Abstract
Friedel–Crafts (FC) acylation reactions were exploited in the preparation of ketone-functionalized aromatics. Environmentally more friendly, solvent-free mechanochemical reaction conditions of this industrially important reaction were developed. Reaction parameters such as FC catalyst, time, ratio of reagents and milling support were studied to establish the optimal reaction conditions. The scope of the reaction was explored by employment of different aromatic hydrocarbons in conjunction with anhydrides and acylation reagents. It was shown that certain FC-reactive aromatics could be effectively functionalized by FC acylations carried out under ball-milling conditions without the presence of a solvent. The reaction mechanism was studied by in situ Raman and ex situ IR spectroscopy.
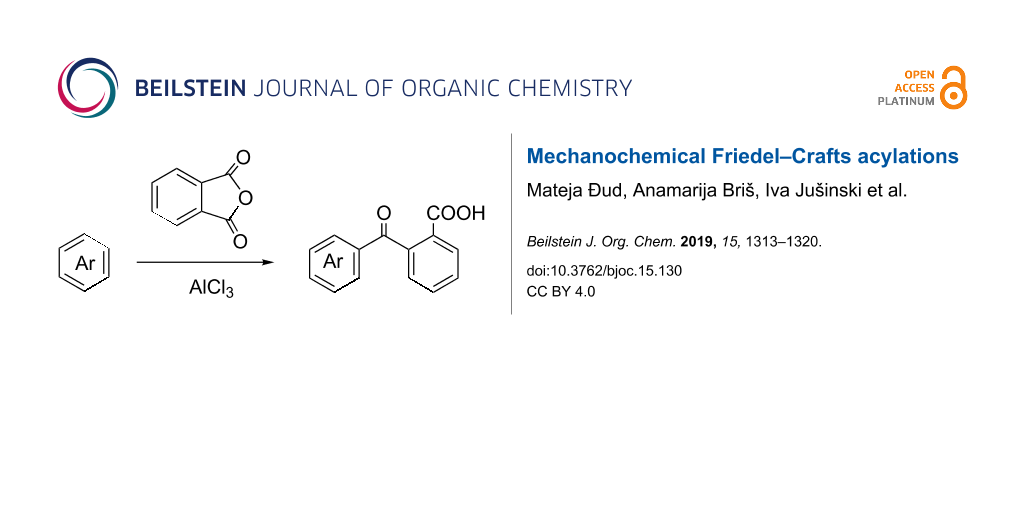
Graphical Abstract
Introduction
The Friedel–Crafts reaction (FCR) is a very powerful tool in organic chemistry for the synthesis of aromatic ketones. It is of great industrial importance and widely used in fine chemicals production [1,2]. In recent years, public awareness of the negative impact of chemical processes on the environment instigates chemists to improve processes by the reduction of waste material, energy consumption and reagents (materials). In this respect, carrying out FCR at room temperature without the use of solvents, which are usually highly toxic (halogenated hydrocarbons) will improve the eco-friendliness of the process. Until now, FCRs have been rarely applied to organic functionalizations which are carried out in solid state by mortar and pestle [3-5]. We are aware of only a few examples of FCRs employing manual grinding: reserpine acylation with AlCl3 [6] and acylation reaction of aromatics [7]. One of the reasons for this scarcity is the hygroscopic nature of the aluminum trichloride catalyst [8-12] when exposed to air humidity. This problem could be easily avoided by conducting the reaction in a closed vessel, by the aid of automated ball milling, which became a very effective synthetic method in recent time [13-18]. The first account on mechanochemical FC alkylation by Borchardt [19] demonstrates the utility of the mechanochemical method in the synthesis of covalent triazine frameworks. Herein, we report related results on solvent-free FC acylation reactions conducted in a ball mill, which is the continuation of our program in organic mechanosynthesis [20-24].
Results and Discussion
Mechanochemical FCR of pyrene (1) and phthalic anhydride (2) producing 1-(o-carboxybenzoyl)pyrene (3) was selected for the optimization of reaction conditions since all reagents and catalyst are solids (Scheme 1, Table 1) [25]. In solution, this reaction is facile and the product could be obtained in quantitative yield (Table 1, entry 15). The results on optimization of reaction conditions in the ball mill indicate that FC acylation could be effectively carried out mechanochemically. The best mechanochemical reaction conditions (Table 1, entry 4): 2 h, equimolar amount of phthalic anhydride and 2.5 equivalents of AlCl3, afforded product 3 in high yield (79%). Identical yields were obtained by the change of reaction time to 1 h and alternative work-up procedures (Table 1, entries 1 and 4). When the catalyst amount was decreased to one equivalent, a significant decrease of yield was attained (Table 1, entry 2). Addition of various grinding additives to improve mass transfer and prevent pasting of the reaction mixture [26-28] (Table 1, entries 5–8) was detrimental to reaction yields. The addition of a small amount of solvents which was reported to facilitate several ball milling reactions (liquid assisted grinding, LAG) [29-32], also decreased yields (Table 1, entries 9 and 10). The reaction carried out in a planetary mill (Table 1, entry 11) afforded yields comparable to the MM400 vibrational mill. We have also performed screening of efficacy of various Lewis acid catalysts [33-38] (Table 1, entries 18–23), which did not lead to formation of products.
Scheme 1: FCR of pyrene and phthalic anhydride.
Scheme 1: FCR of pyrene and phthalic anhydride.
Table 1: Reaction of pyrene with phthalic anhydridea.
| Entry | Conditions | Work-upb | Yield (%)c |
| 1 | 1 h | B | 78 |
| 2 | 1 h, ratio 1:1:1 | A | 44 |
| 3 | 1 h | A | 76 |
| 4 | 2 h | A | 79 |
| 5 | 1 h, silicagel 1 g | A | n.r. |
| 6 | 1 h, dry silicagel 0.5 g | A | 42 |
| 7 | 1 h, dry NaCl 0.5 g | A | 37 |
| 8 | 1 h, dry Na2SO4 0.5 g | A | 43 |
| 9 | 1 h, LAG dry DCM | A | 51 |
| 10 | 1 h, LAG dry THF | A | 16 |
| 11 | 1 h, planetary milld | A | 79 |
| 12 | 1 h, teflon jar | A | 71 |
| 13 | 3 h, reflux, dry DCM | B | 94 [39] |
| 14 | 1 h, reflux, dry DCM | B | 98 |
| 15 | 1 h, reflux, dry DCM | A | 83 |
| 16 | 1 h, rt, dry DCM | A | 99 |
| 17 | 10 min, melt, 180 °C, dry NaCl | C [40] | n.r.e |
| 18 | 1 h, FeCl3 | A | n.r. |
| 19 | 1 h, ZnCl2 | A | n.r. |
| 20 | 1 h, ZnI2 | A | n.r. |
| 21 | 1 h, ZnBr2 | A | n.r. |
| 22 | 1 h, CuBr2 | A | n.r. |
| 23 | 1 h, CuCl2 | A | n.r. |
| 24 | 3 h, scale-up | A | 73f |
aRetsch MM400 ball mill, 16 mL stainless steel vial, 1 × 12 mm stainless steel ball, 30 Hz, substrate/anhydride/AlCl3 ratio 1:1:2.5; bWork-up A: mixture suspended in H2O, pH adjusted with conc. HCl, chromatography; work-up B: identical to work-up A, but recrystallisation from AcOH instead of chromatography; work-up C: suspended in aq oxalic acid, extracted with DCM, chromatography; cisolated yields; dRetsch planetary ball mill PM-200, 500 rpm, 25 mL stainless steel vial, 30 × 3 mm steel balls; emelted in open flask; fscaled up to 500 mg of pyrene.
Experiments collected in Table 1 demonstrate that a FC acylation reaction could be effectively carried out under ball-milling conditions at room temperature without the use of solvent. This reaction could be easily scaled up from 94 to 500 mg of pyrene without the decrease in yield (Table 1, entry 24) [41,42]. To investigate the scope of the reaction, several acylation reagents were employed in conjunction with pyrene (Scheme 2) and a variety of aromatic substrates was subjected to FC acylation (Scheme 3).
Scheme 2: Scope of acylation reagents in FCR under mechanochemical activation conditions and comparison with other reaction conditions (isolated yields); aconversion from NMR analysis; bsolution reaction in flask, substrate/acylation reagent/AlCl3 ratio is 1:1:2.5; ball-milling details are given in Table 1.
Scheme 2: Scope of acylation reagents in FCR under mechanochemical activation conditions and comparison with ...
Scheme 3: Scope of aromatic substrates in FCR under mechanochemical activation conditions. aIsolated yields.
Scheme 3: Scope of aromatic substrates in FCR under mechanochemical activation conditions. aIsolated yields.
Acylation reagents shown in Scheme 2 were less reactive in comparison to phthalic anhydride. Benzoic anhydride was used as a substitute for benzoyl chloride and the reaction proceeded in better yield. The observed disparity in reactivity might be associated with the difference in the physical state of the reagents. Furthermore, succinic anhydride poorly reacted with pyrene, but the reaction proceeds well with the more reactive biphenyl (69%, see Supporting Information File 1). Di-tert-butyl dicarbonate and 4-nitrobenzoyl chloride were unreactive under ball-milling conditions. Similary unreactive was 4-nitrophthalic anhydride, which only in forced conditions (by melting at 200 °C) reacted sluggishly with pyrene affording mixture of regioisomeric products 6 and 7. The advantage of the employment of mechanochemical conditions is evidenced by solid state milling of pyrene with succinic anhydride which showed remarkably better performance than the reaction carried out in solution (40% vs 6% yield).
The screening of substrates showed disparate reactivities, ranging from quantitative to low (Scheme 3). Most rewarding are reactions of toluene, o-xylene, naphthalene and tetralin. Interestingly, ball milling of 4-ethylanisole provided phenol 12, in which acylation was accompanied with the cleavage of the methoxy group [43-45]. A striking advantage of the automated ball milling over manual grinding [46] is evident in the reaction of anthracene with phthalic anhydride which gave no product by manual grinding and the yield of the toluene reaction is increased from 68% to 92%.
When anthracene was subjected to a milling reaction with succinic anhydride, 9-substituted product 22 was obtained in low yield, and accompanied with a small amount of 2-acylated product 23 (Scheme 4), with same regioselectivity to that reported in the literature [47,48]. FC acylation at the 2-position of anthracene was achieved by Levy by the employment of 9,10-dihydroanthracene and subsequent oxidation to anthracene. To direct the acylation towards the 2-position, we devised the use of anthracene photodimer 19 [49] for the protection of 9,10-positions. The photodimer would act as 9,10-dihydroanthracene, and 2-acylated product should be regioselectively formed, which could be converted by thermal retrocyclization via flash vacuum pyrolysis (FVP) [50,51] to 23. However, ball milling of 19 with 20 provided 95% conversion of 19 to anthracene, with a small amount (<5%) of 22. This result indicates that rapid [4π – 4π] cycloreversion of 19 takes place, even in solid state ball-milling conditions at room temperature. Produced anthracene then subsequently participates in FCR. In control reaction of milling of photodimer 19 itself for 1 h was converted to anthracene in 95% yield. This [4π + 4π] cycloreversion in mechanochemical conditions is analogous to previously described dissociation of labile anthracene/C60 cycloadduct [52]. When the reaction of 19 with 20 was carried out in solution (DCM, overnight), 60% of dimer was converted to anthracene, and traces of FC product 22 were observed. Further attempts were made to lower the reaction temperature by cryomilling [53] (reaction vessel was cooled down by dipping into liquid nitrogen every 3–5 min, and ball milled for 30 min in total). This procedure partially suppressed cycloreversion and led to the mixture of 19 and 18 (3:2 ratio), accompanied with a small amount of 22.
Scheme 4: Mechanochemical regiodirected FCR of anthracene dimer and succinic anhydride.
Scheme 4: Mechanochemical regiodirected FCR of anthracene dimer and succinic anhydride.
As a substitute for dianthracene 19, thermally more stable substrate, anthracene-N-methyl maleimide adduct 25 [54] was prepared by Diels–Alder reaction under high pressure conditions as well as by microwave-assisted reaction and mechanochemically (Scheme 5). In this molecule, N-methylmaleimide could be used as protection of the 9,10-positions of anthracene and then removed by FVP. We thought that the maleimide moiety will not be affected in the FC acylation, since the precedencies exist in the literature on imide moiety withstanding the FC reaction [55,56]. However, mechanochemical reaction of 25 with succinic anhydride and 2.5 equiv of AlCl3 showed no reaction and the increase of the excess of catalyst to 5 equiv gave a very complex mixture.
Scheme 5: Regioselectivity direction by protection of 9,10-anthracene ring positions.
Scheme 5: Regioselectivity direction by protection of 9,10-anthracene ring positions.
Phthaloyl chloride was applied in mechanochemical FCR with the goal of obtaining a double reaction leading to the anthraquinone core in a single reaction pot in solid state [57,58]. Indeed, milling of p-xylene, AlCl3 and phthaloyl chloride led to the formation of a mixture of 10 and intramolecular FC product 29 [59] in a 1:3 ratio (Scheme 6). The ratio of 1,4-dimethylanthraquinone (29) did not increase in the presence of 5 equivalents of AlCl3. Formation and ratio of these two products could be conveniently established by 1H NMR analysis, due to a difference in the symmetry of products: there are two methyl signals for 10 and a single methyl line at δ 2.81 ppm in the case of 29. Pyrene and naphthalene were less reactive under the same ball milling conditions and reactions stopped at the stage of formation of product 3 and 15. One-step preparation of quinone 30 [39], was achieved by melting reactants at 140 °C for 10 min. Under these conditions, a mixture of adducts 3 and 30 (1.5:1 ratio) was obtained. The product ratio was established by 1H NMR analysis of the characteristic H-10 proton signal of product 3 (peak resonance doublet at δ 9.2 ppm), which is shifted towards lower magnetic field in quinone 30 (δ 10.0 ppm), and concurrent appearance of singlet for H-3 at δ 9.1 ppm. These experiments demonstrate that quinones could be prepared by simple one-pot FC protocols in the case of reactive aromatics.
Scheme 6: Double FCR of phthaloyl chloride and aromatics.
Scheme 6: Double FCR of phthaloyl chloride and aromatics.
In situ Raman spectroscopy [60] was applied to study mechanistic aspects of the solid state reaction of phthalic anhydride with p-xylene. Raman spectra were simulated and positions of signals for transient reactive intermediates were predicted by density functional theory method B3LYP/6-31G* (Supporting Information File 1) [61]. The stretching of the +C≡O bond of the acylium ion was predicted to be at about 2300 cm−1. Raman spectroscopy revealed that the complexation of phthalic anhydride with AlCl3 is rapid, and within 3 minutes of milling all anhydride is consumed (Figure 1). After 3 minutes of milling, high fluorescence prevents further following of the reaction progress. These spectra indicate that rapid complexation of anhydride with AlCl3 takes place, whereas the formation of the acylium ion intermediate could not be unequivocally verified.
![[1860-5397-15-130-1]](/bjoc/content/figures/1860-5397-15-130-1.png?scale=2.0&max-width=1024&background=FFFFFF)
Figure 1: In situ Raman monitoring of reaction of phthalic anhydride with p-xylene.
Figure 1: In situ Raman monitoring of reaction of phthalic anhydride with p-xylene.
Similar conclusions could be drawn from ex situ IR spectroscopy [62] which indicates rapid complexation and disappearance of phthalic anhydride (Supporting Information File 1, Figures S43 and S44). A further study was carried on complexation of phthalic anhydride with AlCl3 (Supporting Information File 1, Figure S45). Although there are weak signals at 2300 and 3050 cm−1 which could be associated with the acylium ion and the intermediate cation, the raise of intensities of these signals over the time is quite unlikely to come from reactive species (time needed to transfer sample from ball mill to IR spectrophotometer and acquire spectra are within several minutes, which could be detrimental to reactive species to survive in the open air). These signals are not visible after the standard acidic work-up and further study would require the use of in situ IR spectroscopy in solution [63].
Conclusion
In conclusion, the experimental results demonstrate that Friedel–Crafts acylations could be effectively carried out under solid state ball-milling conditions. The reaction takes place by the initial complexation of the carbonyl group of the acylation reagent with aluminium trichloride.
Supporting Information
| Supporting Information File 1: Details of experimental procedures, spectroscopic characterization data of compounds and computational procedures. | ||
| Format: PDF | Size: 3.0 MB | Download |
References
-
Olah, G. A.; Reddy, V. P.; Prakash, G. K. S. Friedel-Crafts Reactions. Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: New York, 2000. doi:10.1002/0471238961.0618090515120108.a01
Return to citation in text: [1] -
Sartori, G.; Maggi, R. Advances in Friedel-Crafts Acylation Reactions: Catalytic and Green Processes; CRC Press: Boca Raton, FL, U.S.A., 2009. doi:10.1201/9781420067934
Return to citation in text: [1] -
Toda, F., Ed. Organic Solid-State Reactions; Topics in Current Chemistry, Vol. 254; Springer: Heidelberg, Germany, 2005. doi:10.1007/b98357
Return to citation in text: [1] -
Tanaka, K.; Toda, F. Chem. Rev. 2000, 100, 1025–1074. doi:10.1021/cr940089p
Return to citation in text: [1] -
Tanaka, K. Solvent-free Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2003. doi:10.1002/3527601821
Return to citation in text: [1] -
Begum, S.; Zehra, S. Q.; Siddiqui, B. S. Synth. Commun. 2006, 36, 3203–3224. doi:10.1080/00397910600908900
Return to citation in text: [1] -
Matlack, A. Introduction to Green Chemistry; CRC Press: Boca Raton, 2010; pp 219 ff.
Return to citation in text: [1] -
Pivsa-Art, S.; Okuro, K.; Miura, M.; Murata, S.; Nomura, M. J. Chem. Soc., Perkin Trans. 1 1994, 1703–1707. doi:10.1039/p19940001703
AlCl3 is the most common Lewis acid employed in FCR. Other LA catalysts were applied in solution reactions. See this reference for: InCl3, SbCl5, TiCl4, FeCl3, SnCl4, ZnCl2.
Return to citation in text: [1] -
Garkhedkar, A. M.; Senadi, G. C.; Wang, J.-J. Org. Lett. 2017, 19, 488–491. doi:10.1021/acs.orglett.6b03642
See for ZnBr2.
Return to citation in text: [1] -
Makarov, A. S.; Kekhvaeva, A. E.; Hall, C. J. J.; Price, D. R.; Trushkov, I. V.; Uchuskin, M. G. Tetrahedron 2017, 73, 7042–7053. doi:10.1016/j.tet.2017.10.054
See for CuBr2.
Return to citation in text: [1] -
Ichikawa, K.; Chano, K.; Inoue, M.; Sugita, T. Bull. Chem. Soc. Jpn. 1982, 55, 3039–3040. doi:10.1246/bcsj.55.3039
See for CuCl2.
Return to citation in text: [1] -
Peng, C.; Zhang, J.; Xue, J.; Li, S.; Wang, X.-N.; Chang, J. J. Org. Chem. 2018, 83, 9256–9266. doi:10.1021/acs.joc.8b01255
See for ZnI2.
Return to citation in text: [1] -
Ball Milling Towards Green Synthesis: Applications, Projects, Challenges; Stolle, A.; Ranu, B., Eds.; RSC Green Chemistry, Vol. 31; Royal Society of Chemistry: Cambridge, UK, 2015. doi:10.1039/9781782621980
Return to citation in text: [1] -
Wang, G.-W. Chem. Soc. Rev. 2013, 42, 7668–7700. doi:10.1039/c3cs35526h
Return to citation in text: [1] -
Stolle, A.; Szuppa, T.; Leonhardt, S. E. S.; Ondruschka, B. Chem. Soc. Rev. 2011, 40, 2317–2329. doi:10.1039/c0cs00195c
Return to citation in text: [1] -
James, S. L.; Adams, C. J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K. D. M.; Hyett, G.; Jones, W.; Krebs, A.; Mack, J.; Maini, L.; Orpen, A. G.; Parkin, I. P.; Shearouse, W. C.; Steed, J. W.; Waddell, D. C. Chem. Soc. Rev. 2012, 41, 413–447. doi:10.1039/c1cs15171a
Return to citation in text: [1] -
Kaupp, G. CrystEngComm 2009, 11, 388–403. doi:10.1039/b810822f
Return to citation in text: [1] -
Margetić, D.; Štrukil, V. Practical Considerations in Mechanochemical Organic Synthesis; Mechanochemical Organic Synthesis; Elsevier: Amsterdam, Netherlands, 2016; pp 1–54. doi:10.1016/b978-0-12-802184-2.00001-7
Return to citation in text: [1] -
Troschke, E.; Grätz, S.; Lübken, T.; Borchardt, L. Angew. Chem., Int. Ed. 2017, 56, 6859–6863. doi:10.1002/anie.201702303
Return to citation in text: [1] -
Briš, A.; Đud, M.; Margetić, D. Beilstein J. Org. Chem. 2017, 13, 1745–1752. doi:10.3762/bjoc.13.169
Return to citation in text: [1] -
Glasovac, Z.; Trošelj, P.; Jušinski, I.; Margetić, D.; Eckert-Maksić, M. Synlett 2013, 24, 2540–2544. doi:10.1055/s-0033-1339876
Return to citation in text: [1] -
Štrukil, V.; Sajko, I. Chem. Commun. 2017, 53, 9101–9104. doi:10.1039/c7cc03510a
Return to citation in text: [1] -
Portada, T.; Margetić, D.; Štrukil, V. Molecules 2018, 23, No. 3163. doi:10.3390/molecules23123163
Return to citation in text: [1] -
Đud, M.; Margetić, D. Int. J. Org. Chem. 2017, 7, 140–144. doi:10.4236/ijoc.2017.72011
Return to citation in text: [1] -
Caution: Aluminium trichloride dust is very irritant and corrosive and reacts violently with water. For its handling appropriate protection measures should be implemented (Supporting Information File 1).
Return to citation in text: [1] -
Howard, J. L.; Sagatov, Y.; Browne, D. L. Tetrahedron 2018, 74, 3118–3123. doi:10.1016/j.tet.2017.11.066
Return to citation in text: [1] -
Yu, J.; Hong, Z.; Yang, X.; Jiang, Y.; Jiang, Z.; Su, W. Beilstein J. Org. Chem. 2018, 14, 786–795. doi:10.3762/bjoc.14.66
Return to citation in text: [1] -
Su, W.; Yu, J.; Li, Z.; Jiang, Z. J. Org. Chem. 2011, 76, 9144–9150. doi:10.1021/jo2015533
Return to citation in text: [1] -
Friščić, T.; Trask, A. V.; Jones, W.; Motherwell, W. D. S. Angew. Chem., Int. Ed. 2006, 45, 7546–7550. doi:10.1002/anie.200603235
Return to citation in text: [1] -
Denlinger, K. L.; Ortiz-Trankina, L.; Carr, P.; Benson, K.; Waddell, D. C.; Mack, J. Beilstein J. Org. Chem. 2018, 14, 688–696. doi:10.3762/bjoc.14.57
Return to citation in text: [1] -
Gonnet, L.; Tintillier, T.; Venturini, N.; Konnert, L.; Hernandez, J.-F.; Lamaty, F.; Laconde, G.; Martinez, J.; Colacino, E. ACS Sustainable Chem. Eng. 2017, 5, 2936–2941. doi:10.1021/acssuschemeng.6b02439
Return to citation in text: [1] -
Howard, J. L.; Brand, M. C.; Browne, D. L. Angew. Chem., Int. Ed. 2018, 57, 16104–16108. doi:10.1002/anie.201810141
Return to citation in text: [1] -
Liu, M.; Wu, L. Faming Zhuanli Shenqing 106905136, Jun 30, 2017. See for FeCl3 employed in FCR acylations with phthalic anhydride in solution.
Return to citation in text: [1] -
Clar, E. Chem. Ber. 1948, 81, 169–175. doi:10.1002/cber.19480810215
See for ZnCl2 employed in FCR acylations with phthalic anhydride in solution.
Return to citation in text: [1] -
Kehoe, T. D.; Sabnis, R. W.; Balchunis, R. J. Oral care compositions with color changing indicator. PCT Int. Appl. WO2006105260, Oct 5, 2006.
Chem. Abstr. 2006, 145, 397365. See for ZnCl2 employed in FCR acylations with phthalic anhydride in solution.
Return to citation in text: [1] -
Nakamura, H.; Tanaka, N.; Matsuhashi, H. J. Jpn. Pet. Inst. 2010, 53, 276–282. doi:10.1627/jpi.53.276
See for sulfated ZrO2 employed in FC acylations with phthalic anhydride in solution.
Return to citation in text: [1] -
Madje, B. R.; Shelke, K. F.; Sapkal, S. B.; Kakade, G. K.; Shingare, M. S. Green Chem. Lett. Rev. 2010, 3, 269–273. doi:10.1080/17518251003776877
See for sulfated ZrO2 employed in FCR acylations with phthalic anhydride in solution.
Return to citation in text: [1] -
Maeimi, H.; Brojerdi, S. S. Polycyclic Aromat. Compd. 2014, 34, 504–517. doi:10.1080/10406638.2014.910238
See for SiO2/sulfuric acid employed in FCR acylations with phthalic anhydride in solution.
Return to citation in text: [1] -
Casas-Solvas, J. M.; Mooibroek, T. J.; Sandramurthy, S.; Howgego, J. D.; Davis, A. P. Synlett 2014, 25, 2591–2594. doi:10.1055/s-0034-1379026
Return to citation in text: [1] [2] -
Arcamone, F.; Bernardi, L.; Patelli, B.; Giardino, P.; Di Marco, A.; Casazza, A. M.; Soranzo, C.; Pratesi, G. Experientia 1978, 34, 1255–1257. doi:10.1007/bf01981401
Return to citation in text: [1] -
Andersen, J.; Mack, J. Green Chem. 2018, 20, 1435–1443. doi:10.1039/c7gc03797j
Return to citation in text: [1] -
Kaupp, G.; Funk, B.; Benz, H. U.; Heupel, A.; Zoz, H. Conference paper APMA-2017. The 4th International Conference on Powder Metallurgy in Asia, Hsinchu, Taiwan, April 9–11, 2017.
Return to citation in text: [1] -
Sato, H.; Dan, T.; Onuma, E.; Tanaka, H.; Aoki, B.; Koga, H. Chem. Pharm. Bull. 1991, 39, 1760–1772. doi:10.1248/cpb.39.1760
Methyl ether cleavage is a common process in Friedel–Crafts reactions with AlCl3, when acylation occurs at the ortho-position. See also references [40,44,45].
Return to citation in text: [1] -
Sato, H.; Kuromaru, K.; Ishizawa, T.; Aoki, B.; Koga, H. Chem. Pharm. Bull. 1992, 40, 2597–2601. doi:10.1248/cpb.40.2597
Return to citation in text: [1] [2] -
Saha, K.; Lajis, N. H.; Abas, F.; Naji, N. A.; Hamzah, A. S.; Shaari, K. Aust. J. Chem. 2008, 61, 821–825. doi:10.1071/ch08084
Return to citation in text: [1] [2] -
Ghiaci, M.; Asghari, J. Synth. Commun. 1998, 28, 2213–2220. doi:10.1080/00397919808007036
Return to citation in text: [1] -
Wiznycia, A. V.; Desper, J.; Levy, C. J. Dalton Trans. 2007, 1520–1527. doi:10.1039/b700001d
Return to citation in text: [1] -
Schoental, R. J. Chem. Soc. 1952, 4403–4406. doi:10.1039/jr9520004403
Return to citation in text: [1] -
Breton, G. W.; Vang, X. J. Chem. Educ. 1998, 75, 81–82. doi:10.1021/ed075p81
Return to citation in text: [1] -
Margetić, D.; Butler, D. N.; Warrener, R. N.; Murata, Y. Tetrahedron 2011, 67, 1580–1588. doi:10.1016/j.tet.2010.12.032
Return to citation in text: [1] -
Margetić, D.; Butler, D. N.; Warrener, R. N. Synlett 2013, 24, 2609–2613. doi:10.1055/s-0033-1339879
Return to citation in text: [1] -
Murata, Y.; Kato, N.; Fujiwara, K.; Komatsu, K. J. Org. Chem. 1999, 64, 3483–3488. doi:10.1021/jo990013z
Return to citation in text: [1] -
Waddell, D. C.; Mack, J. Green Chem. 2009, 11, 79–82. doi:10.1039/b810714a
Return to citation in text: [1] -
Alibert, S.; Santelli-Rouvier, C.; Castaing, M.; Berthelot, M.; Spengler, G.; Molnar, J.; Barbe, J. Eur. J. Med. Chem. 2003, 38, 253–263. doi:10.1016/s0223-5234(03)00018-7
Return to citation in text: [1] -
Reifenrath, W. G.; Bertelli, D. J.; Micklus, M. J.; Fries, D. S. Tetrahedron Lett. 1976, 17, 1959–1962. doi:10.1016/s0040-4039(00)78089-0
Return to citation in text: [1] -
Xu, Q.; Wang, G.; Wang, X.; Wu, T.; Pan, X.; Chan, A. S. C.; Yang, T.-K. Tetrahedron: Asymmetry 2000, 11, 2309–2314. doi:10.1016/s0957-4166(00)00193-2
Return to citation in text: [1] -
Reference [2], p. 43: Product 29 was also prepared by double acylation reaction of p-xylene with phthalic anhydride or with phthaloyl chloride using TfOH.
Return to citation in text: [1] -
Sartori, G.; Casnati, G.; Bigi, F.; Foglio, F. Gazz. Chim. Ital. 1990, 120, 13–19.
Return to citation in text: [1] -
Rosenfeld, S.; VanDyke, S. J. Chem. Educ. 1991, 68, 691–692. doi:10.1021/ed068p691
Return to citation in text: [1] -
Gracin, D.; Štrukil, V.; Friščić, T.; Halasz, I.; Užarević, K. Angew. Chem., Int. Ed. 2014, 53, 6193–6197. doi:10.1002/anie.201402334
Return to citation in text: [1] -
Comparison of signals obtained experimentally was performed with Raman spectra calculated at the B3LYP/6-31G* level and corrected by scaling factor of 0.9614.
Return to citation in text: [1] -
Đud, M.; Glasovac, Z.; Margetić, D. Tetrahedron 2019, 75, 109–115. doi:10.1016/j.tet.2018.11.038
Return to citation in text: [1] -
Huang, Z.; Jin, L.; Han, H.; Lei, A. Org. Biomol. Chem. 2013, 11, 1810–1814. doi:10.1039/c3ob27094g
Further study would require in situ IR spectroscopy in solution. See for details.
Return to citation in text: [1]
| 60. | Gracin, D.; Štrukil, V.; Friščić, T.; Halasz, I.; Užarević, K. Angew. Chem., Int. Ed. 2014, 53, 6193–6197. doi:10.1002/anie.201402334 |
| 61. | Comparison of signals obtained experimentally was performed with Raman spectra calculated at the B3LYP/6-31G* level and corrected by scaling factor of 0.9614. |
| 62. | Đud, M.; Glasovac, Z.; Margetić, D. Tetrahedron 2019, 75, 109–115. doi:10.1016/j.tet.2018.11.038 |
| 1. | Olah, G. A.; Reddy, V. P.; Prakash, G. K. S. Friedel-Crafts Reactions. Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: New York, 2000. doi:10.1002/0471238961.0618090515120108.a01 |
| 2. | Sartori, G.; Maggi, R. Advances in Friedel-Crafts Acylation Reactions: Catalytic and Green Processes; CRC Press: Boca Raton, FL, U.S.A., 2009. doi:10.1201/9781420067934 |
| 8. |
Pivsa-Art, S.; Okuro, K.; Miura, M.; Murata, S.; Nomura, M. J. Chem. Soc., Perkin Trans. 1 1994, 1703–1707. doi:10.1039/p19940001703
AlCl3 is the most common Lewis acid employed in FCR. Other LA catalysts were applied in solution reactions. See this reference for: InCl3, SbCl5, TiCl4, FeCl3, SnCl4, ZnCl2. |
| 9. |
Garkhedkar, A. M.; Senadi, G. C.; Wang, J.-J. Org. Lett. 2017, 19, 488–491. doi:10.1021/acs.orglett.6b03642
See for ZnBr2. |
| 10. |
Makarov, A. S.; Kekhvaeva, A. E.; Hall, C. J. J.; Price, D. R.; Trushkov, I. V.; Uchuskin, M. G. Tetrahedron 2017, 73, 7042–7053. doi:10.1016/j.tet.2017.10.054
See for CuBr2. |
| 11. |
Ichikawa, K.; Chano, K.; Inoue, M.; Sugita, T. Bull. Chem. Soc. Jpn. 1982, 55, 3039–3040. doi:10.1246/bcsj.55.3039
See for CuCl2. |
| 12. |
Peng, C.; Zhang, J.; Xue, J.; Li, S.; Wang, X.-N.; Chang, J. J. Org. Chem. 2018, 83, 9256–9266. doi:10.1021/acs.joc.8b01255
See for ZnI2. |
| 41. | Andersen, J.; Mack, J. Green Chem. 2018, 20, 1435–1443. doi:10.1039/c7gc03797j |
| 42. | Kaupp, G.; Funk, B.; Benz, H. U.; Heupel, A.; Zoz, H. Conference paper APMA-2017. The 4th International Conference on Powder Metallurgy in Asia, Hsinchu, Taiwan, April 9–11, 2017. |
| 7. | Matlack, A. Introduction to Green Chemistry; CRC Press: Boca Raton, 2010; pp 219 ff. |
| 43. |
Sato, H.; Dan, T.; Onuma, E.; Tanaka, H.; Aoki, B.; Koga, H. Chem. Pharm. Bull. 1991, 39, 1760–1772. doi:10.1248/cpb.39.1760
Methyl ether cleavage is a common process in Friedel–Crafts reactions with AlCl3, when acylation occurs at the ortho-position. See also references [40,44,45]. |
| 44. | Sato, H.; Kuromaru, K.; Ishizawa, T.; Aoki, B.; Koga, H. Chem. Pharm. Bull. 1992, 40, 2597–2601. doi:10.1248/cpb.40.2597 |
| 45. | Saha, K.; Lajis, N. H.; Abas, F.; Naji, N. A.; Hamzah, A. S.; Shaari, K. Aust. J. Chem. 2008, 61, 821–825. doi:10.1071/ch08084 |
| 6. | Begum, S.; Zehra, S. Q.; Siddiqui, B. S. Synth. Commun. 2006, 36, 3203–3224. doi:10.1080/00397910600908900 |
| 39. | Casas-Solvas, J. M.; Mooibroek, T. J.; Sandramurthy, S.; Howgego, J. D.; Davis, A. P. Synlett 2014, 25, 2591–2594. doi:10.1055/s-0034-1379026 |
| 3. | Toda, F., Ed. Organic Solid-State Reactions; Topics in Current Chemistry, Vol. 254; Springer: Heidelberg, Germany, 2005. doi:10.1007/b98357 |
| 4. | Tanaka, K.; Toda, F. Chem. Rev. 2000, 100, 1025–1074. doi:10.1021/cr940089p |
| 5. | Tanaka, K. Solvent-free Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2003. doi:10.1002/3527601821 |
| 40. | Arcamone, F.; Bernardi, L.; Patelli, B.; Giardino, P.; Di Marco, A.; Casazza, A. M.; Soranzo, C.; Pratesi, G. Experientia 1978, 34, 1255–1257. doi:10.1007/bf01981401 |
| 25. | Caution: Aluminium trichloride dust is very irritant and corrosive and reacts violently with water. For its handling appropriate protection measures should be implemented (Supporting Information File 1). |
| 29. | Friščić, T.; Trask, A. V.; Jones, W.; Motherwell, W. D. S. Angew. Chem., Int. Ed. 2006, 45, 7546–7550. doi:10.1002/anie.200603235 |
| 30. | Denlinger, K. L.; Ortiz-Trankina, L.; Carr, P.; Benson, K.; Waddell, D. C.; Mack, J. Beilstein J. Org. Chem. 2018, 14, 688–696. doi:10.3762/bjoc.14.57 |
| 31. | Gonnet, L.; Tintillier, T.; Venturini, N.; Konnert, L.; Hernandez, J.-F.; Lamaty, F.; Laconde, G.; Martinez, J.; Colacino, E. ACS Sustainable Chem. Eng. 2017, 5, 2936–2941. doi:10.1021/acssuschemeng.6b02439 |
| 32. | Howard, J. L.; Brand, M. C.; Browne, D. L. Angew. Chem., Int. Ed. 2018, 57, 16104–16108. doi:10.1002/anie.201810141 |
| 2. | Sartori, G.; Maggi, R. Advances in Friedel-Crafts Acylation Reactions: Catalytic and Green Processes; CRC Press: Boca Raton, FL, U.S.A., 2009. doi:10.1201/9781420067934 |
| 20. | Briš, A.; Đud, M.; Margetić, D. Beilstein J. Org. Chem. 2017, 13, 1745–1752. doi:10.3762/bjoc.13.169 |
| 21. | Glasovac, Z.; Trošelj, P.; Jušinski, I.; Margetić, D.; Eckert-Maksić, M. Synlett 2013, 24, 2540–2544. doi:10.1055/s-0033-1339876 |
| 22. | Štrukil, V.; Sajko, I. Chem. Commun. 2017, 53, 9101–9104. doi:10.1039/c7cc03510a |
| 23. | Portada, T.; Margetić, D.; Štrukil, V. Molecules 2018, 23, No. 3163. doi:10.3390/molecules23123163 |
| 24. | Đud, M.; Margetić, D. Int. J. Org. Chem. 2017, 7, 140–144. doi:10.4236/ijoc.2017.72011 |
| 33. | Liu, M.; Wu, L. Faming Zhuanli Shenqing 106905136, Jun 30, 2017. See for FeCl3 employed in FCR acylations with phthalic anhydride in solution. |
| 34. |
Clar, E. Chem. Ber. 1948, 81, 169–175. doi:10.1002/cber.19480810215
See for ZnCl2 employed in FCR acylations with phthalic anhydride in solution. |
| 35. |
Kehoe, T. D.; Sabnis, R. W.; Balchunis, R. J. Oral care compositions with color changing indicator. PCT Int. Appl. WO2006105260, Oct 5, 2006.
Chem. Abstr. 2006, 145, 397365. See for ZnCl2 employed in FCR acylations with phthalic anhydride in solution. |
| 36. |
Nakamura, H.; Tanaka, N.; Matsuhashi, H. J. Jpn. Pet. Inst. 2010, 53, 276–282. doi:10.1627/jpi.53.276
See for sulfated ZrO2 employed in FC acylations with phthalic anhydride in solution. |
| 37. |
Madje, B. R.; Shelke, K. F.; Sapkal, S. B.; Kakade, G. K.; Shingare, M. S. Green Chem. Lett. Rev. 2010, 3, 269–273. doi:10.1080/17518251003776877
See for sulfated ZrO2 employed in FCR acylations with phthalic anhydride in solution. |
| 38. |
Maeimi, H.; Brojerdi, S. S. Polycyclic Aromat. Compd. 2014, 34, 504–517. doi:10.1080/10406638.2014.910238
See for SiO2/sulfuric acid employed in FCR acylations with phthalic anhydride in solution. |
| 19. | Troschke, E.; Grätz, S.; Lübken, T.; Borchardt, L. Angew. Chem., Int. Ed. 2017, 56, 6859–6863. doi:10.1002/anie.201702303 |
| 63. |
Huang, Z.; Jin, L.; Han, H.; Lei, A. Org. Biomol. Chem. 2013, 11, 1810–1814. doi:10.1039/c3ob27094g
Further study would require in situ IR spectroscopy in solution. See for details. |
| 13. | Ball Milling Towards Green Synthesis: Applications, Projects, Challenges; Stolle, A.; Ranu, B., Eds.; RSC Green Chemistry, Vol. 31; Royal Society of Chemistry: Cambridge, UK, 2015. doi:10.1039/9781782621980 |
| 14. | Wang, G.-W. Chem. Soc. Rev. 2013, 42, 7668–7700. doi:10.1039/c3cs35526h |
| 15. | Stolle, A.; Szuppa, T.; Leonhardt, S. E. S.; Ondruschka, B. Chem. Soc. Rev. 2011, 40, 2317–2329. doi:10.1039/c0cs00195c |
| 16. | James, S. L.; Adams, C. J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K. D. M.; Hyett, G.; Jones, W.; Krebs, A.; Mack, J.; Maini, L.; Orpen, A. G.; Parkin, I. P.; Shearouse, W. C.; Steed, J. W.; Waddell, D. C. Chem. Soc. Rev. 2012, 41, 413–447. doi:10.1039/c1cs15171a |
| 17. | Kaupp, G. CrystEngComm 2009, 11, 388–403. doi:10.1039/b810822f |
| 18. | Margetić, D.; Štrukil, V. Practical Considerations in Mechanochemical Organic Synthesis; Mechanochemical Organic Synthesis; Elsevier: Amsterdam, Netherlands, 2016; pp 1–54. doi:10.1016/b978-0-12-802184-2.00001-7 |
| 26. | Howard, J. L.; Sagatov, Y.; Browne, D. L. Tetrahedron 2018, 74, 3118–3123. doi:10.1016/j.tet.2017.11.066 |
| 27. | Yu, J.; Hong, Z.; Yang, X.; Jiang, Y.; Jiang, Z.; Su, W. Beilstein J. Org. Chem. 2018, 14, 786–795. doi:10.3762/bjoc.14.66 |
| 28. | Su, W.; Yu, J.; Li, Z.; Jiang, Z. J. Org. Chem. 2011, 76, 9144–9150. doi:10.1021/jo2015533 |
| 40. | Arcamone, F.; Bernardi, L.; Patelli, B.; Giardino, P.; Di Marco, A.; Casazza, A. M.; Soranzo, C.; Pratesi, G. Experientia 1978, 34, 1255–1257. doi:10.1007/bf01981401 |
| 44. | Sato, H.; Kuromaru, K.; Ishizawa, T.; Aoki, B.; Koga, H. Chem. Pharm. Bull. 1992, 40, 2597–2601. doi:10.1248/cpb.40.2597 |
| 45. | Saha, K.; Lajis, N. H.; Abas, F.; Naji, N. A.; Hamzah, A. S.; Shaari, K. Aust. J. Chem. 2008, 61, 821–825. doi:10.1071/ch08084 |
| 46. | Ghiaci, M.; Asghari, J. Synth. Commun. 1998, 28, 2213–2220. doi:10.1080/00397919808007036 |
| 47. | Wiznycia, A. V.; Desper, J.; Levy, C. J. Dalton Trans. 2007, 1520–1527. doi:10.1039/b700001d |
| 48. | Schoental, R. J. Chem. Soc. 1952, 4403–4406. doi:10.1039/jr9520004403 |
| 59. | Rosenfeld, S.; VanDyke, S. J. Chem. Educ. 1991, 68, 691–692. doi:10.1021/ed068p691 |
| 39. | Casas-Solvas, J. M.; Mooibroek, T. J.; Sandramurthy, S.; Howgego, J. D.; Davis, A. P. Synlett 2014, 25, 2591–2594. doi:10.1055/s-0034-1379026 |
| 55. | Reifenrath, W. G.; Bertelli, D. J.; Micklus, M. J.; Fries, D. S. Tetrahedron Lett. 1976, 17, 1959–1962. doi:10.1016/s0040-4039(00)78089-0 |
| 56. | Xu, Q.; Wang, G.; Wang, X.; Wu, T.; Pan, X.; Chan, A. S. C.; Yang, T.-K. Tetrahedron: Asymmetry 2000, 11, 2309–2314. doi:10.1016/s0957-4166(00)00193-2 |
| 57. | Reference [2], p. 43: Product 29 was also prepared by double acylation reaction of p-xylene with phthalic anhydride or with phthaloyl chloride using TfOH. |
| 58. | Sartori, G.; Casnati, G.; Bigi, F.; Foglio, F. Gazz. Chim. Ital. 1990, 120, 13–19. |
| 54. | Alibert, S.; Santelli-Rouvier, C.; Castaing, M.; Berthelot, M.; Spengler, G.; Molnar, J.; Barbe, J. Eur. J. Med. Chem. 2003, 38, 253–263. doi:10.1016/s0223-5234(03)00018-7 |
| 50. | Margetić, D.; Butler, D. N.; Warrener, R. N.; Murata, Y. Tetrahedron 2011, 67, 1580–1588. doi:10.1016/j.tet.2010.12.032 |
| 51. | Margetić, D.; Butler, D. N.; Warrener, R. N. Synlett 2013, 24, 2609–2613. doi:10.1055/s-0033-1339879 |
| 52. | Murata, Y.; Kato, N.; Fujiwara, K.; Komatsu, K. J. Org. Chem. 1999, 64, 3483–3488. doi:10.1021/jo990013z |
© 2019 Đud et al.; licensee Beilstein-Institut.
This is an Open Access article under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0). Please note that the reuse, redistribution and reproduction in particular requires that the authors and source are credited.
The license is subject to the Beilstein Journal of Organic Chemistry terms and conditions: (https://www.beilstein-journals.org/bjoc)