/s-0030-1260107 |
| 32. | Wu, C.; Mooring, A. M.; Yardley, J. T. UV photoresist containing polycyclic cyclopentanediazodione derivative. Eur. Pat. Appl. EP405957A1, Jan 2, 1991. |
| 33. | Korobitsyna, I. K.; Rodina, L. L.; Nikolaev, V. A. The method of producing bicyclic diazocyclopentanediones. Patent of the USSR 1004359, March 15, 1983. |
| 59. | Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Crystallogr. 2008, 64, 112–122. doi:10.1107/s0108767307043930 |
| 27. | Rodina, L. L.; Baranovskii, V. I.; Galkina, O. S.; Nikolaev, V. A.; Tonogina, N. L.; Povolotskiy, A. V. J. Org. Chem. 2017, 82, 11399–11405. doi:10.1021/acs.joc.7b01848 |
| 30. | Galkina, O. S. Ph.D. Thesis, Saint-Petersburg State University, 2015. |
| 40. | Csizmadia, I. G.; Houlden, S. A.; Meresz, O.; Yates, P. Tetrahedron 1969, 25, 2121–2138. doi:10.1016/s0040-4020(01)82763-0 |
| 60. | Sheldrick, G. M. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. doi:10.1107/s2053273314026370 |
© 2018 Rodina et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)