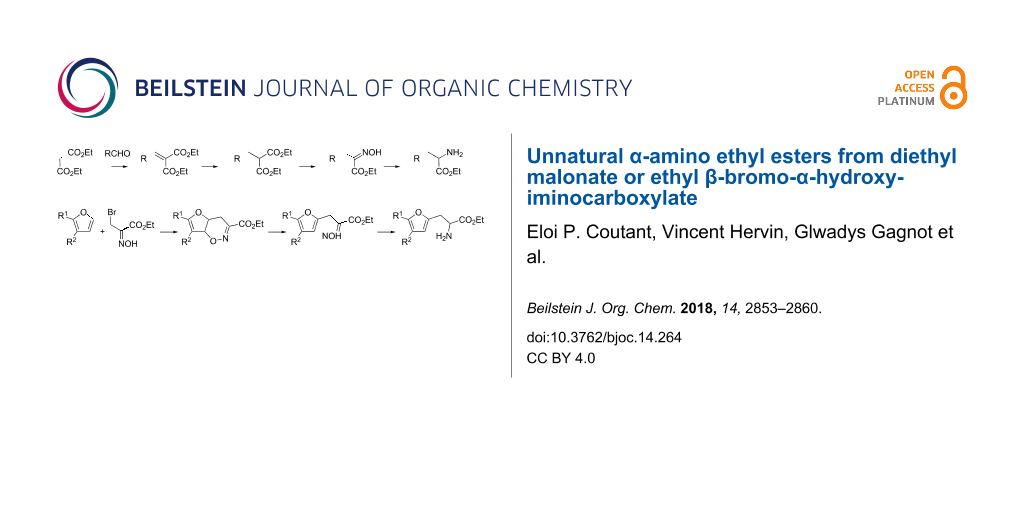
Abstract
We have explored here the scope of the age-old diethyl malonate-based accesses to α-amino esters involving Knoevenagel condensations of diethyl malonate on aldehydes, reductions of the resulting alkylidenemalonates, the preparation of the corresponding α-hydroxyimino esters and their final reduction. This synthetic pathway turned out to be general although some unexpected limitations were encountered. The synthetic modifications of some of the intermediates – using Suzuki–Miyaura coupling or cycloadditions – before undertaking the oximation step – provided accesses to further α-amino esters. Moreover, other pathways to α-hydroxyimino esters were explored including an attempt to improve the cycloadditions between ethyl β-bromo-α-hydroxyiminocarboxylate and various alkylfuranes.
Graphical Abstract
Introduction
Our current work on the chemistry of imidazo[1,2-a]pyrazin-3(7H)-one luciferins [1] has led us to require a large variety of α-amino esters as starting material. In view of the limitations we encountered in the use of ethyl nitroacetate to reach such variety [2], we have focused here on diethyl malonate-based methods as an alternative. Indeed, as we reviewed recently [3], this approach was used for the historic preparation of racemic lysine from diethyl malonate and γ-chlorobutyronitrile [4] and appears to be of a very large scope. The key α-hydroxyimino esters 2 precursors to the α-amino esters 1 are usually prepared by oximation of substituted malonates 3, themselves made, for instance, from diethyl malonate (4) and an alkylation reaction or a Knoevenagel condensation–reduction sequence (Scheme 1).
Scheme 1: Malonate-based retrosynthesis of α-amino esters.
Scheme 1: Malonate-based retrosynthesis of α-amino esters.
Results and Discussion
As depicted in Table 1, Knoevenagel condensations of diethyl malonate (4) and aldehydes 5a–al followed by reduction of the intermediate alkylidenemalonates 6a–al led to the substituted malonates 3a–al. A simple and general procedure was adopted for the condensation using fairly concentrated ethanolic solutions of diethyl malonate (4) and aldehydes 5a–al along with catalytic amounts of piperidine and acetic acid as well as some 4 Å molecular sieves to trap the water formed in situ. The 1H NMR monitoring of crude samples pointed out a complete conversion, most often overnight at 60 °C, and the resulting solutions of alkylidenemalonates 6a–al were then directly reduced to give the malonates 3a–al. For this reduction step, palladium-based catalytic hydrogenation was preferably used although, when incompatible with the substrates, sodium borohydride was employed. In most cases, large proportion of the expected substituted malonates 3a–al were observed by 1H NMR. Thus, in order to simplify even further this procedure, these crude malonates were subjected to the next step after a minimal work-up. Accordingly, they were dissolved in ethanol and treated first with sodium ethoxide followed by isoamyl nitrite (iAmONO) to give the corresponding α-hydroxyimino esters 2a–al. These resulting compounds were then purified and isolated in yields reported in Table 1. These values usually reflected the eventual problems encountered in the course of the reduction or the oximation steps since the condensation was very often more than 98% complete. As further commented on and depicted in Scheme 2, in few instances (preparations of 2t–v and 2ae), explanations were found for the low yield observed and could be sometimes acted upon. In any case, the final reduction of the α-hydroxyimino esters 2b–al using zinc and hydrochloric acid in ethanol gave the α-amino esters 1b–al, which were usually of a high purity grade without recourse to a chromatography. In few cases, only a sample of the intermediate α-hydroxyimino esters was purified for analytical purposes. For instance, pure α-amino esters 1m and 1y were obtained in four steps from the corresponding aldehydes 5m and 5y without the recourse to any chromatography in quite acceptable overall yields.
Table 1: Synthesis of α-amino esters 1b–al via α-hydroxyimino esters 2a–al.
|
|
|||
| Ar | R | % 2a | % 1 |
| a | Ph | 60 | nd |
| b | 2-MeC6H4 | 61 | 85 |
| c | 3-MeC6H4 | 63 | 85 |
| d | 4-MeC6H4 | 60 | 87 |
| e | 4-iPrC6H4 | 48 | 91 |
| f | cyclopentyl | 35 | 85 |
| g | cyclohexyl | 50 | 92 |
| h | 2-CF3C6H4 | 39 | 86 |
| i | 3-CF3C6H4 | 41 | 78 |
| j | 4-CF3C6H4 | 62 | 91 |
| k | 2-FC6H4 | 57 | 88 |
| l | 3-FC6H4 | 65 | 89 |
| m | 4-FC6H4 | – | 81b |
| n | 2,4-F2C6H3 | 50 | 85 |
| o | 2,6-F2C6H3 | 54 | 94 |
| p | 2,3-F2C6H3 | 51 | 92 |
| q | 2,5-F2C6H3 | 50 | 95 |
| r | 3,5-F2C6H3 | 46 | 84 |
| s | 2,3,5-F3C6H2 | 35 | 94 |
| t | 2-ClC6H4 | 23/49c | 94 |
| u | 3-ClC6H4 | 26/59c | 92 |
| v | 4-ClC6H4 | 37/62c | 85 |
| w | 4-BrC6H4 | 42 | 86 |
| x | 2-MeOC6H4 | 60 | 83 |
| y | 3-MeOC6H4 | – | 75b |
| z | 2-BnOC6H4 | 48 | 91 |
| aa | 3-BnOC6H4 | 37 | 92 |
| ab | 4-BnOC6H4 | 40 | 94 |
| ac | 2-pyridyl | 49 | 56 |
| ad | 3-pyridyl | 43 | 63 |
| ae | furan-2-yl | 33/47d | 89 |
| af | 5-methylfuran-2-yl | 33 | 88 |
| ag | 4,5-dimethylfuran-2-yl | 10/23e | 90 |
| ah | 5-ethylfuran-2-yl | 34 | 94 |
| ai | 5-trifluoromethylfuran-2-yl | 36 | 95 |
| aj | 5-ethylthiophen-2-yl | 29 | 92 |
| ak | 3-methylthiophen-2-yl | 32 | 89 |
| al | 4,5-dimethylthiophen-2-yl | 31 | 87 |
aIsolated yield from aldehydes 5. bOverall isolated yield from aldehydes 5m or 5y. cReactions run in isopropanol and NMe4BH4 used as a reductant. dReduction run at –10 °C for 90 minutes. eSame as note d but using a THF/ethanol mixture.
Out of these results, we first sought an explanation for the modest yields observed for the preparation of the 2, 3 or 4-chlorophenyl-α-hydroxyimino esters 2t–v. As depicted in Scheme 2, the 1H NMR monitoring of the ethanolic solution of the alkylidenemalonate 6u pointed out a slow but steady 1,4-addition of ethanol to give compound 7 (a 30% conversion was observed over 3 weeks). Moreover, even if a fresh solution of compound 6u was immediately treated with sodium borohydride, a proportion of this 1,4-adduct was seen in the reaction mixture amongst other side products. We also tried to allow for an eventual reversal of the ethanol addition and thus increased the reaction time of the reduction step, but compound 7 remained unaffected and even more side products arose. To avoid this 1,4-addition, we then resorted to use a less nucleophilic solvent, and tried isopropanol instead of ethanol. However, even if the ensuing reduction of 6u using sodium borohydride led to a somewhat better yield of compound 3u (as estimated by 1H NMR), side products resulting from over-reductions of the ester moieties were still observed. It is only when switching to tetramethylammonium borohydride that this was alleviated and the yields of α-hydroxyimino esters 2t–v reached 49, 59 and 62% from aldehydes 5t–v, respectively. Of note is that we also tried this reducing agent to improve the overall yield of the benzyloxy-bearing oxime 2ab, but to no avail. We next focused on the furan-bearing α-hydroxyimino esters such as compound 2ae. First of all, reduction of the alkylidenemalonate 6ae using a palladium-based catalytic hydrogenation also led to the concomitant hydrogenation of the furan ring to give compound 8. As depicted, this actually allowed us to prepare to the 2-tetrahydrofuranyl-bearing α-aminoester 10 in a 35% overall yield via α-hydroxyimino ester 9, but it also forced us to use borohydrides to avoid the furan ring hydrogenation. Accordingly, an extensive study of the reduction of compound 6ae with borohydrides was made. Trials included reactions run at 0 °C overnight, the use of wet ethanol or dry THF, the addition of less than one equivalent of the sodium borohydride as well as the use of tetramethylammonium borohydride or sodium cyanoborohydride, but none were overly successful. Indeed, a representative assay (dry THF, 24 hours at 0 °C, 0.7 equivalents of NaBH4) led to the isolation of 49% of compound 3ae. Moreover, extensive purification procedures of the complex reaction mixtures led to the full characterization of compound 11 and 12 stemming from over-reduction. Interestingly, the latter one has actually been reported to occur upon a 48 hour long sodium borohydride reduction of S,S-diethyl 2-(furan-2-ylmethylene)propanebis(thioate), the bisthioester homolog of compound 6ae [5]. Finally, the oximation of the pure malonate 3ae only led to a 64% yield of the corresponding α-hydroxyimino ester 2ae, thus accounting for the 33% overall yield reported in Table 1 when we proceeded without purification. It is only later that we found that shortening the reaction of the sodium borohydride reduction of 6ae down to 1.5 hours and lowering the temperature to –10 °C led to a sizable improvement in the overall yield (47% instead of 33%). However, these conditions were a trade-off between reduction of 6ae into 3ae and over-reduction since 1H NMR analysis of this trial pointed out the coexistence of unreacted material 6ae along with (traces) of the over-reduced compounds 11 and 12. We applied this finding to the reduction of 6ag and had to allow for the low solubility of this compound in ethanol and thus run the reaction in a mixture of ethanol and THF. In any case, we could obtain more of the corresponding α-hydroxyimino ester 2ag but in a still very modest 23% overall yield. This last result, likely due to additional problems at the oximation stage, is thus only emphasizing the vagaries of this synthetic access that will sometime require a specific study.
Scheme 2: Some side products and synthesis of α-amino ester 10.
Scheme 2: Some side products and synthesis of α-amino ester 10.
As shown in Scheme 3, the lack or the cost of some more exotic aldehydes 5 were circumvented as we found out that Suzuki–Miyaura carbon–carbon coupling reactions could be achieved with the brominated alkylidenemalonates 13 or 17 as well as the 5-bromofuran derivative 29. Indeed, coupling these compounds with cyclopropylboronic acid gave the corresponding cyclopropyl derivatives 14, 18 or 30 in 60, 47 and 75% yield, respectively. The palladium-based reduction of compound 14 and 18 also led to the hydrogenation of their cyclopropyl ring into a propyl side chain to give compounds 15 and 19. Alternatively, the recourse to sodium borohydride reduced these compounds as well as the furan-bearing derivative 30 into the target cyclopropyl-bearing derivatives 16, 20 and 31. The ensuing oximation of all these compounds provided the α-hydroxyimino esters 21, 23, 25, 27 and 32 in 31–88% overall yield. These were then reduced into the corresponding α-amino esters 22, 24, 26, 28 and 33 using zinc and hydrochloric acid. We also focused on the preparation of the β-methylated furan-bearing α-hydroxyimino ester 35. The introduction of the methyl group was achieved using the previously reported methylmagnesium chloride 1,4-addition on diethyl furfurylidenemalonate (6ae) [6]. Quite unexpectedly, repeated attempts to obtain the α-hydroxyimino ester 35 from the resulting β-methylated derivative 34 failed. Trials were made not only with sodium ethoxide but also with the stronger lithium diisopropylamide. We do not have an explanation for this observation, although such oximation was achieved (in a low yield) when starting from the phenyl-bearing analogue 36 as described below. We suggest a somehow forbidding cation chelation by the oxygen of the furan ring which would prevent its reaction with isoamyl nitrite. Interestingly, a literature search for similar reactions starting from β,β-disubstituted malonates, led to two reports [7,8], whereas from β,β-substituted acetoacetates three examples were reported [9-11], but only one substrate [7] out of these features an aromatic substituent.
Scheme 3: Syntheses of α-amino esters 22, 24, 26, 28 and 33.
Scheme 3: Syntheses of α-amino esters 22, 24, 26, 28 and 33.
As depicted in Scheme 4 and contrary to our attempt to prepare the furan-bearing β-methyl oxime 35, the phenyl-bearing β-methyl aminoester 38 was obtained via the substituted malonate 36 to give upon oximation the α-hydroxyimino ester 37 (although in only a 14% yield). A slightly different approach was used for the preparation of the even more hindered β,β-dimethyl aminoester 41. Treatment of the monoester 39 [12,13] with lithium diisopropylamide and isoamyl nitrite overnight was once again not really successful as a rather poor 15% yield of the corresponding α-hydroxyimino ester 40 was isolated. Nevertheless, the ensuing reduction gave the desired α-amino esters 38 and 41. The model reduction of the α-hydroxyimino ester 2a into the corresponding N-hydroxylamino ester 42 was also studied. Initial trials using reported procedures [14-16] pointed out an exceedingly slow reaction requiring repeated additions of trimethylamine–borane complex on a solution of compound 2a in diethyl ether saturated with hydrogen chloride (ether containing trifluoromethanesulfonic acid was also tried). This led only to a 40% yield probably because of the occurrence of stable boron-based complexes. In an attempt to improve this, we applied the reported use of triethylsilane for reducing oximes into N-hydroxylamines [17] to the case of the α-hydroxyimino ester 2a. However, bringing the reduction of 2a into the N-hydroxylamino derivative 42 to completion with this reagent still required a repeated addition of triethylsilane over a week and led to a 69% isolated yield. Interestingly, further literature search pointed out a very different synthetic access to such N-hydroxylamine derivative (via nitrones) which appears to be far more efficient [18-22]. The isoxazole-bearing α-amino esters 46a,b were also prepared via α-hydroxyimino esters. Their preparation started with the alkylation of diethyl malonate (4) with propargyl bromide to give compound 43 (which could not be separated from unreacted malonate and the bis-alkylated derivative). In the next step, [2 + 3] cycloaddition reactions with nitroethane or 1-nitropropane gave the isoxazole-bearing compounds 44a,b in 23 and 33% yield, respectively. Then, oximation of these compounds gave the α-hydroxyimino esters 45a,b in 50 and 58% and upon their reduction, the expected α-amino esters 46a,b.
Scheme 4: Syntheses of α-amino esters 38, 41 and 46a,b.
Scheme 4: Syntheses of α-amino esters 38, 41 and 46a,b.
As depicted in Table 2, in order to avoid the recourse to the sometime expensive furan-bearing aldehydes 5 and reach an even more diverse set of α-amino esters, we investigated the use of ethyl β-bromo-α-hydroxyiminocarboxylate 47 which has been developed by Gilchrist for the synthesis of many types of α-hydroxyimino esters [23-26] and then extensively used to prepare a wide range of amino acids [3,27]. This proceeds via a [2 + 4] cycloaddition between ethyl nitrosoacrylate, generated in situ from the reaction of sodium carbonate and furan 48, to give the cycloadduct 49. Then, upon heating, a rearrangement leads to the furan-bearing α-hydroxyimino esters 2. Reports have thus described the preparation of 2ae in a 46% yield from furan (48ae) [24] and compound 2af in a 50% yield from 2-methylfuran (48af) [25]. For our part, we first made extensive trials to improve such yields using 2-methylfuran (48af) as a model substrate. It turned out that this [2 + 4] cycloaddition can proceed under a wide range of conditions. The original conditions stirring compound 47, an excess of 2-methylfuran (48af) and solid sodium carbonate in dichloromethane for 24 hours gave the expected cycloadduct 49af but we found out that a chromatography was enough to achieve its isomerisation into 2af. Interestingly, we also found out that the excess 2-methylfuran (48af) was not required and that the reaction proceeded much faster and led directly to the α-hydroxyimino ester 2af if tetrabutylammonium bromide was added as a phase transfer catalyst. For instance, (note b in Table 2), stirring a 1:1.1 proportion of compounds 47 and 48af, sodium carbonate, and 0.01 equiv tetrabutylammonium bromide in toluene for 1.5 hours led to a 42% yield of the α-hydroxyimino ester 2af. On the other hand, such catalysis was not required when stirring a 1:1.1 proportion of compounds 47 and 48af in a mixture of ethyl acetate and water and an array of bases such as ammonium bicarbonate (41% yield, note c in Table 2). We tried under these conditions 1:2 or 2:1 proportions of compounds 47 and 48af but the isolated yields of α-hydroxyimino ester 2af were of (only) 56% and 54%, respectively. Later on, when using a 1:1 proportion of compounds 47 and 48af, a slightly improved yield of 50% was achieved when switching from ammonium bicarbonate to lithium carbonate (note d in Table 2) or in the case of the preparation of compound 2ag switching to sodium carbonate (note e in Table 2). Unfortunately, if the 1H NMR analysis of another chromatographic fraction obtained when purifying these trials pointed out the occurrence of inseparable mixture of aliphatic compounds, these could never be properly purified and identified. A mechanistic explanation for these average yields could be the occurrence upon the base reaction with compound 47 of cis and trans conformations of the nitrosoacrylate. The cis one would be susceptible to undergo an immediate [2 + 4] cycloaddition with the furan whereas the trans form would not, and since the equilibration between the two forms was reported [28] to be a slow process, it would potentially lead to decomposition materials. In any case, as depicted in Table 2, from the commercially available furans 48af–an or from crude solutions of the less accessible (and volatile) compounds 48ao–ar (their preparation greatly benefitted from the reported [29] synthesis of compound 48ap and is fully described in the experimental section), we could isolate the corresponding α-hydroxyimino esters using a variety of conditions which were then reduced into the α-amino esters. Of note is that extensive decomposition was observed when starting from 2-methoxyfuran and as seen by 1H NMR, no oxime occurrence was observed from 2,3,4-trimethylfuran.
Table 2: Synthesis of furan-bearing α-amino esters by a [2 + 4] cycloaddition.
|
|
||||
| Ar | R1 | R2 | % 2a | % 1a |
| ae | H | H | – | – |
| af | Me | H | 42b/41c/50d | – |
| ag | Me | Me | 38b/48c/50e | – |
| ah | Et | H | 43b | – |
| am | n-Pr | H | 40b | 79 |
| an | n-pentyl | H | 32c | 90 |
| ao | Me | Et | 21c,f | 82 |
| ap | (CH2)4 | 40b,g | 82 | |
| aq | Et | Me | 25c, h | 90 |
| ar | iPr | Me | 30c,i | 86 |
aIsolated yield. bToluene, NBu4Br (cat.), Na2CO3. cH2O/AcOEt, NH3/HCO3H. dH2O/AcOEt, Li2CO3. eH2O/AcOEt, Na2CO3. fOverall yield from 1-(2-methylfuran-3-yl)ethan-1-one. gOverall yield from 6,7-dihydrobenzofuran-4(5H)-one. hOverall yield from ethyl 2-ethylfuran-3-carboxylate. iOverall yield from ethyl 2-isopropylfuran-3-carboxylate.
Finally, as described in Scheme 5, we also prepared the dioxolane-bearing α-amino esters 53 and 58. An approach involving a key Curtius reaction was first tried for the preparation of 53. As reported [30], alkylation of diethyl malonate (4) with 2-(bromomethyl)-1,3-dioxolane (50) gave the diester 51. A controlled saponification led to the mono acid 52 and its reaction with diphenylphosphoryl azide [31-33] gave 16% of the target aminoester 53 upon treatment with hydrochloric acid. Alternatively, oximation of the diester 51 gave 28% (from diethyl malonate) of the α-hydroxyimino ester 54, which turned out to be not stable in CDCl3 because of hydrogen chloride traces. For this reason, no attempt was made to reduce its oxime function under the acidic conditions used above (hydrochloric and powdered zinc) and we successfully used instead the much milder [34] mixture of ammonium formate and zinc in ethanol at room temperature to give 43% of the target α-amino ester 53. The same strategy gave the corresponding α-amino ester homolog 58 from the readily available dioxane derivative 56 [35] in a 46% overall yield. However, the final oxime reduction turned out to be much improved when using hydrogen and palladium over charcoal in acetic acid.
Scheme 5: Syntheses of α-amino esters 53 and 58.
Scheme 5: Syntheses of α-amino esters 53 and 58.
Conclusion
As in our previous report [2], the goal of this investigation was to reach a very large variety of racemic α-amino esters. In the course of this study, we could point out that, in comparison with ethyl nitroacetate [2], the use of diethyl malonate (4) is at least as efficient and far more general, especially for the initial condensation step. However, as for the reduction of nitroacrylates [2], the use of borohydrides to reduce some of the resulting alkylidenemalonates 6 turned out to be limiting, even in some cases, tetramethylammonium borohydride instead of sodium borohydride could alleviate this limitation. Besides, out of the many preparations of α-hydroxyimino esters described above, another limitation was encountered when steric hindrance became a previously unreported limiting factor for the oximation of compound 34, and to a lesser degree compounds 36 or 39. Finally, the use of the ethyl β-bromo-α-hydroxyimino carboxylate 47 in a [2 + 4] cycloaddition reaction led us to suggest a simple, reliable and robust alternative for the preparation of many furan-bearing α-amino esters.
Supporting Information
| Supporting Information File 1: Characterization of all the compounds described, scans of 1H and 13C NMR spectra. | ||
| Format: PDF | Size: 18.0 MB | Download |
Acknowledgements
This work was supported by the Agence Nationale de la Recherche (ANR), grant ANR-11-CRNT-0004, in the context of the investment program ‘GLOBAL CARE’, an association of the Instituts Carnot ‘Pasteur-Maladies Infectieuses’, ‘Curie-Cancer’, ‘Voir et Entendre’, ‘Institut du Cerveau et de la moelle Épinière’ and the ‘Consortium pour l’Accélération de l’Innovation et de son Transfert dans le domaine du Lymphome’ (CALYM). This project also benefited from the Valoexpress funding call of the Institut Pasteur. Prof. Christian Bréchot, Dr. Muriel Delepierre and Dr. Daniel Larzul, from the Institut Pasteur are acknowledged for their interest and support. The Amgen foundation is acknowledged for the benefit of an Amgen Scholars Program fellowship for CF.
References
-
Coutant, E. P.; Janin, Y. L. Chem. – Eur. J. 2015, 21, 17158–17171. doi:10.1002/chem.201501531
Return to citation in text: [1] -
Gagnot, G.; Hervin, V.; Coutant, E. P.; Desmons, S.; Baatallah, R.; Monnot, V.; Janin, Y. L. Beilstein J. Org. Chem. 2018, 14, 2846–2852. doi:10.3762/bjoc.14.263
Return to citation in text: [1] [2] [3] [4] -
Hervin, V.; Coutant, E. P.; Gagnot, G.; Janin, Y. L. Synthesis 2017, 49, 4093–4110. doi:10.1055/s-0036-1589506
Return to citation in text: [1] [2] -
Fischer, E.; Weigert, F. Ber. Dtsch. Chem. Ges. 1902, 35, 3772–3778. doi:10.1002/cber.190203503211
Return to citation in text: [1] -
Rose, P. A.; Liu, H.-J. Synth. Commun. 1991, 21, 2089–2095. doi:10.1080/00397919108019815
Return to citation in text: [1] -
Holmberg, G. A.; Karlsson, M.; Ulfstedt, O.; Olli, M. Acta Chem. Scand. 1972, 26, 3483–3491. doi:10.3891/acta.chem.scand.26-3483
Return to citation in text: [1] -
Harington, C. R. J. Biol. Chem. 1925, 64, 29–39.
Return to citation in text: [1] [2] -
Wieland, T.; Georgi, V. Justus Liebigs Ann. Chem. 1966, 700, 133–148. doi:10.1002/jlac.19667000117
Return to citation in text: [1] -
Sen, H. K. Biochem. Z. 1923, 143, 195–200.
Chem. Abstr. 1924, 17, 16327.
Return to citation in text: [1] -
Hamlin, K. E.; Hartung, W. H. J. Biol. Chem. 1942, 145, 349–357.
Return to citation in text: [1] -
Fischer, R.; Wieland, T. Chem. Ber. 1960, 93, 1387–1389. doi:10.1002/cber.19600930623
Return to citation in text: [1] -
Andersen, R.; Piers, E.; Nieman, J.; Coleman, J.; Roberge, M. Hemiasterlin analogs. U.S. Patent US 20090264487, Oct 22, 2009.
Return to citation in text: [1] -
Roberts, D. D. J. Org. Chem. 1964, 29, 2714–2717. doi:10.1021/jo01032a058
Return to citation in text: [1] -
Herscheid, J. D. M.; Ottenheijm, H. C. J. Tetrahedron Lett. 1978, 19, 5143–5144. doi:10.1016/s0040-4039(01)85833-0
Return to citation in text: [1] -
Herscheid, J. D. M.; Colstee, J. H.; Ottenheijm, H. C. J. J. Org. Chem. 1981, 46, 3346–3348. doi:10.1021/jo00329a044
Return to citation in text: [1] -
Tijhuis, M. W.; Herscheid, J. D. M.; Ottenheijm, H. C. J. Synthesis 1980, 890–893. doi:10.1055/s-1980-29255
Return to citation in text: [1] -
Fujita, M.; Oishi, H.; Hiyama, T. Chem. Lett. 1986, 15, 837–838. doi:10.1246/cl.1986.837
Return to citation in text: [1] -
Emmons, W. D. J. Am. Chem. Soc. 1957, 79, 5739–5754. doi:10.1021/ja01578a043
Return to citation in text: [1] -
Połński, T.; Chimiak, A. Tetrahedron Lett. 1974, 15, 2453–2456. doi:10.1016/s0040-4039(01)92284-1
Return to citation in text: [1] -
Wovkulich, P. M.; Uskoković, M. R. Tetrahedron 1985, 41, 3455–3462. doi:10.1016/s0040-4020(01)96700-6
Return to citation in text: [1] -
Grundke, G.; Keese, W.; Rimpler, M. Synthesis 1987, 1115–1116. doi:10.1055/s-1987-28189
Return to citation in text: [1] -
Tokuyama, H.; Kuboyama, T.; Amano, A.; Yamashita, T.; Fukuyama, T. Synthesis 2000, 1299–1304. doi:10.1055/s-2000-6428
Return to citation in text: [1] -
Gilchrist, T. L.; Lingham, D. A.; Roberts, T. G. J. Chem. Soc., Chem. Commun. 1979, 1089–1090. doi:10.1039/c39790001089
Return to citation in text: [1] -
Gilchrist, T. L.; Roberts, T. G. J. Chem. Soc., Perkin Trans. 1 1983, 1283–1292. doi:10.1039/p19830001283
Return to citation in text: [1] [2] -
Gilchrist, T. L.; Hughes, D.; Stretch, W.; Chrystal, E. J. T. J. Chem. Soc., Perkin Trans. 1 1987, 2505–2509. doi:10.1039/p19870002505
Return to citation in text: [1] [2] -
Chrystal, E. J. T.; Gilchrist, T. L.; Stretch, W. J. Chem. Res., Synop. 1987, 180–181.
Return to citation in text: [1] -
Gallos, J. K.; Sarli, V. C.; Massen, Z. S.; Varvogli, A. C.; Papadoyanni, C. Z.; Papaspyrou, S. D.; Argyropoulos, N. G. Tetrahedron 2005, 61, 565–574. doi:10.1016/j.tet.2004.11.009
Return to citation in text: [1] -
Sakaizumi, T.; Tanaka, H.; Hirano, K.; Kuze, N.; Ohashi, O. J. Mol. Spectrosc. 1999, 194, 79–86. doi:10.1006/jmsp.1998.7774
Return to citation in text: [1] -
Lautens, M.; Fillion, E. J. Org. Chem. 1997, 62, 4418–4427. doi:10.1021/jo9701593
Return to citation in text: [1] -
Aïssa, C. J. Org. Chem. 2006, 71, 360–363. doi:10.1021/jo051693a
Return to citation in text: [1] -
Shioiri, T.; Ninomiya, K.; Yamada, S. J. Am. Chem. Soc. 1972, 94, 6203–6205. doi:10.1021/ja00772a052
Return to citation in text: [1] -
Ninomiya, K.; Shioiri, T.; Yamada, S. Chem. Pharm. Bull. 1974, 22, 1398–1404. doi:10.1248/cpb.22.1398
Return to citation in text: [1] -
Scriven, E. F. V.; Turnbull, K. Chem. Rev. 1988, 88, 297–368. doi:10.1021/cr00084a001
Return to citation in text: [1] -
Abiraj, K.; Gowda, D. C. J. Chem. Res., Synop. 2003, 332–333. doi:10.3184/030823403103174281
Return to citation in text: [1] -
Yamanaka, E.; Narushima, M.; Inukai, K.; Sakai, S.-I. Chem. Pharm. Bull. 1986, 34, 77–81. doi:10.1248/cpb.34.77
Return to citation in text: [1]
| 1. | Coutant, E. P.; Janin, Y. L. Chem. – Eur. J. 2015, 21, 17158–17171. doi:10.1002/chem.201501531 |
| 5. | Rose, P. A.; Liu, H.-J. Synth. Commun. 1991, 21, 2089–2095. doi:10.1080/00397919108019815 |
| 3. | Hervin, V.; Coutant, E. P.; Gagnot, G.; Janin, Y. L. Synthesis 2017, 49, 4093–4110. doi:10.1055/s-0036-1589506 |
| 27. | Gallos, J. K.; Sarli, V. C.; Massen, Z. S.; Varvogli, A. C.; Papadoyanni, C. Z.; Papaspyrou, S. D.; Argyropoulos, N. G. Tetrahedron 2005, 61, 565–574. doi:10.1016/j.tet.2004.11.009 |
| 4. | Fischer, E.; Weigert, F. Ber. Dtsch. Chem. Ges. 1902, 35, 3772–3778. doi:10.1002/cber.190203503211 |
| 24. | Gilchrist, T. L.; Roberts, T. G. J. Chem. Soc., Perkin Trans. 1 1983, 1283–1292. doi:10.1039/p19830001283 |
| 3. | Hervin, V.; Coutant, E. P.; Gagnot, G.; Janin, Y. L. Synthesis 2017, 49, 4093–4110. doi:10.1055/s-0036-1589506 |
| 18. | Emmons, W. D. J. Am. Chem. Soc. 1957, 79, 5739–5754. doi:10.1021/ja01578a043 |
| 19. | Połński, T.; Chimiak, A. Tetrahedron Lett. 1974, 15, 2453–2456. doi:10.1016/s0040-4039(01)92284-1 |
| 20. | Wovkulich, P. M.; Uskoković, M. R. Tetrahedron 1985, 41, 3455–3462. doi:10.1016/s0040-4020(01)96700-6 |
| 21. | Grundke, G.; Keese, W.; Rimpler, M. Synthesis 1987, 1115–1116. doi:10.1055/s-1987-28189 |
| 22. | Tokuyama, H.; Kuboyama, T.; Amano, A.; Yamashita, T.; Fukuyama, T. Synthesis 2000, 1299–1304. doi:10.1055/s-2000-6428 |
| 2. | Gagnot, G.; Hervin, V.; Coutant, E. P.; Desmons, S.; Baatallah, R.; Monnot, V.; Janin, Y. L. Beilstein J. Org. Chem. 2018, 14, 2846–2852. doi:10.3762/bjoc.14.263 |
| 23. | Gilchrist, T. L.; Lingham, D. A.; Roberts, T. G. J. Chem. Soc., Chem. Commun. 1979, 1089–1090. doi:10.1039/c39790001089 |
| 24. | Gilchrist, T. L.; Roberts, T. G. J. Chem. Soc., Perkin Trans. 1 1983, 1283–1292. doi:10.1039/p19830001283 |
| 25. | Gilchrist, T. L.; Hughes, D.; Stretch, W.; Chrystal, E. J. T. J. Chem. Soc., Perkin Trans. 1 1987, 2505–2509. doi:10.1039/p19870002505 |
| 26. | Chrystal, E. J. T.; Gilchrist, T. L.; Stretch, W. J. Chem. Res., Synop. 1987, 180–181. |
| 14. | Herscheid, J. D. M.; Ottenheijm, H. C. J. Tetrahedron Lett. 1978, 19, 5143–5144. doi:10.1016/s0040-4039(01)85833-0 |
| 15. | Herscheid, J. D. M.; Colstee, J. H.; Ottenheijm, H. C. J. J. Org. Chem. 1981, 46, 3346–3348. doi:10.1021/jo00329a044 |
| 16. | Tijhuis, M. W.; Herscheid, J. D. M.; Ottenheijm, H. C. J. Synthesis 1980, 890–893. doi:10.1055/s-1980-29255 |
| 9. |
Sen, H. K. Biochem. Z. 1923, 143, 195–200.
Chem. Abstr. 1924, 17, 16327. |
| 10. | Hamlin, K. E.; Hartung, W. H. J. Biol. Chem. 1942, 145, 349–357. |
| 11. | Fischer, R.; Wieland, T. Chem. Ber. 1960, 93, 1387–1389. doi:10.1002/cber.19600930623 |
| 17. | Fujita, M.; Oishi, H.; Hiyama, T. Chem. Lett. 1986, 15, 837–838. doi:10.1246/cl.1986.837 |
| 7. | Harington, C. R. J. Biol. Chem. 1925, 64, 29–39. |
| 8. | Wieland, T.; Georgi, V. Justus Liebigs Ann. Chem. 1966, 700, 133–148. doi:10.1002/jlac.19667000117 |
| 6. | Holmberg, G. A.; Karlsson, M.; Ulfstedt, O.; Olli, M. Acta Chem. Scand. 1972, 26, 3483–3491. doi:10.3891/acta.chem.scand.26-3483 |
| 12. | Andersen, R.; Piers, E.; Nieman, J.; Coleman, J.; Roberge, M. Hemiasterlin analogs. U.S. Patent US 20090264487, Oct 22, 2009. |
| 13. | Roberts, D. D. J. Org. Chem. 1964, 29, 2714–2717. doi:10.1021/jo01032a058 |
| 29. | Lautens, M.; Fillion, E. J. Org. Chem. 1997, 62, 4418–4427. doi:10.1021/jo9701593 |
| 25. | Gilchrist, T. L.; Hughes, D.; Stretch, W.; Chrystal, E. J. T. J. Chem. Soc., Perkin Trans. 1 1987, 2505–2509. doi:10.1039/p19870002505 |
| 28. | Sakaizumi, T.; Tanaka, H.; Hirano, K.; Kuze, N.; Ohashi, O. J. Mol. Spectrosc. 1999, 194, 79–86. doi:10.1006/jmsp.1998.7774 |
| 2. | Gagnot, G.; Hervin, V.; Coutant, E. P.; Desmons, S.; Baatallah, R.; Monnot, V.; Janin, Y. L. Beilstein J. Org. Chem. 2018, 14, 2846–2852. doi:10.3762/bjoc.14.263 |
| 2. | Gagnot, G.; Hervin, V.; Coutant, E. P.; Desmons, S.; Baatallah, R.; Monnot, V.; Janin, Y. L. Beilstein J. Org. Chem. 2018, 14, 2846–2852. doi:10.3762/bjoc.14.263 |
| 2. | Gagnot, G.; Hervin, V.; Coutant, E. P.; Desmons, S.; Baatallah, R.; Monnot, V.; Janin, Y. L. Beilstein J. Org. Chem. 2018, 14, 2846–2852. doi:10.3762/bjoc.14.263 |
| 34. | Abiraj, K.; Gowda, D. C. J. Chem. Res., Synop. 2003, 332–333. doi:10.3184/030823403103174281 |
| 35. | Yamanaka, E.; Narushima, M.; Inukai, K.; Sakai, S.-I. Chem. Pharm. Bull. 1986, 34, 77–81. doi:10.1248/cpb.34.77 |
| 31. | Shioiri, T.; Ninomiya, K.; Yamada, S. J. Am. Chem. Soc. 1972, 94, 6203–6205. doi:10.1021/ja00772a052 |
| 32. | Ninomiya, K.; Shioiri, T.; Yamada, S. Chem. Pharm. Bull. 1974, 22, 1398–1404. doi:10.1248/cpb.22.1398 |
| 33. | Scriven, E. F. V.; Turnbull, K. Chem. Rev. 1988, 88, 297–368. doi:10.1021/cr00084a001 |
© 2018 Coutant et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)